Abstract
Wastewater contains large amounts of pharmaceuticals, pathogens, and antimicrobial resistance determinants. Only a little is known about the dissemination of resistance determinants and changes in soil microbial communities affected by wastewater irrigation. Community DNAs from Mezquital Valley soils under irrigation with untreated wastewater for 0 to 100 years were analyzed by quantitative real-time PCR for the presence of sul genes, encoding resistance to sulfonamides. Amplicon sequencing of bacterial 16S rRNA genes from community DNAs from soils irrigated for 0, 8, 10, 85, and 100 years was performed and revealed a 14% increase of the relative abundance of Proteobacteria in rainy season soils and a 26.7% increase in dry season soils for soils irrigated for 100 years with wastewater. In particular, Gammaproteobacteria, including potential pathogens, such as Pseudomonas, Stenotrophomonas, and Acinetobacter spp., were found in wastewater-irrigated fields. 16S rRNA gene sequencing of 96 isolates from soils irrigated with wastewater for 100 years (48 from dry and 48 from rainy season soils) revealed that 46% were affiliated with the Gammaproteobacteria (mainly potentially pathogenic Stenotrophomonas strains) and 50% with the Bacilli, whereas all 96 isolates from rain-fed soils (48 from dry and 48 from rainy season soils) were affiliated with the Bacilli. Up to six types of antibiotic resistance were found in isolates from wastewater-irrigated soils; sulfamethoxazole resistance was the most abundant (33.3% of the isolates), followed by oxacillin resistance (21.9% of the isolates). In summary, we detected an increase of potentially harmful bacteria and a larger incidence of resistance determinants in wastewater-irrigated soils, which might result in health risks for farm workers and consumers of wastewater-irrigated crops.
INTRODUCTION
Along with pharmaceuticals, wastewater can contain pathogenic microorganisms, including bacteria resistant to antimicrobial substances, and also antimicrobial resistance determinants (1–6). In arid and semiarid areas, wastewater is used for irrigation in agricultural production to alleviate water shortages (7–10). The coexistence of antibiotics, pathogens, and antibiotic resistance determinants in wastewater raises concerns that antibiotic resistance genes are mobilized from and disseminated into the environmental collection of antibiotic resistance genes (the environmental resistome) and transferred to bacteria that are potentially pathogenic to humans (11–13). The release of antibiotics together with the human-linked microbiota might be particularly important for the emergence of newly evolving antibiotic-resistant pathogens (1, 14). Environmental reservoirs for antibiotic resistances, especially those affected by anthropogenic activities (e.g., application of manure), can serve as “hot spots” for the spread of antibiotic resistance genes and antibiotic-resistant bacteria through food and water, with unknown consequences for human health (14–16). D'Costa and colleagues indicated that soil could serve as an underestimated reservoir for antibiotic resistance that has already emerged or has the potential to emerge in clinically important bacteria (17). The first report of a putative link between environmental and clinical antibiotic resistance determinants was published in 1973 by Benveniste and Davies. They detected high similarities between enzymes conferring gentamicin resistance from soil-associated Actinomycetes and enzymes that confer the same resistance in human pathogens, such as Escherichia coli and Pseudomonas aeruginosa (18). Recent studies have shown that the CTX-M β-lactamases potentially originated from the environmental bacterium Kluyvera ascorbata (19, 20). Furthermore, the plasmid-carried qnr genes, encoding fluoroquinolone resistance, originated from aquatic bacteria such as Shewanella algae (21–23). Fluoroquinolones are a family of broad-spectrum antibacterial agents that are active against a wide range of Gram-positive and Gram-negative bacteria. They act by inhibition of type II DNA topoisomerases (gyrases) that are required for bacterial DNA replication. Three mechanisms of resistance are known. Some types of efflux pumps act to decrease intracellular quinolone concentrations. In Gram-negative bacteria, plasmid-mediated resistance genes produce proteins that can bind to DNA gyrase, protecting it from the action of quinolones. In addition, mutations at key sites in DNA gyrase or topoisomerase IV can decrease the binding affinity for quinolones, decreasing the effectiveness of the drug (24).
There are strong indications for a link between antibiotic resistance determinants from the environment and those found in hospitals (13). Another problem is the release of antimicrobials into the environment, which might influence the composition of natural bacterial communities and also may change the physiology of environmental bacteria (25). Thus, wastewater irrigation and other anthropogenic activities, e.g., application of manure, might also change the composition of soil bacterial communities. Some studies have shown that a shift of soil bacterial community structure toward a higher abundance of Gammaproteobacteria (8, 26) results from an input of organic carbon sources or irrigation with treated wastewater. Gammaproteobacteria are a class of medically, ecologically, and scientifically important groups of bacteria, such as the Enterobacteriaceae (e.g., E. coli), Vibrionaceae, Pseudomonadaceae, and Xanthomonadaceae (e.g., Stenotrophomonas maltophilia). An exceedingly large number of important pathogens belongs to this class, such as Salmonella (enteritis and typhoid fever), Vibrio cholerae (cholera), Pseudomonas aeruginosa (lung infections), and Klebsiella pneumoniae (pneumonia). S. maltophilia is found in various natural environments, such as soil, water, and plants, but also occurs in the hospital environment and may cause infections that affect the bloodstream, respiratory tract, urinary tract, and surgical sites.
Frenk et al. (8) compared pyrosequencing data on bacterial 16S rRNA genes from soils irrigated with treated wastewater and those from soils irrigated with freshwater. They observed an increase in the proportion of Gammaproteobacteria during the irrigation season (dry season) and a return to the “baseline state” in the rainy season.
However, the influence of long-term irrigation with untreated wastewater on bacterial soil communities has not been studied so far. Here we investigated the effects of wastewater irrigation for different periods on the occurrence of pathogenic bacteria and antibiotic resistance determinants in the affected Mezquital Valley soils and compared wastewater-irrigated soils with rain-fed agriculture in the same area, incorporating possible seasonal effects by sampling the same soils in the rainy and dry seasons. In previous studies, we detected an increase in the relative abundance of sul resistance genes, encoding resistance toward sulfonamides, and an accumulation of antibiotics during long-term wastewater irrigation in the Mezquital Valley soils (27). Sulfonamides are bacteriostatic antibiotics that inhibit conversion of p-aminobenzoic acid to dihydropteroate, which bacteria need for folate synthesis and, ultimately, purine and DNA synthesis. Resistance in Gram-negative enteric bacteria is plasmid borne and is due mainly to the presence of sul1 and sul2 genes, encoding drug resistance variants of the dihydropteroate synthase enzyme in the folic acid pathway (28).
We hypothesize that irrigation with untreated wastewater changes the composition of soil bacterial communities toward increased abundances of potentially harmful bacteria and that wastewater-derived pathogens can survive in the environment, which might pose risks to people living in the area and to consumers of agricultural products from wastewater-irrigated fields.
MATERIALS AND METHODS
Study sites and soil sampling.
Over the past century, the irrigated area of the Mezquital Valley has increased due to the expansion of the Mexico City Metropolitan Area (MCMA). We selected sites with different durations of irrigation with untreated wastewater (nonirrigated control and durations of 8, 10, 85, and 100 years; referred to as the “soil chronosequence”) for our study. All of them were sampled in either August 2009 (rainy season) or March 2011 (dry season). All soils have been irrigated with MCMA wastewater, which has been well mixed, especially over longer periods, because of the extensive pumping and diversion of wastewater within the MCMA and the Mezquital Valley irrigation system. From each field, a sample composed of 48 subsamples distributed equidistantly within the whole field was taken with an auger at a depth of 0 to 30 cm. Soil samples were collected, transported to the laboratory at 4°C, and stored at −20°C until DNA extraction. Soil properties are given in Table 1.
TABLE 1.
Characteristics of the analyzed soil samplesb
| Sample ID | Irrigation time (yr) | Season | pH | TOC (%) | TC (%) | TN (%) | C/N ratio |
|---|---|---|---|---|---|---|---|
| DS0 | 0 | Dry | 6.3 | 0.91 | 0.95 | 0.05 | 17.8 |
| RS0 | 0 | Rainy | 7.3a | 1.53a | 1.62a | 0.15a | 10.8 |
| DS8 | 8 | Dry | 6.7 | 1.16 | 1.21 | 0.10 | 12.4 |
| RS10 | 10 | Rainy | 8.2 | 1.84 | 2.56 | 0.18 | 14.2 |
| DS85 | 85 | Dry | 6.7 | 2.06 | 2.15 | 0.19 | 11.3 |
| RS85 | 85 | Rainy | 6.4 | 2.26 | 2.30 | 0.29 | 7.9 |
| DS100 | 100 | Dry | 6.9 | 3.15 | 3.25 | 0.30 | 10.9 |
| RS100 | 100 | Rainy | 7.4a | 2.43a | 2.56a | 0.25a | 10.2 |
Properties of the soil samples.
To determine soil pH, 10 g of each soil sample was suspended at a soil-to-liquid ratio of 1:2.5 (soil–0.01 M CaCl2). Subsequently, the pH in the supernatant was measured with a glass electrode (31). For determinations of the total organic carbon content (TOC), the total carbon content (TC), and the total nitrogen content (TN), 0.5 g of each composite soil sample was suspended in 100 ml distilled water and homogenized with Ultra-Turrax (T10 Basic; IKA-Werke GmbH & Co. KG, Staufen, Germany). The samples were measured with a TOC analyzer (Shimadzu TOC-VCPN; Shimadzu Deutschland GmbH, Duisburg, Germany). For evaluations of TOC, TC, and TN, standard curves were generated with serial dilutions of the standards and measured five times. For TC measurement, a potassium hydrogen phthalate solution (2.125 g/liter potassium hydrogen phthalate; equivalent to 1 g carbon per liter) was used; for inorganic carbon, a sodium carbonate solution (4.100 g Na2CO3 and 3.500 g NaHCO3 per liter; equivalent to 1 g inorganic carbon per liter) was used; and for TN measurement, a potassium nitrate solution (7.219 g potassium nitrate; equivalent to 1 g nitrogen per liter) was used, following the manufacturer's instructions.
Total DNA extraction from soils.
Total DNAs were extracted from 500-mg soil samples from fields irrigated with wastewater for 0, 8, 10, 85, and 100 years (triplicates of four soil samples from the dry season and four soil samples from the rainy season), using a NucleoSpin Soil kit according to the manufacturer's protocol (Macherey-Nagel, Düren, Germany). Aliquots of total DNA from the soil samples were analyzed by pyrosequencing of the 16S rRNA gene.
Amplification of partial 16S rRNA genes and pyrosequencing.
The V2-V3 region of the 16S rRNA gene was amplified by PCR, using total DNAs from the different soil samples as starting material. Each PCR mixture (50 μl) contained 10 μl 5-fold reaction buffer (Phusion HF buffer; Thermo Fisher Scientific, Inc., Waltham, MA), a 200 μM concentration of each deoxynucleoside triphosphate, 5% dimethyl sulfoxide (DMSO), 0.5 U Phusion hot-start high-fidelity DNA polymerase (Thermo Fisher Scientific, Inc.), 10 to 200 ng DNA as the template, and 4 μM (each) primers. Primers used were 101F, containing Roche 454 pyrosequencing adaptor B, and 515R, containing a sample-specific MID (extended multiplex identifier; 10 nucleotides) and Roche 454 pyrosequencing adaptor A (Table 2). The PCRs were initiated at 98°C (30 s), followed by 25 cycles of 98°C (10 s), 69°C (30 s), and 72°C (20 s) and a final incubation at 72°C for 10 min. All samples were amplified in triplicate, purified using a peqGold gel extraction kit (Peqlab Biotechnologie GmbH, Erlangen, Germany) as recommended by the manufacturer, and pooled in equal amounts. Quantification of PCR products was performed using a Quant-iT dsDNA BR assay kit and a Qubit fluorometer (Life Technologies, Darmstadt, Germany). The sequences of the partial 16S rRNA genes were determined using a Roche GS-FLX 454 pyrosequencer (Roche, Mannheim, Germany) and Titanium chemistry as recommended by the manufacturer.
TABLE 2.
Primer sets used in this study
| Target | Amplicon size (bp) | Oligonucleotide | Sequence (5′ to 3′)a | Tab (°C) | Reference(s) |
|---|---|---|---|---|---|
| sul1 | 158 | sul1-FW | CACCGGAAACATCGCTGCA | 65 | 32 |
| sul1-RV | AAGTTCCGCCGCAAGGCT | 65 | 32 | ||
| sul2 | 190 | sul2-FW | CTCCGATGGAGGCCGGTAT | 65 | 32 |
| sul2-RV | GGGAATGCCATCTGCCTTGA | 65 | 32 | ||
| qnrA | 543 | qnrA-F | GATAAAGTTTTTCAGCAAGAGG | 56 | 33 |
| qnrA-R | ATCCAGATCGGCAAAGGTTA | 56 | 33 | ||
| qnrB | 497 | qnrB-F | AGCGGCACTGAATTTAT | 56 | 33 |
| qnrB-R | GTTTGCTGCTCGCCAGTC | 56 | 33 | ||
| qnrS | 600 | qnrS-F | GGAAACCTACAATCATACATA | 56 | 33 |
| qnrS-R | GTCAGGATAAACAACAATACC | 56 | 33 | ||
| Bacterial 16S rRNA gene | 1,465 | 27F | GAGTTTGATCMTGGCTCAG | 58 | 34 |
| 1492R | GGYTACCTTGTTACGACTT | 58 | 34 | ||
| 1,324 | 63fw | CAGGCCTAACACATGCAAGTC | 56 | 35 | |
| 1387rev | GGGCGGWGTGTACAAGGC | 56 | 35 | ||
| 414 | 101Fc | CCTATCCCCTGTGTGCCTTGGCAGTCTCAGAGTGGCGGACGGGTGAGTAAd | 69 | 36, 37 | |
| 515Rc | CCATCTCATCCCTGCGTGTCTCCGACTCAG-MID-CCGCGGCTGCTGGCACe | 69 | 36, 37 |
Y = C or T.
Annealing temperature.
Primers for pyrosequencing.
Roche 454 pyrosequencing adaptor B is underlined.
Roche 454 pyrosequencing adaptor A is underlined. MID, sample-specific extended multiplex identifier (10 nucleotides).
Pyrosequencing data processing and statistical analysis.
Sequences shorter than 200 bp, as well as those exhibiting an average quality value of <25, more than two primer mismatches, or long homopolymers (>8 bp), were removed from the data set by employing QIIME, version 1.6 (38). All remaining primer sequences were truncated using the cutadapt program (39). Removal of potential chimeric sequences was performed by applying Uchime (40), with the Greengenes Gold data set gold_strains_gg16S_aligned.fasta as a reference (41). The Acacia error-correction tool (42) was used to remove noise introduced by amplicon pyrosequencing. Determination of operational taxonomic units (OTUs) was performed using Uclust (43). To taxonomically classify OTUs, partial 16S rRNA gene sequences were compared with the SILVA SSU Ref NR 115 database (44). A customized script was used to remove all nonbacterial OTUs from the OTU table. Calculations of rarefaction curves, the Chao1 index (45), and the Shannon index (46) were conducted using QIIME.
We used two-sample t test analyses and the Mann-Whitney U test for nonparametric data to compare relative abundances of bacterial groups and diversity and richness estimates between soils collected during the dry and rainy seasons and between wastewater-irrigated and rain-fed soils, using the software package PAST (47). To compare bacterial community compositions across all samples, based on weighted UniFrac (48) measures, principal coordinate analysis was performed by using QIIME. For determination of the phylogenetic metric (weighted UniFrac), a phylogenetic tree was calculated using a PyNAST (49) alignment. This alignment was produced by aligning a representative sequence set (one sequence from each OTU at a genetic distance of 3%) to Greengenes core set core_set_aligned.fasta (41).
Isolation of soil bacteria.
One hundred milligrams of soil per analyzed sample was suspended in 900 μl sodium pyrophosphate (7.5 mM, with 0.05% Tween 80), and subsequently, the bacteria were detached from the soil particles through shaking at 1,000 rpm for 45 min (50). After 5 min of settling, serial dilutions of the bacterial suspensions were transferred onto tryptic soy agar (TSA) plates and incubated for 24 h at 22°C. Single colonies were picked and purified via two passages on TSA plates.
DNA extraction from bacterial soil isolates.
DNA extraction from bacterial soil isolates was performed using a MasterPure Gram-positive DNA purification kit (Biozym Scientific GmbH, Hessisch Oldendorf, Germany) according to the manufacturer's instructions, using 1 ml overnight culture in tryptic soy broth (TSB) incubated at 22°C. The isolated DNA was applied to amplify the 16S rRNA gene and antibiotic resistance genes by PCR.
Amplification and sequencing of 16S rRNA genes of soil isolates.
For amplification of the 16S rRNA gene, each 50-μl PCR mixture contained 2.5 U Taq polymerase and 1× PCR buffer S (Peqlab Biotechnologie GmbH, Erlangen, Germany), 0.2 μM (each) primers (27F and 1492R [Table 2]), a 0.2 mM concentration of each deoxynucleoside triphosphate, 2 mM MgCl2, and 20 ng template DNA (genomic DNA of a bacterial isolate). DNA amplifications were carried out in an Eppendorf thermocycler (Eppendorf Mastercycler for 96-well plates; Eppendorf AG, Hamburg, Germany). The temperature profile consisted of an initial denaturation step at 95°C for 2 min, followed by 30 cycles of denaturation at 95°C for 30 s, primer annealing at 58°C for 45 s, and extension at 72°C for 1 min and an additional 7-min elongation step at 72°C. PCR products were sequenced with primers 63F and 1387R (Table 2) (Beckman Coulter Genomics, Takeley, United Kingdom). Sequences were analyzed by BLASTn searches of the 16S rRNA gene sequence reference database for bacteria and archaea (51; http://blast.ncbi.nlm.nih.gov/Blast.cgi).
Assessment of antibiotic resistance genes by PCR.
PCR assays specific for sul (32) and qnr (33) resistance genes were performed as follows. Each 25-μl PCR mixture contained 12.5 μl KAPA2G Fast ReadyMix with dye (Peqlab Biotechnologie GmbH, Erlangen, Germany), 2 to 3 mM MgCl2, and 20 ng genomic DNA of a bacterial isolate. DNA amplifications were carried out in an Eppendorf thermocycler (Eppendorf Mastercycler for 96-well plates; Eppendorf AG, Hamburg, Germany). The temperature profile consisted of an initial denaturation step at 95°C for 2 min, followed by 30 cycles of denaturation at 95°C for 30 s, primer annealing at 57°C for 45 s for qnr genes and at 65°C for 30 s for sul genes, and extension at 72°C for 1 min, with an additional 7-min elongation step at 72°C (only for qnr genes). Primers used are listed in Table 2. Absolute quantifications of sul1 and sul2 genes were performed with serially diluted exogenous standards that consisted of purified PCR products. Quantification of absolute target gene numbers was carried out using a LightCycler 480 instrument (Roche Diagnostics, Mannheim, Germany) as described previously (27).
Antimicrobial susceptibility testing of bacterial isolates.
Resistance of the bacterial isolates to specific antibiotics was determined by the disc diffusion method, performed according to CLSI guidelines (52), with the following antibiotic discs (Oxoid, Wesel, Germany): ampicillin (25 μg), chloramphenicol (30 μg), erythromycin (10 μg), gentamicin (10 μg), kanamycin (30 μg), oxacillin (5 μg), streptomycin (25 μg), ciprofloxacin (CIP; 5 and 10 μg), doxycycline (30 μg), tetracycline (30 μg), vancomycin (30 μg), and sulfamethoxazole (SMX; 25 μg). Single colonies of bacterial soil isolates were diluted to an OD630 of 0.16 and streaked with swabs according to DIN 58940-3:2007-10 (86). Instead of Mueller-Hinton agar, TSA plates were used and were incubated for 24 h at 22°C.
Nucleotide sequence accession number.
All nucleotide sequences have been deposited in the Sequence Read Archive of the National Center for Biotechnology Information, under accession number SRP037963.
RESULTS AND DISCUSSION
Characteristics of wastewater-irrigated and rain-fed soils.
Irrigation with untreated wastewater releases organic carbon compounds and other nutrients into soils. More nutrients and a higher humidity over the entire year provide better growth conditions for indigenous bacteria, and possibly also for wastewater-derived bacteria, and thus might change the composition of soil bacterial communities. The organic matter content of the analyzed soils increased during long-term irrigation with wastewater (Table 1). In rain-fed soils, TOC ranged from 0.91 to 1.53%, whereas in wastewater-irrigated soils, TOC ranged from 1.06 to 3.35%. The total nitrogen content in the soils varied from 0.05 to 0.15% (rain-fed soils) and from 0.10 to 0.30% (wastewater-irrigated soils). The soil pH values varied between 6.7 and 7.4. An increase of soil organic matter content through wastewater irrigation has also been reported by others (53–58). This results in a rising microbial biomass and microbial activity (53, 59–61). Furthermore, the increased water supply by wastewater irrigation in the dry season seems to provide better conditions for microbial proliferation (53). This might also increase the survival rate of wastewater-derived bacteria.
General analysis of the pyrosequencing-derived data set and overall bacterial diversity and richness.
Pyrosequencing of partial 16S rRNA genes (V2-V3 region) yielded a total of 452,999 sequences across all analyzed soil samples (n = 24). After preprocessing, including quality filtering, denoising, and removal of nonbacterial or chimeric reads, 337,493 sequences, with an average length of 353 bp, were obtained for further analyses (see Table S1 in the supplemental material). Due to the fact that the number of analyzed sequences per sample has an effect on the predicted number of OTUs, OTU-based comparisons between the 24 analyzed soils were performed at the same level of surveying effort (11,320 sequences per sample) (62).
Rarefaction curve, richness, and diversity analyses were based on numbers of OTUs determined at 3 and 20% genetic distances. Comparison of the rarefaction analyses with the number of OTUs calculated by the Chao1 richness estimator revealed that 72.6 to 86.8% (20% genetic distance) and 31.0 to 48.2% (3% genetic distance) of the estimated richness were covered by the sequencing effort (Fig. 1; see Table S2 in the supplemental material). (The Chao1 nonparametric richness estimator was employed to calculate the estimated true OTU diversity of the samples.) Thus, we did not survey the full extent of diversity, but particularly at a 20% genetic distance (phylum level, according to Schloss and Handelsman [63]), a substantial fraction of the bacterial diversity within individual soil samples was assessed. Dry season samples exhibited significantly higher OTU numbers, Chao1 richness estimates, and bacterial diversity levels as assessed by the Shannon index (H′) than those of rainy season samples (for 3% genetic distance, P < 0.001; and for 20% genetic distance, P < 0.05) (Fig. 1; see Table S2), likely due to the larger input of wastewater-derived bacteria during the irrigation season. Wastewater irrigation had no statistically significant impact on overall bacterial diversity and richness (Fig. 1; see Table S2), in agreement with the studies of Frenk et al. (8).
FIG 1.
Rarefaction curves indicating the observed numbers of OTUs at genetic distances of 3 and 20% within the analyzed soil samples. Sample abbreviations: RS0, rainy season rain-fed soil; RS10, rainy season soil with 10 years of wastewater irrigation; RS85, rainy season soil with 85 years of wastewater irrigation; RS100, rainy season soil with 100 years of wastewater irrigation; DS0, dry season rain-fed soil; DS8, dry season soil with 8 years of wastewater irrigation; DS85, dry season soil with 85 years of wastewater irrigation; DS100, dry season soil with 100 years of wastewater irrigation. Triplicates were analyzed (indicated by “a,” “b,” and “c” in sample names).
Community compositions in wastewater-irrigated and rain-fed soils.
Bacterial 16S rRNA gene sequences were affiliated with 23 phyla (see Table S3 in the supplemental material) and 17 candidate divisions (see Table S4). The dominant phyla and proteobacterial classes across all 24 soil samples were Actinobacteria (27.4%), Alphaproteobacteria (14.6%), Acidobacteria (14.0%), Betaproteobacteria (9.5%), Chloroflexi (9.3%), Gammaproteobacteria (8.9%), Firmicutes (5.2%), Deltaproteobacteria (2.7%), Gemmatimonadetes (2.5%), and Planctomycetes (1.9%). These phyla and proteobacterial classes are typically encountered in soil and were also reported in similar relative abundances in a meta-analysis of 32 soil-derived bacterial 16S rRNA gene libraries (64) and in recent metagenomic as well as metatranscriptomic microbial community analyses (65, 66).
The relative abundances of bacterial phyla and proteobacterial classes varied between wastewater-irrigated and rain-fed soils (Fig. 2). A shift of the bacterial community toward a higher relative abundance of Gammaproteobacteria was observed in both seasons (dry and rainy seasons) in the wastewater-irrigated soils compared to the rain-fed soils (P = 0.002). With respect to rain-fed soil samples, 3.2 to 5.5% (rainy season) and 3.4 to 4.2% (dry season) of the bacterial sequences were affiliated with Gammaproteobacteria, whereas relative abundances of gammaproteobacterial sequences determined for wastewater-irrigated soils ranged from 5.8 to 10.3% (rainy season) and 8.5% to 17.7% (dry season) (see Table S3 in the supplemental material). Strikingly, more potentially harmful Gammaproteobacteria were detected in wastewater-irrigated than in rain-fed soils (Fig. 3). Up to 196-, 28-, and 20-fold higher relative abundances of Acinetobacter, Stenotrophomonas, and Pseudomonas, respectively, were detected in wastewater-irrigated soil than in rain-fed soil during the dry season (Fig. 3). Species within these genera, such as Pseudomonas aeruginosa and Acinetobacter baumannii, are representatives of the so-called ESKAPE (Enterococcus faecium, Staphylococcus aureus, Klebsiella species, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species) organisms that frequently cause nosocomial infections (67, 68). They are of main concern due to the high abundance of multiresistance in these organisms. Another emerging organism that causes nosocomial and community-acquired infections is S. maltophilia (69–72). This bacterium can be associated with respiratory tract infections (71, 72), especially in hospitalized patients on mechanical ventilation. The high prevalence of Stenotrophomonas, Pseudomonas, and Acinetobacter in the wastewater-irrigated soils indicates an adaptation of wastewater-associated bacteria to the soil environment. These bacteria serve as carriers of multiresistance and likely increase the dissemination of resistance determinants in the environment and to other, potentially more dangerous bacteria. The large increases in relative abundance of Acinetobacter, Stenotrophomonas, and Pseudomonas in wastewater-irrigated soil determined for the dry season (P < 0.05) were not detected by statistical analysis for the rainy season (Fig. 3). This result might be related to an increased input of wastewater-derived bacteria by wastewater irrigation in the dry season. These findings are in agreement with the studies of Frenk et al., who demonstrated that the relative abundance of Gammaproteobacteria increases in the irrigation season and decreases in the rainy season (8). Like Gammaproteobacteria, Betaproteobacteria were more abundant in all dry season wastewater-irrigated soil samples than in dry season rain-fed soils (P < 0.001) (Fig. 2). However, no medically relevant betaproteobacterial species were detected. Principal coordinate analysis at a 3% genetic distance indicated that rain-fed soil samples harbored similarity in overall bacterial community composition, since they tended to cluster (Fig. 4). An effect of season on overall bacterial community composition was not revealed.
FIG 2.
Relative abundances of dominant phyla and proteobacterial classes determined for the analyzed soil samples. Sample abbreviations: RS0, rainy season rain-fed soil; RS10, rainy season soil with 10 years of wastewater irrigation; RS85, rainy season soil with 85 years of wastewater irrigation; RS100, rainy season soil with 100 years of wastewater irrigation; DS0, dry season rain-fed soil; DS8, dry season soil with 8 years of wastewater irrigation; DS85, dry season soil with 85 years of wastewater irrigation; DS100, dry season soil with 100 years of wastewater irrigation. Data from analysis of triplicates are illustrated using error bars.
FIG 3.

Heat map showing relative abundances of gammaproteobacterial genera as affected by wastewater irrigation during dry as well as rainy season. Triplicates were analyzed (indicated by “a,” “b,” and “c”).
FIG 4.

Weighted UniFrac 2D principal coordinate analysis plot for beta diversity analysis. Sample abbreviations: RS0, rainy season rain-fed soil; RS10, rainy season soil with 10 years of wastewater irrigation; RS85, rainy season soil with 85 years of wastewater irrigation; RS100, rainy season soil with 100 years of wastewater irrigation; DS0, dry season rain-fed soil; DS8, dry season soil with 8 years of wastewater irrigation; DS85, dry season soil with 85 years of wastewater irrigation; DS100, dry season soil with 100 years of wastewater irrigation.
Characterization of soil isolates.
Bacterial isolates from soils irrigated with untreated wastewater for 100 years and from soils which received only rainwater, from both the dry and rainy seasons, were obtained by incubation on TSA plates for 24 to 48 h at 23°C. The incubation temperature was chosen because it was the mean soil temperature in the Mezquital Valley in the dry season. TSA is a rich medium and proved to be appropriate for isolation of diverse environmental bacteria, as already shown by, e.g., Krishnamurthi and Chakrabarti for soil and Yashiro et al. for the phyllosphere (73, 74); the predominantly isolated soil bacteria in their studies were members of the phylum Firmicutes (most of them Bacillus spp.), followed by Actinobacteria and Proteobacteria. In our study, most bacterial isolates from wastewater-irrigated soils (48 isolates from soil samples collected in the dry season and 48 isolates from soil samples collected in the rainy season) belonged to the Bacilli (50%) and the Gammaproteobacteria (46%). Only 3% of the isolates belonged to the Actinobacteria, and 1% to the Alphaproteobacteria (Fig. 5A; see Table S6 in the supplemental material). The most abundant genera were Bacillus (47%) and Stenotrophomonas (39%), followed by Pseudomonas (5%) and Acinetobacter (2%) (Fig. 5B). For rain-fed soils, all 96 isolates (48 isolates from soil samples collected in the dry season and 48 isolates from soil samples collected in the rainy season) belonged to the Bacilli and, within this class, to the genus Bacillus (see Table S5). Bacillus is ubiquitous in soil environments (75, 76) and can persist under a variety of conditions due to the ability to form endospores (77, 78).
FIG 5.

(A) Abundances of bacterial classes in isolates from wastewater-irrigated soils. (B) Abundances of different bacterial genera in isolates from wastewater-irrigated soils.
The taxonomic groups detected (Proteobacteria, Actinobacteria, and Bacilli) are typical taxa found in agricultural soils. A rise in the available soil nutrients and moisture in wastewater-irrigated soils leads to an increase in Proteobacteria, particularly Gammaproteobacteria, in agreement with previous studies (8, 26). Consistent with the amplicon data, which revealed a higher relative abundance of Gammaproteobacteria in wastewater-irrigated soils than in rain-fed soils, more isolates belonging to the Gammaproteobacteria (Stenotrophomonas, Pseudomonas, and Acinetobacter) were obtained from wastewater-irrigated soils than from rain-fed soils (46% of the isolates from wastewater-irrigated soils versus no isolates from rain-fed soils). The fact that these microorganisms were derived from samples collected in the dry as well as the rainy season indicates that they have adapted to the wastewater-irrigated soil environment. Some crops, such as maize and several herbs, such as Rumex sp., Malva sp., and Chenopodium mexicanum, that grow in wastewater-irrigated fields are consumed by the people in the Mezquital Valley. In particular, the near-ground herbs are in direct contact with wastewater and wastewater-irrigated soils. This might imply health risks for consumers of insufficiently washed crops containing wastewater-derived bacteria and resistance determinants.
Prevalence of multiantibiotic-resistant isolates from wastewater-irrigated soils.
All isolates from wastewater-irrigated soils and from rain-fed soils were tested for susceptibility to 12 different antibiotics. In addition, the isolates that were resistant to sulfamethoxazole (SMX) or ciprofloxacin (CIP) were analyzed for the presence of sul and qnr resistance genes, which encode resistance to sulfonamides and fluoroquinolones, respectively. These genes were detected in total DNA of the chronosequence soils. The sul1, sul2, qnrA, qnrB, and qnrS genes were not found in total DNA of the isolates. For the qnr genes, this was not surprising, as these genes were rarely found in the chronosequence soils (27). Resistance to fluoroquinolones is often the result of point mutations in target genes, such as gyrA, encoding DNA gyrase, and parC, encoding a type IV topoisomerase (79). Only 3 of 96 isolates from wastewater-irrigated soils (one Stenotrophomonas, one Bacillus, and one Exiguobacterium isolate) and none of the 96 isolates from rain-fed soils were resistant to low concentrations of the fluoroquinolone ciprofloxacin (5 μg) (see Tables S5 and S6 in the supplemental material). Several isolates, i.e., 32 from wastewater-irrigated soils and 18 from rain-fed soils, were resistant to the sulfonamide SMX (25 μg). Interestingly, a considerable number of isolates belonging to the Bacillaceae were resistant to SMX, including 18 of the 96 Bacilli isolates from rain-fed soils and 21 of the 46 Bacilli isolates from wastewater-irrigated soils.
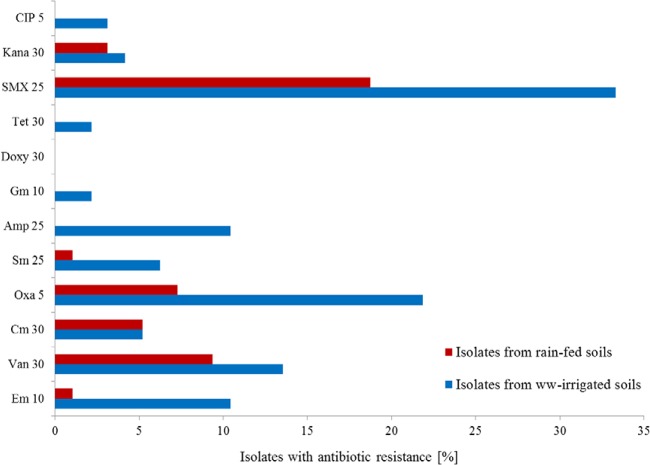
In other studies, SMX-resistant Bacilli were isolated from different environments, such as wastewater, water, and sediments, on selective plates containing between 50 and 200 μg/ml SMX. But even when isolated under selective pressure, not all resistant isolates contained the sul1, sul2, or sul3 gene (80). Sulfonamide resistance can also occur by other mechanisms, such as modification of the antibiotic target, e.g., by mutations of the chromosomal dihydropteroate synthase gene (81). Sulfonamide resistance (often also in combination with trimethoprim resistance) has been described for several Bacillus species (82). From wastewater-irrigated soils, 33% of the isolates (n = 96) were resistant to SMX. Twenty-one of the resistant isolates were Bacillus spp., and the remaining 11 belonged to the genera Stenotrophomonas, Pseudomonas, and Acinetobacter. In wastewater irrigation fields, more isolates (51%) were resistant to at least one antibiotic than in rain-fed soils (34%). In particular, the presence of multiresistant bacteria (resistance to ≥2 antibiotics) was more pronounced in wastewater-irrigated soils (25%) than in rain-fed soils (6%) (see Tables S5 and S6).
In the present study, resistance to oxacillin, erythromycin, vancomycin, and ampicillin was frequently found in isolates from wastewater-irrigated soils. Other resistances were less frequent (<10%), and no isolate resistant to doxycycline was found (summarized in Fig. 6; see Tables S5 and S6 in the supplemental material). The higher abundance of multiresistant isolates from wastewater-irrigated fields is likely related to the different types of bacteria isolated from the two irrigation regimens. In wastewater-irrigated soils, three isolates were resistant to three antibiotics, and nine were resistant to more than three antibiotics. For the isolates from rain-fed soils, only two isolates showed resistance to three different antibiotics, and none to more than three antibiotics. The majority of multiresistant bacteria belonged to the genus Stenotrophomonas (see Table S6). 16S rRNA gene sequences of the Stenotrophomonas isolates showed highest identities (96 to 99%) to S. maltophilia, an opportunistic bacterial pathogen of environmental origin that is associated with several human diseases (69, 70, 72). Treatment proves difficult due to this species' intrinsic antibiotic resistance (69). An increase of the relative abundance of Stenotrophomonas spp. in soils which have been treated by sulfadiazine-amended manure was observed by Ding et al. (83).
FIG 6.
Percentages of antibiotic-resistant isolates from wastewater-irrigated soils and rain-fed soils. CIP, ciprofloxacin (5 μg); Kana, kanamycin (30 μg); SMX, sulfamethoxazole (25 μg); Tet, tetracycline (30 μg); Doxy, doxycycline (30 μg); Gm, gentamicin (10 μg); Amp, ampicillin (25 μg); Sm, streptomycin (25 μg); Oxa, oxacillin (5 μg); Cm, chloramphenicol (30 μg); Van, vancomycin (30 μg); Em, erythromycin (10 μg); ww, wastewater.
For the clinically relevant species S. maltophilia and P. aeruginosa, several studies have reported that multiple-antibiotic resistance is due to the overexpression of multidrug efflux pumps (e.g., SmeDEF or MexA-MexB-OprM) (84, 85). For several environmental Pseudomonas isolates, intrinsic multidrug resistance has been reported. Malik and Aleem demonstrated a high prevalence of antibiotic resistance in Pseudomonas isolates from water and soil (5). They showed that 87.5% of the Pseudomonas isolates from wastewater-irrigated soils were resistant to the sulfonamide sulfadiazine. Furthermore, they revealed that isolates from groundwater-irrigated soils were less resistant to antibiotics than isolates from wastewater-irrigated soils, which is consistent with our data (5).
Finally, our data reveal a higher prevalence of Gammaproteobacteria, in particular of harmful and multiresistant bacteria, such as S. maltophilia, in wastewater-irrigated soil. To the best of our knowledge, this is the first report on the high incidence of Stenotrophomonas spp. in wastewater-irrigated soils. Most of the bacterial isolates from wastewater-irrigated soils were resistant to several antibiotics (up to five different antibiotic classes). The higher incidence of multiple-antibiotic-resistant bacteria in wastewater-irrigated soils indicates survival of wastewater-derived bacteria in the environment and thus represents an increased risk of antibiotic resistance dissemination in the environment. A major health issue is related to the observation that near-ground crops that are in direct contact with soil and wastewater are consumed raw by the people in the Mezquital Valley.
Supplementary Material
ACKNOWLEDGMENTS
We thank R. Brämer and A. Henninger from the University of Applied Sciences Offenburg for support with the measurement of the chemical soil parameters.
This work was supported by grants GR1792/4-1 and GR1792/4-2 from the German Research Foundation and by the Mexican Consejo Nacional de Ciencia y Tecnología (CONACYT) (grants CB 83767 and I 0110-193-10).
Footnotes
Published ahead of print 20 June 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.01295-14.
REFERENCES
- 1.Baquero F, Martínez J, Cantón R. 2008. Antibiotics and antibiotic resistance in water environments. Curr. Opin. Biotechnol. 19:260–265. 10.1016/j.copbio.2008.05.006 [DOI] [PubMed] [Google Scholar]
- 2.Bruchmann J, Kirchen S, Schwartz T. 2013. Sub-inhibitory concentrations of antibiotics and wastewater influencing biofilm formation and gene expression of multi-resistant Pseudomonas aeruginosa wastewater isolates. Environ. Sci. Pollut. Res. 20:3539–3549. 10.1007/s11356-013-1521-4 [DOI] [PubMed] [Google Scholar]
- 3.Chávez A, Maya C, Gibson R, Jiménez B. 2011. The removal of microorganisms and organic micropollutants from wastewater during infiltration to aquifers after irrigation of farmland in the Tula Valley, Mexico. Environ. Pollut. 159:1354–1362. 10.1016/j.envpol.2011.01.008 [DOI] [PubMed] [Google Scholar]
- 4.Levantesi C, La Mantia R, Masciopinto C, Böckelmann U, Ayuso-Gabella MN, Salgot M, Tandoi V, van Houtte E, Wintgens T, Grohmann E. 2010. Quantification of pathogenic microorganisms and microbial indicators in three wastewater reclamation and managed aquifer recharge facilities in Europe. Sci. Total Environ. 408:4923-4930. 10.1016/j.scitotenv.2010.07.042 [DOI] [PubMed] [Google Scholar]
- 5.Malik A, Aleem A. 2011. Incidence of metal and antibiotic resistance in Pseudomonas spp. from the river water, agricultural soil irrigated with wastewater and groundwater. Environ. Monit. Assess. 178:293–308. 10.1007/s10661-010-1690-2 [DOI] [PubMed] [Google Scholar]
- 6.Rizzo L, Manaia C, Merlin C, Schwartz T, Dagot C, Ploy IM, Fatta-Kassinos D. 2013. Urban wastewater treatment plants as hotspots for antibiotic resistant bacteria and genes spread into the environment: a review. Sci. Total Environ. 447:345–360. 10.1016/j.scitotenv.2013.01.032 [DOI] [PubMed] [Google Scholar]
- 7.Elifantz H, Kautsky L, Mor-Yosef M, Tarchitzky J, Bar-Tal A, Chen Y, Minz D. 2011. Microbial activity and organic matter dynamics during 4 years of irrigation with treated wastewater. Microb. Ecol. 62:973–981. 10.1007/s00248-011-9867-y [DOI] [PubMed] [Google Scholar]
- 8.Frenk S, Hadar Y, Minz D. 2014. Resilience of soil bacterial community to irrigation with water of different qualities under Mediterranean climate. Environ. Microbiol. 16:559–569. 10.1111/1462-2920.12183 [DOI] [PubMed] [Google Scholar]
- 9.Siebe C, Cifuentes E. 1995. Environmental impact of wastewater irrigation in central Mexico: an overview. Int. J. Environ. Health Res. 5:161–173. 10.1080/09603129509356845 [DOI] [Google Scholar]
- 10.Jimenez B, Chávez A. 2004. Quality assessment of an aquifer recharged with wastewater for its potential use as drinking source: “El Mezquital Valley” case. Water Sci. Technol. 50:269–276 [PubMed] [Google Scholar]
- 11.Baquero F, Blázquez J. 1997. Evolution of antibiotic resistance. Trends Ecol. Evol. (Amst.) 12:482–487. 10.1016/S0169-5347(97)01223-8 [DOI] [PubMed] [Google Scholar]
- 12.Cantón R. 2009. Antibiotic resistance genes from the environment: a perspective through newly identified antibiotic resistance mechanisms in the clinical setting. Clin. Microbiol. Infect. 15:20–25. 10.1111/j.1469-0691.2008.02679.x [DOI] [PubMed] [Google Scholar]
- 13.Wright GD. 2010. Antibiotic resistance in the environment: a link to the clinic. Curr. Opin. Microbiol. 13:589–594. 10.1016/j.mib.2010.08.005 [DOI] [PubMed] [Google Scholar]
- 14.Martinez JL. 2009. The role of natural environments in the evolution of resistance traits in pathogenic bacteria. Proc. Biol. Sci. 276:2521–2530. 10.1098/rspb.2009.0320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gatica J, Cytryn E. 2013. Impact of treated wastewater irrigation on antibiotic resistance in the soil microbiome. Environ. Sci. Pollut. Res. 20:3529–3538. 10.1007/s11356-013-1505-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang X, Zhang T, Fang HHP. 2009. Antibiotic resistance genes in water environment. Appl. Microbiol. Biotechnol. 82:397–414. 10.1007/s00253-008-1829-z [DOI] [PubMed] [Google Scholar]
- 17.D'Costa VM, McGrann KM, Hughes DW, Wright GD. 2006. Sampling the antibiotic resistome. Science 311:374–377. 10.1126/science.1120800 [DOI] [PubMed] [Google Scholar]
- 18.Benveniste R, Davies J. 1973. Aminoglycoside antibiotic-inactivating enzymes in actinomycetes similar to those present in clinical isolates of antibiotic-resistant bacteria. Proc. Natl. Acad. Sci. U. S. A. 70:2276–2280. 10.1073/pnas.70.8.2276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cantón R, Coque TM. 2006. The CTX-M beta-lactamase pandemic. Curr. Opin. Microbiol. 9:466–475. 10.1016/j.mib.2006.08.011 [DOI] [PubMed] [Google Scholar]
- 20.Humeniuk C, Arlet G, Gautier V, Grimont P, Labia R, Philippon A. 2002. Beta-lactamases of Kluyvera ascorbata, probable progenitors of some plasmid-encoded CTX-M types. Antimicrob. Agents Chemother. 46:3045–3049. 10.1128/AAC.46.9.3045-3049.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Poirel L, Liard A, Rodriguez-Martinez J, Nordmann P. 2005. Vibrionaceae as a possible source of Qnr-like quinolone resistance determinants. J. Antimicrob. Chemother. 56:1118–1121. 10.1093/jac/dki371 [DOI] [PubMed] [Google Scholar]
- 22.Poirel L, Rodriguez-Martinez J, Mammeri H, Liard A, Nordmann P. 2005. Origin of plasmid-mediated quinolone resistance determinant QnrA. Antimicrob. Agents Chemother. 49:3523–3525. 10.1128/AAC.49.8.3523-3525.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sánchez MB, Hernández A, Rodríguez-Martínez JM, Martínez-Martínez L, Martínez JL. 2008. Predictive analysis of transmissible quinolone resistance indicates Stenotrophomonas maltophilia as a potential source of a novel family of Qnr determinants. BMC Microbiol. 8:148. 10.1186/1471-2180-8-148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robicsek A, Jacoby GA, Hooper DC. 2006. The worldwide emergence of plasmid-mediated quinolone resistance. Lancet Infect. Dis. 6:629–640. 10.1016/S1473-3099(06)70599-0 [DOI] [PubMed] [Google Scholar]
- 25.Martinez JL. 2009. Environmental pollution by antibiotics and by antibiotic resistance determinants. Environ. Pollut. 157:2893–2902. 10.1016/j.envpol.2009.05.051 [DOI] [PubMed] [Google Scholar]
- 26.Eilers KG, Lauber CL, Knight R, Fierer N. 2010. Shifts in bacterial community structure associated with inputs of low molecular weight carbon compounds to soil. Soil Biol. Biochem. 42:896–903. 10.1016/j.soilbio.2010.02.003 [DOI] [Google Scholar]
- 27.Dalkmann P, Broszat M, Siebe C, Willaschek E, Sakinc T, Huebner J, Amelung W, Grohmann E, Siemens J. 2012. Accumulation of pharmaceuticals, Enterococcus, and resistance genes in soils irrigated with wastewater for zero to 100 years in central Mexico. PLoS One 7:e45397. 10.1371/journal.pone.0045397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sköld O. 2000. Sulfonamide resistance: mechanisms and trends. Drug Resist. Updat. 3:155–160. 10.1054/drup.2000.0146 [DOI] [PubMed] [Google Scholar]
- 29.Chapela LM. 2010. Variabilidad temporal en el contenido de metales pesados en suelos regados con aguas resiudales en el Valle del Mezquital: México. Master's thesis Universidad Nacional Autónoma de México, Mexico City, Mexico [Google Scholar]
- 30.Schlichting E, Blume HP, Stahr K. 1995. Bodenkundliches Praktikum. Eine Einführung in pedologisches Arbeiten für Oekologen, insbesondere Land- und Forstwirte, und für Geowissenschaftler. Blackwell Wissenschaft, Berlin, Germany [Google Scholar]
- 31.Will C, Thürmer A, Wollherr A, Nacke H, Herold N, Schrumpf M, Gutknecht J, Wubet T, Buscot F, Daniel R. 2010. Horizon-specific bacterial community composition of German grassland soils, as revealed by pyrosequencing-based analysis of 16S rRNA genes. Appl. Environ. Microbiol. 76:6751–6759. 10.1128/AEM.01063-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luo Y, Mao D, Rysz M, Zhou Q, Zhang H, Xu L, Alvarez PJJ. 2010. Trends in antibiotic resistance genes occurrence in the Haihe River, China. Environ. Sci. Technol. 44:7220–7225. 10.1021/es100233w [DOI] [PubMed] [Google Scholar]
- 33.Guillard T, Cavallo JD, Cambau E, Duval V, Bajolet O, Brasme L, de Champs C, Vernet-Garnier V. 2010. Mise au point d'une technique de PCR en temps réel pour la détection rapide des gènes qnr chez des entérobactéries productrices de bêta-lactamases à spectre étendu. Pathol. Biol. 58:430–433. 10.1016/j.patbio.2009.03.003 [DOI] [PubMed] [Google Scholar]
- 34.Fredriksson NJ, Hermansson M, Wilén B-M. 2013. The choice of PCR primers has great impact on assessments of bacterial community diversity and dynamics in a wastewater treatment plant. PLoS One 8:e76431. 10.1371/journal.pone.0076431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marchesi JR, Sato T, Weightman AJ, Martin TA, Fry JC, Hiom SJ, Dymock D, Wade WG. 1998. Design and evaluation of useful bacterium-specific PCR primers that amplify genes coding for bacterial 16S rRNA. Appl. Environ. Microbiol. 64:795–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lane DJ. 1991. 16S/23S rRNA sequencing, p 115–175 In Stackebrandt E, Goodfellow M. (ed), Nucleic acid techniques in bacterial systematics. Modern microbiological methods. Wiley, Chichester, New York [Google Scholar]
- 37.Schmalenberger A, Schwieger F, Tebbe CC. 2001. Effect of primers hybridizing to different evolutionarily conserved regions of the small-subunit rRNA gene in PCR-based microbial community analyses and genetic profiling. Appl. Environ. Microbiol. 67:3557–3563. 10.1128/AEM.67.8.3557-3563.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Peña AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JF, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R. 2010. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 7:335–336. 10.1038/nmeth.f.303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martin M. 2011. Cutadapt removes adapter sequences from high-throughput 699 sequencing reads. EMBnet J. 17:10–12. 10.14806/ej.17.1.200 [DOI] [Google Scholar]
- 40.Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R. 2011. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 27:2194–2200. 10.1093/bioinformatics/btr381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.DeSantis TZ, Hugenholtz P, Larsen N, Rojas M, Brodie EL, Keller K, Huber T, Dalevi D, Hu P, Andersen GL. 2006. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl. Environ. Microbiol. 72:5069–5072. 10.1128/AEM.03006-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bragg L, Stone G, Imelfort M, Hugenholtz P, Tyson GW. 2012. Fast, accurate error-correction of amplicon pyrosequences using Acacia. Nat. Methods 9:425–426. 10.1038/nmeth.1990 [DOI] [PubMed] [Google Scholar]
- 43.Edgar RC. 2010. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26:2460–2461. 10.1093/bioinformatics/btq461 [DOI] [PubMed] [Google Scholar]
- 44.Pruesse E, Quast C, Knittel K, Fuchs BM, Ludwig W, Peplies J, Glöckner FO. 2007. SILVA: a comprehensive online resource for quality checked and aligned ribosomal RNA sequence data compatible with ARB. Nucleic Acids Res. 35:7188–7196. 10.1093/nar/gkm864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chao A, Bunge J. 2002. Estimating the number of species in a stochastic abundance model. Biometrics 58:531–539. 10.1111/j.0006-341X.2002.00531.x [DOI] [PubMed] [Google Scholar]
- 46.Shannon CE. 1948. A mathematical theory of communication. Bell Syst. Tech. J. 27:379–423. 10.1002/j.1538-7305.1948.tb01338.x [DOI] [Google Scholar]
- 47.Hammer Ø, Harper DAT, Ryan PD. 2001. PAST: paleontological statistics software package for education and data analysis. Palaeontol. Electron. 4:1–9 [Google Scholar]
- 48.Lozupone C, Knight R. 2005. UniFrac: a new phylogenetic method for comparing microbial communities. Appl. Environ. Microbiol. 71:8228–8235. 10.1128/AEM.71.12.8228-8235.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Caporaso JG, Bittinger K, Bushman FD, DeSantis TZ, Andersen GL, Knight R. 2010. PyNAST: a flexible tool for aligning sequences to a template alignment. Bioinformatics 26:266–267. 10.1093/bioinformatics/btp636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Böckelmann U, Szewzyk U, Grohmann E. 2003. A new enzymatic method for the detachment of particle associated soil bacteria. J. Microbiol. Methods 55:201–211. 10.1016/S0167-7012(03)00144-1 [DOI] [PubMed] [Google Scholar]
- 51.Zhang Z, Schwartz S, Wagner L, Miller W. 2000. A greedy algorithm for aligning DNA sequences. J. Comput. Biol. 7:203–214. 10.1089/10665270050081478 [DOI] [PubMed] [Google Scholar]
- 52.CLSI. 2012. Performance standards for antimicrobial susceptibility testing; 22nd informational supplement CLSI document M100–S22. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 53.Friedel JK, Langer T, Siebe C, Stahr K. 2000. Effects of long-term waste water irrigation on soil organic matter, soil microbial biomass and its activities in central Mexico. Biol. Fertil. Soils 31:414–421. 10.1007/s003749900188 [DOI] [Google Scholar]
- 54.Saber M. 1986. Prolonged effect of land disposal of human wastes on soil conditions. Water Sci. Technol. 18:371–374 [Google Scholar]
- 55.Mañas P, Castro E, de Las Heras J. 2009. Irrigation with treated wastewater: effects on soil, lettuce (Lactuca sativa L.) crop and dynamics of microorganisms. J. Environ. Sci. Health 44:1261–1273. 10.1080/10934520903140033 [DOI] [PubMed] [Google Scholar]
- 56.Kiziloglu FM, Turan M, Sahin U, Angin I, Anapali O, Okuroglu M. 2007. Effects of wastewater irrigation on soil and cabbageplant (Brassica olereacea var. capitate cv. Yavola-1) chemical properties. J. Plant Nutr. Soil Sci. 170:166–172. 10.1002/jpln.200621971 [DOI] [Google Scholar]
- 57.Jueschke E, Marschner B, Tarchitzky J, Chen Y. 2008. Effects of treated wastewater irrigation on the dissolved and soil organic carbon in Israeli soils. Water Sci. Technol. 57:727–733. 10.2166/wst.2008.173 [DOI] [PubMed] [Google Scholar]
- 58.Siebe C. 1994. Akkumulation, Mobilität und Verfügbarkeit von Schwermetallen in langjährig mit städtischen Abwässern bewässerten Böden in Zentralmexiko. Hohenheimer Bodenkundliche Hefte 17. Universität Hohenheim, Stuttgart, Germany [Google Scholar]
- 59.Filip Z, Kanazawa S, Berthelin J. 1999. Characterization of effects of a long-term wastewater irrigation on soil quality by microbiological and biochemical parameters. J. Plant Nutr. Soil Sci. 162:409–413. [DOI] [Google Scholar]
- 60.Filip Z, Kanazawa S, Berthelin J. 2000. Distribution of microorganisms, biomass ATP, and enzyme activities in organic and mineral particles of a long-term wastewater irrigated soil. J. Plant Nutr. Soil Sci. 163:143–150. [DOI] [Google Scholar]
- 61.Hidri Y, Bouziri L, Maron P, Anane M, Jedidi N, Hassan A, Ranjard L. 2010. Soil DNA evidence for altered microbial diversity after long-term application of municipal wastewater. Agron. Sustain. Dev. 30:423–431. 10.1051/agro/2009038 [DOI] [Google Scholar]
- 62.Morales SE, Cosart TF, Johnson JV, Holben WE. 2009. Extensive phylogenetic analysis of a soil bacterial community illustrates extreme taxon evenness and the effects of amplicon length, degree of coverage, and DNA fractionation on classification and ecological parameters. Appl. Environ. Microbiol. 75:668–675. 10.1128/AEM.01757-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schloss PD, Handelsman J. 2005. Introducing DOTUR, a computer program for defining operational taxonomic units and estimating species richness. Appl. Environ. Microbiol. 71:1501–1506. 10.1128/AEM.71.3.1501-1506.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Janssen PH. 2006. Identifying the dominant soil bacterial taxa in libraries of 16S rRNA and 16S rRNA genes. Appl. Environ. Microbiol. 72:1719–1728. 10.1128/AEM.72.3.1719-1728.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fierer N, Leff JW, Adams BJ, Nielsen UN, Bates ST, Lauber CL, Owens S, Gilbert JA, Wall DH, Caporaso JG. 2012. Cross-biome metagenomic analyses of soil microbial communities and their functional attributes. Proc. Natl. Acad. Sci. U. S. A. 109:21390–21395. 10.1073/pnas.1215210110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nacke H, Fischer C, Thürmer A, Meinicke P, Daniel R. 2014. Land use type significantly affects microbial gene transcription in soil. Microb. Ecol. 67:919–930. 10.1007/s00248-014-0377-6 [DOI] [PubMed] [Google Scholar]
- 67.Boucher HW, Talbot GH, Bradley JS, Edwards JE, Gilbert D, Rice LB, Scheld M, Spellberg B, Bartlett J. 2009. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin. Infect. Dis. 48:1–12. 10.1086/595011 [DOI] [PubMed] [Google Scholar]
- 68.Rice LB. 2008. Federal funding for the study of antimicrobial resistance in nosocomial pathogens: no ESKAPE. J. Infect. Dis. 197:1079–1081. 10.1086/533452 [DOI] [PubMed] [Google Scholar]
- 69.Sanchez MB, Hernandez A, Martinez JL. 2009. Stenotrophomonas maltophilia drug resistance. Future Microbiol. 4:655–660. 10.2217/fmb.09.45 [DOI] [PubMed] [Google Scholar]
- 70.Denton M, Kerr KG. 1998. Microbiological and clinical aspects of infection associated with Stenotrophomonas maltophilia. Clin. Microbiol. Rev. 11:57–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brooke JS. 2012. Stenotrophomonas maltophilia: an emerging global opportunistic pathogen. Clin. Microbiol. Rev. 25:2–41. 10.1128/CMR.00019-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang L, Li XZ, Poole K. 2000. Multiple antibiotic resistance in Stenotrophomonas maltophilia: involvement of a multidrug efflux system. Antimicrob. Agents Chemother. 44:287–293. 10.1128/AAC.44.2.287-293.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yashiro E, Spear RN, McManus PS. 2011. Culture-dependent and culture-independent assessment of bacteria in the apple phyllosphere. J. Appl. Microbiol. 110:1284–1296. 10.1111/j.1365-2672.2011.04975.x [DOI] [PubMed] [Google Scholar]
- 74.Krishnamurthi S, Chakrabarti T. 2013. Diversity of bacteria and archaea from a landfill in Chandigarh, India as revealed by culture-dependent and culture-independent molecular approaches. Syst. Appl. Microbiol. 36:56–68. 10.1016/j.syapm.2012.08.009 [DOI] [PubMed] [Google Scholar]
- 75.Furlong MA, Singleton DR, Coleman DC, Whitman WB. 2002. Molecular and culture-based analyses of prokaryotic communities from an agricultural soil and the burrows and casts of the earthworm Lumbricus rubellus. Appl. Environ. Microbiol. 68:1265–1279. 10.1128/AEM.68.3.1265-1279.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McKillip JL. 2000. Prevalence and expression of enterotoxins in Bacillus cereus and other Bacillus spp., a literature review. Antonie Van Leeuwenhoek 77:393–399. 10.1023/A:1002706906154 [DOI] [PubMed] [Google Scholar]
- 77.Nicholson WL, Law JF. 1999. Method for purification of bacterial endospores from soils: UV resistance of natural Sonoran desert soil populations of Bacillus spp. with reference to B. subtilis strain 168. J. Microbiol. Methods 35:13–21. 10.1016/S0167-7012(98)00097-9 [DOI] [PubMed] [Google Scholar]
- 78.Benardini JN, Sawyer J, Venkateswaran K, Nicholson WL. 2003. Spore UV and acceleration resistance of endolithic Bacillus pumilus and Bacillus subtilis isolates obtained from Sonoran desert basalt: implications for lithopanspermia. Astrobiology 3:709–717. 10.1089/153110703322736033 [DOI] [PubMed] [Google Scholar]
- 79.Hooper DC. 1999. Mechanisms of fluoroquinolone resistance. Drug Resist. Updat. 2:38–55. 10.1054/drup.1998.0068 [DOI] [PubMed] [Google Scholar]
- 80.Hoa PTP, Managaki S, Nakada N, Takada H, Shimizu A, Anh DH, Viet PH, Suzuki S. 2011. Antibiotic contamination and occurrence of antibiotic-resistant bacteria in aquatic environments of northern Vietnam. Sci. Total Environ. 409:2894–2901. 10.1016/j.scitotenv.2011.04.030 [DOI] [PubMed] [Google Scholar]
- 81.Phuong Hoa PT, Nonaka L, Hung Viet P, Suzuki S. 2008. Detection of the sul1, sul2, and sul3 genes in sulfonamide-resistant bacteria from wastewater and shrimp ponds of north Vietnam. Sci. Total Environ. 405:377–384. 10.1016/j.scitotenv.2008.06.023 [DOI] [PubMed] [Google Scholar]
- 82.Luna VA, King DS, Gulledge J, Cannons AC, Amuso PT, Cattani J. 2007. Susceptibility of Bacillus anthracis, Bacillus cereus, Bacillus mycoides, Bacillus pseudomycoides and Bacillus thuringiensis to 24 antimicrobials using Sensititre automated microbroth dilution and Etest agar gradient diffusion methods. J. Antimicrob. Chemother. 60:555–567. 10.1093/jac/dkm213 [DOI] [PubMed] [Google Scholar]
- 83.Ding GC, Radl V, Schloter-Hai B, Jechalke S, Heuer H, Smalla K, Schloter M. 2014. Dynamics of soil bacterial communities in response to repeated application of manure containing sulfadiazine. PLoS One 9:e92958. 10.1371/journal.pone.0092958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Alonso A. 2004. Overexpression of the multidrug efflux pump SmeDEF impairs Stenotrophomonas maltophilia physiology. J. Antimicrob. Chemother. 53:432–434. 10.1093/jac/dkh074 [DOI] [PubMed] [Google Scholar]
- 85.Livermore DM. 2001. Of Pseudomonas, porins, pumps and carbapenems. J. Antimicrob. Chemother. 47:247–250. 10.1093/jac/47.3.247 [DOI] [PubMed] [Google Scholar]
- 86.German Institute for Standardization 2007. DIN 58940-3:2007-10 Medical microbiology—susceptibility testing of microbial pathogens to antimicrobial agents. Part 3: agar diffusion test. German Institute for Standardization, Berlin, Germany [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.