Abstract
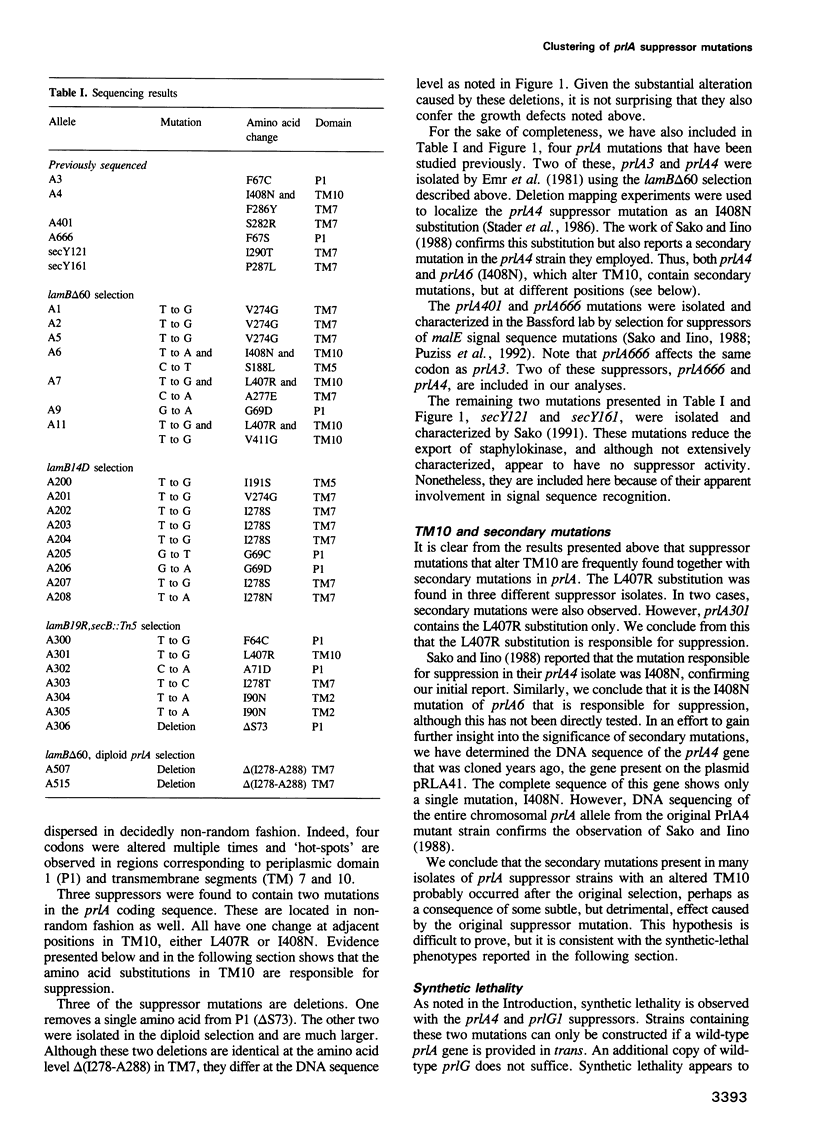
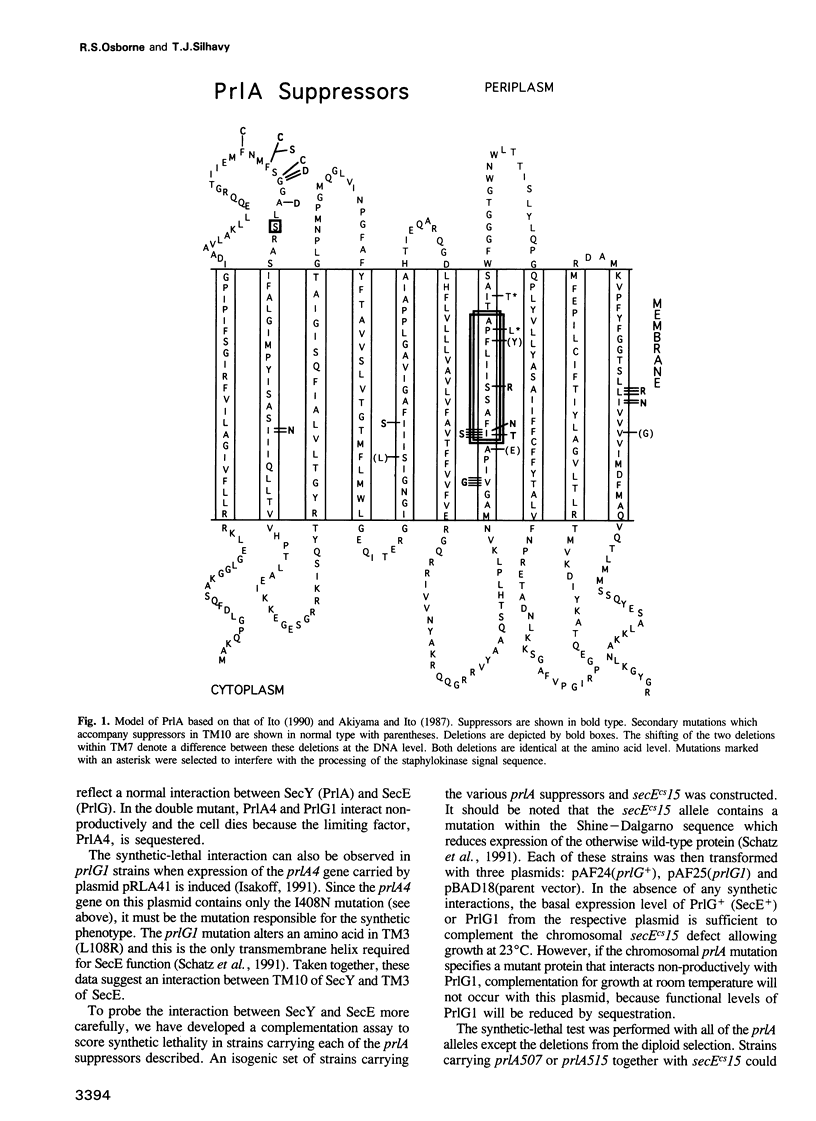
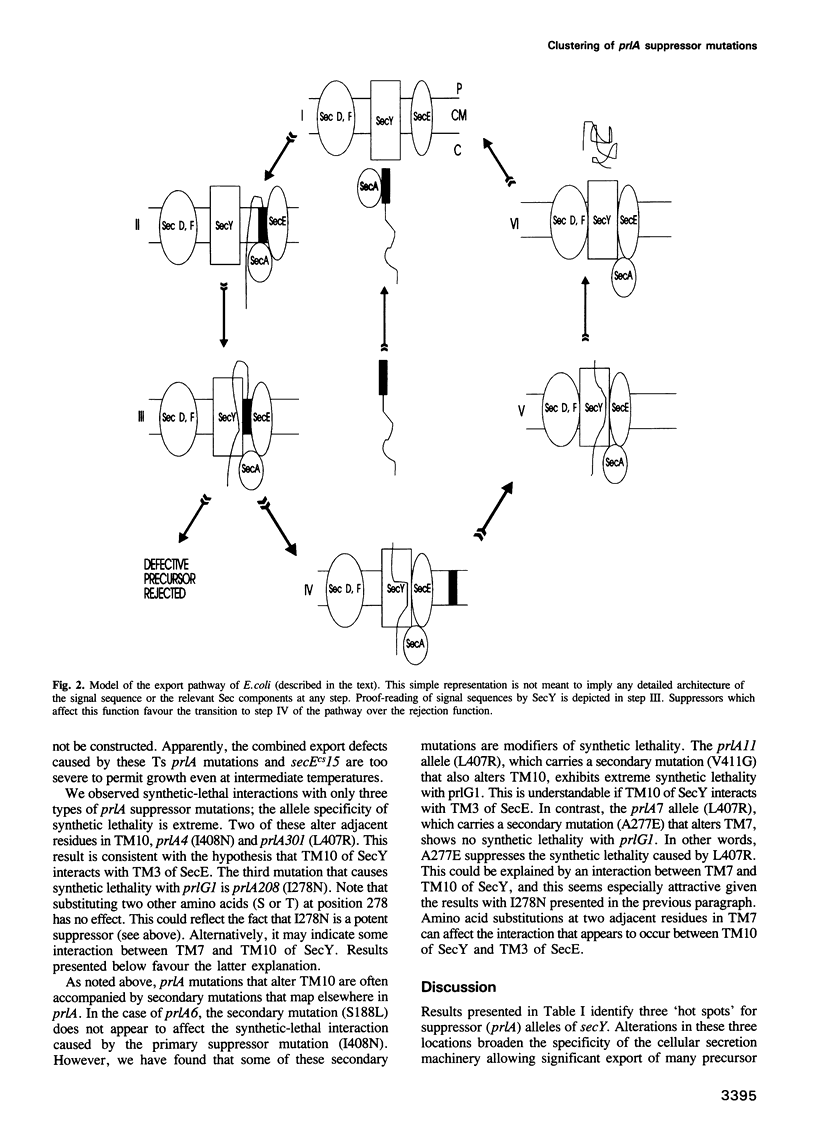
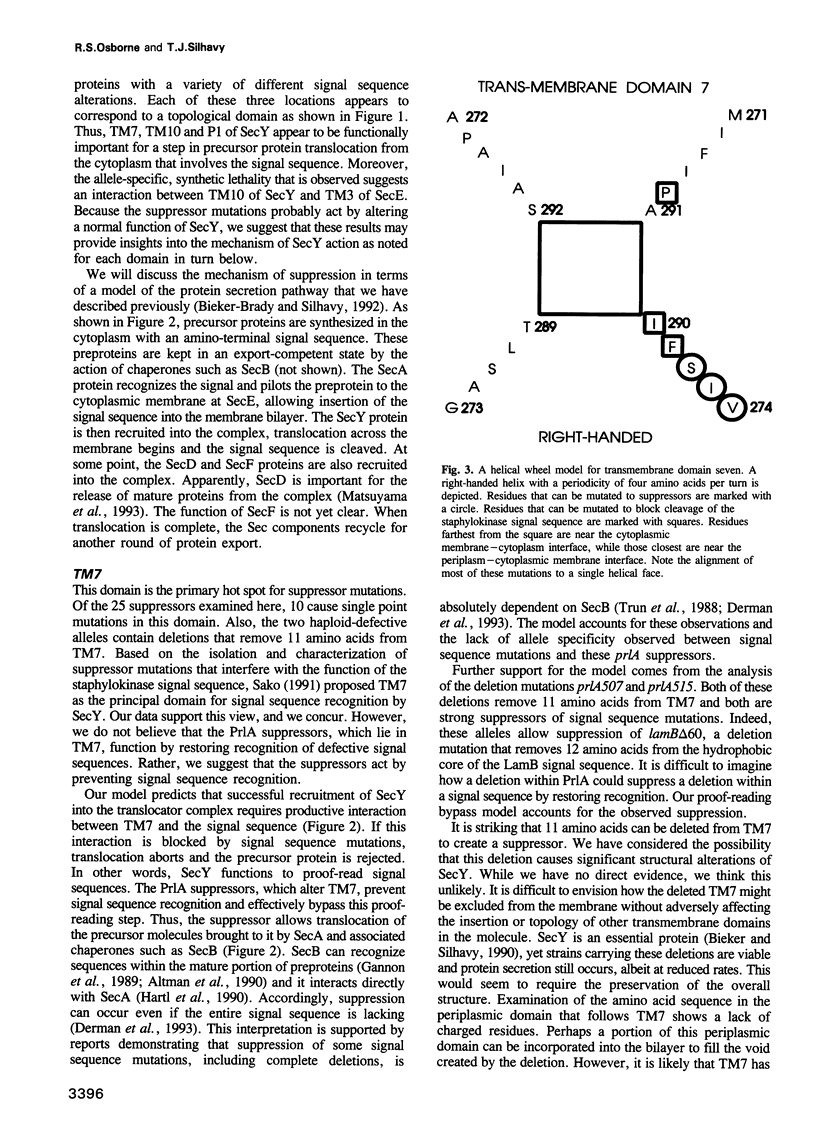
The SecY protein of Escherichia coli and its homologues in other organisms, are integral components of the cellular protein translocation machinery. Suppressor mutations that alter SecY (the prlA alleles) broaden the specificity of this machinery and allow secretion of precursor proteins with defective signal sequences. Twenty-five prlA alleles have been characterized. These suppressor mutations were found to cluster in regions corresponding to three distinct topological domains of SecY. Based on the nature and position of the prlA mutations, we propose that transmembrane domain 7 of SecY functions in signal sequence recognition. Results suggest that this interaction may involve a right-handed supercoil of alpha-helices. Suppressor mutations that alter this domain appear to prevent signal sequence recognition, and this novel mechanism of suppression suggests a proofreading function for SecY. We propose that suppressor mutations that alter a second domain of SecY, transmembrane helix 10, also affect this proof-reading function, but indirectly. Based on the synthetic phenotypes exhibited by double mutants, we propose that these mutations strengthen the interaction with another component of the translocation machinery, SecE. Suppressor mutations were also found to cluster in a region corresponding to an amino-terminal periplasmic domain. Possible explanations for this unexpected finding are discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama Y., Ito K. Topology analysis of the SecY protein, an integral membrane protein involved in protein export in Escherichia coli. EMBO J. 1987 Nov;6(11):3465–3470. doi: 10.1002/j.1460-2075.1987.tb02670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman E., Bankaitis V. A., Emr S. D. Characterization of a region in mature LamB protein that interacts with a component of the export machinery of Escherichia coli. J Biol Chem. 1990 Oct 25;265(30):18148–18153. [PubMed] [Google Scholar]
- Arndt E. The genes for ribosomal protein L15 and the protein equivalent to secY in the archaebacterium Haloarcula (Halobacterium) marismortui. Biochim Biophys Acta. 1992 Feb 28;1130(1):113–116. doi: 10.1016/0167-4781(92)90474-e. [DOI] [PubMed] [Google Scholar]
- Auer J., Spicker G., Böck A. Presence of a gene in the archaebacterium Methanococcus vannielii homologous to secY of eubacteria. Biochimie. 1991 Jun;73(6):683–688. doi: 10.1016/0300-9084(91)90048-6. [DOI] [PubMed] [Google Scholar]
- Bieker-Brady K., Silhavy T. J. Suppressor analysis suggests a multistep, cyclic mechanism for protein secretion in Escherichia coli. EMBO J. 1992 Sep;11(9):3165–3174. doi: 10.1002/j.1460-2075.1992.tb05393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieker K. L., Phillips G. J., Silhavy T. J. The sec and prl genes of Escherichia coli. J Bioenerg Biomembr. 1990 Jun;22(3):291–310. doi: 10.1007/BF00763169. [DOI] [PubMed] [Google Scholar]
- Bieker K. L., Silhavy T. J. PrlA (SecY) and PrlG (SecE) interact directly and function sequentially during protein translocation in E. coli. Cell. 1990 Jun 1;61(5):833–842. doi: 10.1016/0092-8674(90)90193-i. [DOI] [PubMed] [Google Scholar]
- Bieker K. L., Silhavy T. J. PrlA is important for the translocation of exported proteins across the cytoplasmic membrane of Escherichia coli. Proc Natl Acad Sci U S A. 1989 Feb;86(3):968–972. doi: 10.1073/pnas.86.3.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derman A. I., Puziss J. W., Bassford P. J., Jr, Beckwith J. A signal sequence is not required for protein export in prlA mutants of Escherichia coli. EMBO J. 1993 Mar;12(3):879–888. doi: 10.1002/j.1460-2075.1993.tb05728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshaies R. J., Schekman R. A yeast mutant defective at an early stage in import of secretory protein precursors into the endoplasmic reticulum. J Cell Biol. 1987 Aug;105(2):633–645. doi: 10.1083/jcb.105.2.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas S. E. A secY homologue is found in the plastid genome of Cryptomonas phi. FEBS Lett. 1992 Feb 17;298(1):93–96. doi: 10.1016/0014-5793(92)80029-g. [DOI] [PubMed] [Google Scholar]
- Emr S. D., Hanley-Way S., Silhavy T. J. Suppressor mutations that restore export of a protein with a defective signal sequence. Cell. 1981 Jan;23(1):79–88. doi: 10.1016/0092-8674(81)90272-5. [DOI] [PubMed] [Google Scholar]
- Flachmann R., Michalowski C. B., Löffelhardt W., Bohnert H. J. SecY, an integral subunit of the bacterial preprotein translocase, is encoded by a plastid genome. J Biol Chem. 1993 Apr 5;268(10):7514–7519. [PubMed] [Google Scholar]
- Gannon P. M., Li P., Kumamoto C. A. The mature portion of Escherichia coli maltose-binding protein (MBP) determines the dependence of MBP on SecB for export. J Bacteriol. 1989 Feb;171(2):813–818. doi: 10.1128/jb.171.2.813-818.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman L. M., Barondess J. J., Beckwith J. FtsL, an essential cytoplasmic membrane protein involved in cell division in Escherichia coli. J Bacteriol. 1992 Dec;174(23):7716–7728. [PMC free article] [PubMed] [Google Scholar]
- Görlich D., Prehn S., Hartmann E., Kalies K. U., Rapoport T. A. A mammalian homolog of SEC61p and SECYp is associated with ribosomes and nascent polypeptides during translocation. Cell. 1992 Oct 30;71(3):489–503. doi: 10.1016/0092-8674(92)90517-g. [DOI] [PubMed] [Google Scholar]
- Hartl F. U., Lecker S., Schiebel E., Hendrick J. P., Wickner W. The binding cascade of SecB to SecA to SecY/E mediates preprotein targeting to the E. coli plasma membrane. Cell. 1990 Oct 19;63(2):269–279. doi: 10.1016/0092-8674(90)90160-g. [DOI] [PubMed] [Google Scholar]
- Ito K. Structure, function, and biogenesis of SecY, an integral membrane protein involved in protein export. J Bioenerg Biomembr. 1990 Jun;22(3):353–367. doi: 10.1007/BF00763172. [DOI] [PubMed] [Google Scholar]
- Joly J. C., Wickner W. The SecA and SecY subunits of translocase are the nearest neighbors of a translocating preprotein, shielding it from phospholipids. EMBO J. 1993 Jan;12(1):255–263. doi: 10.1002/j.1460-2075.1993.tb05651.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S. K., Kudo T., Horikoshi K. Molecular cloning and characterization of an alkalophilic Bacillus sp. C125 gene homologous to Bacillus subtilis sec Y. J Gen Microbiol. 1992 Jul;138(7):1365–1370. doi: 10.1099/00221287-138-7-1365. [DOI] [PubMed] [Google Scholar]
- Koivula T., Palva I., Hemilä H. Nucleotide sequence of the secY gene from Lactococcus lactis and identification of conserved regions by comparison of four SecY proteins. FEBS Lett. 1991 Aug 19;288(1-2):114–118. doi: 10.1016/0014-5793(91)81015-z. [DOI] [PubMed] [Google Scholar]
- Kumamoto C. A., Beckwith J. Evidence for specificity at an early step in protein export in Escherichia coli. J Bacteriol. 1985 Jul;163(1):267–274. doi: 10.1128/jb.163.1.267-274.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmon M. A., Flanagan J. M., Treutlein H. R., Zhang J., Engelman D. M. Sequence specificity in the dimerization of transmembrane alpha-helices. Biochemistry. 1992 Dec 29;31(51):12719–12725. doi: 10.1021/bi00166a002. [DOI] [PubMed] [Google Scholar]
- Matsuyama S., Fujita Y., Mizushima S. SecD is involved in the release of translocated secretory proteins from the cytoplasmic membrane of Escherichia coli. EMBO J. 1993 Jan;12(1):265–270. doi: 10.1002/j.1460-2075.1993.tb05652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müsch A., Wiedmann M., Rapoport T. A. Yeast Sec proteins interact with polypeptides traversing the endoplasmic reticulum membrane. Cell. 1992 Apr 17;69(2):343–352. doi: 10.1016/0092-8674(92)90414-8. [DOI] [PubMed] [Google Scholar]
- Nakamura K., Nakamura A., Takamatsu H., Yoshikawa H., Yamane K. Cloning and characterization of a Bacillus subtilis gene homologous to E. coli secY. J Biochem. 1990 Apr;107(4):603–607. doi: 10.1093/oxfordjournals.jbchem.a123093. [DOI] [PubMed] [Google Scholar]
- Ohkubo S., Muto A., Kawauchi Y., Yamao F., Osawa S. The ribosomal protein gene cluster of Mycoplasma capricolum. Mol Gen Genet. 1987 Dec;210(2):314–322. doi: 10.1007/BF00325700. [DOI] [PubMed] [Google Scholar]
- Pugsley A. P. The complete general secretory pathway in gram-negative bacteria. Microbiol Rev. 1993 Mar;57(1):50–108. doi: 10.1128/mr.57.1.50-108.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puziss J. W., Strobel S. M., Bassford P. J., Jr Export of maltose-binding protein species with altered charge distribution surrounding the signal peptide hydrophobic core in Escherichia coli cells harboring prl suppressor mutations. J Bacteriol. 1992 Jan;174(1):92–101. doi: 10.1128/jb.174.1.92-101.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo F. D., Slauch J. M., Silhavy T. J. Mutations that affect separate functions of OmpR the phosphorylated regulator of porin transcription in Escherichia coli. J Mol Biol. 1993 May 20;231(2):261–273. doi: 10.1006/jmbi.1993.1281. [DOI] [PubMed] [Google Scholar]
- Sako T., Iino T. Distinct mutation sites in prlA suppressor mutant strains of Escherichia coli respond either to suppression of signal peptide mutations or to blockage of staphylokinase processing. J Bacteriol. 1988 Nov;170(11):5389–5391. doi: 10.1128/jb.170.11.5389-5391.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sako T. Novel prlA alleles defective in supporting staphylokinase processing in Escherichia coli. J Bacteriol. 1991 Apr;173(7):2289–2296. doi: 10.1128/jb.173.7.2289-2296.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders S. L., Whitfield K. M., Vogel J. P., Rose M. D., Schekman R. W. Sec61p and BiP directly facilitate polypeptide translocation into the ER. Cell. 1992 Apr 17;69(2):353–365. doi: 10.1016/0092-8674(92)90415-9. [DOI] [PubMed] [Google Scholar]
- Scaramuzzi C. D., Stokes H. W., Hiller R. G. Characterisation of a chloroplast-encoded secY homologue and atpH from a chromophytic alga. Evidence for a novel chloroplast genome organisation. FEBS Lett. 1992 Jun 15;304(2-3):119–123. doi: 10.1016/0014-5793(92)80601-c. [DOI] [PubMed] [Google Scholar]
- Schatz P. J., Bieker K. L., Ottemann K. M., Silhavy T. J., Beckwith J. One of three transmembrane stretches is sufficient for the functioning of the SecE protein, a membrane component of the E. coli secretion machinery. EMBO J. 1991 Jul;10(7):1749–1757. doi: 10.1002/j.1460-2075.1991.tb07699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shultz J., Silhavy T. J., Berman M. L., Fiil N., Emr S. D. A previously unidentified gene in the spc operon of Escherichia coli K12 specifies a component of the protein export machinery. Cell. 1982 Nov;31(1):227–235. doi: 10.1016/0092-8674(82)90422-6. [DOI] [PubMed] [Google Scholar]
- Simon S. M., Blobel G. Signal peptides open protein-conducting channels in E. coli. Cell. 1992 May 15;69(4):677–684. doi: 10.1016/0092-8674(92)90231-z. [DOI] [PubMed] [Google Scholar]
- Stader J., Benson S. A., Silhavy T. J. Kinetic analysis of lamB mutants suggests the signal sequence plays multiple roles in protein export. J Biol Chem. 1986 Nov 15;261(32):15075–15080. [PubMed] [Google Scholar]
- Stirling C. J., Rothblatt J., Hosobuchi M., Deshaies R., Schekman R. Protein translocation mutants defective in the insertion of integral membrane proteins into the endoplasmic reticulum. Mol Biol Cell. 1992 Feb;3(2):129–142. doi: 10.1091/mbc.3.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh J. W., Boylan S. A., Thomas S. M., Dolan K. M., Oliver D. B., Price C. W. Isolation of a secY homologue from Bacillus subtilis: evidence for a common protein export pathway in eubacteria. Mol Microbiol. 1990 Feb;4(2):305–314. doi: 10.1111/j.1365-2958.1990.tb00597.x. [DOI] [PubMed] [Google Scholar]
- Trun N. J., Silhavy T. J. Characterization and in vivo cloning of prlC, a suppressor of signal sequence mutations in Escherichia coli K12. Genetics. 1987 Aug;116(4):513–521. doi: 10.1093/genetics/116.4.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trun N. J., Stader J., Lupas A., Kumamoto C., Silhavy T. J. Two cellular components, PrlA and SecB, that recognize different sequence determinants are required for efficient protein export. J Bacteriol. 1988 Dec;170(12):5928–5930. doi: 10.1128/jb.170.12.5928-5930.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner W., Driessen A. J., Hartl F. U. The enzymology of protein translocation across the Escherichia coli plasma membrane. Annu Rev Biochem. 1991;60:101–124. doi: 10.1146/annurev.bi.60.070191.000533. [DOI] [PubMed] [Google Scholar]



