Abstract
Azithromycin has shown high efficacy in randomized trials when used for treating infectious syphilis in Africa. However, its use in clinical practice has been limited by the development of antimicrobial drug resistance. Resistance has not previously been reported from Australasia. The aim of this study was to determine the prevalence of and risk factors for azithromycin-resistant syphilis-causing strains in Sydney, Australia. We evaluated 409 samples that were PCR positive for Treponema pallidum DNA collected between 2004 and 2011 for the presence of the A2058G mutation, which confers resistance to macrolide antibiotics such as azithromycin. Overall, 84% of samples harbored the mutation. The prevalence of the mutation increased during the study period (P trend, 0.003). We also collected clinical and demographic data on 220 patients from whom these samples had been collected to determine factors associated with the A2058G mutation; 97% were from men who have sex with men. Reporting sex in countries other than Australia was associated with less macrolide resistance (adjusted odds ratio, 0.25; 95% confidence interval, 0.09 to 0.66; P = 0.005), with other study factors showing no association (age, HIV status, recent macrolide use, stage of syphilis, or history of prior syphilis). Azithromycin cannot be recommended as an alternative treatment for syphilis in Sydney.
INTRODUCTION
Syphilis, caused by the spirochete Treponema pallidum subsp. pallidum, causes over 10 million infections worldwide each year (1). The public health importance of this condition relates not only to the early and late manifestations in infected adults, but also the particularly devastating consequences of congenital syphilis (2). Although endemic in the heterosexual population in many low- and middle-income countries, many high-income countries, as well as China (3), have witnessed a resurgence of syphilis over the last decade, predominantly among homosexually active men (4), where it may contribute to ongoing transmission of HIV (5–6). In Sydney, Australia, just 16 cases of infectious syphilis were reported in 1999, compared to 422 cases in 2011 (7).
A highly effective and inexpensive cure for infectious syphilis in the form of a single dose of benzathine penicillin G has been available for over 65 years, yet universal delivery of this treatment to those in need has not been achieved (8). Trials in Africa of a single oral dose of 2 g of azithromycin have shown equivalent treatment efficacy to a single dose of benzathine penicillin (9, 10). Oral azithromycin has the advantage of easier injection-free administration, a low likelihood of serious adverse events, and the potential for enhanced public health interventions, such as mass treatment and patient-delivered partner therapy.
Azithromycin therapy for syphilis was adopted by some clinicians in several high-income settings, such as the United States, during syphilis epidemics among homosexually active men (11). However, clinical treatment failures were soon reported, followed by the identification of rising rates of the 23S ribosomal mutation A2058G, which confers resistance to macrolide antibiotics. This mutation has now been identified in the United States (11, 12), Ireland (13), the United Kingdom (14), China (15), and Canada (16), but it was not found at significant levels in Madagascar (17), South Africa (18), or Taiwan (19).
Despite local interest in its potential use, Australia has never adopted azithromycin as a first-line treatment for syphilis. Macrolide antibiotics are, however, widely used in the treatment of sexually transmissible diseases and other infections (20).
The aim of this study was to determine the prevalence of and risk factors for the A2058G mutation in T. pallidum among patients diagnosed with syphilis in Sydney and also whether azithromycin could be considered an alternative treatment option for some patients.
(These data were presented in part at the Australasian Sexual Health Conference 2012.)
MATERIALS AND METHODS
Study design.
This was a retrospective observational study that involved laboratory testing on stored samples combined with medical note review.
Setting.
This study was conducted in Sydney, the largest city in New South Wales, Australia. Clinical data were obtained from 1 publically funded sexual health clinic (Sydney Sexual Health Centre) and 3 general medical practices (Taylor Square Private Clinic, East Sydney Doctors, and Holdsworth House Medical Practice). These clinics are located in the suburbs of Sydney that are most commonly listed during syphilis notifications. All requests for T. pallidum PCR analysis in Sydney are sent to a single laboratory.
Laboratory testing.
Since 2004, a PCR test for direct detection of T. pallidum has been available at the Centre for Infectious Diseases and Microbiology (CIDM), Institute for Clinical Pathology and Medical Research (ICPMR), Westmead Hospital, Sydney, Australia. This PCR test targets the tpp47 gene and has been used on mucocutaneous lesion swabs and cerebrospinal fluid samples. Public and private doctors and pathology providers throughout Sydney had access to this test during the 2004 to 2011 study period. Details of the test and its validation in the local population have been previously published (21). All samples testing positive by PCR for the tpp47 target during this period were stored at −20°C until required.
Nucleic acid was reextracted from stored samples by using the NucliSENS easyMAG system (bioMérieux) according to the manufacturer's recommendations. Samples were retested for the presence of T. pallidum DNA by using the tpp47 gene before further testing to detect the mutation in the 23S rRNA gene.
A 628-bp fragment of the 23S rRNA gene was amplified by using a PCR previously described by Lukehart et al. in 2004 (13). In brief, 10 μl of extracted DNA was combined with HotStarTaq master mix (Qiagen), 3.5 mM MgCl2, and 200 nM each primer and denatured at 95°C for 10 min, followed by 50 cycles of 95°C for 1 min, 68°C for 1 min, and 72°C for 1 min, with a final extension step of 72°C for 5 min with a conventional thermal cycler (Eppendorf MasterCycler gradient). PCR products were detected by using agarose gel electrophoresis with SYBR Safe staining.
Restriction digestion using the enzyme MboII (New England BioLabs, Massachusetts) was performed at 37°C overnight, and then products were separated by agarose gel electrophoresis. Samples containing the A2058G resistance mutation produced two bands of sizes 440 bp and 188 bp, while samples without this mutation produced a single band at 628 bp (13).
Clinical data collection.
Positive T. pallidum PCR samples were grouped according to referring clinic. Clinical data were obtained from the medical notes from the four study clinics for patients who had been diagnosed with syphilis by T. pallidum PCR testing. These clinics are all located within a 3-km radius in inner Sydney and provide health services to large numbers of homosexually active men.
Extracted data included age at diagnosis, sex, gender of sexual partners, stage of syphilis, history of previous syphilis, aboriginality, HIV status, sex associated with overseas travel within the prior 12 months, and macrolide antibiotic use within the preceding 12 months. Serological results for syphilis taken at the time of PCR testing were obtained. Multiple public and private pathology providers performed these tests. Patients without a history of previous syphilis were initially tested with a treponema-specific test, such as an enzyme immune assay (EIA) or chemiluminescence assay (CMIA). For patients with a history of previous syphilis, a rapid plasma reagin test (RPR) result indicating a 4-fold change (two tube dilutions) from the baseline titer was considered evidence of a new infection. Treatment information was also obtained.
Statistical analysis.
Odds ratios (ORs) for the presence of the A2058G mutation were calculated, and any variables with significance at a P level of <0.2 were included in the multivariate model. Data were analyzed by using Stata version 12.0 (Statacorp).
Ethics.
Ethical approval for this retrospective study was obtained by the South Eastern Sydney Local Health District—Northern Sector Human research ethics committee (11/131).
RESULTS
Overall.
From 2004 through 2011, the CIDM laboratory recorded 409 positive PCR results for syphilis. This represented 13.5% of the 3,037 cases of infectious syphilis reported to the New South Wales Ministry of Health during the study period (7) (all doctors and laboratories are mandated to provide notification for syphilis to the Ministry of Health via their local Public Health Unit.) The specimens included 401 lesion swabs, 5 cerebrospinal fluid samples, 1 vitreous humor sample, and 2 tissue biopsy specimens. DNA reextraction and subsequent PCR for the azithromycin resistance-causing mutation was successful for 353 (86%) of these specimens.
Prevalence of the A2058G mutation.
Overall, the prevalence of the A2058G mutation was 84.4% (95% confidence interval [CI], 80.2 to 88.0), increasing from a baseline of 66.7% (95% CI, 22.9 to 92.5) in 2004 to 82.5% in 2011 (95% CI, 70.9 to 91.0) (P trend, 0.003) (Table 1 and Fig. 1).
TABLE 1.
Characteristics of subjects in whom syphilis was diagnosed by PCR (2004 to 2011) from four Sydney clinicsa
| Factor | Group | Value |
|---|---|---|
| Age at diagnosis (median, range) | 41 (21–71) | |
| Yr of diagnosis | 2004 | 3 |
| 2005 | 7 | |
| 2006 | 5 | |
| 2007 | 26 | |
| 2008 | 38 | |
| 2009 | 53 | |
| 2010 | 44 | |
| 2011 | 44 | |
| Aboriginal or Torres Strait Islander | No | 157 (71.4%) |
| Yes | 0 (0.0%) | |
| Missing | 63 (28.6%) | |
| Gender | Male | 219 (99.5%) |
| Female | 1 (0.5%) | |
| Gender of sex partners | Male only | 200 (91.0%) |
| Male and female | 3 (1.3%) | |
| Female only | 3 (1.3%) | |
| Missing | 14 (6.4%) | |
| HIV status at diagnosis | Positive | 118 (53.6%) |
| Negative | 102 (46.7%) | |
| Stage of syphilis | Primary | 155 (70.5%) |
| Secondary | 37 (16.8%) | |
| Missing | 28 (12.7%) | |
| Evidence of neurological syphilis | Yes | 7 (3.2%) |
| No | 213 (96.8%) | |
| Previous syphilis | Yes | 65 (29.5%) |
| No | 134 (61.0%) | |
| Missing | 21 (9.5%) | |
| Sex overseas | No | 187 (85.0%) |
| Yes | 33 (15.0%) | |
| Macrolide antibiotic in previous 12 mo | Yes | 47 (21.4%) |
| No | 173 (78.6%) |
In total, there were 220 study subjects.
FIG 1.
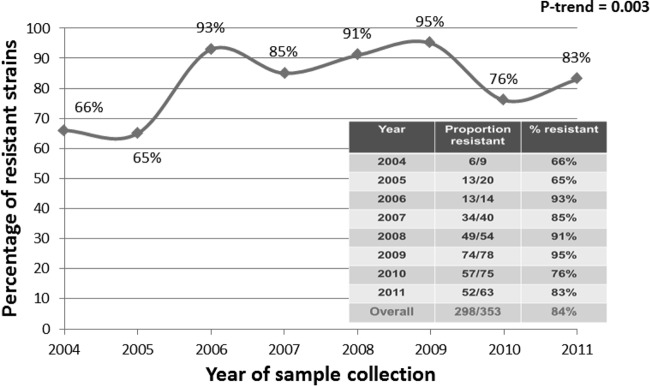
Azithromycin resistance over time.
Of the 409 original T. pallidum-positive samples, 220 originated from one of the four study sites and therefore had clinical data available (Sydney Sexual Health Centre, 63 cases; Taylor Square Private Clinic, 83 cases; East Sydney Doctors, 28 cases; Holdsworth House Medical Practice, 46 cases). At the time of T. pallidum PCR positivity, 201/220 cases had at least one specific or nonspecific serological test for syphilis that was positive or equivocal. Due to the multitude of different syphilis serology tests used at the different pathology evaluators, it was not possible to analyze specific serological results. Ten of 211 (0.5%) cases were negative by syphilis serology testing (usually an enzyme immune assay) at the time of PCR diagnosis, and 5 of these 10 showed serological evidence of seroconversion at first follow-up. Nine cases did not have serology testing simultaneous with PCR testing.
PCR testing for the presence of the A2058G mutation was successful in 198/220 (90%) of the specimens with available clinical data from the four study clinics (Fig. 2). There were no significant differences in the patient clinical or demographic variables for those for whom DNA reextraction was or was not successful, nor for data between clinic sites (data not shown).
FIG 2.
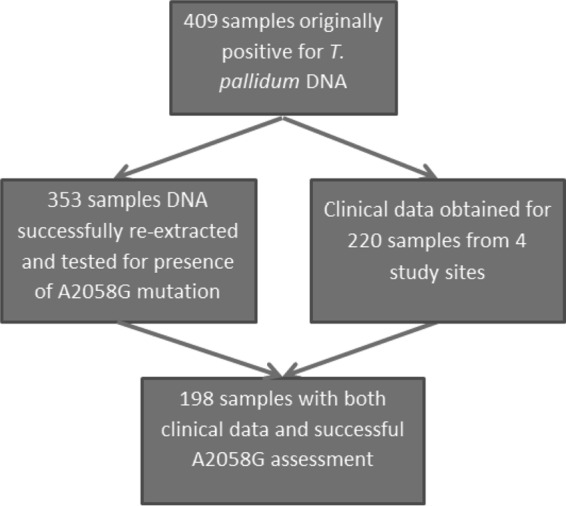
Specimen and study flow chart.
Patient characteristics.
Of the 220 cases, the median age at diagnosis was 41 years (range, 21 to 71 years); 219 (99.5%) were male, 203 (92.3%) reported sex with men, and 118 (53.6%) were known to be HIV positive at the time of syphilis diagnosis. A history of prior syphilis was recorded for 65 subjects (29.5%; a median of 1.6 years prior to the current diagnosis). Thirty-three (15%) reported a history of overseas travel in the 12 months prior to diagnosis, with the most common locations being Asia (n = 16) and Europe (n = 8). Forty-seven (21.4%) had received at least one course of macrolide antibiotics in the preceding 12 months, including 39 on azithromycin, 5 on roxithromycin, and 3 on azithromycin and roxithromycin (Table 1). Forty of these antibiotic prescriptions were provided and/or dispensed by the medical practice at which syphilis was diagnosed, and for the other seven a history of antibiotic use was reported in the medical notes but the prescription originated from a medical practice elsewhere. All previous azithromycin was prescribed for previous confirmed or suspected chlamydial conditions, and roxithromycin was prescribed for upper or lower respiratory illness.
Risk factors for the A2058G mutation.
In our univariate analysis, the year of diagnosis from 2008 onwards was associated with the mutation (OR, 2.44; 95% CI, 1.03 to 5.77; P = 0.043). Reporting sex overseas was a protective factor (OR, 0.30; 95% CI, 0.12 to 0.75; P = 0.01). Prior prescription of macrolide antibiotics (OR, 1.22; 95% CI, 0.97 to 1.54; P = 0.085) was included in the multivariate analysis since all factors with a significance with a P level of <0.2 were included in the multivariate model. Prior syphilis or HIV status was not significantly associated with the resistance mutation.
Based on our multivariate analysis, the only factors that were associated with the presence of the A2058G mutation were diagnosis in the later years of the study (2008 to 2011), adjusted OR (AOR) of 3.2 (95% CI, 1.28 to 8.07; P = 0.013) and reporting recent sex overseas, which remained protective (AOR, 0.25; 95% CI, 0.09 to 0.66; P = 0.005) (Table 2).
TABLE 2.
Factors associated with azithromycin resistancea
| Factor | Proportion resistant (%) | Univariate OR (95% CI) | P value | Multivariate ORb (95% CI) | P value |
|---|---|---|---|---|---|
| Age at diagnosis (yrs) | |||||
| ≤29 | 14/15 (93%) | 1.0 | |||
| 30–39 | 46/54 (85%) | 0.41 (0.05–3.57) | 0.420 | ||
| 40–49 | 81/93 (87%) | 0.48 (0.06–4.00) | 0.500 | ||
| ≥50 | 28/36 (78%) | 0.25 (0.03–2.20) | 0.212 | ||
| Yr of diagnosis | |||||
| Pre-2008 | 30/40 (75%) | 1.0 | |||
| 2008 and onwards | 139/158 (88%) | 2.44 (1.03–5.77) | 0.043 | 3.20 (1.28–8.07) | 0.013 |
| HIV status | |||||
| Negative | 76/90 (84%) | 1.0 | |||
| Positive | 93/108 (86%) | 1.14 (0.52–2.51) | 0.741 | ||
| Stage of syphilis | |||||
| Primary | 119/141 (84%) | 0.92 (0.32–2.67) | 0.885 | ||
| Secondary | 25/30 (83%) | 1.0 | |||
| Missing | 25/27 (93%) | 2.31 (0.51–10.46) | 0.277 | ||
| Previous syphilis | |||||
| No | 104/121 (86%) | 1.74 (0.51–5.94) | 0.371 | ||
| Yes | 51/59 (86%) | 1.82 (0.48–6.94) | 0.380 | ||
| Missing | 14/18 (78%) | 1.0 | |||
| Sex overseas | |||||
| No | 149/169 (88%) | 1.0 | |||
| Yes | 20/29 (69%) | 0.30 (0.12–0.75) | 0.010 | 0.25 (0.09–0.66) | 0.005 |
| Macrolide in last 12 mo | |||||
| No | 129/155 (83%) | 1.0 | 2.82 (0.79–10.05) | 0.110 | |
| Yes | 40/43 (93%) | 1.22 (0.97–1.54) | 0.085 |
There were 198 subjects for which there were resistance data; results are given for samples where data were available. Data for aboriginality, gender, gender of partner, and neurosyphilis were not analyzed due to small sample sizes.
Multivariate analysis was employed for factors where P was <0.2 in the univariate analysis.
DISCUSSION
This study demonstrates, for the first time, that mutations conferring azithromycin resistance in Treponema pallidum are extremely common among homosexually active men in urban Sydney. The A2058G mutation was already well established in 2004, at a prevalence of 66%. Studies in similar populations in the United States demonstrated a rapid increase in the prevalence of this mutation at the beginning of the 21st century. In both Seattle and San Francisco, a prevalence of 0% was reported in 2000 but this rose to to 50% by 2004 (11, 22). Since we do not have stored samples that were obtained prior to 2004, it was not possible to determine if the mutation was always present or, as in the United States, had developed more recently through either antimicrobial drug pressure or the introduction of an imported resistant strain.
We also noted a significant increase in macrolide resistance over time (Fig. 1). The overall prevalence of the A2058G mutation in Sydney remained at 84% across the study period. Again, this is in agreement with the high levels observed in the United States (80% in Seattle by 2010 [12]), United Kingdom (66.6% by 2008 [14]), and mainland China (91.9% from 2008 to 2011 [15]), but the high prevalence is in sharp contrast to levels reported from studies in South Africa (1% [18]), Madagascar (0% [17]), and Taiwan (0% [19]). The reason for the large differences in resistance prevalence rates between countries is not clear but is likely to represent differences in exposure of the at-risk population to macrolide antibiotics. The discovery of clinical treatment failures associated with the A2058G mutation and the rising prevalence of this mutation coincided with the use of azithromycin as a treatment option for established and incubating cases of syphilis in some U.S. cities (11). However, in China and the United Kingdom, where azithromycin treatment has never been a first-line treatment for syphilis, similar levels of mutant variants have been observed, suggesting that, overall, community exposure to macrolide antibiotics is likely to provide the drug pressure for the development of this mutation, rather than treatment of syphilis per se. This seems particularly likely given the long, often asymptomatic infectious period for syphilis and the opportunity for undiagnosed cases to receive antibiotics for other medical conditions. In our study, the majority of previous macrolide prescriptions were for suspected or confirmed sexually transmitted infections. Multivariate analysis in our study did not find a significant association between prior use of macrolide antibiotics and the presence of the A2058G mutation (AOR, 2.8; 95% CI, 0.79 to 10.05; P = 0.110); however, an association was observed in Seattle (AOR, 2.2) (12, 22) and China (OR, 19.65) (15).
One important difference between these other studies and ours is that in both Seattle and China, patients were specifically interviewed about macrolide use at enrollment in the study, whereas we relied on retrospective medical note review. It is therefore possible that some patients in our study received macrolide antibiotics from a different health service, which would have led to an underassessment of macrolide consumption. Despite this potential for underreporting, it is notable that over 20% of patients had been prescribed a macrolide antibiotic in the last 12 months, underlining the high exposure of the population to this antibiotic class. In vitro studies of macrolide exposure with other treponemal species, such as T. denticola, have demonstrated ready selection of resistance mutations in this chromosomal region under such drug pressure and that these organisms retain replicative capacity (23).
A lower proportion of patients in our study who reported sex outside of Australia harbored the resistance mutation (69% vs 88%; AOR, 0.25; 95% CI, 0.09 to 0.66; P = 0.005). Half (16) of these patients had visited Northeast or Southeast Asian countries in relative proximity to Australia, such as Thailand, Malaysia, or Indonesia. With the exception of China (15, 24) and Taiwan (19), no studies for this geographic region have investigated the prevalence of macrolide-resistant syphilis-causing strains, but our data suggest that it may be lower than in Australia and warrants investigation.
Over half of our sample (53.6%) of patients were known to be HIV positive, in keeping with the known epidemiology of syphilis in Sydney (25). However, similar to a study in the United Kingdom (14), there was no association between HIV status and macrolide resistance. Subtyping information has been reported for a sample (n = 90) of syphilis cases in Melbourne, Australia, and revealed multiple diverse subtypes yet no association between HIV status and subtype (26). Testing for macrolide-associated resistance mutations was not performed in the Melbourne study. In both the United Kingdom study (13) and San Francisco study (26), no significant association was found between T. pallidum subtype and azithromycin resistance, but in a larger longitudinal study from Seattle, azithromycin resistance mutations showed some association with subtype and over time discrete subtypes appeared to acquire such mutations (12). Additional molecular typing of clinical isolates would be required to address this question in our population.
A limitation of this study is that the A2058G is not the only potential pathway for macrolide resistance in T. pallidum. Indeed, the first report of an alternative macrolide resistance mutation, A2059G, was published in 2009 and was associated with clinical treatment failure in the Czech Republic (28). Subsequent investigation showed it was present in 14.6% of T. pallidum samples from that country (29). It was not detected in South African or Taiwanese T. pallidum specimens (18, 19), but it has been found at relatively low levels (10 to 13.2%) in the United States (12, 30) and in 5.6% of samples in London, United Kingdom (14). Of note in these studies, no samples were simultaneously positive for both A2058G and A2059G. This suggests that with a current prevalence of 83% in Sydney for the A2058G mutation, the ecological niche for organisms harboring the A2059G mutation is small, its contribution to macrolide-resistant syphilis in Sydney is unlikely to be significant, and its presence would have little impact on the interpretation of this study. Finally, although the majority of cases of syphilis in Sydney are clustered in the catchment area of the study clinics, there is considerable homogeneity between the patients attending these clinics, and therefore it is not possible to extrapolate our findings to culturally and geographically diverse settings in Australia, such as aboriginal or Torres Strait Islander populations, where there are also high rates of syphilis.
In conclusion, the mutation A2058G, which is associated with azithromycin resistance in T. pallidum, is common and increasing among homosexually active men. Azithromycin should not be considered a treatment option for incubating or proven syphilis, and it has no role in disease control in Sydney.
ACKNOWLEDGMENTS
We acknowledge the clinical staff at East Sydney Doctors, Taylor Square Private Clinic, Holdsworth House Medical Practice, and Sydney Sexual Health Centre for facilitating and assisting with data collection.
Footnotes
Published ahead of print 21 May 2014
REFERENCES
- 1.World Health Organization. 2012. Global incidence and prevalence of selected curable sexually transmitted infections—2008. World Health Organization, Geneva, Switzerland [Google Scholar]
- 2.Read P, Donovan B. 2012. Clinical aspects of adult syphilis. Int. Med. J. 42:614–620. 10.1111/j.1445-5994.2012.02814.x [DOI] [PubMed] [Google Scholar]
- 3.Wu Z, Xu J, Liu E, Mao Y, Xiao Y, Sun X, Liu Y, Jiang Y, McGoogan JM, Dou Z, Mi G, Wang N, Sun J, Liu Z, Wang L, Rou K, Pang L, Xing W, Xu J, Wang S, Cui Y, Li Z, Bulterys M, Lin W, Zhao J, Yip R, Wu Y, Hao Y, Wang Y, National MSM Survey Group 2013. HIV and syphilis prevalence among men who have sex with men: a cross-sectional survey of 61 cities in China. Clin. Infect. Dis. 57:298–309. 10.1093/cid/cit210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fenton K, Breben R, Vardavas R. 2008. Infectious syphilis in high-income settings in the 21st century. Lancet Infect. Dis. 8:244–253. 10.1016/S1473-3099(08)70065-3 [DOI] [PubMed] [Google Scholar]
- 5.Stamm WE, Handsfield HH, Rompalo AM, Ashley RL, Roberts PL, Corey L. 1988. The association between genital ulcer disease and acquisition of HIV infection in homosexual men. JAMA 260:1429–1433. 10.1001/jama.1988.03410100119036 [DOI] [PubMed] [Google Scholar]
- 6.Guy RJ, Spelman T, Stoove M, El-Hayek C, Goller J, Fairley CK, Leslie D, Tee B, Roth N, Grulich AE, Hellard ME. 2011. Risk factors for HIV seroconversion in men who have sex with men in Victoria, Australia: results from a sentinel surveillance system. Sex Health 8:319–329. 10.1071/SH10095 [DOI] [PubMed] [Google Scholar]
- 7.KirbyInstitute 2013. HIV/AIDS, hepatitis C and sexually transmissible infections in Australia: annual surveillance report. University of New South Wales, Sydney, Australia [Google Scholar]
- 8.Low N, Broutet N, Adu-Sarkodie Barton P, Hossain M, Hawkes S. 2006. Global control of sexually transmitted infections. Lancet 368:2001–2016. 10.1016/S0140-6736(06)69482-8 [DOI] [PubMed] [Google Scholar]
- 9.Riedner G, Rusizoka M, Todd J, Maboko L, Hoelscher M, Mmbando D, Samky E, Lyamuya E, Mabey D, Grosskurth H, Hayes R. 2005. Single-dose azithromycin versus penicillin G benzathine for the treatment of early syphilis. N. Engl. J. Med. 353:1236–1244. 10.1056/NEJMoa044284 [DOI] [PubMed] [Google Scholar]
- 10.Hook EW, III, Behets F, Van Damme K, Ravelomanana N, Leone P, Sena AC, Martin D, Langley C, McNeil L, Wolff M. 2010. A phase III equivalence trial of azithromycin versus benzathine penicillin for treatment of early syphilis. J. Infect. Dis. 201:1729–1735. 10.1086/652239 [DOI] [PubMed] [Google Scholar]
- 11.Mitchell S, Engelman J, Kent C, Lukehart S, Godornes C, Klausner J. 2006. Azithromycin-resistant syphilis infection: San Francisco, California, 2000–2004. Clin. Infect. Dis. 42:337–345. 10.1086/498899 [DOI] [PubMed] [Google Scholar]
- 12.Grimes M, Sahi SK, Godornes BC, Tantalo LC, Roberts N, Bostick D, Marra CM, Lukehart SA. 2012. Two mutations associated with macrolide resistance in Treponema pallidum: increasing prevalence and correlation with molecular strain type in Seattle, Washington. Sex. Transm. Dis. 39:954–958. 10.1097/OLQ.0b013e31826ae7a8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lukehart SA, Godornes C, Molini BJ, Sonnett P, Hopkins S, Mulcahy F, Engelman J, Mitchell SJ, Rompalo AM, Marra CM, Klausner JD. 2004. Macrolide resistance in Treponema pallidum in the United States and Ireland. N. Engl. J. Med. 351:154–158. 10.1056/NEJMoa040216 [DOI] [PubMed] [Google Scholar]
- 14.Tipple C, McClure M, Taylor G. 2011. High prevalence of macrolide resistant Treponema pallidum strains in a London centre. Sex. Transm. Infect. 87:486–448. 10.1136/sextrans-2011-050082 [DOI] [PubMed] [Google Scholar]
- 15.Chen XS, Yin YP, Wei WH, Wang HC, Peng RR, Zheng HP, Zhang JP, Zhu BY, Liu QZ, Huang SJ. 2013. High prevalence of azithromycin resistance to Treponema pallidum in geographically different areas in China. Clin. Microbiol. Infect. 19:975–979. 10.1111/1469-0691.12098 [DOI] [PubMed] [Google Scholar]
- 16.Morshed M, Jones H. 2006. Treponema pallidum macrolide resistance in BC. CMAJ 174:349. 10.1503/cmaj.1050256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Damme K, Behets F, Ravelomanana N, Godornes C, Khan M, Randrianasolo B, Rabenja NL, Lukehart S, Cohen M, Hook E. 2009. Evaluation of azithromycin resistance in Treponema pallidum specimens from Madagascar. Sex. Transm. Dis. 36:775–776. 10.1097/OLQ.0b013e3181bd11dd [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muller E, Paz-Bailey G, Lewis D. 2012. Macrolide resistance testing and molecular subtyping of Treponema pallidum strains from southern Africa. Sex. Transm. Infect. 88:470–474. 10.1136/sextrans-2011-050322 [DOI] [PubMed] [Google Scholar]
- 19.Wu H, Chang SY, Lee NY, Huang WC, Wu BR, Yang CJ, Liang SH, Lee CH, Ko WC, Lin HH, Chen YH, Liu WC, Su YC, Hsieh CY, Wu PY, Hung CC. 2012. Evaluation of macrolide resistance and enhanced molecular typing of Treponema pallidum in patients with syphilis in Taiwan: a prospective multicenter study. J. Clin. Microbiol. 50:2299–2304. 10.1128/JCM.00341-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Antibiotic Expert Group. 2010. Antibiotic version 14, 2010. Therapeutic Guidelines Ltd., Melbourne, Australia [Google Scholar]
- 21.Shields M, Guy R, Jeoffreys N, Finlayson R, Donovan B. 2012. A longitudinal evaluation of Treponema pallidum PCR testing in early syphilis. BMC Infect. Dis. 12:353. 10.1186/1471-2334-12-353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marra C, Colina A, Gordones C, Tantalo LC, Puray M, Centurion-Lara A, Lukehart SA. 2006. Antibiotic selection may contribute to increases in macrolide-resistant Treponema pallidum. J. Infect. Dis. 194:1771–1773. 10.1086/509512 [DOI] [PubMed] [Google Scholar]
- 23.Lee SY, Ning Y, Fenno JC. 2002. 23S rRNA point mutation associated with erythromycin resistance in Treponema denticola. FEMS Microbiol. Lett. 207:39–42. 10.1111/j.1574-6968.2002.tb11025.x [DOI] [PubMed] [Google Scholar]
- 24.Martin I, Gu W, Yang Y, Tsang R. 2009. Macrolide resistance and molecular types of Treponema pallidum causing primary syphilis in Shanghai, China. Clin. Infect. Dis. 49:515–521. 10.1086/600878 [DOI] [PubMed] [Google Scholar]
- 25.Jin F, Prestage GP, Kippax SC, Pell CM, Donovan BJ, Kaldor JM, Grulich AE. 2005. Epidemic syphilis among homosexually active men in Sydney. Med. J. Aust. 183:179–183 https://www.mja.com.au/journal/2005/183/4/epidemic-syphilis-among-homosexually-active-men-sydney [DOI] [PubMed] [Google Scholar]
- 26.Azzato F, Ryan N, Fyfe J, Leslie D. 2012. Molecular subtyping of Treponema pallidum during a local syphilis epidemic in men who have sex with men in Melbourne, Australia. J. Clin. Microbiol. 50:1895–1899. 10.1128/JCM.00083-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Katz KA, Pillay A, Ahrens K, Kohn RP, Hermanstyne K, Bernstein KT, Ballard RC, Klausner JD. 2010. Molecular epidemiology of syphilis: San Francisco, 2004–2007. Sex. Transm. Dis. 37:660–663. 10.1097/OLQ.0b013e3181e1a77a [DOI] [PubMed] [Google Scholar]
- 28.Matejková P, Flasarová M, Zákoucká H, Borek M, Kremenová S, Arenberger P, Woznicová V, Weinstock GM, Smajs D. 2009. Macrolide treatment failure in a case of secondary syphilis: a novel A2059G mutation in the 23S rRNA gene of Treponema pallidum subsp. pallidum. J. Med. Microbiol. 58:832–836. 10.1099/jmm.0.007542-0 [DOI] [PubMed] [Google Scholar]
- 29.Flasarova M, Pospisilova P, Mikalova L, Valisova Z, Dastychova E, Strnadel R, Kuklova I, Woznicova V, Zakoucka H, Smajs D. 2012. Sequencing-based molecular typing of Treponema pallidum strains in the Czech Republic: all identified genotypes are related to the sequence of the SS14 strain. Acta Derm. Venereol. 92:669–674. 10.2340/00015555-1335 [DOI] [PubMed] [Google Scholar]
- 30.Chen CY, Chi KH, Pillay A, Nachamkin E, Su JR, Ballard RC. 2013. Detection of the A2058G and A2059G 23S rRNA gene point mutations associated with azithromycin resistance in Treponema pallidum by use of a TaqMan real-time multiplex PCR assay. J. Clin. Microbiol. 51:908–913. 10.1128/JCM.02770-12 [DOI] [PMC free article] [PubMed] [Google Scholar]


