Abstract
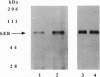
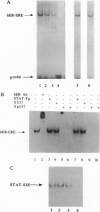
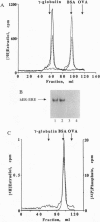
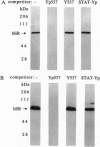
We have previously identified tyrosine-537 as a constitutively phosphorylated site on the human estrogen receptor (hER). A 12-amino acid phosphotyrosyl peptide containing a selected sequence surrounding tyrosine-537 was used to investigate the function of phosphotyrosine-537. The phosphotyrosyl peptide completely blocked the binding of the hER to an estrogen response element (ERE) in a gel mobility shift assay. Neither the nonphosphorylated tyrosyl peptide nor an unrelated phosphotyrosyl peptide previously shown to inhibit the signal transducers and activators of transcription factor (STAT) blocked binding of the hER to the ERE. The hER phosphotyrosyl peptide was shown by molecular sizing chromatography to dissociate the hER dimer into monomers. The hER specifically bound the 32P-labeled phosphotyrosyl peptide, indicating that the inhibition of ERE binding was caused by the phosphotyrosyl peptide binding directly to the hER and blocking dimerization. These data suggest that the phosphorylation of tyrosine-537 is a necessary step for the formation of the hER dimer. In addition, we propose that the dimerization of the hER occurs by a previously unrecognized Src homology 2 domain (SH2)-like phosphotyrosyl coupling mechanism. Consequently, the phosphotyrosyl peptide represents a class of antagonists that inhibits estrogen action by a mechanism other than interacting with the receptor's hormone binding site.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amster-Choder O., Wright A. Modulation of the dimerization of a transcriptional antiterminator protein by phosphorylation. Science. 1992 Sep 4;257(5075):1395–1398. doi: 10.1126/science.1382312. [DOI] [PubMed] [Google Scholar]
- Arnold S. F., Obourn J. D., Jaffe H., Notides A. C. Phosphorylation of the human estrogen receptor on tyrosine 537 in vivo and by src family tyrosine kinases in vitro. Mol Endocrinol. 1995 Jan;9(1):24–33. doi: 10.1210/mend.9.1.7539106. [DOI] [PubMed] [Google Scholar]
- Arnold S. F., Obourn J. D., Yudt M. R., Carter T. H., Notides A. C. In vivo and in vitro phosphorylation of the human estrogen receptor. J Steroid Biochem Mol Biol. 1995 Feb;52(2):159–171. doi: 10.1016/0960-0760(94)00166-j. [DOI] [PubMed] [Google Scholar]
- Beck C. A., Weigel N. L., Moyer M. L., Nordeen S. K., Edwards D. P. The progesterone antagonist RU486 acquires agonist activity upon stimulation of cAMP signaling pathways. Proc Natl Acad Sci U S A. 1993 May 15;90(10):4441–4445. doi: 10.1073/pnas.90.10.4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaikie P., Immanuel D., Wu J., Li N., Yajnik V., Margolis B. A region in Shc distinct from the SH2 domain can bind tyrosine-phosphorylated growth factor receptors. J Biol Chem. 1994 Dec 23;269(51):32031–32034. [PubMed] [Google Scholar]
- Darnell J. E., Jr, Kerr I. M., Stark G. R. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994 Jun 3;264(5164):1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- Du W., Maniatis T. The high mobility group protein HMG I(Y) can stimulate or inhibit DNA binding of distinct transcription factor ATF-2 isoforms. Proc Natl Acad Sci U S A. 1994 Nov 22;91(24):11318–11322. doi: 10.1073/pnas.91.24.11318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. M. The steroid and thyroid hormone receptor superfamily. Science. 1988 May 13;240(4854):889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawell S. E., Lees J. A., White R., Parker M. G. Characterization and colocalization of steroid binding and dimerization activities in the mouse estrogen receptor. Cell. 1990 Mar 23;60(6):953–962. doi: 10.1016/0092-8674(90)90343-d. [DOI] [PubMed] [Google Scholar]
- Forman B. M., Yang C. R., Au M., Casanova J., Ghysdael J., Samuels H. H. A domain containing leucine-zipper-like motifs mediate novel in vivo interactions between the thyroid hormone and retinoic acid receptors. Mol Endocrinol. 1989 Oct;3(10):1610–1626. doi: 10.1210/mend-3-10-1610. [DOI] [PubMed] [Google Scholar]
- Furlow J. D., Murdoch F. E., Gorski J. High affinity binding of the estrogen receptor to a DNA response element does not require homodimer formation or estrogen. J Biol Chem. 1993 Jun 15;268(17):12519–12525. [PubMed] [Google Scholar]
- Hou J., Schindler U., Henzel W. J., Ho T. C., Brasseur M., McKnight S. L. An interleukin-4-induced transcription factor: IL-4 Stat. Science. 1994 Sep 16;265(5179):1701–1706. doi: 10.1126/science.8085155. [DOI] [PubMed] [Google Scholar]
- Jordan V. C., Robinson S. P. Species-specific pharmacology of antiestrogens: role of metabolism. Fed Proc. 1987 Apr;46(5):1870–1874. [PubMed] [Google Scholar]
- Klein-Hitpass L., Schorpp M., Wagner U., Ryffel G. U. An estrogen-responsive element derived from the 5' flanking region of the Xenopus vitellogenin A2 gene functions in transfected human cells. Cell. 1986 Sep 26;46(7):1053–1061. doi: 10.1016/0092-8674(86)90705-1. [DOI] [PubMed] [Google Scholar]
- Koszewski N. J., Notides A. C. Phosphate-sensitive binding of the estrogen receptor to its response elements. Mol Endocrinol. 1991 Aug;5(8):1129–1136. doi: 10.1210/mend-5-8-1129. [DOI] [PubMed] [Google Scholar]
- Kumar V., Chambon P. The estrogen receptor binds tightly to its responsive element as a ligand-induced homodimer. Cell. 1988 Oct 7;55(1):145–156. doi: 10.1016/0092-8674(88)90017-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Landschulz W. H., Johnson P. F., McKnight S. L. The leucine zipper: a hypothetical structure common to a new class of DNA binding proteins. Science. 1988 Jun 24;240(4860):1759–1764. doi: 10.1126/science.3289117. [DOI] [PubMed] [Google Scholar]
- Lees J. A., Fawell S. E., White R., Parker M. G. A 22-amino-acid peptide restores DNA-binding activity to dimerization-defective mutants of the estrogen receptor. Mol Cell Biol. 1990 Oct;10(10):5529–5531. doi: 10.1128/mcb.10.10.5529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin K. H., Ashizawa K., Cheng S. Y. Phosphorylation stimulates the transcriptional activity of the human beta 1 thyroid hormone nuclear receptor. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7737–7741. doi: 10.1073/pnas.89.16.7737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer R. A., Notides A. C. Identification of an estrogen-responsive element from the 5'-flanking region of the rat prolactin gene. Mol Cell Biol. 1987 Dec;7(12):4247–4254. doi: 10.1128/mcb.7.12.4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendel D. B., Khavari P. A., Conley P. B., Graves M. K., Hansen L. P., Admon A., Crabtree G. R. Characterization of a cofactor that regulates dimerization of a mammalian homeodomain protein. Science. 1991 Dec 20;254(5039):1762–1767. doi: 10.1126/science.1763325. [DOI] [PubMed] [Google Scholar]
- Migliaccio A., Rotondi A., Auricchio F. Estradiol receptor: phosphorylation on tyrosine in uterus and interaction with anti-phosphotyrosine antibody. EMBO J. 1986 Nov;5(11):2867–2872. doi: 10.1002/j.1460-2075.1986.tb04581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murre C., McCaw P. S., Baltimore D. A new DNA binding and dimerization motif in immunoglobulin enhancer binding, daughterless, MyoD, and myc proteins. Cell. 1989 Mar 10;56(5):777–783. doi: 10.1016/0092-8674(89)90682-x. [DOI] [PubMed] [Google Scholar]
- Notides A. C., Lerner N., Hamilton D. E. Positive cooperativity of the estrogen receptor. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4926–4930. doi: 10.1073/pnas.78.8.4926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notides A. C., Nielsen S. The molecular mechanism of the in vitro 4 S to 5 S transformation of the uterine estrogen receptor. J Biol Chem. 1974 Mar 25;249(6):1866–1873. [PubMed] [Google Scholar]
- Obourn J. D., Koszewski N. J., Notides A. C. Hormone- and DNA-binding mechanisms of the recombinant human estrogen receptor. Biochemistry. 1993 Jun 22;32(24):6229–6236. doi: 10.1021/bi00075a016. [DOI] [PubMed] [Google Scholar]
- Pawson T., Schlessingert J. SH2 and SH3 domains. Curr Biol. 1993 Jul 1;3(7):434–442. doi: 10.1016/0960-9822(93)90350-w. [DOI] [PubMed] [Google Scholar]
- Rochette-Egly C., Gaub M. P., Lutz Y., Ali S., Scheuer I., Chambon P. Retinoic acid receptor-beta: immunodetection and phosphorylation on tyrosine residues. Mol Endocrinol. 1992 Dec;6(12):2197–2209. doi: 10.1210/mend.6.12.1283441. [DOI] [PubMed] [Google Scholar]
- Shuai K., Horvath C. M., Huang L. H., Qureshi S. A., Cowburn D., Darnell J. E., Jr Interferon activation of the transcription factor Stat91 involves dimerization through SH2-phosphotyrosyl peptide interactions. Cell. 1994 Mar 11;76(5):821–828. doi: 10.1016/0092-8674(94)90357-3. [DOI] [PubMed] [Google Scholar]
- Skafar D. F., Notides A. C. Modulation of the estrogen receptor's affinity for DNA by estradiol. J Biol Chem. 1985 Oct 5;260(22):12208–12213. [PubMed] [Google Scholar]
- Tora L., White J., Brou C., Tasset D., Webster N., Scheer E., Chambon P. The human estrogen receptor has two independent nonacidic transcriptional activation functions. Cell. 1989 Nov 3;59(3):477–487. doi: 10.1016/0092-8674(89)90031-7. [DOI] [PubMed] [Google Scholar]
- Wagner B. J., Hayes T. E., Hoban C. J., Cochran B. H. The SIF binding element confers sis/PDGF inducibility onto the c-fos promoter. EMBO J. 1990 Dec;9(13):4477–4484. doi: 10.1002/j.1460-2075.1990.tb07898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]