Abstract
Toxoplasma gondii is an obligate intracellular parasite of clinical importance, especially in immunocompromised patients. Investigations into the immune response to the parasite found that T cells are the primary effector cells regulating gamma interferon (IFN-γ)-mediated host resistance. However, recent studies have revealed a critical role for the innate immune system in mediating host defense independently of the T cell responses to the parasite. This body of knowledge is put into perspective by the unifying theme that immunity to the protozoan parasite requires a strong IFN-γ host response. In the following review, we discuss the role of IFN-γ-producing cells and the signals that regulate IFN-γ production during T. gondii infection.
INTRODUCTION
Toxoplasma gondii is an obligate intracellular protozoan parasite that infects at least a third of the world's population. Infection with the parasite is divided into a limited acute stage followed by a persistent chronic stage. In the chronic stage, T. gondii forms cysts, found mainly in brain and muscle tissues, which can persist for the lifetime of the host. The disease state, known as toxoplasmosis, generally occurs during the chronic stage after the host becomes immunocompromised, allowing the encysted T. gondii to reactivate. Toxoplasmosis is fatal if untreated, and yet treatments are ineffective at eliminating the encysted parasites, so patients are typically placed on antitoxoplasmosis drugs for as long as they are immunocompromised.
Basic research into the immune response to T. gondii established immunocompromised mouse models during the chronic stage of infection to model clinical observations. It was demonstrated that combined depletions of CD4+ and CD8+ T cells during chronic infection in mice resulted in parasite reactivation and host mortality (1). Additionally, SCID mice, which lack T cells, are susceptible during the transition from the acute to chronic stage of infection, which illustrates the requirement for T cell responses to control the infection.
While the adaptive response is ultimately required for host survival, components of the innate immune system determine initial susceptibility and the outcome of the infection independently of T cell-activation status (2). Mice deficient in myeloid differentiation factor 88 (MyD88), the downstream adaptor protein of most Toll-like receptors (TLRs), are extremely susceptible to T. gondii infection (3). Subsequently, it was shown in mice that MyD88-dependent responses to the parasite were primarily mediated by TLR11 (4), which recognizes a protein component of T. gondii known as profilin (4, 5). Innate responses through TLR11 via MyD88 result in the production of interleukin-12 (IL-12) and gamma interferon (IFN-γ) (3, 4). Dendritic cells (DCs) are the primary source of MyD88-dependent IL-12 (2, 6). However, IL-12 is not the only important effector for initiating the adaptive immune response; administration of IL-12 to MyD88-deficient mice does not rescue their acute susceptibility despite partially restoring Th1 responses (2, 7). These data suggest that there are additional MyD88-dependent factors necessary for full initiation of Th1 responses and host survival.
IFN-γ-PRODUCING CELLS: FROM T TO NK CELLS AND NEUTROPHILS
The cytokine IFN-γ is critical for survival during T. gondii infection. IFN-γ- or IFN-γ-receptor-deficient mice show extreme susceptibility to the parasite (1, 8–10) similar to that observed in MyD88-deficient mice. Additionally, it was shown that IL-12-deficient mice are extremely susceptible to T. gondii and that IL-12 enhances IFN-γ production (11, 12). While IL-12 is known to regulate IFN-γ, it has no protective role independent of IFN-γ-mediated pathogen resistance (9). These data established T. gondii as a model pathogen of Th1 immunity (13). A discussion of the individual cell types involved in producing this critical cytokine is fundamental to our understanding of host immunity to T. gondii.
T cells.
Both CD4+ and CD8+ T cells seem to have an important synergistic role to play during T. gondii infection, because individual depletions of CD4+ or CD8+ T cells do not recapitulate the phenotype of double-depletion or T cell-deficient mice (1, 14). Mice lacking all T cells do not survive into the chronic stage of infection (15), and depletion of T cells in the chronic stage leads to reactivation of the disease state (1).
CD4+ T cells.
The role of CD4+ T cells during intracellular infections is the production of IFN-γ. Adoptive transfer of immune CD4+ T cells into athymic nude mice during a T. gondii reactivation model partially rescues their survival (16). The importance of CD4+ T cells during infection is explained by the effector mechanisms induced by IFN-γ. It is known that IFN-γ made by CD4+ cells is needed to prime macrophages to produce potent antimicrobial responses (17). IFN-γ regulates inducible nitric oxide synthase (iNOS) expression, which is ultimately needed for control of the parasite, as iNOS-deficient mice succumb at 3 to 4 weeks postinfection (18). Production of nitric oxide interacts with diverse metabolic pathways, and, as a free radical, it is toxic to bacteria and intracellular parasites. Mouse macrophages can also use their respiratory burst to generate toxic reactive-oxygen species (ROS) in response to T. gondii, and this response is dependent on IFN-γ (19). Additionally, IFN-γ induces indoleamine 2,3-dioxygense (IDO), which is an enzyme that is involved in tryptophan catabolism and that degrades cellular tryptophan. T. gondii is a tryptophan auxotroph, and induction of IDO-1 inhibits parasite growth in human cells (20, 21). Recently, it was found that inhibiting both IDO-1 and IDO-2 during T. gondii infection in mice also results in increased parasite burden and increased susceptibility in the chronic phase of infection (22). There are additional IFN-γ-induced proteins in mice known as immunity-related GTPases (IRG); however, this large family of proteins is not represented in the human genome (23). The complex phenotypes of IRG knockout mice have been recently reviewed elsewhere (24). The importance for IFN-γ-mediated effector mechanisms is further illustrated by recent evidence that T. gondii actively inhibits IFN-γ signaling by inhibiting the turnover rate of its downstream transcription factor STAT1 in human cell lines (25). Removal of any one of the IFN-γ-induced mechanisms to control T. gondii leads to higher parasite burdens in mice, but acute survival is not impaired, in contrast to the extreme susceptibility of IFN-γ-deficient mice. However, in the absence of CD4+ T cells, an extended IL-12-dependent NK cell response is capable of producing adequate amounts of IFN-γ to allow survival (26). This survival depends on the NK cells helping to generate CD8+ T cell immunity to the parasite during the chronic phase of infection (26). Yet in the normal C57BL/6 model, IFN-γ is predominantly made by CD4+ T cells rather than by CD8+ T cells.
CD8+ T cells.
CD8+ T cells have a complex role to play in control of T. gondii infection: they secrete IFN-γ and they can specifically kill infected cells. It has been shown by several groups that CD8+ T cells specific for parasite antigens can directly kill infected cells. For example, this can be illustrated with CD8+ T cells specific for an immunodominant T. gondii epitope, P30, which were found to be capable of killing both extracellular parasites and infected macrophages in vitro (27, 28). Additionally, CD8+ T cells generated from mice vaccinated with a temperature-sensitive mutant of T. gondii (ts-4) are cytotoxic in vitro for parasite-infected or antigen-pulsed cells in a major histocompatibility complex class I (MHC-I)-restricted manner (29). It is now established in mice that there are genetic variations in susceptibility to reactivation of T. gondii in the chronic stage of the infection: C57BL/6 mice, MHC-I H-2b haplotype, have the H-2Lnull allele and are susceptible to toxoplasmic encephalitis (TE), but BALB/c mice, MHC-I H-2d haplotype, have the H-2Ld allele and are genetically resistant to TE (30–32). This information explains why CD8+ T cells are the dominant T cell responders in BALB/c models but are minor players in C57BL/6 mouse models. A subsequent and perhaps unsurprising finding associated with this homozygosity of the MHC loci in inbred mice is that BALB/c mice have a corresponding TCR restriction, Vβ8 CD8+ cells, which are abundant in the brain of infected mice (33). Adoptive transfer experiments showed that Vβ8 CD8+ cells produced the most IFN-γ and were more protective than Vβ8 CD4+ cells in response to parasite infection, leading the authors to speculate that this restriction allows for the best recognition of T. gondii antigens presented via MHC-I H-2Ld on infected cells (33). CD8+ T cell responses to epitopes of several identified antigenic T. gondii proteins, including P30 (SAG1), GRA4, GRA6, and ROP7 in BALB/c mice (27, 28, 34, 35), GRA1, GRA7, and ROP2 in C3H mice (36–38), or Tgd057 in C57BL/6 mice (39), could potentially be generated, and while vaccination with one of these proteins, such as GRA1, provides some protection from lethal T. gondii challenge to a C3H mouse, this vaccination does not protect BALB/c or C57BL/6 mice despite generation of antigen-specific CD8+ T cells (40). Surprisingly, the exact T. gondii protein used for vaccination seems to be less important than the location of the epitope processed for antigen presentation within the original protein sequence (41), further illustrating that the specific MHC-I haplotype of the host, and consequent bias in peptide loading for antigen presentation, is critical for determining the contribution of CD8+ T cells to protection from the parasite.
While CD8+ T cells play an important role in BALB/c mice during the chronic stage of the infection, there is a considerable amount of conflicting literature as to whether this protection is mediated by secreted IFN-γ or by their direct cytolytic ability. For example, perforin-deficient mice lacking cytolytic ability survive acute T. gondii infection and have an unimpaired level of IFN-γ, but they have a higher cyst burden and slightly increased susceptibility at later time points of the infection. These results indicate that the cytolytic abilities of CD8+ T cells contribute to control of encysted parasites (42). In contrast, adoptive transfer of perforin-deficient CD8+ T cells was still effective at preventing TE in a chronic reactivation model utilizing sulfadiazine-treated athymic nude mice (43). Although adoptive transfer of perforin-deficient CD8+ T cells is effective at preventing TE, recent work supports the model of cytolytic cyst control, as transferred IFN-γ-deficient CD8+ T cells are able to greatly reduce cyst burden in a chronic reactivation model (44). The multifaceted effects of IFN-γ on the immune system make it difficult to separate the contribution of CD8+ T cell IFN-γ from that of other sources of this cytokine. IFN-γ itself increases expression of endothelial vascular cell adhesion molecule 1 (VCAM-1) to aid in recruitment of CD8+ T cells to the brain of chronically infected mice and thus enhances any effects of CD8+ T cells on immunity to T. gondii (45). It seems likely that both IFN-γ and cytolytic functions of CD8+ T cells are contributing to host resistance to pathogenesis (46).
NK cells.
Early experiments into T. gondii infection during T cell deficiency revealed that NK cells were also a source of IFN-γ. Splenocytes from SCID mice were able to produce significant amounts of IFN-γ in response to the parasite (47, 48). In mice lacking CD8+ T cells, NK cells dramatically expand in numbers, produce IFN-γ, and mediate protection against T. gondii (48). Simultaneously, it was shown that NK cells are not directly cytotoxic for T. gondii-infected cells but that NK cell protection is mediated by IFN-γ (15, 48). IFN-γ from NK cells can be enhanced with the early administration of IL-12 during infection and this increases the survival of susceptible SCID mice (12); IL-12 is both required and sufficient for triggering IFN-γ production by NK cells (2). This IL-12 is produced by dendritic cells (DCs), which are discussed in more detail in a subsequent section.
The effect of DC-derived IL-12 on NK cell IFN-γ production triggers a positive-feedback loop that modulates the innate inflammatory environment. T. gondii-infected mice blocked for IFN-γ or depleted of NK cells demonstrate impaired maturation of DCs and inflammatory monocytes, increased numbers of resident macrophages, and decreased IL-12 production by DCs (49). The ability of monocytes to respond to IFN-γ priming is necessary for increased activation of monocytes and for IL-12 production by monocyte-derived DCs in response to T. gondii infection (49, 50). On the molecular level, it has been shown that IFN-γ enhances the production and transcription of IL-12 via induction of regulatory transcription factor 8 (IRF8), a major transcriptional factor regulating IL-12p40 and IL-12p35 transcription (50, 51). Together, these results indicate that NK cells are a critical effector component of the innate immune response—able to effectively prime monocytes and DCs for parasite recognition while mediating T cell-independent IFN-γ-dependent host protection from the parasite.
Neutrophils.
An unforeseen player in the production of IFN-γ during toxoplasmosis is the neutrophil. Recent evidence illustrates that in both wild-type (WT) and TLR11-deficient mice, neutrophils are the dominant cell type responsible for early IFN-γ responses (52). The level of IFN-γ observed in each neutrophil is low compared to that of activated NK or T cells (52), and yet neutrophils are known to congregate in large numbers at the site of acute toxoplasma infection (49, 52–54). Depletion of neutrophils in TLR11-deficient mice resulted in acute mortality that can be delayed with injections of recombinant IFN-γ (52). These data revealed that neutrophil-derived IFN-γ is responsible for the partial survival of TLR11-deficient mice during the acute stage of the infection. In WT mice, both TLR11- and IL-12-dependent NK cell IFN-γ and TLR11-independent neutrophil IFN-γ are involved in resistance to T. gondii during the acute stage of the infection.
Neutrophils and their confusion with inflammatory monocytes.
The consequences of IFN-γ production by neutrophils during T. gondii infection are both in agreement with and in contrast to previous data describing a role for neutrophils in host resistance to the parasite. Original studies on the importance of neutrophils were accomplished by utilizing the antibody Gr-1 to deplete neutrophils, and exacerbation of disease was observed (55, 56). The Gr-1 antibody recognizes two distinctly expressed but highly related surface receptors, Ly-6C and Ly-6G, which are expressed on both monocytes and neutrophils or on neutrophils alone, respectively (57). Conclusions about the importance of neutrophils drawn from experiments utilizing Gr-1 for depletion were confounded by simultaneous depletion of both monocytes and neutrophils. The individual roles these two cell types play during T. gondii infection began to be elucidated with chemokine and chemokine receptor knockout mice. CCR2- and MCP-1-deficient mice fail to recruit inflammatory monocytes, and the results are a loss of parasite control and acute susceptibility to T. gondii infection (58). These data suggest that inflammatory monocytes are essential for parasite elimination at the site of the infection. Yet both CCR2- and MCP-1-deficient mice have unimpaired IFN-γ levels in the serum and peritoneal lavage fluid (58). Furthermore, CD4+ T cells isolated from CCR2- and MCP-1-deficient mice produced similar amounts of IFN-γ in response to T. gondii antigens (58). These results indicate that IFN-γ production and generation of Th1 responses to the parasite are independent of the presence of inflammatory monocytes but that inflammatory monocyte effector mechanisms for parasite elimination are indispensable for the host protection. In contrast to CCR2- and MCP-deficient mice, CXCR2-deficient mice have impaired neutrophil recruitment during infection. CXCR2-deficient mice were found to harbor a higher parasite burden, but these mice did not display increased mortality during the acute stage of the infection (59). Unlike CCR2- and MCP-1-deficient mice, CXCR2-deficient mice did have dramatically reduced serum IFN-γ and T cell IFN-γ responses, implicating neutrophils as early players in influencing IFN-γ levels (59).
The recent availability of an antibody specific for Ly-6G (1A8) allowed further insight into the roles of neutrophils during infection. In an oral infection model, greater than 65% of WT mice treated with 1A8 survived infection, whereas CCR2-deficient mice and WT mice treated with Gr-1 succumb during the acute phase (60). The authors concluded that monocytes and not neutrophils were important for parasite control in part because administration of 1A8 had no effect on the percentage of monocyte recruitment to the site of infection. Interestingly, in a pathogen-free subcutaneous air pouch model, it has been shown that depletion of neutrophils significantly decreases the recruitment of inflammatory monocytes (61). In fact, neutropenia reduces monocyte recruitment in a number of models, as reviewed in reference 62. Thus, in the absence of neutrophils, there may be lower total numbers of inflammatory monocytes, and yet they could represent a percentage of the remaining cells in vivo similar to that seen with the nondepleted condition.
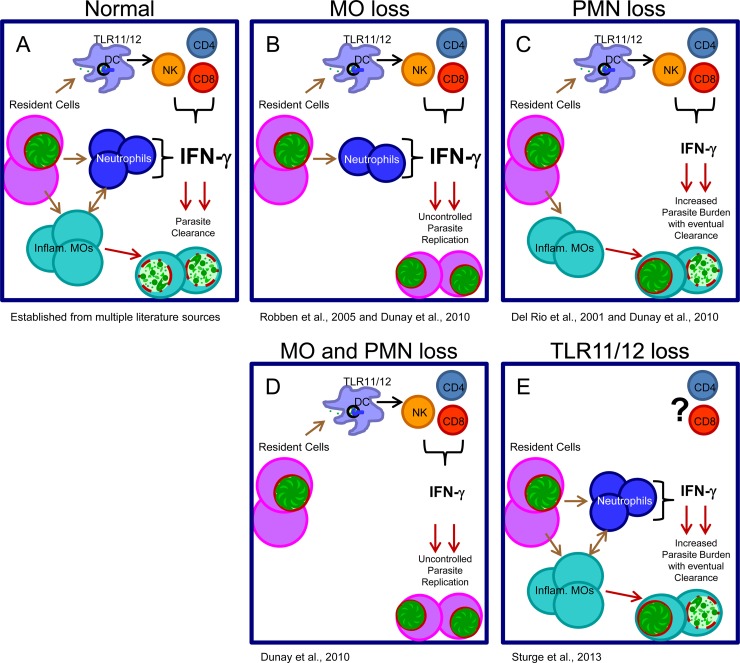
It is important to emphasize that the relative contributions of monocytes and neutrophils were largely examined in mice which have an intact TLR11 recognition system responsible for IL-12-dependent NK cell IFN-γ priming of inflammatory monocytes. But in the absence of inflammatory monocytes, such as in cases of CCR2 deficiency, MCP-1 deficiency, or depletion with anti-Gr-1, sufficient IFN-γ levels become irrelevant. TLR11-deficient mice still have significant amounts of IFN-γ present, initially provided by neutrophils, to prime monocytes, and these mice usually survive infection (4, 63). In the absence of both TLR11 and neutrophils, the early IFN-γ response is severely compromised and inflammatory monocyte recruitment may be reduced, resulting in acute susceptibility (Fig. 1).
FIG 1.
Hypothesized relationship between various immunodeficiencies during acute parasite infection in a murine model of toxoplasmosis. (A) Resident cells become infected or sense danger products from infection and recruit dendritic cells, inflammatory monocytes (Inflam. MOs), and neutrophils (PMNs), allowing IFN-γ production from multiple sources and parasite destruction by inflammatory monocytes. (B) Loss of inflammatory monocytes (MCP-1 deficiency, CCR2 deficiency) results in sufficient IFN-γ production but is not accompanied by the ability to clear parasites effectively, resulting in host mortality (58, 60). (C) Loss of neutrophils alone (CXCR2 deficiency, depletion with Ly-6G [1A8]) results in loss of neutrophil-derived IFN-γ. However, the dominance of the TLR11/TLR12-mediated recognition pathway allows sufficient IFN-γ production for eventual clearance and survival into the chronic stage (59, 60). (D) Loss of both monocytes and neutrophils (depletion with anti-Gr-1) leads to uncontrolled parasite replication and, similarly to monocyte depletion, leads to acute mortality (60). (E) Loss of TLR11/TLR12-mediated recognition results in loss of DC IL-12 and a subsequent delay in IFN-γ production by NK and T cells. Neutrophil-derived IFN-γ affords limited control of the parasite burden, resulting in most or mixed numbers of surviving mice (52, 60).
The large variety of cell types capable of making IFN-γ illustrates the importance of this cytokine in immunity. The contribution of individual cell types to IFN-γ production is a complex subject, but the generation of conditional IFN-γ- or IFN-γ receptor-deficient mouse models may provide the opportunity to dissect this dilemma.
KNOWN AND UNKNOWN REGULATORS OF IFN-γ PRODUCTION: IL-12, IL-1β, AND TNF
DC IL-12 production is essential for IFN-γ production by NK cells.
DCs are the major IL-12-producing cells in response to T. gondii infection. It was observed that IL-12 is initially produced by CD8α+ DCs upon exposure to T. gondii profilin in a TLR11- and MyD88-dependent manner (2, 4, 6). CD8α+ DCs produce large amounts of IL-12 but not other proinflammatory cytokines such as TNF and IL-1β (50). The explanation for this cytokine specificity was recently explained by the finding that TLR11 induction of IL-12 requires activation of the transcriptional factor IRF8 which is selectively expressed in naive CD8α+ DCs (50, 64). At the same time, recognition of T. gondii profilin by CD8α+ DCs fails to trigger substantial activation of NF-κB, which explains their relative lack of production of TNF and IL-1β (50), two cytokines regulated via the NF-κB-signaling pathway. After the initial recognition of the parasite and cytokine production, IFN-γ is produced early in infection by neutrophils and NK cells. Early IFN-γ production induces expression of IRF8 in macrophages and CD8α− DCs which enables them to respond to T. gondii infection, reinforcing the IL-12 response (50).
Impairment of parasite recognition in DCs, with DC-specific inactivation of MyD88 in mice, results in a delayed and reduced, but not abolished, IFN-γ response from NK cells, suggesting that additional sources of IL-12 also contribute during infection (2). Nevertheless, delayed NK cell IFN-γ production has a major impact on the outcome of the infection; mice with DC-specific inactivation of MyD88 are very susceptible to the parasitic infection, with a survival rate below 50% (2). Mice surviving infection with DC-specific inactivation of MyD88 have dramatically elevated cyst burdens during the chronic stages of the infection (2). Thus, even though NK cell IFN-γ production is largely initiated during the acute stage of the infection, it has long-term consequences seen weeks after initial T. gondii infection. It should be noted that this TLR11-dependent IL-12 response regulates IFN-γ production from NK cells (2, 15) and from effector CD8+ T cells (65) but is dispensable for neutrophil IFN-γ production (52). While much is known about TLR11-mediated immunity to T. gondii, these and other data suggest that TLR11 is not the entirety of the story for T. gondii recognition or regulation of cytokine production by immune cells.
TLR12 coordinates with TLR11 in parasite recognition.
Pattern recognition receptors, such as TLRs, are innate immune receptors involved in recognizing conserved molecules present on bacteria, viruses, and parasites which are not expressed by the host cells. In mice, recognition of T. gondii profilin is mediated by TLR11, which directly binds profilin, forms a heterodimer with TLR12, and recruits the TLR adaptor protein MyD88 (4, 5, 50). Recent data suggest that TLR12 can function independently of TLR11 in plasmacytoid DCs (pDCs). pDCs are a specialized subset of DCs that produce large quantities of type 1 interferons. They are most often associated with viral infections but have also been shown to be involved in both bacterial and parasitic infections. pDCs were specifically observed to produce IL-12 and IFN-α in response to profilin, but in contrast to the TLR11 and TLR12 heterodimer complex that usually regulates IL-12 production, the TLR12 homodimer was sufficient (66). However, the conclusion by Koblansky et al. is inconsistent with an earlier analysis which found that pDCs fail to produce IL-12 or IFN-α in the absence of TLR11 (67); therefore, their further observations attributing NK cell IFN-γ to pDC IL-12 should be viewed with some skepticism. This discrepancy regarding the TLR dependence of pDC IL-12 needs to be clarified, as does the observed dependence of IFN-α on TLR12. Furthermore, how TLR12 can trigger cytokine production in the absence of TLR11 is a mystery because, in contrast to TLR11, TLR12 fails to biochemically interact with MyD88 (50).
Regulation and production of IL-1β and TNF.
IL-1β is a powerful mediator of the inflammatory response. This cytokine is synthesized in a pro-form in response to TLR signals in a MyD88-dependent manner and then typically cleaved by caspase-1 and secreted in response to danger signals. Daily injections of IL-1β or TNF into T. gondii-infected BALB/c mice significantly prolong their survival, and administration of a single dose of IL-1β and TNF combined greatly enhances this survival (68). Furthermore, production of IFN-γ by SCID mouse splenocytes stimulated with IL-12 plus TNF was completely ablated by administering anti-IL-1β (69). As a pleotropic mediator of inflammation, IL-1β mediates its protective effects via multiple mechanisms, including enhancing expression of the TNF receptor, which may explain the synergistic effect of IL-1β and TNF (70). While TNF alone is unable to significantly increase the antiparasitic activity of macrophages, TNF synergizes with IFN-γ to enhance it (68). Additionally, both TNF and IL-1β are crucial regulators of neutrophil-derived IFN-γ, as blocking either TNF or IL-1β decreased the observed IFN-γ levels (52). IL-1R-deficient mice have almost no detectable neutrophil-derived IFN-γ, which is a stronger phenotype than blocking IL-1β and is in contrast to the resistance phenotype of caspase-1-deficient mice (52, 63). Possible explanations for the discrepancy between IL-1β blocking and IL-1R-deficient mice include incomplete blocking of IL-1β, the dominance of the TLR11 recognition system in IL-1R-deficient mice, and the fact that neutrophils are capable of caspase-1-independent IL-1β production (71). The dominant source of TNF and IL-1β is monocytes, but since neutrophils themselves are capable of making both of these cytokines, this may be an important feedback loop during the initial response (52). While it seems likely that TLRs are involved in the recognition system regulating TNF, it is possible that there is an unknown recognition system or danger signal(s) regulating IL-1β.
MyD88 dependence of host resistance independent of TLR11.
A likely explanation for residual resistance to T. gondii infection in the absence of TLR11 is the cooperation of additional endosomal TLRs and IL-1β in activation of MyD88. In 3d mice, which lack functional UNC93B1—a chaperone protein that controls trafficking of TLRs from the endoplasmic reticulum to the endolysosome—resistance to T. gondii is lost because all endosomal TLRs, including TLR11, TLR12, TLR7, and TLR9, are incorrectly localized in these mice (50, 72–76). Interestingly, the severe acute susceptibility seen in 3d mice is nearly identical to that of MyD88-deficient mice, suggesting that there are additional UNC93B1- and MyD88-dependent TLRs involved (74, 75). TLR3-, TLR7-, or TLR9-deficient mice exhibit little to no phenotype during T. gondii infection (63, 74) even when the deficiencies are combined, as is the case of TLR3/TLR7/TLR9 triple deficiency (76). These data illustrate the dominant effect of TLR11/TLR12 profilin recognition in generating a Th1 immune response to the parasite in a mouse model. However, since humans do not have functional TLR11 or TLR12 (77), the importance of other TLRs must be considered. It has been found that TLR7 can recognize T. gondii RNA and that TLR9 can recognize many CpG motifs in T. gondii DNA (76), and a further discussion of these data, and other TLRs, has been recently reviewed (24). These additional intracellular TLRs likely contribute to the susceptibility of both MyD88 and 3d mice, as TLR3/TLR7/TLR9/TLR11 quadruple-deficient mice had almost no IL-12 or IFN-γ observed in the first week of infection (76).
Yet there is a striking difference between the survivability of 3d mice and MyD88-deficient mice during T. gondii infection in that administration of IL-12 to 3d mice rescues their survival but IL-12-treated MyD88-deficient mice are as susceptible to infection as nontreated mice despite partially restored Th1 responses (2, 7). This suggests that MyD88 is important beyond IL-12-dependent innate immune cell responses. At least some of this may be explained by the role of MyD88 in IL-1β production and neutrophil IFN-γ as discussed above. Additionally, MyD88 may be involved in the priming event in inflammasome activation. The inflammasome is a complex of proteins that assemble in response to intracellular danger signals, often mediated by proteins from the ‘nucleotide-binding domain and leucine-rich repeat-containing gene family, with a pyrin domain' (NLRPs), capable of activating caspase proteins to cleave pro-IL-1β or pro-IL-18 into their mature forms for secretion. Before IL-1β and IL-18 can be cleaved, they need to be expressed, and priming through TLRs via MyD88 is one way to induce production of the pro-form of these cytokines. It was recently shown that T. gondii can activate NLRP1 (78, 79) and NLRP3 (79) inflammasome complexes to stimulate IL-1β or IL-18 cleavage and secretion. It is known that, in oral infection models of T. gondii, commensal bacteria can provide the ligands for TLR2, TLR4, and TLR9 stimulation (63). These bacterially derived TLR ligands could provide the priming signal necessary for successful inflammasome responses. However, T. gondii itself can also provide additional TLR ligands, as mentioned above, and these data are supported by the fact that LPS priming was not required in vitro for bone marrow-derived macrophages to produce IL-1β in response to T. gondii (79) and by the fact that T. gondii infection in germfree mice had a robust IL-1β response (78). These data indicate that MyD88 is necessary for a successful inflammasome response to T. gondii infection, although the precise signaling events leading to caspase activation upstream as well as downstream of MyD88 are not fully defined.
Additionally, experiments in conditional MyD88-deficient mice showed that removing MyD88 in T cells alone resulted in a dramatic abrogation of Th1 response to the parasite (80). Whether the mechanisms underlying T cell-intrinsic MyD88-dependent loss of IFN-γ in CD4+ T cells can be attributed to TLR- or IL-1-dependent activation of MyD88 remains to be identified. However, it is clear that the acute susceptibility of completely MyD88-deficient mice to T. gondii infection stems from multiple defects in activation of IFN-γ production by neutrophils and by NK and T cells: deficiency in IL-1β signaling impairs neutrophil IFN-γ production, DC-specific loss of TLR signaling results in deficient NK cell IFN-γ production, and T cell-intrinsic MyD88 deficiency abrogates Th1 responses to the parasite.
CONCLUDING REMARKS
This review has given a broad overview of IFN-γ during T. gondii infection and serves to illustrate the blurring of the line between innate and adaptive responses: MyD88 is important for the IFN-γ immune responses from neutrophils and from NK and T cells (Fig. 2). Much is now known about the immune responses to T. gondii in murine models, including the dominance of TLR11-mediated recognition and IRGs. Humans lack these systems and yet have a robust immune response which includes the production of IFN-γ. Therefore, future work in the field will likely be focused on identification and clarification of the roles of innate sensors and resistance mechanisms important for T. gondii recognition in humans.
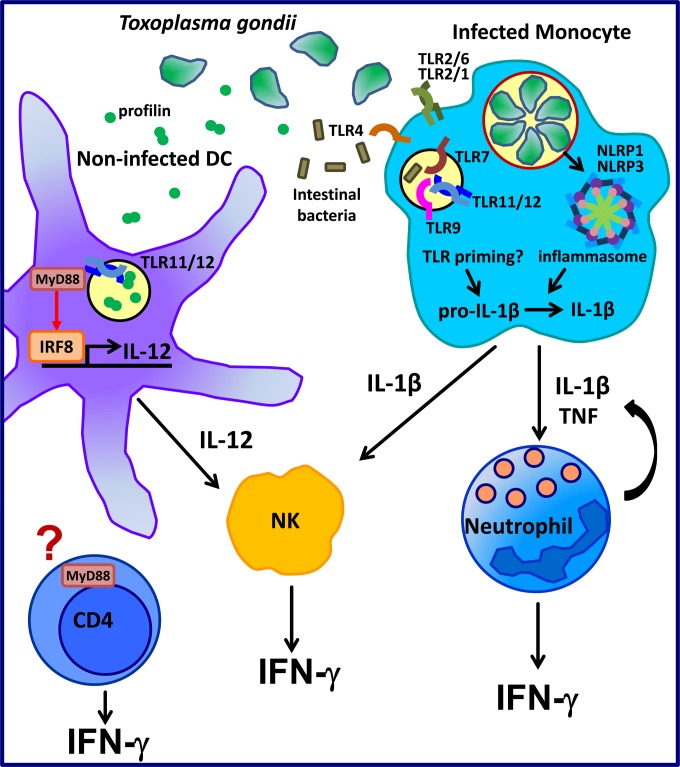
FIG 2.
Current model of IFN-γ-mediated resistance to T. gondii. When T. gondii infects a mouse, the endosomal TLR11/TLR12 complex recognizes a parasitic protein profilin. TLR11 via its adaptor protein MyD88 initiates downstream signaling, leading to activation of the transcription factor IRF8. This allows DCs to produce IL-12 in response to T. gondii infection. IL-12 mediates activation of NK cells and their IFN-γ production. Infected macrophages and monocytes may be able to respond to T. gondii infection through NLRP1 and NLRP3 inflammasomes. Priming by TLRs is needed to induce pro-IL-1β expression prior to its subsequent cleavage by the inflammasome complex and secretion in its mature form. While the specific TLRs involved in priming are not known, the process is likely initiated by two different events based on the following facts: (i) T. gondii itself has ligands for endosomal TLR7, TLR9, TLR11, and TLR12 and for surface-expressed TLR2 and (ii) intestinal bacteria can pass the mucosal barrier during T. gondii-mediated inflammation and contain ligands for TLR2, TLR4, and TLR9. IL-1β and TNF are involved in IFN-γ as seen from neutrophils, and neutrophils themselves can produce IL-1β and TNF, resulting in potential positive feedback. IL-1β production can also enhance the response of NK cells. It was recently shown that T cell IFN-γ is largely dependent on the presence of T cell-intrinsic MyD88 rather than MyD88 as seen in other cell types. Whether this MyD88 is dependent on TLRs or the IL-1 family receptors has yet to be investigated.
Footnotes
Published ahead of print 27 May 2014
REFERENCES
- 1.Gazzinelli R, Xu Y, Hieny S, Cheever A, Sher A. 1992. Simultaneous depletion of CD4+ and CD8+ T lymphocytes is required to reactivate chronic infection with Toxoplasma gondii. J. Immunol. 149:175–180. [PubMed] [Google Scholar]
- 2.Hou B, Benson A, Kuzmich L, DeFranco AL, Yarovinsky F. 2011. Critical coordination of innate immune defense against Toxoplasma gondii by dendritic cells responding via their Toll-like receptors. Proc. Natl. Acad. Sci. U. S. A. 108:278–283. 10.1073/pnas.1011549108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scanga CA, Aliberti J, Jankovic D, Tilloy F, Bennouna S, Denkers EY, Medzhitov R, Sher A. 2002. Cutting edge: MyD88 is required for resistance to Toxoplasma gondii infection and regulates parasite-induced IL-12 production by dendritic cells. J. Immunol. 168:5997–6001. 10.4049/jimmunol.168.12.5997. [DOI] [PubMed] [Google Scholar]
- 4.Yarovinsky F, Zhang D, Andersen JF, Bannenberg GL, Serhan CN, Hayden MS, Hieny S, Sutterwala FS, Flavell RA, Ghosh S, Sher A. 2005. TLR11 activation of dendritic cells by a protozoan profilin-like protein. Science 308:1626–1629. 10.1126/science.1109893. [DOI] [PubMed] [Google Scholar]
- 5.Plattner F, Yarovinsky F, Romero S, Didry D, Carlier MF, Sher A, Soldati-Favre D. 2008. Toxoplasma profilin is essential for host cell invasion and TLR11-dependent induction of an interleukin-12 response. Cell Host Microbe 3:77–87. 10.1016/j.chom.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 6.Mashayekhi M, Sandau MM, Dunay IR, Frickel EM, Khan A, Goldszmid RS, Sher A, Ploegh HL, Murphy TL, Sibley LD, Murphy KM. 2011. CD8alpha(+) dendritic cells are the critical source of interleukin-12 that controls acute infection by Toxoplasma gondii tachyzoites. Immunity 35:249–259. 10.1016/j.immuni.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.LaRosa DF, Stumhofer JS, Gelman AE, Rahman AH, Taylor DK, Hunter CA, Turka LA. 2008. T cell expression of MyD88 is required for resistance to Toxoplasma gondii. Proc. Natl. Acad. Sci. U. S. A. 105:3855–3860. 10.1073/pnas.0706663105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suzuki Y, Orellana MA, Schreiber RD, Remington JS. 1988. Interferon-gamma: the major mediator of resistance against Toxoplasma gondii. Science 240:516–518. 10.1126/science.3128869. [DOI] [PubMed] [Google Scholar]
- 9.Scharton-Kersten TM, Wynn TA, Denkers EY, Bala S, Grunvald E, Hieny S, Gazzinelli RT, Sher A. 1996. In the absence of endogenous IFN-gamma, mice develop unimpaired IL-12 responses to Toxoplasma gondii while failing to control acute infection. J. Immunol. 157:4045–4054. [PubMed] [Google Scholar]
- 10.Yap GS, Sher A. 1999. Effector cells of both nonhemopoietic and hemopoietic origin are required for interferon (IFN)-gamma- and tumor necrosis factor (TNF)-alpha-dependent host resistance to the intracellular pathogen, Toxoplasma gondii. J. Exp. Med. 189:1083–1092. 10.1084/jem.189.7.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gazzinelli RT, Hieny S, Wynn TA, Wolf S, Sher A. 1993. Interleukin 12 is required for the T-lymphocyte-independent induction of interferon gamma by an intracellular parasite and induces resistance in T-cell-deficient hosts. Proc. Natl. Acad. Sci. U. S. A. 90:6115–6119. 10.1073/pnas.90.13.6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khan IA, Matsuura T, Kasper LH. 1994. Interleukin-12 enhances murine survival against acute toxoplasmosis. Infect. Immun. 62:1639–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jankovic D, Kullberg MC, Hieny S, Caspar P, Collazo CM, Sher A. 2002. In the absence of IL-12, CD4(+) T cell responses to intracellular pathogens fail to default to a Th2 pattern and are host protective in an IL-10(-/-) setting. Immunity 16:429–439. 10.1016/S1074-7613(02)00278-9. [DOI] [PubMed] [Google Scholar]
- 14.Suzuki Y, Remington JS. 1988. Dual regulation of resistance against Toxoplasma gondii infection by Lyt-2+ and Lyt-1+, L3T4+ T cells in mice. J. Immunol. 140:3943–3946. [PubMed] [Google Scholar]
- 15.Hunter CA, Subauste CS, Van Cleave VH, Remington JS. 1994. Production of gamma interferon by natural killer cells from Toxoplasma gondii-infected SCID mice: regulation by interleukin-10, interleukin-12, and tumor necrosis factor alpha. Infect. Immun. 62:2818–2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kang H, Suzuki Y. 2001. Requirement of non-T cells that produce gamma interferon for prevention of reactivation of Toxoplasma gondii infection in the brain. Infect. Immun. 69:2920–2927. 10.1128/IAI.69.5.2920-2927.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cohen SB, Maurer KJ, Egan CE, Oghumu S, Satoskar AR, Denkers EY. 2013. CXCR3-dependent CD4(+) T cells are required to activate inflammatory monocytes for defense against intestinal infection. PLoS Pathog. 9:e1003706. 10.1371/journal.ppat.1003706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scharton-Kersten TM, Yap G, Magram J, Sher A. 1997. Inducible nitric oxide is essential for host control of persistent but not acute infection with the intracellular pathogen Toxoplasma gondii. J. Exp. Med. 185:1261–1273. 10.1084/jem.185.7.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arsenijevic D, Bilbao FD, Giannakopoulos P, Girardier L, Samec S, Richard D. 2001. A role for interferon-gamma in the hypermetabolic response to murine toxoplasmosis. Eur. Cytokine Netw. 12:518–527. [PubMed] [Google Scholar]
- 20.Pfefferkorn ER. 1984. Interferon gamma blocks the growth of Toxoplasma gondii in human fibroblasts by inducing the host cells to degrade tryptophan. Proc. Natl. Acad. Sci. U. S. A. 81:908–912. 10.1073/pnas.81.3.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gupta SL, Carlin JM, Pyati P, Dai W, Pfefferkorn ER, Murphy MJ., Jr 1994. Antiparasitic and antiproliferative effects of indoleamine 2,3-dioxygenase enzyme expression in human fibroblasts. Infect. Immun. 62:2277–2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Divanovic S, Sawtell NM, Trompette A, Warning JI, Dias A, Cooper AM, Yap GS, Arditi M, Shimada K, Duhadaway JB, Prendergast GC, Basaraba RJ, Mellor AL, Munn DH, Aliberti J, Karp CL. 2012. Opposing biological functions of tryptophan catabolizing enzymes during intracellular infection. J. Infect. Dis. 205:152–161. 10.1093/infdis/jir621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Howard JC, Hunn JP, Steinfeldt T. 2011. The IRG protein-based resistance mechanism in mice and its relation to virulence in Toxoplasma gondii. Curr. Opin. Microbiol. 14:414–421. 10.1016/j.mib.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 24.Yarovinsky F. 2014. Innate immunity to Toxoplasma gondii infection. Nat. Rev. Immunol. 14:109–121. 10.1038/nri3598. [DOI] [PubMed] [Google Scholar]
- 25.Rosowski EE, Nguyen QP, Camejo A, Spooner E, Saeij JP. 2014. Toxoplasma gondii inhibits gamma interferon (IFN-gamma)- and IFN-beta-induced host cell STAT1 transcriptional activity by increasing the association of STAT1 with DNA. Infect. Immun. 82:706–719. 10.1128/IAI.01291-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Combe CL, Curiel TJ, Moretto MM, Khan IA. 2005. NK cells help to induce CD8(+)-T-cell immunity against Toxoplasma gondii in the absence of CD4(+) T cells. Infect. Immun. 73:4913–4921. 10.1128/IAI.73.8.4913-4921.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khan IA, Smith KA, Kasper LH. 1988. Induction of antigen-specific parasiticidal cytotoxic T cell splenocytes by a major membrane protein (P30) of Toxoplasma gondii. J. Immunol. 141:3600–3605. [PubMed] [Google Scholar]
- 28.Kasper LH, Khan IA, Ely KH, Buelow R, Boothroyd JC. 1992. Antigen-specific (p30) mouse CD8+ T cells are cytotoxic against Toxoplasma gondii-infected peritoneal macrophages. J. Immunol. 148:1493–1498. [PubMed] [Google Scholar]
- 29.Hakim FT, Gazzinelli RT, Denkers E, Hieny S, Shearer GM, Sher A. 1991. CD8+ T cells from mice vaccinated against Toxoplasma gondii are cytotoxic for parasite-infected or antigen-pulsed host cells. J. Immunol. 147:2310–2316. [PubMed] [Google Scholar]
- 30.Brown CR, McLeod R. 1990. Class I MHC genes and CD8+ T cells determine cyst number in Toxoplasma gondii infection. J. Immunol. 145:3438–3441. [PubMed] [Google Scholar]
- 31.Suzuki Y, Joh K, Kwon OC, Yang Q, Conley FK, Remington JS. 1994. MHC class I gene(s) in the D/L region but not the TNF-alpha gene determines development of toxoplasmic encephalitis in mice. J. Immunol. 153:4649–4654. [PubMed] [Google Scholar]
- 32.Brown CR, Hunter CA, Estes RG, Beckmann E, Forman J, David C, Remington JS, McLeod R. 1995. Definitive identification of a gene that confers resistance against Toxoplasma cyst burden and encephalitis. Immunology 85:419–428. [PMC free article] [PubMed] [Google Scholar]
- 33.Wang X, Claflin J, Kang H, Suzuki Y. 2005. Importance of CD8(+)Vbeta8(+) T cells in IFN-gamma-mediated prevention of toxoplasmic encephalitis in genetically resistant BALB/c mice. J. Interferon Cytokine Res. 25:338–344. 10.1089/jir.2005.25.338. [DOI] [PubMed] [Google Scholar]
- 34.Frickel EM, Sahoo N, Hopp J, Gubbels MJ, Craver MP, Knoll LJ, Ploegh HL, Grotenbreg GM. 2008. Parasite stage-specific recognition of endogenous Toxoplasma gondii-derived CD8+ T cell epitopes. J. Infect. Dis. 198:1625–1633. 10.1086/593019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blanchard N, Gonzalez F, Schaeffer M, Joncker NT, Cheng T, Shastri AJ, Robey EA, Shastri N. 2008. Immunodominant, protective response to the parasite Toxoplasma gondii requires antigen processing in the endoplasmic reticulum. Nat. Immunol. 9:937–944. 10.1038/ni.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Duquesne V, Auriault C, Gras-Masse H, Boutillon C, Darcy F, Cesbron-Delauw MF, Tartar A, Capron A. 1991. Identification of T cell epitopes within a 23-kD antigen (P24) of Toxoplasma gondii. Clin. Exp. Immunol. 84:527–534. [PMC free article] [PubMed] [Google Scholar]
- 37.Saavedra R, de Meuter F, Decourt JL, Herion P. 1991. Human T cell clone identifies a potentially protective 54-kDa protein antigen of Toxoplasma gondii cloned and expressed in Escherichia coli. J. Immunol. 147:1975–1982. [PubMed] [Google Scholar]
- 38.Jacobs D, Vercammen M, Saman E. 1999. Evaluation of recombinant dense granule antigen 7 (GRA7) of Toxoplasma gondii for detection of immunoglobulin G antibodies and analysis of a major antigenic domain. Clin. Diagn. Lab. Immunol. 6:24–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilson DC, Grotenbreg GM, Liu K, Zhao Y, Frickel EM, Gubbels MJ, Ploegh HL, Yap GS. 2010. Differential regulation of effector- and central-memory responses to Toxoplasma gondii infection by IL-12 revealed by tracking of Tgd057-specific CD8+ T cells. PLoS Pathog. 6:e1000815. 10.1371/journal.ppat.1000815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vercammen M, Scorza T, Huygen K, De Braekeleer J, Diet R, Jacobs D, Saman E, Verschueren H. 2000. DNA vaccination with genes encoding Toxoplasma gondii antigens GRA1, GRA7, and ROP2 induces partially protective immunity against lethal challenge in mice. Infect. Immun. 68:38–45. 10.1128/IAI.68.1.38-45.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Feliu V, Vasseur V, Grover HS, Chu HH, Brown MJ, Wang J, Boyle JP, Robey EA, Shastri N, Blanchard N. 2013. Location of the CD8 T cell epitope within the antigenic precursor determines immunogenicity and protection against the Toxoplasma gondii parasite. PLoS Pathog. 9:e1003449. 10.1371/journal.ppat.1003449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Denkers EY, Yap G, Scharton-Kersten T, Charest H, Butcher BA, Caspar P, Heiny S, Sher A. 1997. Perforin-mediated cytolysis plays a limited role in host resistance to Toxoplasma gondii. J. Immunol. 159:1903–1908. [PubMed] [Google Scholar]
- 43.Wang X, Kang H, Kikuchi T, Suzuki Y. 2004. Gamma interferon production, but not perforin-mediated cytolytic activity, of T cells is required for prevention of toxoplasmic encephalitis in BALB/c mice genetically resistant to the disease. Infect. Immun. 72:4432–4438. 10.1128/IAI.72.8.4432-4438.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Suzuki Y, Wang X, Jortner BS, Payne L, Ni Y, Michie SA, Xu B, Kudo T, Perkins S. 2010. Removal of Toxoplasma gondii cysts from the brain by perforin-mediated activity of CD8+ T cells. Am. J. Pathol. 176:1607–1613. 10.2353/ajpath.2010.090825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang X, Michie SA, Xu B, Suzuki Y. 2007. Importance of IFN-gamma-mediated expression of endothelial VCAM-1 on recruitment of CD8+ T cells into the brain during chronic infection with Toxoplasma gondii. J. Interferon Cytokine Res. 27:329–338. 10.1089/jir.2006.0154. [DOI] [PubMed] [Google Scholar]
- 46.Suzuki Y, Sa Q, Gehman M, Ochiai E. 2011. Interferon-gamma- and perforin-mediated immune responses for resistance against Toxoplasma gondii in the brain. Expert Rev. Mol. Med. 13:e31. 10.1017/S1462399411002018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sher A, Oswald IP, Hieny S, Gazzinelli RT. 1993. Toxoplasma gondii induces a T-independent IFN-gamma response in natural killer cells that requires both adherent accessory cells and tumor necrosis factor-alpha. J. Immunol. 150:3982–3989. [PubMed] [Google Scholar]
- 48.Denkers EY, Gazzinelli RT, Martin D, Sher A. 1993. Emergence of NK1.1+ cells as effectors of IFN-gamma dependent immunity to Toxoplasma gondii in MHC class I-deficient mice. J. Exp. Med. 178:1465–1472. 10.1084/jem.178.5.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goldszmid RS, Caspar P, Rivollier A, White S, Dzutsev A, Hieny S, Kelsall B, Trinchieri G, Sher A. 2012. NK cell-derived interferon-gamma orchestrates cellular dynamics and the differentiation of monocytes into dendritic cells at the site of infection. Immunity 36:1047–1059. 10.1016/j.immuni.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Raetz M, Kibardin A, Sturge CR, Pifer R, Li H, Burstein E, Ozato K, Larin S, Yarovinsky F. 2013. Cooperation of TLR12 and TLR11 in the IRF8-dependent IL-12 response to Toxoplasma gondii profilin. J. Immunol. 191:4818–4827. 10.4049/jimmunol.1301301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ma X, Chow JM, Gri G, Carra G, Gerosa F, Wolf SF, Dzialo R, Trinchieri G. 1996. The interleukin 12 p40 gene promoter is primed by interferon gamma in monocytic cells. J. Exp. Med. 183:147–157. 10.1084/jem.183.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sturge CR, Benson A, Raetz M, Wilhelm CL, Mirpuri J, Vitetta ES, Yarovinsky F. 2013. TLR-independent neutrophil-derived IFN-gamma is important for host resistance to intracellular pathogens. Proc. Natl. Acad. Sci. U. S. A. 110:10711–10716. 10.1073/pnas.1307868110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Coombes JL, Charsar BA, Han SJ, Halkias J, Chan SW, Koshy AA, Striepen B, Robey EA. 2013. Motile invaded neutrophils in the small intestine of Toxoplasma gondii-infected mice reveal a potential mechanism for parasite spread. Proc. Natl. Acad. Sci. U. S. A. 110:E1913–E1922. 10.1073/pnas.1220272110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Abi Abdallah DS, Lin C, Ball CJ, King MR, Duhamel GE, Denkers EY. 2012. Toxoplasma gondii triggers release of human and mouse neutrophil extracellular traps. Infect. Immun. 80:768–777. 10.1128/IAI.05730-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bliss SK, Gavrilescu LC, Alcaraz A, Denkers EY. 2001. Neutrophil depletion during Toxoplasma gondii infection leads to impaired immunity and lethal systemic pathology. Infect. Immun. 69:4898–4905. 10.1128/IAI.69.8.4898-4905.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sayles PC, Johnson LL. 1996. Exacerbation of toxoplasmosis in neutrophil-depleted mice. Nat. Immun. 15:249–258. [PubMed] [Google Scholar]
- 57.Fleming TJ, Fleming ML, Malek TR. 1993. Selective expression of Ly-6G on myeloid lineage cells in mouse bone marrow. RB6–8C5 mAb to granulocyte-differentiation antigen (Gr-1) detects members of the Ly-6 family. J. Immunol. 151:2399–2408. [PubMed] [Google Scholar]
- 58.Robben PM, LaRegina M, Kuziel WA, Sibley LD. 2005. Recruitment of Gr-1+ monocytes is essential for control of acute toxoplasmosis. J. Exp. Med. 201:1761–1769. 10.1084/jem.20050054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Del Rio L, Bennouna S, Salinas J, Denkers EY. 2001. CXCR2 deficiency confers impaired neutrophil recruitment and increased susceptibility during Toxoplasma gondii infection. J. Immunol. 167:6503–6509. 10.4049/jimmunol.167.11.6503. [DOI] [PubMed] [Google Scholar]
- 60.Dunay IR, Fuchs A, Sibley LD. 2010. Inflammatory monocytes but not neutrophils are necessary to control infection with Toxoplasma gondii in mice. Infect. Immun. 78:1564–1570. 10.1128/IAI.00472-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Soehnlein O, Zernecke A, Eriksson EE, Rothfuchs AG, Pham CT, Herwald H, Bidzhekov K, Rottenberg ME, Weber C, Lindbom L. 2008. Neutrophil secretion products pave the way for inflammatory monocytes. Blood 112:1461–1471. 10.1182/blood-2008-02-139634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Soehnlein O, Lindbom L, Weber C. 2009. Mechanisms underlying neutrophil-mediated monocyte recruitment. Blood 114:4613–4623. 10.1182/blood-2009-06-221630. [DOI] [PubMed] [Google Scholar]
- 63.Benson A, Pifer R, Behrendt CL, Hooper LV, Yarovinsky F. 2009. Gut commensal bacteria direct a protective immune response against Toxoplasma gondii. Cell Host Microbe 6:187–196. 10.1016/j.chom.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tailor P, Tamura T, Morse HC, III, Ozato K. 2008. The BXH2 mutation in IRF8 differentially impairs dendritic cell subset development in the mouse. Blood 111:1942–1945. 10.1182/blood-2007-07-100750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wilson DC, Matthews S, Yap GS. 2008. IL-12 signaling drives CD8+ T cell IFN-gamma production and differentiation of KLRG1+ effector subpopulations during Toxoplasma gondii infection. J. Immunol. 180:5935–5945. 10.4049/jimmunol.180.9.5935. [DOI] [PubMed] [Google Scholar]
- 66.Koblansky AA, Jankovic D, Oh H, Hieny S, Sungnak W, Mathur R, Hayden MS, Akira S, Sher A, Ghosh S. 2013. Recognition of profilin by Toll-like receptor 12 is critical for host resistance to Toxoplasma gondii. Immunity 38:119–130. 10.1016/j.immuni.2012.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pepper M, Dzierszinski F, Wilson E, Tait E, Fang Q, Yarovinsky F, Laufer TM, Roos D, Hunter CA. 2008. Plasmacytoid dendritic cells are activated by Toxoplasma gondii to present antigen and produce cytokines. J. Immunol. 180:6229–6236. 10.4049/jimmunol.180.9.6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chang HR, Grau GE, Pechere JC. 1990. Role of TNF and IL-1 in infections with Toxoplasma gondii. Immunology 69:33–37. [PMC free article] [PubMed] [Google Scholar]
- 69.Hunter CA, Chizzonite R, Remington JS. 1995. IL-1 beta is required for IL-12 to induce production of IFN-gamma by NK cells. A role for IL-1 beta in the T cell-independent mechanism of resistance against intracellular pathogens. J. Immunol. 155:4347–4354. [PubMed] [Google Scholar]
- 70.Saperstein S, Chen L, Oakes D, Pryhuber G, Finkelstein J. 2009. IL-1beta augments TNF-alpha-mediated inflammatory responses from lung epithelial cells. J. Interferon Cytokine Res. 29:273–284. 10.1089/jir.2008.0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Guma M, Ronacher L, Liu-Bryan R, Takai S, Karin M, Corr M. 2009. Caspase 1-independent activation of interleukin-1beta in neutrophil-predominant inflammation. Arthritis Rheum. 60:3642–3650. 10.1002/art.24959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tabeta K, Hoebe K, Janssen EM, Du X, Georgel P, Crozat K, Mudd S, Mann N, Sovath S, Goode J, Shamel L, Herskovits AA, Portnoy DA, Cooke M, Tarantino LM, Wiltshire T, Steinberg BE, Grinstein S, Beutler B. 2006. The Unc93b1 mutation 3d disrupts exogenous antigen presentation and signaling via Toll-like receptors 3, 7 and 9. Nat. Immunol. 7:156–164. 10.1038/ni1297. [DOI] [PubMed] [Google Scholar]
- 73.Brinkmann MM, Spooner E, Hoebe K, Beutler B, Ploegh HL, Kim YM. 2007. The interaction between the ER membrane protein UNC93B and TLR3, 7, and 9 is crucial for TLR signaling. J. Cell Biol. 177:265–275. 10.1083/jcb.200612056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Melo MB, Kasperkovitz P, Cerny A, Konen-Waisman S, Kurt-Jones EA, Lien E, Beutler B, Howard JC, Golenbock DT, Gazzinelli RT. 2010. UNC93B1 mediates host resistance to infection with Toxoplasma gondii. PLoS Pathog. 6:e1001071. 10.1371/journal.ppat.1001071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pifer R, Benson A, Sturge CR, Yarovinsky F. 2011. UNC93B1 is essential for TLR11 activation and IL-12-dependent host resistance to Toxoplasma gondii. J. Biol. Chem. 286:3307–3314. 10.1074/jbc.M110.171025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Andrade WA, Souza Mdo C, Ramos-Martinez E, Nagpal K, Dutra MS, Melo MB, Bartholomeu DC, Ghosh S, Golenbock DT, Gazzinelli RT. 2013. Combined action of nucleic acid-sensing Toll-like receptors and TLR11/TLR12 heterodimers imparts resistance to Toxoplasma gondii in mice. Cell Host Microbe 13:42–53. 10.1016/j.chom.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Roach JC, Glusman G, Rowen L, Kaur A, Purcell MK, Smith KD, Hood LE, Aderem A. 2005. The evolution of vertebrate Toll-like receptors. Proc. Natl. Acad. Sci. U. S. A. 102:9577–9582. 10.1073/pnas.0502272102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ewald SE, Chavarria-Smith J, Boothroyd JC. 2014. NLRP1 is an inflammasome sensor for Toxoplasma gondii. Infect. Immun. 82:460–468. 10.1128/IAI.01170-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gorfu G, Cirelli KM, Melo MB, Mayer-Barber K, Crown D, Koller BH, Masters S, Sher A, Leppla SH, Moayeri M, Saeij JP, Grigg ME. 2014. Dual role for inflammasome sensors NLRP1 and NLRP3 in murine resistance to Toxoplasma gondii. mBio. 5:e01117–13. 10.1128/mBio.01117-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Raetz M, Hwang SH, Wilhelm CL, Kirkland D, Benson A, Sturge CR, Mirpuri J, Vaishnava S, Hou B, Defranco AL, Gilpin CJ, Hooper LV, Yarovinsky F. 2013. Parasite-induced TH1 cells and intestinal dysbiosis cooperate in IFN-gamma-dependent elimination of Paneth cells. Nat. Immunol. 14:136–142. [DOI] [PMC free article] [PubMed] [Google Scholar]