Abstract
Rapid diagnostic testing with matrix-assisted laser desorption ionization–time of flight (MALDI-TOF) decreases the time to organism identification by 24 to 36 h compared to the amount of time required by conventional methods. However, there are limited data evaluating the impact of MALDI-TOF with real-time antimicrobial stewardship team (AST) review and intervention on antimicrobial prescribing and outcomes for patients with bacteremia and blood cultures contaminated with coagulase-negative Staphylococcus (CoNS). A quasiexperimental study was conducted to analyze the impact of rapid diagnostic testing with MALDI-TOF plus AST review and intervention for adult hospitalized patients with blood cultures positive for CoNS. Antibiotic prescribing patterns and clinical outcomes were compared before and after implementation of MALDI-TOF with AST intervention for patients with CoNS bacteremia and CoNS contamination. A total of 324 patients with a positive CoNS blood culture were included; 246 were deemed to have contaminated cultures (117 in the preintervention group and 129 in AST the intervention group), and 78 patients had bacteremia (46 in the preintervention group and 32 in the AST intervention group). No differences in demographics were seen between the groups, and similar rates of contamination occurred between the preintervention and AST intervention groups (64.3% versus 72.6%, P = 0.173). Patients with bacteremia were initiated on optimal therapy sooner in the AST intervention group (58.7 versus 34.4 h, P = 0.030), which was associated with a similarly decreased mortality (21.7% versus 3.1%, P = 0.023). Patients with CoNS-contaminated cultures had similar rates of mortality, lengths of hospitalization, recurrent bloodstream infections, and 30-day hospital readmissions, but the AST intervention group had a decreased duration of unnecessary antibiotic therapy (1.31 versus 3.89 days, P = 0.032) and a decreased number of vancomycin trough assays performed (0.88 versus 1.95, P < 0.001). In patients with CoNS bacteremia, rapid pathogen identification integrated with real-time stewardship interventions improved timely organism identification and initiation of antibiotic therapy. Patients in the AST group with blood cultures contaminated with CoNS had decreased inappropriate antimicrobial prescribing and decreased unnecessary serum vancomycin trough assays.
INTRODUCTION
Coagulase-negative Staphylococcus (CoNS) is the most common pathogen associated with hospital-acquired central line infections and the most common organism responsible for contaminated blood cultures (1). The National Healthcare Safety Network's (NHSN′s) recommended criteria for the detection of a CoNS-contaminated blood culture include detection of a single positive culture from multiple samples from a patient without hypotension, fever, or chills in the acute care setting (2). However, differentiation of true bacteremia from contamination can be challenging and should involve detailed patient evaluation (3).
Matrix-assisted laser desorption ionization–time of flight (MALDI-TOF) utilizes mass spectrometry to rapidly identify organisms following isolation from clinical specimens. MALDI-TOF accurately and promptly identifies most bacterial and yeast species and represents an attractive alternative to more time-consuming conventional testing methods (4, 5). Integration of MALDI-TOF into the clinical microbiology work flow decreases the time to organism identification by 1.2 to 1.5 days compared to the amount of time required for conventional methods (6–9). However, there are limited data evaluating the impact of MALDI-TOF implementation on patient outcomes (6, 7). Furthermore, previous studies demonstrate that antimicrobial stewardship teams (ASTs) providing real-time review and intervention improve patient outcomes compared to those achieved from reporting of microbiology results alone (10–12). Therefore, the objective of the initiative described here was to evaluate clinical outcomes and the impact on antibiotic prescribing following antimicrobial stewardship review and intervention for patients with blood cultures positive for CoNS identified by MALDI-TOF.
MATERIALS AND METHODS
Study design.
This single-center, quasiexperimental study was conducted at the University of Michigan Hospitals and Health System and received investigational review board approval. Adult patients greater than 18 years of age with a CoNS blood culture identified via MALDI-TOF over a 3-month period (1 September 2012 to 30 November 2012) were compared to a historical control group with CoNS identified by conventional methods over the same three calendar months in the previous year (1 September 2011 to 30 November 2011). Patients were divided into 4 categories: patients with CoNS bacteremia before and after implementation of MADLI-TOF plus AST intervention and patients with CoNS contamination before and after MADLI-TOF plus AST intervention. Patients transferred from an outside hospital were excluded.
Microbiology work flow.
Blood cultures were performed using FAN medium on the BacT/Alert system (bioMérieux, Durham, NC). Aliquots from bottles that signaled positive were Gram stained and subcultured, and the results of the Gram stain were communicated directly to the ordering clinicians during both study periods and were posted to the clinical information system once verbal notification was performed. Coagulase-negative Staphylococcus identification and antimicrobial susceptibility testing results were routinely reported between 6:00 a.m. and 11:30 p.m. during both the intervention and control periods. Susceptibilities for CoNS were performed for patients with more than one positive culture, and testing occurred via the Vitek 2 system (bioMérieux, Durham, NC).
During both the control and intervention study periods, positive blood cultures were subcultured to solid medium and organism identification was performed following overnight incubation. During the control period, identification of isolates was performed using conventional methods (e.g., coagulase testing and/or the Vitek 2 system). During the intervention period, isolates recovered from positive blood cultures were identified by MALDI-TOF mass spectrometry using a Bruker Microflex instrument, Biotyper software (v. 3.0), and the Biotyper database (v. 3.1.0) (Bruker Daltonik, Bremen, Germany). Isolates were processed by either direct transfer (with or without formic acid overlay) or manual formic acid extraction procedures, as recommended by the manufacturer, and Biotyper scores were interpreted as previously reported (11, 13). All isolates were initially spotted in duplicate onto the manufacturer's reusable steel target plates, with testing of Gram-positive isolates occurring after the addition of matrix and formic acid overlays.
MALDI-TOF with stewardship intervention study period.
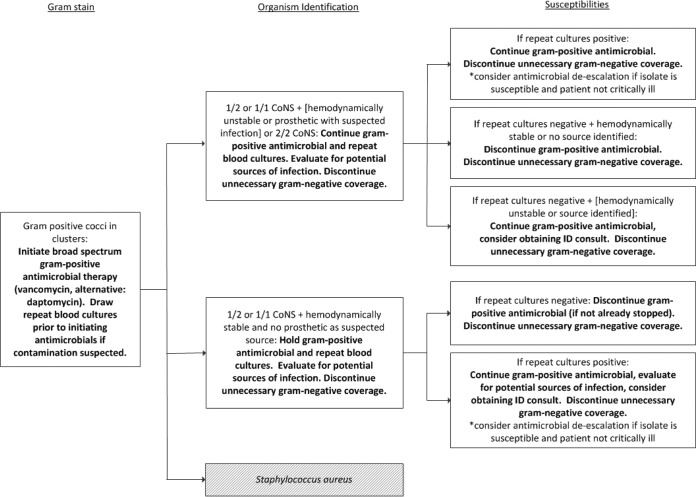
The AST at the University of Michigan hospitals consists of two infectious diseases physicians, two infectious diseases pharmacists, and an infectious diseases pharmacy resident. The AST developed an evidence-based algorithm for the management of patients with CoNS from blood cultures which involved evaluation of the blood samples for culture drawn, potential sources of infection, and clinical status (Fig. 1). An AST member received real-time notification for all patients with positive blood cultures and provided prescribers with preestablished, evidence-based antibiotic recommendations in accordance with the CoNS algorithm and hospital guidelines. The AST utilized clinical decision support computer software (TheraDoc, v. 4.4; Hospira, Lake Forest, IL) to receive real-time electronic pages between 6:00 a.m. and 11:30 p.m. In addition to real-time pages, AST members also received email notification 24 hours per day, and Gram stain results reported between 11:30 p.m. and 6:00 a.m. were reviewed on the following morning.
FIG 1.
Antimicrobial stewardship approach to CoNS cultures following organism Gram stain, identification, and susceptibility determination. Bold text indicates the corresponding AST action.
Preintervention (control) study period.
During the preintervention (control) study period, CoNS identification was performed via conventional methods, primarily utilizing the Vitek 2 system (bioMérieux). Prescribers were immediately notified of positive Gram stain results from blood cultures by a microbiology technologist. The AST did not intervene for positive bacterial cultures in real time during this time period. The AST reviewed daily reports from Monday through Friday for all patients receiving restricted antimicrobials (carbapenems, daptomycin, linezolid, quinupristin-dalfopristin, ceftaroline, tigecycline, voriconazole, posaconazole, and liposomal amphotericin B) and recommended therapy changes on the basis of institutional guidelines and clinical judgment when appropriate, and the patients for whom these changes were recommended may have included patients with blood cultures positive for CoNS. All stewardship activities, except for the addition of real-time alerts for positive blood cultures during the intervention period, remained unchanged during the study time frame.
Outcomes.
The study objective was to analyze clinical outcomes and the impact on antibiotic prescribing following rapid identification of CoNS isolates via MALDI-TOF in combination with AST intervention, including 30-day all-cause mortality, hospital and intensive care unit (ICU) length of stay following blood culture positivity, microbiologic clearance, recurrent bacteremia within 30 days of discontinuing antimicrobial therapy, and 30-day readmission for recurrent bacteremia with the same organism. For patients with CoNS bacteremia, the time to effective therapy and the time to optimal antimicrobial therapy were recorded. For patients with CoNS contamination, the duration of antibiotic therapy, the number of vancomycin serum assays, and the rate of Clostridium difficile infection were measured. Time to effective therapy was defined to be from the time at which the blood sample for culture was drawn to the time of administration of the first antimicrobial to which the organism had known susceptibility per the microbiology report. Time to optimal therapy was defined as the time from the blood culture draw to the time the patient received appropriate antibiotic therapy, which included deescalation based on known susceptibility results, concomitant infections, antibiotic allergies or intolerances, discontinuation of antimicrobials if the pathogen in the blood culture was determined to be a contaminant, and discontinuation of unnecessary antibiotic coverage targeting other organisms (for example, discontinuation of therapy against a Gram-negative organism for a patient with CoNS bacteremia).
The AST recorded all recommended interventions and the prescriber acceptance rate, as well as the timing of the intervention in relation to the time of the Gram stain result, organism identification, and antimicrobial susceptibility testing during the intervention period. Interventions were classified as broadening or initiating coverage, narrowing antimicrobial coverage to target the isolated organism, discontinuing therapy targeting organisms not isolated, or other, which included modification of antibiotic coverage for multiple infections or a specific disease state.
Statistical analysis.
All statistical analyses were performed using SPSS software (v. 20.0; SPSS, Inc., Chicago, IL). Demographic data were analyzed by descriptive statistics, outcomes with continuous data were analyzed by 2-tailed Student's t test, and dichotomous data were analyzed by Pearson's chi-square test.
RESULTS
Patients with a positive blood culture (n = 324) with CoNS were included in the evaluation; 175 were in the preintervention group, and 149 were in the intervention group, and similar proportions of patients with true bacteremia were detected in the two groups (26.3% versus 21.5%, P = 0.362). Demographics, comorbidities, immunosuppression, site of acquisition of bacteremia, and disease severity were similar among the patients in the preintervention and intervention groups with CoNS bacteremia and CoNS contamination (Table 1). MALDI-TOF identified CoNS quicker than traditional methods (83.4 versus 57.0 h, P < 0.001) (Table 2). Patients with true CoNS bacteremia were commonly empirically started on broad-spectrum antimicrobials with activity against Gram-positive bacteria, and there was no significant difference in the time to effective therapy (37.7 versus 23.0 h, P = 0.064). However, optimal therapy, which included discontinuation of unnecessary coverage against Gram-positive and Gram-negative bacteria in patients with CoNS contamination, was achieved significantly sooner in the intervention group (58.7 versus 34.4 h, P = 0.030). Patients with CoNS bacteremia in the preintervention and intervention groups also had similar clinical outcomes, which included length of hospitalization, length of ICU stay, recurrent bacteremia, and hospital readmission (Table 2), but patients with CoNS bacteremia in the intervention group had lower rates of mortality (21.7 versus 3.1%, P = 0.023).
TABLE 1.
Patient demographics
| Characteristica | CoNS bacteremia group |
CoNS contamination group |
||||
|---|---|---|---|---|---|---|
| Preintervention group (n = 46) | AST intervention group (n = 32) | P value | Preintervention group (n = 129) | AST intervention group (n = 117) | P value | |
| Demographics | ||||||
| No. (%) females | 18 (39.1) | 13 (40.6) | >0.99 | 65 (50.4) | 56 (47.9) | 0.704 |
| Mean age ± SD (yr) | 55.6 ± 15.1 | 60.4 ± 10.8 | 0.128 | 56 ± 15.6 | 59.1 ± 15.6 | 0.112 |
| No. (%) of patients with the following comorbidity: | ||||||
| Malignancy | 20 (43.5) | 19 (59.4) | 0.25 | 46 (35.7) | 47 (40.2) | 0.551 |
| Solid organ transplantation | 2 (4.3) | 3 (9.4) | 0.396 | 12 (9.3) | 11 (9.4) | >0.99 |
| Bone marrow transplantation | 8 (17.4) | 8 (25.0) | 0.57 | 11 (8.5) | 15 (12.8) | 0.304 |
| HIV infection | 0 (0.0) | 0 (0.0) | >0.99 | 0 (0.0) | 1 (0.9) | 0.478 |
| Chronic lung disease | 9 (19.6) | 3 (9.4) | 0.34 | 26 (20.2) | 20 (17.1) | 0.624 |
| Chronic heart disease | 19 (41.3) | 14 (43.8) | >0.99 | 35 (27.1) | 32 (27.4) | >0.99 |
| Chronic kidney disease | 13 (28.3) | 10 (31.3) | 0.805 | 32 (27.4) | 22 (25.9) | 0.283 |
| Chronic liver disease | 1 (2.2) | 2 (6.3) | 0.568 | 9 (7.0) | 15 (12.8) | 0.137 |
| No. (%) of patients with the following immunosuppression: | ||||||
| Antirejection medications | 8 (17.4) | 10 (31.3) | 0.18 | 23 (17.8) | 23 (19.7) | 0.745 |
| Chronic corticosteroids | 3 (6.5) | 6 (18.8) | 0.15 | 17 (13.2) | 17 (14.5) | 0.854 |
| Chemotherapy within 90 days | 4 (8.7) | 7 (21.9) | 0.184 | 12 (9.3) | 15 (12.8) | 0.419 |
| ANC, <500/mm3 | 1 (2.2) | 1 (3.1) | >0.99 | 1 (0.8) | 4 (3.4) | 0.194 |
| CD4 count, <200/mm3 | 0 (0.0) | 0 (0.0) | >0.99 | 0 (0.0) | 1 (0.9) | 0.478 |
| No. (%) of patients with the following site of acquisition: | ||||||
| Community acquired | 10 (21.7) | 7 (21.9) | >0.99 | 34 (26.4) | 39 (33.3) | 0.264 |
| Health care associated | 22 (47.9) | 16 (50.0) | >0.99 | 63 (48.8) | 44 (37.6) | 0.094 |
| Hospital acquired | 14 (30.4) | 9 (28.1) | >0.99 | 32 (24.8) | 34 (29.1) | 0.474 |
| Clinical status | ||||||
| ICU admission | 13 (28.3) | 5 (15.6) | 0.42 | 36 (27.9) | 27 (23.1) | 0.465 |
| Hemodynamic instability requiring vasopressor therapy | 4 (8.7) | 4 (12.5) | 0.71 | 8 (6.2) | 14 (12.0) | 0.124 |
Abbreviations: human immunodeficiency virus; ANC, absolute neutrophil count.
TABLE 2.
Outcomes for patients with CoNS bacteremia
| Characteristic | Preintervention group (n = 46) | AST intervention group (n = 32) | P value |
|---|---|---|---|
| Time to organism identificationa (h) | 83.4 ± 29.5 | 57.0 ± 32.3 | <0.001 |
| Time to effective therapya (h) | 37.7 ± 40.1 | 23.0 ± 10.7 | 0.064 |
| Time to optimal therapya (h) | 58.7 ± 56.4 | 34.4 ± 29.9 | 0.030 |
| No. (%) of patients with 30-day all-cause mortality | 10 (21.7) | 1 (3.1) | 0.023 |
| Length of hospitalizationa,b (days) | 14 ± 22 | 15 ± 14 | 0.954 |
| Length of ICU staya,b (days) | 28 ± 33 | 11 ± 11 | 0.188 |
| No. (%) of patients with recurrent bacteremia | 6 (13.0) | 0 (0.0) | 0.076 |
| No. (%) of patients with 30-day readmission with CoNS bacteremia | 2 (4.3) | 0 (0.0) | 0.51 |
Data are means ± standard deviations.
Lengths of hospitalization and ICU stay were defined as the time from blood culture positivity to the time of discharge.
Patients with CoNS contamination in the preintervention and intervention groups had similar clinical outcomes (Table 3). However, the stewardship intervention group had a decreased duration of inappropriate antibiotic administration with vancomycin or daptomycin (4.4 versus 3.0 days, P = 0.015) and a decreased number of vancomycin serum assays (2.0 versus 1.0 orders, P < 0.001). Despite a reduction in vancomycin utilization, the rates of Clostridium difficile colitis within 30 days following detection of CoNS by culture were similar between groups (8.4 versus 4.7%, P = 0.367). The AST provided 52 interventions: 6 interventions for patients with CoNS bacteremia and 46 interventions for patients with CoNS contamination. The majority of interventions involved the stewardship pharmacist discontinuing antibiotic therapy following organism identification in 37 patients in the CoNS contamination group. The AST recommended initiation of vancomycin therapy in all 6 patients with true bacteremia.
TABLE 3.
Antimicrobial use and outcomes for patients with CoNS contamination
| Characteristic | Preintervention group (n = 83) | AST intervention group (n = 85) | P value |
|---|---|---|---|
| Duration of CoNS antibiotic therapya (days) | 4.4 ± 4.2 | 3.0 ± 1.6 | 0.015 |
| Vancomycin utilizationa (g) | 4.8 ± 6.3 | 3.0 ± 3.9 | 0.038 |
| Daptomycin utilizationa (g) | 2.88 | 0 | 0.243 |
| No. of vancomycin serum assays obtaineda | 2.0 ± 2.2 | 0.9 ± 1.4 | <0.001 |
| No. (%) of patients with 30-day all-cause mortality | 9 (10.8) | 10 (11.8) | >0.99 |
| Length of hospitalizationa (days) | 14.6 ± 22.9 | 15.8 ± 18.6 | 0.7 |
| No. (%) of patients with recurrent bacteremia | 3 (3.6) | 2 (2.4) | 0.68 |
| No. (%) of patients with 30-day readmission with CoNS bacteremia | 2 (2.4) | 1 (1.2) | 0.618 |
| No. (%) of patients Clostridium difficile colitis | 7 (8.4) | 4 (4.7) | 0.367 |
Data are means ± standard deviations.
DISCUSSION
This study demonstrated that real-time AST review of CoNS blood cultures resulted in decreases in the unnecessary utilization of antimicrobials with activity against Gram-positive bacteria in patients with contaminated blood cultures. Additionally, a decrease in the mean number of vancomycin serum drug assays obtained was observed, despite the fact that the AST was not responsible for ordering therapeutic drug monitoring and did not provide recommendations to limit unnecessary ordering of vancomycin serum assays.
The emergence of rapid diagnostic testing with MALDI-TOF, peptide nucleic acid-fluorescence in situ hybridization (PNA-FISH), and nucleic acid microarrays provides clinical microbiology laboratories with tools to dramatically reduce the time to organism identification. Wong and colleagues conducted a similar before-and-after quasiexperimental study evaluating real-time stewardship intervention for CoNS bloodstream contamination in 53 patients but utilized rapid diagnostic testing with PNA-FISH during both study periods (14). They reported a 32-h reduction in the time to the cessation of unnecessary vancomycin therapy (57.7 versus 25.7 h, P = 0.005), which is remarkably similar to the reduction of 1.4 days (33.6 h) reported in this study. They also reported a decrease in infection-related length of hospitalization following the time of availability of culture results (10 versus 5.5 days, P = 0.018), which was not evaluated in this study. Additionally, similar results were noted for patients with true bacteremia, in which Wong and colleagues initiated timely vancomycin therapy in 7 patients (14), whereas timely vancomycin therapy was initiated for 6 patients in the current study. The results of both studies suggest that real-time pharmacist review of blood cultures combined with rapid diagnostic testing can help facilitate the timely initiation of antibiotic therapy for patients with CoNS bacteremia and reduce unnecessary antibiotic therapy for patients with CoNS contamination. MALDI-TOF offers an advantage over PNA-FISH, as rapid detection of many organisms, which can help facilitate the timely initiation of antibiotic therapy without setting up additional testing platforms, is possible. MALDI-TOF plus AST intervention can improve the time to effective antibiotic therapy and is associated with decreased mortality, a decreased length of hospitalization, and decreased costs when utilized for patients with true bacteremia or candidemia (6, 7).
This study noted a reduction in mortality for patients with CoNS bacteremia. However, the sample size for patients with true infection was small, which limits the ability to perform additional statistical testing to evaluate the impact of the AST intervention while controlling for other factors that may impact mortality. Another limitation was our inability to perform MALDI-TOF directly from positive blood culture bottles; our study used MALDI-TOF to identify isolates from subculture, which delayed identification by another 12 to 24 h. Multiple studies have demonstrated the analytical benefits of rapid identification of pathogens directly from positive blood culture bottles (13, 15–17). Although our current blood culture bottles (FAN; bioMérieux) are not compatible with MALDI-TOF- and amplification-based methodologies for testing directly from positive bottles, additional benefits may be realized if we were to utilize different vendors for blood culture bottles and were able to perform MALDI sooner. Additionally, we demonstrated similar demographics, comorbidities, and rates of ICU admission for the patient populations, but undocumented differences in the patient populations could exist, which is a limitation of quasiexperimental studies. Other limitations in this study include the lack of randomization, which may not take into account changes in care over the previous year that could improve mortality. The study periods were selected to minimize the chronologic bias of seasonal variations in the incidence of pathogens and minimize the potential maturation bias of medical interns, residents, and fellows at our large academic teaching institution by performing the study at the same time points during training. Furthermore, there were limitations in the time to effective therapy and the time to optimal therapy, as antimicrobial administration times were recorded only for inpatients. Antimicrobial administration time in patients who received treatment prior to admission (i.e., in the emergency department) was not assessed. Thus, the time to effective therapy may actually be sooner than that reported in the results. The interventions were not performed overnight (11:30 p.m. to 6:00 a.m.), which may limit the clinical impact compared to that which would be achieved with a true 24-hour-per-day, 7-day-per-week review. Finally, this is a single-center study, and the ability to reproduce our results in other institutions may be limited.
In conclusion, rapid diagnostic testing with real-time AST intervention can reduce the time to identification of CoNS from blood cultures, improve the time to optimal therapy for patients with true bacteremia, decrease unnecessary antibiotic therapy, and decrease the need for additional vancomycin therapeutic drug monitoring in patients with blood cultures contaminated with CoNS.
Footnotes
Published ahead of print 28 May 2014
REFERENCES
- 1.Sievert DM, Edwards JR, Schneider A, Patel J, Srinivasan A, Kallen A, Limbago B, Fridkin S. 2013. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2009-2010. Infect. Control Hosp. Epidemiol. 34:1–14. 10.1086/668770 [DOI] [PubMed] [Google Scholar]
- 2.Horan TC, Andrus M, Dudeck MA. 2008. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am. J. Infect. Control 36:309–332. 10.1016/j.ajic.2008.03.002 [DOI] [PubMed] [Google Scholar]
- 3.Rahkonen M, Luttienen S, Koskela M, Hautala T. 2012. True bacteremias caused by coagulase negative Staphylococcus are difficult to distinguish from blood culture contaminants. Eur. J. Clin. Microbiol. Infect. Dis. 31:2639–2644. 10.1007/s10096-012-1607-9 [DOI] [PubMed] [Google Scholar]
- 4.Stevenson LG, Drake SK, Murray PR. 2010. Rapid identification of bacteria in positive blood culture broths by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J. Clin. Microbiol. 48:444–447. 10.1128/JCM.01541-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marklein G, Josten M, Klanke U, Müller E, Horré R, Maier T, Wenzel T, Kostrzewa M, Bierbaum G, Hoerauf A, Sahl HG. 2009. Matrix-assisted laser desorption ionization–time of flight mass spectrometry for fast and reliable identification of clinical yeast isolates. J. Clin. Microbiol. 47:2912–2917. 10.1128/JCM.00389-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang AM, Newton D, Kunapuli A, Gandhi TN, Washer LL, Isip J, Collins CD, Nagel JL. 2013. Impact of rapid organism identification via matrix-assisted laser desorption ionization time-of-flight combined with antimicrobial stewardship team intervention in adult patients with bacteremia and candidemia. Clin. Infect. Dis. 57:1237–1245. 10.1093/cid/cit498 [DOI] [PubMed] [Google Scholar]
- 7.Perez KK, Olsen RJ, Musick WL, Cernoch PL, Davis JR, Land GA, Peterson LE, Musser JM. 2013. Integrating rapid pathogen identification and antimicrobial stewardship significantly decreases hospital costs. Arch. Pathol. Lab. Med. 137:1247–1254. 10.5858/arpa.2012-0651-OA [DOI] [PubMed] [Google Scholar]
- 8.Tan KE, Ellis BC, Lee R, Stamper PD, Zhang SX, Carroll KC. 2012. Prospective evaluation of a matrix-assisted laser desorption ionization-time of flight mass spectrometry system in a hospital clinical microbiology laboratory for identification of bacteria and yeasts: a bench-by-bench study for assessing the impact on time to identification and cost-effectiveness. J. Clin. Microbiol. 50:3301–3308. 10.1128/JCM.01405-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vlek AL, Bonten MJ, Boel CH. 2012. Direct matrix-assisted laser desorption ionization time-of-flight mass spectrometry improves appropriateness of antibiotic treatment of bacteremia. PLoS One 7:e32589. 10.1371/journal.pone.0032589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ly T, Gulia J, Pyrgos V, Waga M, Shoham S. 2008. Impact upon clinical outcomes of transplant of PNA FISH-generated laboratory data from the clinical microbiology bench to bedside in real time. Ther. Clin. Risk Manag. 4:637–640. 10.2147/TCRM.S2838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carver PL, Lin S, DePestel DD, Newton DW. 2008. Impact of mecA gene testing and intervention by infectious disease clinical pharmacists on time to optimal antimicrobial therapy for Staphylococcus aureus bacteremia at a university hospital. J. Clin. Microbiol. 46:2381–2383. 10.1128/JCM.00801-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holtzman C, Whitney D, Barlam T, Miller NS. 2011. Assessment of impact of peptide nucleic acid fluorescence in situ hybridization for rapid identification of coagulase-negative staphylococci in the absence of antimicrobial stewardship intervention. J. Clin. Microbiol. 49:1581–1582. 10.1128/JCM.02461-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Samuel LP, Tibbetts RJ, Agotesku A, Fey M, Hensley R, Meier FA. 2013. Evaluation of a microarray-based assay for rapid identification of gram-positive organisms and resistance markers in positive blood cultures. J. Clin. Microbiol. 51:1188–1192. 10.1128/JCM.02982-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wong JR, Bauer KA, Mangino JE, Goff DA. 2012. Antimicrobial stewardship pharmacist interventions for coagulase-negative Staphylococcus positive blood cultures using rapid polymerase chain reaction. Ann. Pharmacother. 46:1484–1490. 10.1345/aph.1R439 [DOI] [PubMed] [Google Scholar]
- 15.Lagacé-Wiens PRS, Adam HJ, Karlowsky JA, Nichol KA, Pang PF, Guenther J, Webb AA, Miller C, Alfa MJ. 2012. Identification of blood culture isolates directly from positive blood cultures by use of matrix-assisted laser desorption ionization–time of flight mass spectrometry and a commercial extraction system: analysis of performance, cost, and turnaround time. J. Clin. Microbiol. 50:3324–3328. 10.1128/JCM.01479-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blaschke AJ, Heyrend C, Byington CL, Fisher MA, Barker E, Garrone NF, Thatcher SA, Pavia AT, Barney T, Alger GD, Daly JA, Ririe KM, Ota I, Poritz MA. 2012. Rapid identification of pathogens from positive blood cultures by multiplex PCR using the FilmArray system. Diagn. Microbiol. Infect. Dis. 74:349–355. 10.1016/j.diagmicrobio.2012.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oliveira K, Procop GW, Wilson D, Coull J, Stender H. 2002. Rapid identification of Staphylococcus aureus directly from blood cultures by fluorescence in situ hybridization with peptide nucleic acid probes. J. Clin. Microbiol. 40:247–251. 10.1128/JCM.40.1.247-251.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]