Abstract
Moraxella catarrhalis is a common respiratory tract pathogen that causes otitis media in children and infections in adults with chronic obstructive pulmonary disease. Since the introduction of the pneumococcal conjugate vaccines with/without protein D of nontypeable Haemophilus influenzae, M. catarrhalis has become a high-priority pathogen in otitis media. For the development of antibacterial vaccines and therapies, substrate binding proteins of ATP-binding cassette transporters are important targets. In this study, we identified and characterized a substrate binding protein, SBP2, of M. catarrhalis. Among 30 clinical isolates tested, the sbp2 gene sequence was highly conserved. In 2 different analyses (whole-cell enzyme-linked immunosorbent assay and flow cytometry), polyclonal antibodies raised to recombinant SBP2 demonstrated that SBP2 expresses epitopes on the bacterial surface of the wild type but not the sbp2 mutant. Mice immunized with recombinant SBP2 showed significantly enhanced clearance of M. catarrhalis from the lung compared to that in the control group at both 25-μg and 50-μg doses (P < 0.001). We conclude that SBP2 is a novel, attractive candidate as a vaccine antigen against M. catarrhalis.
INTRODUCTION
Moraxella catarrhalis is a human-specific Gram-negative diplococcus frequently found as a commensal of the upper respiratory tract (1, 2). Although this bacterium has been overlooked, it is now recognized as a respiratory tract pathogen of serious public health concern, particularly as a cause of otitis media in children and infections in adults with chronic obstructive pulmonary disease (COPD) (3, 4).
Otitis media mainly consists of 2 clinical forms, including acute otitis media (AOM) and otitis media with effusion. AOM is one of the most common forms of bacterial infection in children, with an incidence of 0.5 to 1.2 episodes per person-year during the first 2 years of life (5). AOM imposes a large burden on both children and their parents, with 13 million episodes annually and an annual cost of more than $3 billion in U.S. children aged 0 to 4 years (6). Approximately 10 to 30% of children develop recurrent AOM and are diagnosed as otitis prone (3 separate episodes in 6 months or 4 episodes in 1 year) (7–9). Otitis media with effusion is diagnosed by the presence of nonpurulent middle ear fluid without symptoms of acute infection. Approximately 2.2 million episodes are diagnosed as otitis media with effusion annually in the United States, and its annual cost is estimated at $4 billion (10). PCR detection methods for bacterial DNA in middle ear effusion reveal that otopathogens may play a role in otitis media with effusion (11, 12).
The most important complication of otitis media is hearing impairment that causes delayed speech and language development (13). M. catarrhalis is the third leading cause of AOM (10 to 20%) following Streptococcus pneumoniae and nontypeable Haemophilus influenzae (4, 14, 15) and the second leading otopathogen detected from otitis media with effusion (9 to 24%) (12, 16, 17). Since the introduction of the pneumococcal conjugate vaccines, several studies have shown a shift of otopathogens in AOM. The proportion of M. catarrhalis detected has increased to up to 30%, resulting in the bacterium being the second most common cause of AOM (12, 17) and otitis media with effusion (12, 16, 17).
COPD is a chronic debilitating disease in adults and is a major global concern (18, 19). In the United States, COPD caused 133,575 deaths (63.1 per 100,000) and affected 6.5% of adults (approximately 13.7 million) in 2010 (20). M. catarrhalis is the second most common bacterial cause of exacerbations of COPD after nontypeable H. influenzae, accounting for 2 to 4 million episodes annually in the United States (21–23).
Recently, a pneumococcal-nontypeable H. influenzae protein D conjugate vaccine has been licensed in many countries in addition to the original version of pneumococcal conjugate vaccines, to prevent 2 important causes of otitis media. On the other hand, vaccine development for M. catarrhalis is lagging behind. We previously identified surface-exposed proteins, including a substrate binding protein of an ATP-binding cassette (ABC) transporter, as attractive candidates for vaccine antigens through a genome-mining approach (24, 25). Based on their functions in bacterial virulence and antigenicity, substrate binding proteins of ABC transporters have been recognized as potential targets for the development of antibacterial vaccines (26).
In this study, we identified a gene cluster in the M. catarrhalis genome with characteristics of a putative ABC transporter. The gene cluster contains genes that encode 3 substrate binding proteins, named SBP1, -2, and -3. We report here that SBP2 has great potential as a vaccine antigen against M. catarrhalis, supported by several lines of evidence.
MATERIALS AND METHODS
Bacterial strains and growth.
M. catarrhalis strain O35E was provided by Eric Hansen. Clinical strains 135, 555, 3584, 4223, 5191, 5193, 5488, 6349, 6955, and 9483 were middle ear fluid isolates obtained via tympanocentesis, and strains 556, 2017, 2528, 2886, 2952, 3583, 3615, 5192, 6350, and 6954 were nasopharyngeal isolates from Buffalo, NY, provided by Diane Dryja and Howard Faden. These clinical isolates include 5 pairs of a middle ear fluid isolate and a nasopharyngeal isolate detected from patients with AOM. M. catarrhalis strains 10P66B1, 14P30B1, 19P54B1, 39P33B1, and 47P31B1 were isolated from the sputum of adults with COPD experiencing an exacerbation as part of a prospective study in Buffalo, NY (22). M. catarrhalis strains M2, M3, M4, M5, and M6, provided by Daniel Musher, were isolated from the sputum of adults with COPD experiencing an exacerbation. M. catarrhalis strains were grown on brain heart infusion (BHI) plates at 35°C with 5% CO2 or in BHI broth with shaking at 37°C. Chemically competent Escherichia coli strains TOP10 and BL21(DE3) were obtained from Invitrogen (Carlsbad, CA) and were grown at 37°C on Luria-Bertani plates, in Luria-Bertani broth, or in Terrific broth.
Nucleotide sequence analysis.
The sequences of sbp2 genes amplified from strain O35E and 30 clinical isolates of M. catarrhalis were determined at the Roswell Park Cancer Institute DNA sequencing facility with sequencing primers SBP3kanmutup5′ and P14-S2-R, listed in Table S1 in the supplemental material. These sequences were aligned and analyzed with BLAST (http://blast.ncbi.nlm.nih.gov/Blast.cgi).
Expression and purification of recombinant SBP1, SBP2, and SBP3 proteins.
The sbp1, sbp2, and sbp3 genes were individually cloned into the plasmid pCATCH, which allows for expression of recombinant lipoproteins in E. coli (25, 27, 28). Oligonucleotide primers corresponding to the 5′ end starting after the predicted cysteine codon and the 3′ end of the genes were designed with NcoI and BamHI restriction sites (see Table S1 in the supplemental material). The genes were amplified by PCR from genomic DNA of M. catarrhalis strain O35E. The resultant PCR products were ligated into pCATCH and transformed into E. coli TOP10 cells. Colonies were picked and grown in broth, and plasmids were purified. PCR and sequencing confirmed the insertion of each gene into plasmids pSBP1, pSBP2, and pSBP3.
The same method was used for expression and purification of all 3 proteins. The plasmids were transformed into E. coli BL21(DE3) for expression, and cultures were grown in 50 ml of Terrific broth containing 75 μg/ml kanamycin with shaking at 37°C to an optical density at 600 nm (OD600) of 0.6 to 1.0. IPTG (isopropyl-β-d-thiogalactopyranoside) was added to a concentration of 1 mM, and cells were incubated with shaking at 37°C for 20 min. Rifampin was added to a concentration of 0.5 mg/ml, and cells were incubated with shaking at 37°C for 3.5 to 4 h. Bacteria were harvested by centrifugation at 4,000 × g for 15 min at 4°C. The pellet was frozen at −20°C.
The pellet was suspended in 25 ml of phosphate-buffered saline (PBS) plus 1% Triton X-100 and 1× Protein Arrest (G-Biosciences, St. Louis, MO), and the suspension was sonicated (Branson Sonifier 450 at setting 4) 3 times for 30 s on ice. The suspension was mixed by nutation for 20 min at room temperature and then centrifuged at 15,000 × g for 20 min at 4°C, and the supernatant (bacterial lysate) was saved.
An aliquot of 2 ml of BD Talon resin (BD Biosciences, Palo Alto, CA) was centrifuged at 750 × g for 5 min at 4°C, suspended in PBS plus 1% Triton X-100 and 1× Protein Arrest, and centrifuged again. The resin was suspended in 25 ml of bacterial lysate and mixed by nutation for 30 min at room temperature. The suspension was centrifuged at 750 × g for 5 min at 4°C. The resin containing bound protein was washed in PBS plus 1% Triton X-100 and 1× Protein Arrest and was then suspended in 0.01 M Tris (pH 7.4), 0.15 M NaCl, 1% NDSB-201 [3-(1-pyrodino)-1 propane sulfonate] (G-Biosciences). To elute recombinant protein, the washed resin was suspended with 1 column volume of the same buffer containing 0.3 M imidazole and mixed by nutation for 10 min at room temperature. The resin was removed by centrifugation, and the supernatant containing purified recombinant protein was collected. The elution was repeated, and the eluates were pooled. The eluates were diafiltrated using a Millipore 10,000 filter with a 5-fold volume of PBS so that the purified proteins were in PBS for subsequent experiments. Protein concentration was measured by the bicinchoninic acid assay (Pierce, Rockford, IL). The purity of the proteins was assessed by SDS-PAGE (sodium dodecyl sulfate-polyacrylamide gel electrophoresis) and Coomassie blue staining.
Development of antiserum to recombinant SBP1, SBP2, and SBP3 proteins.
Purified recombinant proteins were sent to Covance (Denver, PA) for antibody production in New Zealand White rabbits using a 59-day protocol. Briefly, 250 μg of purified proteins was individually emulsified 1:1 in complete Freund's adjuvant for initial subcutaneous immunization. Subsequent immunization followed a 3-week cycle of boosts with 125 μg of protein emulsified 1:1 in incomplete Freund's adjuvant. Serum was collected 2 weeks after the second boost.
SDS-PAGE and immunoblot assay.
SDS-PAGE and immunoblot assays were performed as described previously (25). For immunoblot assays of purified recombinant SBP1, SBP2, and SBP3 (1 μg each), the membrane was incubated with a 1:105 dilution of antiserum raised to the corresponding SBP proteins. For immunoblotting of whole-cell lysates of wild-type M. catarrhalis and corresponding mutants, the primary antibodies were assayed at a 1:40,000 dilution.
Construction of mutants.
Mutants were constructed in M. catarrhalis strain O35E in which the sbp1, sbp2, and sbp3 genes were individually knocked out. A mutant in which all 3 genes were knocked out was also constructed. Mutant construction was accomplished by using overlap extension PCR (29) and homologous recombination as described previously (25, 29, 30). Briefly, the transforming DNA for the mutants was composed of 3 overlapping fragments that included ~1 kb upstream of the gene being knocked out (fragment 1), the nonpolar kanamycin resistance cassette amplified from plasmid pUC18K (31) (fragment 2), and ~1 kb downstream of the gene (fragment 3) using the oligonucleotide primers listed in Table S1 in the supplemental material. Mutants were constructed by transformation of strain O35E with a fragment composed of fragments 1, 2, and 3 and selection on BHI plates containing 50 μg/ml of kanamycin. The insert and surrounding sequences of each of the mutants were confirmed by sequence analysis.
Mutants in which sbp2 was knocked out were constructed in 10 additional middle ear fluid strains by transforming each strain with a fragment that consisted of ∼1 kb upstream of sbp2 from strain O35E, the kanamycin resistance cassette from plasmid pUC18K, and ~1 kb downstream of sbp2 from strain O35E as described above. The insert and surrounding sequences of each of the mutants were confirmed by sequence analysis.
Assessment of bacterial growth.
Growth curves were performed using the Bioscreen C automated growth curve analysis system (Oy Growth Curves AB Ltd., Helsinki, Finland) according to the manufacturer's instructions. For M. catarrhalis strains, OD600 was determined at 30-min intervals with the Bioscreen C system at 37°C with constant shaking (machine settings: fast speed, high amplitude).
Whole-cell ELISA.
The whole-cell enzyme-linked immunosorbent assay (ELISA) was done as described previously (25). M. catarrhalis strain O35E and the mutant strains were grown in BHI broth to an OD600 of 0.2, harvested by centrifugation, and resuspended in PBS. A volume of 100 μl of the suspension was added to each well of a 96-well microtiter Immulon 4 plate (Thermo Labsystems, Franklin, MA) to coat the wells with bacterial cells. Wells with PBS alone were included as controls. The plate was washed once with PBST (0.5% Tween 20 in PBS) and blocked with 3% bovine serum albumin in PBS for 1 h at room temperature, after which the plate was washed 3 times with PBST. Paired rabbit antisera to SBP1, SBP2, and SBP3 (preimmune and immune) described above were diluted 1:4,000 in diluent buffer (1% bovine serum albumin in PBST) and added to the sham-coated control wells and each whole-bacterial-cell sample well in parallel. After incubation for 2 h at 37°C, the plate was washed 3 times with PBST and a 1:3,000 dilution of peroxidase-labeled secondary antibody, anti-rabbit IgG (KPL, Gaithersburg, MD). After another 1 h of incubation at room temperature, the plate was washed 3 times with PBST and developing reagent was added to the wells. The reaction was allowed to proceed in the dark for 10 min and was stopped with 2 M sulfuric acid. The absorbance at 450 nm was determined using a Bio-Rad model 3550-UV microplate reader (Hercules, CA).
The percent change between paired samples was calculated with the following formula: [(value of SBP-immunized serum − value of preimmune serum)/value of preimmune serum] × 100. Whole-cell ELISAs were repeated in five independent experiments for strain O35E and the mutant strains and in three independent experiments for AOM strains and the sbp2 mutants. The mean and standard deviation (SD) of the percent change of absorbance value were calculated. The values of whole-cell ELISA between wild type and sbp mutants were compared using an unpaired Student t test. A P value of ≤0.05 was considered significant.
Flow cytometry.
Flow cytometry was done as described previously (25). M. catarrhalis was grown to mid-logarithmic phase, and 100 μl of culture was centrifuged at 4,000 × g for 5 min. The bacterial pellet was resuspended in an appropriate dilution of rabbit serum in PBS. Appropriate dilutions were determined by performing initial experiments at multiple 2-fold dilutions to identify the dilution of serum that yielded the greatest signal-to-noise ratio. Bacteria incubated in PBS alone were used as a negative control, while bacteria incubated with polyclonal antiserum from rabbits immunized with whole bacterial cells of strain O35E were used as a positive control. Bacteria were incubated with antisera for 1 h at 37°C. The bacteria were centrifuged, and the bacterial pellets were resuspended in a 1:100 to 1:600 dilution of anti-rabbit IgG conjugated to R-phycoerythrin (SouthernBiotech, Birmingham, AL). After 30 min of incubation at 37°C, the sample was diluted with 900 μl PBS and fluorescence was detected with a FACSCalibur flow cytometer using Cell Quest 3.1 software (BD Biosciences, San Jose, CA). The percent change of geometric mean between paired samples was calculated using the same formula as that for whole-cell ELISA. Flow cytometry for O35E and the sbp mutants was repeated in three independent experiments. The mean and SD of the percent change of geometric mean were calculated. The values of flow cytometry were compared between wild type and sbp mutants using an unpaired Student t test. A P value of ≤0.05 was considered significant.
Immunization of mice.
All studies were reviewed and approved by the University at Buffalo Institutional Animal Care and Use Committee. Systemic immunization was accomplished with groups of 6 BALB/c mice that were immunized subcutaneously with 25 μg or 50 μg of purified recombinant SBP2 emulsified in incomplete Freund's adjuvant. Additional controls were immunized with either PBS plus adjuvant (negative control, n = 6) or formalin-killed M. catarrhalis O35E emulsified in incomplete Freund's adjuvant (positive control, n = 6). Injections were repeated at 15 and 28 days after the initial immunization. Mice were challenged on day 35 as described below.
Mouse pulmonary clearance model.
To determine if immunization with SBP2 induces potentially protective responses in vivo, the mouse pulmonary clearance model was used as described previously (25, 32). Overnight culture of M. catarrhalis O35E was inoculated into 100 ml BHI broth to bring the OD600 to 0.05 and grown to an OD600 of 0.3. Bacteria were collected by centrifugation and resuspended in 10 ml PCGM buffer (4.3 mM NaHPO4, 1.4 mM KH2PO4, 137 mM NaCl, 2.7 mM KCl, 5 mM CaCl2, 0.5 mM MgCl2, 0.1% gelatin, pH 7.3). An aliquot of suspension was diluted and plated to determine the starting concentration of bacteria. Ten milliliters of the bacterial suspension (109 CFU/ml) was placed in the nebulizer of a Glas-Col inhalational exposure system, model 099C A4212 (Glas-Col, Terre Haute, IN). The groups of immunized mice described above (n = 6 each) were challenged using this inhalation system with the following settings: 10-min preheating, 40-min nebulization, 30-min cloud decay, 10-min decontamination, vacuum flow meter at 60 cubic feet/h, and compressed airflow meter at 10 cubic feet/h.
Three hours postchallenge, the mice were euthanized by inhalation of isoflurane. Lungs were then harvested and homogenized on ice in 5 ml PCGM buffer using a tissue homogenizer. Aliquots (20 μl) of undiluted and 1:10 diluted lung homogenate were plated in duplicate and incubated at 35°C with 5% CO2 overnight. Colonies were counted the following day. Statistical significance of colony counts between groups of immunized and sham-immunized mice was determined by two-tailed t tests. A P value of ≤0.05 was considered significant.
RESULTS
Identification of the sbp gene cluster.
As part of a genome-mining approach to identify putative vaccine antigens of M. catarrhalis, the genome of strain ATCC 43617 was analyzed (accession numbers AX067426 through AX067466) to identify open reading frames (ORFs) that were predicted to be potentially surface exposed (24). Of a total of 348 ORFs that were predicted to be potentially surface exposed, 14 had homology to substrate binding proteins of ABC transporter systems. In previous work, we identified oligopeptide binding protein A (OppA), the substrate binding protein of the oligopeptide permease ABC transporter system, using this genome-mining approach (25). OppA expresses epitopes on the bacterial surface and induces potentially protective immune responses to M. catarrhalis (25). Based on this previous work showing that the substrate binding protein of an ABC transporter system has features of a promising vaccine antigen, in the current study we characterized the substrate binding proteins, SBP1, SBP2, and SBP3, of a predicted ABC transporter system identified in the M. catarrhalis genome.
Figure 1 shows the gene cluster from M. catarrhalis BBH18 that includes 3 ORFs that are predicted to encode SBP1, SBP2, and SBP3, which are the focus of this study (33, 34). Based on homology, the gene cluster has 7 ORFs that are predicted to encode the components of an ABC transporter system, including an ATPase, 3 substrate binding proteins, and 2 permeases. In addition, a small ORF (156 bp) that is predicted to encode a hypothetical protein that has no known homology is present downstream of the ORF that encodes SBP3. This potential ORF is predicted to be transcribed in the opposite direction from the other genes in the gene cluster. The M. catarrhalis SBPs are homologous with amino acid transport substrate binding proteins of other Gram-negative bacteria in the range of 50% identity and 70% similarity based on BLAST searches. The SBPs have amino acid sequence similarity with one another (Table 1).
FIG 1.
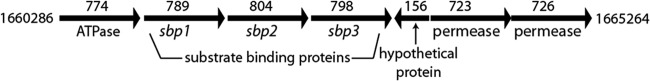
Schematic illustration of the sbp gene cluster from the genome of M. catarrhalis strain BBH18. The numbers above each gene show the size of open reading frames in base pairs. Numbers to the left and right of the gene cluster indicate the initial and terminal nucleotide positions of the gene cluster in GenBank (accession number CP002005).
TABLE 1.
Characteristics of predicted substrate binding proteins encoded by sbp1, sbp2, and sbp3 of M. catarrhalis strain O35E
| Protein | No. of amino acidsa | Leader peptideb | % amino acid identity |
% amino acid similarity |
||||
|---|---|---|---|---|---|---|---|---|
| SBP1 | SBP2 | SBP3 | SBP1 | SBP2 | SBP3 | |||
| SBP1 | 262 | 17 | 45.2 | 39.5 | 64.4 | 57.6 | ||
| SBP2 | 267 | 21 | 45.2 | 38.7 | 64.4 | 60.9 | ||
| SBP3 | 265 | 20 | 39.5 | 38.7 | 57.6 | 60.9 | ||
Open reading frame including signal sequence.
Predicted by LipoP 1.0.
BLAST analysis of 10 strains of M. catarrhalis whose whole-genome shotgun contigs are deposited in GenBank (35) revealed that the genomes of all 10 strains each contain the 7 ORFs in the predicted SBP gene cluster. The predicted ATPase, 2 permeases, and 3 substrate binding proteins (Fig. 1) are 99 to 100% identical in predicted amino acid sequence to the ORFs in strain BBH18 (33, 34). The hypothetical protein downstream of sbp3 is present in all 10 strains and is 96 to 100% identical in predicted amino acid sequence to the corresponding ORF in strain BBH18.
Characterization of purified SBPs.
The M. catarrhalis sbp genes are predicted to encode proteins of 262 amino acids (SBP1), 267 amino acids (SBP2), and 265 amino acids (SBP3). Based on the prediction program LipoP (36), which discriminates between lipoprotein signal peptides and other signal peptides of Gram-negative bacteria, all 3 are predicted lipoproteins. Figure 2A shows a Coomassie blue-stained gel of purified recombinant SBP1, SBP2, and SBP3.
FIG 2.
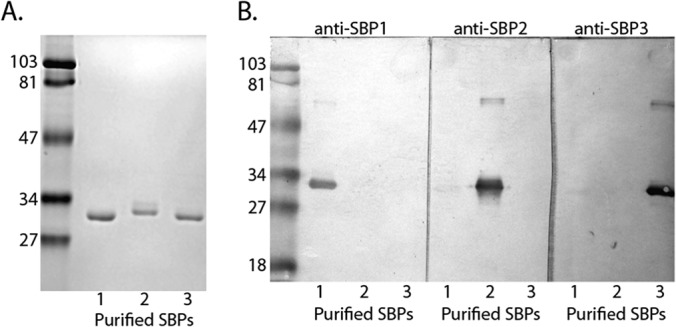
Analysis of purified recombinant SBP1, SBP2, and SBP3. (A) Coomassie blue-stained SDS gel of purified recombinant SBP1, SBP2, and SBP3 as noted at the bottom of the gel. Molecular mass markers are noted in kilodaltons on the left. (B) Immunoblot assays of purified recombinant SBP1, SBP2, and SBP3 probed with antiserum raised to the corresponding proteins (dilution, 1:105). Molecular mass markers are noted in kDa on the left. Antisera detect proteins with molecular masses of ∼30 kDa and faint dimers of ∼60 kDa.
Characterization of antisera to SBPs.
Individual rabbit antisera raised to recombinant SBP1, SBP2, and SBP3 were assayed in immunoblot assays. Figure 2B shows that each antiserum shows binding to its corresponding purified recombinant protein. Under the conditions of this immunoblot assay, antiserum to each individual SBP binds to the homologous purified protein at the highest reactivity and shows no or low-level binding to the other SBPs encoded by sbp genes in the gene cluster.
To assess the binding of antibodies raised to recombinant proteins of M. catarrhalis SBPs, whole-cell lysates of the wild type and corresponding mutants were subjected to immunoblot assays. Figure 3 shows that antisera to recombinant proteins bind M. catarrhalis proteins. The absence of binding to the corresponding mutant further confirms that each antiserum recognizes its corresponding SBP with the highest affinity.
FIG 3.
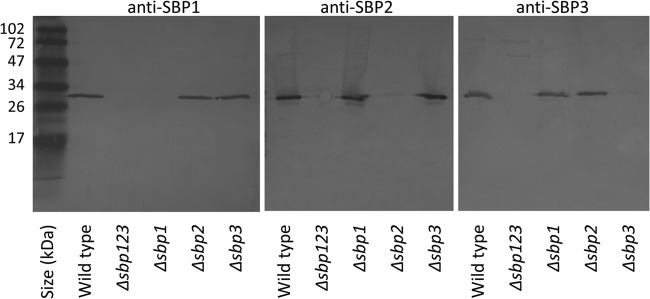
Immunoblot assays. Whole-cell lysates of wild-type M. catarrhalis and corresponding mutants are noted at the bottom. Blots were probed with rabbit antisera to recombinant SBP proteins (anti-SBP1, anti-SBP2, and anti-SBP3) as noted at the top of each panel. Molecular mass markers are noted in kDa on the left.
Growth characteristics of sbp mutants.
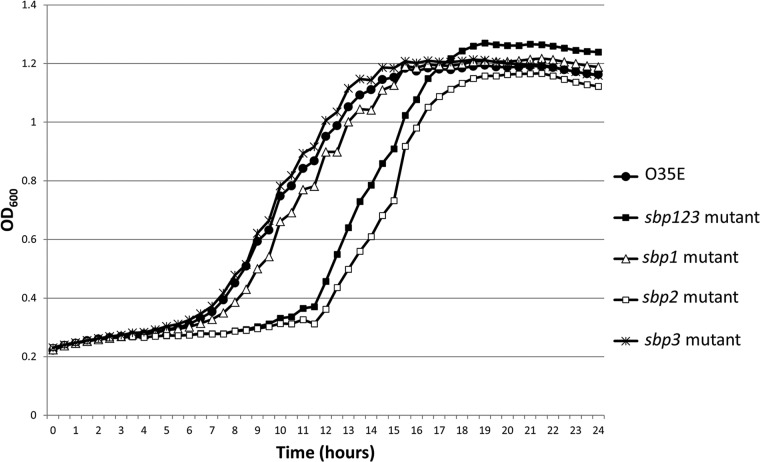
In order to characterize the growth of the sbp mutants in laboratory media (BHI), we performed growth curve experiments. Compared to the wild-type strain O35E, the sbp2 mutant and sbp123 mutant revealed a longer lag phase, while the sbp1 and sbp3 mutants had growth characteristics similar to those of the wild-type strain (Fig. 4). PCR using sbp-specific primers showed that the genes were absent in the sbp2 mutant and the sbp123 mutant after 24 h of growth, excluding revertant or mutant populations of bacteria to account for the longer lag phase of growth. These results indicate that SBP2 plays a role in bacterial growth.
FIG 4.
Growth curve results of M. catarrhalis strain O35E and its sbp mutants. The x axis shows time (hours), and the y axis shows optical density at 600 nm. The sbp2 mutant and sbp123 mutant revealed a longer lag phase (11 to 12 h) than that of the wild-type strain O35E. The sbp1 and sbp3 mutants had growth characteristics similar to those of the wild-type strain. The peak optical density values are not significantly different between the wild-type strain and mutants.
Surface exposure of SBP epitopes. (i) Whole-cell ELISA.
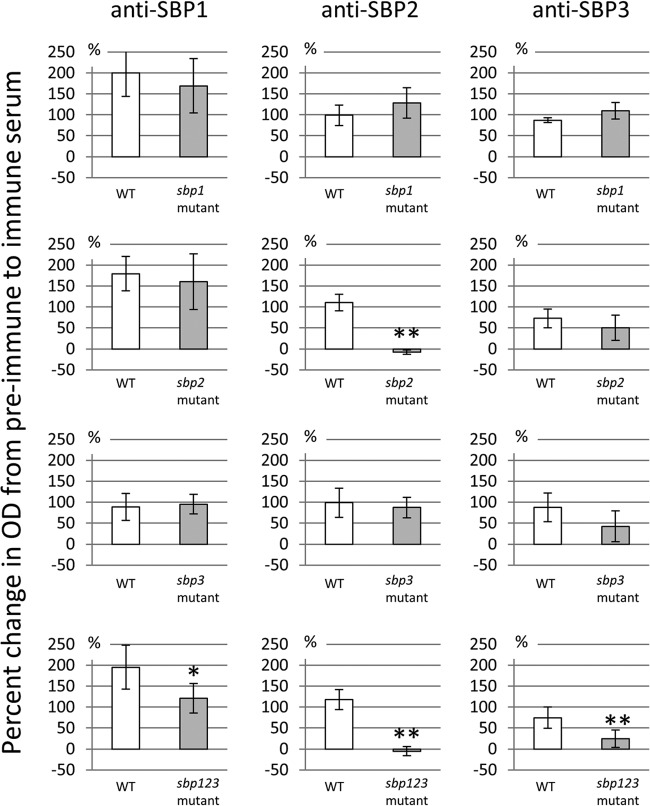
Surface exposure is an important feature of vaccine antigens. In the whole-cell ELISA using polyclonal antibodies to SBP2 compared to preimmune serum, the mean ± SD of the percent change was 110.5% ± 19.8% for wild-type O35E, compared to −7.8% ± 5.0% for the sbp2 mutant (P < 0.01) (Fig. 5). The sbp123 mutant yielded a similar result. In contrast, the other whole-cell ELISAs using polyclonal antibodies to SBP1 and SBP3 revealed no significant differences in reactivity between the wild type and corresponding mutants. These results demonstrated that SBP2 expresses epitopes on the bacterial cell surface of M. catarrhalis strain O35E.
FIG 5.
Results of whole-cell ELISA with antisera to SBPs. The y axes show percent change of ELISA values between preimmune and immune sera. The percent change between paired samples was calculated by the equation [(value of SBP-immunized serum − value of preimmune serum)/value of preimmune serum] × 100. Error bars indicate the standard deviations of the results from five independent experiments. *, P < 0.05; **, P < 0.01. There are significant differences in percent changes of ELISA values for anti-SBP2 serum between O35E and the sbp2 mutant (P < 0.01) and the sbp123 mutant (P < 0.01).
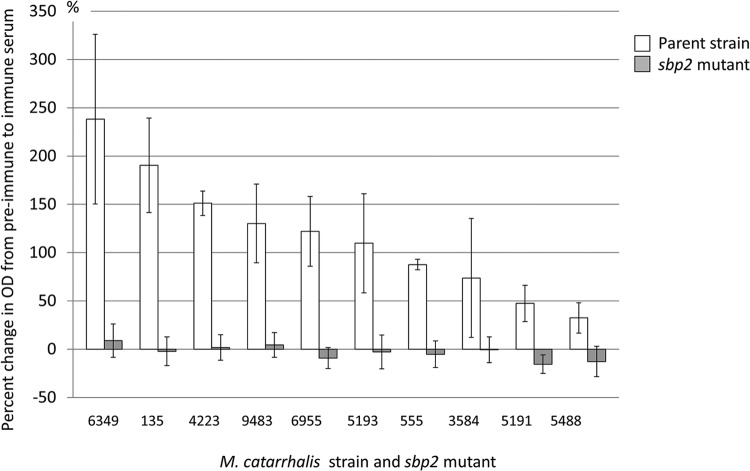
To determine whether the SBP2 proteins of other clinical isolates also express epitopes on the bacterial cell surface, we constructed mutants by knocking out the sbp2 gene in 10 middle ear fluid isolates of M. catarrhalis. Whole-cell ELISAs were performed using anti-SBP2 antiserum with the 10 isolates and their corresponding sbp2 mutants. The percent change of ELISA values between preimmune and immune serum among the 10 AOM strains ranged from 238.3% (strain 6349) to 32.4% (strain 5488). Conversely, sbp2 mutants showed no significant change, ranging from −15.5% to 8.9% (Fig. 6). We conclude that SBP2 proteins of all 10 clinical isolates express surface epitopes.
FIG 6.
Results of whole-cell ELISA with antiserum to SBP2 with acute otitis media strains and their sbp2 mutants. The x axis shows strain names; the y axis shows percent changes of ELISA values between preimmune and immune sera. White bars indicate the parent strain isolated from a patient with acute otitis media, and gray bars indicate the sbp2 mutant of the parent strain. Error bars indicate the standard deviations of results from three independent experiments. The percent changes of ELISA values among the 10 acute otitis media strains ranged from 238.3% (strain 6349) to 32.4% (strain 5488). Conversely, sbp2 mutants showed no significant change, with their ELISA values ranging from −15.5% to 8.9%.
(ii) Flow cytometry.
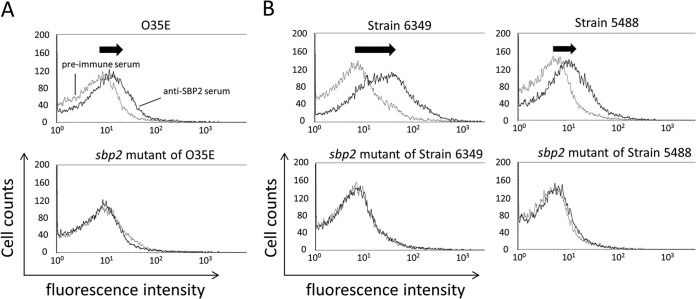
To assess the exposure of SBP2 epitopes on the bacterial surface, flow cytometry was performed. Comparing preimmune serum and anti-SBP2 serum with strain O35E, median fluorescence intensity increased as shown by a shift of the curve to the right (Fig. 7A, top). In contrast, an assay of the same antiserum to the sbp2 mutant showed no shift to the right (Fig. 7A, bottom). The mean ± SD of the percent change in geometric mean was 83.0% ± 16.9% for wild-type strain O35E, compared to −3.1% ± 5.1% for the sbp2 mutant (P < 0.01). We conclude that SBP2 immune serum contains SBP2-specific antibodies to epitopes on the bacterial surface of strain O35E.
FIG 7.
Results of flow cytometry with anti-SBP2 serum. The x axes show fluorescence intensity, and the y axes show cell counts. (A) Wild-type strain O35E (top panel) and sbp2 mutant (bottom panel) assayed with rabbit antiserum to recombinant purified SBP2 and preimmune serum. The mean ± SD of the percent change in geometric mean was 83.0% ± 16.9% for O35E, compared to −3.1% ± 5.1% for the sbp2 mutant (P < 0.01). (B) Representative data from flow cytometry among acute otitis media strains and their sbp2 mutants. Representative strains were strain 6349, which showed the highest percent change, and strain 5488, which showed the lowest percent change in whole-cell ELISA values of the 10 acute otitis media strains.
Ten additional clinical isolates of M. catarrhalis were subjected to flow cytometry with anti-SBP2 immune serum. The results in Fig. 7B are representative data of flow cytometry experiments among AOM strains and their sbp2 mutants. Strain 6349, which showed the highest percent change in whole-cell ELISA, showed a large peak shift. In contrast, strain 5488, which showed the lowest percent change in whole-cell ELISA of the 10 strains, indicated a relatively minor peak shift. The mean ± SD of the percent change in geometric mean was 185.7% ± 67.8% for the 10 AOM strains, compared to 9.1% ± 18.8% for their corresponding sbp2 mutants (P < 0.01).
These results indicate that cells of the sbp2 mutant strain lack affinity for the anti-SBP2 antibody and that the binding of anti-SBP2 antibody to cells of the wild-type strain was specifically mediated by the SBP2 protein. Based on the results of whole-cell ELISA and flow cytometry assays, we conclude that the SBP2 protein has epitopes on the bacterial surface of 11 of 11 strains of M. catarrhalis tested and that these epitopes are accessible to antibody binding. This characteristic suggests that SBP2 has potential as a protective immunogen.
Sequence conservation of sbp2 among strains of M. catarrhalis.
Among 30 clinical isolates, nucleotide variations were present at only 4 discrete positions (see Table S2 in the supplemental material) distributed over the 804 bases of the full-length sbp2 gene compared to strain O35E. Nucleotide variation at one of these positions was silent, and those at the other 3 positions gave rise to one amino acid change (A to T). Strains 555 and 9483 have an identical sbp2 sequence compared to that of strain O35E. Strains 5193 and 6955 have A82T and A257T substitutions, respectively. The other 6 AOM isolates have an A33T substitution compared to strain O35E. The gene identity scores among these strains range from 99.8% to 100%. These data indicate that the sbp2 gene is highly conserved among M. catarrhalis strains.
Induction of mouse pulmonary clearance.
Mice immunized with SBP2 showed significantly enhanced clearance of bacteria from the lung compared to that in the control group at both 25-μg and 50-μg doses (Fig. 8). Subcutaneous immunization with SBP2 resulted in colony counts approximately 50 to 70% lower than those of the sham-immunized control, an effect comparable to that induced by immunization with killed whole bacteria. The experiment depicted in Fig. 8 was repeated 2 additional times with similar results. We conclude that immunization of mice with purified recombinant SBP2 induces enhanced clearance of M. catarrhalis in the mouse pulmonary clearance model.
FIG 8.
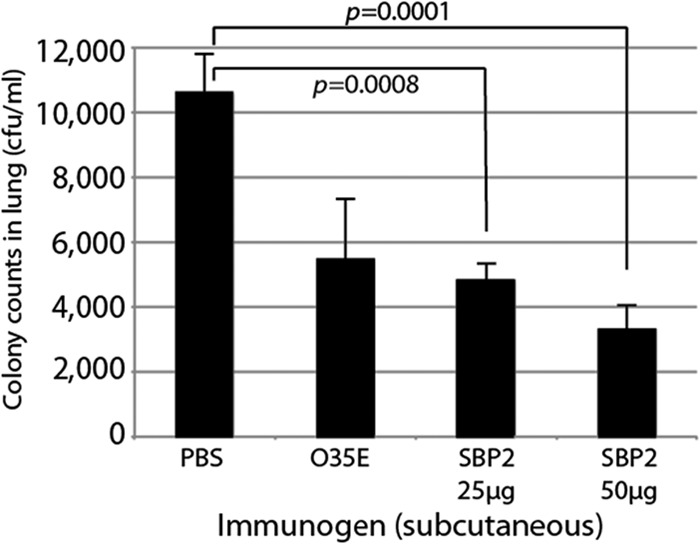
Results of mouse pulmonary clearance after aerosol challenge. Mice were challenged with M. catarrhalis O35E following subcutaneous immunization with PBS, formalin-killed M. catarrhalis O35E, and recombinant SBP2 (25 μg and 50 μg). At 3 h postchallenge, bacteria present in mouse lung homogenate were plated and incubated overnight. Colony counts were plotted. Error bars represent the standard errors of the means (n = 6).
DISCUSSION
M. catarrhalis contributes substantially to the heavy burden of otitis media and COPD both as a primary pathogen and as a copathogen. Almost all strains of M. catarrhalis express β-lactamase to inactivate β-lactam antibiotics such as amoxicillin (37), which is used widely to treat otitis media. Thus, coinfection of M. catarrhalis with S. pneumoniae and H. influenzae may lead to treatment failure (38–43). The bacterium may facilitate infection by other pathogens through outer membrane vesicles (44) and quorum signaling (45). Furthermore, it is likely to have a role in more chronic, indolent forms of otitis media based on results of PCR of middle ear fluid (12, 16, 17). Therefore, preventing M. catarrhalis infections by an effective vaccine will have a broader impact than the estimated burden based on cultures of middle ear fluid in AOM. This concept will be an important consideration in the design of clinical trials to assess efficacy of vaccines for M. catarrhalis.
Inspection of the annotated genome of M. catarrhalis revealed that the genome has approximately 22 predicted full or partial ABC transporter systems (33, 34). The sbp genes were discovered as genes encoding substrate binding proteins, which are components of a predicted amino acid ABC transporter system. Although substrate binding proteins are characteristically present within the periplasm in Gram-negative bacteria (46), a number of these proteins have been shown to be immunogenic and to induce potentially protective immune responses (26). For example, the substrate binding protein OppA of M. catarrhalis (25), Borrelia burgdorferi (47), and Yersinia pestis (48) induces immune protection against infection in mice. Therefore, we decided to test the substrate binding proteins encoded by sbp genes as possible vaccine antigens.
In evaluating vaccine antigens, the ideal one should meet several characteristics: (i) present on the surface of all strains, (ii) antigenically conserved among strains, and (iii) capable of inducing a protective immune responses (4, 49). In this study, we showed that SBP2 has excellent potential as a vaccine antigen against M. catarrhalis, having essentially all of these characteristics. SBP2 expresses abundant surface epitopes on the bacterial surface, based on the results of 2 independent methods (whole-cell ELISA and flow cytometry) using an sbp2 mutant as a control in each experiment (Fig. 5 to 7). The nucleotide sequence of sbp2 is highly conserved among 30 M. catarrhalis strains, including nasopharyngeal isolates, middle ear isolates, and sputum isolates from adults with COPD. Interestingly, single nucleotide variations were present at only 4 discrete positions in the 30 strains and 3 of the variations gave rise to an amino acid change from alanine to threonine. The results of whole-cell ELISA and flow cytometry indicated that the amino acid changes in SBP2 found in this study did not affect the expression of epitopes on the bacterial surface, although some variability is observed with degree of surface exposure among strains. Although the function of the sbp gene cluster is not known, we found that knocking out sbp2 alters growth (Fig. 4). The function of the SBP proteins will be a focus of future study to advance vaccine development.
The observation that SBP2 of M. catarrhalis expresses epitopes on the bacterial surface, as does OppA (25), raises the question of how a predicted periplasmic substrate binding protein exposes epitopes on the bacterial surface. We speculate that epitopes are intermittently exposed when the protein binds its substrate from the environment for transport across the periplasm. An alternative speculation is that, in addition to its periplasmic location, the protein may also be inserted in the outer membrane (25). The observation that SBP2 is a predicted lipoprotein is consistent with an outer membrane location.
At the outset of the present study, we assessed the surface exposure of SBP1, -2, and -3, which are all predicted substrate binding proteins in the same gene cluster. In whole-cell ELISA, SBP1 and SBP3 appear not to express epitopes on the bacterial surface in a comparison of the wild type to the corresponding mutants (Fig. 5). However, experiments comparing the wild type with the sbp123 mutant suggest a possibility that SBP1 and SBP3 also express surface epitopes. In addition to nonspecific responses by the polyclonal antibodies, we suspect that cross-reactivity of SBP1 and SBP3 with SBP2 may account for this observation, given the amino acid similarity between the proteins (Table 1). Furthermore, recombinant SBP1 and SBP3 proteins may have been denatured during purification, resulting in destruction of conformational surface epitopes. Therefore, caution must be used in dismissing SBP1 and SBP3 as potential vaccine antigens based on these data. Additional studies to assess surface exposure using methods that do not rely solely on antibodies to recombinant proteins will be important in elucidating the antigenic structures of the SBPs in the bacterial cell wall.
To determine if immunization with SBP2 induced a protective immune response, in vivo experiments using an animal model were performed. The mouse pulmonary clearance model is the most widely used model to test potential vaccine antigens of M. catarrhalis (4, 49). Subcutaneous immunization of mice with purified recombinant SBP2 induced enhanced pulmonary clearance following aerosol pulmonary challenge (Fig. 8). Up to 70% clearance in the mouse pulmonary clearance model is consistent with the level of clearance observed with other vaccine antigens (25, 32, 50–52). A limitation of the model is that mice do not develop infections (e.g., pneumonia) that simulate human infection. Rather, the endpoint is rate of clearance of bacteria from the lungs. However, the model is simple and reproducible, is performed in multiple centers, and measures a functional response (4, 49). We conclude that SBP2 induces a potentially protective immune response in the mouse pulmonary clearance model.
In summary, we showed that SBP2 meets 3 critical conditions of a vaccine antigen: (i) expression of epitopes on the bacterial surface, (ii) a high degree of conservation among strains, and (iii) induction of a potentially protective immune response. Thus, SBP2 is a novel, attractive candidate as a vaccine antigen against M. catarrhalis. Further development of SBP2 and other promising M. catarrhalis vaccine antigens will be important along with vaccines for S. pneumoniae and nontypeable H. influenzae to maximize the likelihood of a successful vaccine to prevent otitis media in children.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by NIH grant DC012200 (T.F.M.) and the Uehara Memorial Foundation (T.O.).
Footnotes
Published ahead of print 9 June 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.01832-14.
REFERENCES
- 1.Johnson MA, Drew WL, Roberts M. 1981. Branhamella (Neisseria) catarrhalis—a lower respiratory tract pathogen? J. Clin. Microbiol. 13:1066–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Faden H, Harabuchi Y, Hong JJ. 1994. Epidemiology of Moraxella catarrhalis in children during the first 2 years of life: relationship to otitis media. J. Infect. Dis. 169:1312–1317. 10.1093/infdis/169.6.1312. [DOI] [PubMed] [Google Scholar]
- 3.Verduin CM, Hol C, Fleer A, van Dijk H, van Belkum A. 2002. Moraxella catarrhalis: from emerging to established pathogen. Clin. Microbiol. Rev. 15:125–144. 10.1128/CMR.15.1.125-144.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murphy TF, Parameswaran GI. 2009. Moraxella catarrhalis, a human respiratory tract pathogen. Clin. Infect. Dis. 49:124–131. 10.1086/599375. [DOI] [PubMed] [Google Scholar]
- 5.Otsuka T, Kitami O, Kondo K, Ota H, Oshima S, Tsuchiya A, Shirai T, Fujii K, Nakamure M, Shoji Y, Nakamura H, Masuda Y, Komiyama K, Yoshida K, Ishikawa Y, Iwaya A, Takahashi S, Okazaki M, Hotomi M, Yamanaka N. 2013. Incidence survey of acute otitis media in children in Sado Island, Japan—Sado Otitis Media Study (SADOMS). PLoS One 8:e68711. 10.1371/journal.pone.0068711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O'Brien MA, Prosser LA, Paradise JL, Ray GT, Kulldorff M, Kurs-Lasky M, Hinrichsen VL, Mehta J, Colborn DK, Lieu TA. 2009. New vaccines against otitis media: projected benefits and cost-effectiveness. Pediatrics 123:1452–1463. 10.1542/peds.2008-1482. [DOI] [PubMed] [Google Scholar]
- 7.Teele DW, Klein JO, Rosner B. 1989. Epidemiology of otitis media during the first seven years of life in children in greater Boston: a prospective, cohort study. J. Infect. Dis. 160:83–94. 10.1093/infdis/160.1.83. [DOI] [PubMed] [Google Scholar]
- 8.Faden H. 2001. The microbiologic and immunologic basis for recurrent otitis media in children. Eur. J. Pediatr. 160:407–413. 10.1007/s004310100754. [DOI] [PubMed] [Google Scholar]
- 9.Poehling KA, Szilagyi PG, Grijalva CG, Martin SW, LaFleur B, Mitchel E, Barth RD, Nuorti JP, Griffin MR. 2007. Reduction of frequent otitis media and pressure-equalizing tube insertions in children after introduction of pneumococcal conjugate vaccine. Pediatrics 119:707–715. 10.1542/peds.2006-2138. [DOI] [PubMed] [Google Scholar]
- 10.American Academy of Family Physicians, American Academy of Otolaryngology-Head and Neck Surgery, American Academy of Pediatrics Subcommittee on Otitis Media with Effusion. 2004. Otitis media with effusion. Pediatrics 113:1412–1429. 10.1542/peds.113.5.1412. [DOI] [PubMed] [Google Scholar]
- 11.Hendolin PH, Markkanen A, Ylikoski J, Wahlfors JJ. 1997. Use of multiplex PCR for simultaneous detection of four bacterial species in middle ear effusions. J. Clin. Microbiol. 35:2854–2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holder RC, Kirse DJ, Evans AK, Peters TR, Poehling KA, Swords WE, Reid SD. 2012. One third of middle ear effusions from children undergoing tympanostomy tube placement had multiple bacterial pathogens. BMC Pediatr. 12:87. 10.1186/1471-2431-12-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Teele DW, Klein JO, Chase C, Menyuk P, Rosner BA. 1990. Otitis media in infancy and intellectual ability, school achievement, speech, and language at age 7 years. Greater Boston Otitis Media Study Group. J. Infect. Dis. 162:685–694. [DOI] [PubMed] [Google Scholar]
- 14.Grubb MS, Spaugh DC. 2010. Microbiology of acute otitis media, Puget Sound region, 2005–2009. Clin. Pediatr. 49:727–730. 10.1177/0009922810361365. [DOI] [PubMed] [Google Scholar]
- 15.Casey JR, Kaur R, Friedel VC, Pichichero ME. 2013. Acute otitis media otopathogens during 2008 to 2010 in Rochester, New York. Pediatr. Infect. Dis. J. 32:805–809. 10.1097/INF.0b013e31828d9acc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aydin E, Tastan E, Yucel M, Aydogan F, Karakoc E, Arslan N, Kantekin Y, Demirci M. 2012. Concurrent assay for four bacterial species including Alloiococcus otitidis in middle ear, nasopharynx and tonsils of children with otitis media with effusion: a preliminary report. Clin. Exp. Otorhinolaryngol. 5:81–85. 10.3342/ceo.2012.5.2.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stol K, Verhaegh SJ, Graamans K, Engel JA, Sturm PD, Melchers WJ, Meis JF, Warris A, Hays JP, Hermans PW. 2013. Microbial profiling does not differentiate between childhood recurrent acute otitis media and chronic otitis media with effusion. Int. J. Pediatr. Otorhinolaryngol. 77:488–493. 10.1016/j.ijporl.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lopez AD, Shibuya K, Rao C, Mathers CD, Hansell AL, Held LS, Schmid V, Buist S. 2006. Chronic obstructive pulmonary disease: current burden and future projections. Eur. Respir. J. 27:397–412. 10.1183/09031936.06.00025805. [DOI] [PubMed] [Google Scholar]
- 19.Mannino DM, Buist AS. 2007. Global burden of COPD: risk factors, prevalence, and future trends. Lancet 370:765–773. 10.1016/S0140-6736(07)61380-4. [DOI] [PubMed] [Google Scholar]
- 20.Ford ES, Croft JB, Mannino DM, Wheaton AG, Zhang X, Giles WH. 2013. COPD surveillance—United States, 1999–2011. Chest 144:284–305. 10.1378/chest.13-0809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sethi S, Evans N, Grant BJ, Murphy TF. 2002. New strains of bacteria and exacerbations of chronic obstructive pulmonary disease. N. Engl. J. Med. 347:465–471. 10.1056/NEJMoa012561. [DOI] [PubMed] [Google Scholar]
- 22.Murphy TF, Brauer AL, Grant BJ, Sethi S. 2005. Moraxella catarrhalis in chronic obstructive pulmonary disease. Burden of disease and immune response. Am. J. Respir. Crit. Care Med. 172:195–199. 10.1164/rccm.200412-1747OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sethi S, Murphy TF. 2008. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N. Engl. J. Med. 359:2355–2365. 10.1056/NEJMra0800353. [DOI] [PubMed] [Google Scholar]
- 24.Ruckdeschel EA, Kirkham C, Lesse AJ, Hu Z, Murphy TF. 2008. Mining the Moraxella catarrhalis genome: identification of potential vaccine antigens expressed during human infection. Infect. Immun. 76:1599–1607. 10.1128/IAI.01253-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang M, Johnson A, Murphy TF. 2011. Characterization and evaluation of the Moraxella catarrhalis oligopeptide permease A as a mucosal vaccine antigen. Infect. Immun. 79:846–857. 10.1128/IAI.00314-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garmory HS, Titball RW. 2004. ATP-binding cassette transporters are targets for the development of antibacterial vaccines and therapies. Infect. Immun. 72:6757–6763. 10.1128/IAI.72.12.6757-6763.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cullen PA, Lo M, Bulach DM, Cordwell SJ, Adler B. 2003. Construction and evaluation of a plasmid vector for the expression of recombinant lipoproteins in Escherichia coli. Plasmid 49:18–29. 10.1016/S0147-619X(02)00150-6. [DOI] [PubMed] [Google Scholar]
- 28.Adlowitz DG, Sethi S, Cullen P, Adler B, Murphy TF. 2005. Human antibody response to outer membrane protein G1a, a lipoprotein of Moraxella catarrhalis. Infect. Immun. 73:6601–6607. 10.1128/IAI.73.10.6601-6607.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shevchuk NA, Bryksin AV, Nusinovich YA, Cabello FC, Sutherland M, Ladisch S. 2004. Construction of long DNA molecules using long PCR-based fusion of several fragments simultaneously. Nucleic Acids Res. 32:e19. 10.1093/nar/gnh014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murphy TF, Brauer AL, Kirkham C, Johnson A, Koszelak-Rosenblum M, Malkowski MG. 2013. Role of the zinc uptake ABC transporter of Moraxella catarrhalis in persistence in the respiratory tract. Infect. Immun. 81:3406–3413. 10.1128/IAI.00589-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Menard R, Sansonetti PJ, Parsot C. 1993. Nonpolar mutagenesis of the ipa genes defines IpaB, IpaC, and IpaD as effectors of Shigella flexneri entry into epithelial cells. J. Bacteriol. 175:5899–5906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ruckdeschel EA, Brauer AL, Johnson A, Murphy TF. 2009. Characterization of proteins Msp22 and Msp75 as vaccine antigens of Moraxella catarrhalis. Vaccine 27:7065–7072. 10.1016/j.vaccine.2009.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Vries SP, van Hijum SA, Schueler W, Riesbeck K, Hays JP, Hermans PW, Bootsma HJ. 2010. Genome analysis of Moraxella catarrhalis strain RH4, a human respiratory tract pathogen. J. Bacteriol. 192:3574–3583. 10.1128/JB.00121-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Vries SP, van Hijum SA, Schueler W, Riesbeck K, Hays JP, Hermans PW, Bootsma HJ. 2010. Genome analysis of Moraxella catarrhalis strain BBH18, [corrected] a human respiratory tract pathogen. J. Bacteriol. 192:3574–3583. 10.1128/JB.00121-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davie JJ, Earl J, de Vries SP, Ahmed A, Hu FZ, Bootsma HJ, Stol K, Hermans PW, Wadowsky RM, Ehrlich GD, Hays JP, Campagnari AA. 2011. Comparative analysis and supragenome modeling of twelve Moraxella catarrhalis clinical isolates. BMC Genomics 12:70. 10.1186/1471-2164-12-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Juncker AS, Willenbrock H, Von Heijne G, Brunak S, Nielsen H, Krogh A. 2003. Prediction of lipoprotein signal peptides in Gram-negative bacteria. Protein Sci. 12:1652–1662. 10.1110/ps.0303703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khan MA, Northwood JB, Levy F, Verhaegh SJ, Farrell DJ, Van Belkum A, Hays JP. 2010. bro β-lactamase and antibiotic resistances in a global cross-sectional study of Moraxella catarrhalis from children and adults. J. Antimicrob. Chemother. 65:91–97. 10.1093/jac/dkp401. [DOI] [PubMed] [Google Scholar]
- 38.Wardle JK. 1986. Branhamella catarrhalis as an indirect pathogen. Drugs 31(Suppl 3):S93–S96. [DOI] [PubMed] [Google Scholar]
- 39.Brook I. 1989. The concept of indirect pathogenicity by beta-lactamase production, especially in ear, nose and throat infection. J. Antimicrob. Chemother. 24(Suppl B):63–72. 10.1093/jac/24.suppl_B.63. [DOI] [PubMed] [Google Scholar]
- 40.Hol C, Van Dijke EE, Verduin CM, Verhoef J, van Dijk H. 1994. Experimental evidence for Moraxella-induced penicillin neutralization in pneumococcal pneumonia. J. Infect. Dis. 170:1613–1616. 10.1093/infdis/170.6.1613. [DOI] [PubMed] [Google Scholar]
- 41.Budhani RK, Struthers JK. 1998. Interaction of Streptococcus pneumoniae and Moraxella catarrhalis: investigation of the indirect pathogenic role of beta-lactamase-producing moraxellae by use of a continuous-culture biofilm system. Antimicrob. Agents Chemother. 42:2521–2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pettigrew MM, Gent JF, Revai K, Patel JA, Chonmaitree T. 2008. Microbial interactions during upper respiratory tract infections. Emerg. Infect. Dis. 14:1584–1591. 10.3201/eid1410.080119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Verhaegh SJ, Snippe ML, Levy F, Verbrugh HA, Jaddoe VW, Hofman A, Moll HA, van Belkum A, Hays JP. 2011. Colonization of healthy children by Moraxella catarrhalis is characterized by genotype heterogeneity, virulence gene diversity and co-colonization with Haemophilus influenzae. Microbiology 157:169–178. 10.1099/mic.0.042929-0. [DOI] [PubMed] [Google Scholar]
- 44.Tan TT, Morgelin M, Forsgren A, Riesbeck K. 2007. Haemophilus influenzae survival during complement-mediated attacks is promoted by Moraxella catarrhalis outer membrane vesicles. J. Infect. Dis. 195:1661–1670. 10.1086/517611. [DOI] [PubMed] [Google Scholar]
- 45.Armbruster CE, Hong W, Pang B, Weimer KE, Juneau RA, Turner J, Swords WE. 2010. Indirect pathogenicity of Haemophilus influenzae and Moraxella catarrhalis in polymicrobial otitis media occurs via interspecies quorum signaling. mBio 1(3):e00102-10. 10.1128/mBio.00102-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van der Heide T, Poolman B. 2002. ABC transporters: one, two or four extracytoplasmic substrate-binding sites? EMBO Rep. 3:938–943. 10.1093/embo-reports/kvf201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nowalk AJ, Gilmore RD, Jr, Carroll JA. 2006. Serologic proteome analysis of Borrelia burgdorferi membrane-associated proteins. Infect. Immun. 74:3864–3873. 10.1128/IAI.00189-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tanabe M, Atkins HS, Harland DN, Elvin SJ, Stagg AJ, Mirza O, Titball RW, Byrne B, Brown KA. 2006. The ABC transporter protein OppA provides protection against experimental Yersinia pestis infection. Infect. Immun. 74:3687–3691. 10.1128/IAI.01837-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Murphy TF. 2009. Vaccine development for Moraxella catarrhalis: rationale, approaches and challenges. Expert Rev. Vaccines 8:655–658. 10.1586/erv.09.28. [DOI] [PubMed] [Google Scholar]
- 50.Forsgren A, Brant M, Riesbeck K. 2004. Immunization with the truncated adhesin Moraxella catarrhalis immunoglobulin D-binding protein (MID764-913) is protective against M. catarrhalis in a mouse model of pulmonary clearance. J. Infect. Dis. 190:352–355. 10.1086/422155. [DOI] [PubMed] [Google Scholar]
- 51.Becker PD, Bertot GM, Souss D, Ebensen T, Guzman CA, Grinstein S. 2007. Intranasal vaccination with recombinant outer membrane protein CD and adamantylamide dipeptide as the mucosal adjuvant enhances pulmonary clearance of Moraxella catarrhalis in an experimental murine model. Infect. Immun. 75:1778–1784. 10.1128/IAI.01081-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu DF, McMichael JC, Baker SM. 2007. Moraxella catarrhalis outer membrane protein CD elicits antibodies that inhibit CD binding to human mucin and enhance pulmonary clearance of M. catarrhalis in a mouse model. Infect. Immun. 75:2818–2825. 10.1128/IAI.00074-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.