Abstract
Bacterial lipopolysaccharides (LPS) are structural components of the outer membranes of Gram-negative bacteria and also are potent inducers of inflammation in mammals. Higher vertebrates are extremely sensitive to LPS, but lower vertebrates, like fish, are resistant to their systemic toxic effects. However, the effects of LPS on the fish intestinal mucosa remain unknown. Edwardsiella ictaluri is a primitive member of the Enterobacteriaceae family that causes enteric septicemia in channel catfish (Ictalurus punctatus). E. ictaluri infects and colonizes deep lymphoid tissues upon oral or immersion infection. Both gut and olfactory organs are the primary sites of invasion. At the systemic level, E. ictaluri pathogenesis is relatively well characterized, but our knowledge about E. ictaluri intestinal interaction is limited. Recently, we observed that E. ictaluri oligo-polysaccharide (O-PS) LPS mutants have differential effects on the intestinal epithelia of orally inoculated catfish. Here we evaluate the effects of E. ictaluri O-PS LPS mutants by using a novel catfish intestinal loop model and compare it to the rabbit ileal loop model inoculated with Salmonella enterica serovar Typhimurium LPS. We found evident differences in rabbit ileal loop and catfish ileal loop responses to E. ictaluri and S. Typhimurium LPS. We determined that catfish respond to E. ictaluri LPS but not to S. Typhimurium LPS. We also determined that E. ictaluri inhibits cytokine production and induces disruption of the intestinal fish epithelia in an O-PS-dependent fashion. The E. ictaluri wild type and ΔwibT LPS mutant caused intestinal tissue damage and inhibited proinflammatory cytokine synthesis, in contrast to E. ictaluri Δgne and Δugd LPS mutants. We concluded that the E. ictaluri O-PS subunits play a major role during pathogenesis, since they influence the recognition of the LPS by the intestinal mucosal immune system of the catfish. The LPS structure of E. ictaluri mutants is needed to understand the mechanism of interaction.
INTRODUCTION
The genus Edwardsiella, which consists of the four species E. tarda, E. hoshinae, E. piscicida, and E. ictaluri, is one of the most primitive members of the Enterobacteriaceae family (1). E. ictaluri is one of the most important pathogens of commercially raised channel catfish (Ictalurus punctatus) (2), which account for more than 80% of U.S. aquaculture production, in spite of the recent production decrease (3, 4). E. ictaluri infects and colonizes catfish internal lymphoid tissues upon oral or bath infection, making it a promising strain to develop effective live attenuated recombinant vaccines for the catfish industry. Both gut and olfactory organs are the primary sites of invasion of E. ictaluri in natural outbreaks (5). E. ictaluri crosses the intestinal mucosa of channel catfish in 15 min after oral inoculation with 109 CFU (6). Although there are substantial descriptive data relative to the invasion, spread, and persistence of E. ictaluri in channel catfish (6–8), little is known about the molecular mechanisms of E. ictaluri fish intestinal pathogenicity and the pathogen-associated molecular patterns (PAMPs) recognized by fish.
One of the most studied PAMPs is the lipopolysaccharide (LPS) that in mammals is recognized by the Toll-like receptor 4 (TLR-4) (9–11). LPS is the major component of the external layer of the outer membrane of Gram-negative bacteria. LPS is composed of three distinct parts: carbohydrate subunits or oligo-polysaccharides (O-PS), the oligosaccharide core region, and lipid A, which is responsible for the activation of the innate immune response in mammals and confers the endotoxic properties of the LPS (12). On the other hand, fish, in contrast to mammals, are remarkable resistant to the toxic effects of LPS (13–15).
LPS is an important virulence factor for E. ictaluri (16–18). The E. ictaluri LPS gene cluster has been identified by transposon mutagenesis (16, 17) and was recently fully described (18). E. ictaluri LPS O-PS mutants exhibited different levels of virulence, tissue colonization, and intestinal gut inflammation in orally inoculated catfish (18). Indeed, the E. ictaluri wild type causes diarrhea-like symptoms in orally infected fish, in contrast to E. ictaluri LPS mutant strains (18). This observation correlates with the current idea that fish recognize the O-PS of the LPS instead of the lipid A (19) and that fish recognize LPS at the intestinal level.
Ligated ileal loops have been used to evaluate the contribution of LPS to intestinal bacterial colitis in rabbits, mice, and calves (20–23). Initially ligated loops of rabbit small intestine were used as a model to assess the contribution of putative virulence factors to bacterial pathogen-induced diarrhea. This model was used for Vibrio cholerae, where the injection of whole cultures (24, 25), culture supernatants (26), and cell extracts (27) caused dilation of the loop due to fluid accumulation. The rabbit ileal loop model also has been used to study pathogenesis of Escherichia coli (28–30), Salmonella (20, 31, 32), Shigella (33), Pseudomonas aeruginosa (34), Clostridium perfringens (35), Vibrio parahaemolyticus, Vibrio alcaligenes (36), and Bacillus cereus (37). In the search for a model that mimics the human intestinal bacterial infection and inflammatory responses, murine and bovine ligated ileal loops also have been used (38–40).
The complete LPS structure of E. ictaluri has not been elucidated. Nevertheless, the composition and structure of the E. ictaluri O-PS have been reported (41). The E. ictaluri typical O-chain was found to be an unbranched linear polymer of a repeating tetrasaccharide unit composed of d-glucose, 2-acetamido-2-deoxy-d-galactose, and d-galactose in a 1:2:1 ratio having the structure [→4)-α-d-Glcp-(1→4)-α-d-GalpNAc-(1→3)-β-d-GalpNAc-(1→4)-β-d-Galp-(1→]n (41). The E. ictaluri O-PS biosynthesis enzymes are encoded by four genes, wibT, galF, gne, and ugd, located in the O-PS gene cluster (17, 18). As mentioned previously, we determined that catfish orally inoculated with the E. ictaluri wild type developed diarrhea-like symptoms, in contrast to fish inoculated with LPS-defective (ΔwibT, Δgne, and Δugd) mutants (18). Intestinal diseases often lead to disruption of the intestinal epithelial barrier either through attachment and internalization-mediated effector molecule release or through stimulation of host inflammatory responses that ultimately compromise junctional integrity (42). Several studies have begun to explore the cellular and molecular compositions of mucosal surfaces in salmonids (43, 44), carp (45), cod (46), flounder (47), and catfish (48–50). Recently, it has been suggested that E. ictaluri survives in intestinal macrophages (18) and causes intestinal barrier disruption and immune suppression (48). Using a novel catfish intestinal loop model, we corroborated that E. ictaluri caused intestinal barrier disruption and immunosuppression in an LPS O-PS-dependent fashion. Furthermore, we determined that E. ictaluri LPS O-PS plays a major role during catfish intestinal infection and immune protective stimulation by a live attenuated E. ictaluri vaccine.
MATERIALS AND METHODS
Ethics statement.
All research involving fish was conducted as per protocol 09-1042R, approved by the Arizona State University Institutional Animal Care and Use Committee.
Bacterial strains, media, and reagents.
The bacterial strains and plasmids used in this study are listed in Table 1. Bacteriological media and components are from Difco (Franklin Lakes, NJ). Antibiotics and reagents are from Sigma (St. Louis, MO). LB broth (tryptone, 10 g; yeast extract, 5 g; NaCl, 10 g; dextrose, 1 g; double-distilled water [ddH2O], 1 liter) (51) and Bacto brain heart infusion (BHI) were used routinely. When required, the media were supplemented with 1.5% agar or colistin sulfate (Col) (12.5 μg/ml). Bacterial growth was monitored spectrophotometrically and/or by plating.
TABLE 1.
Bacterial strains used in this study
| Strain | Relevant characteristic(s) | Source or reference |
|---|---|---|
| S. Typhimurium χ3761 | Wild-type UK-1 | 91, 92 |
| E. ictaluri | ||
| J100 | Wild-type 2003/c, isolated from channel catfish (Ictalurus punctatus); pEI1+ pEI2+; API20E 40040057, 100% E. ictaluri; smooth LPS; Colr Pmbr Pror; H2S− and H2O2+; ox bile and sodium deoxycholate resistance, >60 mg/ml | 93, 94 |
| J124 | ΔwibT90, J100 derivative; pEI1+ pEI2+; API20E 40040057, 100% E. ictaluri; rough LPS; Colr Pmbr Pror; H2S− and H2O2+; ox bile and sodium deoxycholate resistance, >60 mg/ml | 18 |
| J126 | Δgne-31 J100 derivative; pEI1+ pEI2+; API20E 40040057, 100% E. ictaluri; rough LPS; Colr Pmbr Pror; H2S− and H2O2+; ox bile and sodium deoxycholate resistance, >60 mg/ml | 18 |
| J135 | Δugd-11 J100 derivative; pEI1+ pEI2+; API20E 40040057, 100% E. ictaluri; rough LPS; Cols Pmbs Pros; H2S− and H2O2+; ox bile and sodium deoxycholate resistance, >60 mg/ml | 18 |
Bacterial inoculate preparation.
Bacterial strains were grown overnight standing, and then the cultures were diluted 1:20 in prewarmed BHI broth and grown with mild aeration (180 rpm) at 28°C to an optical density at 600 nm (OD600) of 0.8 to 0.9 (∼108 CFU/ml). Bacteria were sedimented for 10 min by centrifugation (7,000 rpm) at room temperature and resuspended in saline (NaCl, 0.85%) to appropriate densities for inoculation.
Bile sensitivity.
Sensitivity to bile was determined by the microplate serial dilution assay. This assay was performed using flat-bottom 96-well clear microtiter plates. Ox bile and sodium deoxycholate were serially diluted in BHI broth and then inoculated with mid-log-phase cultures of the E. ictaluri strains. The plates were incubated for 48 h at 28°C.
LPS purification and analysis.
LPS extraction was performed by using TRI reagent (Sigma) as described previously (52). The LPS profile was evaluated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and visualized by silver staining (53, 54). Protein contamination was evaluated by SYPRO Ruby (Invitrogene) staining and UV scanning visualization (Typhoon Trio multimode imager; GE Healthcare) compared to commercial LPS (Sigma).
OMP preparation and purification.
Sarkosyl-insoluble outer membrane proteins (OMPs) were obtained as previously described (55). The outer membrane proteins were LPS detoxified and normalized to 25 μg/μl by using a Nanodrop ND-1000 spectrophotometer and separated by 10% (wt/vol) SDS-PAGE for verification. Coomassie blue staining was performed to visualize proteins. The residual LPS was removed from the OMPs by Detoxi-Gel endotoxin columns (Thermo) and verified by LPS profiles.
Rabbit surgery.
The rabbits were fasted (but provided water ad lib) overnight (maximum of 16 h) and then premedicated with 30 mg/kg of body weight ketamine and 6 mg/kg xylazine intramuscularly (i.m.). Then, while masked with isoflurane in oxygen to a surgical level of anesthesia (as indicated by lack of toe pinch reflex, ear twitch reflexes, and stable heart rate via pulse oximetry), the ventral surface of the neck was shaved and disinfected by sequential washes with chlorhexidine and alcohol. A cut-down tracheotomy was performed, and an endotracheal tube was placed to ensure a patent airway throughout the procedure. At this point, mask administration of isoflurane was discontinued, and further administration was delivered via the endotracheal tube. Additionally, an intravenous catheter was placed in an ear vessel to deliver lactated Ringer's solution. The rabbits were monitored for depth of anesthesia using pulse oximetry (heart rate and peripheral capillary oxygen saturation [SpO2]), ventilatory rate, and the lack of a toe pinch reflex. Abdominal hair was removed along the mid-ventral body wall, and the skin was disinfected using sequential washes with chlorhexidine and alcohol. An approximately 10-cm midline incision was made through the skin and body wall. The most distal region of the ileum was isolated, and a 4- to 6-cm section was double ligated at each end. Working proximally up the intestine, 6 more intestinal loops were created, each 1 to 2 cm apart. Throughout the procedure, sterile saline and moist gauze were used to keep the viscera and body walls moist. After creation of all loops, 1.0 ml of LPS (100 μg), bacterial culture (about 107 CFU), or sterilized saline (NaCl, 0.85%) as a negative control was injected into each loop. The body wall and skin were separately closed using a simple continuous suture pattern. The rabbit was anesthetized for 6 h, while being continuously observed. After 6 h, the rabbit was euthanized using an overdose of sodium pentobarbital (150 mg/kg) injected intravenously. The intestinal loops were then removed, the length of each segment was measured, and the intestinal fluid was extracted with the volume-to-length (V:L) ratio in milliliters per centimeter for each loop recorded. A piece of the intestinal tissue was fixed in 10% formalin and subjected to histopathological study. Each slide was graded on the basis of degree of mucosal disruption, cellularity, and vascular congestion.
Channel catfish surgery.
Thirty-five outbred channel catfish that were specific-pathogen free and with a mean weight of 2 kg ± 10 g were used. The animals were acclimatized for 1 week prior to surgery in 100-liter tanks at 26 ± 1°C. Each tank is equipped with a recirculating, biofiltered, mechanically filtered, and UV water-treated system with a 12-h light cycle per day. The fish were fed daily with commercial Aquamax (Purina Mills, Inc., St. Louis, MO). The water quality was monitored for pH, NO2, and NO3 with standard kits. Two days prior to surgery, the animals were fastened. The fish were anesthetized with buffered tricaine methanesulfonate (pH 7.5) (56). Four anesthesia doses were used: a fish handling dose (15 mg/liter, 25 to 26°C), a fish surgery dose (100 mg/liter, 20 to 22°C), a recovery dose (30 mg/liter, 20 to 22°C), and a euthanasia dose (300 mg/liter, 10 to 15°C). From the acclimatization tanks, the fish was moved to the handling anesthesia dose for at least 20 min, and then the fish was moved to the surgery platform in a supine position (Fig. 1) and connected to the surgical anesthesia dose. The fish platform developed here is a modified version of previously described fish surgery platforms (57, 58). The body is partially submerged in water (the surgical site will remain above the water line), and the recirculating water flows continuously through the mouth and over the gills (Fig. 1A and B). This permitted the fish to be in position for the surgery while effectively ventilating the fish. Once the fish was fully anesthetized, its body wall was cut, and the coelomic cavity was entered via blunt dissection (Fig. 1C). The lower small intestine was isolated, and up to 6 sections of 3 cm each were prepared by double ligation (Fig. 1D). One hundred micrograms of purified LPS in 500 μl of phosphate-buffered saline (PBS) or 500 μl of a 107-CFU/ml dose of E. ictaluri strains was injected into each intestinal section (Fig. 1E). The control consisted of loops inoculated with PBS, OMPs (100 μg), or peptidoglycan from Staphylococcus aureus (100 μg; Sigma). After injection, the body wall was sutured closed (Fig. 1F), and the fish was moved to a bath containing a recovery anesthesia dose, where it remained for up to 6 h to allow time for the intestine to respond to the inoculate (Fig. 1G). After this period, the fish was euthanized with a high concentration of buffered tricaine (300 mg/liter) followed by the harvesting of vital organs as a secondary method. The intestinal loops were then removed, the length of each segment was measured, and the intestinal fluid extracted with the volume-to-length (V:L) ratio in milliliters per centimeter for each loop was recorded. A piece of the intestinal tissue was fixed in 10% formalin and subjected to histopathological study. Each slide was graded on the basis of degree of mucosal disruption, cellularity, and vascular congestion.
FIG 1.
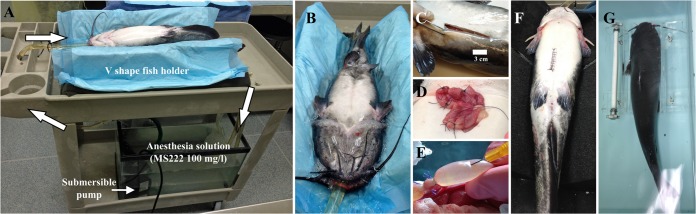
Fish intestinal loop surgery process. (A) Fish surgery platform. The arrows indicate the water flow. (B) Fish anesthesia application. (C) Incision in the catfish to enter the coelomic cavity. (D) Catfish intestinal loops. (E) Injection of intestinal loop. (F) Suture of the incision. (G) Recovery bath (MS222, 30 mg/liter).
qRT-PCR.
Gut samples were frozen in liquid nitrogen prior to grinding. Total RNA was isolated using TRIzol LS reagent (Invitrogen) according to the manufacturer's instructions. Extracted RNA was quantified by UV absorption using a NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies). The RNA was stored at −80°C before use in cDNA synthesis. Double-stranded cDNA was synthesized using a SuperScript III 1st strand synthesis kit (Invitrogen) in a final volume of 20 μl containing 2 μg of total RNA, 50 ng of random hexamers, 1 μl (10 mM) deoxynucleoside triphosphate (dNTP), 2 μl (0.1 M) dithiothreitol (DTT), 2 μl 10× reverse transcription (RT) buffer, 4 μl (25 mM) MgCl2, 1 μl (40 U/μl) RNase-out, and 1 μl (200 U/μl) SuperScript III reverse transcriptase and incubated at 50°C for 50 min and at 85°C for 5 min to terminate the reactions. One microliter of cDNA was subsequently used as the template in quantitative real-time reverse transcription-PCR (qRT-PCR) using the catfish-specific inflammatory cytokine primers listed in Table 2. RT-PCRs were performed using the iQ SYBR green supermix (Bio-Rad) on the Bio-Rad multicolor real-time PCR detection system with programmed thermal cycling conditions consisting of 40 cycles of 95°C for 10 s, 60°C for 30 s, and 72°C for 30 s. Each sample was normalized to the equivalent of the β-actin housekeeping gene. The relative expression of the target gene was estimated from the threshold cycles (CT) according to the 2−ΔΔCT method (59). Statistical analyses were performed using GraphPad Prism version 6.00 for Windows (Graph-Pad Software). Statistical comparison was performed using unpaired Student's t test. The significance level of the Student t test was set at P < 0.05.
TABLE 2.
Primers used for qRT-PCR in this study
| Gene product | Primer |
Reference | |
|---|---|---|---|
| Orientation | Sequence | ||
| β-Actin | Forward | AGAGAGAAATTGTCCGTGACATC | 62 |
| Reverse | CTCCGATCCAGACAGAGTATTTG | ||
| TNF-α | Forward | GGCCTCTACTTCGTCTAC | 61 |
| Reverse | GCAGCAGCTTCTCGTCCAT | ||
| IL-1βa | Forward | CGGCAGATGTGACCTGCACA | 60 |
| Reverse | CAGAGTAAAAGCCAGCAGAAG | ||
| IL-8 | Forward | CACCACGATGAAGGCTGCAACTC | 62 |
| Reverse | TGTCCTTGGTTTCCTTCTGG | ||
Statistics.
Analysis of variance (ANOVA) (SPSS Software) analysis followed by the least significant difference (LSD) method was used to evaluate differences in bacterial titers discerned to 95% confidence intervals. P < 0.05 was considered statistically significant.
RESULTS
E. ictaluri bile sensitivity.
Bile is one of the main antibacterial components of the intestinal fluids. Thus, we evaluate whether the E. ictaluri LPS (Fig. 2) mutant strains are sensitive to ox bile and sodium deoxycholate (Table 1). All of the strains used in this study were highly resistant to bile, suggesting that the LPS does not play role on bile resistance in E. ictaluri.
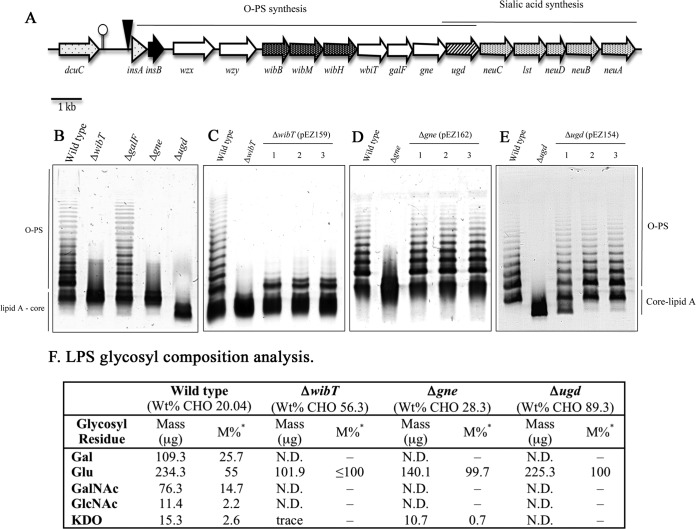
FIG 2.
Phenotype of E. ictaluri O-PS mutants. (A) Map of E. ictaluri oligo-polysaccharide genes. (B) LPS profile of E. ictaluri O-PS mutants. (C to E) Complementation of O-PS mutants. (F) Table of LPS glycosyl composition analysis. Wt, wild type; *M%, values expressed as mol% of total carbohydrate; N.D., not detected; Gal, galactose; Glu, glucose; GalNAc, N-acetylgalactosamine; GlcNAc, N-acetylglucosamine; KDO, 2-keto-3-deoxyoctonic acid. Adapted from reference 20.
Rabbit intestinal ileal loops inoculated with purified LPS.
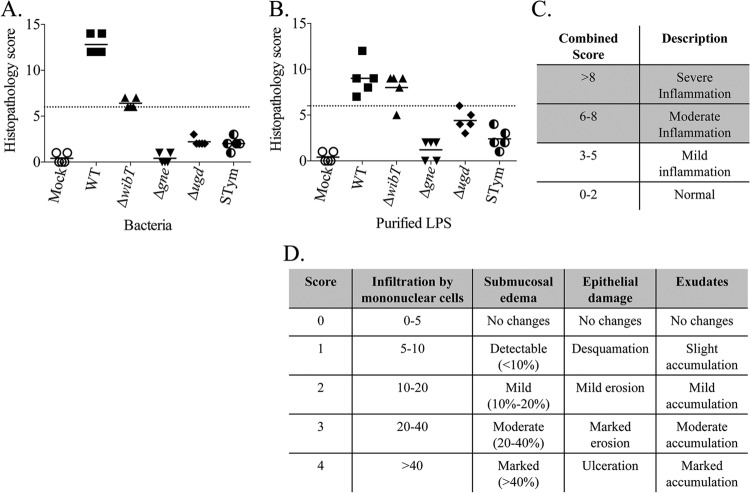
Salmonella enterica serovar Typhimurium LPS triggered significant fluid secretion with the presence of blood (Fig. 3A), which correlated with the intestinal inflammation and tissue damage (Fig. 4). In contrast, E. ictaluri wild-type LPS triggered low fluid secretion (Fig. 3A) and mild inflammation of the intestinal epithelia without severe tissue damage (Fig. 4). E. ictaluri LPS mutants, including the ΔwibT, Δgne, and Δugd LPS, did not trigger fluid secretion (Fig. 3A). E. ictaluri ΔwibT and Δgne LPS triggered mild epithelial damage (Fig. 4). In contrast, E. ictaluri Δugd LPS did not cause tissue damage on the rabbit ligated loops (Fig. 4). Loops injected with OMPs or peptidoglycan did not show any differences compared to the control.
FIG 3.
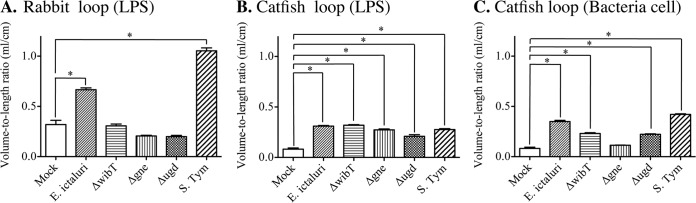
Catfish intestine loops and fluid secretion. (A) Rabbit loops 6 h postinoculation with 100 μg of LPS. (B) Catfish loops 6 h postinoculation with 100 μg of LPS. (C) Catfish fluid secretion from the intestinal loops 6 h postinoculation with 107 CFU/ml of E. ictaluri. S. Typhimurium was used as a control (108 CFU/ml). The number of animals per group is 3. The experiment was repeated 3 times independently. The total number of animals used per group was 9. The error bars indicate the standard deviation. *, significant difference versus mock control (P < 0.05).
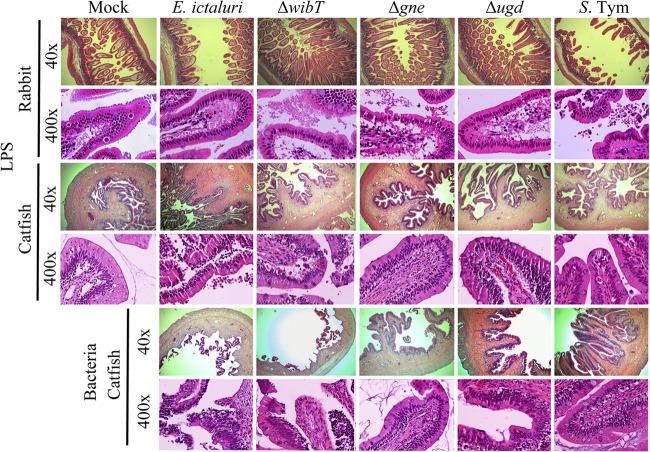
FIG 4.
Comparative intestinal loop histology 6 h postinoculation with 100 μg of LPS or a 107-CFU dose of bacteria. Mock intestinal loops were injected with saline (NaCl, 0.85%). The ileal loops were injected with 1 ml of the respective sample. These experiments were repeated 2 to 3 times independently. S. Tym, Salmonella enterica serovar Typhimurium.
Catfish intestinal loops inoculated with LPS.
All purified LPS molecules from S. Typhimurium or E. ictaluri triggered similar and significant levels of fluid secretion in the catfish gut (Fig. 3B). However, S. Typhimurium LPS did not cause evident tissue damage, in contrast to E. ictaluri wild-type LPS, which caused massive tissue damage and gut inflammation (Fig. 4 and 5). Purified LPS from the E. ictaluri ΔwibT mutant caused notorious tissue damage, in contrast to E. ictaluri Δgne LPS, which did not cause tissue damage or significant inflammation (Fig. 4 and 5). E. ictaluri Δugd LPS triggered a mild inflammation with evident epithelial cell-cell junction disruption (Fig. 4 and 5). Loops injected with OMPs or peptidoglycan did not show differences with respect to the control. It has been established that injection of the fish intracoelomic cavity with LPS or fish macrophages exposed to LPS does not generate an inflammatory immune response (19). However, fish macrophages exposed to peptidoglycan mount an inflammatory immune response (19). Catfish intestinal loops injected with purified peptidoglycan (100 μg/dose) did not show differences compared to the control (Fig. 6).
FIG 5.
Histopathology scores. (A) Whole bacteria. (B) Purified LPS. (C) Combined score. (D) Scoring table.
FIG 6.
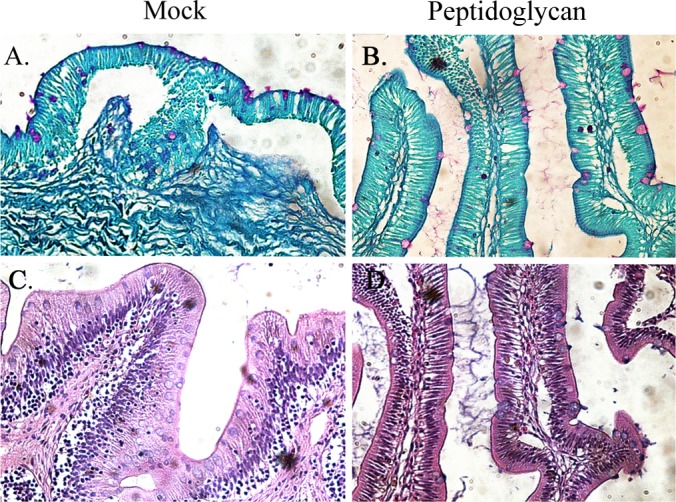
Catfish intestinal loop histology 6 h postinoculation with peptidoglycan. (A) Mock, periodic acid-Schiff (PAS) stained. (B) Pepetidoglycan, PAS stained. (C) Mock, hematoxylin and eosin (HE) stained. (D) Peptidoglycan, HE stained. (A and C) Mock intestinal loops were injected with 1 ml of saline (NaCl, 0.85%). (B and D) Intestinal loops injected with 1 ml of peptidoglycan (100 μg). These experiments were repeated 2 to 3 times independently.
Catfish intestinal loops inoculated with E. ictaluri strains.
The E. ictaluri wild-type strain and S. Typhimurium triggered significant fluid secretion, in contrast with the mock negative control (Fig. 3C and 4). However, the E. ictaluri wild type caused extensive tissue damage and inflammation, in contrast with S. Typhimurium, which did not cause tissue damage or inflammation (Fig. 4 and 5). We noted that purified LPS from the E. ictaluri Δgne and Δugd mutants and S. Typhimurium stimulates goblet cells and mucus secretion (Fig. 7). Both E. ictaluri and S. Typhimurium were recovered from the intestinal fluids and tissue at similar levels. The E. ictaluri wild type and S. Typhimurium were able to grow in the intestinal milieu (Fig. 8A) and colonized intestinal epithelial tissues (Fig. 8B).
FIG 7.
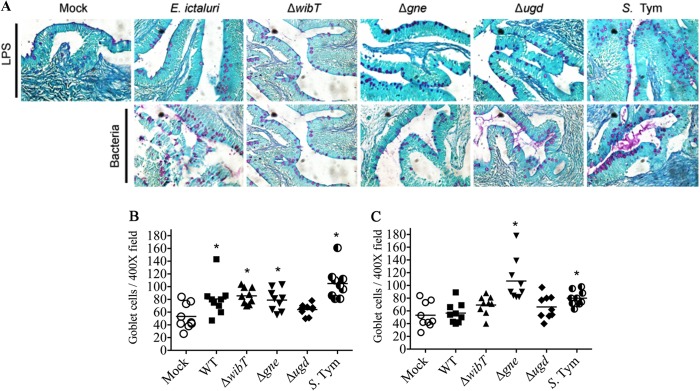
Comparative intestinal loop histology 6 h postinoculation with 100 μg of LPS or a 107-CFU dose of bacteria. (A) Catfish loop, periodic acid-Schiff (PAS) stained. S. Tym, Salmonella enterica serovar Typhimurium. (B) Number of goblet cells in a bacterium-injected loop. (C) Number of goblet cells in an LPS-injected loop. Mock intestinal loops were injected with saline (NaCl, 0.85%). Catfish intestinal loops were injected with 1 ml of the respective LPS sample (100 μg). These experiments were repeated 3 to 4 times independently. Each symbol represents a field. *, significant difference (P < 0.05).
FIG 8.
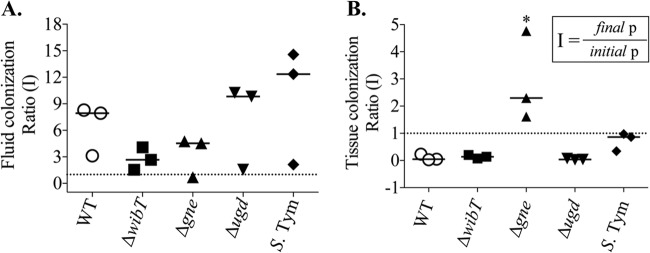
E. ictaluri colonization ratio in intestinal loop histology 6 h postinoculation with a 107-CFU dose of bacteria. (A) Fluid colonization. S. Tym, Salmonella enterica serovar Typhimurium. (B) Tissue colonization. *, significant difference versus wild type (P < 0.05). Each symbol represents an independent experiment. A total of 9 animals were used. The error bars indicate the standard deviation.
The E. ictaluri ΔwibT mutant caused significant levels of fluid secretion (Fig. 3C) and significant tissue damage, similar to the E. ictaluri wild type (Fig. 4 and 5). We noted that E. ictaluri Δgne and Δugd mutants and S. Typhimurium stimulate goblet cells and mucus secretion without causing tissue damage (Fig. 4, 5, and 7). E. ictaluri ΔwibT mutant titers increased 1 log fold in the intestinal milieu (Fig. 8A), and the mutant strain colonized epithelial tissues similar to the E. ictaluri wild-type strain (Fig. 8B). The E. ictaluri Δgne mutant generated significant low fluid secretion, causing a mild inflammation with low tissue damage (Fig. 3C, 5, and 6). The E. ictaluri Δgne mutant grows in the intestinal fluids at similar levels to the E. ictaluri wild type (Fig. 8A). However, the E. ictaluri Δgne mutant was recovered in higher numbers from the intestinal epithelium than the E. ictaluri wild type (Fig. 8B). These results are in concordance with the low tissue damage observed (Fig. 4 and 5) where epithelial cells containing E. ictaluri are not in the intestinal fluids like the loops infected with the E. ictaluri wild type and ΔwibT mutant. Previous studies suggested that the E. ictaluri Δgne mutant is attenuated and immunoprotective when orally administered to the fish, triggering a mild intestinal inflammation without significant tissue damage (18). These results are coincident with our results, suggesting that the Δgne mutant is a good candidate for oral live attenuated vaccine development. E. ictaluri Δugd mutant titers recovered from the intestinal fluids and intestinal tissue were similar to those of the E. ictaluri wild type (Fig. 8A and B).
Cytokine expression.
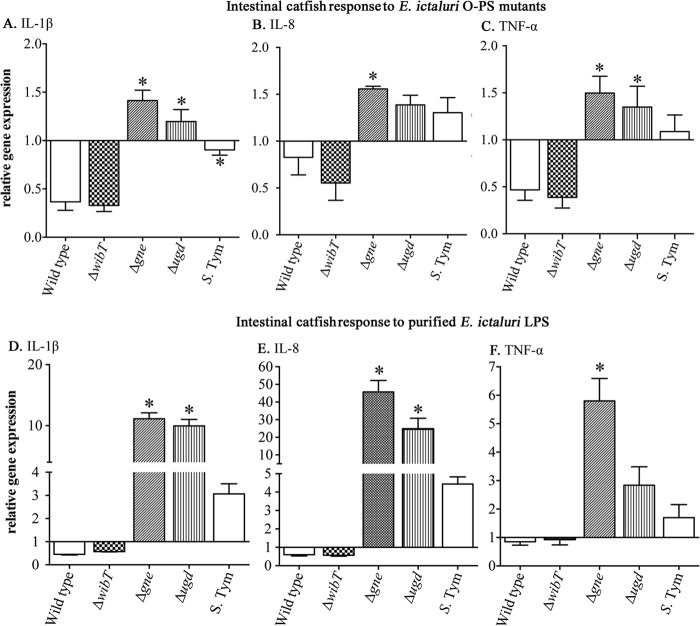
Cytokines play a major role in inflammatory responses. Interleukins (IL) and tumor necrosis factors (TNFs) are a large group of cytokines involved in innate immunity. In catfish, only IL-1βa, IL-1βb, TNF-α, and IL-8 have been identified (60–62). IL-1β plays a pivotal role in early proinflammatory cytokines that enable the fish to respond to infection and enhance the immune response induced by vaccines (63). Also, it has been reported that in salmonid species, IL-1β and TNF-α are induced in the presence of LPS and Gram-negative bacteria (64, 65), triggering synthesis of IL-8 (66). However, these studies focused on internal tissues, like spleen, liver, and head kidney, and few studies have focused on the fish gut. Recently, we found that oral inoculation of catfish with E. ictaluri LPS O-PS mutants triggers lower inflammatory symptoms, in contrast to the symptoms in the wild-type-inoculated catfish (18), suggesting that LPS plays a role in inflammation at the intestinal level in fish. Thus, here we evaluated expression of IL-1βa, TNF-α, and IL-8 in the intestinal tissues 6 h postinoculation. Under qRT-PCR settings, we detected that the primers described for IL-1βb generate two bands—one with the described molecular weight and other just with the same sizes of β-actin amplification fragment. Therefore, IL-1βb was not measured here. We found that the E. ictaluri wild type and ΔwibT mutant downregulated IL-1βa, IL-8, and TNF-α, in contrast to the Δgne and Δugd mutants, which upregulated these cytokines (Fig. 9A to C). S. Typhimurium slightly downregulated IL-βa in a similar fashion to the E. ictaluri wild type, but with a significant difference between the fish and nonfish bacterial pathogens (Fig. 9C). Although, TNF-α and IL-8 levels were slightly upregulated in S. Typhimurium-inoculated loops, no significant differences were observed, in contrast to the E. ictaluri wild type (Fig. 9B and C). These results suggest that E. ictaluri wild type and ΔwibT mutant have the ability to inhibit early innate immunity detection. Results from the loops inoculated with the Δgne and Δugd mutants indicate that this inhibitory ability might be LPS mediated. Therefore, catfish intestinal loops were injected with purified LPS. Similar results, but with an ∼10-fold increase in Δgne and Δugd LPS samples, were found in loops injected with purified LPS (Fig. 9D to F). This suggests that E. ictaluri LPS plays an innate immunity inhibitory role during fish intestinal colonization. It also indicates that E. ictaluri Δgne and Δugd LPS molecules have intestinal immunostimulatory activity in the fish gut, but determination of the structure of these molecules is required to better understand these interactions. Although the significant fold differences between the whole bacteria and the purified LPS are evident, the results under both conditions have the same pattern. This might be due to the fact that in the entire bacterium, the LPS is in the outer membrane, not totally accessible to interaction with its putative receptor, in contrast to the purified LPS.
FIG 9.
qRT-PCT of intestinal catfish cytokines induced by E. ictaluri LPS. (A to C) Intestinal response to E. ictaluri O-PS mutants. The intestinal loops were inoculated with 107 CFU of each mutant. (D to F) Intestinal response to purified E. ictaluri LPS. The intestinal loops were inoculated with 100 μg of LPS from each mutant. The experiment was repeated 2 times independently. The total of animals used per group was 6. The error bars indicate the standard deviation. *, significant difference versus wild type.
DISCUSSION
In mammals, the lipid A portion of the LPS acts as a toxin by overstimulating the TLR-4 innate immune signaling, which induces pathogenic inflammatory responses. The LPS is recognized by the serum circulating protein LBP (LPS-binding protein) (67), which facilitates the transfer of LPS to the costimulatory molecule CD14 (68) and then to myeloid differentiation protein 2 (MD2; also called LY96) (69). MD2 is associated with the Toll-like receptor 4 (TLR4) and specifically recognizes the endotoxic lipid A molecule (70), triggering a downstream signaling that involves several intracellular TIR domain-containing adaptors, like MDy88 and TICAM (71, 72).
On the other hand, it is well established that fish and amphibians are very resistant to the toxic effects of LPS (13). Several reports suggest that fish do not respond to LPS because of the lack of PLB, CD14, LY96, and TCAM2, essential components for the TLR4 function (19). Until today, functional LBP, CD14, MD2, and TCAM2 molecules have not been described in fish. Indeed, more evolutionarily advanced pufferfish lack a TLR4 ortholog (73, 74). Also, it is suggested that LBP, CD14, MD2, and TCAM2 have recently arisen during vertebrate evolution, being limited only to higher vertebrates (19, 75). This is supported by the extant literature indicating that fish are resistant to LPS toxicity (13, 18, 19, 76). It is evident that lower vertebrates interact with a much higher bacterial load in their living environment than land vertebrates, especially through the mucosal tissues. Perhaps due to the environmental selective pressure imposed by the aquatic “bacterial soup,” fish evolution did not favor high sensitivity to LPS.
However, several reports indicate that fish macrophages detect and respond to high doses of LPS (14, 15, 18, 19, 75), but the mechanisms of detention and response are unknown.
In the context of bacterial pathogenesis, recently we observed that catfish orally infected with the E. ictaluri wild type presented diarrhea-like symptoms, excreting mucoid feces with high E. ictaluri titers (104 to 105 CFU/ml of feces) (18). In contrast, catfish orally inoculated with the E. ictaluri ΔwibT, Δgne, or Δugd mutants (O-PS mutants) did not present diarrhea-like symptoms (18). These results suggested that fish respond to LPS at the gut level, influencing E. ictaluri infection. These observations prompted us to investigate the role of E. ictaluri LPS during intestinal inflammation by using the catfish intestinal loop model and comparing it to the rabbit intestinal loop model and S. Typhimurium LPS. Intestinal ligated loops have been used since the 1950s to investigate intestinal interactions with bacterial pathogens and their virulence factors. As mentioned previously, all of these studies have been done with mammals, including mice, rats, rabbits, and calves (21–23, 26). In contrast to mammals, little is known about the fish intestinal interaction with the bacterial pathogen LPS.
We observed that E. ictaluri LPS has effects on fish intestinal inflammation, which depends on O-PS in fish but not in mammals (Fig. 3, 4, and 5). For instance, we determined that purified E. ictaluri wild-type LPS triggers a mild inflammation in rabbit ligated ileal loops, in contrast to S. Typhimurium LPS, which caused massive tissue damage and inflammation (Fig. 4 and 5). The opposite results were observed in catfish intestinal loops inoculated with purified E. ictaluri wild-type LPS. Catfish ligated loops inoculated with S. Typhimurium LPS did not show significant tissue damage and inflammation, in contrast with E. ictaluri LPS, which caused evident inflammation and tissue damage (Fig. 4, 5, and 6). E. ictaluri ΔwibT LPS caused inflammation in both rabbits and fish ligated loops, indicating that the E. ictaluri ΔwibT LPS molecule is recognized by the innate immune systems of fish and mammals. E. ictaluri Δgne LPS triggered reduced epithelial damage in rabbits, but no tissue damage was detected in intestinal fish loops. Although the LPS profiles of the E. ictaluri ΔwibT and Δgne mutants are similar, their glycosyl compositions are different (Fig. 2), suggesting that the structure of the O-PS is relevant to bacterium-fish intestinal epithelial interaction and pathogenesis. E. ictaluri Δugd LPS did not have effects on rabbit ligated loops, but in fish, the LPS triggers a mild inflammation with an epithelial cell-cell junction disruption (Fig. 4 and 5).
Immune stimulants represent a promising tool in aquaculture for enhancing disease and stress resistance in cultured fish. It has been shown that oral administration of LPS prevents disease in fish (77, 78). However, the mechanisms of this oral immune stimulation are unknown. Here we determined that purified LPS from E. ictaluri Δgne and Δugd mutants triggers synthesis of proinflammatory cytokines like IL-1β, IL-8, and TNF-α, in contrast to LPS from the E. ictaluri wild type and ΔwibT mutant (Fig. 9).
IL-1β plays a pivotal role in early proinflammatory cytokines that enable the organism to respond to infection. Also, IL-1β has the potential to enhance the immune response induced by vaccines (63), and recombinant IL-1β has been use as an immunostimulant for vaccines in sheep (79, 80), pigs (81), cattle (82), and sea bass (83, 84). In teleosts, the administration of immunostimulants, such as β-1,3-glucan and peptidoglycan, helps to prevent infections through activation of phagocytes, such as neutrophils, monocytes, and macrophages, suggesting that the activation of innate immunity in teleost fish by immunostimulants is a useful method of disease prevention that can replace the use of antibiotics. Recent studies showed that orally administered LPS from Pantoea agglomerans has a preventive effect against infection in fish such as yellowtail, carp, and ayu (77). Among the possible receptors for LPS in fish, it has been suggested that β2-integrin could play a role in LPS recognition (85, 86). The β2-integrins are one of the most abundant receptors found in macrophages, and they transmit intracellular activation signals through mitogen-activated protein (MAP) kinases and NF-κB (85, 86). The β2-integrins recognize the hydrophilic carbohydrate moiety that is buried in the outer bacterial membrane but not the hydrophobic lipid A (87). Also, the concentrations of LPS required to activate β2-integrin-mediated activation of NF-κB are high (88, 89). We observed that the response to LPS at the gut level is O-PS dependent (Fig. 9), suggesting an interaction with β2-integrin receptors or another carbohydrate receptor. This idea is supported by previous observations in which fish intravenously injected with LPS and fish macrophages inoculated with LPS respond in an O-PS-dependent fashion (19). We observed that LPS derived from E. ictaluri Δgne and Δugd mutants form supramolecules or aggregates, which seem to increase the interaction with fish macrophages (19). This observation correlates with the increased stimulation of IL-1, IL-8, and TNF-α synthesis in the intestinal loops inoculated with Δgne and Δugd LPSs, suggesting their potential utility as immunostimulants for fish.
Currently, there is a need for effective orally delivered vaccines (90). Thus, studies about fish gut-bacterial interaction become important to develop effective oral vaccines for aquaculture. Recently, we determined that an E. ictaluri Δgne strain conferred immune protection to orally immunized fish (18). In contrast to the rest of E. ictaluri LPS mutants study here, only the Δgne mutation confers immune protection to the orally immunized fish (18). We observed that the E. ictaluri Δgne mutant does not cause tissue damage and fluid secretion, increasing its colonization and interaction with lymphoid intestinal cells, positively influencing the immune response to E. ictaluri. Perhaps, the immunostimulatory properties of E. ictauri Δgne LPS, combined with the mutant's increased colonization of intestinal mucosa, lower capability to survive in catfish macrohages, resistance to the antimicrobial peptides, and motility (18), make the E. ictauri Δgne mutant a good candidate for use as a live attenuated vaccine for the catfish aquaculture industry.
Here we have developed a catfish intestinal loop model to study the bacterial interaction with the fish intestinal epithelia. By using this model, we determined that the responses to LPS at the intestinal level could differ, depending on the LPS molecule and the host. For instance, E. ictaluri LPS did not cause inflammation in the rabbit; perhaps its lipid A has a different structure. The fish intestinal loop model is a useful methodology to study pathogenesis and intestinal immunology, but it also could be applied to evaluate feeding diets, probiotics, and therapeutic drugs.
ACKNOWLEDGMENTS
This work was supported by USDA grant CRIS-ARZR-2009-01801 and the Comisión Nacional de Investigación Científica y Tecnológica (CONICYT), Gestión Propia Fellowship, Chile.
We thank Tim Corsi and Joanne Tetens for assistance at the animal facility of The Biodesign Institute, Arizona State University, and Erika Arch, Paul Hartig, and Tina Hartig for logistic support.
Footnotes
Published ahead of print 27 May 2014
REFERENCES
- 1.Abayneh T, Colquhoun DJ, Sorum H. 2013. Edwardsiella piscicida sp. nov., a novel species pathogenic to fish. J. Appl. Microbiol. 114:644–654. 10.1111/jam.12080. [DOI] [PubMed] [Google Scholar]
- 2.Shoemaker CA, Klesius PH, Arias CR, Evans JJ. 2009. Uses of modified live vaccines in aquaculture. J. World Aquacult. Soc. 5:573–585. 10.1111/j.1749-7345.2009.00279.x. [DOI] [Google Scholar]
- 3.Hanson T, Sites D. 2012. 2011 U.S. catfish database. http://www.aces.edu/anr/fish/aquaculture/files/2012/03/2011-catfish-report-whole1.pdf [Google Scholar]
- 4.Harvey D. 2006. Aquaculture outlook. LDP-AQS-23. USDA Economic Research Service; Washington, DC: www.ers.usda.gov. [Google Scholar]
- 5.Shotts EB, Blazer VS, Waltman WD. 1986. Pathogenesis of experimental Edwardsiella ictaluri infections in channel catfish (Ictalurus punctatus). Can. J. Fish Aquat. Sci. 43:36–42. 10.1139/f86-005. [DOI] [Google Scholar]
- 6.Baldwin TJ, Newton JC. 1993. Pathogenesis of enteric septicemia of channel catfish, caused by Edwardsiella ictaluri: bacteriologic and light and electron microscopic findings. J. Aquat. Anim. Health 5:189–198. . [DOI] [Google Scholar]
- 7.Thune RL, Fernandez DH, Benoit JL, Kelly-Smith M, Rogge ML, Booth NJ, Landry CA, Bologna RA. 2007. Signature-tagged mutagenesis of Edwardsiella ictaluri identifies virulence-related genes, including a Salmonella pathogenicity island 2 class of type III secretion systems. Appl. Environ. Microbiol. 73:7934–7946. 10.1128/AEM.01115-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morrison RN, Cooper GA, Koop BF, Rise ML, Bridle AR, Adams MB, Nowak BF. 2006. Transcriptome profiling the gills of amoebic gill disease (AGD)-affected Atlantic salmon (Salmo salar L.): a role for tumor suppressor p53 in AGD pathogenesis? Physiol. Genomics 26:15–34. 10.1152/physiolgenomics.00320.2005. [DOI] [PubMed] [Google Scholar]
- 9.Poltorak A, Peppel K, Beutler B. 1994. Receptor-mediated label-transfer assay (RELAY): a novel method for the detection of plasma tumor necrosis factor at attomolar concentrations. J. Immunol. Methods 169:93–99. 10.1016/0022-1759(94)90128-7. [DOI] [PubMed] [Google Scholar]
- 10.Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, Takeda K, Akira S. 1999. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the LPS gene product. J. Immunol. 162:3749–3752. [PubMed] [Google Scholar]
- 11.Qureshi ST, Lariviere L, Leveque G, Clermont S, Moore KJ, Gros P, Malo D. 1999. Endotoxin-tolerant mice have mutations in Toll-like receptor 4 (Tlr4). J. Exp. Med. 189:615–625. 10.1084/jem.189.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raetz CR, Whitfield C. 2002. Lipopolysaccharide endotoxins. Annu. Rev. Biochem. 71:635–700. 10.1146/annurev.biochem.71.110601.135414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berczi I, Bertok L, Bereznai T. 1966. Comparative studies on the toxicity of Escherichia coli lipopolysaccharide endotoxin in various animal species. Can. J. Microbiol. 12:1070–1071. 10.1139/m66-143. [DOI] [PubMed] [Google Scholar]
- 14.MacKenzie S, Planas JV, Goetz FW. 2003. LPS-stimulated expression of a tumor necrosis factor-alpha mRNA in primary trout monocytes and in vitro differentiated macrophages. Dev. Comp. Immunol. 27:393–400. 10.1016/S0145-305X(02)00135-0. [DOI] [PubMed] [Google Scholar]
- 15.Pelegrin P, Garcia-Castillo J, Mulero V, Meseguer J. 2001. Interleukin-1beta isolated from a marine fish reveals up-regulated expression in macrophages following activation with lipopolysaccharide and lymphokines. Cytokine 16:67–72. 10.1006/cyto.2001.0949. [DOI] [PubMed] [Google Scholar]
- 16.Lawrence M, Banes M, Williams M. 2001. Phenotype and virulence of a transposon-derived lipopolysaccharide O side-chain mutant of Edwardsiella ictaluri. J. Aquat. Anim. Health 13:291–299. . [DOI] [Google Scholar]
- 17.Lawrence ML, Banes MM, Azadi P, Reeks BY. 2003. The Edwardsiella ictaluri O polysaccharide biosynthesis gene cluster and the role of O polysaccharide in resistance to normal catfish serum and catfish neutrophils. Microbiology 149:1409–1421. 10.1099/mic.0.26138-0. [DOI] [PubMed] [Google Scholar]
- 18.Santander J, Martin T, Loh A, Pohlenz C, Gatlin DM, Curtiss R., III 2013. Mechanisms of intrinsic resistance to antimicrobial peptides of Edwardsiella ictaluri and its influence on fish gut inflammation and virulence. Microbiology 159:1471–1486. 10.1099/mic.0.066639-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iliev DB, Roach JC, Mackenzie S, Planas JV, Goetz FW. 2005. Endotoxin recognition: in fish or not in fish? FEBS Lett. 579:6519–6528. 10.1016/j.febslet.2005.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Everest P, Ketley J, Hardy S, Douce G, Khan S, Shea J, Holden D, Maskell D, Dougan G. 1999. Evaluation of Salmonella typhimurium mutants in a model of experimental gastroenteritis. Infect. Immun. 67:2815–2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kong Q, Six DA, Liu Q, Gu L, Wang S, Alamuri P, Raetz CR, Curtiss R., III 2012. Phosphate groups of lipid A are essential for Salmonella enterica serovar Typhimurium virulence and affect innate and adaptive immunity. Infect. Immun. 80:3215–3224. 10.1128/IAI.00123-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Menge C, Blessenohl M, Eisenberg T, Stamm I, Baljer G. 2004. Bovine ileal intraepithelial lymphocytes represent target cells for Shiga toxin 1 from Escherichia coli. Infect. Immun. 72:1896–1905. 10.1128/IAI.72.4.1896-1905.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Menge C, Stamm I, Van Diemen PM, Sopp P, Baljer G, Wallis TS, Stevens MP. 2004. Phenotypic and functional characterization of intraepithelial lymphocytes in a bovine ligated intestinal loop model of enterohaemorrhagic Escherichia coli infection. J. Med. Microbiol. 53:573–579. 10.1099/jmm.0.45530-0. [DOI] [PubMed] [Google Scholar]
- 24.De SN, Ghose ML, Sen A. 1960. Activities of bacteria-free preparations from Vibrio cholerae. J. Pathol. Bacteriol. 79:373–380. 10.1002/path.1700790219. [DOI] [PubMed] [Google Scholar]
- 25.Syngkon A, Elluri S, Koley H, Rompikuntal PK, Saha DR, Chakrabarti MK, Bhadra RK, Wai SN, Pal A. 2010. Studies on a novel serine protease of a ΔhapA ΔprtV Vibrio cholerae O1 strain and its role in hemorrhagic response in the rabbit ileal loop model. PLoS One 5:e13122. 10.1371/journal.pone.0013122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De SN, Chatterje DN. 1953. An experimental study of the mechanism of action of Vibrio cholerae on the intestinal mucous membrane. J. Pathol. Bacteriol. 66:559–562. 10.1002/path.1700660228. [DOI] [PubMed] [Google Scholar]
- 27.Burrows W, Musteikis GM. 1966. Cholera infection and toxin in the rabbit ileal loop. J. Infect. Dis. 116:183–190. 10.1093/infdis/116.2.183. [DOI] [PubMed] [Google Scholar]
- 28.Kohler EM. 1971. Enterotoxic activity of whole cell lysates of Escherichia coli in young pigs. Am. J. Vet. Res. 32:731–737. [PubMed] [Google Scholar]
- 29.Moon HW, Whipp SC, Engstrom GW, Baetz AL. 1970. Response of the rabbit ileal loop to cell-free products from Escherichia coli enteropathogenic for swine. J. Infect. Dis. 121:182–187. 10.1093/infdis/121.2.182. [DOI] [PubMed] [Google Scholar]
- 30.Smith HW, Halls S. 1967. Observations by the ligated intestinal segment and oral inoculation methods on Escherichia coli infections in pigs, calves, lambs and rabbits. J. Pathol. Bacteriol. 93:499–529. 10.1002/path.1700930211. [DOI] [PubMed] [Google Scholar]
- 31.Fromme I, Schlecht S. 1973. Influence of aeration conditions in fermentation cultures on the chemical composition of Salmonella lipopolysaccharides. Zentralbl. Bakteriol. Orig. A 224:331–344 (In German.) [PubMed] [Google Scholar]
- 32.Wallis TS, Paulin SM, Plested JS, Watson PR, Jones PW. 1995. The Salmonella dublin virulence plasmid mediates systemic but not enteric phases of salmonellosis in cattle. Infect. Immun. 63:2755–2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taylor J, Wilkins MP, Payne JM. 1961. Relation of rabbit gut reaction to enteropathogenic Escherichia coli. Br. J. Exp. Pathol. 42:43–52. [PMC free article] [PubMed] [Google Scholar]
- 34.Kubota Y, Liu PV. 1971. An enterotoxin of Pseudomonas aeruginosa. J. Infect. Dis. 123:97–98. 10.1093/infdis/123.1.97. [DOI] [PubMed] [Google Scholar]
- 35.Duncan CL, Sugiyama H, Strong DH. 1968. Rabbit ileal loop response to strains of Clostridium perfringens. J. Bacteriol. 95:1560–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bhattacharya S, Bose AK, Ghosh AK. 1971. Permeability and enterotoxic factors of nonagglutinable vibrios Vibrio alcaligenes and Vibrio parahaemolyticus. Appl. Microbiol. 22:1159–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spira WM, Goepfert JM. 1972. Bacillus cereus-induced fluid accumulation in rabbit ileal loops. Appl. Microbiol. 24:341–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Frost AJ, Bland AP, Wallis TS. 1997. The early dynamic response of the calf ileal epithelium to Salmonella typhimurium. Vet. Pathol. 34:369–386. 10.1177/030098589703400501. [DOI] [PubMed] [Google Scholar]
- 39.Santos RL, Zhang S, Tsolis RM, Baumler AJ, Adams LG. 2002. Morphologic and molecular characterization of Salmonella typhimurium infection in neonatal calves. Vet. Pathol. 39:200–215. 10.1354/vp.39-2-200. [DOI] [PubMed] [Google Scholar]
- 40.Wallis TS, Hawker RJ, Candy DC, Qi GM, Clarke GJ, Worton KJ, Osborne MP, Stephen J. 1989. Quantification of the leucocyte influx into rabbit ileal loops induced by strains of Salmonella typhimurium of different virulence. J. Med. Microbiol. 30:149–156. 10.1099/00222615-30-2-149. [DOI] [PubMed] [Google Scholar]
- 41.Vinogradov E, Nossova L, Perry MB, Kay WW. 2005. The structure of the antigenic O-polysaccharide of the lipopolysaccharide of Edwardsiella ictaluri strain MT104. Carbohydr. Res. 340:1509–1513. 10.1016/j.carres.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 42.Guttman JA, Finlay BB. 2009. Tight junctions as targets of infectious agents. Biochim. Biophys. Acta 1788:832–841. 10.1016/j.bbamem.2008.10.028. [DOI] [PubMed] [Google Scholar]
- 43.Niklasson L, Sundh H, Fridell F, Taranger GL, Sundell K. 2011. Disturbance of the intestinal mucosal immune system of farmed Atlantic salmon (Salmo salar), in response to long-term hypoxic conditions. Fish Shellfish Immunol. 31:1072–1080. 10.1016/j.fsi.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 44.Komatsu K, Tsutsui S, Hino K, Araki K, Yoshiura Y, Yamamoto A, Nakamura O, Watanabe T. 2009. Expression profiles of cytokines released in intestinal epithelial cells of the rainbow trout, Oncorhynchus mykiss, in response to bacterial infection. Dev. Comp. Immunol. 33:499–506. 10.1016/j.dci.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 45.Rombout JH, van der Tuin SJ, Yang G, Schopman N, Mroczek A, Hermsen T, Taverne-Thiele JJ. 2008. Expression of the polymeric immunoglobulin receptor (pIgR) in mucosal tissues of common carp (Cyprinus carpio L.). Fish Shellfish Immunol. 24:620–628. 10.1016/j.fsi.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 46.Rajan B, Fernandes JM, Caipang CM, Kiron V, Rombout JH, Brinchmann MF. 2011. Proteome reference map of the skin mucus of Atlantic cod (Gadus morhua) revealing immune competent molecules. Fish Shellfish Immunol. 31:224–231. 10.1016/j.fsi.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 47.Palaksha KJ, Shin GW, Kim YR, Jung TS. 2008. Evaluation of non-specific immune components from the skin mucus of olive flounder (Paralichthys olivaceus). Fish Shellfish Immunol. 24:479–488. 10.1016/j.fsi.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 48.Li C, Zhang Y, Wang R, Lu J, Nandi S, Mohanty S, Terhune J, Liu Z, Peatman E. 2012. RNA-seq analysis of mucosal immune responses reveals signatures of intestinal barrier disruption and pathogen entry following Edwardsiella ictaluri infection in channel catfish, Ictalurus punctatus. Fish Shellfish Immunol. 32:816–827. 10.1016/j.fsi.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 49.Hebert P, Ainsworth AJ, Boyd B. 2002. Histological enzyme and flow cytometric analysis of channel catfish intestinal tract immune cells. Dev. Comp. Immunol. 26:53–62. 10.1016/S0145-305X(01)00044-1. [DOI] [PubMed] [Google Scholar]
- 50.Skirpstunas RT, Baldwin TJ. 2002. Edwardsiella ictaluri invasion of IEC-6, Henle 407, fathead minnow and channel catfish enteric epithelial cells. Dis. Aquat. Organ. 51:161–167. 10.3354/dao051161. [DOI] [PubMed] [Google Scholar]
- 51.Bertani G. 1951. Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J. Bacteriol. 62:293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yi EC, Hackett M. 2000. Rapid isolation method for lipopolysaccharide and lipid A from gram-negative bacteria. Analyst 125:651–656. 10.1039/b000368i. [DOI] [PubMed] [Google Scholar]
- 53.Hitchcock PJ, Brown TM. 1983. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J. Bacteriol. 154:269–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tsai CM, Frasch CE. 1982. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal. Biochem. 119:115–119. 10.1016/0003-2697(82)90673-X. [DOI] [PubMed] [Google Scholar]
- 55.Santander J, Golden G, Wanda SY, Curtiss R., III 2012. The Fur regulated iron uptake system of Edwardsiella ictaluri and its influence on pathogenesis and immunogenicity in the catfish host. Infect. Immun. 80:2689–2703. 10.1128/IAI.00013-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Popovic NT, Strunjak-Perovic I, Coz-Rakovac R, Barisic J, Jadan M, Persin Berakovic A, Sauerborn Klobucar R. 2012. Tricaine methane-sulfonate (MS-222) application in fish anaesthesia. J. Appl. Ichthyol. 28:553–564. 10.1111/j.1439-0426.2012.01950.x. [DOI] [Google Scholar]
- 57.Brown LA. 1987. Recirculation anaesthesia for laboratory fish. Lab Anim. 21:210–215. 10.1258/002367787781268846. [DOI] [PubMed] [Google Scholar]
- 58.Harms CA. 2005. Surgery in fish research: common procedures and postoperative care. Lab Anim. 34:28–34. 10.1038/laban0105-28. [DOI] [PubMed] [Google Scholar]
- 59.Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408. 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 60.Wang Y, Wang Q, Baoprasertkul P, Peatman E, Liu Z. 2006. Genomic organization, gene duplication, and expression analysis of interleukin-1beta in channel catfish (Ictalurus punctatus). Mol. Immunol. 43:1653–1664. 10.1016/j.molimm.2005.09.024. [DOI] [PubMed] [Google Scholar]
- 61.Zou J, Secombes CJ, Long S, Miller N, Clem LW, Chinchar VG. 2003. Molecular identification and expression analysis of tumor necrosis factor in channel catfish (Ictalurus punctatus). Dev. Comp. Immunol. 27:845–858. 10.1016/S0145-305X(03)00085-5. [DOI] [PubMed] [Google Scholar]
- 62.Chen L, He C, Baoprasertkul P, Xu P, Li P, Serapion J, Waldbieser G, Wolters W, Liu Z. 2005. Analysis of a catfish gene resembling interleukin-8: cDNA cloning, gene structure, and expression after infection with Edwardsiella ictaluri. Dev. Comp. Immunol. 29:135–142. 10.1016/j.dci.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 63.Nash PV, Mastro AM. 1993. Activation of primary lymphocytes requires prolonged lectin stimulation. J. Leukoc. Biol. 53:73–78. [DOI] [PubMed] [Google Scholar]
- 64.Peddie S, Zou J, Secombes CJ. 2002. Immunostimulation in the rainbow trout (Oncorhynchus mykiss) following intraperitoneal administration of Ergosan. Vet. Immunol. Immunopathol. 86:101–113. 10.1016/S0165-2427(02)00019-3. [DOI] [PubMed] [Google Scholar]
- 65.Secombes CJ, Wang T, Hong S, Peddie S, Crampe M, Laing KJ, Cunningham C, Zou J. 2001. Cytokines and innate immunity of fish. Dev. Comp. Immunol. 25:713–723. 10.1016/S0145-305X(01)00032-5. [DOI] [PubMed] [Google Scholar]
- 66.Wang Z, Liu X, Dacanay A, Harrison BA, Fast M, Colquhoun DJ, Lund V, Brown LL, Li JJ, Altman E. 2007. Carbohydrate analysis and serological classification of typical and atypical isolates of Aeromonas salmonicida: a rationale for the lipopolysaccharide-based classification of A. salmonicida. Fish Shellfish Immunol. 23:1095–1106. 10.1016/j.fsi.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 67.Mathison JC, Tobias PS, Wolfson E, Ulevitch RJ. 1992. Plasma lipopolysaccharide (LPS)-binding protein. A key component in macrophage recognition of gram-negative LPS. J. Immunol. 149:200–206. [PubMed] [Google Scholar]
- 68.Tobias PS, Soldau K, Gegner JA, Mintz D, Ulevitch RJ. 1995. Lipopolysaccharide binding protein-mediated complexation of lipopolysaccharide with soluble CD14. J. Biol. Chem. 270:10482–10488. 10.1074/jbc.270.18.10482. [DOI] [PubMed] [Google Scholar]
- 69.Gioannini TL, Teghanemt A, Zhang D, Levis EN, Weiss JP. 2005. Monomeric endotoxin:protein complexes are essential for TLR4-dependent cell activation. J. Endotoxin Res. 11:117–123. 10.1177/09680519050110020801. [DOI] [PubMed] [Google Scholar]
- 70.Akashi S, Saitoh S, Wakabayashi Y, Kikuchi T, Takamura N, Nagai Y, Kusumoto Y, Fukase K, Kusumoto S, Adachi Y, Kosugi A, Miyake K. 2003. Lipopolysaccharide interaction with cell surface Toll-like receptor 4-MD-2: higher affinity than that with MD-2 or CD14. J. Exp. Med. 198:1035–1042. 10.1084/jem.20031076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.O'Neill LA, Bowie AG. 2007. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat. Rev. Immunol. 7:353–364. 10.1038/nri2079. [DOI] [PubMed] [Google Scholar]
- 72.Vogel SN, Fenton M. 2003. Toll-like receptor 4 signalling: new perspectives on a complex signal-transduction problem. Biochem. Soc. Trans. 31:664–668. [DOI] [PubMed] [Google Scholar]
- 73.Oshiumi H, Tsujita T, Shida K, Matsumoto M, Ikeo K, Seya T. 2003. Prediction of the prototype of the human Toll-like receptor gene family from the pufferfish, Fugu rubripes, genome. Immunogenetics 54:791–800. [DOI] [PubMed] [Google Scholar]
- 74.Jault C, Pichon L, Chluba J. 2004. Toll-like receptor gene family and TIR-domain adapters in Danio rerio. Mol. Immunol. 40:759–771. 10.1016/j.molimm.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 75.Sepulcre MP, Alcaraz-Perez F, Lopez-Munoz A, Roca FJ, Meseguer J, Cayuela ML, Mulero V. 2009. Evolution of lipopolysaccharide (LPS) recognition and signaling: fish TLR4 does not recognize LPS and negatively regulates NF-kappaB activation. J. Immunol. 182:1836–1845. 10.4049/jimmunol.0801755. [DOI] [PubMed] [Google Scholar]
- 76.Swain P, Nayak SK, Nanda PK, Dash S. 2008. Biological effects of bacterial lipopolysaccharide (endotoxin) in fish: a review. Fish Shellfish Immunol. 25:191–201. 10.1016/j.fsi.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 77.Kohchi C, Inagawa H, Nishizawa T, Yamaguchi T, Nagai S, Soma G. 2006. Applications of lipopolysaccharide derived from Pantoea agglomerans (IP-PA1) for health care based on macrophage network theory. J. Biosci. Bioeng. 102:485–496. 10.1263/jbb.102.485. [DOI] [PubMed] [Google Scholar]
- 78.Kadowaki T, Yasui Y, Nishimiya O, Takahashi Y, Kohchi C, Soma G, Inagawa H. 2013. Orally administered LPS enhances head kidney macrophage activation with down-regulation of IL-6 in common carp (Cyprinus carpio). Fish Shellfish Immunol. 34:1569–1575. 10.1016/j.fsi.2013.03.372. [DOI] [PubMed] [Google Scholar]
- 79.Elhay MJ, Andersen P. 1997. Immunological requirements for a subunit vaccine against tuberculosis. Immunol. Cell Biol. 75:595–603. 10.1038/icb.1997.94. [DOI] [PubMed] [Google Scholar]
- 80.Lofthouse SA, Andrews AE, Barcham GJ, Nash AD. 1995. Parameters related to the application of recombinant ovine interleukin-1 beta as an adjuvant. Vaccine 13:1277–1287. 10.1016/0264-410X(95)00074-B. [DOI] [PubMed] [Google Scholar]
- 81.Blecha F, Reddy DN, Chitko-McKown CG, McVey DS, Chengappa MM, Goodband RD, Nelssen JL. 1995. Influence of recombinant bovine interleukin-1 beta and interleukin-2 in pigs vaccinated and challenged with Streptococcus suis. Vet. Immunol. Immunopathol. 44:329–346. 10.1016/0165-2427(94)05301-8. [DOI] [PubMed] [Google Scholar]
- 82.Reddy DN, Reddy PG, Minocha HC, Fenwick BW, Baker PE, Davis WC, Blecha F. 1990. Adjuvanticity of recombinant bovine interleukin-1 beta: influence on immunity, infection, and latency in a bovine herpesvirus-1 infection. Lymphokine Res. 9:295–307. [PubMed] [Google Scholar]
- 83.Buonocore F, Mazzini M, Forlenza M, Randelli E, Secombes CJ, Zou J, Scapigliati G. 2004. Expression in Escherichia coli and purification of sea bass (Dicentrarchus labrax) interleukin 1beta, a possible immunoadjuvant in aquaculture. Mar. Biotechnol. 6:53–59. 10.1007/s10126-003-0011-y. [DOI] [PubMed] [Google Scholar]
- 84.Buonocore F, Forlenza M, Randelli E, Benedetti S, Bossu P, Meloni S, Secombes CJ, Mazzini M, Scapigliati G. 2005. Biological activity of sea bass (Dicentrarchus labrax L.) recombinant interleukin-1beta. Mar. Biotechnol. 7:609–617. 10.1007/s10126-004-5131-5. [DOI] [PubMed] [Google Scholar]
- 85.Ingalls RR, Golenbock DT. 1995. CD11c/CD18, a transmembrane signaling receptor for lipopolysaccharide. J. Exp. Med. 181:1473–1479. 10.1084/jem.181.4.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ingalls RR, Arnaout MA, Delude RL, Flaherty S, Savedra R, Jr, Golenbock DT. 1998. The CD11/CD18 integrins: characterization of three novel LPS signaling receptors. Prog. Clin. Biol. Res. 397:107–117. [PubMed] [Google Scholar]
- 87.Chateau MT, Caravano R. 1997. The oxidative burst triggered by Salmonella typhimurium in differentiated U937 cells requires complement and a complete bacterial lipopolysaccharide. FEMS Immunol. Med. Microbiol. 17:57–66. 10.1016/S0928-8244(96)00105-8. [DOI] [PubMed] [Google Scholar]
- 88.Santos DR, Calixto JB, Souza GE. 2003. Effect of a kinin B2 receptor antagonist on LPS- and cytokine-induced neutrophil migration in rats. Br. J. Pharmacol. 139:271–278. 10.1038/sj.bjp.0705236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Aurell CA, Wistrom AO. 1998. Critical aggregation concentrations of gram-negative bacterial lipopolysaccharides (LPS). Biochem. Biophys. Res. Commun. 253:119–123. 10.1006/bbrc.1998.9773. [DOI] [PubMed] [Google Scholar]
- 90.Plant KP, Lapatra SE. 2011. Advances in fish vaccine delivery. Dev. Comp. Immunol. 35:1256–1262. 10.1016/j.dci.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 91.Hassan JO, Curtiss R., III 1990. Control of colonization by virulent Salmonella typhimurium by oral immunization of chickens with avirulent delta cya delta crp S. Typhimurium. Res. Microbiol. 141:839–850. 10.1016/0923-2508(90)90119-B. [DOI] [PubMed] [Google Scholar]
- 92.Luo Y, Kong Q, Yang J, Golden G, Wanda SY, Jensen RV, Ernst PB, Curtiss R., III 2012. Complete genome sequence of the universal killer Salmonella enterica serovar Typhimurium UK-1 (ATCC 68169). J. Bacteriol. 193:4035–4036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Santander J, Xin W, Yang Z, Curtiss R., III 2010. The aspartate-semialdehyde dehydrogenase of Edwardsiella ictaluri and its use as balanced-lethal system in fish vaccinology. PLoS One 5:e15944. 10.1371/journal.pone.0015944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Petrie-Hanson L, Romano CL, Mackey RB, Khosravi P, Hohn CM, Boyle CR. 2007. Evaluation of zebrafish Danio rerio as a model for enteric septicemia of catfish (ESC). J. Aquat. Anim. Health 19:151–158. 10.1577/H06-026.1. [DOI] [PubMed] [Google Scholar]