Abstract
Calmodulin has long been suspected to be involved in calcium-regulated exocytosis but its precise site(s) of action has not yet been identified. In Paramecium, a genetic approach to the problem is possible as in vivo-selected mutations in the calmodulin gene that prevent the activation of some channels have been characterized. Three of these calmodulin mutants were examined for exocytotic capacity and the mutant cam1 was found to be defective for exocytosis at 35 degrees C. The loss of exocytotic capacity in cam1 cells can be restored by transformation with the wild-type calmodulin gene, demonstrating that its exocytotic lesion is indeed due to the mutation in the calmodulin gene. The cam1 mutant displays abnormal exocytotic sites at the non-permissive temperature: it lacks the links ('rosettes' of intramembranous particles in the plasma membrane and the fibrous 'connecting material') which normally connect plasma and trichocyst membranes. Upon shift of cam1 cells from the permissive to a non-permissive temperature, performed sites remain functional. These results demonstrate that calmodulin is necessary for the assembly of these links at the exocytotic site. These results do not, however, exclude the possibility of calmodulin also being involved in Ca(2+)-dependent steps of the stimulus-exocytosis coupling.
Full text
PDF
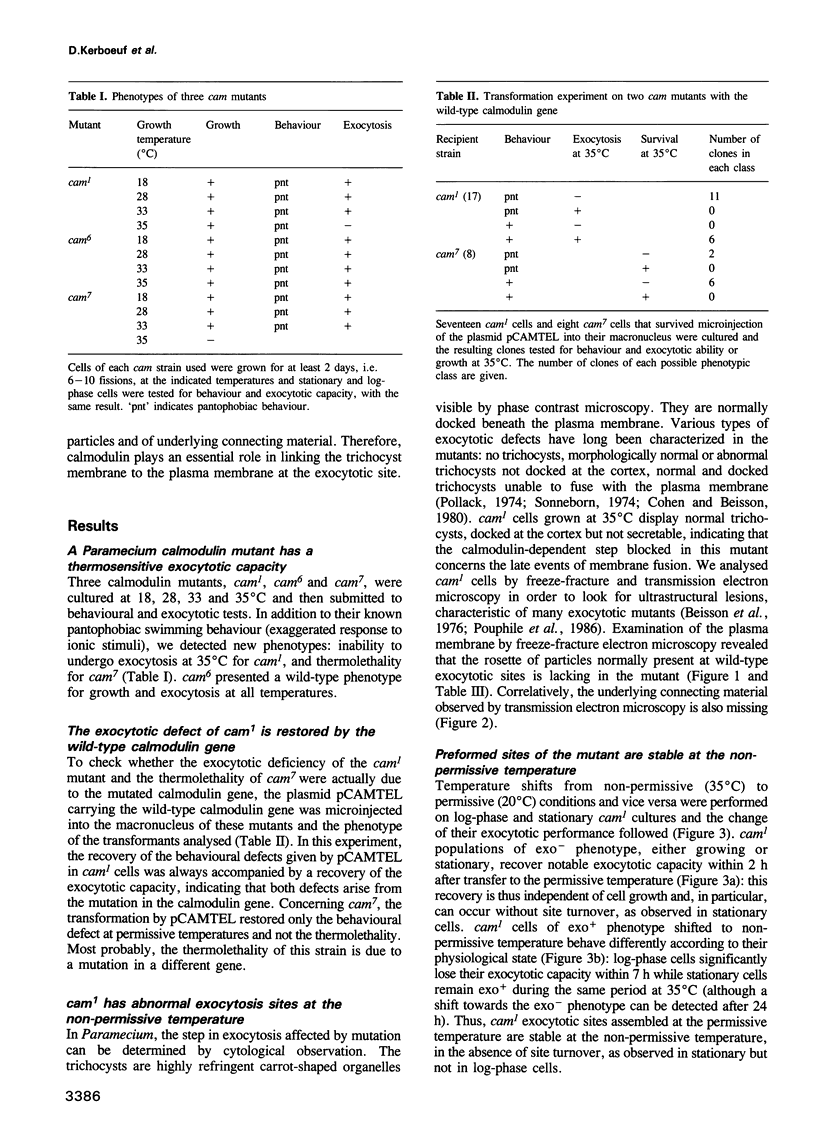

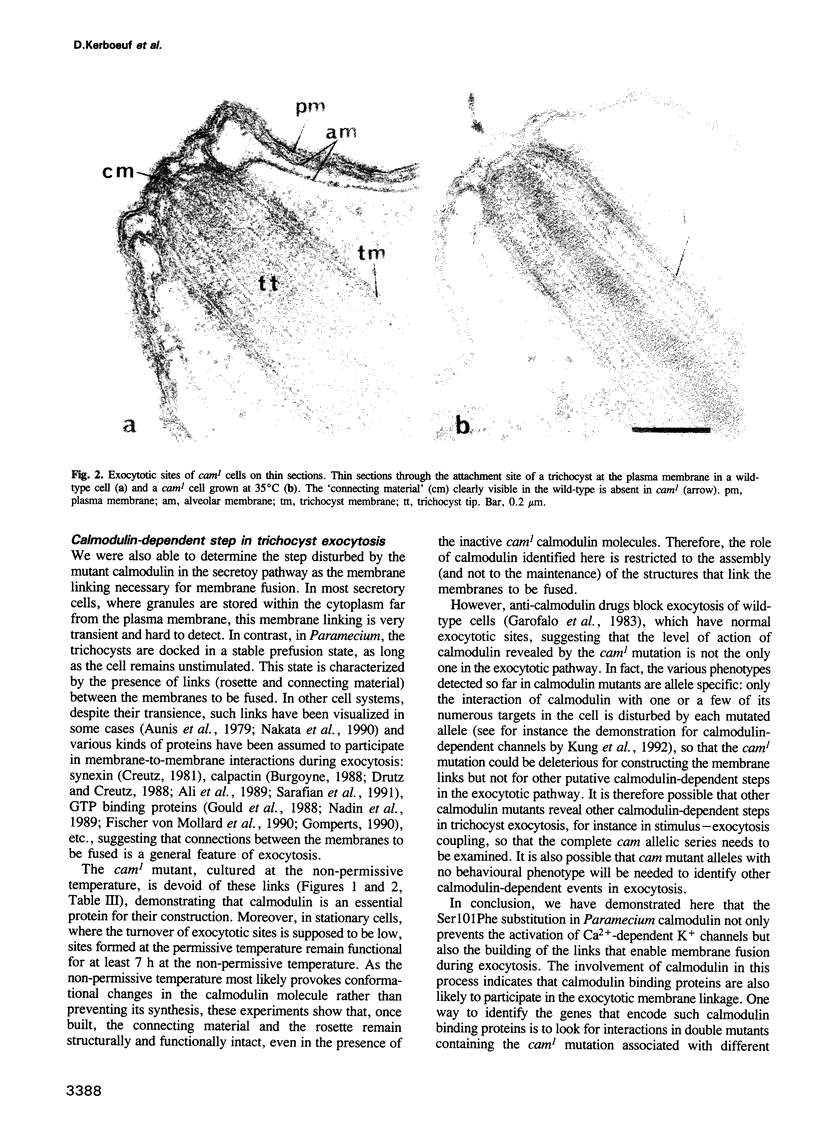
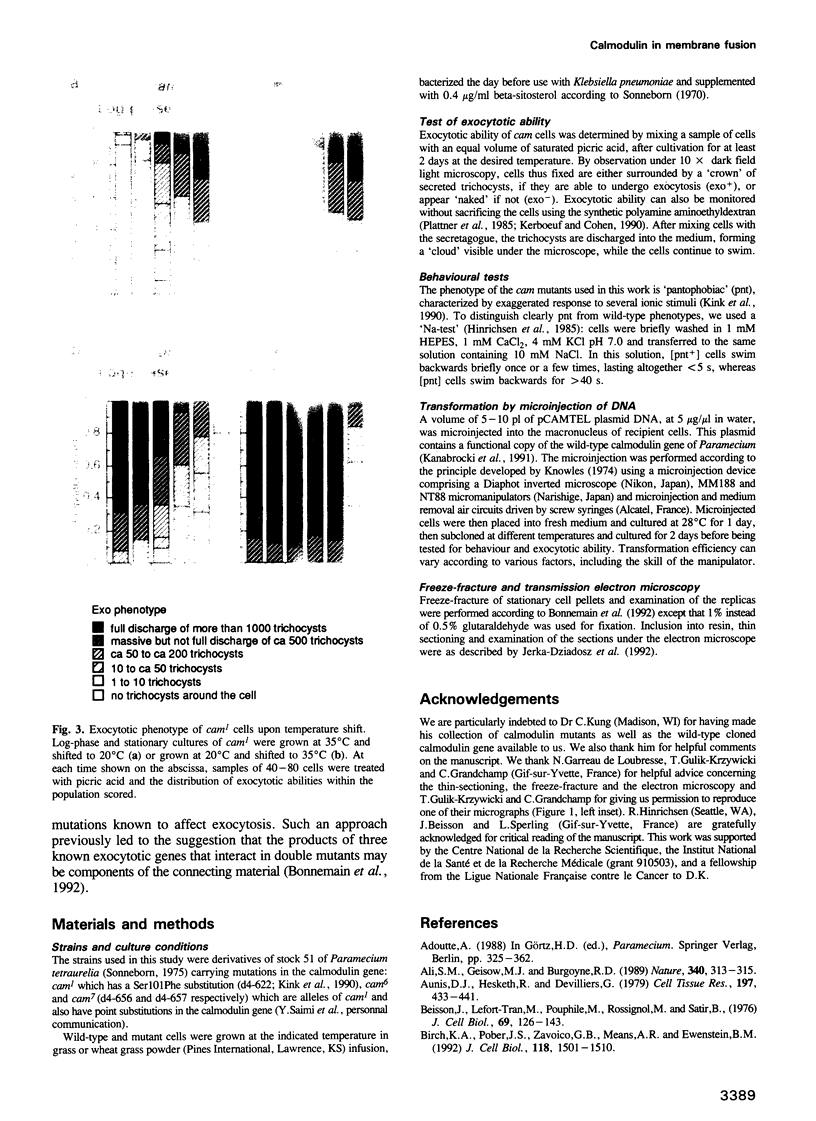

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ali S. M., Geisow M. J., Burgoyne R. D. A role for calpactin in calcium-dependent exocytosis in adrenal chromaffin cells. Nature. 1989 Jul 27;340(6231):313–315. doi: 10.1038/340313a0. [DOI] [PubMed] [Google Scholar]
- Aunis D., Hesketh J. E., Devilliers G. Freeze-fracture study of the chromaffin cell during exocytosis: evidence for connections between the plasma membrane and secretory granules and for movements of plasma membrane-associated particles. Cell Tissue Res. 1979 Apr 12;197(3):433–441. doi: 10.1007/BF00233568. [DOI] [PubMed] [Google Scholar]
- Beisson J., Lefort-Tran M., Pouphile M., Rossignol M., Satir B. Genetic analysis of membrane differentiation in Paramecium. Freeze-fracture study of the trichocyst cycle in wild-type and mutant strains. J Cell Biol. 1976 Apr;69(1):126–143. doi: 10.1083/jcb.69.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch K. A., Pober J. S., Zavoico G. B., Means A. R., Ewenstein B. M. Calcium/calmodulin transduces thrombin-stimulated secretion: studies in intact and minimally permeabilized human umbilical vein endothelial cells. J Cell Biol. 1992 Sep;118(6):1501–1510. doi: 10.1083/jcb.118.6.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnemain H., Gulik-Krzywicki T., Grandchamp C., Cohen J. Interactions between genes involved in exocytotic membrane fusion in paramecium. Genetics. 1992 Mar;130(3):461–470. doi: 10.1093/genetics/130.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgoyne R. D. Calpactin in exocytosis. Nature. 1988 Jan 7;331(6151):20–20. doi: 10.1038/331020a0. [DOI] [PubMed] [Google Scholar]
- Burgoyne R. D., Geisow M. J., Barron J. Dissection of stages in exocytosis in the adrenal chromaffin cell with use of trifluoperazine. Proc R Soc Lond B Biol Sci. 1982 Aug 23;216(1202):111–115. doi: 10.1098/rspb.1982.0064. [DOI] [PubMed] [Google Scholar]
- Cheek T. R. Spatial aspects of calcium signalling. J Cell Sci. 1989 Jun;93(Pt 2):211–216. doi: 10.1242/jcs.93.2.211. [DOI] [PubMed] [Google Scholar]
- Cohen J., Beisson J. Genetic analysis of the relationships between the cell surface and the nuclei in Paramecium tetraurella. Genetics. 1980 Aug;95(4):797–818. doi: 10.1093/genetics/95.4.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creutz C. E. cis-Unsaturated fatty acids induce the fusion of chromaffin granules aggregated by synexin. J Cell Biol. 1981 Oct;91(1):247–256. doi: 10.1083/jcb.91.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W. Involvement of calcium in exocytosis and the exocytosis--vesiculation sequence. Biochem Soc Symp. 1974;(39):1–28. [PubMed] [Google Scholar]
- Douglas W. W. Stimulus-secretion coupling: the concept and clues from chromaffin and other cells. Br J Pharmacol. 1968 Nov;34(3):451–474. doi: 10.1111/j.1476-5381.1968.tb08474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drust D. S., Creutz C. E. Aggregation of chromaffin granules by calpactin at micromolar levels of calcium. Nature. 1988 Jan 7;331(6151):88–91. doi: 10.1038/331088a0. [DOI] [PubMed] [Google Scholar]
- Fischer von Mollard G., Mignery G. A., Baumert M., Perin M. S., Hanson T. J., Burger P. M., Jahn R., Südhof T. C. rab3 is a small GTP-binding protein exclusively localized to synaptic vesicles. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1988–1992. doi: 10.1073/pnas.87.5.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguly A., Chiou S., Fineberg N. S., Davis J. S. Greater importance of Ca(2+)-calmodulin in maintenance of ang II- and K(+)-mediated aldosterone secretion: lesser role of protein kinase C. Biochem Biophys Res Commun. 1992 Jan 15;182(1):254–261. doi: 10.1016/s0006-291x(05)80138-x. [DOI] [PubMed] [Google Scholar]
- Garofalo R. S., Gilligan D. M., Satir B. H. Calmodulin antagonists inhibit secretion in Paramecium. J Cell Biol. 1983 Apr;96(4):1072–1081. doi: 10.1083/jcb.96.4.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilligan D. M., Satir B. H. Stimulation and inhibition of secretion in Paramecium: role of divalent cations. J Cell Biol. 1983 Jul;97(1):224–234. doi: 10.1083/jcb.97.1.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomperts B. D. GE: a GTP-binding protein mediating exocytosis. Annu Rev Physiol. 1990;52:591–606. doi: 10.1146/annurev.ph.52.030190.003111. [DOI] [PubMed] [Google Scholar]
- Goud B., Salminen A., Walworth N. C., Novick P. J. A GTP-binding protein required for secretion rapidly associates with secretory vesicles and the plasma membrane in yeast. Cell. 1988 Jun 3;53(5):753–768. doi: 10.1016/0092-8674(88)90093-1. [DOI] [PubMed] [Google Scholar]
- Hinrichsen R. D., Amberger E., Saimi Y., Burgess-Cassler A., Kung C. Genetic analysis of mutants with a reduced Ca2+-dependent K+ current in Paramecium tetraurelia. Genetics. 1985 Nov;111(3):433–445. doi: 10.1093/genetics/111.3.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinrichsen R. D., Fraga D., Reed M. W. 3'-modified antisense oligodeoxyribonucleotides complementary to calmodulin mRNA alter behavioral responses in Paramecium. Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8601–8605. doi: 10.1073/pnas.89.18.8601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerka-Dziadosz M., Garreau de Loubresse N., Beisson J. Development of surface pattern during division in Paramecium. II. Defective spatial control in the mutant kin241. Development. 1992 May;115(1):319–335. doi: 10.1242/dev.115.1.319. [DOI] [PubMed] [Google Scholar]
- Kanabrocki J. A., Saimi Y., Preston R. R., Haynes W. J., Kung C. Efficient transformation of cam2, a behavioral mutant of Paramecium tetraurelia, with the calmodulin gene. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10845–10849. doi: 10.1073/pnas.88.23.10845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerboeuf D., Cohen J. A Ca2+ influx associated with exocytosis is specifically abolished in a Paramecium exocytotic mutant. J Cell Biol. 1990 Dec;111(6 Pt 1):2527–2535. doi: 10.1083/jcb.111.6.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kink J. A., Maley M. E., Preston R. R., Ling K. Y., Wallen-Friedman M. A., Saimi Y., Kung C. Mutations in paramecium calmodulin indicate functional differences between the C-terminal and N-terminal lobes in vivo. Cell. 1990 Jul 13;62(1):165–174. doi: 10.1016/0092-8674(90)90250-i. [DOI] [PubMed] [Google Scholar]
- Knight D. E., von Grafenstein H., Athayde C. M. Calcium-dependent and calcium-independent exocytosis. Trends Neurosci. 1989 Nov;12(11):451–458. doi: 10.1016/0166-2236(89)90095-7. [DOI] [PubMed] [Google Scholar]
- Knowles J. K. An improved microinjection technique in Paramecium aurelia. Transfer of mitochondria conferring erythromycin-resistance. Exp Cell Res. 1974 Sep;88(1):79–87. doi: 10.1016/0014-4827(74)90620-x. [DOI] [PubMed] [Google Scholar]
- Krausz Y., Wollheim C. B., Siegel E., Sharp G. W. Possible role for calmodulin in insulin release. Studies with trifluoperazine in rat pancreatic islets. J Clin Invest. 1980 Sep;66(3):603–607. doi: 10.1172/JCI109893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kung C., Preston R. R., Maley M. E., Ling K. Y., Kanabrocki J. A., Seavey B. R., Saimi Y. In vivo Paramecium mutants show that calmodulin orchestrates membrane responses to stimuli. Cell Calcium. 1992 Jun-Jul;13(6-7):413–425. doi: 10.1016/0143-4160(92)90054-v. [DOI] [PubMed] [Google Scholar]
- Matt H., Bilinski M., Plattner H. Adenosinetriphosphate, calcium and temperature requirements for the final steps of exocytosis in Paramecium cells. J Cell Sci. 1978 Aug;32:67–86. doi: 10.1242/jcs.32.1.67. [DOI] [PubMed] [Google Scholar]
- Matt H., Plattner H., Reichel K., Lefort-Tran M., Beisson J. Genetic dissection of the final exocytosis steps in Paramecium tetraurelia cells: trigger analyses. J Cell Sci. 1980 Dec;46:41–60. doi: 10.1242/jcs.46.1.41. [DOI] [PubMed] [Google Scholar]
- Means A. R., Dedman J. R. Calmodulin--an intracellular calcium receptor. Nature. 1980 May 8;285(5760):73–77. doi: 10.1038/285073a0. [DOI] [PubMed] [Google Scholar]
- Means A. R. Molecular mechanisms of action of calmodulin. Recent Prog Horm Res. 1988;44:223–262. doi: 10.1016/b978-0-12-571144-9.50012-0. [DOI] [PubMed] [Google Scholar]
- Momayezi M., Kersken H., Gras U., Vilmart-Seuwen J., Plattner H. Calmodulin in Paramecium tetraurelia: localization from the in vivo to the ultrastructural level. J Histochem Cytochem. 1986 Dec;34(12):1621–1638. doi: 10.1177/34.12.3097118. [DOI] [PubMed] [Google Scholar]
- Momayezi M., Lumpert C. J., Kersken H., Gras U., Plattner H., Krinks M. H., Klee C. B. Exocytosis induction in Paramecium tetraurelia cells by exogenous phosphoprotein phosphatase in vivo and in vitro: possible involvement of calcineurin in exocytotic membrane fusion. J Cell Biol. 1987 Jul;105(1):181–189. doi: 10.1083/jcb.105.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadin C. Y., Rogers J., Tomlinson S., Edwardson J. M. A specific interaction in vitro between pancreatic zymogen granules and plasma membranes: stimulation by G-protein activators but not by Ca2+. J Cell Biol. 1989 Dec;109(6 Pt 1):2801–2808. doi: 10.1083/jcb.109.6.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakata T., Sobue K., Hirokawa N. Conformational change and localization of calpactin I complex involved in exocytosis as revealed by quick-freeze, deep-etch electron microscopy and immunocytochemistry. J Cell Biol. 1990 Jan;110(1):13–25. doi: 10.1083/jcb.110.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okabe T., Sugimoto N., Matsuda M. Calmodulin is involved in catecholamine secretion from digitonin-permeabilized bovine adrenal medullary chromaffin cells. Biochem Biophys Res Commun. 1992 Jul 31;186(2):1006–1011. doi: 10.1016/0006-291x(92)90846-d. [DOI] [PubMed] [Google Scholar]
- Plattner H. Intramembraneous changes on cationophore-triggered exocytosis in Paramecium. Nature. 1974 Dec 20;252(5485):722–724. doi: 10.1038/252722a0. [DOI] [PubMed] [Google Scholar]
- Plattner H., Lumpert C. J., Knoll G., Kissmehl R., Höhne B., Momayezi M., Glas-Albrecht R. Stimulus-secretion coupling in Paramecium cells. Eur J Cell Biol. 1991 Jun;55(1):3–16. [PubMed] [Google Scholar]
- Plattner H., Miller F., Bachmann L. Membrane specializations in the form of regular membrane-to-membrane attachment sites in Paramecium. A correlated freeze-etching and ultrathin-sectioning analysis. J Cell Sci. 1973 Nov;13(3):687–719. doi: 10.1242/jcs.13.3.687. [DOI] [PubMed] [Google Scholar]
- Plattner H., Pape R., Haacke B., Olbricht K., Westphal C., Kersken H. Synchronous exocytosis in Paramecium cells. VI. Ultrastructural analysis of membrane resealing and retrieval. J Cell Sci. 1985 Aug;77:1–17. doi: 10.1242/jcs.77.1.1. [DOI] [PubMed] [Google Scholar]
- Pollack S. Mutations affecting the trichocysts in Paramecium aurelia. I. Morphology and description of the mutants. J Protozool. 1974 May;21(2):352–362. doi: 10.1111/j.1550-7408.1974.tb03669.x. [DOI] [PubMed] [Google Scholar]
- Sarafian T., Pradel L. A., Henry J. P., Aunis D., Bader M. F. The participation of annexin II (calpactin I) in calcium-evoked exocytosis requires protein kinase C. J Cell Biol. 1991 Sep;114(6):1135–1147. doi: 10.1083/jcb.114.6.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satir B. H., Oberg S. G. Paramecium fusion rosettes: possible function as Ca2+ gates. Science. 1978 Feb 3;199(4328):536–538. doi: 10.1126/science.341312. [DOI] [PubMed] [Google Scholar]
- Steinhardt R. A., Alderton J. M. Calmodulin confers calcium sensitivity on secretory exocytosis. Nature. 1982 Jan 14;295(5845):154–155. doi: 10.1038/295154a0. [DOI] [PubMed] [Google Scholar]
- Stoclet J. C., Gérard D., Kilhoffer M. C., Lugnier C., Miller R., Schaeffer P. Calmodulin and its role in intracellular calcium regulation. Prog Neurobiol. 1987;29(4):321–364. doi: 10.1016/0301-0082(87)90018-9. [DOI] [PubMed] [Google Scholar]
- Tiggemann R., Plattner H. Possible involvement of a calmodulin regulated Ca2+ -ATPase in exocytosis performance in Paramecium tetraurelia cells. FEBS Lett. 1982 Nov 8;148(2):226–230. doi: 10.1016/0014-5793(82)80812-0. [DOI] [PubMed] [Google Scholar]