Abstract
In Europe, human infections with “Candidatus Neoehrlichia mikurensis” have mainly been restricted to immunocompromised patients. We report here the first cases of asymptomatic “Ca. Neoehrlichia mikurensis” infection in immunocompetent humans (5/316 [1.6%] were infected). Due to the potential threats of infections with “Ca. Neoehrlichia mikurensis” in healthy persons to the safety of the blood supply, further study of this phenomenon is required.
TEXT
In 2004, “Candidatus Neoehrlichia mikurensis” was isolated by a Japanese group from naturally infected wild rats (Rattus norvegicus) and Ixodes ovatus (1). This obligate intracellular pathogen was proposed as a new species within the family Anaplasmataceae. Like the other two pathogenic Anaplasmataceae of the genera Anaplasma and Ehrlichia, Neoehrlichia is transmitted by Ixodidae ticks. “Ca. Neoehrlichia mikurensis” has been found in Ixodes ricinus ticks in several countries in Europe (2–5) and Ixodes persulcatus ticks in eastern Asia (6). It is one of the most prevalent pathogens in I. ricinus, second only to Borrelia afzelii (7). Wild rodents have been proposed as potential reservoir hosts for “Ca. Neoehrlichia mikurensis” (8, 9). However, the pathogenic role of this bacterium in humans remains unclear (10). Since 2007, a total of eight human cases of “Ca. Neoehrlichia mikurensis,” mainly in immunocompromised patients, have been described in Europe (2, 10–13). Infections have also been detected in humans from Asia, including a recent report of seven cases in China (14). In Europe, most patients experienced severe fever illnesses, cough, headaches, arthralgia, nausea, vomiting, or diarrhea. The purpose of this study was to investigate the prevalence of and risk for “Ca. Neoehrlichia mikurensis” infection in healthy professional foresters from northeastern Poland, where tick-borne diseases are endemic.
A total of 404 blood samples were collected from 316 foresters (236 male, 80 female; mean age, 44.1 years) employed in the 5 regions of the Warmińsko-Mazurskie (Czerwony Dwór [N54°7′51″ E22°11′32″], Gołdap [N54°18′25″ E22°18′13″]) and Podlaskie (Suwałki [N54°6′41″ E22°55′51″], Rudka [N52°43′28″ E22°43′41″], and Nurzec [N52°26′45″ E23°5′55″]) provinces in northeastern Poland. The samples were taken twice: in July and November 2012 from foresters in 3 regions (Czerwony Dwór, Gołdap, and Suwałki), and once in July 2012 from those in the other 2 regions (Rudka and Nurzec). Genomic DNA was isolated from whole blood using the DNeasy blood and tissue kit (Qiagen, Crawley, United Kingdom) and used as the template in specific nested-PCRs for the 16S rRNA gene (430 bp [7]) and groESL heat shock operon (920 bp [14]) to screen for “Ca. Neoehrlichia mikurensis” infection. In PCR-positive samples, species assessment was carried out by sequencing the amplified fragments in both directions. DNA sequence alignments and phylogenetic analysis were conducted using MEGA version 5.0 (15). After testing the data for the best substitution model, phylogenetic trees were obtained using the maximum likelihood method as the tree construction method and the Hasegawa-Kishino-Yano parameter algorithm as a distance method. For comparison, the sequences of “Ca. Neoehrlichia mikurensis” obtained from GenBank (www.ncbi.nlm.nih.gov) were included in the sequence alignment.
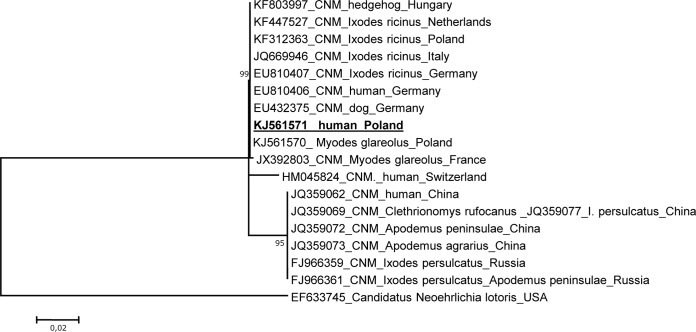
Among the 316 studied foresters, 5 cases (1.6%) of asymptomatic infection with “Ca. Neoehrlichia mikurensis” were diagnosed. The positive blood samples were collected in early July 2012 from 4 men and one woman working in the Czerwony Dwór, Gołdap (Warmińsko-Mazurskie), and Suwałki (Podlaskie) regions. Infection with this bacterium was also diagnosed from the sample taken in early November 2012 from one of the same men from Czerwony Dwór. No “Ca. Neoehrlichia mikurensis” morulae were observed on the Giemsa-stained blood smears. The nucleotide sequences of all of the amplified 16S rRNA gene fragments (430 bp; nucleotide positions 5′-3′ of amplified fragment, 133 to 563; number corresponds to the nucleotide positions relative to the sequence of the 16S rRNA gene [1,426 bp] of “Ca. Neoehrlichia mikurensis” [GenBank accession no. EU810404]) were identical to those of the “Ca. Neoehrlichia mikurensis” isolates obtained from infected humans in Switzerland (GQ501090), Germany (EU810404), and China (JQ359045), as well as from I. ricinus collected in Slovakia (JN378917) and Germany (EU810405), I. persulcatus collected in Russia (FJ966362), and Apodemus spp. collected in Japan (AB196305). Since the 16S rRNA gene is too highly conserved for an analysis of genetic heterogeneity, partial groESL operon fragments (920 bp; nucleotide positions 5′-3′ of amplified fragment, 248 to 1168; number corresponds to the nucleotide positions relative to the sequence of the groESL [1,233 bp] of “Ca. Neoehrlichia mikurensis” [EU810406]) amplified from all positive samples were also sequenced. The nucleotide sequences of all 5 isolates were identical and showed 100% identity to the groESL sequences of the “Ca. Neoehrlichia mikurensis” isolates obtained from I. ricinus ticks, rodents, a dog, and a human from Europe (Fig. 1). The DNA sequences amplified from our human “Ca. Neoehrlichia mikurensis” isolates were also identical to those obtained from ticks and bank voles collected in Warmińsko-Mazurskie province in our previous studies (5). However, the phylogenetic tree of the partial groESL operon sequences showed that two European “Ca. Neoehrlichia mikurensis” isolates from a bank vole and a human (Switzerland) differed by 3 and 13 nucleotides, respectively. Phylogenetic analysis revealed that differences in the groESL sequence were sufficient to differentiate the European and Chinese variants of the species “Ca. Neoehrlichia mikurensis” (Fig. 1). Similar molecular analyses for Babesia, Anaplasma, Ehrlichia, Rickettsia (5), and Borrelia (7) species did not demonstrate any active coinfection in our 5 “Ca. Neoehrlichia mikurensis”-positive foresters.
FIG 1.
Phylogenetic tree (maximum likelihood [ML]) of the “Ca. Neoehrlichia” isolates identified in foresters from northeast Poland and selected isolates from GenBank, based on a fragment of the groESL heat shock operon. The numbers at the nodes of the tree indicate bootstrap values (1,000 replicates). The accession number of the newly reported sequence in this study is in bold and underlined.
Prior to this study, 8 cases of human infection with “Ca. Neoehrlichia mikurensis” had been described in Europe (2), and 7 more were recently reported in China (14). Little is known about the distribution, risk areas, and zoonotic reservoir of this bacterium, and its pathogenic mechanisms remain largely uncharacterized. Like all members of the family Anaplasmataceae,“Ca. Neoehrlichia mikurensis” is an obligate intracellular pathogen that resides within the cytoplasm of endothelial cells (1). The symptoms produced by infections with this pathogen in immunocompromised patients may be severe and can include aneurysm, thromboembolic complications, subcutaneous hemorrhages, and erythematous rashes (10–13). Until now, asymptomatic infection in immunocompetent people had not been reported, although most Ehrlichia infections are known to be either asymptomatic or mild self-limiting diseases (16). The present study describes five new asymptomatic human cases of “Ca. Neoehrlichia mikurensis” infection in healthy foresters working in the northeastern part of Poland, where I. ricinus ticks are endemic, and all of the infected individuals recalled a recent tick bite. Human infections caused by this bacterium have been recognized as an emerging problem in the last decade, and this is most likely due to ecological changes and the resulting expansion of Ixodidae tick populations. Due to the potentially serious nature of disease caused by “Ca. Neoehrlichia mikurensis” in immunocompromised individuals, further study of this bacterium, its tick vectors, and reservoir hosts is urgently required, as is the establishment of a European ecoepidemiological monitoring program for this infection. It should also be emphasized that the identification of new blood-borne infectious agents, especially in immunocompetent humans, demands further research in order to determine the potential threat they may pose to the blood supply (17). Eight transfusion-transmitted cases of Anaplasma phagocytophilum infection (18) and a single case of ehrlichiosis acquired from a transfusion of blood products have been documented in the literature to date (19). However, A. phagocytophilum and Ehrlichia spp. remain viable under refrigeration conditions at 4°C for up to 18 days, enabling the potential transmission of infection by blood transfusion (20). Thus, the increasing spread of vectors and reservoirs make the new and well-known tick-borne pathogens important to monitor and a future target for possible donation screening or inactivation by pathogen reduction technologies.
Nucleotide sequence accession numbers.
The new 16S rRNA gene and groESL operon sequences from CNM present in the blood samples of healthy humans in Poland have been deposited in GenBank under accession no. KJ123754 and KJ561571.
ACKNOWLEDGMENT
This study is part of project NN 404 795240, supported by the Ministry of Science and Higher Education in Poland.
Footnotes
Published ahead of print 4 June 2014
REFERENCES
- 1.Kawahara M, Rikihisa Y, Isogai E, Takahashi M, Misumi H, Suto C, Shibata S, Zhang C, Tsuji M. 2004. Ultrastructure and phylogenetic analysis of “Candidatus Neoehrlichia mikurensis” in the family Anaplasmataceae, isolated from wild rats and found in Ixodes ovatus ticks. Int. J. Syst. Evol. Microbiol. 54:1837–1843. 10.1099/ijs.0.63260-0 [DOI] [PubMed] [Google Scholar]
- 2.Maurer FP, Keller PM, Beuret C, Joha C, Achermann Y, Gubler J, Bircher D, Karrer U, Fehr J, Zimmerli L, Bloemberg GV. 2013. Close geographic association of human neoehrlichiosis and tick populations carrying “Candidatus Neoehrlichia mikurensis” in eastern Switzerland. J. Clin. Microbiol. 51:169–176. 10.1128/JCM.01955-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andersson M, Bartkova S, Lindestad O, Råberg L. 2013. Co-infection with “Candidatus Neoehrlichia mikurensis” and Borrelia afzelli in Ixodes ricinus ticks in southern Sweden. Vector Borne Zoonotic Dis. 13:438–442. 10.1089/vbz.2012.1118 [DOI] [PubMed] [Google Scholar]
- 4.Pangrácová L, Derdáková M, Pekárik L, Hviščová I, Víchová B, Stanko M, Hlavatá H, Pet'ko B. 2013. Ixodes ricinus abundance and its infection with the tick-borne pathogens in urban and suburban areas of eastern Slovakia. Parasit. Vectors 6:238. 10.1186/1756-3305-6-238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Welc-Falęciak R, Kowalec M, Karbowiak G, Bajer A, Behnke JM, Siński E. 2014. Rickettsiaceae and Anaplasmataceae infections in Ixodes ricinus ticks from urban and natural areas of Poland. Parasit. Vectors 7:121. 10.1186/1756-3305-7-121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tabara K, Arai S, Kawabuchi T, Itagaki A, Ishihara C, Satoh H, Okabe N, Tsuji M. 2007. Molecular survey of Babesia microti, Ehrlichia species and Candidatus Neoehrlichia Mikurensis in wild rodents from Shimane Prefecture, Japan. Microbiol. Immunol. 51:359–367. 10.1111/j.1348-0421.2007.tb03923.x [DOI] [PubMed] [Google Scholar]
- 7.Richter D, Matuschka FR. 2012. “ CandidatusNeoehrlichia mikurensis,” Anaplasma phagocytophilum, and Lyme disease spirochetes in questing European vector ticks and in feeding ticks removed from people. J. Clin. Microbiol. 50:943–947. 10.1128/JCM.05802-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andersson M, Råberg L. 2011. Wild rodents and novel human pathogen Candidatus Neoehrlichia mikurensis, southern Sweden. Emerg. Infect. Dis. 17:1716–1718. 10.3201/eid1709.101058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jahfari S, Fonville M, Hengeveld P, Reusken C, Scholte EJ, Takken W, Heyman P, Medlock J, Heylen D, Kleve J, Sprong H. 2012. Prevalence of Neoehrlichia mikurensis in ticks and rodents from North-west Europe. Parasit. Vectors 5:74. 10.1186/1756-3305-5-74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.von Loewenich FD, Geissdörfer W, Disqué C, Matten J, Schett G, Sakka SG, Bogdan C. 2010. Detection of “Candidatus Neoehrlichia mikurensis” in two patients with severe febrile illnesses: evidence for a European sequence variant. J. Clin. Microbiol. 48:2630–2635. 10.1128/JCM.00588-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Welinder-Olsson C, Kjellin E, Vaht K, Jacobsson S, Wennerås C. 2010. First case of human “Candidatus Neoehrlichia mikurensis” infection in a febrile patient with chronic lymphocytic leukemia. J. Clin. Microbiol. 48:1956–1959. 10.1128/JCM.02423-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fehr JS, Bloemberg GV, Ritter C, Hombach M, Lüscher TF, Weber R, Keller PM. 2010. Septicemia caused by tick-borne bacterial pathogen Candidatus Neoehrlichia mikurensis. Emerg. Infect. Dis. 16:1127–1129. 10.3201/eid1607.091907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pekova S, Vydra J, Kabickova H, Frankova S, Haugvicova R, Mazal O, Cmejla R, Hardekopf DW, Jancuskova T, Kozak T. 2011. Candidatus Neoehrlichia mikurensis infection identified in 2 hemato-oncologic patients: benefit of molecular techniques for rare pathogen detection. Diagn. Microbiol. Infect. Dis. 69:266–270. 10.1016/j.diagmicrobio.2010.10.004 [DOI] [PubMed] [Google Scholar]
- 14.Li H, Jiang JF, Liu W, Zheng YC, Huo QB, Tang K, Zuo SY, Liu K, Jiang BG, Yang H, Cao WC. 2012. Human infection with Candidatus Neoehrlichia mikurensis, China. Emerg. Infect. Dis. 18:1636–1639. 10.3201/eid1810.120594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. 2011. MEGA5: Molecular Evolutionary Genetics Analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28:2731–2739. 10.1093/molbev/msr121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ismail N, Bloch KC, McBride JW. 2010. Human ehrlichiosis and anaplasmosis. Clin. Lab. Med. 30:261–292. 10.1016/j.cll.2009.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chamberland ME. 2002. Emerging infectious agents: do they pose a risk to the safety of transfused blood and blood products? Clin. Infect. Dis. 34:797–805. 10.1086/338787 [DOI] [PubMed] [Google Scholar]
- 18.Townsend RL, Moritz ED, Fialkow LB, Berardi V, Stramer SL. 2014. Probable transfusion-transmission of Anaplasma phagocytophilum by leukoreduced platelets. Transfusion 10.1111/trf.12675 [DOI] [PubMed] [Google Scholar]
- 19.Regan J, Matthias J, Green-Murphy A, Stanek D, Bertholf M, Pritt BS, Sloan LM, Kelly AJ, Singleton J, McQuiston JH, Hocevar SN, Whittle JP. 2013. A confirmed Ehrlichia ewingii infection likely acquired through platelet transfusion. Clin. Infect. Dis. 56:e105–e107. 10.1093/cid/cit177 [DOI] [PubMed] [Google Scholar]
- 20.Kalantarpour F, Chowdhury I, Wormser GP, Aguero-Rosenfeld ME. 2000. Survival of the human granulocytic ehrlichiosis agent under refrigeration conditions. J. Clin. Microbiol. 38:2398–2399 [DOI] [PMC free article] [PubMed] [Google Scholar]