Abstract
Multilocus sequence analysis (MLSA) is an important method for identification of taxa that are not well differentiated by 16S rRNA gene sequences alone. In this procedure, concatenated sequences of selected genes are constructed and then analyzed. The effects that the number and the order of genes used in MLSA have on reconstruction of phylogenetic relationships were examined. The recA, rpoA, gapA, 16S rRNA gene, gyrB, and ftsZ sequences from 56 species of the genus Vibrio were used to construct molecular phylogenies, and these were evaluated individually and using various gene combinations. Phylogenies from two-gene sequences employing recA and rpoA in both possible gene orders were different. The addition of the gapA gene sequence, producing all six possible concatenated sequences, reduced the differences in phylogenies to degrees of statistical (bootstrap) support for some nodes. The overall statistical support for the phylogenetic tree, assayed on the basis of a reliability score (calculated from the number of nodes having bootstrap values of ≥80 divided by the total number of nodes) increased with increasing numbers of genes used, up to a maximum of four. No further improvement was observed from addition of the fifth gene sequence (ftsZ), and addition of the sixth gene (gyrB) resulted in lower proportions of strongly supported nodes. Reductions in the numbers of strongly supported nodes were also observed when maximum parsimony was employed for tree construction. Use of a small number of gene sequences in MLSA resulted in accurate identification of Vibrio species.
INTRODUCTION
The genus Vibrio consists of more than 100 validly described species of Gram-negative, mainly marine bacteria. Many additional candidate species have been noted (e.g., see reference 1) but have not yet been described in the literature (2, 3). Most vibrios are versatile and fast-growing chemoheterotrophs. Several species are also diazotrophic, contributing combined nitrogen to marine ecosystems (4–6). In addition to their participation in nutrient cycling, many vibrios engage in very close relationships with higher organisms. These interactions range from the bioluminescence symbiosis of Vibrio fischeri with the Hawaiian bobtail squid, Euprymna scolopes (7–9), to the many pathogenic interactions between a variety of Vibrio species and marine fauna. For example, Vibrio alginolyticus and Vibrio splendidus are bivalve-associated pathogens (10–12), Vibrio vulnificus causes vibriosis in eels (13–15), Vibrio ordalii is a pathogen of fishes (16, 17), and V. harveyi and Vibrio campbellii are pathogenic to shrimp (18, 19). Several Vibrio species are also important opportunistic human pathogens. The best known of these are Vibrio cholerae (20–22), Vibrio parahaemolyticus (23, 24), and Vibrio vulnificus (15, 25), but Vibrio cincinnatiensis (26), Vibrio fluvialis (27–29), Vibrio furnissii (30), Vibrio metschnikovii (31–33), and Vibrio mimicus (34–36) can also cause infections in human hosts. These organisms are obviously of great interest, as are additional Vibrio species that have been shown to carry an array of virulence-related genes (1).
In general, relatively little divergence of 16S rRNA gene sequences occurs among many Vibrio species (37), complicating species identification. Molecular phylogenetics is particularly problematic in the case of Vibrio species that are known or potential pathogens. All such species are very closely related to more benign species, often making correct identification of pathogenic isolates difficult (1, 38–40). Genes other than 16S rRNA genes, including the recombinase alpha subunit (recA) (41, 42), have been employed to differentiate species and to construct phylogenies, but the phylogenetic resolution that can be obtained from any single gene is perforce limited. This has led to the widespread use of multilocus sequence analysis (MLSA), which employs a number of housekeeping genes, joined end to end to construct concatenated sequences for phylogenetic analysis (42–44). The advantages of this approach include extensive databases of useful reference sequences, the low cost of DNA sequencing relative to that of detailed physiological and immunological characterizations, and the speed, ease, and accuracy of data collection and analysis. Typically, housekeeping genes that encode proteins essential to cellular reproduction are employed, as these genes are sufficiently conservative to allow accurate and facile sequence comparisons while also encompassing sufficient sequence variation to provide the needed resolution of different species (43, 44). Genes commonly employed in MLSA include recA (41, 42) and genes for RNA polymerase alpha subunit (rpoA) (42, 43), glyceraldehyde-3-phosphate dehydrogenase alpha subunit (gapA) (42, 45), cell division protein (ftsZ) (46), and DNA gyrase beta subunit (gyrB) (47), along with several others (42, 44, 48).
A broad range of gene sequences are available, and as sequencing of whole genomes continues to expand, many more are becoming available. Thus, it becomes necessary to address how many gene sequences should be employed and how they should be employed to produce efficient, economical, and robust MLSA results. The assumption implicit in many Vibrio phylogenetic studies is that addition of more genes to the analysis results in more accurate representation of the relationships of species (2, 3, 42, 50), but this assumption has not yet been subjected to rigorous testing. In addition to the genes used, the impact of gene order has not been established. The objective of this study was to determine the impacts of gene numbers and orders in the concatenated sequences on the accuracy and precision of MLSA of vibrios. We have established that that the sensitivity of MLSA saturates for concatenated sequences only a few genes in length and that addition of more sequences can in fact compromise the reliability of the method.
MATERIALS AND METHODS
Gene sequences for the 16S rRNA gene, ftsZ, gapA, gyrB, recA, and rpoA were downloaded from the NCBI GenBank database before February 2012. Sequences of a given gene that were 65% shorter than the mean sequence length (see Table S1 in the supplemental material) were excluded from the analysis. Sequences from each Vibrio species, unless otherwise noted, are from the type strain or from a well-characterized reference strain.
Individual gene sequences were validated by alignment using ClustalW in MEGA5 with the default alignment parameters (51). The alignments were checked manually, and any alignment and translation errors were corrected. Gene sequences were then combined to produce concatenated sequences for MLSA. All possible combinations of two or three genes (2 and 6 combinations, respectively) were constructed, and then gene sequences were added to the 6 three-gene concatemers to yield higher-order concatemers. Concatenated sequences were aligned and checked for alignment errors in the same manner as the individual genes. The lengths of sequences for a given gene varied, as each gene sequence set included both full-length sequences and shorter sequences derived from PCR amplicons (Table 1). Since a particular gene sequence might not be available for all species, the addition of gene sequences to concatemers reduced the number of species that could be included in the analysis. The number of species for which single gene sequences were available varied from 89 species for the 16S rRNA gene to 58 species for gyrB (see Table S1 in the supplemental material). Seventy species could be included in analyses based on the initial two-gene concatemers, but only 41 species were represented in the 6-gene concatemers (see Table S1).
TABLE 1.
Settings used in MEGA5 to construct phylogenetic trees for the maximum likelihood, minimum parsimony, minimum evolution, and neighbor joining methodsa
| Model | No. of bootstrap replicates | Substitution model | Model | Substitutions to include | Rates among sites | Gaps/missing data treatment | Select codon positions | Heuristic method(s) |
|---|---|---|---|---|---|---|---|---|
| Minimum evolution | 1,000 | Nucleotide | K-2 | TT | γ = 4 | Complete deletion | All sites | NA |
| Neighbor joining | 1,000 | Nucleotide | K-2 | TT | γ = 4 | Complete deletion | All sites | NA |
| Maximum likelihood | 1,000 | Nucleotide | K-2 | NA | γ = 4 | Complete deletion | All sites | NNI |
| NJ-BioNJ | ||||||||
| VS | ||||||||
| NT = 1 | ||||||||
| Maximum parsimony | 1,000 | Nucleotide | NA | NA | NA | Complete deletion | All sites | SPR |
| NTRA = 10 | ||||||||
| SL = 1 | ||||||||
| MT = 100 |
NA, not applicable; K-2, Kimura 2-parameter; TT, transitions and transversions; NNI, nearest neighbor interchange; NJ-BioNJ, make initial tree automatically (default NJ/BioNJ); VS, branch swap filter (very strong); NT, number of threads; SPR, subtree pruning regrafting; SL, search level; MT, maximum number of trees to retain.
Phylogenetic trees were constructed using the maximum likelihood, maximum parsimony, minimum evolution, and neighbor joining methods using MEGA5 (51). Settings utilized in the construction of the phylogenetic trees for the individual methods are given in Table 1. The clades and tree topologies from the various methods were compared for commonalities and differences. Bootstrap analysis was employed to quantitatively compare the trees. The use of bootstrap analysis in the reconstruction of phylogenetic trees provides a statistical validation through random resampling of the topology presented in the consensus tree (52–54). The number of nodes having bootstrap values greater than 80 were tallied and divided by the total number of nodes present in the tree to yield a reliability score for that tree. Statistical evaluation of variability in these scores employed the Ryan-Einot-Gabriel-Welsh F test (REGW F) in the statistical package SPSS (55), and differences were deemed statistically significant for a P value of ≤0.05.
RESULTS AND DISCUSSION
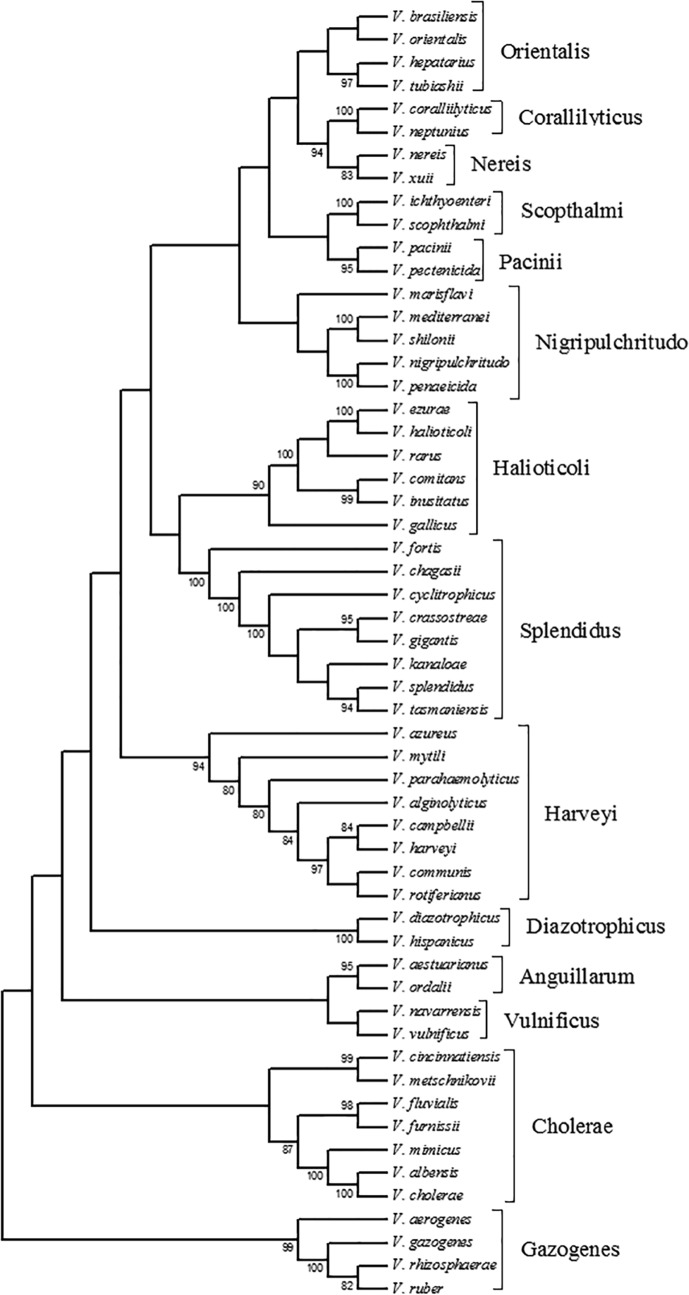
The construction of phylogenetic trees from the selected gene sequences using different models resulted in some differences in phylogenies. The most extensive changes occurred between the minimum evolution and neighbor joining methods. These changes included differences in the bifurcations present in the phylogenetic trees and most frequently occurred at bifurcations supported by bootstrap values less than 80. Phylogenetic trees constructed using maximum likelihood included many of the clades previously described by Sawabe et al. (2, 3) (Fig. 1), with additional species added to some clades.
FIG 1.
Unrooted phylogenetic reconstruction of the genus Vibrio constructed using the Kimura 2-parameter model with the maximum likelihood method, bootstrapped 1,000 times, with concatenated gene sequence order of 16S rRNA gene-gapA-recA-rpoA. Bootstrap values below 80 are not shown. Clades shown were defined by Sawabe et al. (2).
The model employed to reconstruct phylogenetic trees also had an impact on the phylogram mean reliability score. The maximum likelihood, minimum evolution, and neighbor joining models produced mean reliability scores that were not significantly different (Table 2) and phylogenies that had greater reliability than those of the maximum parsimony trees. The highest reliability scores from each method employing a single concatemer were 0.623, 0.604, 0.623, and 0.558 for maximum likelihood (16S rRNA gene-gapA-recA-rpoA), neighbor joining (16S rRNA gene-gapA-recA-rpoA and 16S rRNA gene-rpoA-gapA-recA), minimum evolution (16S rRNA gene-gapA-rpoA-recA), and maximum parsimony (16S rRNA gene-gapA-recA-rpoA-gyrB), respectively.
TABLE 2.
Mean reliability scores in phylogenetic reconstructionsa
| No. of genes in concatemers | Reliability score, mean ± SD |
|||
|---|---|---|---|---|
| Maximum likelihood | Minimum evolution | Neighbor joining | Maximum parsimony | |
| 1 | 0.27 ± 0.09 | 0.31 ± 0.10 | 0.31 ± 0.10 | 0.22 ± 0.09 |
| 2 | 0.43 ± 0.01 | 0.40 ± 0.04 | 0.42 ± 0.01 | 0.41 ± 0.01 |
| 3 | 0.53 ± 0.02 | 0.54 ± 0.01 | 0.53 ± 0.02 | 0.44 ± 0.04 |
| 4 | 0.59 ± 0.02 | 0.60 ± 0.02 | 0.59 ± 0.02 | 0.47 ± 0.02 |
| 5 | 0.57 ± 0.01 | 0.57 ± 0.01 | 0.57 ± 0.01 | 0.51 ± 0.03 |
| 6 | 0.55 ± 0.01 | 0.56 ± 0.01 | 0.55 ± 0.01 | 0.47 ± 0.01 |
Each reliability score is the number of nodes with bootstrap values greater than or equal to 80 divided by the total number of nodes. Standard deviations represent 1 standard deviation from the mean. The genes used in the concatemers are as follows: 16S rRNA gene, ftsZ, gapA, gyrB, recA, or rpoA in 1-gene concatemers, recA and rpoA in 2-gene concatemers, recA, rpoA, and gapA in 3-gene concatemers, 16S rRNA gene, recA, rpoA, and gapA in 4-gene concatemers, 16S rRNA gene, recA, rpoA, gapA, and gyrB in 5-gene concatemers, and 16S rRNA gene, recA, rpoA, gapA, gyrB, and ftsZ in 6-gene concatemers.
The number of gene sequences present in the concatemer also influenced the reliability score. The addition of genes to the concatemers, followed by use of the maximum likelihood, minimum evolution, and neighbor joining models, resulted in increased reliability through the addition of the fourth gene (Table 2). Addition of the fifth gene did not significantly increase reliability, and addition of the sixth gene decreased the reliability of the phylogenetic analysis. The reliability scores for trees constructed using maximum parsimony increased with addition of genes to the concatemers up to a maximum of five genes. The addition of the sixth gene caused the score to decrease to that obtained using four genes.
The phylogenetic trees constructed with two genes, recA and rpoA, included 13 of the 14 clades previously described by Sawabe et al. (2), and this was not affected by the order of the two genes. The two phylogenetic reconstructions had similar topologies, although there were changes in bifurcations having bootstrap support lower than 80. The addition of gapA to the concatemers, in all six gene order combinations with recA and rpoA, had minimal impact on the tree topology. Thirteen of the 14 clades were retained in phylogenetic trees using these concatemers, with the Vulnificus clade decomposing and the placement of V. navarrensis in the Gazogenes clade when three of the six concatemers were employed (gapA-recA-rpoA, recA-gapA-rpoA, and rpoA-recA-gapA). The Vulnificus clade had low bootstrap support when it was observed. The addition of the 16S rRNA gene to the six order combinations of gapA, recA, and rpoA had minimal effect on tree topology, with changes occurring only at bifurcations having low support. The addition of the 16S rRNA gene to the previous concatemers retained all 14 clades, although three clades (Orientalis, Nigripulchritudo, and Vulnificus) had low bootstrap support. The addition of the fourth gene to the concatemers allowed the closely related species V. harveyi, V. campbellii, and V. rotiferianus to be differentiated with significant bootstrap values in all models except for maximum parsimony.
Thirteen of the 14 clades were resolved following addition of the gyrB gene to the concatemers containing the 16S rRNA gene, gapA, recA, and rpoA. The Orientalis clade decomposed with V. tubiashii grouping with the Coralliilyticus clade (with significant bootstrap support) in five of the concatemers. Bootstrap support for the Coralliilyticus clade was not significant for the tree produced using the 16S rRNA gene-gapA-rpoA-recA-gyrB concatemer. Minor topological changes occurred at bifurcations having low bootstrap support. The addition of the ftsZ gene resulted in decomposition of the Orientalis clade, as well as minor topological changes at nodes having low bootstrap support. Clade support decreased with the addition of ftsZ in three of the six combinations, resulting in decreases below a bootstrap value of 80 for the Cholerae and Orientalis clades. These concatemers produced the only phylogenetic reconstructions in which bootstrap support for the Cholerae clade was less than 80.
Gene order had differing effects on the reconstruction of phylogenetic relationships, based on the model used. Phylogenetic trees reconstructed using the minimum evolution method appeared to be most susceptible to changes in gene order when using two genes. In the recA-rpoA concatemer the number of nodes having bootstrap scores greater than 80 was 29, and in the rpoA-recA order the number of strongly supported nodes was only 25. Another notable change occurred when using the maximum parsimony model. In the case of concatemers containing three genes in the order rpoA-recA-gapA, 28 nodes had bootstrap support greater than or equal to 80, while the average number of nodes with values greater than or equal to 80 for the remaining permutations was 23.1 ± 2.5. The order of genes used in the maximum parsimony model appears to have an impact on the number of nodes that are statistically supported. The variation driven by gene order appears to be dampened with an increase in the number of genes utilized, with most variation occurring in the two-gene concatemers and that variation decreasing with each gene addition, with the previously stated exceptions. The variation of the reliability scores yielded by all four methods suggests that gene order can have an impact on the outcome of the analysis, with the greatest impact occurring in the maximum parsimony method.
The reliability of phylogenetic reconstructions employing concatemers having different numbers of genes varied depending on the model employed. The REGW F test, when applied to reliability scores for trees built from concatemers employing from three genes up to six genes, defined two subsets at P values of ≤0.05. The first subset included only trees built using the maximum parsimony method, which produced mean reliability scores that were significantly lower in all cases than those from the other models. The second subset included the maximum likelihood, minimum evolution, and neighbor joining models, which were not significantly different from each other (Table 3).
TABLE 3.
Intermodel comparison of mean reliability scoresa
| No. of genes | Model | Reliability score for subset |
|
|---|---|---|---|
| 1 | 2 | ||
| 3 | Maximum parsimony | 0.437 | |
| Maximum likelihood | 0.538 | ||
| Minimum evolution | 0.528 | ||
| Neighbor joining | 0.541 | ||
| 4 | Maximum parsimony | 0.475 | |
| Maximum likelihood | 0.588 | ||
| Minimum evolution | 0.597 | ||
| Neighbor joining | 0.585 | ||
| 5 | Maximum parsimony | 0.512 | |
| Maximum likelihood | 0.574 | ||
| Minimum evolution | 0.570 | ||
| Neighbor joining | 0.574 | ||
| 6 | Maximum parsimony | 0.467 | |
| Maximum likelihood | 0.549 | ||
| Minimum evolution | 0.557 | ||
| Neighbor joining | 0.545 | ||
Each reliability score is the number of nodes with bootstrap values greater than or equal to 80 divided by total number of nodes of phylogenetic trees reconstructed using the maximum parsimony, maximum likelihood, minimum evolution, and neighbor joining methods grouped into subsets (groups of homogenous means) with a P value of 0.05 for confidence level using the Ryan-Einot-Gabriel-Welsch F test. The genes used in the concatemers are as follows: 16S rRNA gene, ftsZ, gapA, gyrB, recA, or rpoA in 1-gene concatemers, recA and rpoA in 2-gene concatemers, recA, rpoA, and gapA in 3-gene concatemers, 16S rRNA gene, recA, rpoA, and gapA in 4-gene concatemers, 16S rRNA gene, recA, rpoA, gapA, and gyrB in 5-gene concatemers, and 16S rRNA gene, recA, rpoA, gapA, gyrB, and ftsZ in 6-gene concatemers.
The optimum number of genes employed in the concatemers is influenced by the phylogenetic model used (Table 4). The REGW F test results grouped the maximum likelihood and neighbor joining products into three subsets. Concatemers utilizing five or four genes yielded higher reliability scores than those containing two, three, and six genes. The grouping of the four- and five-gene concatemers indicates that these two means are not significantly different at a P value of 0.05. The minimum evolution model produced four subsets, with the four-gene concatemers grouped separately, indicating that the highest reliability score occurs in the minimum evolution model with four genes. Maximum parsimony grouped the concatemers into three subsets that overlapped extensively, and the highest reliability score was obtained using four genes.
TABLE 4.
Intramodel comparison of mean reliability scoresa
| Model | No. of genes | Reliability score for subset |
|||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||
| Maximum likelihood | 2 | 0.425 | |||
| 3 | 0.538 | ||||
| 6 | 0.549 | ||||
| 5 | 0.574 | ||||
| 4 | 0.588 | ||||
| Neighbor joining | 2 | 0.418 | |||
| 3 | 0.528 | ||||
| 6 | 0.545 | ||||
| 5 | 0.574 | ||||
| 4 | 0.585 | ||||
| Minimum evolution | 2 | 0.403 | |||
| 3 | 0.541 | ||||
| 6 | 0.557 | 0.557 | |||
| 5 | 0.570 | ||||
| 4 | 0.597 | ||||
| Maximum parsimony | 2 | 0.410 | |||
| 3 | 0.437 | 0.437 | |||
| 6 | 0.467 | 0.467 | 0.467 | ||
| 4 | 0.475 | 0.475 | |||
| 5 | 0.512 | ||||
Each reliability score is the number of nodes with bootstrap values greater than or equal to 80 divided by total number of nodes of phylogenetic trees reconstructed using the maximum likelihood, neighbor joining, minimum evolution, and maximum parsimony methods grouped into subsets. A group of homogenous means is listed from lowest to highest, with a P value of 0.05 for confidence level using the Ryan-Einot-Gabriel-Welsh F test. The genes used in the concatemers are as follows: 16S rRNA gene, ftsZ, gapA, gyrB, recA, or rpoA in 1-gene concatemers, recA and rpoA in 2-gene concatemers, recA, rpoA, and gapA in 3-gene concatemers, 16S rRNA gene, recA, rpoA, and gapA in 4-gene concatemers, 16S rRNA gene, recA, rpoA, gapA, and gyrB in 5-gene concatemers, and 16S rRNA gene, recA, rpoA, gapA, gyrB, and ftsZ in 6-gene concatemers.
This analysis showed that the specific genes employed in MLSA, the numbers of these genes, the orders of genes in concatemers, and the model used to reconstruct phylogenetic trees for species in the genus Vibrio all have impacts on the reliability of the analysis. The order of genes appeared to matter most when either very short concatemers or the maximum parsimony model was used. The maximum parsimony model removes from the analysis all sites that do not affect tree topology, therefore reducing the amount of data that is available for phylogenetic reconstruction (56) and likely contributing to the impact of gene order. The number of genes used in the concatemers appears to have a strong impact on the reliability of the final phylogenetic tree, but not necessarily the impact expected. For the maximum likelihood, minimum evolution, maximum parsimony, and neighbor joining models, the optimum number of genes that should be employed is four genes, as four-gene concatemers yielded the highest mean reliability scores. Including more genes in concatemers in an effort to increase the accuracy of the MLSA method may not yield the expected result but does require more sequencing and a greater time commitment to concatemer construction and quality control, and it is more computationally intensive. It should be noted that the concatemers used in this study yielded the most reliable phylogenetic reconstructions for species of Vibrio, but the use of different genes may yield different results and use of the genes employed here may yield different results for other bacterial genera. When looking at genera that include more divergent species, the number of genes required to accurately define species could be fewer than for genera consisting of less divergent species. The model employed can also impact the overall reliability of the MLSA phylogenetic reconstructions.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by South Carolina SeaGrant Sub-Award N156 to C.R.L. M.W.G. acknowledges support from an American Society for Microbiology undergraduate research fellowship.
We thank Jay Pinckney for statistical assistance.
Footnotes
Published ahead of print 20 June 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.01206-14.
REFERENCES
- 1.Klein SL, Guitierrez West CK, Mejia DM, Lovell CR. 2014. Genes similar to the Vibrio parahaemolyticus virulence-related genes tdh, tlh, and vscC2 occur in other Vibrionaceae species isolated from a pristine estuary. Appl. Environ. Microbiol. 80:595–602. 10.1128/AEM.02895-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sawabe T, Kita-Tsukamoto K, Thompson FL. 2007. Inferring the evolutionary history of vibrios by means of multilocus sequence analysis. J. Bacteriol. 189:7932–7936. 10.1128/JB.00693-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sawabe T, Ogura Y, Matsumura Y, Feng G, Amin AKMR, Mino S, Nakagawa S, Sawabe T, Kumar R, Fukui Y, Santomi M, Matsushima R, Thompson FL, Gomez-Gil B, Christian R, Maruyama F, Kurokawa K, Hayashi T. 2013. Updating the Vibrio clades defined by multilocus sequence phylogeny: proposal of eight new clades, and the novel description of Vibrio tritonius sp. nov. Front. Microbiol. 4:414. 10.3389/fmicb.2013.00414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown MM, Friez MJ, Lovell CR. 2003. Expression of nifH genes by diazotrophic bacteria in the rhizosphere of short form Spartina alterniflora. FEMS Microbiol. Ecol. 43:411–417. 10.1111/j.1574-6941.2003.tb01081.x [DOI] [PubMed] [Google Scholar]
- 5.Criminger JD, Hazen TH, Sobecky PA, Lovell CR. 2007. Nitrogen fixation by Vibrio parahaemolyticus and its implications for a new ecological niche. Appl. Environ. Microbiol. 73:5959–5961. 10.1128/AEM.00981-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Urdaci MC, Stal LJ, Marchand M. 1988. Occurrence of nitrogen fixation among Vibrio spp. Arch. Microbiol. 150:224–229. 10.1007/BF00407784 [DOI] [Google Scholar]
- 7.Miyashiro T, Ruby EG. 2012. Shedding light on bioluminescence regulation in Vibrio fischeri. Mol. Microbiol. 84:795–806. 10.1111/j.1365-2958.2012.08065.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ruby EG. 1996. Lessons from a cooperative, bacterial-animal association: the Vibrio fischeri-Euprymna scolopes light organ symbiosis. Annu. Rev. Microbiol. 50:591–624. 10.1146/annurev.micro.50.1.591 [DOI] [PubMed] [Google Scholar]
- 9.Stabb EV. 2006. The Vibrio fischeri-Euprymna scolopes light organ symbiosis, p 204–218 In Thompson FL, Austin B, Swings J. (ed), The biology of vibrios. ASM Press, Washington DC [Google Scholar]
- 10.Gómez-León J, Villamil L, Lemos ML, Novoa B, Figueras A. 2005. Isolation of Vibrio alginolyticus and Vibrio splendidus from aquacultured carpet shell clam (Ruditapes decussatus) larvae associated with mass mortalities. Appl. Environ. Microbiol. 71:98–104. 10.1128/AEM.71.1.98-104.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sugumar G, Nakai T, Hirata Y, Matsubara D, Muroga K. 1998. Vibrio splendidus biovar II as the causative agent in bacillary necrosis of Japanese oyster Crassostrea gigas larvae. Dis. Aquat. Org. 33:111–118. 10.3354/dao033111 [DOI] [PubMed] [Google Scholar]
- 12.Luna-González A, Maeda-Martinez AN, Sainz JC, Ascencio-Valle F. 2002. Comparative susceptibility of veliger larvae of four bivalve mollusks to a Vibrio alginolyticus strain. Dis. Aquat. Org. 49:221–226. 10.3354/dao049221 [DOI] [PubMed] [Google Scholar]
- 13.Amaro C, Biosca EG. 1996. Vibrio vulnificus biotype 2, pathogenic for eels, is also an opportunistic pathogen for humans. Appl. Environ. Microbiol. 62:1454–1457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tison DL, Nishibuchi M, Greenwood JD, Seidler RJ. 1982. Vibrio vulnificus biogroup 2: new biogroup pathogenic for eels. Appl. Environ. Microbiol. 44:640–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones MK, Oliver JD. 2009. Vibrio vulnificus: disease and pathogenesis. Infect. Immun. 77:1723–1733. 10.1128/IAI.01046-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Toranzo AE, Magariños B, Romalde JL. 2005. A review of the main bacterial fish diseases in mariculture systems. Aquaculture 246:37–61. 10.1016/j.aquaculture.2005.01.002 [DOI] [Google Scholar]
- 17.Schiewe MH, Trust TJ, Crosa JH. 1981. Vibrio ordalii sp. nov.: a causative agent of vibriosis in fish. Curr. Microbiol. 6:343–348. 10.1007/BF01567009 [DOI] [Google Scholar]
- 18.Soto-Rodriguez SA, Gomez-Gil B, Lozano R. 2010. ‘Bright-red' syndrome in Pacific white shrimp Litopenaeus vannamei is caused by Vibrio harveyi. Dis. Aquat. Org. 92:11–19. 10.3354/dao02274 [DOI] [PubMed] [Google Scholar]
- 19.Haldar S, Chatterjee S, Sugimoto N, Das S, Chowdhury N, Hinenoyu A, Asakura M, Yamasaki S. 2011. Identification of Vibrio campbellii isolated from diseased farm-shrimp from south India and establishment of its pathogenic potential in an Artemia model. Microbiology 157:179–188. 10.1099/mic.0.041475-0 [DOI] [PubMed] [Google Scholar]
- 20.Colwell RR, Brayton PR, Grimes DJ, Roszak DB, Huq SA, Palmer LM. 1985. Viable but non-culturable Vibrio cholerae and related pathogens in the environment: implications for release of genetically engineered microorganisms. Nat. Biotechnol. 3:817–820. 10.1038/nbt0985-817 [DOI] [Google Scholar]
- 21.Cottingham KL, Chiavelli DA, Taylor RK. 2003. Environmental microbe and human pathogen: the ecology and microbiology of Vibrio cholerae. Front. Ecol. Environ. 1:80–86. 10.1890/1540-9295(2003)001[0080:EMAHPT]2.0.CO;2 [DOI] [Google Scholar]
- 22.Dziejman M, Balon E, Boyd D, Fraser CM, Heidelberg JF, Mekalanos JJ. 2002. Comparative genomic analysis of Vibrio cholerae: genes that correlate with cholera endemic and pandemic disease. Proc. Natl. Acad. Sci. U. S. A. 99:1556–1561. 10.1073/pnas.042667999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Daniels NA, MacKinnon L, Bishop R, Altekruse S, Ray B, Hammond RM, Thompson S, Wilson S, Bean NH, Griffin PM, Slutsker L. 2000. Vibrio parahaemolyticus infections in the United States, 1973–1998. J. Infect. Dis. 181:1661–1666. 10.1086/315459 [DOI] [PubMed] [Google Scholar]
- 24.Velazquez-Roman J, León-Sicairos N, Flores-Villaseñor H, Villafaña-Rauda S, Canizalez-Roman A. 2012. Association of pandemic Vibrio parahaemolyticus O3:K6 present in the coastal environment of northwest Mexico with cases of recurrent diarrhea between 2004 and 2010. Appl. Environ. Microbiol. 78:1794–1803. 10.1128/AEM.06953-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Strom MS, Paranjpye RN. 2000. Epidemiology and pathogenesis of Vibrio vulnificus. Microbes Infect. 2:177–188. 10.1016/S1286-4579(00)00270-7 [DOI] [PubMed] [Google Scholar]
- 26.Brayton PR, Bode RB, Colwell RR, MacDonell MT, Hall HL, Grimes DJ, West PA, Bryant TN. 1986. Vibrio cincinnatiensis sp. nov., a new human pathogen. J. Clin. Microbiol. 23:104–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Igbinosa EO, Okoh AL. 2010. Vibrio fluvialis: an unusual enteric pathogen of increasing public health concern. Int. J. Environ. Res. Public Health 7:3628–3643. 10.3390/ijerph7103628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chakraborty R, Chakraborty S, De K, Sinha S, Mukhopadhyay AK, Khanam J, Ramamurthy T, Takeda Y, Bhattacharya SK, Nair GB. 2005. Cytotoxic and cell vacuolating activity of Vibrio fluvialis isolated from paediatric patients with diarrhoea. J. Med. Microbiol. 54:707–716. 10.1099/jmm.0.45820-0 [DOI] [PubMed] [Google Scholar]
- 29.Chowdhury G, Pazhani GP, Dutta D, Guin S, Dutta S, Ghosh S, Izumiya H, Asakura M, Yamasaki S, Takeda Y, Arakawa E, Wantanabe H, Mukhpadhyay AK, Bhattacharya MK, Rajendran K, Nair GB, Ramamurthy T. 2012. Vibrio fluvialis in patients with diarrhea, Kolkata, India. Emerg. Infect. Dis. 18:1868–1871. 10.3201/eid1811.120520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brenner DJ, Hickman-Brenner FW, Lee JV, Steigerwalt AG, Fanning GR, Hollis DG, Farmer JJ, III, Weaver RE, Joseph SW, Seidler RJ. 1983. Vibrio furnissii (formerly aerogenic biogroup of Vibrio fluvialis), a new species isolated from human feces and the environment. J. Clin. Microbiol. 18:816–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Linde H-J, Kobuch R, Jayasinghe S, Reischal V, Lehn N, Kaulfuss S, Beutin L. 2004. Vibrio metschnikovii, a rare cause of wound infection. J. Clin. Microbiol. 42:4909–4911. 10.1128/JCM.42.10.4909-4911.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dalsgaard A, Alarcon A, Lanata CF, Jensen T, Hansen HJ, Delgado F, Gil AI, Penny ME, Taylor D. 1996. Clinical manifestations and molecular epidemiology of five cases of diarrhoea in children associated with Vibrio metschnikovii in Arequipa, Peru. J. Med. Microbiol. 45:494–500. 10.1099/00222615-45-6-494 [DOI] [PubMed] [Google Scholar]
- 33.Wallet F, Tachon M, Nseir S, Courcol RJ, Roussel-Delvallez M. 2005. Vibrio metschnikovii pneumonia. Emerg. Infect. Dis. 11:1641–1642. 10.3201/eid1110.050177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vieira VV, Teixeira LFM, Vicente ACP, Momen H, Salles CA. 2001. Differentiation of environmental and clinical isolates of Vibrio mimicus from Vibrio cholerae by multilocus enzyme electrophoresis. Appl. Environ. Microbiol. 67:2360–2364. 10.1128/AEM.67.5.2360-2364.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Campos E, Bolaños H, Acuña MT, Diaz G, Matamoros MC, Raventós H, Sánchez LM, Sánchez O, Barquero C. 1996. Vibrio mimicus diarrhea following ingestion of raw turtle eggs. Appl. Environ. Microbiol. 62:1141–1144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chitov T, Kirikaew P, Yungyune P, Ruengprapan N, Sontikun K. 2009. An incidence of large foodborne outbreak associated with Vibrio mimicus. Eur. J. Clin. Microbiol. Infect. Dis. 28:421–424. 10.1007/s10096-008-0639-7 [DOI] [PubMed] [Google Scholar]
- 37.Dorsch M, Lane D, Stackebrandt E. 1992. Towards a phylogeny of the Genus Vibrio based on 16S rRNA sequences. Int. J. Syst. Evol. Microbiol. 42:58–63 [DOI] [PubMed] [Google Scholar]
- 38.Farmer JJ, III, Janda JM, Brenner FM, Cameron DN, Birkhead KM. 2005. Genus I. Vibrio Pacini 1854, 411AL, p 494–546 In Brenner DJ, Kreig NR, Staley JT, Garrity GM. (ed), Bergey's manual of systematic bacteriology, 2nd ed, vol 2, part B Springer, New York, NY [Google Scholar]
- 39.Stackebrandt E, Goebel BM. 1994. Taxonomic note: a place for DNA-DNA reassociation and 16S rRNA sequence analysis in the present species definition in bacteriology. Int. J. Syst. Evol. Microbiol. 44:846–849 [Google Scholar]
- 40.Ruimy R, Breittmayer V, Elbaze P, Lafay B, Boussemart O, Gauthier M, Christen R. 1994. Phylogenetic analysis and assessment of the genera Vibrio, Photobacterium, Aeromonas, and Plesiomonas deduced from small-subunit rRNA sequences. Int. J. Syst. Evol. Microbiol. 44:416–426 [DOI] [PubMed] [Google Scholar]
- 41.Thompson CC, Thompson FL, Vandemeulebroecke K, Hoste B, Dawyndt P, Swings J. 2004. Use of recA as an alternative phylogenetic marker in the family Vibrionaceae. Int. J. Syst. Evol. Microbiol. 54:919–924. 10.1099/ijs.0.02963-0 [DOI] [PubMed] [Google Scholar]
- 42.Thompson FL, Gomez-Gil B, Vasconcelos ATR, Sawabe T. 2007. Multilocus sequence analysis reveals that Vibrio harveyi and V. campbellii are distinct species. Appl. Environ. Microbiol. 73:4279–4285. 10.1128/AEM.00020-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thompson FL, Gevers D, Thompson CC, Dawyndt P, Naser S, Hoste B, Munn CB, Swings J. 2005. Phylogeny and molecular identification of vibrios on the basis of multilocus sequence analysis. Appl. Environ. Microbiol. 71:5107–5115. 10.1128/AEM.71.9.5107-5115.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martens M, Dawyndt P, Coopman R, Gillis M, De Vos P, Willems A. 2008. Advantages of multilocus sequence analysis for taxonomic studies: a case study using 10 housekeeping genes in the genus Ensifer (including former Sinorhizobium). Int. J. Syst. Evol. Microbiol. 58:200–214. 10.1099/ijs.0.65392-0 [DOI] [PubMed] [Google Scholar]
- 45.Nishiguchi MK, Nair VS. 2003. Evolution of symbiosis in the Vibrionaceae: a combined approach using molecules and physiology. Int. J. Syst. Evol. Microbiol. 53:2019–2026. 10.1099/ijs.0.02792-0 [DOI] [PubMed] [Google Scholar]
- 46.Tracz DM, Backhouse PG, Olson AB, McCrea JK, Walsh JA, Ng LK, Gilmour MW. 2007. Rapid detection of Vibrio species using liquid microsphere arrays and real-time PCR targeting the ftsZ locus. J. Med. Microbiol. 56:56–65. 10.1099/jmm.0.46759-0 [DOI] [PubMed] [Google Scholar]
- 47.Le Roux F, Gay M, Lambert C, Nicolas JL, Gouy M, Berthe F. 2004. Phylogenetic study and identification of Vibrio splendidus-related strains based on gyrB gene sequences. Dis. Aquat. Org. 58:143–150. 10.3354/dao058143 [DOI] [PubMed] [Google Scholar]
- 48.Gevers D, Vandepoele K, Simillion C, Van de Peer Y. 2004. Gene duplication and biased functional retention of paralogs in bacterial genomes. Trends Micriobiol. 12:148–154. 10.1016/j.tim.2004.02.007 [DOI] [PubMed] [Google Scholar]
- 49. Reference deleted.
- 50.Pascual J, Macián MC, Arahal DR, Garay E, Pujalte MJ. 2010. Multilocus sequence analysis of the central clade of the genus Vibrio by using 16S rRNA, recA, pyrH, rpoD, gyrB, rctB and toxR genes. Int. J. Syst. Evol. Microbiol. 60:154–165. 10.1099/ijs.0.010702-0 [DOI] [PubMed] [Google Scholar]
- 51.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. 2011. MEGA5: molecular evolutionary genetics using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28:2731–2739. 10.1093/molbev/msr121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Felsenstein J. 1985. Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39:783–791. 10.2307/2408678 [DOI] [PubMed] [Google Scholar]
- 53.Alfaro ME, Zoller S, Lutzoni F. 2003. Bayes or bootstrap? A simulation study comparing the performance of Bayesian Markov chain Monte Carlo sampling and bootstrapping in assessing phylogenetic confidence. Mol. Biol. Evol. 20:255–266. 10.1093/molbev/msg028 [DOI] [PubMed] [Google Scholar]
- 54.Hedges SB. 1992. The number of replications needed for accurate estimation of the bootstrap P value in phylogenetic studies. Mol. Biol. Evol. 9:366–369 [DOI] [PubMed] [Google Scholar]
- 55.IBM Corp. 2012. IBM SPSS statistics for Windows, version 21.0. IBM Corp, Armonk, NY [Google Scholar]
- 56.Stewart CB. 1993. The powers and pitfalls of parsimony. Nature 361:603–607. 10.1038/361603a0 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.