Abstract
In patients infected with the fungus Aspergillus fumigatus, Th1 responses are considered protective, while Th2 responses are associated with increased morbidity and mortality. How host-pathogen interactions influence the development of these protective or detrimental immune responses is not clear. We compared lung immune responses to conidia from two fungal isolates that expressed different levels of the fungal cell wall component chitin. We observed that repeated aspirations of the high-chitin-expressing isolate Af5517 induced increased airway eosinophilia in the lungs of recipient mice compared to the level of eosinophilia induced by isolate Af293. CD4+ T cells in the bronchoalveolar lavage fluid (BALF) of Af5517-aspirated mice displayed decreased gamma interferon secretion and increased interleukin-4 transcription. In addition, repeated aspirations of Af5517 induced lung transcription of the Th2-associated chemokines CCL11 (eotaxin-1) and CCL22 (macrophage-derived chemokine). Eosinophil recruitment in response to conidial aspiration was correlated with the level of chitin exposure during germination and was decreased by constitutive lung chitinase expression. Moreover, eosinophil-deficient mice subjected to multiple aspirations of Af5517 prior to neutrophil depletion and infection exhibited decreased morbidity and fungal burden compared to the levels of morbidity and fungal burden found in wild-type mice. These results suggest that exposure of chitin in germinating conidia promotes eosinophil recruitment and ultimately induces Th2-skewed immune responses after repeated aspiration. Furthermore, our results suggest that eosinophils should be examined as a potential therapeutic target in patients that mount poorly protective Th2 responses to A. fumigatus infection.
INTRODUCTION
Immune-deficient individuals are at risk for developing disseminated fungal infections that cause significant morbidity and mortality, and the risk has increased concurrently with immune-suppressive or myeloablative therapies (1). As a result, interest in understanding the mechanisms of immune protection from infection has also increased. As with several other fungal pathogens, T helper type 1 (Th1) responses to the human pathogen Aspergillus fumigatus are considered protective, while Th2 responses increase morbidity and mortality (2–4). Th1 responses to fungi are generated by dendritic cell (DC) recognition of fungal pathogen-associated molecular patterns (PAMPs) by Toll-like and c-type lectin receptors, resulting in DC production of the Th1-promoting cytokine interleukin-12 (IL-12) (4). However, how host-pathogen interactions drive protective or detrimental responses is less clear. In dormant A. fumigatus conidia, fungal PAMPs are hidden from immune recognition behind an inert hydrophobic rodlet layer (5). As conidia swell and germinate, immune-stimulatory molecules, such as β-glucan, become exposed (6). Immune recognition of β-glucan provides protection from A. fumigatus infection (7) and promotes Th17 responses (8), which may either promote or inhibit immune protection (7, 9). Chitin, another critical cell wall component, forms microfibrils that are covalently attached to β-(1,3)-glucan in the cell walls of almost all fungi (10). The immune response to chitin appears to be complex and depends on several factors, such as the size of the chitin particles and the presence or timing of allergic sensitization in experimental animals (11–14). Purified chitin or Aspergillus-derived mycelial extracts containing chitin mediated lung eosinophil recruitment in laboratory mice; however, recruitment was decreased in the presence of constitutive lung expression of acidic mammalian chitinase (AMCase) (12, 14). Although these results also suggest a role for chitin in directing immune responses to chitin-containing pathogens, immune recognition of chitin in models of infection or in response to Aspergillus conidia has not been examined.
Eosinophils are antimicrobial effector cells that are recruited in response to parasitic helminths and contribute to pathology in asthma and allergy (15). Recruitment of eosinophils is mediated by Th2-associated cytokines and chemokines, including IL-5 and CCL11 (eotaxin-1) (16–18). Treatment of asthmatic patients with anti-IL-5 resulted in decreased eosinophil recruitment and decreased the frequency of exacerbations in a subset of patients (19). In sensitized mice deficient in IL-5 or eosinophils, allergic inflammation was significantly decreased (16, 20). Eosinophil recruitment in response to lung exposure to A. fumigatus was dependent on IL-5 and IL-17 (21, 22). Although eosinophils are also recruited to the lungs in a mouse model of invasive pulmonary aspergillosis (IPA) (23) and may play a protective role in immunocompetent mice through direct killing of fungi (24, 25), the role of eosinophils in Th2-mediated host pathology in invasive infection has not been examined.
In order to develop the most effective therapy for patients that respond poorly to A. fumigatus infection, the roles of cell wall components that have the potential to limit or inhibit protective immunity require investigation. We have observed increased cell wall chitin in an environmental isolate (Af5517) of A. fumigatus compared to the level in other clinical and environmental isolates. Identification of a high-chitin-expressing isolate provided the opportunity to study the role of chitin in eosinophil recruitment and the pathological role of these cells in A. fumigatus infection. In this study, we report that lung immune responses to repeated aspiration of Af5517 conidia are Th2 skewed in comparison to the response to clinical isolate Af293. We also observed that exposure to the chitin in germinating conidia promotes eosinophil recruitment and the absence of these cells results in decreased morbidity and fungal burdens in neutropenic Af5517-infected mice.
MATERIALS AND METHODS
Growth and handling of fungi.
The clinical isolate Af293 was obtained from the Fungal Genetics Stock Center, and the environmental isolate NRRL5517 (Af5517) was obtained from the United States Agricultural Research Service. Fungi were grown on malt extract agar (MEA) plates at 22°C or 37°C, where specified, and fungal conidia were isolated from plates using glass beads as previously described (26). In some experiments, conidia were allowed to germinate in RPMI 1640 (Life Technologies) for 5 or 24 h and then fixed for 4 to 8 h in BD Cytofix buffer (BD Biosciences) (5). Chitin exposure was assessed by staining with 1:500 to 1:1,000 wheat germ agglutinin (WGA)-fluorescein isothiocyanate (FITC; Invitrogen) for 1 h in fluorescence-activated cell sorter (FACS) buffer with 10% rat serum on ice in the dark.
Mouse aspiration and sacrifice.
BALB/c or C57BL/6 (B6) mice were obtained from Harlan Laboratories. Eosinophil-deficient (ΔdblGATA) mice (BALB/c mouse background) (27) were obtained from The Jackson Laboratory. SPAM and 4get mice (BALB/c mouse background) were generated previously (12, 28). Conidial suspensions were delivered by involuntary aspiration of 2 × 106 conidia for single or repeated aspirations (Fig. 1A). Mice were sacrificed 2 to 3 days after the final challenge, as previously described (26), or were continuously monitored for disease progression until they were moribund or at 10 days postinfection. All animal procedures were approved by the Animal Care and Use Committee of Indiana State University.
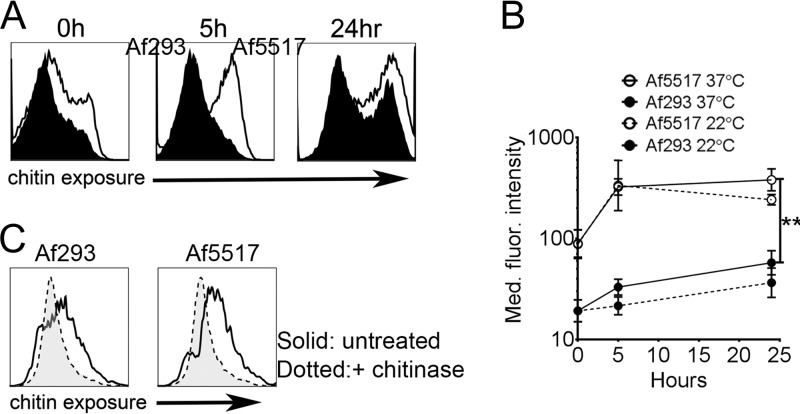
FIG 1.
Surface chitin exposure during germination is increased in isolate Af5517 in comparison to that in Af293. (A) Conidia were left dormant or were germinated at 37°C for 5 or 24 h and then analyzed by flow cytometry for changes in binding of WGA-FITC. Representative histograms from three independent experiments are depicted. (B) Summary of the results of three experiments showing changes in the median fluorescence (Med. fluor.) intensity of WGA-FITC binding at 0, 5, and 24 h at 22°C (dotted lines) and 37°C (solid lines). The differences at 5 and 24 h at 37°C were statistically significant. (C) Conidia were allowed to germinate in the presence or absence of chitinase for 24 h and then stained with WGA-FITC. **, P < 0.01.
Flow cytometric analysis of BALF cells.
Bronchoalveolar lavage fluid (BALF) cell composition was determined by flow cytometric analysis of recovered lavage cells in suspension as previously described (26). All flow cytometry reagents were obtained from BD Biosciences or eBioscience, unless otherwise specified. Populations of cells were evaluated by flow cytometric analysis on a Guava EasyCyte 8HT benchtop flow cytometer (EMD Millipore). Neutrophils were defined as CD45hi Ly6G+, eosinophils were defined as CD45hi Ly6G− CD11c− SiglecF+, and alveolar macrophages were defined as CD45hi Ly6G− CD11c+ SiglecF+. CD4+ T cells were defined on the basis of forward and side scatter and surface CD4 expression. The number of cytokine-producing CD4+ T cells was determined by fluorescent intracellular cytokine staining (ICS) with antibodies specific for gamma interferon (IFN-γ) and IL-17A. CD4+ T cells that were positive for the IL-4 transcript were identified by use of IL-4–green fluorescent protein (GFP) reporter mice (4get) in combination with surface CD4 staining (28).
Analysis of lung cytokine transcription using quantitative reverse transcription-PCR (RT-PCR).
All reagents for analysis of lung cytokine transcription were purchased from SA Biosciences, unless specified otherwise. Mouse lungs were perfused, removed, and homogenized in Tri Reagent 3 days after the final aspiration (Sigma-Aldrich). Total RNA was extracted from lung tissue and purified using an RNA tissue prep kit (Qiagen). Lung mRNA was transcribed into cDNA, and the gene expression level was analyzed with cytokine-specific primers using a custom cytokine array kit (SA Biosciences).
Mouse model of invasive pulmonary aspergillosis.
Neutropenic BALB/c or ΔdblGATA mice were infected with 5 × 106 or 1 × 107 A. fumigatus isolate Af5517 conidia using a protocol based on well-established models of IPA (29, 30). Briefly, mice (Fig. 1A) were depleted of neutrophils using 0.5 mg of anti-Ly6G (1A8 [31]; Bio-X-Cell) 1 day before and after infection. Neutrophil depletion was verified by flow cytometric analysis of peripheral blood. Infected mice were monitored for changes in disease using a five-point scale: 0, healthy; 1, minimal disease (e.g., ruffled fur); 2, moderate disease (e.g., ungroomed, hunched); 3, severe disease (e.g., severely hunched, changes in eye color, low motility); and 4, moribund or deceased. In an independent assessment, disease scores were found to be correlated with weight loss. Mice were sacrificed when they were determined to be moribund. Some mice were sacrificed 3 days after infection to assess the lung fungal burden by measuring the fungal 18S rDNA content by quantitative PCR as previously described (32, 33) using an Mx3005p quantitative PCR (qPCR) system (Agilent Technologies). All qPCR samples were run in triplicate with at least 5 technical replicates. For histological analysis, mice were sacrificed at 3 days postinfection and the lungs were perfused with 5 ml of saline and then 5 ml of 10% formalin-buffered saline followed by inflation using a tracheal catheter insertion and injection of 1 ml of formalin-buffered saline. The lungs were removed and fixed overnight in formalin-buffered saline, followed by tissue embedding, processing, and staining of sections with hematoxylin-eosin (H&E) and Gomori's methenamine silver (GMS) stains. All histological sample processing and staining were performed by the Terre Haute Regional Hospital Pathology Laboratory.
Data analysis methods.
Analysis of flow cytometric samples was performed with FlowJo software (TreeStar). Prism software (GraphPad) was used for generation of graphs and for statistical analyses. Unpaired t tests were used to measure statistical significance, and those that resulted in a P value of less than 0.05 were considered significant. When results for more than two groups and/or time points were analyzed, one- or two-way analyses of variance (ANOVAs) were used, along with Tukey's or Holm-Sidak's posttest for multiple comparisons, respectively. For quantification of fungal growth using histological sections, the mean of four representative fields of GMS-positive (GMS+) staining for each sample was calculated using ImageJ software (National Institutes of Health).
RESULTS
Surface chitin exposure is increased in A. fumigatus isolate Af5517.
In previous studies, chitin-mediated lung eosinophil recruitment was induced in response to chitin beads or extracts derived from Aspergillus mycelia (12, 14). However, the role of chitin in shaping immune responses to conidia is still unclear. Surface exposure of chitin is likely temporal and similar to that of β-glucan, and, thus, chitin is masked behind a hydrophobic rodlet layer in dormant conidia and is exposed during swelling of the conidia and subsequent germling formation (5, 6, 34). To examine the relationship between fungal cell wall chitin exposure and eosinophil recruitment, we used FITC-conjugated wheat germ agglutinin as a chitin-specific probe to compare changes in exposure during germination. At 0, 5, and 24 h, chitin exposure was increased in Af5517 conidia compared to that in Af293 conidia (Fig. 1A and B). When conidia were allowed to germinate in the presence of chitinase, surface staining was decreased in both isolates compared to that in the untreated controls (Fig. 1C), thus demonstrating the specificity of the chitin-binding probe and the sensitivity of surface chitin to chitinase-mediated degradation.
Increased airway eosinophil recruitment and altered IFN-γ/IL-4 expression in response to repeated aspiration of A. fumigatus isolate Af5517.
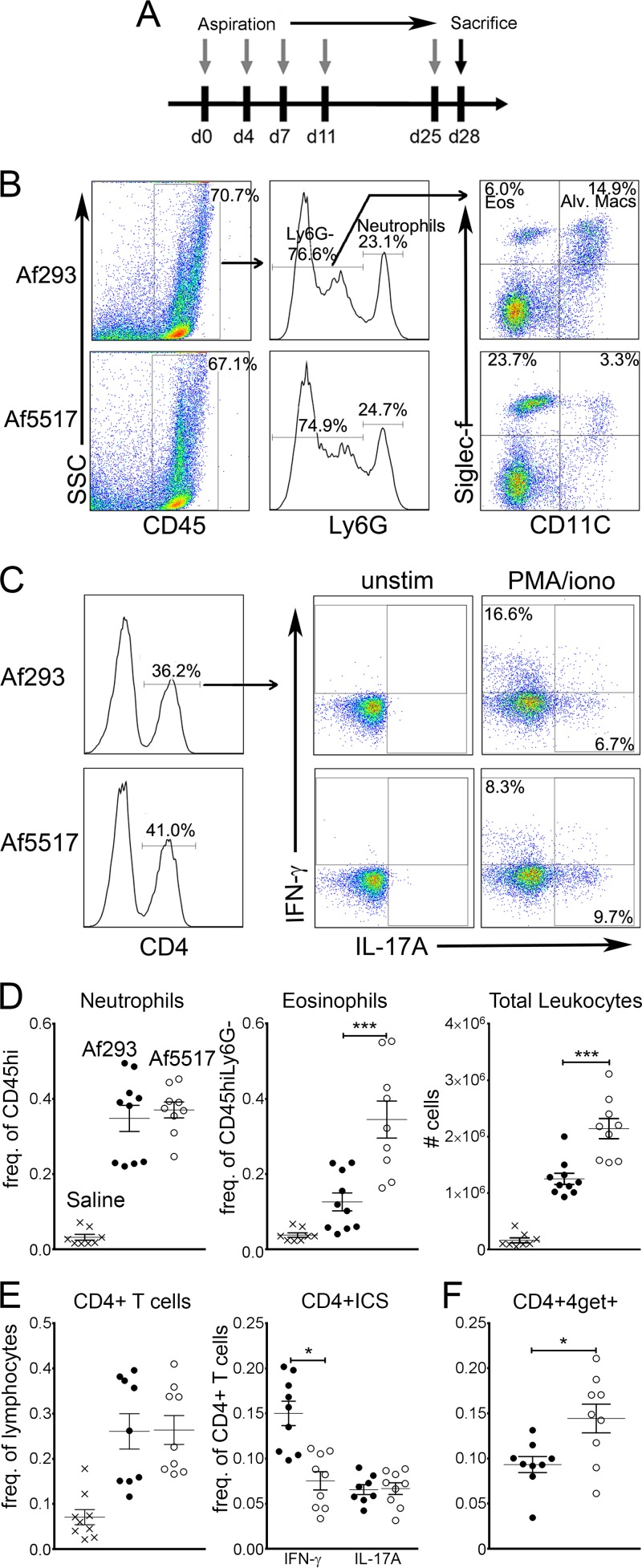
We used isolates Af293 and Af5517 to compare airway immune responses to multiple aspirations of conidia from low- and high-chitin-expressing strains of A. fumigatus in BALB/c mice (the schedule of aspiration is shown in Fig. 2A). Three days after the final aspiration, we observed a marked increase in airway (BALF) eosinophils in response to Af5517 compared to that in response to Af293 (Fig. 2B and D). In contrast, neutrophil recruitment was equivalent in response to both isolates. Due to infiltration of inflammatory cells, the frequency of alveolar macrophages was decreased in response to both isolates compared to that for the saline-treated controls, although Af5517-aspirated mice displayed a greater decrease (Fig. 2B and data not shown). The frequency of lung CD4 T cells and IL-17A-producing CD4 T cells was equivalent in response to each of the isolates (Fig. 2C and E). However, IFN-γ-producing CD4 T cells were significantly decreased in response to Af5517. In addition to the differences in BALF cells that we observed, similar differences in eosinophil and CD4+ IFN-γ-positive cell recruitment were observed in lung homogenates (see Fig. S1A and B in the supplemental material). Although we were unable to detect IL-4-producing airway CD4+ T cells in Af293- or Af5517-aspirated mice (data not shown), we did observe an increase in IL-4 reporter-expressing CD4+ T cells in response to Af5517 (Fig. 2F). IL-4 reporter mice (4get) were generated previously and have been used to enumerate IL-4-competent or Th2-skewed cells that do not necessarily secrete high levels of cytokine (28, 35). Despite the increase in IL-4 expression in CD4+ T cells, total IgE was not increased in response to Af5517 (see Fig. S1C in the supplemental material). These results indicate that repeated aspiration of Af5517 conidia is characterized by increased eosinophilia and a Th2-skewed IFN-γ/IL-4 expression profile compared to the findings obtained after repeated aspiration of Af293 conidia.
FIG 2.
Increased airway eosinophil recruitment and concomitant Th1/Th2 shift in response to repeated aspiration of A. fumigatus isolate Af5517 by BALB/c mice. BALF was harvested 72 h after the final aspiration. (A) Schedule of repeated aspiration of A. fumigatus conidia. d, day. (B) Flow cytometric analysis of airway leukocytes in BALB/c mice that repeatedly aspirated a low-chitin-expressing (Af293) or high-chitin-expressing (Af5517) isolate of A. fumigatus. Results for representative samples are depicted, with arrows showing parent and daughter gating of cell populations. SSC, side scatter; Eos, eosinophils; Alv. Macs, alveolar macrophages. (C) Representative FACS plots of intracellular staining of airway CD4+ T cells for IFN-γ and IL-17A. BALF cells were left unstimulated (unstim) or were stimulated for 4 h at 37°C with phorbol myristate acetate-ionomycin (PMA/iono) to stimulate cytokine production. (D) Total number of leukocytes and frequency of airway neutrophils and eosinophils in mice that repeatedly aspirated saline, Af293 conidia, or Af5517 conidia. (E) Frequency (Freq.) of airway cytokine-producing CD4 T cells. (F) Frequency of GFP-positive (IL-4 transcript-positive) CD4 T cells from 4get mice that repeatedly aspirated Af293 or Af5517 conidia. The data in panels D to F are shown as a summary of the data from two experiments. *, P < 0.05; ***, P < 0.001.
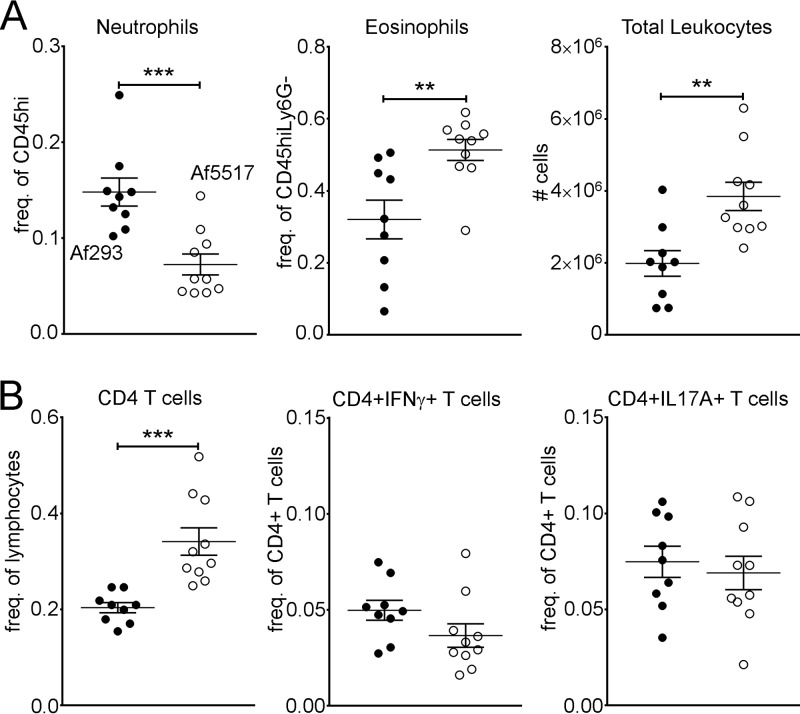
In response to repeated aspiration of A. fumigatus conidia, BALB/c and B6 mice exhibited mouse strain-dependent differences characterized by increased airway eosinophils in B6 mice and increased neutrophils in BALB/c mice (36). To examine the mouse strain-dependent responses in our model, we also compared the airway responses to repeated aspiration of Af293 or Af5517 conidia in B6 mice. As with the BALB/c strain, B6 mice exhibited a marked increase in airway eosinophil recruitment after repeated Af5517 aspiration (Fig. 3A, middle). However, in contrast to the response in BALB/c mice, increased eosinophilia was accompanied by decreases in neutrophils (Fig. 3A, left) and increases in CD4+ T cells (Fig. 3B, left) in B6 mice. Furthermore, IFN-γ-producing CD4 T cells were not significantly decreased in response to Af5517 (Fig. 3B, middle). Therefore, although differences were observed between BALB/c and B6 mice, increased airway eosinophilia in response to Af5517 conidia was not mouse strain dependent.
FIG 3.
Increased eosinophil recruitment in response to Af5517 is not mouse strain dependent. C57BL/6 mice were subjected to multiple aspirations of A. fumigatus conidia, as depicted in Fig. 2A. (A) Frequencies of BALF neutrophils and eosinophils and the total number of leukocytes; (B) frequencies of CD4+ T cells and IFN-γ- and IL-17A-producing CD4+ T cells. Data are shown as a summary of the data from two experiments. **, P < 0.01; ***, P < 0.001.
Increased lung Th2 chemokine and decreased IFN-γ transcription in response to repeated aspiration of Af5517.
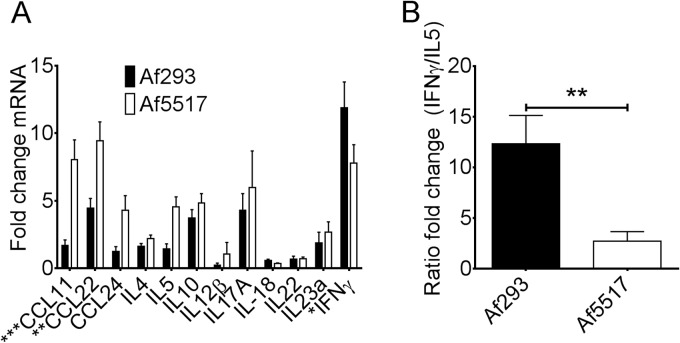
We further examined the ability of Af5517 conidia to promote Th2 responses by comparing the levels of mRNA of selected cytokines in the lung tissue of BALB/c mice aspirated with Af293 or Af5517. Repeated aspiration of Af5517 resulted in an increase in Th2 cytokine and chemokine transcription in mouse lung tissue (Fig. 4A). Among these cytokines and chemokines, the transcription of CCL11 (eotaxin-1) and CCL22 (macrophage-derived chemokine) was significantly increased. In contrast, IFN-γ transcription was decreased in the lungs of Af5517-aspirated mice. The levels of transcription of other cytokines that are involved in eosinophil recruitment and responses, such as IL-17 and IL-18 (21, 37, 38), were equivalent in response to both isolates. These data demonstrate that repeated aspiration with a high-chitin-expressing isolate results in a shift from a Th1-type to a Th2-type expression profile in the lungs, further illustrated as the ratio of IFN-γ transcription/IL-5 transcription in Af293- and Af5517-immune mice (Fig. 4B).
FIG 4.
Increased lung Th2 chemokine transcription and decreased IFN-γ transcription in response to Af5517. (A) BALB/c mouse lung mRNA was analyzed by quantitative RT-PCR for cytokine transcription, which is shown as the fold change over that for the saline-treated controls. Data depict the results of 9 mice per group. (B) Data from panel A are depicted as a ratio of the fold change in IFN-γ expression/fold change in IL-5 expression. ***, P < 0.001; **, P < 0.01; *, P < 0.05.
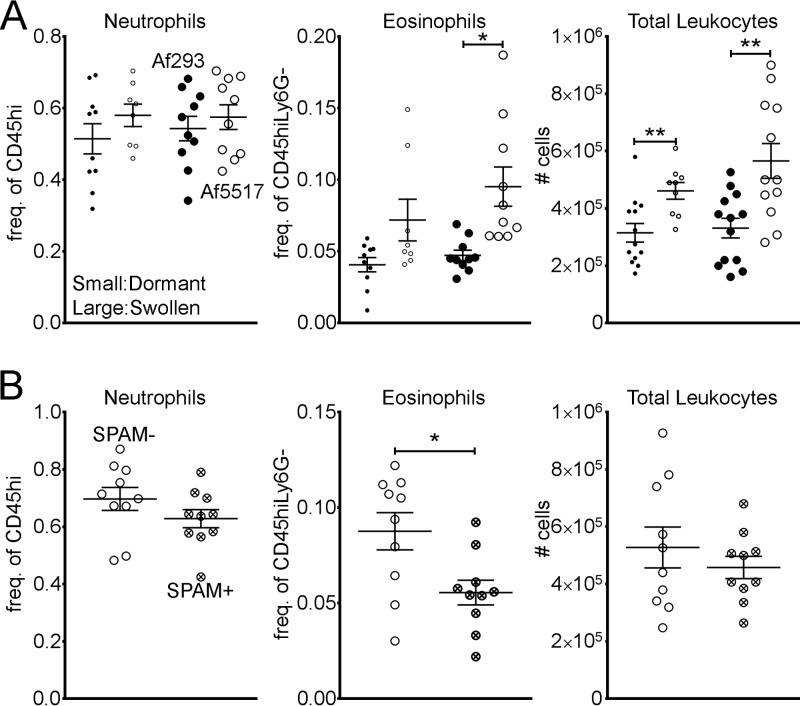
Eosinophil recruitment is associated with surface chitin exposure during germination and is sensitive to chitin degradation.
In order to determine if increased chitin exposure (Fig. 1) was correlated with increased eosinophil recruitment, we gave BALB/c mice a single aspiration of dormant or swollen conidia (which were obtained at 0 or 5 h postgermination, respectively) that were fixed with paraformaldehyde to prevent further germination. Regardless of the state of germination, the recruitment of neutrophils was identical in response to Af293 and Af5517 conidia (Fig. 5A). However, eosinophils were significantly increased in response to swollen Af5517 conidia. To determine if in vivo chitin degradation reduced eosinophil recruitment, mice that constitutively express AMCase in the lungs (SPAM mice, BALB/c mouse background) aspirated swollen Af5517 conidia. In comparison to wild-type mice, SPAM mice displayed a marked reduction in eosinophil recruitment, while neutrophil recruitment was less affected (Fig. 5B). Thus, eosinophil recruitment in response to A. fumigatus conidia is sensitive to chitin degradation.
FIG 5.
Chitin promotes eosinophil recruitment in response to A. fumigatus conidia. BALB/c mice were given a single aspiration of 2 × 106 Af293 or Af5517 conidia, and BALF was harvested 48 h later. (A) Frequencies of airway cells from mice that aspirated dormant (small circles) or swollen (large circles) conidia that had been fixed to prevent further germination. (B) Frequencies of airway cells from wild-type or SPAM mice that aspirated swollen (fixed) Af5517 conidia. Data are displayed as a summary of the results of two experiments. *, P < 0.05; **, P < 0.01.
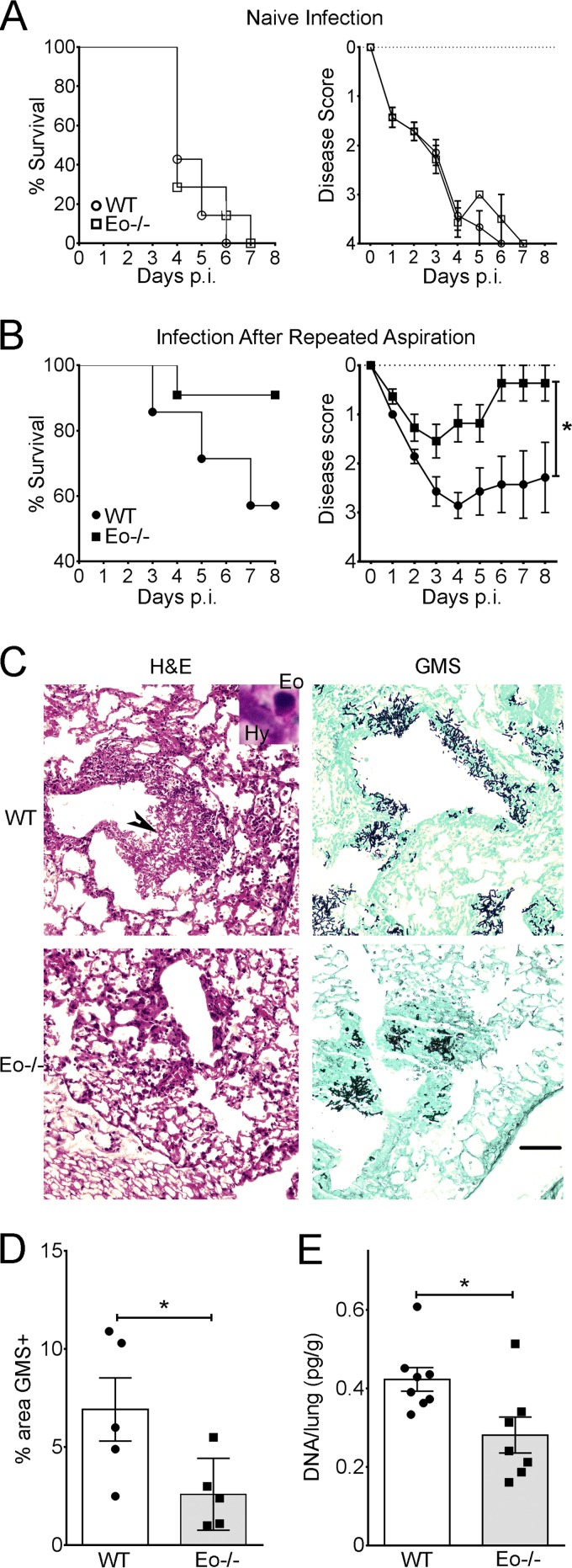
Decreased morbidity and fungal burden in the absence of eosinophils in a model of invasive pulmonary aspergillosis.
The increased chitin-mediated eosinophil recruitment observed in response to Af5517 provided an opportunity to examine the role of these cells in Th2 responses to invasive infection. In order to accomplish this, we infected eosinophil-deficient (ΔdblGATA) or wild-type BALB/c mice that had repeatedly aspirated Af5517 and thus had developed Th2-skewed responses to A. fumigatus. In naive mice with no previous exposure, no differences in morbidity or mortality were apparent between wild-type and eosinophil-deficient mice (Fig. 6A). In both groups, repeated exposures to Af5517 provided protection from infection, although disease severity was significantly increased when eosinophils were present (Fig. 6B). In both groups of previously exposed mice, we observed significant fungal growth in or near bronchoalveolar spaces, accompanied by significant infiltration of inflammatory cells (Fig. 6C). In wild-type mice, it was possible to observe eosinophils within bronchoalveolar inflammatory foci in direct association with A. fumigatus hyphae (Fig. 6C, inset, top left). When the lung fungal burden was measured at 3 days postinfection by quantification of GMS+ areas in histological sections (Fig. 6D) or by qPCR of fungal DNA in lung tissue (Fig. 6E), the burden in eosinophil-deficient A. fumigatus-immune mice was significantly decreased. Therefore, our results suggest that eosinophils contribute to increased morbidity and decreased fungal clearance associated with Th2 responses to invasive A. fumigatus infection.
FIG 6.
Eosinophil-deficient (Eo−/−) mice exhibit decreased morbidity and fungal burden in invasive A. fumigatus infection. (A) Naive neutropenic wild-type (WT) or eosinophil-deficient (ΔdblGATA) BALB/c mice were infected by aspiration with Af5517 conidia. (Left) Survival; (right) disease scores recorded for each animal, as described in Materials and Methods. p.i. postinfection. (B) Neutropenic wild-type or eosinophil-deficient BALB/c mice were infected after being repeatedly aspirated with Af5517 conidia using the schedule depicted in Fig. 2A. Data are displayed as a summary of the results of two experiments with 7 to 11 mice per group. *, the results for days 4 and 6 to 8 were found to be statistically significant. (C) Representative histopathology of Af5517 infection in wild-type and eosinophil-deficient mice. Black arrowhead, area of fungal growth and airway infiltration of leukocytes. (Inset) Eosinophil interaction with a fungal hypha (Hy) in an adjacent area. Magnification, ×100. Bar, 100 μm. (D) GMS staining from four representative GMS+ fields per sample was quantified using ImageJ software. (E) The fungal burden in the lung tissue of mice infected with the indicated dose of conidia was measured by quantitative PCR at day 3 postinfection. *, P < 0.05.
DISCUSSION
Our results indicate that the chitin associated with fungal germination promotes lung Th2-type cytokine/chemokine production and eosinophil recruitment. How this occurs is not entirely clear. An immune receptor that recognizes and signals in response to the specific binding of chitin has not been completely characterized, although NOD2, Toll-like receptor 9 (TLR9), and mannose receptor have recently been shown to be required for chitin-induced IL-10 macrophage secretion in vitro (39). In macrophages, TLR2 was required for the chitin-mediated induction of IL-17A, although direct TLR2-chitin interactions were not observed (40). It is possible that the Th2-skewed immune responses to Af5517 conidia in our model are dependent on binding of chitin to one or more of these candidate receptors.
We observed that multiple aspirations of isolate Af5517 increased the lung transcription of CCL11 and CCL22 and decreased IFN-γ (Fig. 4). CCL11 and IL-5 are potent inducers of eosinophil development and/or chemotaxis (15), while dendritic cell-produced CCL22 is increased in patients with allergic rhinitis or atopic dermatitis and induces Th2 chemotaxis (41–43). However, other cytokines might also play a role in the increased eosinophil recruitment and pathology observed in our study. In two recent reports, it was shown that IL-17A promoted eosinophil recruitment in response to A. fumigatus inhalation and impaired protection from infection (9, 21). The roles of IL-17A and other cytokines related to eosinophil recruitment, the development of Th2 responses, and A. fumigatus pathology are important factors to consider in future studies.
We observed increased surface chitin exposure in Af5517 conidia compared to that in isolate Af293, and this difference was enhanced in swollen conidia (Fig. 5A and B). In addition to chitin, β-glucan is also exposed at the surface of swollen and germling conidia (6, 34). Thus, immune recognition of chitin and β-glucan is occurring simultaneously, and this corecognition likely plays a role in shaping the lung immune responses observed in our studies. Although we observed that eosinophil recruitment was partly sensitive to chitin degradation (Fig. 5C), the contribution of corecognition of β-glucan is still unknown and requires further examination.
Despite the Th2 shift in response to Af5517, memory/effector T cell responses to previous exposures to Af5517 conidia still provided some protection from infection, as naive mice succumbed to infection with Af5517 at the doses that we used in this study (Fig. 6A). However, the virulence of Af5517 was decreased relative to that of Af293 (45), and BALB/c mice are less susceptible to IPA than other strains (29). It will be of interest to induce Th2 responses to more virulent isolates and include more susceptible mouse strains in future studies to observe the effect of these experimental manipulations on the role of eosinophils in Th2-mediated pathology.
Although eosinophils were previously implicated in the pathology of asthma and allergy (19, 20), the results of this study suggest that eosinophils should be further examined as a potential target to enhance treatment of IPA, particularly in individuals that mount poorly protective Th2 responses. Eosinophils are immune effector cells that secrete inflammatory cytokines and granule proteins that are highly toxic to parasitic helminths but also contribute to the pathology of allergy and asthma (15). In naive immunocompetent mice, eosinophils provide some protection from A. fumigatus infection (25). However, our results suggest that in the context of Th2 responses, eosinophils have the potential to exacerbate lung inflammation and worsen disease. It is possible that the presence or absence of neutrophils determines the ability of eosinophils to enhance or impair protection from infection. Additionally, the absence of both cell types might result in the recruitment of other effector cells that ultimately enhance protection. For example, in neutropenic mice infected with A. fumigatus, DC recruitment and maturation were enhanced (44), and it is possible that an additional eosinophil deficiency would stimulate further enhancement. Therefore, an important direction for further study is evaluation of the role of eosinophils and other host effector cells and molecules that ultimately drive protective or pathogenic immune responses to fungal infection.
Supplementary Material
ACKNOWLEDGMENTS
We thank Angar Tsoggerel, Henry Owegi, and Keith Dietel for technical assistance, Joe Lewis for animal care, and Mark Kaplan for helpful discussions.
This work was supported by an Indiana University School of Medicine research enhancement grant (REG).
Footnotes
Published ahead of print 19 May 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.01990-14.
REFERENCES
- 1.Hohl TM, Feldmesser M. 2007. Aspergillus fumigatus: principles of pathogenesis and host defense. Eukaryot. Cell 6:1953–1963. 10.1128/EC.00274-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cenci E, Mencacci A, Del Sero G, Bacci A, Montagnoli C, d'Ostiani CF, Mosci P, Bachmann M, Bistoni F, Kopf M, Romani L. 1999. Interleukin-4 causes susceptibility to invasive pulmonary aspergillosis through suppression of protective type I responses. J. Infect. Dis. 180:1957–1968. 10.1086/315142. [DOI] [PubMed] [Google Scholar]
- 3.Perruccio K, Tosti A, Burchielli E, Topini F, Ruggeri L, Carotti A, Capanni M, Urbani E, Mancusi A, Aversa F, Martelli MF, Romani L, Velardi A. 2005. Transferring functional immune responses to pathogens after haploidentical hematopoietic transplantation. Blood 106:4397–4406. 10.1182/blood-2005-05-1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Romani L. 2011. Immunity to fungal infections. Nat. Rev. Immunol. 11:275–288. 10.1038/nri2939. [DOI] [PubMed] [Google Scholar]
- 5.Aimanianda V, Bayry J, Bozza S, Kniemeyer O, Perruccio K, Elluru SR, Clavaud C, Paris S, Brakhage AA, Kaveri SV, Romani L, Latge JP. 2009. Surface hydrophobin prevents immune recognition of airborne fungal spores. Nature 460:1117–1121. 10.1038/nature08264. [DOI] [PubMed] [Google Scholar]
- 6.Hohl TM, Van Epps HL, Rivera A, Morgan LA, Chen PL, Feldmesser M, Pamer EG. 2005. Aspergillus fumigatus triggers inflammatory responses by stage-specific beta-glucan display. PLoS Pathog. 1:e30. 10.1371/journal.ppat.0010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Werner JL, Metz AE, Horn D, Schoeb TR, Hewitt MM, Schwiebert LM, Faro-Trindade I, Brown GD, Steele C. 2009. Requisite role for the dectin-1 beta-glucan receptor in pulmonary defense against Aspergillus fumigatus. J. Immunol. 182:4938–4946. 10.4049/jimmunol.0804250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rivera A, Hohl TM, Collins N, Leiner I, Gallegos A, Saijo S, Coward JW, Iwakura Y, Pamer EG. 2011. Dectin-1 diversifies Aspergillus fumigatus-specific T cell responses by inhibiting T helper type 1 CD4 T cell differentiation. J. Exp. Med. 208:369–381. 10.1084/jem.20100906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zelante T, De Luca A, Bonifazi P, Montagnoli C, Bozza S, Moretti S, Belladonna ML, Vacca C, Conte C, Mosci P, Bistoni F, Puccetti P, Kastelein RA, Kopf M, Romani L. 2007. IL-23 and the Th17 pathway promote inflammation and impair antifungal immune resistance. Eur. J. Immunol. 37:2695–2706. 10.1002/eji.200737409. [DOI] [PubMed] [Google Scholar]
- 10.Fontaine T, Simenel C, Dubreucq G, Adam O, Delepierre M, Lemoine J, Vorgias CE, Diaquin M, Latge JP. 2000. Molecular organization of the alkali-insoluble fraction of Aspergillus fumigatus cell wall. J. Biol. Chem. 275:27594–27607. 10.1074/jbc.M909975199. [DOI] [PubMed] [Google Scholar]
- 11.Lenardon MD, Munro CA, Gow NA. 2010. Chitin synthesis and fungal pathogenesis. Curr. Opin. Microbiol. 13:416–423. 10.1016/j.mib.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reese TA, Liang HE, Tager AM, Luster AD, Van Rooijen N, Voehringer D, Locksley RM. 2007. Chitin induces accumulation in tissue of innate immune cells associated with allergy. Nature 447:92–96. 10.1038/nature05746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shibata Y, Foster LA, Bradfield JF, Myrvik QN. 2000. Oral administration of chitin down-regulates serum IgE levels and lung eosinophilia in the allergic mouse. J. Immunol. 164:1314–1321. 10.4049/jimmunol.164.3.1314. [DOI] [PubMed] [Google Scholar]
- 14.Van Dyken SJ, Garcia D, Porter P, Huang X, Quinlan PJ, Blanc PD, Corry DB, Locksley RM. 2011. Fungal chitin from asthma-associated home environments induces eosinophilic lung infiltration. J. Immunol. 187:2261–2267. 10.4049/jimmunol.1100972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rothenberg ME, Hogan SP. 2006. The eosinophil. Annu. Rev. Immunol. 24:147–174. 10.1146/annurev.immunol.24.021605.090720. [DOI] [PubMed] [Google Scholar]
- 16.Foster PS, Hogan SP, Ramsay AJ, Matthaei KI, Young IG. 1996. Interleukin 5 deficiency abolishes eosinophilia, airways hyperreactivity, and lung damage in a mouse asthma model. J. Exp. Med. 183:195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kopf M, Brombacher F, Hodgkin PD, Ramsay AJ, Milbourne EA, Dai WJ, Ovington KS, Behm CA, Kohler G, Young IG, Matthaei KI. 1996. IL-5-deficient mice have a developmental defect in CD5+ B-1 cells and lack eosinophilia but have normal antibody and cytotoxic T cell responses. Immunity 4:15–24. 10.1016/S1074-7613(00)80294-0. [DOI] [PubMed] [Google Scholar]
- 18.Rankin SM, Conroy DM, Williams TJ. 2000. Eotaxin and eosinophil recruitment: implications for human disease. Mol. Med. Today 6:20–27. 10.1016/S1357-4310(99)01635-4. [DOI] [PubMed] [Google Scholar]
- 19.Haldar P, Brightling CE, Hargadon B, Gupta S, Monteiro W, Sousa A, Marshall RP, Bradding P, Green RH, Wardlaw AJ, Pavord ID. 2009. Mepolizumab and exacerbations of refractory eosinophilic asthma. N. Engl. J. Med. 360:973–984. 10.1056/NEJMoa0808991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jacobsen EA, Ochkur SI, Pero RS, Taranova AG, Protheroe CA, Colbert DC, Lee NA, Lee JJ. 2008. Allergic pulmonary inflammation in mice is dependent on eosinophil-induced recruitment of effector T cells. J. Exp. Med. 205:699–710. 10.1084/jem.20071840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murdock BJ, Falkowski NR, Shreiner AB, Sadighi Akha AA, McDonald RA, White ES, Toews GB, Huffnagle GB. 2012. Interleukin-17 drives pulmonary eosinophilia following repeated exposure to Aspergillus fumigatus conidia. Infect. Immun. 80:1424–1436. 10.1128/IAI.05529-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shreiner AB, Murdock BJ, Sadighi Akha AA, Falkowski NR, Christensen PJ, White ES, Hogaboam CM, Huffnagle GB. 2012. Repeated exposure to Aspergillus fumigatus conidia results in CD4+ T cell-dependent and -independent pulmonary arterial remodeling in a mixed Th1/Th2/Th17 microenvironment that requires interleukin-4 (IL-4) and IL-10. Infect. Immun. 80:388–397. 10.1128/IAI.05530-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cenci E, Mencacci A, Fe d'Ostiani C, Del Sero G, Mosci P, Montagnoli C, Bacci A, Romani L. 1998. Cytokine- and T helper-dependent lung mucosal immunity in mice with invasive pulmonary aspergillosis. J. Infect. Dis. 178:1750–1760. 10.1086/314493. [DOI] [PubMed] [Google Scholar]
- 24.Yoon J, Ponikau JU, Lawrence CB, Kita H. 2008. Innate antifungal immunity of human eosinophils mediated by a beta 2 integrin, CD11b. J. Immunol. 181:2907–2915. 10.4049/jimmunol.181.4.2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lilly LM, Scopel M, Nelson MP, Burg AR, Dunaway CW, Steele C. 2014. Eosinophil deficiency compromises lung defense against Aspergillus fumigatus. Infect. Immun. 82:1315–1325. 10.1128/IAI.01172-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Templeton SP, Buskirk AD, Law B, Green BJ, Beezhold DH. 2011. Role of germination in murine airway CD8+ T-cell responses to Aspergillus conidia. PLoS One 6:e18777. 10.1371/journal.pone.0018777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu C, Cantor AB, Yang H, Browne C, Wells RA, Fujiwara Y, Orkin SH. 2002. Targeted deletion of a high-affinity GATA-binding site in the GATA-1 promoter leads to selective loss of the eosinophil lineage in vivo. J. Exp. Med. 195:1387–1395. 10.1084/jem.20020656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mohrs M, Shinkai K, Mohrs K, Locksley RM. 2001. Analysis of type 2 immunity in vivo with a bicistronic IL-4 reporter. Immunity 15:303–311. 10.1016/S1074-7613(01)00186-8. [DOI] [PubMed] [Google Scholar]
- 29.Stephens-Romero SD, Mednick AJ, Feldmesser M. 2005. The pathogenesis of fatal outcome in murine pulmonary aspergillosis depends on the neutrophil depletion strategy. Infect. Immun. 73:114–125. 10.1128/IAI.73.1.114-125.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mehrad B, Strieter RM, Moore TA, Tsai WC, Lira SA, Standiford TJ. 1999. CXC chemokine receptor-2 ligands are necessary components of neutrophil-mediated host defense in invasive pulmonary aspergillosis. J. Immunol. 163:6086–6094. [PubMed] [Google Scholar]
- 31.Daley JM, Thomay AA, Connolly MD, Reichner JS, Albina JE. 2008. Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice. J. Leukoc. Biol. 83:64–70. 10.1189/jlb.0407247. [DOI] [PubMed] [Google Scholar]
- 32.Feng X, Krishnan K, Richie DL, Aimanianda V, Hartl L, Grahl N, Powers-Fletcher MV, Zhang M, Fuller KK, Nierman WC, Lu LJ, Latge JP, Woollett L, Newman SL, Cramer RA, Jr, Rhodes JC, Askew DS. 2011. HacA-independent functions of the ER stress sensor IreA synergize with the canonical UPR to influence virulence traits in Aspergillus fumigatus. PLoS Pathog. 7:e1002330. 10.1371/journal.ppat.1002330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bowman JC, Abruzzo GK, Anderson JW, Flattery AM, Gill CJ, Pikounis VB, Schmatz DM, Liberator PA, Douglas CM. 2001. Quantitative PCR assay to measure Aspergillus fumigatus burden in a murine model of disseminated aspergillosis: demonstration of efficacy of caspofungin acetate. Antimicrob. Agents Chemother. 45:3474–3481. 10.1128/AAC.45.12.3474-3481.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steele C, Rapaka RR, Metz A, Pop SM, Williams DL, Gordon S, Kolls JK, Brown GD. 2005. The beta-glucan receptor dectin-1 recognizes specific morphologies of Aspergillus fumigatus. PLoS Pathog. 1:e42. 10.1371/journal.ppat.0010042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mohrs K, Wakil AE, Killeen N, Locksley RM, Mohrs M. 2005. A two-step process for cytokine production revealed by IL-4 dual-reporter mice. Immunity 23:419–429. 10.1016/j.immuni.2005.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fei M, Bhatia S, Oriss TB, Yarlagadda M, Khare A, Akira S, Saijo S, Iwakura Y, Fallert Junecko BA, Reinhart TA, Foreman O, Ray P, Kolls J, Ray A. 2011. TNF-alpha from inflammatory dendritic cells (DCs) regulates lung IL-17A/IL-5 levels and neutrophilia versus eosinophilia during persistent fungal infection. Proc. Natl. Acad. Sci. U. S. A. 108:5360–5365. 10.1073/pnas.1015476108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kumano K, Nakao A, Nakajima H, Hayashi F, Kurimoto M, Okamura H, Saito Y, Iwamoto I. 1999. Interleukin-18 enhances antigen-induced eosinophil recruitment into the mouse airways. Am. J. Respir. Crit. Care Med. 160:873–878. 10.1164/ajrccm.160.3.9805026. [DOI] [PubMed] [Google Scholar]
- 38.Wild JS, Sigounas A, Sur N, Siddiqui MS, Alam R, Kurimoto M, Sur S. 2000. IFN-gamma-inducing factor (IL-18) increases allergic sensitization, serum IgE, Th2 cytokines, and airway eosinophilia in a mouse model of allergic asthma. J. Immunol. 164:2701–2710. 10.4049/jimmunol.164.5.2701. [DOI] [PubMed] [Google Scholar]
- 39.Wagener J, Malireddi RK, Lenardon MD, Koberle M, Vautier S, Maccallum DM, Biedermann T, Schaller M, Netea MG, Kanneganti TD, Brown GD, Brown AJ, Gow NA. 2014. Fungal chitin dampens inflammation through IL-10 induction mediated by NOD2 and TLR9 activation. PLoS Pathog. 10:e1004050. 10.1371/journal.ppat.1004050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Da Silva CA, Hartl D, Liu W, Lee CG, Elias JA. 2008. TLR-2 and IL-17A in chitin-induced macrophage activation and acute inflammation. J. Immunol. 181:4279–4286. 10.4049/jimmunol.181.6.4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hashimoto S, Nakamura K, Oyama N, Kaneko F, Tsunemi Y, Saeki H, Tamaki K. 2006. Macrophage-derived chemokine (MDC)/CCL22 produced by monocyte derived dendritic cells reflects the disease activity in patients with atopic dermatitis. J. Dermatol. Sci. 44:93–99. 10.1016/j.jdermsci.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 42.Yanai M, Sato K, Aoki N, Takiyama Y, Oikawa K, Kobayashi H, Kimura S, Harabuchi Y, Tateno M. 2007. The role of CCL22/macrophage-derived chemokine in allergic rhinitis. Clin. Immunol. 125:291–298. 10.1016/j.clim.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 43.Cronshaw DG, Owen C, Brown Z, Ward SG. 2004. Activation of phosphoinositide 3-kinases by the CCR4 ligand macrophage-derived chemokine is a dispensable signal for T lymphocyte chemotaxis. J. Immunol. 172:7761–7770. 10.4049/jimmunol.172.12.7761. [DOI] [PubMed] [Google Scholar]
- 44.Park SJ, Burdick MD, Mehrad B. 2012. Neutrophils mediate maturation and efflux of lung dendritic cells in response to Aspergillus fumigatus germ tubes. Infect. Immun. 80:1759–1765. 10.1128/IAI.00097-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Amarsaikhan N, O'Dea EM, Tsoggerel A, Owegi H, Gillenwater J, Templeton SP. 2014. Isolate-dependent growth, virulence, and cell wall composition in the human pathogen Aspergillus fumigatus. PLoS One 9:e100430. 10.1371/journal.pone.0100430. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.