Abstract
Carbapenem-resistant Enterobacteriaceae (CRE) have spread globally and represent a serious and growing threat to public health. Rapid methods for tracking plasmids carrying carbapenemase genes could greatly benefit infection control efforts. Here, we demonstrate that real-time, direct tracking of a single plasmid in a bacterial strain responsible for an outbreak is possible using a commercial matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) system. In this case, we retrospectively tracked the blaKPC carbapenemase gene-bearing pKpQIL plasmid responsible for a CRE outbreak that occurred at the NIH Clinical Center in 2011. An ∼11,109-Da MS peak corresponding to a gene product of the blaKPC pKpQIL plasmid was identified and characterized using a combination of proteomics and molecular techniques. This plasmid peak was present in spectra from retrospectively analyzed K. pneumoniae outbreak isolates, concordant with results from whole-genome sequencing, and absent from a diverse control set of blaKPC-negative clinical Enterobacteriaceae isolates. Notably, the gene characterized here is located adjacent to the blaKPC Tn4401 transposon on the pKpQIL plasmid. Sequence analysis demonstrates the presence of this gene in other blaKPC Tn4401-containing plasmids and suggests that this signature MS peak may be useful in tracking other plasmids conferring carbapenem resistance. Plasmid identification using this MALDI-TOF MS method was accomplished in as little as 10 min from isolated colonies and 30 min from positive (spiked) blood cultures, demonstrating the potential clinical utility for real-time plasmid tracking in an outbreak.
INTRODUCTION
Carbapenem-resistant Enterobacteriaceae (CRE) are Gram-negative bacteria that are resistant to most or all currently available beta-lactam antibiotics. In 2013, the U.S. Centers for Disease Control and Prevention assigned the highest threat level to these organisms and declared that they require urgent public health attention (1, 2). A more complete understanding of the mechanisms of CRE dissemination is essential to combating their spread. Hospital CRE outbreaks often involve patient-to-patient transmission of genetically clonal populations of resistant bacteria. In particular, Klebsiella pneumoniae sequence type 258 (ST258) and its derivatives carrying plasmid-borne blaKPC carbapenemase genes have spread to cause outbreaks globally (3–10). Some evidence suggests that CRE outbreaks could alternatively involve the primary transmission of carbapenemase-encoding plasmids or other mobile elements among genetically heterogeneous populations of bacteria, although this has not been demonstrated to be a dominant epidemiologic mechanism to date (11–18). In areas of high endemicity, it is also possible for multiple unrelated plasmids mediating carbapenem resistance to be present concurrently among bacterial strains circulating during a given outbreak, leading to potentially complex transmission chains.
Classical strain-typing techniques, such as pulsed-field gel electrophoresis (PFGE) and multilocus sequence typing (MLST), primarily detect features of chromosomal similarity among linked isolates in an outbreak (19, 20). While these methods have been used extensively in epidemiologic investigations, they are time-consuming and may prove inadequate for the construction of transmission maps that involve the movement of mobile resistance elements among genetically unrelated organisms. PCR- and sequencing-based methods for tracking signatures of individual resistance plasmids or transposable elements can be used in these scenarios but are difficult to integrate into the daily diagnostic workflow of a clinical microbiology laboratory. Additionally, reliable plasmid data are often not obtained with next-generation short-read sequencing methods due to the difficulty of plasmid sequence assembly. Rapid methods to detect and track individual resistance plasmids as part of routine data analysis could therefore greatly benefit infection control efforts.
Matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) has demonstrated substantial utility in the clinical microbiology laboratory for rapid organism identification (21). Fast and accurate taxonomic classification is accomplished by comparison of whole-organism MALDI-TOF MS data to a spectral library of characterized strains. With this technology, species level identifications can be made in as little as 10 min from isolated colonies, and diagnostic spectral data can be acquired directly from fluids such as blood (22) and urine (23). Two commercial MALDI-TOF MS instruments to date have received clearance from relevant regulatory bodies (the U.S. Food and Drug Administration and the European Conformité Européenne) for clinical use in microbiology, and the technology is rapidly making its way into hospital laboratories around the world. The speed and accuracy of commercial MALDI-TOF MS systems may also prove useful in clinical epidemiology if spectral features can be found that identify plasmid, transposon, or clone level characteristics of isolates. Automated computational mining of routinely acquired MALDI-TOF MS data for such epidemiological information could have great value. Although methods have been developed for the differentiation of certain bacterial strains, such as methicillin-susceptible and -resistant strains of Staphylococcus aureus (24–27), MALDI-TOF MS-based epidemiologic techniques have not yet been broadly applied to routine practice.
Here, we demonstrate that real-time, direct tracking of a single plasmid responsible for a hospital outbreak is possible using a commercial MALDI-TOF MS system in a manner that could be implemented for routine screening. In this case, we retrospectively tracked the blaKPC carbapenemase gene-bearing pKpQIL plasmid responsible for a CRE outbreak that occurred at the National Institutes of Health (NIH) Clinical Center in 2011 (3).
MATERIALS AND METHODS
MALDI-TOF MS.
For protein extraction, fresh bacterial isolates (<24 h old) were resuspended in 1 ml 70% ethanol, vortexed for 1 min, and centrifuged at 13,000 rpm for 2 min. The supernatant was removed completely, and the sample was vortexed for 10 s with 50 μl of 70% formic acid (FA) (Baker; 90% stock) and 50 μl acetonitrile (ACN). After 2 min centrifugation at 13,000 rpm, 1 μl of supernatant was spotted onto the target plate and overlaid with 2 μl of alpha-cyano-4-hydroxycinnamic acid (α-CHCA). MALDI-TOF MS analysis was performed on a Bruker MicroFlex LT mass spectrometer (Bruker Daltonics, Billerica, MA). Spectra were acquired as described previously (28). FlexAnalysis and ClinProtTools software (v3.0; Bruker Daltonics, Inc.) was used for peak analysis. Baseline subtraction was performed on spectral data using the built-in FlexAnalysis functions.
For direct-spotting experiments, whole bacteria were spotted directly onto the plate and overlaid with 1 μl of 70% formic acid and 2 μl of α-CHCA. Spectral acquisition and analysis were the same as described above.
blaKPC gene PCR.
TaqMan PCR for the blaKPC gene was performed on both single bacterial colonies and plasmid preparations using an ABI 7500 Fast real-time PCR instrument (Applied Biosystems, Inc., Foster City, CA). Primer and probe sets were adapted from those published by the CDC (http://www.cdc.gov/HAI/pdfs/labSettings/KPC-NDM-protocol-2011.pdf) and described previously (29).
Antimicrobial susceptibility testing.
Susceptibility testing was performed with a combination of standard Kirby-Bauer disk diffusion-based testing and automated broth microdilution MIC testing using a BD Phoenix instrument (Becton, Dickinson and Company, Sparks, MD). The carbapenems tested included ertapenem, imipenem, meropenem, and doripenem. Susceptibility breakpoints were interpreted according to the 2013 Clinical and Laboratory Standards Institute document M100-S23 (30).
Plasmid isolation.
Plasmid DNA was extracted from blaKPC-positive K. pneumoniae isolates using the Qiafilter Plasmid Midi-Prep kit (Qiagen, Valencia, CA) according to the manufacturer's instructions. The presence of the blaKPC gene in the extracted plasmid DNA was confirmed by PCR as described above.
Plasmid elimination experiments.
K. pneumoniae was passaged twice daily in tryptic soy broth (Remel, Lenexa, KS) at 42°C. After 8 days, cultures were diluted and plated onto sheep blood agar (Remel). Individual colonies were screened for loss of the blaKPC plasmid by growth inhibition on RambaChrom KPC (Gibson Biosciences, Lexington, KY), for loss of blaKPC by colony PCR, and for loss of carbapenem resistance by antimicrobial susceptibility testing.
Plasmid transformation experiments.
Two electrocompetent cured (previously blaKPC-positive) K. pneumoniae strains and one blaKPC-negative, carbapenem-susceptible K. pneumoniae strain were transformed by electroporation with extracted plasmid DNA and selected on RambaChrom KPC agar. A 10-beta chemically competent Escherichia coli strain (New England Biolabs [NEB], Ipswich, MA) (Table 1) was transformed with extracted plasmid DNA according to the manufacturer's instructions and selected on RambaChrom KPC agar. Colonies were confirmed to have the blaKPC gene by PCR and resistance to carbapenems by antimicrobial susceptibility testing.
TABLE 1.
Commercial strains used
| Strain | Description | Source |
|---|---|---|
| ATCC BAA-1705 | blaKPC-positive K. pneumoniae strain | ATCC |
| ATCC BAA-1706 | blaKPC-negative K. pneumoniae strain | ATCC |
| 10-beta E. coli | Δ(ara-leu)7697 araD139 fhuA ΔlacX74 galK16 galE15 e14-ϕ80dlacZΔM15 recA1 relA1 endA1 nupG rpsL (Strr) rph spoT1 Δ(mrr-hsdRMS-mcrBC) chemically competent cells | NEB |
Spiked blood culture experiments.
To simulate the detection of pKpQIL_p019 directly from positive blood cultures, 50 CFU from two 2011 K. pneumoniae outbreak isolates were inoculated into two separate Bactec Standard aerobic bottles (BD Diagnostic Systems, Sparks, MD) that each contained 10 ml of blood. The bottles were incubated at 37°C on a Bactec FX instrument (BD Diagnostic Systems, Sparks, MD). Time to positivity ranged from 10 to 11 h. Once positive, 3 ml of blood culture was drawn into a serum separator clot activator tube (Greiner Bio-One, Monroe, NC). Proteins were extracted from 2 ml of blood for MALDI-TOF MS using the SepsiTyper Kit (Bruker Daltonics, Billerica, MA), according to the manufacturer's instructions. MALDI-TOF MS was performed on the resulting extracted proteins as described above.
Protein profiling by Q-TOF LC-MS.
Intact-protein profiling to identify the ∼11,109-Da peak by quadrupole time of flight (Q-TOF) liquid chromatography (LC)-MS was performed on K. pneumoniae ATCC BAA-1705 (blaKPC-positive) and K. pneumoniae ATCC BAA-1706 (blaKPC-negative) strains, two additional blaKPC-positive 2011 K. pneumoniae outbreak isolates, and two blaKPC-negative clinical K. pneumoniae isolates. An Agilent 1200 series LC system (Agilent, Santa Clara, CA) coupled to an Agilent 6540 Q-TOF system was used for intact-protein profiling. An Agilent Poroshell 2.1- by 75-mm SB300 5-Å C18 column was used with 0.1% FA H2O-ACN mobile phases. Agilent's maximum-entropy algorithm was used to deconvolute LC-MS data to determine the molecular weight of the intact protein.
Protein isolation.
A high-performance liquid chromatography (HPLC) protocol in LC-MS was optimized to isolate the 11,108.4-Da protein, and the fractions of HPLC between retention times of 5 and 6 min were collected manually in steps of 0.1 min. HPLC separation was repeated 4 times, and a total 40 fractions were collected. MALDI-TOF MS was used for screening fractions containing the desired 11-kDa protein. The fractions containing 3,733-Da [M+3H]3+, 5,607-Da [M+2H]2+, and 11,175-Da [M+H]+ peaks in the MALDI-TOF MS spectra were combined and used for protein identification. A portion of the combined fractions was injected back into LC-MS to confirm the presence of the 11,108.4-Da protein and its high level of purity. The mass shifts in the MALDI-TOF MS spectra were presumed to represent formation of an uncharacterized adduct, as no molecular mass shifts were observed in the Q-TOF LC-MS measurement, and the presumed adduct peaks were missing from the Q-TOF LC-MS spectra.
N-terminal Edman sequencing.
Automated N-terminal Edman protein sequencing was performed on the collected HPLC fraction using a Sequanator model Procise 494 cLC connected to an online UV detector Series 200 (Life Technologies, Foster City, CA). A C18 capillary column (5 μm; 250 by 0.8 mm) was used for the separation of the phenylthiohydantoin (PTH) amino acids. Standard sequencing protocols were used according to the manufacturer's recommendations.
Top-down proteomics.
Speed-Vac-dried protein from the HPLC fraction was dissolved in 30% acetonitrile and 0.1% formic acid buffer and directly infused at a 300-nl/min flow rate through a fused silica capillary (inside diameter [ID], 75 μm; tip ID, 10 μm) into the Thermo Orbitrap Elite LC-tandem mass spectrometry (MS-MS) instrument. Both the full-mass scans and the MS-MS scans were acquired in the Orbitrap at 60,000 mass resolution. The charge state 14+ was picked for fragmentation and MS-MS spectrum collection. The collision energy was optimized at 25 eV for high-energy collisional dissociation (HCD) and 20 eV for collision-induced dissociation (CID). The resulting MS-MS spectra were extracted in Thermo Xcalibur 2.2 (Thermo Fisher Scientific, San Jose, CA) and searched using ProSight PC 3.0 (Thermo Fisher Scientific, San Jose, CA) against a custom 5,451-protein K. pneumoniae protein database that included plasmid sequences from the 2011 outbreak K. pneumoniae isolates (3).
Bottom-up proteomics.
The protein sample was digested with trypsin at an approximately 1:30 enzyme/substrate ratio overnight at 37°C in 25 mM triethylammonium bicarbonate (TEA-Bic) (Sigma) buffer. The digest was analyzed on a Thermo Orbitrap Elite LC–MS-MS instrument (Thermo Fisher Scientific, San Jose, CA) coupled with an Eksigent nanoLC-Ultra 1D plus system (Dublin, CA). Peptides were separated on a PicoFrit analytical column (250 mm long; ID, 75 μm; tip ID, 10 μm; packed with BetaBasic 5-μm 300-Å particles; New Objective, Woburn, MA) using a 30-min linear gradient of 5 to 35% ACN in 0.1% FA at a flow rate of 250 nl/min. Mass analysis was carried out in data-dependent analysis mode, where MS1 scanned the full MS mass range from m/z 300 to 2,000 at 60,000 mass resolution, and 10 CID MS2 scans were sequentially carried out in the Orbitrap and the ion trap.
The LC-MS data were searched against the custom K. pneumoniae database described above using the Mascot server (version 2.3) (Matrix Science, London, United Kingdom). The searching parameters were set with precursor mass tolerance at 20 ppm, fragment ion mass tolerance at 0.8 Da, and trypsin enzyme with 2 miscleavages.
RESULTS
Identification of a MALDI-TOF MS peak associated with blaKPC.
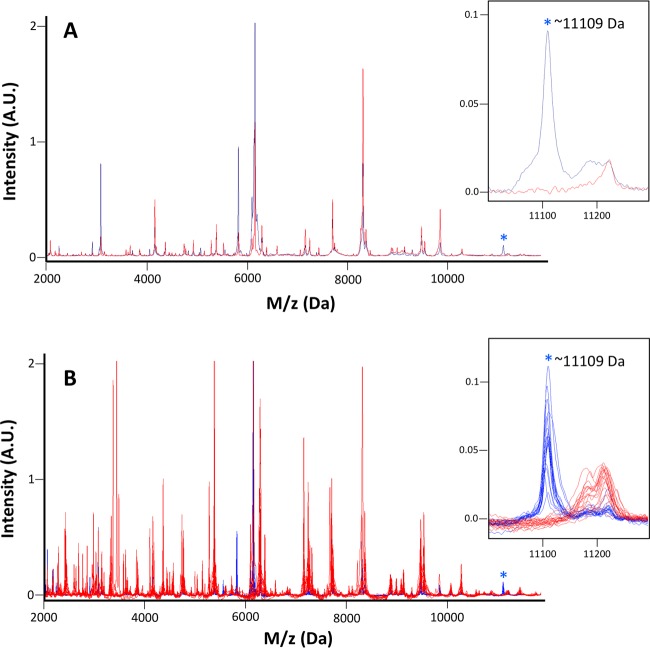
To identify a MALDI-TOF MS peak associated with the blaKPC gene, we compared spectra from blaKPC-positive and blaKPC-negative K. pneumoniae isolates that were acquired with the Bruker MicroFlex LT (Bruker Daltonics, Billerica, MA). The blaKPC-positive isolate set included K. pneumoniae ATCC BAA-1705 (ATCC, Manassas, VA) and 18 clinical K. pneumoniae isolates from an outbreak that occurred in the NIH Clinical Center in 2011 (3). It was known from whole-genome sequencing of the 2011 outbreak isolates that the blaKPC gene was carried on a pKpQIL plasmid (3). The blaKPC-negative set included K. pneumoniae ATCC BAA-1706 and 18 blaKPC-negative K. pneumoniae clinical isolates cultured from NIH Clinical Center patients. Visual comparison of the traces in the two sets identified a strong MALDI-TOF MS peak located at approximately 11,109 Da that was present in the majority of the blaKPC-positive isolates and absent in all of the blaKPC-negative isolates (Fig. 1A). This peak was pursued as a candidate blaKPC plasmid-identifying MALDI-TOF MS signature.
FIG 1.
Identification of a MALDI-TOF MS peak associated with blaKPC. (A) The MALDI-TOF MS spectra of blaKPC-positive K. pneumoniae ATCC BAA-1705 (blue) demonstrate a peak at ∼11,109 Da (asterisk) that is absent from the spectra of blaKPC-negative K. pneumoniae ATTC BAA-1706 (red). Peak intensities are displayed in arbitrary units (A.U.), and peak data are truncated at 2 A.U. for display. The inset shows the ∼11,109-Da peak magnified. (B) Overlaid MALDI-TOF MS spectra from 18 recultured blaKPC-positive K. pneumoniae isolates from the 2011 NIH Clinical Center outbreak (blue) and 18 blaKPC-negative clinical K. pneumoniae isolates (red). Peak data are truncated at 2 A.U. for display. The ∼11,109-Da peak (asterisk) is present in all recultured outbreak isolates and absent from all blaKPC-negative isolates. The inset shows the ∼11,109-Da peak present in 18 (blue) isolates and absent from 18 (red) isolates.
Analysis of the 2011 NIH Clinical Center outbreak isolates.
Initial identification of the candidate blaKPC plasmid signature relied on examination of the original MALDI-TOF MS spectra acquired during the 2011 NIH Clinical Center outbreak. The ∼11,109-Da peak was identified clearly in 14 isolates and with a low-intensity signal relative to noise in two isolates; the MS peak was missing from the spectra of the two remaining isolates. We therefore recultured all 18 isolates from frozen stocks and reacquired MALDI-TOF data. The ∼11,109-Da peak was strongly present in all 18 recultured isolates from the outbreak (Fig. 1B) in this data set. Direct comparison of the original (2011) and reacquired (2014) spectra suggested that the 2011 spectra were of generally lower quality and that the differences in spectral quality might be attributed, in part, to improvements in the MALDI-TOF MS protein extraction technique between 2011 and 2014 and/or operator training and expertise. A companion peak at ∼5,555 Da was observed in some spectra, along with the ∼11,109-Da peak, consistent with a double-charged species. A set of 26 additional clinical blaKPC-negative, but carbapenem-resistant Enterobacteriaceae isolates representing K. pneumoniae, E. coli, Serratia marcescens, Citrobacter freundii complex, and Enterobacter sp. was examined for the ∼11,109-Da peak (see Table S1 in the supplemental material). Isolates were required to test intermediate or resistant to two or more carbapenems for inclusion in the set, and carbapenem resistance was presumed to be due to mechanisms other than a plasmid-mediated blaKPC carbapenemase. None of the isolates in this additional control set demonstrated the ∼11,109-Da peak (data not shown).
High-resolution measurement of candidate MS peak mass using Q-TOF LC-MS.
To confirm the presence of the observed MS peak by a second technique, and to obtain a more exact mass than that provided by the clinical Bruker MicroFlex LT MALDI-TOF mass spectrometer, intact protein profiling was performed using a Q-TOF LC-MS system on K. pneumoniae ATCC BAA-1705 containing blaKPC and K. pneumoniae ATCC BAA-1706 lacking blaKPC. An abundant 11,108.4-Da protein was detected in protein extracts from the ATCC BAA-1705 strain near a retention time of 5.6 to 5.7 min, and no such protein (within an estimated maximum MALDI-TOF MS measurement error range of 11,108.4 ± 5.0 Da) was detected in extracts from ATCC BAA-1706. The 11,108.4-Da protein detected by Q-TOF LC-MS was therefore assumed to represent the same protein as the ∼11,109-Da peak detected by MALDI-TOF MS. Intact-protein profiling using Q-TOF LC-MS was additionally performed on protein extracts from two K. pneumoniae isolates from the 2011 NIH Clinical Center outbreak known to contain blaKPC on a pKpQIL plasmid and two blaKPC-negative clinical K. pneumoniae isolates. The 11,108.4-Da peak was present in the blaKPC-pKpQIL-positive isolates and absent in the blaKPC-negative isolates, consistent with the MALDI-TOF MS findings.
Purification of the protein underlying the candidate MS peak.
As LC-MS intact-protein profiling provides both the mass spectra and the retention times of all protein peaks detected, it was possible to optimize an HPLC protocol on the LC-MS instrument to isolate the identified 11,108.4-Da protein from positive isolates. To accomplish this, LC-MS was used to monitor the protein separation, and fractions near the retention time of the 11,108.4-Da protein were collected. MALDI-TOF MS was employed for screening fractions that contained the ∼11,109-Da protein. Fractions containing triple-charged 3,733-Da [M+3H]3+, double-charged 5,607-Da [M+2H]2+, and single-charged 11,175-Da [M+H]+ peaks in the MALDI-TOF MS spectra were combined and used for protein identification. It was noted that the masses of these species as measured by MALDI-TOF MS shifted following HPLC separation. However, no molecular mass shift was observed when this purified protein was analyzed in the LC-MS instrument that was used to confirm the presence and purity of the 11,108.4-Da protein. This suggests that the difference in mass may represent an uncharacterized adduct from the matrix used for the MALDI-TOF MS.
Identification of the purified protein as pKpQIL_p019.
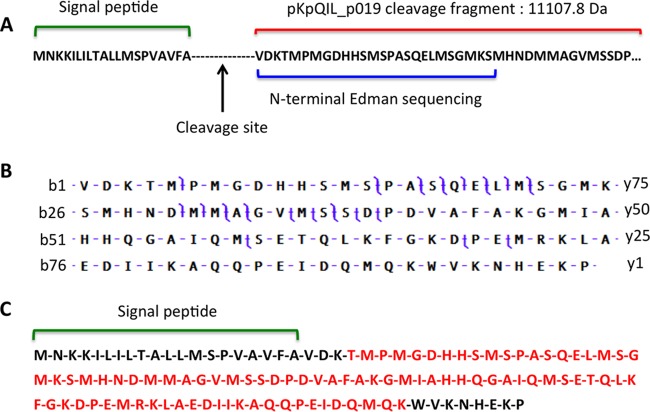
To identify the purified protein, N-terminal Edman sequencing was performed on the selected and pooled HPLC fractions, and a 26-amino-acid sequence (VDKTMPMGDHHSMSPASQELMSGMKS) was identified by this method. An initial protein BLAST search against NCBI's BLAST site (http://blast.ncbi.nlm.nih.gov/) using this 26-amino-acid sequence identified YP_003560396.1, a hypothetical protein encoded by plasmid pKpQIL and annotated as pKpQIL_p019 (31). The predicted pKpQIL_p019 protein is 119 amino acids in length, and the 26 amino acids detected by N-terminal Edman sequencing correspond to positions 21 to 46 in the primary sequence (Fig. 2A). The theoretical molecular mass of the pKpQIL_p019 fragment comprising amino acids 21 to 119 is 11,107.8 Da, which corresponds well to the measured 11,108.4 Da by Q-TOF LC-MS and ∼11,109 Da [M+H]+ by MALDI-TOF MS (this molecular mass difference is consistent with the mass accuracy of the instruments). Top-down proteomics using Thermo Orbitrap Elite LC–MS-MS and bottom-up proteomics using trypsin digestion confirmed the results of N-terminal Edman protein sequencing (Fig. 2B and C).
FIG 2.
Identification of pKpQIL_p019 protein using three proteomics methods. (A) N-terminal Edman sequencing was used to define a 26-amino-acid sequence from the purified 11,109-Da protein (blue). Protein BLAST identified this sequence as a fragment representing amino acids 21 to 46 of the 119-amino-acid protein pKpQIL_p019, encoded by the pKpQIL plasmid. The mass of amino acids 21 to 119 (red) is 11,107.8 Da, corresponding to the identified MALDI-TOF MS peak (note that only a partial protein sequence is shown). The first 20 amino acids (green) are predicted to represent a signal peptide with a cleavage site between amino acids 20 and 21. (B) Top-down proteomics analysis was performed using CID by Thermo Orbitrap Elite. LC–MS-MS confirmed the identity of the protein as pKpQIL_p019. Cleavage sites used for identification are shown in purple. A total of 13 b-ion fragments and 15 y-ion fragments were detected. (C) Bottom-up proteomics performed using trypsin digestion also confirmed the identity of the protein as pKpQIL_p019. Tryptic digest analysis yielded 89% coverage (shown in red) of the purified protein cleavage fragment (amino acids 21 to 119) with a total Mascot score of 4,600 at a 1% false discovery rate (FDR).
Analysis performed with the SignaIP-4.1 signal peptide prediction program (32) identified the first 20 amino acids in pKpQIL_p019 as a potential signal peptide, with a predicted cleavage site between amino acids 20 and 21, consistent with the experimental proteomics findings.
pKpQIL plasmid elimination and transformation experiments.
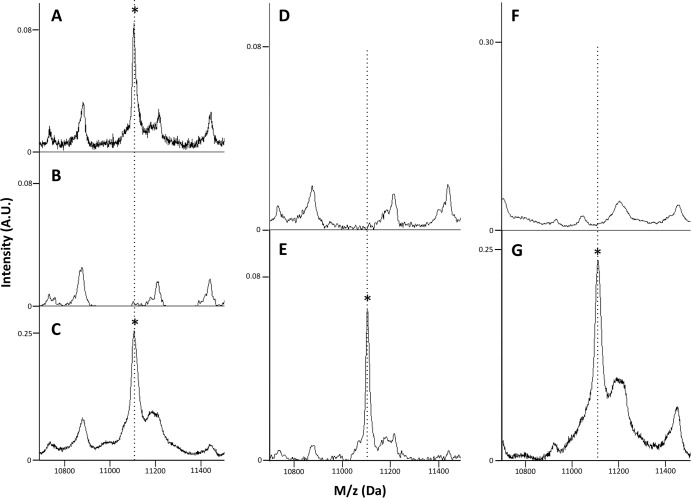
Plasmid elimination and transformation experiments were performed to demonstrate that the observed ∼11,109-Da MS peak was associated with the presence of the pKpQIL plasmid in the tested isolates. Two blaKPC pKpQIL-containing isolates from the NIH Clinical Center outbreak set were cured of the blaKPC plasmid by serial transfer at elevated temperature. These isolates lost blaKPC gene PCR positivity and were rendered susceptible to the carbapenems (see Table S1 in the supplemental material), and the ∼11,109-Da pKpQIL_p019 MALDI-TOF MS peak was absent following plasmid elimination (Fig. 3A and B). These two pKpQIL-cured isolates were then transformed with a pKpQIL-containing plasmid preparation derived from the 2011 K. pneumoniae outbreak strain (confirmed to contain the pKpQIL plasmid by blaKPC gene PCR). Plasmid transformation with pKpQIL coordinately restored blaKPC gene PCR positivity, carbapenem resistance, and the ∼11,109-Da pKpQIL_p019 MALDI-TOF MS peak in multiple recovered transformants on carbapenem-containing media (Fig. 3C).
FIG 3.
Plasmid elimination and transformation experiments. (A) blaKPC-positive 2011 NIH Clinical Center outbreak K. pneumoniae isolate MALDI-TOF MS trace (the asterisk denotes the pKpQIL_p019 MS peak). (B) Plasmid cure eliminates the pKpQIL_p019 MS peak. (C) Transformation of the plasmid-cured strain with a pKpQIL-containing plasmid preparation restores the pKpQIL_p019 MS peak (asterisk). (D) A blaKPC-negative clinical K. pneumoniae isolate lacks the pKpQIL_p019 MS peak. (E) Transformation with a pKpQIL plasmid preparation yields a pKpQIL_p019 MS peak (asterisk), demonstrating that the ability to express pKpQIL_p019 is not unique to the 2011 NIH outbreak isolates. (F) A competent 10-beta E. coli strain lacks the pKpQIL_p019 MS peak. (G) Transformation with a pKpQIL plasmid preparation yields a pKpQIL_p019 MS peak (asterisk), demonstrating that the ability to express pKpQIL_p019 is not specific to the genus Klebsiella.
Further experiments were carried out in a blaKPC-negative clinical K. pneumoniae strain to confirm that the ability to express pKpQIL_p019 was not a unique feature of the 2011 NIH outbreak isolates. Plasmid transformation of this susceptible isolate with a preparation containing pKpQIL conferred blaKPC gene PCR positivity, carbapenem resistance (see Table S1 in the supplemental material), and the presence of the pKpQIL_p019 MALDI-TOF MS peak (Fig. 3D and E).
To determine whether pKpQIL_p019 could be expressed in a different genus of Enterobacteriaceae, a competent 10-beta E. coli strain was transformed with the pKpQIL-containing plasmid preparation. Plasmid transformation in this isolate conferred blaKPC PCR positivity and the presence of the pKpQIL_p019 MALDI-TOF MS peak (Fig. 3F to G). Interestingly, this transformation conferred cephalosporin resistance, but incomplete carbapenem resistance (ertapenem susceptible, imipenem resistant, and meropenem intermediate) (see Table S1 in the supplemental material), a feature that is not fully understood but that could conceivably be related to lower blaKPC gene expression levels in the 10-beta E. coli strain. Together, these experiments demonstrated that the pKpQIL_p019 MS peak was eliminated by plasmid cure in the tested isolates and that expression of the pKpQIL_p019 protein was not limited to the 2011 outbreak isolates or to the genus Klebsiella.
Analysis of the pKpQIL_p019 sequence.
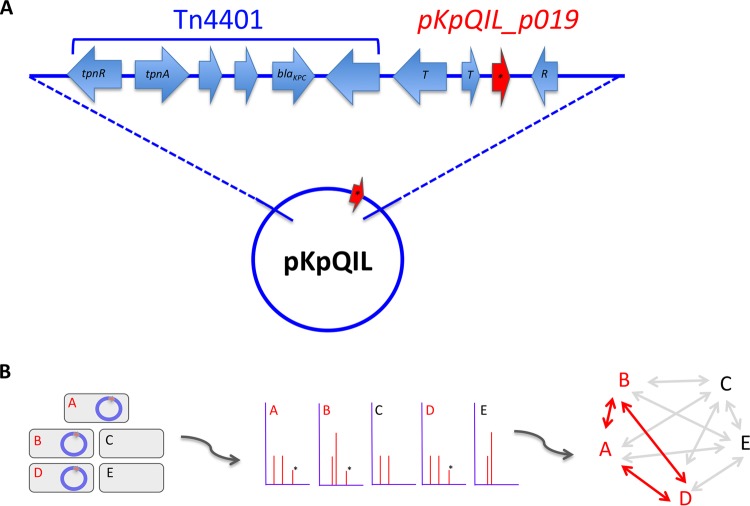
The proteomics and molecular analysis described above confirmed the ∼11,109-Da MALDI-TOF MS peak to be a marker of the blaKPC-containing pKpQIL plasmid. Review of the pKpQIL plasmid sequence revealed that the pKpQIL_p019 open reading frame is located adjacent to the blaKPC-Tn4401 transposon between transposase and resolvase genes (31) (Fig. 4A). Further analysis identified the pKpQIL_p019 coding sequence in 9 additional plasmids highly related to pKpQIL (with 90 to 100% identities to pKpQIL): pKpQIL-IT (33), pKpQIL-LS6 (34), pKP1504-kpc (NCBI reference sequence NC_023903.1), pKP1780-kpc (NCBI reference sequence NC_023904.1), pKP1870-kpc (NCBI reference sequence NC_023905.1), pKP3913-kpc (NCBI reference sequence NC_023906.1), pKpQIL-234, pKpQIL-Ec, and pKpQIL-10 (35). The pKpQIL_p01 sequence is also present in at least four other blaKPC-Tn4401-containing plasmids that are less closely related to pKpQIL: pBK32179 (9), pKPN101-IT (8), KPC-NY79 (32), and pSLMT (GenBank accession number HQ589350.1). All of these plasmids contain the blaKPC gene.
FIG 4.
pKpQIL_p019 and single-plasmid tracking in outbreaks. (A) Schematic diagram demonstrating the location of pKpQIL_p019 (red arrow) within pKpQIL family plasmids. The pKpQIL_p019 gene is located adjacent to the blaKPC-containing Tn4401 transposon in at least four other plasmids. T, transposase; R, resolvase. (B) Illustration demonstrating how MALDI-TOF plasmid tracking could facilitate real-time epidemiologic mapping in an outbreak. Isolates A, B, and D contain a plasmid tracked by a unique MS peak (marked by asterisks), allowing the linkage of these isolates in the transmission map.
Plasmid tracking directly from positive blood cultures.
As a final test of potential clinical applications of the MALDI-TOF MS method, we sought to determine whether tracking of pKpQIL_p019 would be possible directly from positive blood cultures. Two blood culture bottles spiked with 2011 outbreak isolates were cultured on a Bactec FX instrument following standard clinical laboratory techniques and directly analyzed by MALDI-TOF MS after the instrument signaled culture positivity. The ∼11,109-Da MALDI-TOF MS peak was present in the spectra from both positive blood bottles with a total technical time of less than 30 min. This demonstrates that rapid plasmid tracking should be possible in cases of culture-positive bacteremia hours before isolated colonies are available for testing.
Real-time MALDI-TOF MS plasmid tracking.
We have since incorporated our MALDI-TOF MS plasmid peak-tracking method into our routine analysis of carbapenem-resistant Enterobacteriaceae, and automated MS peak analysis software is in development. Integration of automated peak detection and analysis of patient metadata may facilitate epidemiologic transmission mapping in real time during an outbreak (Fig. 4B).
DISCUSSION
In this proof-of-principle study, we show that rapid tracking of a single plasmid responsible for a hospital outbreak is possible using a commercial MALDI-TOF MS technology that is becoming increasingly available in clinical microbiology laboratories. To demonstrate the feasibility of this method, we retrospectively tracked the blaKPC-containing pKpQIL plasmid in isolates from a 2011 NIH Clinical Center CRE outbreak (3). This was accomplished by identifying an ∼11,109-Da MALDI-TOF MS peak corresponding to the cleavage product of pKpQIL_p019, a protein encoded by the pKpQIL plasmid. Plasmid elimination and transformation experiments confirmed the association of the ∼11,109-Da MALDI-TOF MS peak with the presence of the plasmid in the tested K. pneumoniae isolates and also demonstrated that pKpQIL_p019 could be expressed in E. coli following plasmid transformation. The pKpQIL_p019 MALDI-TOF MS peak was present in the spectra of 18 recultured clinical K. pneumoniae outbreak isolates analyzed retrospectively, consistent with the results of genome and plasmid sequencing (3). The pKpQIL_p019 peak was absent from 44 control blaKPC-negative clinical Enterobacteriaceae isolates. We additionally demonstrated that detection of the pKpQIL_p019 MALDI-TOF MS peak directly from simulated (spiked) positive blood cultures is possible. This approach provides the basis for real-time analysis for any future CRE hospital outbreak and for ongoing surveillance of plasmid prevalence.
While the primary aim of this work was to demonstrate the feasibility of MALDI-TOF MS-based tracking of an individual blaKPC-containing plasmid in a well-studied CRE outbreak, sequence analysis suggests that the pKpQIL_p019 signature ∼11,109-Da plasmid peak may have utility for identifying plasmids beyond the one involved in the outbreak analyzed here. Members of the pKpQIL plasmid family have been responsible globally for a number of CRE outbreaks (3, 5, 7, 31, 33–36). In pKpQIL and related plasmids, the blaKPC gene is located within a Tn4401 transposon sequence (4, 37, 38). The pKpQIL_p019 gene, in turn, is located adjacent to the defined Tn4401 transposon sequence between transposase and resolvase genes and therefore appears to be fairly closely linked to the blaKPC gene.
Analysis of the pKpQIL_p019 protein sequence revealed that it is present under different annotations in at least four other blaKPC-Tn4401-containing plasmids that are not closely related to the pKpQIL family: pBK32179 (9), pKPN101-IT (8), KPC-NY79 (39), and pSLMT (GenBank accession number HQ589350.1), and possibly others yet to be sequenced or assembled. Notably, pKpQIL_p019 appears to have followed the blaKPC-Tn4401 transposon into plasmid backbones that span at least two incompatibility groups (IncFIIk and IncX). These findings suggest that the pKpQIL_p019 MS peak identified in this work may have significantly broader utility in early detection of outbreaks involving other blaKPC-containing plasmids (although the MS peak would fail to distinguish individual plasmids in cases where multiple pKpQIL_p019-containing plasmids may be circulating in different strains concurrently).
In considering the analytical sensitivity of the MALDI-TOF MS method described here, it should be noted that the MS peak was expressed with variable intensity in the tested isolates and was not identifiable (or not distinguishable from noise) in some retrospectively analyzed spectral traces of some isolates. Notably, the peak was not strongly discernible from noise in 2 and absent entirely from another 2 of the 18 original spectra acquired during the 2011 outbreak. However, on reculture from frozen stocks, the MS peak was strongly present and clearly identifiable in all 18 isolates. Differences in data quality apparent by direct comparison of the original (2011) and reacquired (2014) spectra suggest that these differences may be attributed in part to improvements in the MALDI-TOF MS protein extraction technique between 2011 and 2014 and/or improvements in operator training and expertise. However, we cannot rule out run-to-run variability in expression or detection of the protein as an explanation for the differences in detection.
Although the pKpQIL_p019 MALDI-TOF MS peak was identified in all 18 recultured outbreak isolates studied here, they do not constitute a statistical validation set, and our data do not allow us to extrapolate the quantitative sensitivity of this method for identifying the pKpQIL plasmid in isolates that were not part of this study. We present an analysis linking an identified MALDI-TOF MS peak to a specific plasmid-borne gene, with general implications for the feasibility of MALDI-TOF MS-based plasmid tracking. We expect that natural variability in plasmid gene expression may affect the sensitivity of the MALDI-TOF MS peak as a marker for the presence of the plasmid. While the primary MALDI-TOF data analyzed for this study were obtained using bacterial protein extracts, the pKpQIL_p019 MALDI-TOF MS peak was also observed in samples using a direct bacterial spotting technique with 70% formic acid overlay (data not shown). Although the relative sensitivities of the two methods have not been rigorously compared, our experience with direct spotting suggests that this method would allow reliable identification of the ∼11,109-Da peak.
Rapid tracking of individual plasmids may augment classical epidemiologic or sequencing-based methods to facilitate the construction of more complete outbreak transmission maps in real time. The analysis presented here suggests that routine screening of Enterobacteriaceae MALDI-TOF MS spectra for the pKpQIL_p019 peak could reveal the presence of a blaKPC-containing plasmid significantly before results of PCR, sequencing, or other epidemiologic testing are available. This could allow earlier recognition of a new potential plasmid in the hospital or of a transmission cluster before a larger outbreak is present and may provide actionable information implicating specific transmission intermediates.
It should be cautioned that while plasmid MS peak information may be available before the results of susceptibility testing, blaKPC carbapenemase expression and carbapenem resistance cannot be rigorously inferred from the MALDI-TOF MS peak characterized here. For instance, mutations or other genetic events in the plasmid may change the association of the pKpQIL_p019 MALDI-TOF MS peak with the presence of functional carbapenemase protein. As such, while the MS peak provides a rapid method for detecting plasmids in isolates and an association with carbapenem resistance is expected, it does not replace antimicrobial susceptibility testing. It should be noted in this context that other MALDI-TOF MS and LC–MS-MS methods have been developed that assay phenotypic carbapenem hydrolysis activity (40–45). We envision that the plasmid-tracking technique described here will complement the above-mentioned approaches.
In this study, identification of the plasmid-associated MALDI-TOF MS peak relied on visual detection by a human. Work is under way to develop automated systems and software to identify and extract other plasmid-associated MALDI-TOF MS peaks. Ideally, MALDI-TOF MS data collected as part of the routine workflow for organism identification in the clinical microbiology laboratory could be analyzed in real time for known and new plasmid signatures. Furthermore, the results of such analysis could guide the automated mining of patient metadata specifying locations, providers, procedures, and other relevant parameters for automated epidemiologic mapping in outbreaks.
Supplementary Material
ACKNOWLEDGMENTS
We thank Julie Segre and Sean Conlan for their thoughtful discussions and advice.
The work was supported by the Intramural Research Program of the National Institutes of Health.
The content is solely our responsibility and does not necessarily represent the official views of the National Institutes of Health.
A.F.L., S.K.D., K.M.F., and J.P.D. have been involved in a collaborative agreement with Bruker Daltonics, Inc., to develop organism databases for MALDI-TOF MS. Bruker Daltonics, Inc., had no involvement at any stage in the planning, execution, or interpretation of the experiments in this study or in writing of the manuscript. We have no other competing interests.
Requests for bacterial isolates used in this study require a material transfer agreement (MTA).
Footnotes
Published ahead of print 21 May 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JCM.00694-14.
REFERENCES
- 1.Centers for Disease Control and Prevention. 2013. Antibiotic resistance threats in the United States, 2013. Centers for Disease Control and Prevention, Atlanta, GA [Google Scholar]
- 2.Jacob JT, Klein E, Laxminarayan R, Kallen AJ, Ricks P, Edwards J, Srinivasan A, Fridkin S, Rasheed JK, Lonsway D, Bulens S, Herrera R, McDonald LC, Patel J, Limbago B, Bell M, Cardo D. 2013. Vital signs: carbapenem-resistant Enterobacteriaceae. MMWR Morb. Mortal. Wkly. Rep. 62:165–170 [PMC free article] [PubMed] [Google Scholar]
- 3.Snitkin ES, Zelazny AM, Thomas PJ, Stock F, Henderson DK, Palmore TN, Segre JA. 2012. Tracking a hospital outbreak of carbapenem-resistant Klebsiella pneumoniae with whole-genome sequencing. Sci. Transl. Med. 4:148ra116. 10.1126/scitranslmed.3004129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patel G, Bonomo RA. 2013. “Stormy waters ahead”: global emergence of carbapenemases. Front. Microbiol. 4:48. 10.3389/fmicb.2013.00048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leavitt A, Chmelnitsky I, Ofek I, Carmeli Y, Navon-Venezia S. 2010. Plasmid pKpQIL encoding Kpc-3 and Tem-1 confers carbapenem resistance in an extremely drug-resistant epidemic Klebsiella pneumoniae strain. J. Antimicrob. Chemother. 65:243–248. 10.1093/jac/dkp417 [DOI] [PubMed] [Google Scholar]
- 6.Cuzon G, Naas T, Truong H, Villegas MV, Wisell KT, Carmeli Y, Gales AC, Venezia SN, Quinn JP, Nordmann P. 2010. Worldwide diversity of Klebsiella pneumoniae that produce beta-lactamase blakpc-2 gene. Emerg. Infect. Dis. 16:1349–1356. 10.3201/eid1609.091389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Richter SN, Frasson I, Franchin E, Bergo C, Lavezzo E, Barzon L, Cavallaro A, Palu G. 2012. KPC-mediated resistance in Klebsiella pneumoniae in two hospitals in Padua, Italy, June 2009-December 2011: massive spreading of a Kpc-3-encoding plasmid and involvement of non-intensive care units. Gut Pathog. 4:7. 10.1186/1757-4749-4-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frasson I, Lavezzo E, Franchin E, Toppo S, Barzon L, Cavallaro A, Richter SN, Palu G. 2012. Antimicrobial treatment and containment measures for an extremely drug-resistant Klebsiella pneumoniae ST101 isolate carrying pKPN101-IT, a novel fully sequenced blaKpc-2 plasmid. J. Clin. Microbiol. 50:3768–3772. 10.1128/JCM.01892-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen L, Chavda KD, Al Laham N, Melano RG, Jacobs MR, Bonomo RA, Kreiswirth BN. 2013. Complete nucleotide sequence of a blakpc-harboring IncI2 plasmid and its dissemination in New Jersey and New York hospitals. Antimicrob. Agents Chemother. 57:5019–5025. 10.1128/AAC.01397-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deleo FR, Chen L, Porcella SF, Martens CA, Kobayashi SD, Porter AR, Chavda KD, Jacobs MR, Mathema B, Olsen RJ, Bonomo RA, Musser JM, Kreiswirth BN. 2014. Molecular dissection of the evolution of carbapenem-resistant multilocus sequence type 258 Klebsiella pneumoniae. Proc. Natl. Acad. Sci. U. S. A. 111:4988–4993. 10.1073/pnas.1321364111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thomas CM, Nielsen KM. 2005. Mechanisms of, and barriers to, horizontal gene transfer between bacteria. Nat. Rev. Microbiol. 3:711–721. 10.1038/nrmicro1234 [DOI] [PubMed] [Google Scholar]
- 12.Rasheed JK, Biddle JW, Anderson KF, Washer L, Chenoweth C, Perrin J, Newton DW, Patel JB. 2008. Detection of the Klebsiella pneumoniae carbapenemase type 2 carbapenem-hydrolyzing enzyme in clinical isolates of Citrobacter freundii and K. oxytoca carrying a common plasmid. J. Clin. Microbiol. 46:2066–2069. 10.1128/JCM.02038-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sidjabat HE, Silveira FP, Potoski BA, Abu-Elmagd KM, Adams-Haduch JM, Paterson DL, Doi Y. 2009. Interspecies spread of Klebsiella pneumoniae carbapenemase gene in a single patient. Clin. Infect. Dis. 49:1736-1738. 10.1086/648077 [DOI] [PubMed] [Google Scholar]
- 14.Goren MGY, Carmeli Y, Schwaber MJ, Chmelnitsky I, Schechner V, Navon-Venezia S. 2010. Transfer of carbapenem-resistant plasmid from Klebsiella pneumoniae ST258 to Escherichia coli in patient. Emerg. Infect. Dis. 16:1014–1017. 10.3201/eid1606.091671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Richter SN, Frasson I, Bergo C, Parisi S, Cavallaro A, Palu G. 2011. Transfer of Kpc-2 carbapenemase from Klebsiella pneumoniae to Escherichia coli in a patient: first case in Europe. J. Clin. Microbiol. 49:2040–2042. 10.1128/JCM.00133-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mathers AJ, Cox HL, Kitchel B, Bonatti H, Brassinga AK, Carroll J, Scheld WM, Hazen KC, Sifri CD. 2011. Molecular dissection of an outbreak of carbapenem-resistant Enterobacteriaceae reveals intergenus KPC carbapenemase transmission through a promiscuous plasmid. mBio 2:e00204-11. 10.1128/mBio.00204-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carattoli A. 2011. Plasmids in gram negatives: molecular typing of resistance plasmids. Int. J. Med. Microbiol. 301:654–658. 10.1016/j.ijmm.2011.09.003 [DOI] [PubMed] [Google Scholar]
- 18.Tofteland S, Naseer U, Lislevand JH, Sundsfjord A, Samuelsen O. 2013. A long-term low-frequency hospital outbreak of KPC-producing Klebsiella pneumoniae involving intergenus plasmid diffusion and a persisting environmental reservoir. PLoS One 8:e59015. 10.1371/journal.pone.0059015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tenover FC, Arbeit RD, Goering RV, Mickelsen PA, Murray BE, Persing DH, Swaminathan B. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233–2239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maiden MC, Bygraves JA, Feil E, Morelli G, Russell JE, Urwin R, Zhang Q, Zhou J, Zurth K, Caugant DA, Feavers IM, Achtman M, Spratt BG. 1998. Multilocus sequence typing: a portable approach to the identification of clones within populations of pathogenic microorganisms. Proc. Natl. Acad. Sci. U. S. A. 95:3140–3145. 10.1073/pnas.95.6.3140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mitsuma SF, Mansour MK, Dekker JP, Kim J, Rahman MZ, Tweed-Kent A, Schuetz P. 2013. Promising new assays and technologies for the diagnosis and management of infectious diseases. Clin. Infect. Dis. 56:996–1002. 10.1093/cid/cis1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stevenson LG, Drake SK, Murray PR. 2010. Rapid identification of bacteria in positive blood culture broths by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J. Clin. Microbiol. 48:444–447. 10.1128/JCM.01541-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Demarco ML, Burnham CA. 2014. Diafiltration MALDI-TOF mass spectrometry method for culture-independent detection and identification of pathogens directly from urine specimens. Am. J. Clin. Pathol. 141:204–212. 10.1309/AJCPQYW3B6JLKILC [DOI] [PubMed] [Google Scholar]
- 24.Edwards-Jones V, Claydon MA, Evason DJ, Walker J, Fox AJ, Gordon DB. 2000. Rapid discrimination between methicillin-sensitive and methicillin-resistant Staphylococcus aureus by intact cell mass spectrometry. J. Med. Microbiol. 49:295–300 [DOI] [PubMed] [Google Scholar]
- 25.Du Z, Yang R, Guo Z, Song Y, Wang J. 2002. Identification of Staphylococcus aureus and determination of its methicillin resistance by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Anal. Chem. 74:5487–5491. 10.1021/ac020109k [DOI] [PubMed] [Google Scholar]
- 26.Majcherczyk PA, McKenna T, Moreillon P, Vaudaux P. 2006. The discriminatory power of MALDI-TOF mass spectrometry to differentiate between isogenic teicoplanin-susceptible and teicoplanin-resistant strains of methicillin-resistant Staphylococcus aureus. FEMS Microbiol. Lett. 255:233–239. 10.1111/j.1574-6968.2005.00060.x [DOI] [PubMed] [Google Scholar]
- 27.Szabados F, Kaase M, Anders A, Gatermann SG. 2012. Identical MALDI-TOF MS-derived peak profiles in a pair of isogenic Sccmec-harboring and Sccmec-lacking strains of Staphylococcus aureus. J. Infect. 65:400–405. 10.1016/j.jinf.2012.06.010 [DOI] [PubMed] [Google Scholar]
- 28.Lau AF, Drake SK, Calhoun LB, Henderson CM, Zelazny AM. 2013. Development of a clinically comprehensive database and a simple procedure for identification of molds from solid media by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J. Clin. Microbiol. 51:828–834. 10.1128/JCM.02852-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hindiyeh M, Smollen G, Grossman Z, Ram D, Davidson Y, Mileguir F, Vax M, Ben David D, Tal I, Rahav G, Shamiss A, Mendelson E, Keller N. 2008. Rapid detection of blaKPC carbapenemase genes by real-time PCR. J. Clin. Microbiol. 46:2879–2883. 10.1128/JCM.00661-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clinical and Laboratory Standards Institute. 2013. Performance standards for antimicrobial susceptibility testing, 23rd informational supplement: M100-S23. CLSI, Wayne, PA [Google Scholar]
- 31.Petersen TN, Brunak S, von Heijne G, Nielsen H. 2011. Signalp 4.0: discriminating signal peptides from transmembrane regions. Nat. Methods 8:785–786. 10.1038/nmeth.1701 [DOI] [PubMed] [Google Scholar]
- 32.Leavitt A, Chmelnitsky I, Carmeli Y, Navon-Venezia S. 2010. Complete nucleotide sequence of KPC-3-encoding plasmid pKpQIL in the epidemic Klebsiella pneumoniae sequence type 258. Antimicrob. Agents Chemother. 54:4493–4496. 10.1128/AAC.00175-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garcia-Fernandez A, Villa L, Carta C, Venditti C, Giordano A, Venditti M, Mancini C, Carattoli A. 2012. Klebsiella pneumoniae ST258 producing KPC-3 identified in Italy carries novel plasmids and OmpK36/OmpK35 porin variants. Antimicrob. Agents Chemother. 56:2143–2145. 10.1128/AAC.05308-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Villa L, Capone A, Fortini D, Dolejska M, Rodriguez I, Taglietti F, De Paolis P, Petrosillo N, Carattoli A. 2013. Reversion to susceptibility of a carbapenem-resistant clinical isolate of Klebsiella pneumoniae producing KPC-3. J. Antimicrob. Chemother. 68:2482–2486. 10.1093/jac/dkt235 [DOI] [PubMed] [Google Scholar]
- 35.Chen L, Chavda KD, Melano RG, Jacobs MR, Koll B, Hong T, Rojtman AD, Levi MH, Bonomo RA, Kreiswirth BN. 2014. Comparative genomic analysis of KPC-encoding pKpQIL-like plasmids and their distribution in New Jersey and New York hospitals. Antimicrob. Agents Chemother. 58:2871–2877. 10.1128/AAC.00120-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carattoli A. 2013. Plasmids and the spread of resistance. Int. J. Med. Microbiol. 303:298–304. 10.1016/j.ijmm.2013.02.001 [DOI] [PubMed] [Google Scholar]
- 37.Cuzon G, Naas T, Nordmann P. 2011. Functional characterization of Tn4401, a Tn3-based transposon involved in blaKPC gene mobilization. Antimicrob. Agents Chemother. 55:5370–5373. 10.1128/AAC.05202-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gootz TD, Lescoe MK, Dib-Hajj F, Dougherty BA, He W, Della-Latta P, Huard RC. 2009. Genetic organization of transposase regions surrounding blaKPC carbapenemase genes on plasmids from Klebsiella strains isolated in a New York City hospital. Antimicrob. Agents Chemother. 53:1998–2004. 10.1128/AAC.01355-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ho PL, Cheung YY, Lo WU, Li Z, Chow KH, Lin CH, Chan JF, Cheng VC. 2013. Molecular characterization of an atypical IncX3 plasmid pKPC-NY79 carrying blaKPC-2 in a Klebsiella pneumoniae. Curr. Microbiol. 67:493–498. 10.1007/s00284-013-0398-2 [DOI] [PubMed] [Google Scholar]
- 40.Hrabak J, Studentova V, Walkova R, Zemlickova H, Jakubu V, Chudackova E, Gniadkowski M, Pfeifer Y, Perry JD, Wilkinson K, Bergerova T. 2012. Detection of NDM-1, VIM-1, KPC, OXA-48, and OXA-162 carbapenemases by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J. Clin. Microbiol. 50:2441–2443. 10.1128/JCM.01002-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee W, Chung HS, Lee Y, Yong D, Jeong SH, Lee K, Chong Y. 2013. Comparison of matrix-assisted laser desorption ionization-time-of-flight mass spectrometry assay with conventional methods for detection of IMP-6, VIM-2, NDM-1, SIM-1, KPC-1, OXA-23, and OXA-51 carbapenemase-producing Acinetobacter spp., Pseudomonas aeruginosa, and Klebsiella pneumoniae. Diagn. Microbiol. Infect. Dis. 77:227–230. 10.1016/j.diagmicrobio.2013.07.005 [DOI] [PubMed] [Google Scholar]
- 42.Wang L, Han C, Sui W, Wang M, Lu X. 2013. MALDI-TOF MS applied to indirect carbapenemase detection: a validated procedure to clearly distinguish between carbapenemase-positive and carbapenemase-negative bacterial strains. Anal. Bioanal. Chem. 405:5259–5266. 10.1007/s00216-013-6913-2 [DOI] [PubMed] [Google Scholar]
- 43.Alvarez-Buylla A, Picazo JJ, Culebras E. 2013. Optimized method for Acinetobacter species carbapenemase detection and identification by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J. Clin. Microbiol. 51:1589–1592. 10.1128/JCM.00181-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peaper DR, Kulkarni MV, Tichy AN, Jarvis M, Murray TS, Hodsdon ME. 2013. Rapid detection of carbapenemase activity through monitoring ertapenem hydrolysis in Enterobacteriaceae with LC-MS/MS. Bioanalysis 5:147–157. 10.4155/bio.12.310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Burckhardt I, Zimmermann S. 2011. Using matrix-assisted laser desorption ionization-time of flight mass spectrometry to detect carbapenem resistance within 1 to 2.5 hours. J. Clin. Microbiol. 49:3321–3324. 10.1128/JCM.00287-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.