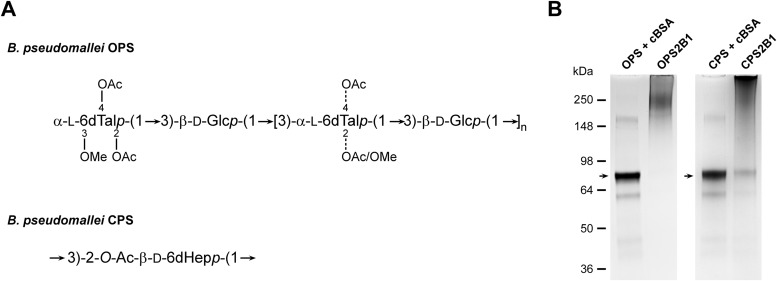Abstract
Burkholderia pseudomallei, the etiologic agent of melioidosis, is a CDC tier 1 select agent that causes severe disease in both humans and animals. Diagnosis and treatment of melioidosis can be challenging, and in the absence of optimal chemotherapeutic intervention, acute disease is frequently fatal. Melioidosis is an emerging infectious disease for which there are currently no licensed vaccines. Due to the potential malicious use of B. pseudomallei as well as its impact on public health in regions where the disease is endemic, there is significant interest in developing vaccines for immunization against this disease. In the present study, type A O-polysaccharide (OPS) and manno-heptose capsular polysaccharide (CPS) antigens were isolated from nonpathogenic, select-agent-excluded strains of B. pseudomallei and covalently linked to carrier proteins. By using these conjugates (OPS2B1 and CPS2B1, respectively), it was shown that although high-titer IgG responses against the OPS or CPS component of the glycoconjugates could be raised in BALB/c mice, only those animals immunized with CPS2B1 were protected against intraperitoneal challenge with B. pseudomallei. Extending upon these studies, it was also demonstrated that when the mice were immunized with a combination of CPS2B1 and recombinant B. pseudomallei LolC, rather than with CPS2B1 or LolC individually, they exhibited higher survival rates when challenged with a lethal dose of B. pseudomallei. Collectively, these results suggest that CPS-based glycoconjugates are promising candidates for the development of subunit vaccines for immunization against melioidosis.
INTRODUCTION
Burkholderia pseudomallei is a Gram-negative, facultative, intracellular pathogen that causes melioidosis, a serious and often fatal disease in humans and animals (1). While the incidence of melioidosis is particularly high in Southeast Asia and northern Australia, recent reports have expanded the region of endemicity to include India and Brazil. Sporadic cases of melioidosis have also been reported in various parts of the Caribbean, Central and South America, the Middle East, and Africa (2, 3). B. pseudomallei can be isolated from environmental niches such as rice paddies, still or stagnant waters, and moist soils, which predominate in the tropics (4), and it is believed that these habitats are the primary reservoirs from which susceptible hosts acquire infections. Routes of infection include inhalation, ingestion, and percutaneous inoculation (5).
Melioidosis has a broad clinical spectrum and may manifest as acute localized infections, acute pulmonary infections, and fulminating septicemias or as chronic disease (6, 7). Remarkably, 20% of all community-acquired septicemias in northeast Thailand and 32% of community-acquired bacteremic pneumonias in northern Australia are due to B. pseudomallei infections (8, 9). Because of the nonspecific presentation, the lack of rapid diagnostic tests, and the intrinsic resistance of B. pseudomallei to commonly used antibiotics, diagnosis and treatment of melioidosis can be challenging. In the absence of optimal chemotherapeutic intervention, mortality rates associated with acute human melioidosis remain unacceptably high (40 to 50% in northeast Thailand and 19% in Australia [9, 10]). At present, there are no human vaccines available for immunization against this emerging infectious disease (for recent reviews, see references 11–13). Due to the high risk of aerosol infection, the severe course of disease, and the potential for malicious use, B. pseudomallei is currently classified as a CDC tier 1 select agent.
Several studies have demonstrated that B. pseudomallei expresses a number of factors that are required for virulence in animal models of infection. Included among these are the Bsa type III secretion system, the VirAG two-component regulatory system, and the cluster 1 type VI secretion system (for recent reviews, see references 5 and 14). Additionally, studies in our laboratories and others have shown that the O-polysaccharide (OPS) component of B. pseudomallei lipopolysaccharides (LPSs) and the manno-heptose capsular polysaccharide (CPS) are both virulence determinants (15–17) and protective antigens (18). Consequently, these carbohydrate moieties have become important components of various subunit vaccines that are currently being developed for immunization against melioidosis (19, 20).
Unlike other Gram-negative pathogens, B. pseudomallei isolates appear to express only a limited repertoire of OPS (21) and CPS (16, 22) antigens. At present, the significance of these observations with regard to virulence and evasion of host immune responses remains to be fully determined. Nevertheless, these attributes bode well from a vaccine development standpoint. Relevant to the current study, a number of studies have shown that monoclonal and polyclonal antibodies specific for OPS and CPS can be used to passively immunize mice against lethal challenges of B. pseudomallei (18, 20, 23–26). Such findings confirm the protective capacity of these surface-exposed antigens and support the rationale for exploring the use of OPS and CPS antigens for active immunization against melioidosis.
Immunologically, antigens can be classified as either T-cell-dependent (TD), T-cell-independent type 1 (TI-1), or TI-2 antigens. Carbohydrates such as capsular antigens and O-polysaccharides are generally considered to be TI-2 antigens (27). Typically, high-molecular-weight TI-2 antigens such as capsular polysaccharides are immunogenic due to their ability to cross-link multiple surface immunoglobulin molecules present on antigen-specific B cells (28), but without the involvement of T helper (Th) cells, TI-2 antigens induce poor immunological memory and only limited affinity maturation and isotype switching (29). Additionally, without dosing at frequent intervals, antibody levels often decline. Efforts to overcome the poor immunogenicity of many clinically relevant polysaccharides have led to the development of glycoconjugate vaccines (30, 31), a number of which are currently licensed for human use (32). Covalent linkage of polysaccharides to carrier proteins promotes Th-cell involvement, which improves immunological memory (33) and increases isotype switching. The affinities of the antibodies elicited by glycoconjugates may also be higher than those produced by polysaccharides alone (29).
In the present study, B. pseudomallei OPS- and CPS-based glycoconjugates were constructed and evaluated for their immunogenic potential and protective capacities by using a murine model of melioidosis. Collectively, the results suggest that CPS-based glycoconjugates are promising candidates for the development of subunit vaccines for immunization against melioidosis.
MATERIALS AND METHODS
Strains and growth conditions.
The bacteria used in this study were B. pseudomallei strains K96243 (34), RR2808 (ΔpurM ΔwcbB) (35), and RR2683 (ΔpurM ΔrmlD) (19) and Escherichia coli BL21 Star(DE3)(pLysS)(pCRT7/NT-TOPO-LolC) (36). B. pseudomallei strains RR2808 and RR2683 are derivatives of the adenine auxotroph Bp82 (37), a select-agent-excluded ΔpurM derivative of strain 1026b. Strain RR2808 (CPS mutant; source of OPS) and RR2683 (OPS mutant; source of CPS) enable the production of highly purified polysaccharides without the requirement for biosafety level 3 (BSL-3) facilities. B. pseudomallei K96243 and E. coli BL21 were cultured in Luria broth (L-broth) and on L-agar at 37°C. B. pseudomallei RR2808 and RR2683 were cultured in L-broth and on L-agar supplemented with thiamine (5 μg/ml) and adenine (100 μg/ml) at 37°C. Bacterial stocks were maintained at −80°C as 20% glycerol suspensions. Wild-type B. pseudomallei K96243 was handled at Advisory Committee for Dangerous Pathogens (ACDP) containment level 3.
For animal challenges, B. pseudomallei K96243 was inoculated from a glycerol stock into 100 ml L-broth and incubated for 24 h at 37°C with shaking (180 rpm). The optical density at 590 nm (OD590) was adjusted to 0.4 (corresponding to approximately 4 × 108 CFU/ml) and diluted in L-broth to the correct concentration for challenge.
OPS and CPS purification.
Broth in 2-liter baffled Erlenmeyer flasks was inoculated with B. pseudomallei RR2808 or RR2683 and incubated overnight at 37°C with vigorous shaking. Cell pellets were obtained by centrifugation and extracted by using a modified hot aqueous phenol procedure (38). Purified OPS and CPS antigens were then obtained essentially as previously described (19, 39).
Glycoconjugate synthesis.
The OPS2B1 and CPS2B1 glycoconjugates used in this study were synthesized by using reductive amination chemistry as previously described (19, 39). Briefly, purified OPS or CPS samples were solubilized at 5 mg/ml in phosphate-buffered saline (PBS) and added to small amber vials. Six milligrams (∼30 mM) of sodium meta-periodate (NaIO4; Pierce) was added to each milliliter of the solution. Once the crystals had dissolved by gentle agitation, the reaction mixtures were gently stirred at room temperature for 40 min. To remove any excess oxidizing agent, the reaction mixtures were applied onto Zeba Desalt Spin columns (Pierce) equilibrated with PBS, and the eluates were collected. To facilitate conjugation of the OPS or CPS antigens to cationized bovine serum albumin (cBSA; Pierce), the activated polysaccharides were added to small amber vials. A total of 0.5 ml of the carrier proteins (5 mg/ml in PBS) was added to each milliliter of the OPS or CPS solution. After mixing by gentle agitation, 10-μl aliquots of a sodium cyanoborohydride stock (1 M NaBH3CN in 10 mM NaOH) were added to each milliliter of the conjugation mixtures, and the reaction mixtures were gently stirred at room temperature for 4 days. Following this, 10-μl aliquots of a sodium borohydride stock (1 M NaBH4 in 10 mM NaOH) were added to each milliliter of the conjugation mixtures, and the reaction mixtures were stirred for 40 min. The conjugate reaction mixtures were then brought to 5 ml with ultrapure water, dialyzed against distilled water, and then lyophilized. The resulting preparations were resuspended in ultrapure water as 1-mg/ml stocks and stored at −20°C until required for use. Bicinchoninic acid (BCA) assays were used to quantitate the protein concentrations of the glycoconjugate stocks (the remaining masses of which were assumed to be polysaccharide).
LolC protein preparation.
LolC protein (encoded by BPSL2277 in B. pseudomallei K96243) was purified from E. coli BL21 Star(DE3)(pLysS)(pCRT7/NT-TOPO-LolC) as previously described (36). Briefly, cells were grown to mid-log phase at 37°C, induced by the addition of 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG), and cooled to 20°C for overnight incubation. Following cell disruption by sonication, LolC was loaded onto HisTrap FF columns (GE Healthcare) and eluted in buffer (40 mM Tris-Cl, 750 mM NaCl, 1% glucose, 5% glycerol [pH 7.5]) by using imidazole in steps to 500 mM. The recovered material was dialyzed against PBS, protein purity was assessed by using SDS-PAGE, and concentrations were determined by using a BCA assay.
SDS-PAGE and Western immunoblotting.
Glycoconjugate samples were analyzed by using SDS-PAGE and Western immunoblotting as previously described (19, 39). Briefly, samples were solubilized in 1× SDS-PAGE sample buffer and heated to 100°C for 5 min prior to electrophoresis on 12% Precise gels (Pierce). Proteins were visualized via staining with Coomassie blue R-250. For Western immunoblot analyses, the glycoconjugate samples and controls were separated on the same 12% gels and electrophoretically transferred onto nitrocellulose membranes before hybridization to either a B. pseudomallei OPS-specific monoclonal antibody (MAb) (Pp-PS-W) or a CPS-specific MAb (3C5).
Animal studies.
All investigations involving animals were carried out according to the requirements of the United Kingdom Animal (Scientific Procedures) Act of 1986. Studies were performed by using 6- to 8-week-old female BALB/c mice (Charles River) randomly allocated into cages of five mice upon arrival. Mice were held under a 12-h light/dark cycle with free access to food and water and were implanted with a microchip to allow tracking of individual mice. After challenge with B. pseudomallei, animals were handled under ACDP containment level 3 conditions within a rigid-wall half-suit isolator. Mice were checked at least twice daily following challenge, and clinical signs were recorded for each mouse. Upon reaching predetermined humane endpoints, mice were culled via cervical dislocation.
Mice were immunized with the various immunogens subcutaneously (s.c.) on days 0, 21, and 35. All immunogens were formulated in 2% Alhydrogel (500 μg/mouse; Brenntag) plus ODN 2006 (CpG) (20 μg/mouse; InvivoGen). The mice received 5 μg per dose of OPS or CPS as a conjugate, 5 μg per dose of unconjugated OPS or CPS, and 10 μg per dose of cBSA or LolC. Challenges with B. pseudomallei K96243 were delivered at day 70 via the intraperitoneal (i.p.) route (the minimal lethal dose [MLD] at day 35 for B. pseudomallei K96243 via this route was calculated to be 744 CFU in our hands). For organ bacterial enumeration, animals were culled, and organs were removed. These organs were then mashed through 40-μm sieves into PBS, serially diluted, and plated onto L-agar.
Analysis of antibody responses.
Approximately 0.1 ml of blood was collected from the tail veins of mice 2 weeks after the final boost was administered. After clotting at 4°C, the serum was removed and stored at −20°C until required for use. Responses directed against the OPS, CPS, or LolC antigen were assessed by an enzyme-linked immunosorbent assay (ELISA) essentially as previously described (40). A reading of twice the background or higher was considered positive, and the titer was determined to be the reciprocal of the final positive dilution.
Statistical analysis.
All graphs were produced by using the GraphPad PRISM V5.0 program. Survival data were analyzed by using a log-rank (Mantel-Cox) test. Bacterial burden data were transformed to the logarithm of 10 and compared by using a Mann-Whitney U test. Antibody data were transformed to the logarithm of 10 and compared by using a Mann-Whitney U test.
RESULTS
Glycoconjugate synthesis.
Polysaccharide-based glycoconjugates represent a rational approach for immunization against melioidosis. However, until recently, isolation of OPS and CPS antigens to develop these vaccine candidates has been hampered by the fact that B. pseudomallei is a select agent that requires specialized handling and containment practices. To address this issue, B. pseudomallei RR2808 and RR2683 (derivatives of the select-agent-excluded strain Bp82, a ΔpurM derivative of 1026b) were created to enable us to produce these polysaccharides in a safer and more cost-effective manner without the requirement for BSL-3 containment (19, 35).
To facilitate the construction of the glycoconjugates described in this study, the OPS and CPS antigens purified from RR2808 and RR2683 were chemically activated with sodium periodate and covalently linked to cBSA via reductive amination to produce OPS2B1 and CPS2B1 (Fig. 1A). Following conjugation, the samples were examined by SDS-PAGE. Results of these analyses demonstrated that in both instances, the polysaccharides had covalently linked to the protein carriers, as indicated by the shifts in molecular weights of the glycoconjugates relative to the molecular weights of the unconjugated cBSA controls (Fig. 1B). Additionally, Western immunoblotting confirmed that the structural integrity/antigenicity of the OPS and CPS moieties remained intact following chemical activation and linkage to the protein carriers based upon their reactivity with MAb Pp-PS-W or 3C5 (data not shown). Further analysis of the constructs revealed that OPS2B1 and CPS2B1 contained 60% (wt/wt) OPS and 53% (wt/wt) CPS, respectively.
FIG 1.
Physical analysis of the B. pseudomallei OPS- and CPS-based glycoconjugates used in this study. (A) Structures of the B. pseudomallei OPS and CPS antigens used to construct the OPS2B1 and CPS2B1 glycoconjugates. OAc, acetate; OMe, methyl. (B) SDS-PAGE and Coomassie blue staining were used to confirm the covalent linkage of the OPS and CPS antigens to cBSA. The OPS + cBSA and CPS + cBSA lanes represent unconjugated controls. Lanes were loaded with equal amounts of protein and carbohydrate to facilitate direct comparisons. Unconjugated cBSA is indicated by black arrowheads.
Immunogenicity of the glycoconjugates.
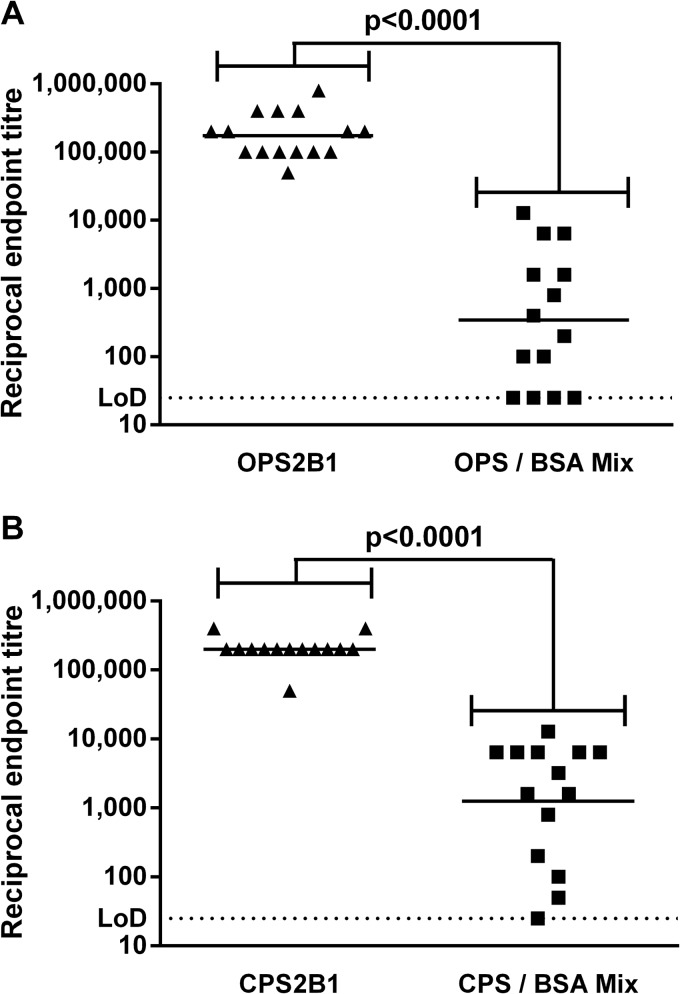
To compare the immunogenicities of the glycoconjugates, groups of BALB/c mice were immunized with OPS2B1 or CPS2B1. For control purposes, groups of mice were also immunized with a matching amount of antigen composed of mixed but unconjugated polysaccharide and cBSA. Two weeks after the final boost, serum was obtained from the mice, and the LPS- and CPS-specific IgG titers were determined by an ELISA (Fig. 2). In both cases, the glycoconjugates induced significantly higher antigen-specific IgG titers than did the unconjugated controls (P < 0.0001). The titers elicited by the two glycoconjugates were similar in magnitude, with an endpoint of ∼1:100,000.
FIG 2.
Characterization of murine immune responses against OPS2B1 and CPS2B1. Mice were immunized with various immunogens in Alhydrogel-CpG via the s.c. route on days 0, 21, and 35. Serum was obtained from mice 14 days after the final boost, and titers of IgG specific for B. pseudomallei LPS (A) or CPS (B) were determined by an ELISA. Individual symbols represent a single immunized mouse. The horizontal black lines represent the geometric means for each group (n = 15 for the OPS2B1 group, n = 14 for the CPS2B1 group, and n = 14 for unconjugated control groups). Significance at the 95% confidence level was determined by using a Mann-Whitney U test. LoD, limit of detection.
Protective capacities of OPS2B1 and CPS2B1.
To assess the protective capacities of OPS2B1 and CPS2B1, mice immunized with the glycoconjugates or with adjuvant only were challenged with B. pseudomallei K96243 5 weeks after the final boost. Since it was previously observed that the first 24 h of infection are critical to the eventual outcome for mice (i.e., reduced counts, especially in the spleen, lead to a better outcome), 5 mice from each of the test and control groups were culled 24 h after challenge to enable the enumeration of bacterial loads in lungs, livers, and spleens. The remaining mice were then monitored for 21 days after challenge for signs of morbidity and mortality.
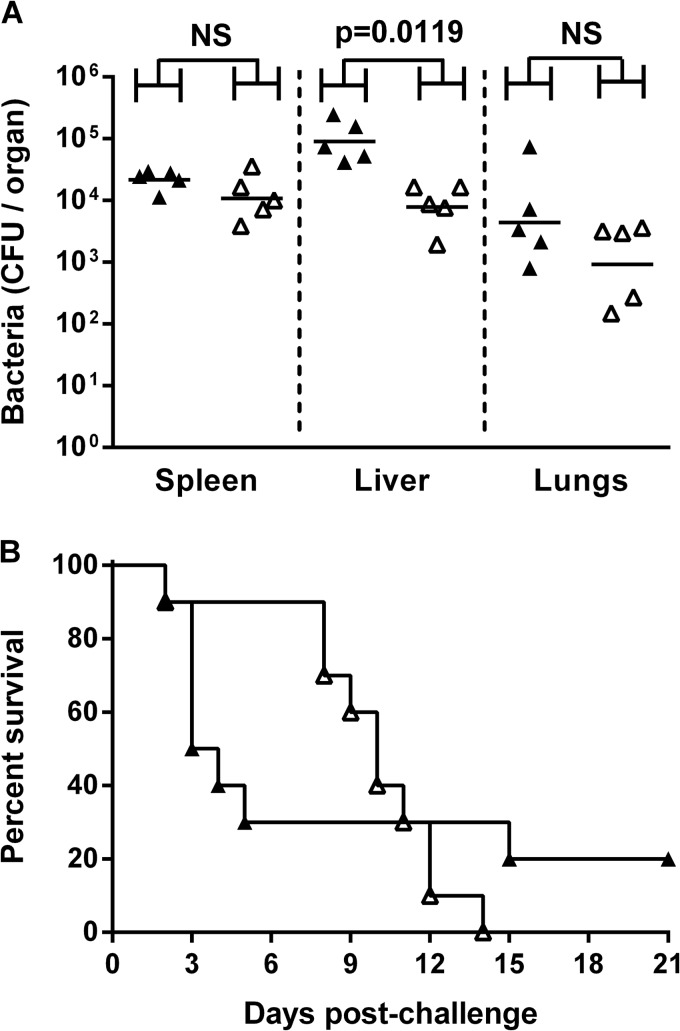
The OPS2B1-immunized mice and adjuvant controls received a challenge of approximately 4.05 × 104 CFU (∼54 MLDs at day 35). Twenty-four hours after challenge, numbers of CFU in the livers from the OPS2B1-immunized mice were significantly lower than those in the livers from the controls (P = 0.0119), whereas CFU in the spleens and lungs were not (P = 0.2222 and P = 0.3095, respectively) (Fig. 3A). Immunization led to an increase in the median time to death (MTTD) from 3.5 days for the controls to 10 days for the OPS2B1-immunized mice (Fig. 3B). However, by day 21 postchallenge, all of the OPS2B1-immunized mice had succumbed to infection, while only 80% of the control mice had succumbed. The survival curves of the OPS2B1- and adjuvant-treated mice were not significantly different (P = 0.8686) (Fig. 3B).
FIG 3.
Protective capacity of OPS2B1. Mice (n = 15 mice per group) were immunized with Alhydrogel-CpG alone (▲) or OPS2B1 formulated with Alhydrogel-CpG (△) via the s.c. route on days 0, 21, and 35. Five weeks after the final boost, mice were challenged i.p. with 4.05 × 104 CFU of B. pseudomallei K96243. (A) Twenty-four hours after challenge, five mice from each group were culled, organs were removed, and bacterial burdens were determined. (B) The remaining 10 mice from each group were monitored until day 21 postchallenge, and survival was plotted. The horizontal black lines in panel A represent the geometric means for each group. Significance at the 95% confidence limit for organ bacterial counts was determined by using a Mann-Whitney U test, and that for survival was determined by using a log-rank (Mantel-Cox) test, as indicated (NS, not significant).
The CPS2B1-immunized mice and adjuvant controls received a lower challenge of approximately 1.06 × 104 CFU (∼14 MLDs at day 35), leading to less acute disease. By 24 h after challenge (Fig. 4A), CPS2B1-immunized mice had significantly lower counts in both the spleens (P = 0.0195) and livers (P = 0.0317), but not in the lungs (P = 0.0952), than controls. The control mice in this study had a median time to death of 12.5 days. It was not possible, however, to calculate a median time to death for the CPS2B1-immunized mice, since by day 21, only 10% had succumbed to infection. The survival curves of the CPS2B1- and adjuvant-treated mice were significantly different (P = 0.0059) (Fig. 4B). Based upon these studies, CPS2B1 appeared to provide superior protection in our murine model of melioidosis compared to OPS2B1. Because of this, a decision was made to further investigate the protective capacity of the CPS2B1 construct.
FIG 4.
Protective capacity of CPS2B1. Mice (n = 15 mice per group) were immunized with Alhydrogel-CpG alone (▲) or CPS2B1 formulated with Alhydrogel-CpG (△) via the s.c. route on days 0, 21, and 35. Five weeks after the final boost, mice were challenged i.p. with 1.06 × 104 CFU of B. pseudomallei K96243. (A) Twenty-four hours after challenge, five mice from each group were culled, organs were removed, and bacterial burdens were determined. (B) The remaining 10 mice from each group were monitored until day 21 postchallenge, and survival was plotted. The horizontal black lines in panel A represent the geometric means for each group. Significance at the 95% confidence limit for organ bacterial counts was determined by using a Mann-Whitney U test, and that for survival was determined by using a log-rank (Mantel-Cox) test, as indicated (NS, not significant).
Protective capacity of CPS2B1 formulated with LolC.
Optimal immunity to B. pseudomallei infections is likely to be complex, requiring both humoral and cellular immune responses. Since the CPS2B1 glycoconjugate would be predicted to provide protection primarily via the production of anti-CPS antibodies, further investigations were carried out to determine whether or not it would be advantageous to coformulate the glycoconjugate with an additional protective antigen known to promote robust cellular immune responses. To facilitate these studies, the CPS2B1 glycoconjugate was mixed with B. pseudomallei LolC, a protein that was previously shown to offer protection against melioidosis and is recognized by gamma interferon-secreting T cells in mice and seropositive humans from areas of endemicity (36, 41, 42).
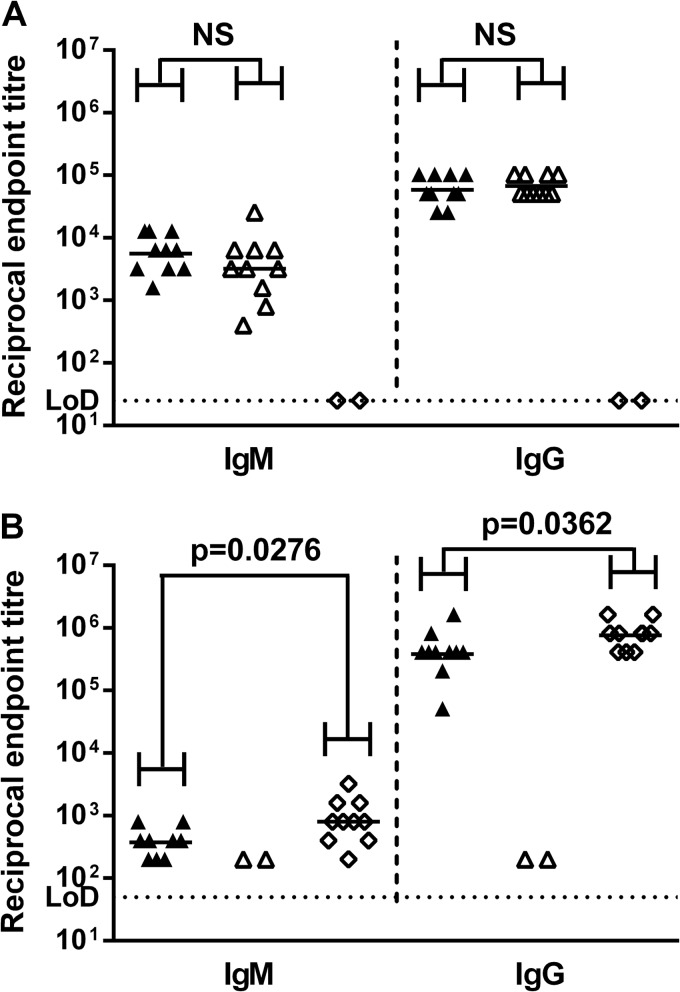
For these studies, mice were immunized with a mixture of CPS2B1 and LolC, LolC alone, CPS2B1 alone, or adjuvant only. Serum was obtained from the immunized mice 14 days after the final boost, and anti-CPS- and anti-LolC-specific IgM and IgG titers were determined by an ELISA. CPS-specific IgM and IgG titers were not significantly different between the group immunized with the mixture of CPS2B1 and LolC and the group immunized with CPS2B1 alone (P = 0.2431 for IgM and P = 0.6445 for IgG) (Fig. 5A). However, LolC-specific IgM and IgG titers were significantly different between the group immunized with the mixture of CPS2B1 and LolC and the group immunized with LolC alone (P = 0.0276 for IgM and P = 0.0362 for IgG) (Fig. 5B).
FIG 5.
Characterization of murine immune responses against CPS2B1 and LolC. Mice were immunized with various immunogens via the s.c. route on days 0, 21, and 35. Serum was obtained from mice 14 days after the final boost with a mixture of CPS2B1 and LolC formulated with Alhydrogel-CpG (▲), CPS2B1 formulated with Alhydrogel-CpG (△), or LolC formulated with Alhydrogel-CpG (◊), and titers of IgM and IgG specific for B. pseudomallei CPS (A) and LolC (B) were determined by an ELISA. Individual symbols represent a single immunized mouse (n = 10 for each group), except where no response was expected (anti-CPS in the LolC group and anti-LolC in the CPS2B1 group), where samples were pooled by cage (n = 2 for these groups). The horizontal black lines represent the geometric means for each group. Significance at the 95% confidence level was determined by using a Mann-Whitney U test and is indicated (NS, not significant).
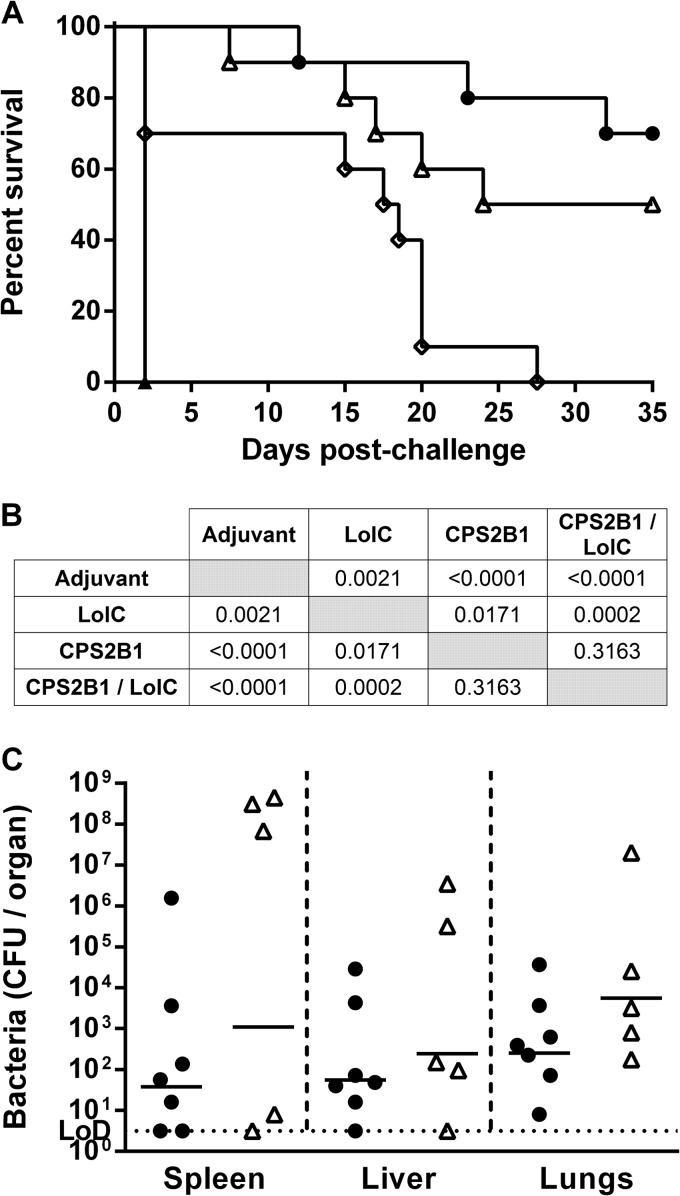
To assess the protective capacities of the various antigen formulations, immunized mice were challenged with B. pseudomallei K96243 5 weeks after the final boost, and signs of morbidity and mortality were monitored for 35 days (Fig. 6A). A higher challenge dose of approximately 8.44 × 104 CFU (113 MLDs at day 35) was used to assess the limits of protection offered by CPS2B1 alone as well as to provide a greater likelihood of observing differences in protection between the mice immunized with the mixture of CPS2B1 and LolC and those immunized with CPS2B1 alone. As anticipated, all of the mice immunized with the adjuvant-only control succumbed to infection by day 2. The mice immunized with the various antigen formulations had survival curves that were significantly different from those of the control mice (Fig. 6B), with a corresponding increase in the median time to death (control, 2 days; LolC, 18 days; CPS2B1, 29.5 days; CPS2B1 and LolC mixture, undefined). As shown in Fig. 6A, immunization with the mixture of CPS2B1 and LolC offered the greatest protection, with 70% of mice surviving for the duration of the study. Although this was a significant improvement over immunization with LolC alone (P = 0.0002), the survival curve was not significantly different from that of immunization with CPS2B1 alone (P = 0.3163). Interestingly, however, there was a difference in the clinical signs observed between the two vaccinated groups. Specifically, the surviving mice immunized with the mixture of CPS2B1 and LolC had no signs of disease throughout the study, whereas three of the five surviving mice immunized with CPS2B1 alone displayed clinical signs of disease (ruffled fur and hunched posture). At the end of the study, surviving mice (n = 7 for the mixture of CPS2B1 and LolC, and n = 5 for CPS2B1) were culled, and lungs, livers, and spleens were removed for enumeration of bacterial colonization (Fig. 6C). Due to the large variation in counts between the groups (particularly in the spleens), no significant differences were observed, although the geometric means were lower in the mice immunized with the mixture of CPS2B1 and LolC than in the mice immunized with CPS2B1 in all organs examined.
FIG 6.
Protective capacity of CPS2B1 mixed with LolC. Mice (n = 10 mice per group) were immunized with a mixture of CPS2B1 and LolC formulated with Alhydrogel-CpG (●), CPS2B1 formulated with Alhydrogel-CpG (△), LolC formulated with Alhydrogel-CpG (◊), or Alhydrogel/CpG alone (▲) via the s.c. route on days 0, 21, and 35. Five weeks after the final boost, mice were challenged i.p. with 8.4 × 104 CFU of B. pseudomallei K96243. (A) The mice were monitored until day 35 postchallenge, and survival was plotted. (B) Significance of survival was determined by using a log-rank (Mantel-Cox) test. (C) At the end of the study, surviving mice were culled (n = 7 for CPS2B1 mixed with LolC and n = 5 for CPS2B1), organs were removed, and bacterial burdens were determined. Due to the large variation in counts between the groups (particularly in the spleens) and the limited number of survivors, significant differences could not be assessed. The horizontal black lines represent the geometric means for each group.
DISCUSSION
Bacterial polysaccharides represent attractive antigens for the development of vaccines. In particular, capsular polysaccharides have been widely used to immunize against diseases caused by Streptococcus pneumoniae, Haemophilus influenzae, Neisseria meningitidis, and Salmonella enterica serovar Typhi (32). B. pseudomallei OPS and CPS antigens also represent attractive candidates to develop melioidosis vaccines, since they have both been identified as protective antigens in animal models using active (18) and passive (18, 20, 23–26) immunization strategies. In this report, two glycoconjugates composed of OPS or CPS linked to a common carrier protein were constructed, and their immunogenic potential and protective capacities were evaluated in a murine model of melioidosis. This work details for the first time the reported use of OPS- or CPS-based glycoconjugates for active immunization against melioidosis.
The two glycoconjugates described in this study were shown to contain roughly equivalent levels of polysaccharide (60% and 53% [wt/wt] for OPS2B1 and CPS2B1, respectively), which enabled direct comparisons between the immunogenic potentials of the constructs. Immunization of BALB/c mice with OPS2B1 and CPS2B1 induced similar levels of antigen-specific IgG, with endpoint titers of approximately 1:100,000. In both cases, the titers induced following immunization with the glycoconjugates were significantly higher than the titers induced by using unconjugated controls. Such findings are consistent with the ability of glycoconjugates to promote high-titer antibody responses against their carbohydrate components (33).
Previous work has shown that mice actively immunized with B. pseudomallei or B. thailandensis LPS were protected against melioidosis (50% survival by day 35 postchallenge [18, 43]). Similarly, both polyclonal and monoclonal antibodies recognizing OPS have been shown to offer significant protection in experimental models of melioidosis when administered passively (18, 20, 23–26). Thus, given the magnitude of the antibody responses raised against OPS2B1, it was surprising that the construct failed to provide protection in our challenge study. In our previous study, it is possible that the endotoxic activity associated with whole LPS antigens may have acted as a more efficient adjuvant to promote protective anti-OPS responses than the Alhydrogel-CpG adjuvant used in the present study. Studies are ongoing to investigate this interesting phenomenon.
In contrast to the lack of protection offered by OPS2B1, CPS2B1 provided excellent protection against challenge, with 90% of the mice surviving to day 21. Although some of the mice displayed signs of disease at this point, the survival curve was significantly different to that of the control mice. Since T-cell immunity directed against the CPS would not be expected to play a large role in this challenge study, it seems reasonable to speculate that the observed protection was likely due to the presence of high-titer CPS-specific antibodies. These results are consistent with previous studies demonstrating the protective capacity of CPS-specific monoclonal and polyclonal antibodies when administered passively to animals (18, 23, 25, 26).
Although the immunological basis of protection remains to be determined, it is clear from our results that CPS2B1 offers better protection than OPS2B1 in our animal model. It is worth noting that the challenge dose of B. pseudomallei used for the CPS2B1-treated mice was slightly lower than that used for the OPS2B1-treated mice and that this resulted in less acute disease in the control mice (MTTD of 12.5 days for control mice in the CPS2B1 study compared to 3.5 days for control mice in the OPS2B1 study). However, by 21 days postchallenge, survival rates of these groups were similar (30% and 20%, respectively), and the survival curves for the mice immunized with adjuvant in these studies were not significantly different (P = 0.4021). Based upon the results of these initial observations, we decided to further investigate the protective capacity of CPS2B1 as well as determine whether or not the protective capacity could be augmented by coformulation with another protective antigen. We decided to coformulate CPS2B1 with a B. pseudomallei protein antigen against which T-cell-mediated responses have been reported. For this, LolC was chosen, since it is a known protective antigen (36) and is recognized by gamma interferon-secreting T cells following immunization of mice with purified protein and by T cells of seropositive humans in areas of endemicity (36, 41, 42).
In initial studies, 90% of mice immunized with CPS2B1 survived to day 21 postchallenge, which would have complicated the ability to observe any differences in protection if the glycoconjugate had been coformulated with LolC. To address this issue, a higher challenge dose was used, and the duration of the second challenge study was extended out to 35 days. This approach also enabled the limits of protection offered by CPS2B1 alone to be further investigated. Using the higher dose, there was 100% mortality in the control mice within 48 h postchallenge. Immunization with LolC offered significant protection and an extended median time to death (18 days) compared to the control mice, but all mice eventually succumbed to infection before the end of the study. These data are consistent with previous reports for this antigen (36). In contrast, CPS2B1 performed significantly better than LolC, with 50% of mice surviving to the end of the study. However, most of the animals displayed external signs of disease, visible organ pathology (e.g., splenic abscesses), and bacterial colonization of their organs. It is interesting that CPS2B1, which would be predicted to stimulate protective anti-CPS humoral responses only (since cBSA is not a B. pseudomallei protein), resulted in greater survival than did B. pseudomallei LolC, which has the potential to elicit both protective humoral and cell-mediated responses. This finding supports recent findings by Silva et al. that suggest that humoral immunity is critically important for vaccine-induced protection against acute disease (44).
Immunization of the mice with the mixture of CPS2B1 and LolC appeared to have an additive effect and provided the highest level of protection in this study (with 70% of mice surviving to 35 days). While protection afforded by the combination was significantly improved compared to that afforded by LolC immunization, it was not significantly improved in comparison to that afforded by CPS2B1 immunization. Importantly, however, the surviving mice immunized with the CPS2B1 and LolC mixture did not display clinical signs of disease throughout the study, whereas three of the surviving CPS2B1 mice displayed signs of disease and would likely have succumbed to infection if the study had been extended. Based upon these findings, it appears that immunization with the mixture of CPS2B1 and LolC provided the greatest degree of protection, as measured by survival and decreased signs of clinical disease in the surviving mice. The specific mechanisms underlying this protection have not yet been elucidated. It is tempting to speculate, however, that the high levels of CPS-specific antibodies protected the mice against the initial acute infection by reducing extracellular bacterial numbers, whereas LolC-specific cell-mediated responses were important for the control of intracellular bacteria later in infection. This possibility is in line with previous data suggesting that both antibody- and cell-mediated mechanisms are important for protection (45) and is currently being investigated by our laboratories.
Collectively, the results of our current study suggest that CPS-based glycoconjugates are promising candidates for the development of subunit vaccines for immunization against melioidosis. Future studies will be required, however, to more thoroughly investigate this possibility as well as better establish immune correlates of protection for this subunit vaccine.
ACKNOWLEDGMENT
This work was supported by funding from the United Kingdom Ministry of Defense.
Footnotes
Published ahead of print 27 May 2014
REFERENCES
- 1.Cheng AC, Currie BJ. 2005. Melioidosis: epidemiology, pathophysiology, and management. Clin. Microbiol. Rev. 18:383–416. 10.1128/CMR.18.2.383-416.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Currie BJ, Dance DAB, Cheng AC. 2008. The global distribution of Burkholderia pseudomallei and melioidosis: an update. Trans. R. Soc. Trop. Med. Hyg. 102:S1–S4. 10.1016/S0035-9203(08)70002-6. [DOI] [PubMed] [Google Scholar]
- 3.Wiersinga WJ, Currie BJ, Peacock SJ. 2012. Melioidosis. N. Engl. J. Med. 367:1035–1044. 10.1056/NEJMra1204699. [DOI] [PubMed] [Google Scholar]
- 4.Dance DAB. 2000. Ecology of Burkholderia pseudomallei and the interactions between environmental Burkholderia spp. and human-animal hosts. Acta Trop. 74:159–168. 10.1016/S0001-706X(99)00066-2. [DOI] [PubMed] [Google Scholar]
- 5.Wiersinga WJ, van der Poll T, White NJ, Day NP, Peacock SJ. 2006. Melioidosis: insights into the pathogenicity of Burkholderia pseudomallei. Nat. Rev. Microbiol. 4:272–282. 10.1038/nrmicro1385. [DOI] [PubMed] [Google Scholar]
- 6.Meumann EM, Cheng AC, Ward L, Currie BJ. 2012. Clinical features and epidemiology of melioidosis pneumonia: results from a 21-year study and review of the literature. Clin. Infect. Dis. 54:362–369. 10.1093/cid/cir808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Currie BJ, Ward L, Cheng AC. 2010. The epidemiology and clinical spectrum of melioidosis: 540 cases from the 20 year Darwin prospective study. PLoS Negl. Trop. Dis. 4:e900. 10.1371/journal.pntd.0000900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chaowagul W, White NJ, Dance DAB, Wattanagoon Y, Naigowit P, Davis TME, Looareesuwan S, Pitakwatchara N. 1989. Melioidosis: a major cause of community-acquired septicemia in Northeastern Thailand. J. Infect. Dis. 159:890–899. 10.1093/infdis/159.5.890. [DOI] [PubMed] [Google Scholar]
- 9.Currie BJ, Fisher DA, Howard DM, Burrow JNC, Lo D, Selva-nayagam S, Anstey NM, Huffam SE, Snelling PL, Marks PJ, Stephens DP, Lum GD, Jacups SP, Krause VL. 2000. Endemic melioidosis in tropical Northern Australia: a 10-year prospective study and review of the literature. Clin. Infect. Dis. 31:981–986. 10.1086/318116. [DOI] [PubMed] [Google Scholar]
- 10.Limmathurotsakul D, Wongratanacheewin S, Teerawattanasook N, Wongsuvan G, Chaisuksant S, Chetchotisakd P, Chaowagul W, Day NPJ, Peacock SJ. 2010. Increasing incidence of human melioidosis in Northeast Thailand. Am. J. Trop. Med. Hyg. 82:1113–1117. 10.4269/ajtmh.2010.10-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Silva EB, Dow SW. 2013. Development of Burkholderia mallei and pseudomallei vaccines. Front. Cell. Infect. Microbiol. 3:10. 10.3389/fcimb.2013.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patel N, Conejero L, De Reynal M, Easton A, Bancroft GJ, Titball RW. 2011. Development of vaccines against Burkholderia pseudomallei. Front. Microbiol. 2:198. 10.3389/fmicb.2011.00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choh L-C, Ong G-H, Vellasamy KM, Kalaiselvam K, Kang W-T, Al-Maleki AR, Mariappan V, Vadivelu J. 2013. Burkholderia vaccines: are we moving forward? Front. Cell. Infect. Microbiol. 3:5. 10.3389/fcimb.2013.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galyov EE, Brett PJ, DeShazer D. 2010. Molecular insights into Burkholderia pseudomallei and Burkholderia mallei pathogenesis. Annu. Rev. Microbiol. 64:495–517. 10.1146/annurev.micro.112408.134030. [DOI] [PubMed] [Google Scholar]
- 15.Atkins T, Prior R, Mack K, Russell P, Nelson M, Prior J, Ellis J, Oyston P, Dougan G, Titball R. 2002. Characterisation of an acapsular mutant of Burkholderia pseudomallei identified by signature tagged mutagenesis. J. Med. Microbiol. 51:539–553 http://jmm.sgmjournals.org/content/51/7/539.long. [DOI] [PubMed] [Google Scholar]
- 16.Reckseidler SL, DeShazer D, Sokol PA, Woods DE. 2001. Detection of bacterial virulence genes by subtractive hybridization: identification of capsular polysaccharide of Burkholderia pseudomallei as a major virulence determinant. Infect. Immun. 69:34–44. 10.1128/IAI.69.1.34-44.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeShazer D, Brett PJ, Woods DE. 1998. The type II O-antigenic polysaccharide moiety of Burkholderia pseudomallei lipopolysaccharide is required for serum resistance and virulence. Mol. Microbiol. 30:1081–1100. [DOI] [PubMed] [Google Scholar]
- 18.Nelson M, Prior JL, Lever MS, Jones HE, Atkins TP, Titball RW. 2004. Evaluation of lipopolysaccharide and capsular polysaccharide as subunit vaccines against experimental melioidosis. J. Med. Microbiol. 53:1177–1182. 10.1099/jmm.0.45766-0. [DOI] [PubMed] [Google Scholar]
- 19.Burtnick MN, Heiss C, Roberts RA, Schweizer HP, Azadi P, Brett PJ. 2012. Development of capsular polysaccharide-based glycoconjugates for immunization against melioidosis and glanders. Front. Cell. Infect. Microbiol. 2:108. 10.3389/fcimb.2012.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brett PJ, Woods DE. 1996. Structural and immunological characterization of Burkholderia pseudomallei O-polysaccharide-flagellin protein conjugates. Infect. Immun. 64:2824–2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anuntagool N, Wuthiekanun V, White NJ, Currie BJ, Sermswan RW, Wongratanacheewin S, Taweechaisupapong S, Chaiyaroj SC, Sirisinha S. 2006. Lipopolysaccharide heterogeneity among Burkholderia pseudomallei from different geographic and clinical origins. Am. J. Trop. Med. Hyg. 74:348–352 http://www.ajtmh.org/content/74/3/348.long. [PubMed] [Google Scholar]
- 22.Zou N, Tsai S, Feng S-H, Newsome T, Kim H-Y, Li B, Zhang S, Lo S-C. 2008. Relationship between antigenicity and pathogenicity for Burkholderia pseudomallei and Burkholderia mallei revealed by a large panel of mouse MAbs. Hybridoma 27:231–240. 10.1089/hyb.2008.0012. [DOI] [PubMed] [Google Scholar]
- 23.Jones S, Ellis J, Russell P, Griffin K, Oyston P. 2002. Passive protection against Burkholderia pseudomallei infection in mice by monoclonal antibodies against capsular polysaccharide, lipopolysaccharide or proteins. J. Med. Microbiol. 51:1055–1062 http://jmm.sgmjournals.org/content/51/12/1055.long. [DOI] [PubMed] [Google Scholar]
- 24.Bryan LE, Wong S, Woods DE, Dance DA, Chaowagul W. 1994. Passive protection of diabetic rats with antisera specific for the polysaccharide portion of the lipopolysaccharide isolated from Pseudomonas pseudomallei. Can. J. Infect. Dis. 5:170–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang S, Feng S-H, Li B, Kim H-Y, Rodriguez J, Tsai S, Lo S-C. 2011. In vitro and in vivo studies of monoclonal antibodies with prominent bactericidal activity against Burkholderia pseudomallei and Burkholderia mallei. Clin. Vaccine Immunol. 18:825–834. 10.1128/CVI.00533-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.AuCoin DP, Reed DE, Marlenee NL, Bowen RA, Thorkildson P, Judy BM, Torres AG, Kozel TR. 2012. Polysaccharide specific monoclonal antibodies provide passive protection against intranasal challenge with Burkholderia pseudomallei. PLoS One 7:e35386. 10.1371/journal.pone.0035386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mond JJ, Lees A, Snapper CM. 1995. T cell-independent antigens type 2. Annu. Rev. Immunol. 13:655–692. 10.1146/annurev.iy.13.040195.003255. [DOI] [PubMed] [Google Scholar]
- 28.Snapper CM, Mond JJ. 1996. A model for induction of T cell-independent humoral immunity in response to polysaccharide antigens. J. Immunol. 157:2229–2233. [PubMed] [Google Scholar]
- 29.Weintraub A. 2003. Immunology of bacterial polysaccharide antigens. Carbohydr. Res. 338:2539–2547. 10.1016/j.carres.2003.07.008. [DOI] [PubMed] [Google Scholar]
- 30.Stein KE. 1992. Thymus-independent and thymus-dependent responses to polysaccharide antigens. J. Infect. Dis. 165:S49–S52. 10.1093/infdis/165-Supplement_1-S49. [DOI] [PubMed] [Google Scholar]
- 31.Lockhart S. 2003. Conjugate vaccines. Expert Rev. Vaccines 2:633–648. 10.1586/14760584.2.5.633. [DOI] [PubMed] [Google Scholar]
- 32.Rappuoli R, Bagnoli F. 2011. Vaccine design. Caister Academic Press, Norfolk, United Kingdom. [Google Scholar]
- 33.Kelly DF, Snape MD, Clutterbuck EA, Green S, Snowden C, Diggle L, Yu L-M, Borkowski A, Moxon ER, Pollard AJ. 2006. CRM197-conjugated serogroup C meningococcal capsular polysaccharide, but not the native polysaccharide, induces persistent antigen-specific memory B cells. Blood 108:2642–2647. 10.1182/blood-2006-01-009282. [DOI] [PubMed] [Google Scholar]
- 34.Holden MTG, Titball RW, Peacock SJ, Cerdeño-Tárraga AM, Atkins T, Crossman LC, Pitt T, Churcher C, Mungall K, Bentley SD, Sebaihia M, Thomson NR, Bason N, Beacham IR, Brooks K, Brown KA, Brown NF, Challis GL, Cherevach I, Chillingworth T, Cronin A, Crossett B, Davis P, DeShazer D, Feltwell T, Fraser A, Hance Z, Hauser H, Holroyd S, Jagels K, Keith KE, Maddison M, Moule S, Price C, Quail MA, Rabbinowitsch E, Rutherford K, Sanders M, Simmonds M, Songsivilai S, Stevens K, Tumapa S, Vesaratchavest M, Whitehead S, Yeats C, Barrell BG, Oyston PCF, Parkhill J. 2004. Genomic plasticity of the causative agent of melioidosis, Burkholderia pseudomallei. Proc. Natl. Acad. Sci. U. S. A. 101:14240–14245. 10.1073/pnas.0403302101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heiss C, Burtnick MN, Roberts RA, Black I, Azadi P, Brett PJ. 2013. Revised structures for the predominant O-polysaccharides expressed by Burkholderia pseudomallei and Burkholderia mallei. Carbohydr. Res. 381:6–11. 10.1016/j.carres.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harland DN, Chu K, Haque A, Nelson M, Walker NJ, Sarkar-Tyson M, Atkins TP, Moore B, Brown KA, Bancroft G, Titball RW, Atkins HS. 2007. Identification of a LolC homologue in Burkholderia pseudomallei, a novel protective antigen for melioidosis. Infect. Immun. 75:4173–4180. 10.1128/IAI.00404-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Propst KL, Mima T, Choi KH, Dow SW, Schweizer HP. 2010. A Burkholderia pseudomallei delta purM mutant is avirulent in immunocompetent and immunodeficient animals: candidate strain for exclusion from select-agent lists. Infect. Immun. 78:3136–3143. 10.1128/IAI.01313-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perry MB, MacLean LL, Schollaardt T, Bryan LE, Ho M. 1995. Structural characterization of the lipopolysaccharide O antigens of Burkholderia pseudomallei. Infect. Immun. 63:3348–3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burtnick MN, Heiss C, Schuler AM, Azadi P, Brett PJ. 2012. Development of novel O-polysaccharide based glycoconjugates for immunization against glanders. Front. Cell. Infect. Microbiol. 2:148. 10.3389/fcimb.2012.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scott AE, Laws TR, D'Elia RV, Stokes MGM, Nandi T, Williamson ED, Tan P, Prior JL, Atkins TP. 2013. Protection against experimental melioidosis following immunization with live Burkholderia thailandensis expressing manno-heptose capsule. Clin. Vaccine Immunol. 20:1041–1047. 10.1128/CVI.00113-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chu KK, Tippayawat P, Walker NJ, Harding SV, Atkins HS, Maillere B, Bancroft GJ, Lertmemongkolchai G, Altmann DM. 2011. CD4+ T-cell immunity to the Burkholderia pseudomallei ABC transporter LolC in melioidosis. Eur. J. Immunol. 41:107–115. 10.1002/eji.201040881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tippayawat P, Saenwongsa W, Mahawantung J, Suwannasaen D, Chetchotisakd P, Limmathurotsakul D, Peacock SJ, Felgner PL, Atkins HS, Titball RW, Bancroft GJ, Lertmemongkolchai G. 2009. Phenotypic and functional characterization of human memory T cell responses to Burkholderia pseudomallei. PLoS Negl. Trop. Dis. 3:e407. 10.1371/journal.pntd.0000407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ngugi SA, Ventura VV, Qazi O, Harding SV, Kitto GB, Estes DM, Dell A, Titball RW, Atkins TP, Brown KA, Hitchen PG, Prior JL. 2010. Lipopolysaccharide from Burkholderia thailandensis E264 provides protection in a murine model of melioidosis. Vaccine 28:7551–7555. 10.1016/j.vaccine.2010.08.058. [DOI] [PubMed] [Google Scholar]
- 44.Silva EB, Goodyear A, Sutherland MD, Podnecky NL, Gonzalez-Juarrero M, Schweizer HP, Dow SW. 2013. Correlates of immune protection following cutaneous immunization with an attenuated Burkholderia pseudomallei vaccine. Infect. Immun. 81:4626–4634. 10.1128/IAI.00915-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Healey GD, Elvin SJ, Morton M, Williamson ED. 2005. Humoral and cell-mediated adaptive immune responses are required for protection against Burkholderia pseudomallei challenge and bacterial clearance postinfection. Infect. Immun. 73:5945–5951. 10.1128/IAI.73.9.5945-5951.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]