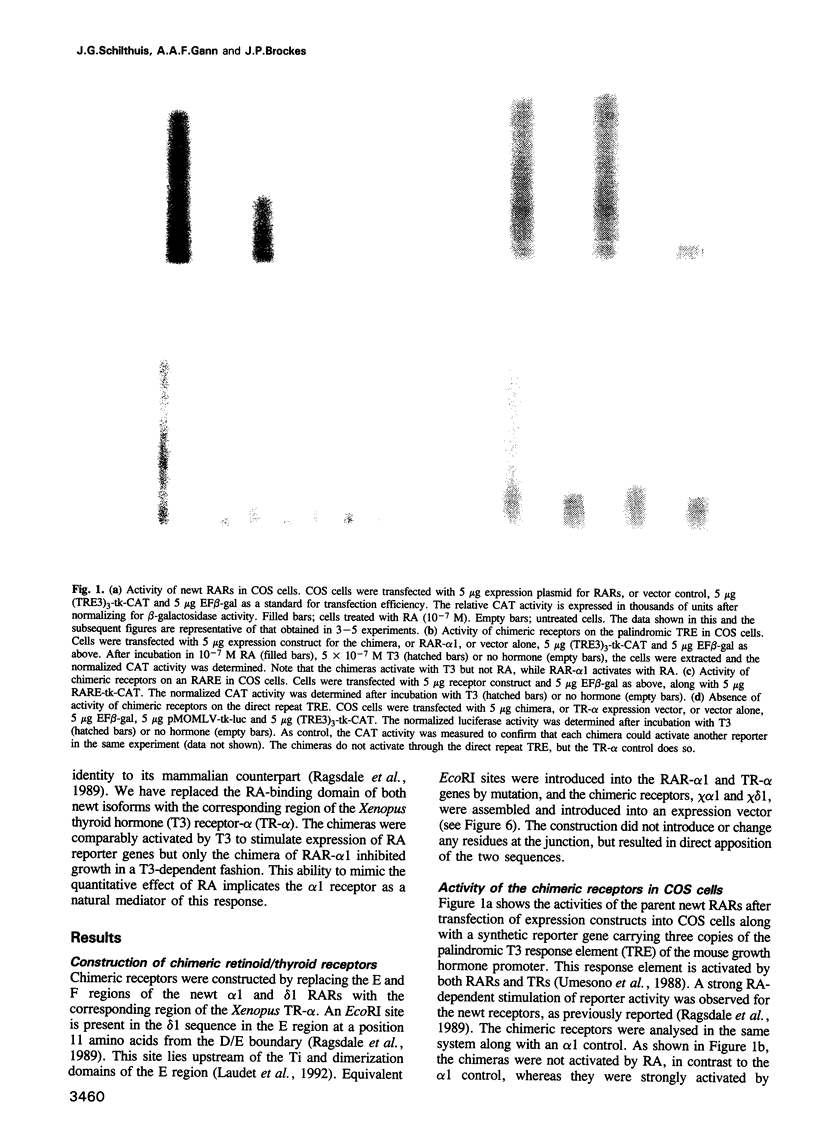
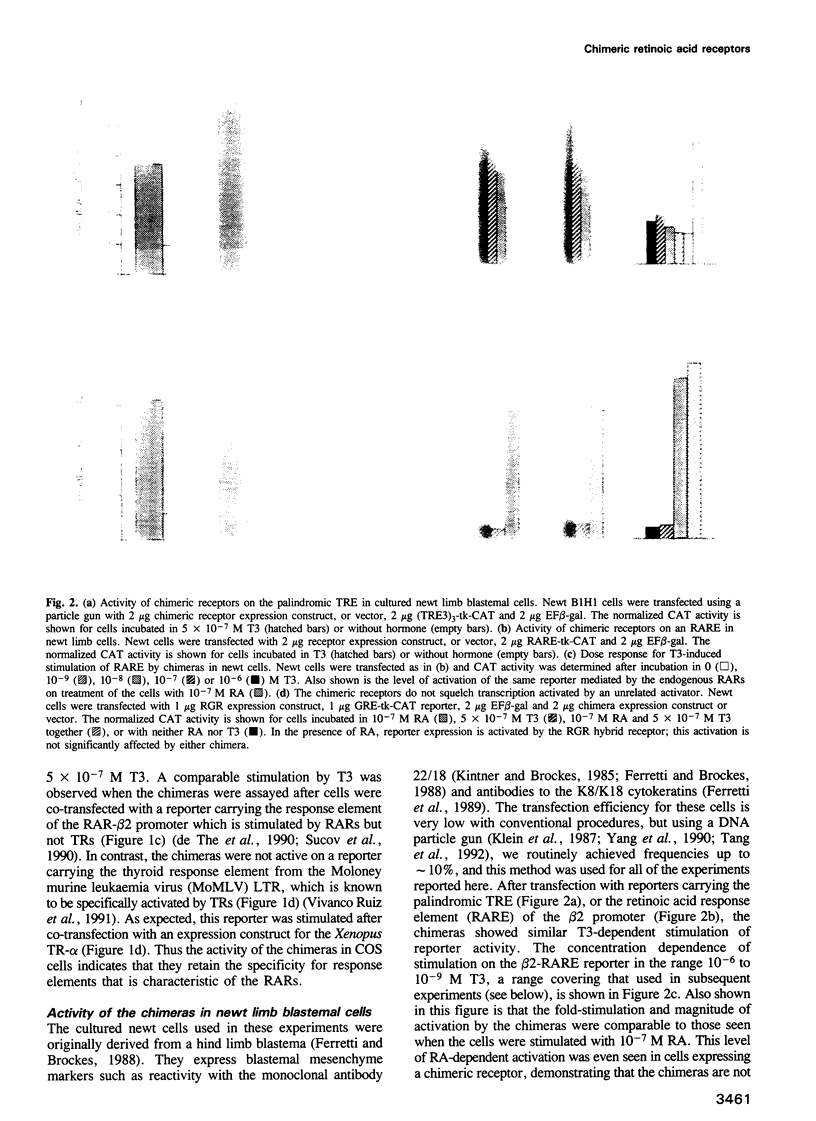
Abstract
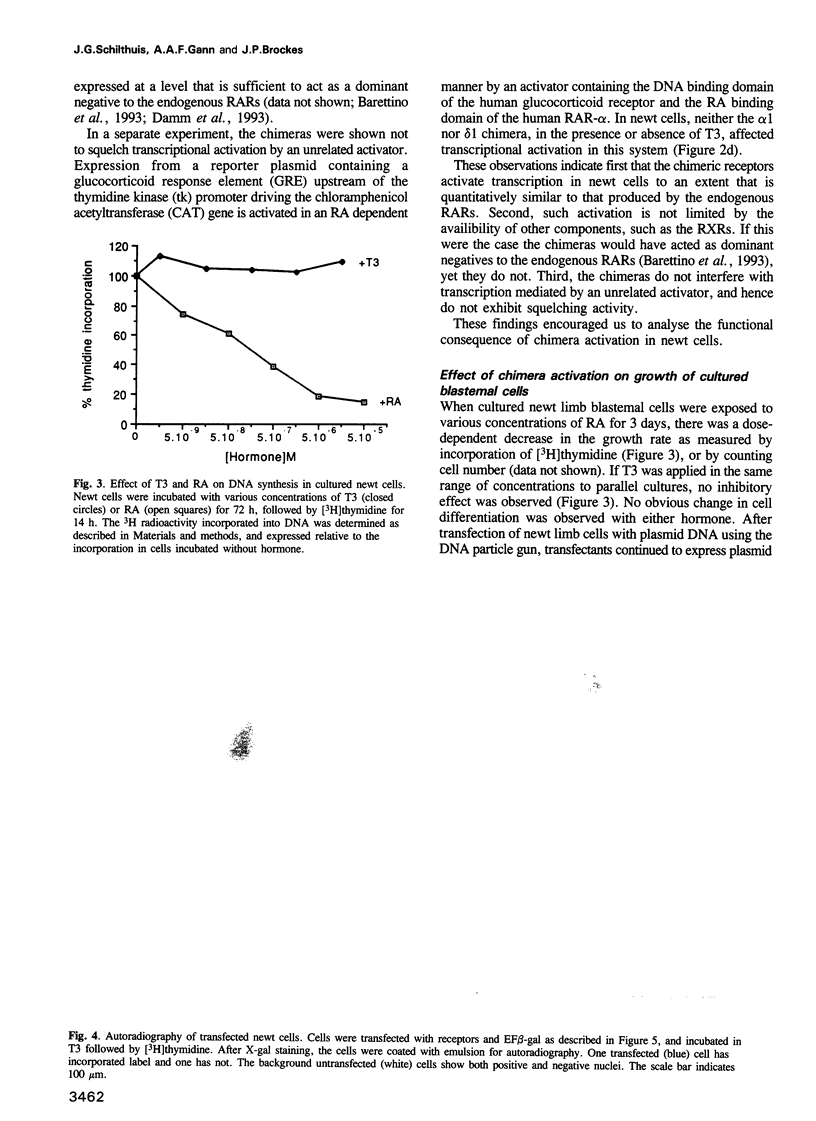
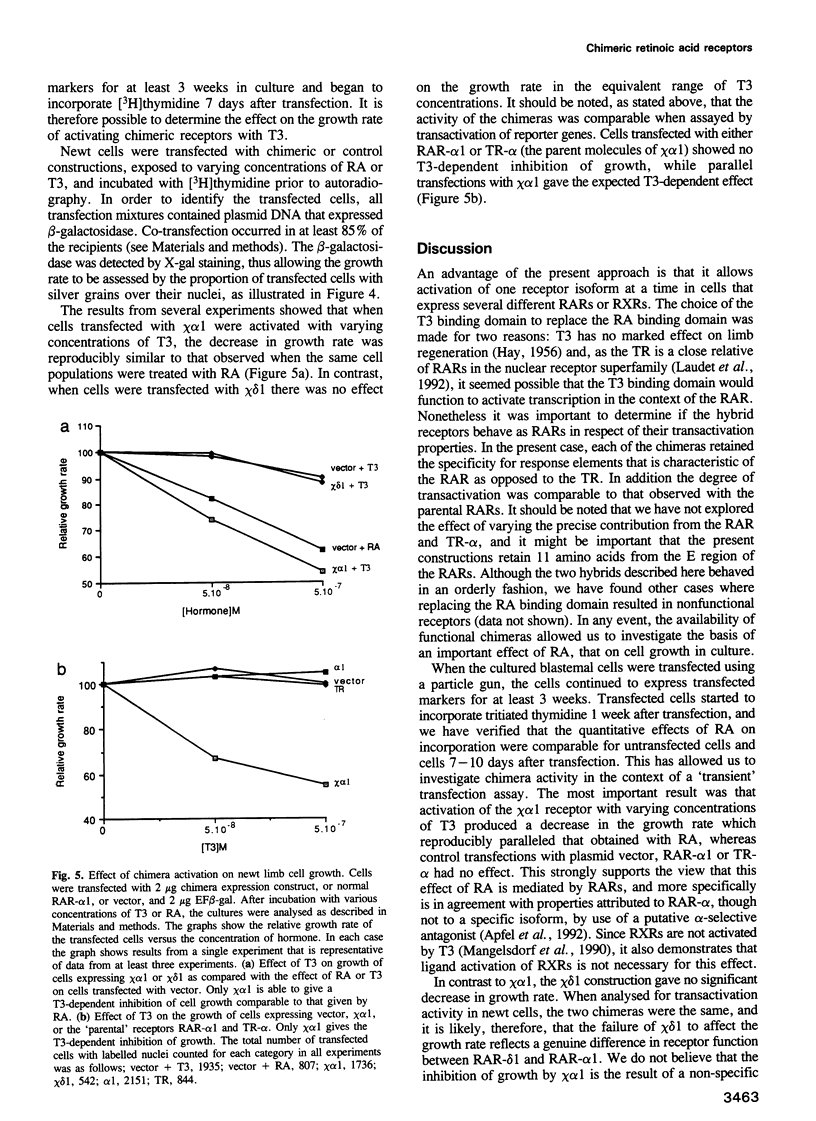
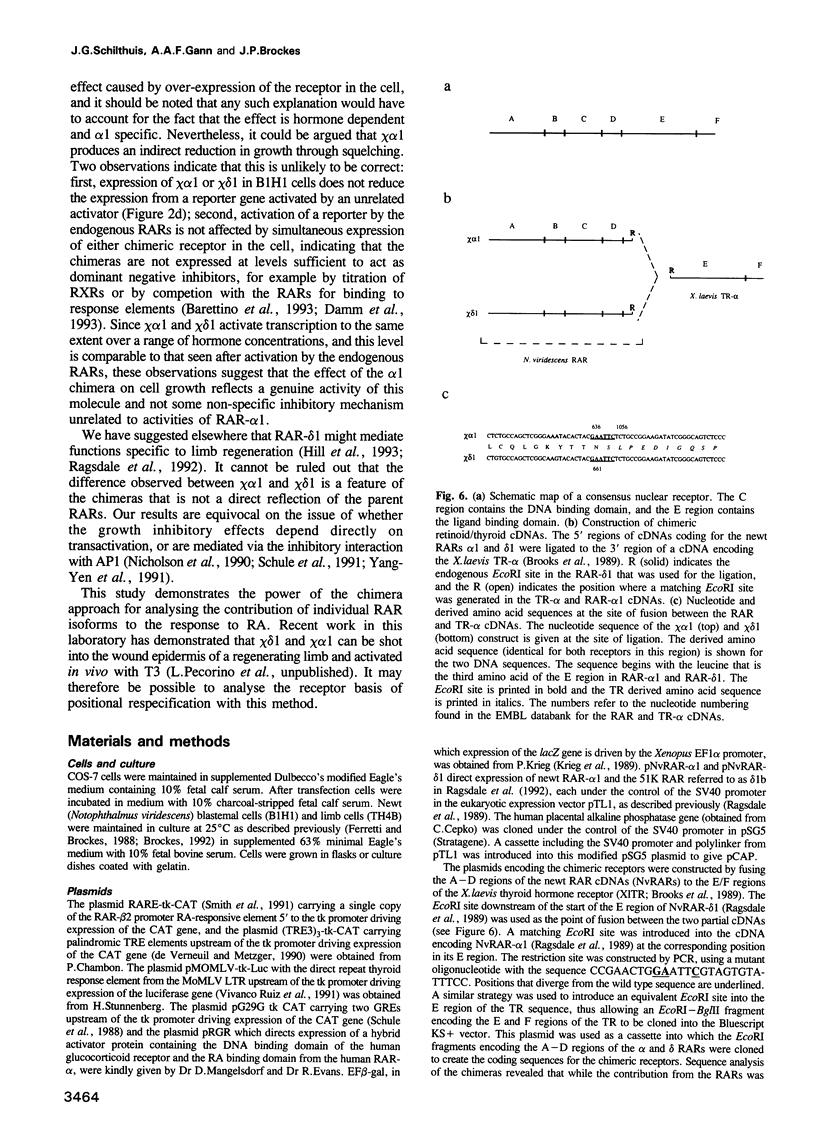
Retinoic acid (RA) affects the growth and differentiation of cells in culture, usually to decrease the growth rate. In amphibian limb regeneration RA has the remarkable ability to affect pattern formation by changing positional identity, but its initial action on the limb is to inhibit division of the blastemal progenitor cells. Newt limb blastemal cells also show this inhibition in culture. In order to investigate the role of different RA receptors (RARs) in the RA response, the hormone binding domain of the newt RARs alpha 1 and delta 1 was replaced with the corresponding region from the Xenopus thyroid hormone receptor-alpha (TR-alpha). In COS cells transfected with each of the chimeras, transcription was activated after exposure to thyroid hormone (T3). Their profile of activity on three different response elements was indicative of RAR specificity and not TR specificity. After transfection of cultured newt blastemal cells with a DNA particle gun, the chimeras were equally active in stimulating T3-dependent transcription of two different synthetic reporter genes. Blastemal cells were transfected with chimeras or control plasmids along with a marker plasmid expressing beta-galactosidase, exposed to RA or T3 and labelled with [3H]thymidine followed by autoradiography. The alpha 1 chimera gave T3-dependent inhibition of growth, comparable to the effect exerted by RA itself, whereas the delta 1 chimera and control plasmids were inactive. The results imply that RAR-alpha 1 mediates the effects of RA on blastemal cell growth.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apfel C., Bauer F., Crettaz M., Forni L., Kamber M., Kaufmann F., LeMotte P., Pirson W., Klaus M. A retinoic acid receptor alpha antagonist selectively counteracts retinoic acid effects. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):7129–7133. doi: 10.1073/pnas.89.15.7129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barettino D., Bugge T. H., Bartunek P., Vivanco Ruiz M. D., Sonntag-Buck V., Beug H., Zenke M., Stunnenberg H. G. Unliganded T3R, but not its oncogenic variant, v-erbA, suppresses RAR-dependent transactivation by titrating out RXR. EMBO J. 1993 Apr;12(4):1343–1354. doi: 10.1002/j.1460-2075.1993.tb05779.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benbrook D., Lernhardt E., Pfahl M. A new retinoic acid receptor identified from a hepatocellular carcinoma. Nature. 1988 Jun 16;333(6174):669–672. doi: 10.1038/333669a0. [DOI] [PubMed] [Google Scholar]
- Brand N., Petkovich M., Krust A., Chambon P., de Thé H., Marchio A., Tiollais P., Dejean A. Identification of a second human retinoic acid receptor. Nature. 1988 Apr 28;332(6167):850–853. doi: 10.1038/332850a0. [DOI] [PubMed] [Google Scholar]
- Breitman T. R., Selonick S. E., Collins S. J. Induction of differentiation of the human promyelocytic leukemia cell line (HL-60) by retinoic acid. Proc Natl Acad Sci U S A. 1980 May;77(5):2936–2940. doi: 10.1073/pnas.77.5.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockes J. P. Introduction of a retinoid reporter gene into the urodele limb blastema. Proc Natl Acad Sci U S A. 1992 Dec 1;89(23):11386–11390. doi: 10.1073/pnas.89.23.11386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockes J. P. Retinoids, homeobox genes, and limb morphogenesis. Neuron. 1989 Apr;2(4):1285–1294. doi: 10.1016/0896-6273(89)90066-4. [DOI] [PubMed] [Google Scholar]
- Brooks A. R., Sweeney G., Old R. W. Structure and functional expression of a cloned Xenopus thyroid hormone receptor. Nucleic Acids Res. 1989 Nov 25;17(22):9395–9405. doi: 10.1093/nar/17.22.9395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant S. V., Gardiner D. M. Retinoic acid, local cell-cell interactions, and pattern formation in vertebrate limbs. Dev Biol. 1992 Jul;152(1):1–25. doi: 10.1016/0012-1606(92)90152-7. [DOI] [PubMed] [Google Scholar]
- Damm K., Heyman R. A., Umesono K., Evans R. M. Functional inhibition of retinoic acid response by dominant negative retinoic acid receptor mutants. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):2989–2993. doi: 10.1073/pnas.90.7.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darrow A. L., Rickles R. J., Strickland S. Maintenance and use of F9 teratocarcinoma cells. Methods Enzymol. 1990;190:110–117. doi: 10.1016/0076-6879(90)90015-s. [DOI] [PubMed] [Google Scholar]
- Dollé P., Ruberte E., Kastner P., Petkovich M., Stoner C. M., Gudas L. J., Chambon P. Differential expression of genes encoding alpha, beta and gamma retinoic acid receptors and CRABP in the developing limbs of the mouse. Nature. 1989 Dec 7;342(6250):702–705. doi: 10.1038/342702a0. [DOI] [PubMed] [Google Scholar]
- Dollé P., Ruberte E., Leroy P., Morriss-Kay G., Chambon P. Retinoic acid receptors and cellular retinoid binding proteins. I. A systematic study of their differential pattern of transcription during mouse organogenesis. Development. 1990 Dec;110(4):1133–1151. doi: 10.1242/dev.110.4.1133. [DOI] [PubMed] [Google Scholar]
- Ferretti P., Brockes J. P. Culture of newt cells from different tissues and their expression of a regeneration-associated antigen. J Exp Zool. 1988 Jul;247(1):77–91. doi: 10.1002/jez.1402470111. [DOI] [PubMed] [Google Scholar]
- Ferretti P., Fekete D. M., Patterson M., Lane E. B. Transient expression of simple epithelial keratins by mesenchymal cells of regenerating newt limb. Dev Biol. 1989 Jun;133(2):415–424. doi: 10.1016/0012-1606(89)90045-6. [DOI] [PubMed] [Google Scholar]
- Giguere V., Ong E. S., Segui P., Evans R. M. Identification of a receptor for the morphogen retinoic acid. Nature. 1987 Dec 17;330(6149):624–629. doi: 10.1038/330624a0. [DOI] [PubMed] [Google Scholar]
- HAY E. D. Effects of thyroxine on limb regeneration in the newt, Triturus viridescens. Bull Johns Hopkins Hosp. 1956 Nov;99(5):262–286. [PubMed] [Google Scholar]
- Hill D. S., Ragsdale C. W., Jr, Brockes J. P. Isoform-specific immunological detection of newt retinoic acid receptor delta 1 in normal and regenerating limbs. Development. 1993 Mar;117(3):937–945. doi: 10.1242/dev.117.3.937. [DOI] [PubMed] [Google Scholar]
- Huang M. E., Ye Y. C., Chen S. R., Chai J. R., Lu J. X., Zhoa L., Gu L. J., Wang Z. Y. Use of all-trans retinoic acid in the treatment of acute promyelocytic leukemia. Blood. 1988 Aug;72(2):567–572. [PubMed] [Google Scholar]
- Ide H., Aono H. Retinoic acid promotes proliferation and chondrogenesis in the distal mesodermal cells of chick limb bud. Dev Biol. 1988 Dec;130(2):767–773. doi: 10.1016/0012-1606(88)90365-x. [DOI] [PubMed] [Google Scholar]
- Kastner P., Krust A., Mendelsohn C., Garnier J. M., Zelent A., Leroy P., Staub A., Chambon P. Murine isoforms of retinoic acid receptor gamma with specific patterns of expression. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2700–2704. doi: 10.1073/pnas.87.7.2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K. H., Stellmach V., Javors J., Fuchs E. Regulation of human mesothelial cell differentiation: opposing roles of retinoids and epidermal growth factor in the expression of intermediate filament proteins. J Cell Biol. 1987 Dec;105(6 Pt 2):3039–3051. doi: 10.1083/jcb.105.6.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kintner C. R., Brockes J. P. Monoclonal antibodies to the cells of a regenerating limb. J Embryol Exp Morphol. 1985 Oct;89:37–55. [PubMed] [Google Scholar]
- Krieg P. A., Varnum S. M., Wormington W. M., Melton D. A. The mRNA encoding elongation factor 1-alpha (EF-1 alpha) is a major transcript at the midblastula transition in Xenopus. Dev Biol. 1989 May;133(1):93–100. doi: 10.1016/0012-1606(89)90300-x. [DOI] [PubMed] [Google Scholar]
- Laudet V., Hänni C., Coll J., Catzeflis F., Stéhelin D. Evolution of the nuclear receptor gene superfamily. EMBO J. 1992 Mar;11(3):1003–1013. doi: 10.1002/j.1460-2075.1992.tb05139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroy P., Krust A., Zelent A., Mendelsohn C., Garnier J. M., Kastner P., Dierich A., Chambon P. Multiple isoforms of the mouse retinoic acid receptor alpha are generated by alternative splicing and differential induction by retinoic acid. EMBO J. 1991 Jan;10(1):59–69. doi: 10.1002/j.1460-2075.1991.tb07921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maden M. The effect of vitamin A on the regenerating axolotl limb. J Embryol Exp Morphol. 1983 Oct;77:273–295. [PubMed] [Google Scholar]
- Maden M. Vitamin A and pattern formation in the regenerating limb. Nature. 1982 Feb 25;295(5851):672–675. doi: 10.1038/295672a0. [DOI] [PubMed] [Google Scholar]
- Mangelsdorf D. J., Ong E. S., Dyck J. A., Evans R. M. Nuclear receptor that identifies a novel retinoic acid response pathway. Nature. 1990 May 17;345(6272):224–229. doi: 10.1038/345224a0. [DOI] [PubMed] [Google Scholar]
- Nagpal S., Saunders M., Kastner P., Durand B., Nakshatri H., Chambon P. Promoter context- and response element-dependent specificity of the transcriptional activation and modulating functions of retinoic acid receptors. Cell. 1992 Sep 18;70(6):1007–1019. doi: 10.1016/0092-8674(92)90250-g. [DOI] [PubMed] [Google Scholar]
- Nicholson R. C., Mader S., Nagpal S., Leid M., Rochette-Egly C., Chambon P. Negative regulation of the rat stromelysin gene promoter by retinoic acid is mediated by an AP1 binding site. EMBO J. 1990 Dec;9(13):4443–4454. doi: 10.1002/j.1460-2075.1990.tb07895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen D. F., Langille R. M., Dress V., Solursh M. Selective stimulation of in vitro limb-bud chondrogenesis by retinoic acid. Differentiation. 1988 Dec;39(2):123–130. doi: 10.1111/j.1432-0436.1988.tb00087.x. [DOI] [PubMed] [Google Scholar]
- Petkovich M., Brand N. J., Krust A., Chambon P. A human retinoic acid receptor which belongs to the family of nuclear receptors. Nature. 1987 Dec 3;330(6147):444–450. doi: 10.1038/330444a0. [DOI] [PubMed] [Google Scholar]
- Ragsdale C. W., Jr, Gates P. B., Hill D. S., Brockes J. P. Delta retinoic acid receptor isoform delta 1 is distinguished by its exceptional N-terminal sequence and abundance in the limb regeneration blastema. Mech Dev. 1993 Jan;40(1-2):99–112. doi: 10.1016/0925-4773(93)90091-b. [DOI] [PubMed] [Google Scholar]
- Ragsdale C. W., Jr, Petkovich M., Gates P. B., Chambon P., Brockes J. P. Identification of a novel retinoic acid receptor in regenerative tissues of the newt. Nature. 1989 Oct 19;341(6243):654–657. doi: 10.1038/341654a0. [DOI] [PubMed] [Google Scholar]
- Robertson K. A., Emami B., Mueller L., Collins S. J. Multiple members of the retinoic acid receptor family are capable of mediating the granulocytic differentiation of HL-60 cells. Mol Cell Biol. 1992 Sep;12(9):3743–3749. doi: 10.1128/mcb.12.9.3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruberte E., Dolle P., Chambon P., Morriss-Kay G. Retinoic acid receptors and cellular retinoid binding proteins. II. Their differential pattern of transcription during early morphogenesis in mouse embryos. Development. 1991 Jan;111(1):45–60. doi: 10.1242/dev.111.1.45. [DOI] [PubMed] [Google Scholar]
- Ruberte E., Dolle P., Krust A., Zelent A., Morriss-Kay G., Chambon P. Specific spatial and temporal distribution of retinoic acid receptor gamma transcripts during mouse embryogenesis. Development. 1990 Feb;108(2):213–222. doi: 10.1242/dev.108.2.213. [DOI] [PubMed] [Google Scholar]
- Schüle R., Muller M., Kaltschmidt C., Renkawitz R. Many transcription factors interact synergistically with steroid receptors. Science. 1988 Dec 9;242(4884):1418–1420. doi: 10.1126/science.3201230. [DOI] [PubMed] [Google Scholar]
- Schüle R., Rangarajan P., Yang N., Kliewer S., Ransone L. J., Bolado J., Verma I. M., Evans R. M. Retinoic acid is a negative regulator of AP-1-responsive genes. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6092–6096. doi: 10.1073/pnas.88.14.6092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seed B., Sheen J. Y. A simple phase-extraction assay for chloramphenicol acyltransferase activity. Gene. 1988 Jul 30;67(2):271–277. doi: 10.1016/0378-1119(88)90403-9. [DOI] [PubMed] [Google Scholar]
- Smith W. C., Nakshatri H., Leroy P., Rees J., Chambon P. A retinoic acid response element is present in the mouse cellular retinol binding protein I (mCRBPI) promoter. EMBO J. 1991 Aug;10(8):2223–2230. doi: 10.1002/j.1460-2075.1991.tb07758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocum D. L., Crawford K. Use of retinoids to analyze the cellular basis of positional memory in regenerating amphibian limbs. Biochem Cell Biol. 1987 Aug;65(8):750–761. doi: 10.1139/o87-098. [DOI] [PubMed] [Google Scholar]
- Stocum D. L. Limb regeneration: a call to arms (and legs). Cell. 1991 Oct 4;67(1):5–8. doi: 10.1016/0092-8674(91)90565-g. [DOI] [PubMed] [Google Scholar]
- Strickland S., Mahdavi V. The induction of differentiation in teratocarcinoma stem cells by retinoic acid. Cell. 1978 Oct;15(2):393–403. doi: 10.1016/0092-8674(78)90008-9. [DOI] [PubMed] [Google Scholar]
- Sucov H. M., Murakami K. K., Evans R. M. Characterization of an autoregulated response element in the mouse retinoic acid receptor type beta gene. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5392–5396. doi: 10.1073/pnas.87.14.5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabin C. J. Retinoids, homeoboxes, and growth factors: toward molecular models for limb development. Cell. 1991 Jul 26;66(2):199–217. doi: 10.1016/0092-8674(91)90612-3. [DOI] [PubMed] [Google Scholar]
- Tang D. C., DeVit M., Johnston S. A. Genetic immunization is a simple method for eliciting an immune response. Nature. 1992 Mar 12;356(6365):152–154. doi: 10.1038/356152a0. [DOI] [PubMed] [Google Scholar]
- Umesono K., Giguere V., Glass C. K., Rosenfeld M. G., Evans R. M. Retinoic acid and thyroid hormone induce gene expression through a common responsive element. Nature. 1988 Nov 17;336(6196):262–265. doi: 10.1038/336262a0. [DOI] [PubMed] [Google Scholar]
- Vivanco Ruiz M. M., Bugge T. H., Hirschmann P., Stunnenberg H. G. Functional characterization of a natural retinoic acid responsive element. EMBO J. 1991 Dec;10(12):3829–3838. doi: 10.1002/j.1460-2075.1991.tb04952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang-Yen H. F., Zhang X. K., Graupner G., Tzukerman M., Sakamoto B., Karin M., Pfahl M. Antagonism between retinoic acid receptors and AP-1: implications for tumor promotion and inflammation. New Biol. 1991 Dec;3(12):1206–1219. [PubMed] [Google Scholar]
- Yang N. S., Burkholder J., Roberts B., Martinell B., McCabe D. In vivo and in vitro gene transfer to mammalian somatic cells by particle bombardment. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9568–9572. doi: 10.1073/pnas.87.24.9568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelent A., Krust A., Petkovich M., Kastner P., Chambon P. Cloning of murine alpha and beta retinoic acid receptors and a novel receptor gamma predominantly expressed in skin. Nature. 1989 Jun 29;339(6227):714–717. doi: 10.1038/339714a0. [DOI] [PubMed] [Google Scholar]
- Zelent A., Mendelsohn C., Kastner P., Krust A., Garnier J. M., Ruffenach F., Leroy P., Chambon P. Differentially expressed isoforms of the mouse retinoic acid receptor beta generated by usage of two promoters and alternative splicing. EMBO J. 1991 Jan;10(1):71–81. doi: 10.1002/j.1460-2075.1991.tb07922.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Thé H., Vivanco-Ruiz M. M., Tiollais P., Stunnenberg H., Dejean A. Identification of a retinoic acid responsive element in the retinoic acid receptor beta gene. Nature. 1990 Jan 11;343(6254):177–180. doi: 10.1038/343177a0. [DOI] [PubMed] [Google Scholar]
- de Verneuil H., Metzger D. The lack of transcriptional activation of the v-erbA oncogene is in part due to a mutation present in the DNA binding domain of the protein. Nucleic Acids Res. 1990 Aug 11;18(15):4489–4497. doi: 10.1093/nar/18.15.4489. [DOI] [PMC free article] [PubMed] [Google Scholar]