Abstract
Infection with Plasmodium falciparum may result in severe disease affecting various organs, including liver, spleen, and brain, resulting in high morbidity and mortality. Plasmodium berghei Anka infection of mice recapitulates many features of severe human malaria. The aryl hydrocarbon receptor (AhR) is an intracellular receptor activated by ligands important in the modulation of the inflammatory response. We found that AhR-knockout (KO) mice infected with P. berghei Anka displayed increased parasitemia, earlier mortality, enhanced leukocyte-endothelial cell interactions in the brain microvasculature, and increased inflammation in brain (interleukin-17 [IL-17] and IL-6) and liver (gamma interferon [IFN-γ] and tumor necrosis factor alpha [TNF-α]) compared to infected wild-type (WT) mice. Infected AhR-KO mice also displayed a reduction in cytokines required for host resistance, including TNF-α, IL-1β, and IFN-γ, in the brain and spleen. Infection of AhR-KO mice resulted in an increase in T regulatory cells and transforming growth factor β, IL-6, and IL-17 in the brain. AhR modulated the basal expression of SOCS3 in spleen and brain, and P. berghei Anka infection resulted in enhanced expression of SOCS3 in brain, which was absent in infected AhR-KO mice. These data suggest that AhR-mediated control of SOCS3 expression is probably involved in the phenotype seen in infected AhR-KO mice. This is, to our knowledge, the first demonstration of a role for AhR in the pathogenesis of malaria.
INTRODUCTION
Plasmodium falciparum malaria is a major cause of morbidity and mortality and is responsible for millions of infections, especially in sub-Saharan Africa. Falciparum malaria may be mild or lead to severe life-threatening complications, such as altered liver function, including fulminant hepatic failure; renal failure; and cerebral malaria (CM). CM is a major life-threatening complication of P. falciparum infection in humans, especially in young children (1). Infection of susceptible mouse strains with Plasmodium berghei Anka is commonly employed as a model of CM, because this model displays many features in common with human CM (2, 3). Use of this P. berghei Anka model has allowed the identification of several important pathogenesis pathways in CM (2, 3). While there has been discussion regarding the utility of this model as a surrogate for human CM (3, 4), it is regarded as an important and valid animal model of human CM (5–7). Moreover, the P. berghei Anka infection model also allows investigations into liver damage, as described in Plasmodium-infected humans (8, 9). Observations from humans with CM due to P. falciparum, as well as those from the CM mouse model using P. berghei Anka, suggest that the pathogenesis of CM is multifactorial and includes sequestration of infected red blood cells (iRBCs), disruption of the blood-brain barrier (BBB), upregulation of the inflammatory pathways, and dysregulation of endothelin pathways (10–13). Previously, we have demonstrated a crucial role for platelet-activating factor in experimental CM infection (10–13). Infection with P. berghei Anka results in a reduction in cerebral blood flow and neuronal dysfunction (14, 15). In addition, low nitric oxide (NO) bioavailability, hypoarginemia, and elevated levels of cell-free hemoglobin (16–20) have also been demonstrated to contribute to the pathogenesis of CM.
As with other infections (21), the inflammatory response triggered by malaria parasite infection is a double-edged sword. The early inflammatory response is required to control parasite replication and promote the clearance of infected erythrocytes. However, excessive levels of proinflammatory cytokines, such as tumor necrosis factor alpha (TNF-α), gamma interferon (IFN-γ), interleukin-1β (IL-1β), and IL-6, may result in cerebral dysfunction (22–24). Therefore, the outcome of infection depends on a delicate balance between appropriate and inappropriate induction of these mediators (25).
Both CD4+ and CD8+ T cells contribute to the pathogenesis of CM. CD8+ T cells are sequestered in the brain following P. berghei Anka infection at the onset of altered neurological signs and symptoms and are postulated to cause damage to the brain endothelium via the production of perforin (26). Sequestration of CD8+ T cells as well as red blood cells is associated with the development of CM (27, 28). T regulatory (Treg) cells play a critical role in the maintenance of immunological self-tolerance, as well as the control of immune responses to pathogens (29). Treg cells mediate their effects by direct cell contact or by secretion of anti-inflammatory cytokines, such as IL-10 and transforming growth factor β (TGF-β). In CM, Treg cells may contribute to the pathogenesis of infection by suppressing antiparasitic immunity (30). The role of Treg cells in the pathogenesis of CM in P. berghei Anka-infected mice has not been fully elucidated (31–33).
The aryl hydrocarbon receptor (AhR) is a ligand-dependent transcription factor and a member of the Per-Arnt-Sim (PAS) superfamily of proteins, which contains proteins involved in the detection of intracellular or environmental changes, sensing of light, oxygen and redox potential, the circadian rhythm, the response to hypoxia, and hormone signaling (34, 35). In the absence of ligands, AhR is inactive and remains in the cytoplasm complexed with accessory proteins. Upon ligand binding, the receptor shifts to the nucleus and heterodimerizes with the AhR nuclear translocator (ARNT). Once transcriptional regulation has occurred, the AhR is exported to the cytosol and degraded by the proteasome (35, 36).
AhR is activated by exogenous compounds, particularly by environmental contaminants, such as 2,3,7,8-tetrachlorobenzo-p-dioxin (TCDD). Activation of the AhR by this environmental pollutant can lead to several toxic changes, including hepatocellular damage, epithelial changes, cancer, birth defects, thymic involution, and immunosuppression (37, 38). There are a variety of endogenous agents capable of activating AhR, such as eicosanoids, bilirubin, the tryptophan photoproduct 6-formylindolo[3,2-b]carbazole (FICZ), kynurenines, and indoleamine 2,3-dioxygenase (IDO) (34, 35, 37, 39). During immune responses, AhR regulates both Treg and Th17 cell differentiation in a ligand-specific fashion (40–42).
AhR has been reported to contribute to the modulation of the inflammatory response to infections with Toxoplasma gondii (43), Listeria monocytogenes (44), and herpes simplex virus (45). During T. gondii infection, AhR deficiency results in a reduction of suppressor of cytokine signaling 2 (SOCS2) expression in the spleen. Suppressors of cytokine signaling (SOCS) are a family of intracellular proteins modulating the immune response, acting in several signaling pathways. Several studies have evaluated the roles of SOCS1, -2, and -3 under steady-state conditions and after infection. SOCS1-deficient mice spontaneously exhibit severe lymphopenia, fatty degeneration of the liver, and macrophage infiltration of major organs. SOCS3-deficient mice have embryonic lethality due to massive expansion of erythroid progenitors. Infection of mice with either T. gondii or Trypanosoma cruzi infection in the absence of SOCS2 leads to alterations in the central nervous and cardiovascular systems. While one study has demonstrated the participation of AhR in the modulation of SOCS2 expression induced by lipoxin in dendritic cells (46), the role of AhR in the modulation of SOCS1 and SOCS3 in dendritic or other cell types is unknown. The role of AhR in malaria has not been explored.
Since inflammation is an important factor in the pathogenesis of CM and AhR is a modulator of inflammation, we examined the role of AhR in P. berghei Anka-infected mice. Herein, we demonstrate that AhR modulates the development of CM and protects against liver damage. These data provide new insights into the pathogenesis of CM.
MATERIALS AND METHODS
Ethics statement.
This study was carried out in strict accordance with the Brazilian guidelines on animal work and the Guide for the Care and Use of Laboratory Animals (47). The animal ethics committee of the Universidade Federal de Minas Gerais, CETEA/UFMG, approved all experiments and procedures, including euthanasia and fluid and organ removal (permit number 202/10). All animal experiments were planned in order to minimize mouse suffering.
Parasitology and pathology.
Wild-type (WT) C57BL/6 mice (6 to 8 weeks old) were obtained from the Animal Care Facilities of the Universidade Federal de Minas Gerais, Belo Horizonte, Minas Gerais, Brazil. AhR-knockout (KO) mice were bred on a C57BL/6 genetic background under pathogen-free conditions at the Instituto de Ciências Biológicas, Universidade Federal de Minas Gerais. Blood stages of P. berghei Anka constitutively expressing green fluorescent protein (GFP) (P. berghei Anka-GFP; the 15cy1 clone) (48), kindly provided by Claudio Marinho (Universidade de São Paulo), were stored in liquid nitrogen. Mice were infected intraperitoneally (i.p.) with 105 P. berghei Anka-infected red cells suspended in 0.2 ml phosphate-buffered saline (PBS). The percent parasitemia was quantified by determination of the frequency of GFP in whole blood using flow cytometry. Briefly, a drop of tail blood from infected and uninfected mice was collected directly into polystyrene tubes containing 2 ml of PBS for flow cytometry analysis. Each sample was analyzed using a flow cytometer (FACSCanto II; Becton, Dickinson, San Jose, CA). GFP frequency was measured using an argon laser (488 nm), and the acquisition was processed using Diva software (Becton, Dickinson, San Jose, CA). Erythrocyte populations were identified on the basis of their morphological characteristics in a dot plot graphic (forward scatter-side scatter) and analyzed for the presence/expression of GFP. A minimum of 100,000 gated events on the erythrocyte population of each sample was acquired for analysis. Mice were observed daily for parasitemia, survival, and clinical signs of CM culminating in ataxia and paralysis, which were assessed by use of the rapid murine coma and behavior scale (RMCBS) (49). For determination of hematocrit, samples of blood were collected at 5 days postinfection (dpi) and placed into heparinized capillary tubes, and the tubes were centrifuged for 10 min in a hematocrit centrifuge (HT, São Paulo, Brazil). For determination of liver function, alanine aminotransferase (ALT) activity was measured using a commercially available kit following the manufacturer's protocol (Quibasa, Bioclin, Belo Horizonte, Brazil). Serum iron levels were determined by colorimetric assay using a commercially available kit (Gold Analisa, Belo Horizonte, Brazil) following the manufacturer's protocol.
At the time points indicated below, mice were euthanized with ketamine-xylazine, the brain and a portion of the liver were removed and immediately fixed in 4% buffered formalin, and tissue fragments were embedded in paraffin. Tissue sections (4 μm thick) were stained with hematoxylin-eosin (H&E) and examined under a light microscope. Sections were captured with a digital camera (DEI-470; Optronics, Goleta, CA) connected to a microscope (IX70; Olympus, Center Valley, PA) with a magnification of ×200. Liver pathology (degeneration and inflammation) was graded from 0 to 4, considering the severity of the lesions (none, 0; minimal, 1; mild, 2; moderate, 3; marked, 4).
Intravital microscopy.
Intravital microscopy of the mouse brain microvasculature was performed at 5 dpi. Mice were anesthetized i.p. with a sterile mixture of 10 mg/kg of body weight xylazine and 150 mg/kg ketamine in PBS. The tail vein was cannulated for administration of fluorescent dyes. A craniotomy was performed with a high-speed drill (Beltec, São Paulo, Brazil), and the dura and arachnoid mater were removed to expose the underlying pia mater vasculature. During the experiment, the mice were maintained at 37°C with a heating pad (Fine Science Tools Inc., North Vancouver, BC, Canada), and the exposed brain was kept moist with artificial cerebrospinal fluid buffer (pH 7.4, 132 mmol/liter NaCl, 2.95 mmol/liter KCl, 1.71 mmol/liter CaCl2, 0.64 mmol/liter MgCl2, 24.6 mmol/liter NaHCO3, 3.71 mmol/liter dextrose, 6.7 mmol/liter urea) at 37°C. To observe leukocyte-endothelium interactions, leukocytes were fluorescently labeled by intravenous administration of rhodamine 6G (0.3/0.5 mg/kg body weight; Sigma-Aldrich, St. Louis, MO). An intravital microscope (Nikon CSRS H550L; Nikon, Japan) with a ×20 objective lens outfitted with a fluorescent light source (epi-illumination at 510 to 560 nm, using a 590-nm emission filter) was used to examine the brain microcirculation. A digital camera (Nikon DS-QiMc) was used to project the images onto a computer monitor, and images were recorded for playback analysis using Imaging software (NIS-Elements; Nikon). The number of rolling and adherent leukocytes was determined offline during video playback analysis. Leukocytes were considered adherent to the venular endothelium if they remained stationary for a minimum of 30 s, and adherent leukocytes were expressed as the number of cells per 100 μm. Rolling leukocytes were defined as white blood cells moving at a velocity lower than that of erythrocytes within a given vessel. The P. berghei Anka-GFP-infected red blood cells were observed in the pia mater vessel using a green fluorescent light source (epi-illumination at 510 to 560 nm, using a 560-nm emission filter).
Assessment of BBB integrity.
The integrity of the blood-brain barrier (BBB) was investigated using Evans blue dye as described previously (11). Briefly, Evans blue solution (20 mg/kg; Sigma-Aldrich) was administered (1 ml/kg) via an eye vein 30 min prior to sacrifice at 6 dpi. One hour later, the mice were sacrificed and the heart was perfused with 5 ml of PBS. Brain samples were removed and weighed, and Evans blue extravasation was evaluated using 1 ml of formamide (24 h at room temperature). The amount of Evans blue in the tissue was obtained by comparing the extracted absorbance with that of a standard curve of Evans blue read at 620 nm in an enzyme-linked immunosorbent assay (ELISA) plate reader as a measurement of capillary permeability. Results are presented as the amount of Evans blue per 100 mg of tissue
Hemodynamic measurements.
Systolic blood pressure (SBP) and heart rate were measured in uninfected and infected mice at 0, 3, 4, and 5 dpi. All mice were acclimated to the blood pressure measurement device for 5 days. SBP and heart rate were determined by the tail-cuff plethysmography method in nonanesthetized mice. The tail-cuff measurements were made in trained mice that had not undergone any invasive procedures, and the intra-arterial measurements were made without the stress of heating and restraints required during the tail-cuff procedure. All data are expressed as the mean ± standard error of the mean (SEM). Changes in SBP and heart rate from the baseline are expressed as absolute values as well as areas under the curves.
Quantification of nitric oxide in brain tissue.
The brain was harvested and homogenized in a PBS buffer containing a protease inhibitor cocktail. Nitric oxide (NO) was quantified as nitrite. Nitrates were reduced to nitrites by enzymatic conversion by nitrate reductase (Sigma). The level of nitrites was determined by the Griess method. The NO2− concentration (μM) was determined by reference to an NaNO2 standard curve (absorbance at 550 nm).
CBA.
Serum cytokine levels were determined using a mouse Th1/Th2/Th17 cytometric bead array (CBA) kit (BD Biosciences, San Diego, CA) and analyzed on a FACSCanto II flow cytometer. Standard curves for each cytokine were determined over a range of 20 to 5,000 pg/ml. The lower limit of detection for the CBA, according to the manufacturer, is 0.1 to 16.8 pg/ml, depending on the analyte. The levels of the cytokines IL-2, IL-6, IL-10, IL-17A, IFN-γ, and TNF-α were measured and analyzed by FCAP Array software (BD Biosciences).
Quantification of tissue cytokines (ELISA).
Brain and spleen tissues were homogenized in a PBS buffer containing a protease inhibitor cocktail to determine the concentrations of the cytokines IL-1β, IL-6, IL-12p70, IL-10, IL-17A, IFN-γ, TGF-β, and TNF-α by ELISA (DuoSet kits; R&D Systems), in accordance with the manufacturer's instructions.
Real-time PCR (qPCR).
Brains and spleen were removed for analysis of the transcript levels of AhR, SOCS1, SOCS2, SOCS3, and FOXP3. Total RNA was obtained using the TRIzol reagent (Invitrogen, Carlsbad, CA) according to the procedure supplied by the manufacturer. Total RNA was reverse transcribed with SuperScript III reverse transcriptase (Invitrogen) as described by the manufacturer, and quantitative PCR (qPCR) was performed on an ABI Prism Step One sequence detection system (Applied Biosystems, Carlsbad, CA), using SYBR green PCR master mix (Applied Biosystems), for the AhR, FOXP3, SOCS1, SOCS2, and SOCS3 genes. The 18S rRNA gene was used as an endogenous control for normalization, according to the manufacturer's protocol, and was tested in a Step One thermal cycler and a 7500 Fast real-time PCR system (Applied Biosystems) with specific primer pairs. The relative expression level of the genes was determined by the 2−ΔΔCT threshold cycle (CT) method, and the data were normalized by the 18S ribosome subunit expression levels. All reactions were replicated. Test samples were expressed as the fold change of transcript abundance in uninfected WT or AhR-KO mice compared with that in infected WT or AhR-KO mice, respectively (mean expression from at least three uninfected mice). The sequences of the primers used in this study were as follows: AhR Forward, GGAGCGCTGCTTCCTCCAC; AhR Reverse, GCTGCCCTTTGGCATCACAACC; FOXP3 Forward, CCCAGGAAAGACAGCAACCTT; FOXP3 Reverse, TCCTCACAACCAGGCCACTTG; SOCS1 Forward, ACACTCACTTCCGCACCTTC; SOCS1 Reverse, GAAGCCATCTCCACGCTG; SOCS2 Forward, CGGCGGTGGAGGCGATCTG; SOCS2 Reverse, CCGAAATGGTGGCGGAGGGG; SOCS3 Forward, GTTGAGCGTCAAGACCCAGT; SOCS3 Reverse, GGGTGGCAAAGAAAAGGAG; 18S Forward, CGTTCCACCAACTAAGAACG; and 18S Reverse, CTCAACACGGGAAACCTCAC.
Flow cytometry of brain lymphocytes.
P. berghei Anka-infected mice were sacrificed at 6 dpi and perfused by intracardiac catheter with PBS to remove circulating RBCs and leukocytes from the brain. The brains were then removed, and lymphocytes were isolated as described previously (27). Briefly, the brains were collected and gently homogenized with a sterile glass tissue grinder in RPMI 1640 medium containing 5% fetal calf serum. Homogenates were passed through a nylon cell strainer (pore size, 70 μm; Becton, Dickinson, San Jose, CA) and then centrifuged at 400 × g for 10 min. The pellet was resuspended in 35% Percoll gradient (Sigma-Aldrich), and this was deposited on a 70% Percoll gradient. After centrifugation (1,100 × g), myelin was aspirated off the top of the 35% Percoll layer and leukocytes were collected at the boundary layer, between the 70% and 35% gradients. Leukocytes were then resuspended in fluorescence-activated cell sorting buffer (PBS containing 1% fetal calf serum and 0.01% NaN3) and counted. At 4 h posttreatment with brefeldin A (10 μg/ml), the cells were fixed and stained with labeled mouse-specific antibodies with the combinations of biotin-CD3 followed by Strep-Cy5, CD4 (phycoerythrin [PE]-Cy7), CD8 (allophycocyanin [APC]), CD25 (APC), FOXP3 (PE), IFN-γ (PE), IL-10 (APC), IL-17A (PE), TNF-α (Alexa Fluor 647), and isotype controls (all from BD Pharmingen). For each sample, 20,000 cells from the lymphocyte population were scored. Data were acquired on a FACSCanto II flow cytometer (Becton, Dickinson) and analyzed by FlowJo (version 7.6) software (Tree Star, Inc.). The gating strategy used is illustrated in Fig. S1A in the supplemental material.
Flow cytometry of liver lymphocytes.
P. berghei Anka-infected mice were sacrificed at 6 dpi and perfused with PBS by an intraportal catheter to remove circulating RBCs and leukocytes from the liver. Liver-derived lymphocytes were isolated using a modified method previously described (50). Briefly, livers were excised and finely minced in digestive medium containing 0.05% collagenase (Life Technologies) and 0.002% DNase I (Sigma-Aldrich) in Hanks' balanced salt solution (Life Technologies). After gentle agitation at 37°C for 30 min, the concentrate was passed through a nylon cell strainer (pore size, 70 μm; Becton, Dickinson, San Jose, CA), washed twice with ice-cold PBS (pH 7.4), and centrifuged at 300 × g for 10 min. Lymphocytes were purified by layering the cell suspension on fluorescence-activated cell sorting buffer (PBS containing 1% fetal calf serum and 0.01% NaN3) and centrifuged at 800 × g for 20 min at room temperature. Lymphocytes were washed in PBS and counted. Liver cells were incubated with brefeldin A (10 μg/ml) for 4 h, followed by fixation and staining with specific antibody combinations: biotin-CD3 followed by Strep-Cy5, CD4 (PE-Cy7), CD8 (APC), CD25 (APC), FOXP3 (PE), IFN-γ (PE), IL-10 (APC), IL-17A (PE), TNF-α (Alexa 647), and isotype controls (all from BD Pharmingen). Data were acquired on a FACSCanto II flow cytometer (Becton, Dickinson), and viable cells were analyzed by flow cytometry using FlowJo software (Tree Star, Inc.). The gating strategy used is illustrated in Fig. S1B in the supplemental material.
Flow cytometry of spleen and thymus lymphocytes.
Spleen and thymus cells were evaluated ex vivo for extracellular molecular expression patterns and for intracellular cytokine expression patterns. Purified splenocytes and thymus cells were removed from infected mice at 6 dpi. Spleen cells were incubated with brefeldin A (10 μg/ml) for 4 h, followed by fixation and staining with specific antibody combinations: biotin-CD3 followed by Strep-Cy5, CD4 (PE-Cy7), CD8 (APC), CD25 (APC), FOXP3 (PE), IFN-γ (PE), IL-10 (APC), IL-17A (PE), TNF-α (Alexa 647), and isotype controls (all from BD Pharmingen). Thymus cells were removed and stained directly with CD3, CD8, CD4, CD25, and FOXP3. Data were acquired on a FACSCanto II flow cytometer (Becton, Dickinson), and viable cells were analyzed by flow cytometry using FlowJo software (Tree Star, Inc.). The gating strategy used is illustrated in Fig. S1C in the supplemental material.
Statistical analysis.
Results are shown as means ± SEMs. Differences were compared by using two-tailed Student's t tests with 95% confidence intervals, analysis of variance (ANOVA), or two-way ANOVA followed by the Bonferroni correction, as needed, for multiple comparisons when parametric assumptions were met. Otherwise, the Mann-Whitney U test was applied. Differences between lethality curves were calculated using the log rank test (GraphPad Prism software, version 5.0). Results with a P value of <0.05 were considered significant. All data are representative of those from at least 2 experiments (n = 5 to 7 mice).
RESULTS
AhR deficiency results in increased parasitemia and mortality in Plasmodium berghei Anka infection.
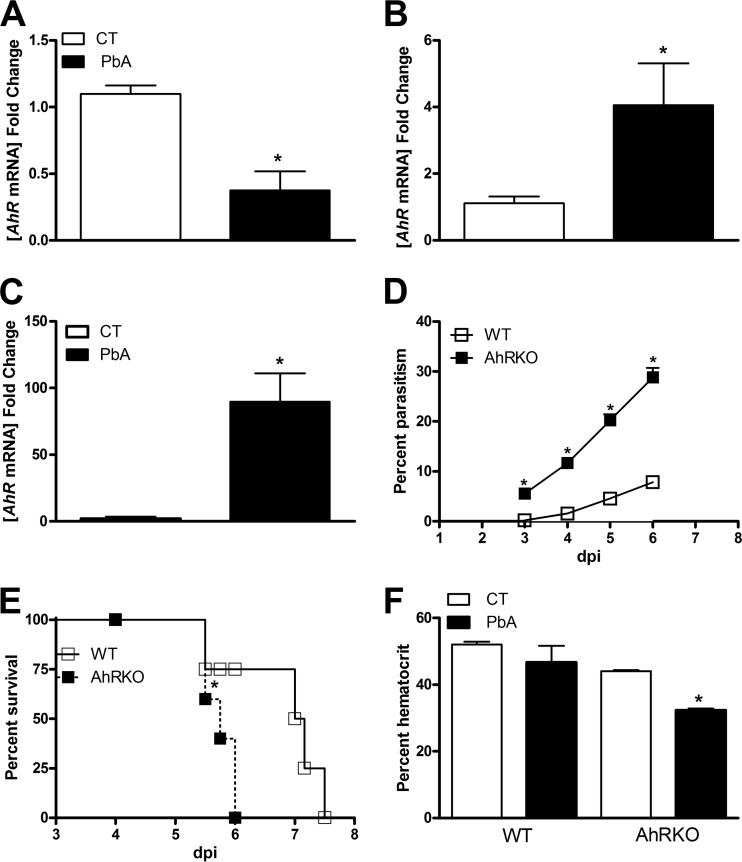
To investigate whether AhR expression was modulated during P. berghei Anka infection, qPCR was performed on brain, liver, and spleen. After infection, there was a significant reduction in AhR expression in the brain compared with that in the brain of uninfected mice (Fig. 1 A), and there was a markedly increased AhR expression in the liver and spleen after infection (Fig. 1B and C). These results suggest that during infection, AhR expression is modulated in an organ-specific fashion. To determine the role of AhR in the pathogenesis of P. berghei Anka infection, AhR-deficient mice were infected and parasitemia (Fig. 1D) and mortality (Fig. 1E) were monitored. The absence of AhR resulted in a significant increase in parasitemia (Fig. 1D) and more rapid mortality (statistical analysis by the log rank test, P < 0.05; Fig. 1E) during infection. Severe neurological signs, including ataxia, paralysis, seizure, rollover, and coma, appeared earlier in infected AhR-KO mice than infected WT mice. The hematocrit at day 5 dpi was significantly lower in infected AhR-KO mice than infected WT mice (Fig. 1F). In agreement with the findings of a previous study, uninfected AhR-KO mice were found to have a slight decrease in hematocrit compared with WT mice (51).
FIG 1.
AhR-KO mice are highly susceptible to Plasmodium berghei Anka (PbA) infection. C57BL/6 (WT) mice were infected i.p. with 105 parasitized erythrocytes. (A to C) Brain (A), liver (B), and spleen (C) were harvested from control and infected WT mice at 6 dpi, followed by RNA extraction, and quantitative reverse transcription-PCR using primers specific for AhR in brain, liver, and spleen was performed. The data are expressed as the fold change in transcript abundance in uninfected WT mice compared with the abundance in infected WT mice. (D to F) WT and AhR-KO mice were infected i.p. with 105 parasitized erythrocytes, and at the indicated time points, the following parameters were assessed: the natural course of infection by flow cytometric analysis (D), the survival curves of P. berghei Anka-infected WT and AhR-KO mice (expressed as the number of days after infection) (E), and hematocrit at 5 dpi (F). The data are representative of those from at least two independent experiments (5 mice/group) and are shown as the means ± SEMs. *, P < 0.05 for the comparison of infected versus uninfected WT mice (A to C) or the comparison of infected WT versus AhR-KO mice (D to F). CT, control.
Alterations in leukocyte-endothelial cell interaction and brain vascular permeability in AhR-KO mice during P. berghei Anka infection.
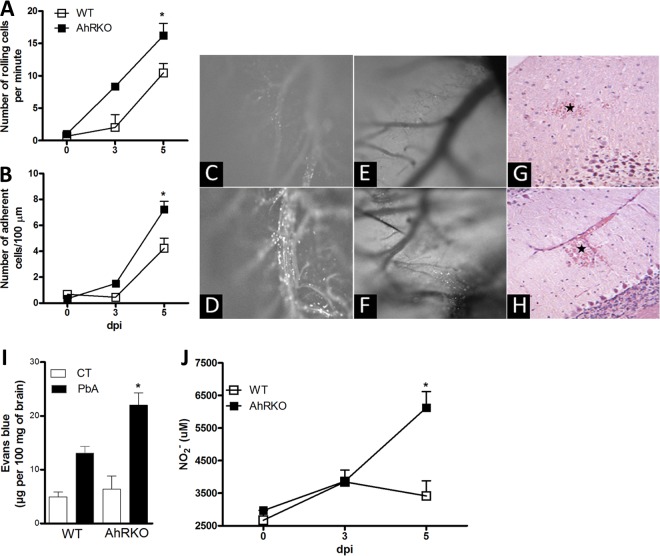
CM is associated with inflammation in the brain, which is characterized by leukocyte adherence in the brain microvasculature, damage to the endothelium, and eventual disruption of the BBB and hemorrhage. The role of AhR in the interaction of leukocytes with the pia mater vasculature was evaluated using intravital microscopy. There was a significant increase in the number of leukocytes rolling and adhering in the vasculature of the pia mater of P. berghei Anka-infected AhR-KO compared with the number of such leukocytes in infected WT mice (Fig. 2A to D). Moreover, the intravital images and histological analyses demonstrated that AhR deficiency increased the hemorrhagic foci in the pia mater and neuropil (Fig. 2E to H).
FIG 2.
Leukocyte-endothelium interaction and vascular leakage in P. berghei Anka-infected AhR-KO brain. WT and AhR-KO mice were infected i.p. with 105 parasitized erythrocytes. (A, B) Intravital microscopy was used to assess the rolling (A) and adhesion (B) of leukocytes on the brain microvasculature in uninfected and infected mice at 3 and 5 dpi. The data are presented as the mean number of rolling cells per minute ± SEM (A) or the mean number of adherent cells per 100 μm ± SEM (B). (C to F) Time-lapse images reveal rolling and adherent cells in WT (C) and AhR-KO (D) mice and the presence of P. berghei Anka-GFP red blood cells in the pia mater of infected WT (E) and AhR-KO (F) mice. (G, H) Representative photomicrographs of H&E-stained cerebellar sections from infected WT (G) and AhR-KO (H) mice at 6 dpi. Infected WT mice displayed mild focal hemorrhage at the molecular layer (asterisk) (G). Infected AhR-KO mouse displayed extensive hemorrhagic areas (asterisk) (H). Magnifications, ×200. Quantitative assessment of brain vascular permeability using Evans blue extravasation in formamide was made by measurement of the absorbance at 650 nm in brain extract on day 6 dpi. (I) Infected AhR-KO mice showed increased vascular permeability compared with that of P. berghei Anka-infected WT mice. (J) The brain was harvested and homogenized, and nitrite levels were assayed by the Griess method. The data are representative of those from one of three independent experiments (5 mice/group) and are shown as the mean ± SEM. *, P < 0.05 for the comparison of infected WT versus AhR-KO mice.
Severe malaria is usually accompanied by vascular sequestration of leukocytes in the microvasculature in a variety of vital organs. In the brain, it may result in functional alterations in the endothelium and in the BBB. Experiments using Evans blue revealed that at 6 dpi AhR-KO mice had an increase in vascular permeability compared to infected WT mice (Fig. 2I), suggesting that P. berghei Anka infection in the absence of AhR is associated with increased vasculature permeability.
NO is an important regulator of vascular tone. It is believed that alterations in cerebral NO levels may contribute to altered neurological signs and symptoms, including coma, during P. berghei Anka infection. We found that AhR-KO-infected mice had increased levels of NO compared to infected WT mice (Fig. 2J). The systolic blood pressure and heart rate in WT and AhR-KO mice, however, were similar during the period of observation (see Fig. S1D and E in the supplemental material).
The absence of AhR results in severe inflammation and hepatic injury during P. berghei Anka infection.
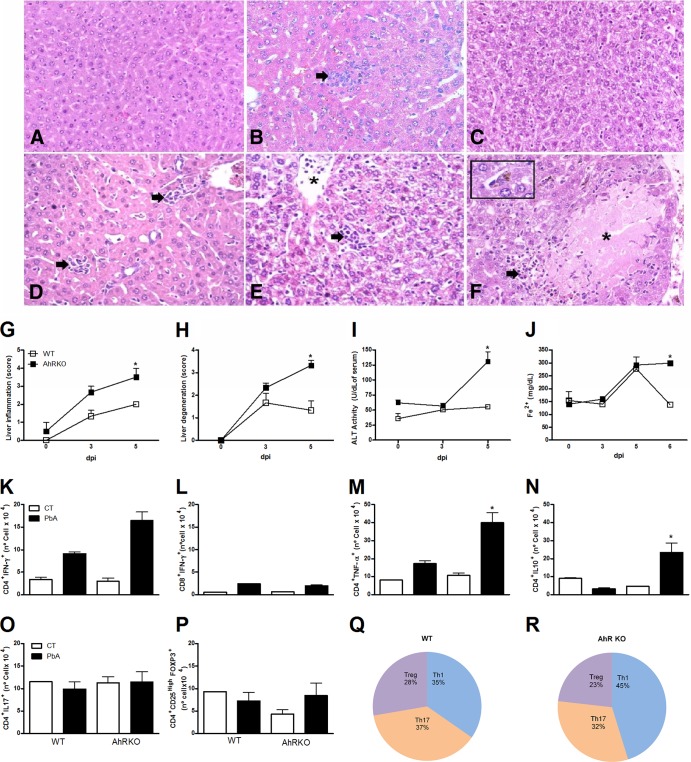
Examination of liver sections revealed increased liver inflammation and necrosis in infected AhR-KO mice compared to infected WT mice (Fig. 3A to H). In addition, P. berghei Anka-infected AhR-KO mice had a significant increase in serum levels of ALT at 5 dpi (Fig. 3I). Hemolysis often accompanies malaria parasite infection, resulting in released free heme catalyzed in iron, an important metabolite for the parasite life cycle. Both infected WT and AhR-KO mice had a significant increase in the levels of iron at 5 dpi. By 6 dpi, WT mice had a reduction in iron to basal levels; however, iron levels remained elevated in AhR-KO mice (Fig. 3J).
FIG 3.
Increased liver injury in P. berghei Anka-infected AhR-KO mice. WT and AhR-KO animals were infected i.p. with 105 parasitized erythrocytes. (A to F) Representative photomicrographs of H&E-stained liver sections from uninfected WT (A) and AhR-KO (D) mice or infected WT (B, C) and AhR-KO (E, F) mice on days 3 (B, E) and 6 (C, F) after infection. (A) Normal histological appearance of liver. (D) Inflammatory foci (arrows) are seen to be scattered in the hepatic parenchyma. (B) focal inflammation revealed in an infected WT mouse (arrow). (E) An AhR-KO mouse displayed moderate degeneration of hepatocytes, accumulation of leukocytes in the lumen of the blood vessel (asterisk), and inflammatory infiltrates (arrow). (C) Infected WT mice exhibited moderate and diffuse hepatocellular degeneration. (F) Focal necrosis (asterisk) partially surrounded by mononuclear cells (arrow) in an AhR-KO mouse. Note the mild Kupffer cell hyperplasia and deposition of malarial pigment (inset). Magnifications, ×200 (A to F) and ×400 (inset). (G to J) At the indicated time points, the following parameters were assessed: liver inflammation (G) and injury (H), ALT levels in serum (I), and the iron concentration in serum by a ferrozine-based colorimetric assay (J). (K to P) Leukocytes were harvested from WT and AhR-KO mouse livers at 0 and 6 dpi, followed by detection of CD4+ IFN-γ+ (K), CD8+ IFN-γ+ (L), CD4+ TNF-α+ (M), CD4+ IL-10+ (N), CD4+ IL-17+ (O), and CD4+ CD25+ FOXP3+ (P) cells by flow cytometry. (Q, R) The CD4+ T cell numbers (infected WT and AhR-KO mice) found by flow cytometry are presented as pie graphs and were used for comparison of the profile/proportion of Th cell responses: Th1 (CD4+ IFN+), Th17 (CD4+ IL-17+), and Treg (CD4+ CD25high FOXP3+) cells. The data are representative of those from two independent experiments (5 mice/group) and are shown as the mean ± SEM. *, P < 0.05 for the comparison of P. berghei Anka-infected WT mice versus AhR-KO mice.
Since immune mediators such as T cells and cytokines have been shown to be crucial for the development of severe malaria, we investigated the phenotype of infiltrating cells found in the liver. Greater numbers of CD4+ IFN-γ-positive (IFN-γ+), CD4+ TNF-α-positive (TNF-α+), and CD4+ IL-10-positive (IL-10+) cells, but not CD8+ IFN-γ+ cells, were observed in the livers of infected AhR-KO mice than in those of infected WT mice (Fig. 3K to N). On the other hand, there were no differences detected in the number of CD4+ IL-17+ and CD4+ CD25+ FOXP3-positive (FOXP3+) cells among all uninfected and infected groups analyzed (Fig. 3O and P). Figures 3Q and R show the proportion of Th1, Th17, and Treg cells in the liver of P. berghei Anka-infected WT and AhR-KO mice.
Inflammatory response to P. berghei Anka infection in the brain of AhR-KO mice.
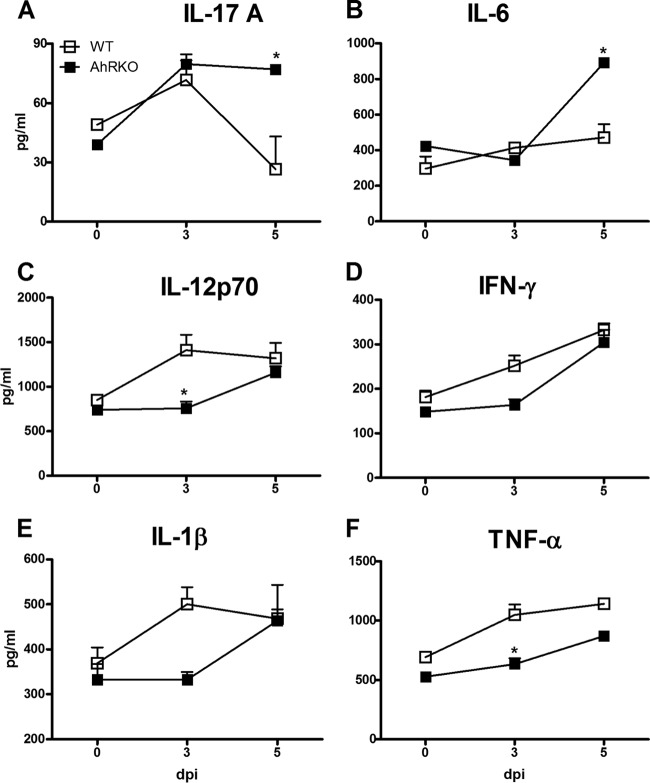
Several parameters of the immune response were evaluated in the brain. At 5 dpi, increased levels of IL-17 and IL-6 were observed in the brain of AhR-KO mice compared with the levels in the brain of WT mice (Fig. 4A and B), while the levels of IL-12p70, IFN-γ, TNF-α, and IL-1β at 3 and 5 dpi were either reduced or unchanged (Fig. 4C to F). The profile of infiltrating cells in the brain was next determined. The absence of AhR did not alter the numbers of infiltrating CD4+ IFN-γ+ and CD8+ IFN-γ+ cells in the brain of infected mice (Fig. 5A and B); however, there was an increase in the number of CD4+ IL-17+ T cells (Fig. 5C).
FIG 4.
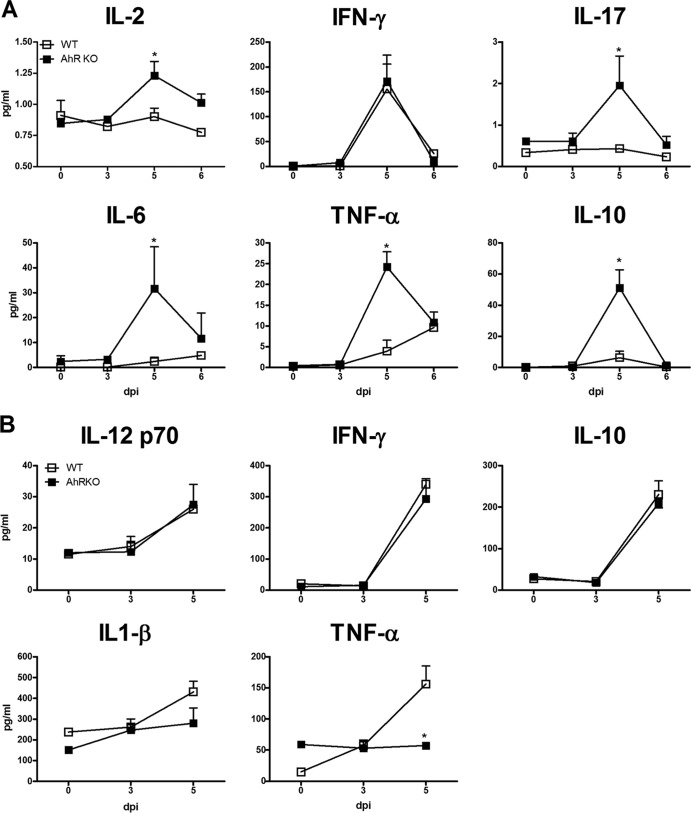
Increased production of IL-17A and IL-6 in the brain of P. berghei Anka-infected AhR-KO mice. WT and AhR-KO animals were infected i.p. with 105 parasitized erythrocytes. Brain was harvested and homogenized, and the kinetics of expression of the following cytokines were measured by ELISA: IL-17A (A), IL-6 (B), IL-12p70 (C), IFN-γ (D), IL-1β (E), and TNF-α (F). The data are representative of those from two independent experiments (5 mice/group) and are shown as the mean ± SEM. *, P < 0.05 for the comparison of infected WT versus AhR-KO mice.
FIG 5.
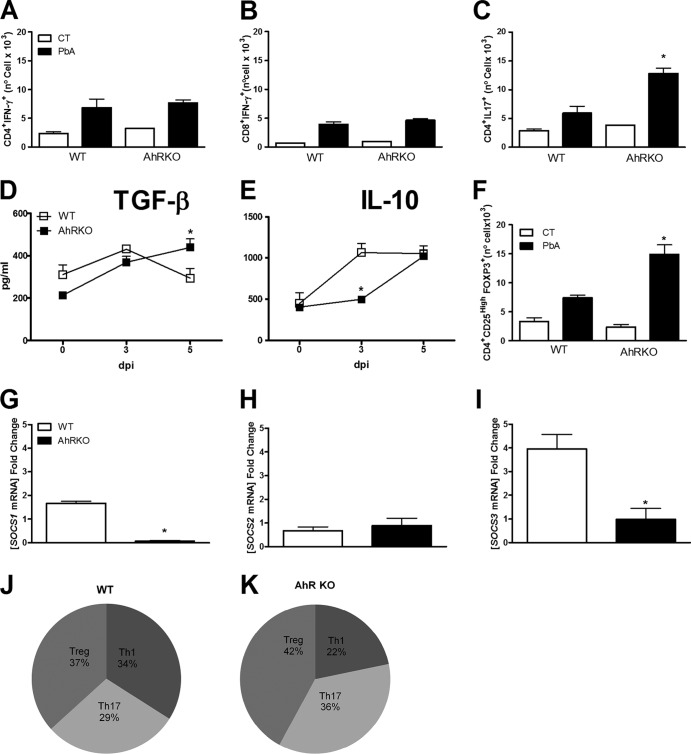
AhR modulates the production of anti-inflammatory mediators in the brain during P. berghei Anka infection. WT and AhR-KO mice were infected by the i.p. route with 105 parasitized erythrocytes. (A to C) Leukocytes were harvested from WT and AhR-KO mouse brain at 0 and 6 dpi, followed by detection of CD4+ IFN-γ+ (A), CD8+ IFN-γ+ (B), and CD4+ IL-17+ (C) cells by flow cytometry. (D, E) Brains were harvested and homogenized, and the kinetics of the expression of TGF-β (D) and IL-10 (E) were measured by ELISA. (F) Leukocytes were harvested from WT and AhR-KO mouse brains at 0 and 6 dpi, followed by detection of CD4+ CD25+ FOXP3+ cells by flow cytometry. (G to I) Brains were harvested from control and infected (6 dpi) WT and KO mice, followed by RNA extraction and quantitative reverse transcription-PCR using primers specific for SOCS1 (G), SOCS2 (H), and SOCS3 (I). Data were normalized to those for uninfected WT or AhR-KO mice. (J, K) The CD4+ T cell numbers (infected WT and AhR-KO mice) were recorded by flow cytometry and are represented as pie graphs used for comparison of the profile/proportion of Th cell responses: Th1 (CD4+ IFN+), Th17 (CD4+ IL-17+), and Treg (CD4+ CD25high FOXP3+) cells. The results are presented as the mean fold change compared to the level of expression in infected WT versus AhR-KO mice. *, P < 0.05 for the comparison of infected WT mice versus infected AhR-KO mice. Data are shown as the mean ± SEM and are representative of those from two independent experiments with at least five animals per group.
Treg cells play an important role in the control of immune responses to several pathogens. At 6 dpi, there were increased levels of TGF-β (Fig. 5D), but not IL-10 (Fig. 5E), and significantly increased numbers of CD4+ CD25+ FOXP3+ cells (Fig. 5F) in the brain of infected AhR-KO mice compared with infected WT mice. The suppressor of cytokine signaling (SOCS) family plays an important role in immune responses by modulating cytokine signaling pathways, and AhR has been shown to modulate SOCS2 expression in the mouse model of T. gondii infection (46). SOCS2 is also induced during T. cruzi infection (52) and was shown to be modulated as a result of aspirin treatment (53). Therefore, we examined SOCS1, -2, and -3 expression in the brain tissue of infected mice. In the brain of infected AhR-KO mice, the expression of SOCS3 was significantly decreased, whereas the expression of SOCS1 was only minimally reduced and the expression of SOCS2 was unchanged compared with that in the brain of infected WT mice (Fig. 5G to I). Figures 5J and K show the proportion of Th1, Th17, and Treg cells in the brain of P. berghei Anka-infected WT and AhR-KO mice.
Systemic and splenic cytokine responses.
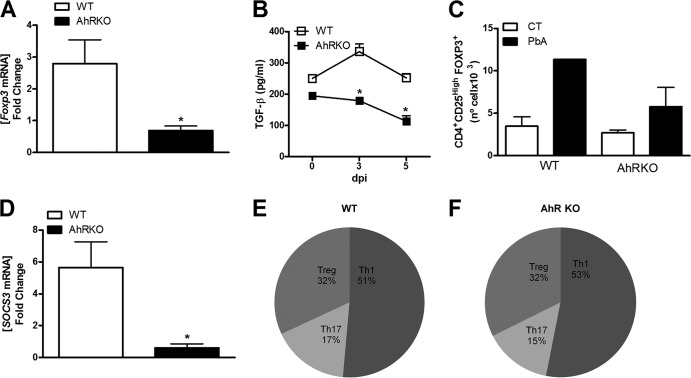
At 5 dpi, infected AhR-KO mice displayed a significant increase in serum levels of IL-2, IL-17, IL-6, TNF-α, and IL-10, but not IFN-γ, compared with those in infected WT mice (Fig. 6A). However, no significant differences in the levels of IL-12p70, IFN-γ, IL-10, and IL-β in the spleen (Fig. 6B) were observed between P. berghei Anka-infected WT and AhR-KO mice. Infection of AhR-deficient mice resulted in an absence of TNF-α induction (Fig. 6B), reduced FOXP3 expression (Fig. 7A), and decreased TGF-β production (Fig. 7B) in the spleen compared with the results for infected WT mice. We also observed a significant increase in the number of CD4+ CD25+ FOXP3+ cells in the spleen of infected WT mice but not in the spleen of infected AhR-KO mice (Fig. 7C).
FIG 6.
Systemic and splenic modulation of the inflammatory response in P. berghei Anka-infected AhR-KO mice. WT and AhR-KO animals were infected i.p. with 105 parasitized erythrocytes. (A) Serum cytokine levels were determined by CBA at the indicated time point. (B) Spleens were harvested and homogenized, and the kinetics of IL-12p70, IFN-γ, IL-10, IL-1β, and TNF-α cytokine expression were measured by ELISA. Data are shown as the mean ± SEM and are representative of those from two independent experiments with at least five animals per group. *, P < 0.05 for the comparison of P. berghei Anka-infected WT versus AhR-KO mice.
FIG 7.
Modulation of TGF-β and SOCS3 in the spleen by AhR during P. berghei Anka infection. WT and AhR-KO mice were infected i.p. with 105 parasitized erythrocytes. (A) Splenocytes were harvested from control and infected WT and AhR-KO mice at 6 dpi, mRNA was extracted, and quantitative reverse transcription-PCR was performed using primers specific for FOXP3. Data were normalized to those for uninfected WT or AhR-KO mice. (B) Spleens were harvested and homogenized, and the kinetics of TGF-β expression were measured by ELISA. (C) Splenocytes were harvested from control and infected WT and AhR-KO mice at 6 dpi, followed by detection of CD4+ CD25+ FOXP3+ cells by flow cytometry. (D) Splenocytes were harvested from control and infected WT and AhR-KO mice at 6 dpi, RNA was extracted, and quantitative reverse transcription-PCR was performed using primers specific for SOCS3. Data were normalized to those for uninfected WT and AhR-KO mice. (E, F) The CD4+ T cell numbers for infected WT and AhR-KO mice found by flow cytometry are represented as pie graphs used for comparison of the profile/proportion of Th cell responses: Th1 (CD4+ IFN+), Th17 (CD4+ IL-17+), and Treg (CD4+ CD25high FOXP3+) cells. The results are presented as the mean fold change compared to the level of expression for infected WT versus AhR-KO mice. Data are shown as the mean ± SEM and are representative of those from two independent experiments. *, P < 0.05, for the comparison of infected WT versus infected AhR-KO mice.
Alterations in the generation and expansion of CD4+ CD25+ FOXP3+ cells were demonstrated in the thymus of AhR-KO mice (see Fig. S2A in the supplemental material). P. berghei Anka infection resulted in a minimal reduction of CD4+ CD25+ FOXP3+ cells in the thymus of WT mice (see Fig. S2A in the supplemental material). Infection of AhR-KO mice resulted in a significant reduction of Treg cells in the thymus and impaired their generation/expansion in the spleen compared with the findings for infected WT mice (Fig. 7C; see Fig. S2A in the supplemental material). Moreover, the generation of T cells in the thymus was altered by the absence of AhR, including an increase in the number of double-positive thymocytes (see Fig. S2B in the supplemental material) and CD4+ CD8low cells (see Fig. S2D in the supplemental material) and a decrease in the numbers of CD8+ CD4− cells (see Fig. S2G in the supplemental material) compared with the results for WT mice. Upon infection, AhR deficiency resulted in decreased numbers of double-positive thymocytes (see Fig. S2B in the supplemental material), CD4+ CD8− cells (see Fig. S2E in the supplemental material), CD8+ CD4low cells (see Fig. S2F in the supplemental material), and CD8+ CD4− cells (see Fig. S2G in the supplemental material) at 6 dpi. In the spleen of infected AhR-KO mice, SOCS3 expression was significantly lower than that in the spleen of WT infected mice (Fig. 7D). Figures 7E and F demonstrate the proportion of Th1, Th17, and Treg cells in the spleen of P. berghei Anka-infected WT and AhR-KO mice.
DISCUSSION
AhR, a highly conserved receptor found in members of the animal kingdom from invertebrates to mammals, participates in a variety of physiological functions (35, 36, 54, 55). Recent studies have identified several endogenous ligands for AhR, suggesting that AhR not only is a master regulator of drug metabolism but also participates in many physiological functions, including cell growth, differentiation, vascular and hematopoietic development, and regulation of immune responses. To our knowledge, this is the first report demonstrating a role for AhR in the pathogenesis of severe malaria caused by P. berghei Anka infection. AhR-KO mice are more susceptible to P. berghei Anka infection, displaying early neurological signs such as ataxia, paralysis, seizure, rollover, and coma. These mice also display anemia, increased parasitemia, mortality, and significant pathological changes in the brain and liver compared with infected WT mice. P. berghei Anka-infected AhR-KO mice displayed increases in serum levels of both pro- and anti-inflammatory mediators, including IL-2, IL-17, IL-6, TNF-α, and IL-10, which are likely associated with the defects in the generation/expansion of T and Treg cells found in the thymus and spleen. These observations underscore a role for the AhR in the control of infection.
A successful response to malaria requires a timely and proportional release of proinflammatory cytokines to minimize parasitemia, while anti-inflammatory cytokines counteract the high level of inflammatory cytokines to control the inflammatory process and minimize the pathology (56). Plasmodium infection is associated with increased levels of cytokines in the blood and brain tissue (10, 56–58). The high levels of proinflammatory cytokines observed in P. berghei Anka-infected AhR-KO mice could induce vascular cell adhesion molecule expression, promoting recruitment of leukocytes to the brain microvasculature and thus contributing to neurovascular endothelial damage and BBB disruption (4, 9, 11, 17, 27, 28, 59).
Intravital microscopy demonstrated that a deficiency of AhR resulted in significant increases in infection-induced rolling and adherent cells in the pia mater vasculature, hemorrhagic foci, and the levels of NO in brain tissue. These may contribute to the disruption of the BBB observed at 6 dpi. These alterations in the BBB during CM could allow cytokines and malaria parasite antigens to enter the brain, from which they are normally excluded, leading to activation of microglia and/or damage to astrocytes (17). Basal levels of some inflammatory cytokines were detected in the brain of uninfected WT and AhR-KO mice, but the levels were markedly enhanced after disease induction. Low levels of cytokines in the brain under basal conditions have also been found in other studies and may reflect normal brain physiology. Indeed, cytokines such as TNF-α, IL-1β, and IL-6 not only are involved in the immune response to infection and/or stress but also have critical roles in normal brain functioning (60). For example, TNF-α is produced by the resident immune cells of the central nervous system, microglia and astrocytes (61), and is essential to maintenance of synaptic scaling, and therefore, it has a role in learning and memory formation (62). Whether cytokines exert physiological or pathological effects depends on their amount and on the spatial and temporal pattern of their production (63).
P. berghei Anka-infected AhR-KO mice displayed marked anemia and increased levels of iron in serum, whereas the infected WT mice were able to reestablish the physiological levels of iron in serum. Iron is required for the proliferation of Plasmodium, which then utilizes diverse mechanisms to acquire iron from hosts. Nevertheless, the precocious higher level of parasitemia found in the AhR-deficient mice could not only be attributed to increased iron, since it was significantly higher than that in WT mice only at 6 dpi. However, the liver damage observed in P. berghei Anka-infected AhR-KO mice could be responsible, in part, for the severe hemolysis observed in these mice, which could then generate free hemoglobin that, in the presence of reactive oxygen species (ROS), would readily be oxidized, releasing free heme, a molecule cytotoxic to the host and parasite (9, 18, 64, 65). Likewise, previous studies have shown that AhR has an endogenous role in the physiology and homeostasis of the liver, preventing the development of fibrosis. Mice lacking AhR had a variable degree of hepatic fibrosis (minimal to mild portal fibrosis) without an inflammatory stimulus. The development of severe hepatic fibrosis is common only in older AhR-KO mice (9 to 11 months of age) (66–68). Infected AhR-KO mice displayed increased serum levels of ALT as well as hemozoin deposits and inflammation and necrosis in the liver, indicating hepatic damage more severe than that in WT mice. The liver injury induced by P. berghei Anka appears to be caused by the inflammatory response driven by the accumulation of parasites in this organ (69). In fact, we observed a greater number of infiltrating CD4+ IFN-γ+ and CD4+ IL-10+ cells and a markedly enhanced number of CD4+ TNF-α+ cells in the liver of infected AhR-KO, suggesting the profile of the inflammatory response which contributed to the liver damage observed in these mice.
A balance between protective immunity and immune-mediated pathology is observed in CM. Recent studies have investigated the role of Treg cells in the regulation of the immune response against Plasmodium. Treg cells suppress the Th1 response, preventing Plasmodium elimination both in humans and in experimental models (31, 70). In P. berghei Anka infection, Treg cells contribute to pathogenesis by modulating the balance of pro- and anti-inflammatory responses (33). The combined induction of Treg and Th1 cells appears to follow a carefully orchestrated immune response, where insufficient or excessive induction of Treg cells at different time points during infection may disrupt this balance and contribute to severe disease (33, 71). P. berghei Anka-infected AhR-KO mice displayed increased levels of TGF-β in the brain (30, 71). In AhR-KO mice, there was a decrease in CD4+ CD25high FOXP3+ cells in the thymus and a higher frequency of CD4+ CD25high FOXP3+ cells in the brain compared with the findings for WT mice. While Treg cells have been involved in the regulation of the immune response to malaria, the precise mechanism by which Treg cells modulate cerebral pathology is still unclear. In different models of infection, Treg cells have been described to be important in the modulation of T cell responses (52, 54, 72). During CM, Treg cells participate in the modulation of T cell recruitment to the brain. CD8+ as well as CD4+ T cells have been shown to contribute to CM pathogenesis (30). CD8+ T cells accumulated in the brain just prior to the onset of signs and are believed to be the primary effectors of experimental CM (73). However, brain sequestration of CD8+ T cells is not sufficient for the development of CM in C57BL/6 mice, and the concomitant presence of parasitized red blood cells appears to play an important role in the onset of neuropathology (27, 28). Our results indicate that a deficiency of AhR does not alter the number of CD8+ T cells and CD4+ T cells accumulated in the brain but results in a higher frequency of Th17 and Treg cells.
The balance between Treg and Th17 cells is essential for immune homeostasis. IL-6, together with TGF-β, has an important role in Th17 cell generation (74), and our results indicate that AhR could participate in this process as a regulator of Th17 as well in Treg cell differentiation (37, 40, 54). TGF-β is necessary for the induction of both Th17 and Treg cells in naive CD4+ T cells. The choice of this response is controlled, in part, by IL-6, which prevents the expression of FOXP3 and initiates the Th17 profile (75). A deficiency of AhR in brain resulted in the increased levels of IL-6 and TGF-β. This change was associated with higher numbers of CD4+ IL-17+ cells in the brain and an induction of Th17 cell differentiation/IL-17 production during P. berghei Anka infection. However, one study using mice that failed to generate an IL-17 profile suggested no role for IL-17 in the development of CM (76). Our results demonstrate that in the absence of AhR, one can still find a generation/stimulation Th17 environment with a greater number of CD4+ IL-17+ cells infiltrated into brain and the production of higher levels of IL-17. In the liver of infected mice, the absence of AhR resulted in an increased Th1 response compared with that in WT mice. Therefore, the absence or reduction of AhR expression/activity during P. berghei Anka infection could be one reason for the inappropriate proportion of Th1/Th17/Treg cell responses. This suggests that the activation of AhR has an important role in the pathogenesis of the immune response.
Infected AhR-KO mice also displayed decreased levels of SOCS1 and SOCS3. These data suggest that during P. berghei Anka infection, IL-17 and IL-6 production in the brain could activate astrocytes, contributing to Th17 differentiation, a process modulated by SOCS3. To this end, a recent study demonstrated that astrocytes can serve as a target of Th17 cells and IL-17 in the central nervous system and that SOCS3 participates as a negative regulator of IL-17 functions in this tissue, reinforcing our hypothesis (77). Further experiments are under way in our laboratory to confirm this hypothesis in the setting of malaria.
Our data demonstrate that AhR activation modulates cytokine expression during murine P. berghei Anka infection. AhR has been regarded as a potential regulator of the immune response (35–37, 39, 54, 78, 79), playing various roles in the differentiation of Th cells, which are differently regulated, depending on their activating ligands (39, 40, 42, 55). For example, the tryptophan photoproduct 6-formylindolo[3,2-b]carbazole and kynurenine, endogenous AhR ligands, increase only the Th17 population (37, 40), while 2,3,7,8-tetrachlorodibenzo-p-dioxin, a high-affinity exogenous AhR ligand, appears to expand only the Treg cell population (preventing experimental autoimmune encephalomyelitis) (42). Additionally, we demonstrated that AhR expression is associated with modulation of the expression of SOCS, a well-known class of molecules contributing to the regulation of cytokine receptor signaling pathways important in the modulation of the immune response against infection (46, 52). In this regard, SOCS1 and SOCS3 expression counteracts the actions of IFN-γ and IL-6 (46, 52, 77), which play important roles in the pathogenesis of CM. Since there was a reduction in the expression of SOCS1 and SOCS3 in infected AhR-KO mice, one would expect that the inflammatory milieu found in the infected brain would have a greater ability to induce CM. However, the precise intracellular role of SOCS as a modulator of CM development still needs to be identified. Currently, we are conducting studies to clarify the role of AhR in the modulation of SOCS3 expression and its participation in the generation/differentiation of Th17 and Treg cells and the pathology resulting from P. berghei Anka infection.
In summary, the current report demonstrates the importance of AhR in the modulation of immune responses and development of disease during P. berghei Anka infection. AhR appears to contribute significantly to the control of parasite replication and the modulation of inflammatory responses, likely via controlling the expression of crucial pathways, such as the SOCS1 and SOCS3 pathways.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by CNPq (to F.S.M., A.L.T., M.M.T.), FAPEMIG (to F.S.M., A.L.T., M.M.T.), NIH grant AI-076248 (to H.B.T.), and the program INCT em Dengue (Brazil).
Footnotes
Published ahead of print 12 May 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.01733-14.
REFERENCES
- 1.WHO. 2011. Malaria report. WHO, Geneva, Switzerland. [Google Scholar]
- 2.de Souza JB, Hafalla JC, Riley EM, Couper KN. 2010. Cerebral malaria: why experimental murine models are required to understand the pathogenesis of disease. Parasitology 137:755–772. 10.1017/S0031182009991715. [DOI] [PubMed] [Google Scholar]
- 3.Schofield L, Grau GE. 2005. Immunological processes in malaria pathogenesis. Nat. Rev. Immunol. 5:722–735. 10.1038/nri1686. [DOI] [PubMed] [Google Scholar]
- 4.Cabrales P, Zanini GM, Meays D, Frangos JA, Carvalho LJ. 2010. Murine cerebral malaria is associated with a vasospasm-like microcirculatory dysfunction, and survival upon rescue treatment is markedly increased by nimodipine. Am. J. Pathol. 176:1306–1315. 10.2353/ajpath.2010.090691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Craig AG, Grau GE, Janse C, Kazura JW, Milner D, Barnwell JW, Turner G, Langhorne J. 2012. The role of animal models for research on severe malaria. PLoS Pathog. 8:e1002401. 10.1371/journal.ppat.1002401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hunt NH, Grau GE, Engwerda C, Barnum SR, van der Heyde H, Hansen DS, Schofield L, Golenser J. 2010. Murine cerebral malaria: the whole story. Trends Parasitol. 26:272–274. 10.1016/j.pt.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 7.Riley EM, Couper KN, Helmby H, Hafalla JC, de Souza JB, Langhorne J, Jarra WB, Zavala F. 2010. Neuropathogenesis of human and murine malaria. Trends Parasitol. 26:277–278. 10.1016/j.pt.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 8.Autino B, Corbett Y, Castelli F, Taramelli D. 2012. Pathogenesis of malaria in tissues and blood. Mediterr. J. Hematol. Infect. Dis. 4:e2012061. 10.4084/MJHID.2012.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haque A, Best SE, Amante FH, Ammerdorffer A, de Labastida F, Pereira T, Ramm GA, Engwerda CR. 2011. High parasite burdens cause liver damage in mice following Plasmodium berghei Anka infection independently of CD8(+) T cell-mediated immune pathology. Infect. Immun. 79:1882–1888. 10.1128/IAI.01210-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Desruisseaux MS, Machado FS, Weiss LM, Tanowitz HB, Golightly LM. 2010. Cerebral malaria: a vasculopathy. Am. J. Pathol. 176:1075–1078. 10.2353/ajpath.2010.091090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lacerda-Queiroz N, Rodrigues DH, Vilela MC, Rachid MA, Soriani FM, Sousa LP, Campos RD, Quesniaux VF, Teixeira MM, Teixeira AL. 2012. Platelet-activating factor receptor is essential for the development of experimental cerebral malaria. Am. J. Pathol. 180:246–255. 10.1016/j.ajpath.2011.09.038. [DOI] [PubMed] [Google Scholar]
- 12.Machado FS, Desruisseaux MS, Nagajyothi Kennan RP, Hetherington HP, Wittner M, Weiss LM, Lee SC, Scherer PE, Tsuji M, Tanowitz HB. 2006. Endothelin in a murine model of cerebral malaria. Exp. Biol. Med. (Maywood) 231:1176–1181. [PubMed] [Google Scholar]
- 13.Dai M, Freeman B, Bruno FP, Shikani HJ, Tanowitz HB, Weiss LM, Reznik SE, Stephani RA, Desruisseaux MS. 2012. The novel ETA receptor antagonist HJP-272 prevents cerebral microvascular hemorrhage in cerebral malaria and synergistically improves survival in combination with an artemisinin derivative. Life Sci. 91:687–692. 10.1016/j.lfs.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pamplona A, Hanscheid T, Epiphanio S, Mota MM, Vigario AM. 2009. Cerebral malaria and the hemolysis/methemoglobin/heme hypothesis: shedding new light on an old disease. Int. J. Biochem. Cell Biol. 41:711–716. 10.1016/j.biocel.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 15.van der Heyde HC, Nolan J, Combes V, Gramaglia I, Grau GE. 2006. A unified hypothesis for the genesis of cerebral malaria: sequestration, inflammation and hemostasis leading to microcirculatory dysfunction. Trends Parasitol. 22:503–508. 10.1016/j.pt.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 16.Gramaglia I, Sobolewski P, Meays D, Contreras R, Nolan JP, Frangos JA, Intaglietta M, van der Heyde HC. 2006. Low nitric oxide bioavailability contributes to the genesis of experimental cerebral malaria. Nat. Med. 12:1417–1422. 10.1038/nm1499. [DOI] [PubMed] [Google Scholar]
- 17.Hunt NH, Golenser J, Chan-Ling T, Parekh S, Rae C, Potter S, Medana IM, Miu J, Ball HJ. 2006. Immunopathogenesis of cerebral malaria. Int. J. Parasitol. 36:569–582. 10.1016/j.ijpara.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 18.Pamplona A, Ferreira A, Balla J, Jeney V, Balla G, Epiphanio S, Chora A, Rodrigues CD, Gregoire IP, Cunha-Rodrigues M, Portugal S, Soares MP, Mota MM. 2007. Heme oxygenase-1 and carbon monoxide suppress the pathogenesis of experimental cerebral malaria. Nat. Med. 13:703–710. 10.1038/nm1586. [DOI] [PubMed] [Google Scholar]
- 19.Martins YC, Carvalho LJ, Daniel-Ribeiro CT. 2009. Challenges in the determination of early predictors of cerebral malaria: lessons from the human disease and the experimental murine models. Neuroimmunomodulation 16:134–145. 10.1159/000180268. [DOI] [PubMed] [Google Scholar]
- 20.Cabrales P, Zanini GM, Meays D, Frangos JA, Carvalho LJ. 2011. Nitric oxide protection against murine cerebral malaria is associated with improved cerebral microcirculatory physiology. J. Infect. Dis. 203:1454–1463. 10.1093/infdis/jir058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garcia CC, Guabiraba R, Soriani FM, Teixeira MM. 2010. The development of anti-inflammatory drugs for infectious diseases. Discov. Med. 10:479–488. [PubMed] [Google Scholar]
- 22.Gimenez F, Barraud de Lagerie S, Fernandez C, Pino P, Mazier D. 2003. Tumor necrosis factor alpha in the pathogenesis of cerebral malaria. Cell. Mol. Life Sci. 60:1623–1635. 10.1007/s00018-003-2347-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walther M, Jeffries D, Finney OC, Njie M, Ebonyi A, Deininger S, Lawrence E, Ngwa-Amambua A, Jayasooriya S, Cheeseman IH, Gomez-Escobar N, Okebe J, Conway DJ, Riley EM. 2009. Distinct roles for FOXP3 and FOXP3 CD4 T cells in regulating cellular immunity to uncomplicated and severe Plasmodium falciparum malaria. PLoS Pathog. 5:e1000364. 10.1371/journal.ppat.1000364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haldar K, Murphy SC, Milner DA, Taylor TE. 2007. Malaria: mechanisms of erythrocytic infection and pathological correlates of severe disease. Annu. Rev. Pathol. 2:217–249. 10.1146/annurev.pathol.2.010506.091913. [DOI] [PubMed] [Google Scholar]
- 25.Malaguarnera L, Musumeci S. 2002. The immune response to Plasmodium falciparum malaria. Lancet Infect. Dis. 2:472–478. 10.1016/S1473-3099(02)00344-4. [DOI] [PubMed] [Google Scholar]
- 26.Belnoue E, Kayibanda M, Vigario AM, Deschemin JC, van Rooijen N, Viguier M, Snounou G, Renia L. 2002. On the pathogenic role of brain-sequestered alphabeta CD8+ T cells in experimental cerebral malaria. J. Immunol. 169:6369–6375. 10.4049/jimmunol.169.11.6369. [DOI] [PubMed] [Google Scholar]
- 27.Baptista FG, Pamplona A, Pena AC, Mota MM, Pied S, Vigario AM. 2010. Accumulation of Plasmodium berghei-infected red blood cells in the brain is crucial for the development of cerebral malaria in mice. Infect. Immun. 78:4033–4039. 10.1128/IAI.00079-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McQuillan JA, Mitchell AJ, Ho YF, Combes V, Ball HJ, Golenser J, Grau GE, Hunt NH. 2011. Coincident parasite and CD8 T cell sequestration is required for development of experimental cerebral malaria. Int. J. Parasitol. 41:155–163. 10.1016/j.ijpara.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 29.Belkaid Y, Rouse BT. 2005. Natural regulatory T cells in infectious disease. Nat. Immunol. 6:353–360. 10.1038/nrm1638. [DOI] [PubMed] [Google Scholar]
- 30.Amante FH, Stanley AC, Randall LM, Zhou Y, Haque A, McSweeney K, Waters AP, Janse CJ, Good MF, Hill GR, Engwerda CR. 2007. A role for natural regulatory T cells in the pathogenesis of experimental cerebral malaria. Am. J. Pathol. 171:548–559. 10.2353/ajpath.2007.061033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hisaeda H, Maekawa Y, Iwakawa D, Okada H, Himeno K, Kishihara K, Tsukumo S, Yasutomo K. 2004. Escape of malaria parasites from host immunity requires CD4+ CD25+ regulatory T cells. Nat. Med. 10:29–30. 10.1038/nm975. [DOI] [PubMed] [Google Scholar]
- 32.Steeg C, Adler G, Sparwasser T, Fleischer B, Jacobs T. 2009. Limited role of CD4+Foxp3+ regulatory T cells in the control of experimental cerebral malaria. J. Immunol. 183:7014–7022. 10.4049/jimmunol.0901422. [DOI] [PubMed] [Google Scholar]
- 33.Wu JJ, Chen G, Liu J, Wang T, Zheng W, Cao YM. 2010. Natural regulatory T cells mediate the development of cerebral malaria by modifying the pro-inflammatory response. Parasitol. Int. 59:232–241. 10.1016/j.parint.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 34.Denison MS, Nagy SR. 2003. Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Annu. Rev. Pharmacol. Toxicol. 43:309–334. 10.1146/annurev.pharmtox.43.100901.135828. [DOI] [PubMed] [Google Scholar]
- 35.Stockinger B, Hirota K, Duarte J, Veldhoen M. 2011. External influences on the immune system via activation of the aryl hydrocarbon receptor. Semin. Immunol. 23:99–105. 10.1016/j.smim.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 36.Esser C, Rannug A, Stockinger B. 2009. The aryl hydrocarbon receptor in immunity. Trends Immunol. 30:447–454. 10.1016/j.it.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 37.Mezrich JD, Fechner JH, Zhang X, Johnson BP, Burlingham WJ, Bradfield CA. 2010. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J. Immunol. 185:3190–3198. 10.4049/jimmunol.0903670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fernandez-Salguero PM. 2010. A remarkable new target gene for the dioxin receptor: the Vav3 proto-oncogene links AhR to adhesion and migration. Cell Adh. Migr. 4:172–175. 10.4161/cam.4.2.10387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Veldhoen M, Duarte JH. 2010. The aryl hydrocarbon receptor: fine-tuning the immune-response. Curr. Opin. Immunol. 22:747–752. 10.1016/j.coi.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 40.Quintana FJ, Basso AS, Iglesias AH, Korn T, Farez MF, Bettelli E, Caccamo M, Oukka M, Weiner HL. 2008. Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature 453:65–71. 10.1038/nature06880. [DOI] [PubMed] [Google Scholar]
- 41.Kimura A, Naka T, Nohara K, Fujii-Kuriyama Y, Kishimoto T. 2008. Aryl hydrocarbon receptor regulates Stat1 activation and participates in the development of Th17 cells. Proc. Natl. Acad. Sci. U. S. A. 105:9721–9726. 10.1073/pnas.0804231105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Veldhoen M, Hirota K, Westendorf AM, Buer J, Dumoutier L, Renauld JC, Stockinger B. 2008. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature 453:106–109. 10.1038/nature06881. [DOI] [PubMed] [Google Scholar]
- 43.Sanchez Y, de Dios Rosado J, Vega L, Elizondo G, Estrada-Muniz E, Saavedra R, Juarez I, Rodriguez-Sosa M. 2010. The unexpected role for the aryl hydrocarbon receptor on susceptibility to experimental toxoplasmosis. J. Biomed. Biotechnol. 2010:505694. 10.1155/2010/505694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shi LZ, Faith NG, Nakayama Y, Suresh M, Steinberg H, Czuprynski CJ. 2007. The aryl hydrocarbon receptor is required for optimal resistance to Listeria monocytogenes infection in mice. J. Immunol. 179:6952–6962. 10.4049/jimmunol.179.10.6952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Veiga-Parga T, Suryawanshi A, Rouse BT. 2011. Controlling viral immuno-inflammatory lesions by modulating aryl hydrocarbon receptor signaling. PLoS Pathog. 7:e1002427. 10.1371/journal.ppat.1002427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Machado FS, Johndrow JE, Esper L, Dias A, Bafica A, Serhan CN, Aliberti J. 2006. Anti-inflammatory actions of lipoxin A4 and aspirin-triggered lipoxin are SOCS-2 dependent. Nat. Med. 12:330–334. 10.1038/nm1355. [DOI] [PubMed] [Google Scholar]
- 47.National Research Council. 2011. Guide for the care and use of laboratory animals, 8th ed. National Academies Press, Washington, DC. [Google Scholar]
- 48.Neres R, Marinho CR, Goncalves LA, Catarino MB, Penha-Goncalves C. 2008. Pregnancy outcome and placenta pathology in Plasmodium berghei Anka infected mice reproduce the pathogenesis of severe malaria in pregnant women. PLoS One 3:e1608. 10.1371/journal.pone.0001608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Carroll RW, Wainwright MS, Kim KY, Kidambi T, Gomez ND, Taylor T, Haldar K. 2010. A rapid murine coma and behavior scale for quantitative assessment of murine cerebral malaria. PLoS One 5:e13124. 10.1371/journal.pone.0013124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tjandra K, Sharkey KA, Swain MG. 2000. Progressive development of a Th1-type hepatic cytokine profile in rats with experimental cholangitis. Hepatology 31:280–290. 10.1002/hep.510310204. [DOI] [PubMed] [Google Scholar]
- 51.Lund AK, Agbor LN, Zhang N, Baker A, Zhao H, Fink GD, Kanagy NL, Walker MK. 2008. Loss of the aryl hydrocarbon receptor induces hypoxemia, endothelin-1, and systemic hypertension at modest altitude. Hypertension 51:803–809. 10.1161/HYPERTENSIONAHA.107.100586. [DOI] [PubMed] [Google Scholar]
- 52.Esper L, Roman-Campos D, Lara A, Brant F, Castro LL, Barroso A, Araujo RR, Vieira LQ, Mukherjee S, Gomes ER, Rocha NN, Ramos IP, Lisanti MP, Campos CF, Arantes RM, Guatimosim S, Weiss LM, Cruz JS, Tanowitz HB, Teixeira MM, Machado FS. 2012. Role of SOCS2 in modulating heart damage and function in a murine model of acute Chagas disease. Am. J. Pathol. 181:130–140. 10.1016/j.ajpath.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mukherjee S, Machado FS, Huang H, Oz HS, Jelicks LA, Prado CM, Koba W, Fine EJ, Zhao D, Factor SM, Collado JE, Weiss LM, Tanowitz HB, Ashton AW. 2011. Aspirin treatment of mice infected with Trypanosoma cruzi and implications for the pathogenesis of Chagas disease. PLoS One 6:e16959. 10.1371/journal.pone.0016959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gandhi R, Kumar D, Burns EJ, Nadeau M, Dake B, Laroni A, Kozoriz D, Weiner HL, Quintana FJ. 2010. Activation of the aryl hydrocarbon receptor induces human type 1 regulatory T cell-like and Foxp3(+) regulatory T cells. Nat. Immunol. 11:846–853. 10.1038/ni.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Veldhoen M. 2010. A toxin-sensitive receptor able to reduce immunopathology. Nat. Immunol. 11:779–781. 10.1038/ni0910-779. [DOI] [PubMed] [Google Scholar]
- 56.Bakir HY, Tomiyama C, Abo T. 2011. Cytokine profile of murine malaria: stage-related production of inflammatory and anti-inflammatory cytokines. Biomed. Res. 32:203–208. 10.2220/biomedres.32.203. [DOI] [PubMed] [Google Scholar]
- 57.de Souza JB, Riley EM. 2002. Cerebral malaria: the contribution of studies in animal models to our understanding of immunopathogenesis. Microbes Infect. 4:291–300. 10.1016/S1286-4579(02)01541-1. [DOI] [PubMed] [Google Scholar]
- 58.Hunt NH, Grau GE. 2003. Cytokines: accelerators and brakes in the pathogenesis of cerebral malaria. Trends Immunol. 24:491–499. 10.1016/S1471-4906(03)00229-1. [DOI] [PubMed] [Google Scholar]
- 59.Combes V, Coltel N, Faille D, Wassmer SC, Grau GE. 2006. Cerebral malaria: role of microparticles and platelets in alterations of the blood-brain barrier. Int. J. Parasitol. 36:541–546. 10.1016/j.ijpara.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 60.Ransohoff RM, Brown MA. 2012. Innate immunity in the central nervous system. J. Clin. Invest. 122:1164–1171. 10.1172/JCI58644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McCoy MK, Tansey MG. 2008. TNF signaling inhibition in the CNS: implications for normal brain function and neurodegenerative disease. J. Neuroinflammation 5:45. 10.1186/1742-2094-5-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Camara ML, Corrigan F, Jaehne EJ, Jawahar MC, Anscomb H, Koerner H, Baune BT. 2013. TNF-alpha and its receptors modulate complex behaviours and neurotrophins in transgenic mice. Psychoneuroendocrinology 38:3102–3114. 10.1016/j.psyneuen.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 63.Arisi GM. 14 February 2014. Nervous and immune systems signals and connections: cytokines in hippocampus physiology and pathology. Epilepsy Behav. 10.1016/j.yebeh.2014.01.017. [DOI] [PubMed] [Google Scholar]
- 64.Epiphanio S, Mikolajczak SA, Goncalves LA, Pamplona A, Portugal S, Albuquerque S, Goldberg M, Rebelo S, Anderson DG, Akinc A, Vornlocher HP, Kappe SH, Soares MP, Mota MM. 2008. Heme oxygenase-1 is an anti-inflammatory host factor that promotes murine Plasmodium liver infection. Cell Host Microbe 3:331–338. 10.1016/j.chom.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 65.Haque A, Engwerda CR. 2011. An antioxidant link between sickle cell disease and severe malaria. Cell 145:335–336. 10.1016/j.cell.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 66.Corchero J, Martin-Partido G, Dallas SL, Fernandez-Salguero PM. 2004. Liver portal fibrosis in dioxin receptor-null mice that overexpress the latent transforming growth factor-beta-binding protein-1. Int. J. Exp. Pathol. 85:295–302. 10.1111/j.0959-9673.2004.00397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fernandez-Salguero P, Pineau T, Hilbert DM, McPhail T, Lee SS, Kimura S, Nebert DW, Rudikoff S, Ward JM, Gonzalez FJ. 1995. Immune system impairment and hepatic fibrosis in mice lacking the dioxin-binding Ah receptor. Science 268:722–726. 10.1126/science.7732381. [DOI] [PubMed] [Google Scholar]
- 68.Fernandez-Salguero PM, Ward JM, Sundberg JP, Gonzalez FJ. 1997. Lesions of aryl-hydrocarbon receptor-deficient mice. Vet. Pathol. 34:605–614. 10.1177/030098589703400609. [DOI] [PubMed] [Google Scholar]
- 69.Amante FH, Haque A, Stanley AC, Rivera de Labastida F, Randall LM, Wilson YA, Yeo G, Pieper C, Crabb BS, de Koning-Ward TF, Lundie RJ, Good MF, Pinzon-Charry A, Pearson MS, Duke MG, McManus DP, Loukas A, Hill GR, Engwerda CR. 2010. Immune-mediated mechanisms of parasite tissue sequestration during experimental cerebral malaria. J. Immunol. 185:3632–3642. 10.4049/jimmunol.1000944. [DOI] [PubMed] [Google Scholar]
- 70.Vigario AM, Gorgette O, Dujardin HC, Cruz T, Cazenave PA, Six A, Bandeira A, Pied S. 2007. Regulatory CD4+ CD25+ Foxp3+ T cells expand during experimental Plasmodium infection but do not prevent cerebral malaria. Int. J. Parasitol. 37:963–973. 10.1016/j.ijpara.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 71.Scholzen A, Minigo G, Plebanski M. 2010. Heroes or villains? T regulatory cells in malaria infection. Trends Parasitol. 26:16–25. 10.1016/j.pt.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 72.Josefowicz SZ, Lu LF, Rudensky AY. 2012. Regulatory T cells: mechanisms of differentiation and function. Annu. Rev. Immunol. 30:531–564. 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Claser C, Malleret B, Gun SY, Wong AY, Chang ZW, Teo P, See PC, Howland SW, Ginhoux F, Renia L. 2011. CD8+ T cells and IFN-gamma mediate the time-dependent accumulation of infected red blood cells in deep organs during experimental cerebral malaria. PLoS One 6:e18720. 10.1371/journal.pone.0018720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kimura A, Kishimoto T. 2011. Th17 cells in inflammation. Int. Immunopharmacol. 11:319–322. 10.1016/j.intimp.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 75.Hirota K, Duarte JH, Veldhoen M, Hornsby E, Li Y, Cua DJ, Ahlfors H, Wilhelm C, Tolaini M, Menzel U, Garefalaki A, Potocnik AJ, Stockinger B. 2011. Fate mapping of IL-17-producing T cells in inflammatory responses. Nat. Immunol. 12:255–263. 10.1038/ni.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Villegas-Mendez A, Greig R, Shaw TN, de Souza JB, Gwyer Findlay E, Stumhofer JS, Hafalla JC, Blount DG, Hunter CA, Riley EM, Couper KN. 2012. IFN-gamma-producing CD4+ T cells promote experimental cerebral malaria by modulating CD8+ T cell accumulation within the brain. J. Immunol. 189:968–979. 10.4049/jimmunol.1200688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ma X, Reynolds SL, Baker BJ, Li X, Benveniste EN, Qin H. 2010. IL-17 enhancement of the IL-6 signaling cascade in astrocytes. J. Immunol. 184:4898–4906. 10.4049/jimmunol.1000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Esser C. 2009. The immune phenotype of AhR null mouse mutants: not a simple mirror of xenobiotic receptor over-activation. Biochem. Pharmacol. 77:597–607. 10.1016/j.bcp.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 79.Stevens EA, Mezrich JD, Bradfield CA. 2009. The aryl hydrocarbon receptor: a perspective on potential roles in the immune system. Immunology 127:299–311. 10.1111/j.1365-2567.2009.03054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.