Abstract
As an immune-privileged site, the eye, and particularly the outer corneal surface, lacks resident mature immune effector cells. Physical barriers and innate mediators are the best-described effectors of immunity in the cornea. When the barriers are breached, infection can result in rapid tissue destruction, leading to loss of visual acuity and frank blindness. To determine the cellular and molecular components needed for effective adaptive immunity on the corneal surface, we investigated which immune system effectors were required for protection against Staphylococcus aureus corneal infections in mice, which are a serious cause of human eye infections. Both systemically injected and topically applied antibodies to the conserved cell surface polysaccharide poly-N-acetylglucosamine (PNAG) were effective at mediating reductions in corneal pathology and bacterial levels. Additional host factors impacting protection included intercellular adhesion molecule 1 (ICAM-1)-dependent polymorphonuclear leukocyte (PMN) recruitment, functional CD4+ T cells, signaling via the interleukin-17 (IL-17) receptor, and IL-22 production. In germfree mice, there was no protective efficacy of antibody to PNAG due to the lack of LY6G+ inflammatory cell coeffector recruitment to the cornea. Protection was manifest after 3 weeks of exposure to conventional mice and acquisition of a resident microbiota. We conclude that in the anterior eye, ICAM-1-mediated PMN recruitment to the infected cornea along with endogenous microbiota-matured CD4+ T cells producing both IL-17 and IL-22 is required for antibody to PNAG to protect against S. aureus infection.
INTRODUCTION
Infections are responsible for a large proportion of blindness worldwide. Major problems are seen in economically underdeveloped parts of the world where diseases such as ocular trachoma (1) and onchocerciasis (river blindness) (2) are prevalent. Additionally, in countries with emerging economies, trauma-associated agricultural work is often a predisposing factor for eye infections causing serious compromises of vision (3). Even in economically developed countries, the use of contact lenses (4) or ocular surgery (5) to correct vision problems is a predisposing factor for infections and loss of visual acuity. The outermost layer of the eye, the avascular cornea, primarily functions in transmitting and refracting light to allow the retina to perceive and form the images of sight. The cornea is made up of ordered 30-nm collagen fibrils separated by 60 nm to keep light from scattering. Apart from the corneal epithelium, there are few resident cells in the cornea, particularly mature immune cells, making it challenging to provide rapid and adequate protection against infection using the cellular and humoral mediators of innate and acquired immunity.
Rapid responses to infection are essential to avoid inflammatory damage to the cornea, which can result in scarring and loss of vision due to a diminished capacity to transmit and refract light (6). Understanding the basis for proper activation of innate and acquired immunity in this tissue is critical in order to design and test immunotherapeutic interventions such as immunomodulators, vaccines, antibodies, and cellular effectors that maximize clearance of infectious agents while minimizing inflammatory damage. In this context, the lack of mature resident immune cells in the cornea poses the question as to what role extracorneal cells play in mediating acquired immunity and how this is impacted by our current understanding of immune cell maturation driven by the normal microbial constituents of a mammalian host.
To investigate this issue, we used a mouse model of corneal keratitis caused by Staphylococcus aureus, a well-documented etiology of community-acquired and nosocomial infections (7) and a leading cause of infectious keratitis (8, 9). Local and systemic effects on immunity and the need for microbiome-matured cellular cofactors in the cornea were investigated to define the mechanisms by which antibody to the conserved β-1-6-linked poly-N-acetylglucosamine (PNAG) surface polysaccharide synthesized by most S. aureus strains (10), as well as many other microbial pathogens that can be causes of eye infections (11), is able to clear bacterial cells and prevent corneal scarring. While both polyclonal antibody and a human monoclonal antibody (MAb) to PNAG were highly effective in ameliorating the consequences of S. aureus ulcerative keratitis, the therapeutic efficacy of the MAb was negated if mice were unable to recruit polymorphonuclear leukocytes (PMNs) to the cornea or were deficient in CD4+ T cells, interleukin-22 (IL-22) production, or IL-17 receptors (IL-17Rs). Importantly, there was no antibody-mediated protective immunity to ocular infection in germfree mice due to lack of recruitment of LY6+ inflammatory cells, but protection was induced after 3 weeks of exposure of young germfree mice to a normal mouse microbiota. Overall, microbiome-matured immune cell function appears essential for antibody-mediated resistance of the eye to infection.
MATERIALS AND METHODS
Bacterial strains.
S. aureus strains NCTC 10833, 15981, Newman, and MN8 and isogenic Δica mutants were obtained or produced as described previously (12), as was a chromosomally complemented variant of the initial Δica 10833 strain (13). S. aureus strain LAC (a USA300 methicillin-resistant S. aureus [MRSA] strain) and its isogenic ica-deficient mutant were obtained from the Network on Antimicrobial Resistance in S. aureus (NARSA). S. aureus strains were grown overnight on Trypticase soy agar (TSA) and then inoculated into either Trypticase soy broth (TSB) plus 1% glucose or TSB plus 2% NaCl to induce PNAG synthesis, grown to late log/early stationary phase, and used to prepare an inoculum in phosphate-buffered saline (PBS) for infecting eyes. Inocula were ∼1 × 107 CFU/eye.
Mice and mouse manipulations.
A/J and C57BL/6 mice 6 to 8 weeks old were purchased from Jackson Laboratories or Taconic Farms. A/J mice were used to establish basic parameters of the infection system as they have enhanced susceptibility to S. aureus infections (14), whereas C57BL/6 mice were used as controls for transgenic strains backcrossed into this genetic background. Germfree C57BL/6 mice were obtained from the Harvard Digestive Diseases Center Germ Free and Gnotobiotic Microbiology Core. Germfree mice were removed from the barrier for the 48-h period of the corneal infection experiment and kept in sterile microisolator cages with filter tops and sterile bedding, food, and water until sacrifice. Colons were analyzed for the acquisition of Gram-negative bacterial fecal organisms after euthanasia as an indicator of environmental acquisition of gastrointestinal (GI) organisms. Conventionalization of germfree mice was achieved by housing 3- to 4-week-old initially germfree mice with specific-pathogen-free C57BL/6 mice for 3 weeks prior to use in animal infection experiments. IL-17 receptor knockout (IL-17R KO) mice were provided by Amgen. Intercellular adhesion molecule 1 (ICAM-1) KO mice (full gene deletion) were provided by Daniel Bullard of the University of Alabama at Birmingham (15). Mice deficient in recombinase activating gene 1 (RAG1), ICAM-1, IL-22, or the IL-17 receptor were bred using homozygous gene KO pairs in our animal facility. Depletion of T cells used 500-μg/mouse intraperitoneal (i.p.) injections of rat antibodies to mouse CD4, clone GK1.5 (BioXCell), or mouse CD8 (obtained from Brigham and Women's Hospital, Brigham Research Institute Antibody Core Facility). Controls received normal rat IgG. Confirmation of T cell depletion was determined by taking spleens from mice after euthanasia, recovering single cells, and staining them for analysis by fluorescence-activated cell sorting (FACS) with the depleting or control MAbs and secondary antibody to rat IgG conjugated to AF488.
Antibodies to PNAG.
Polyclonal antibody to PNAG was raised in rabbits using a synthetic oligosaccharide of polyglucosamine, 9GlcNH2, conjugated to the carrier protein tetanus toxoid (9GlcNH2-TT) (16). Additionally, an extensively characterized fully human IgG1 MAb to PNAG, MAb F598, was used (17). Controls received normal rabbit serum (NRS) or human IgG1 MAb F429 specific to Pseudomonas aeruginosa alginate (18), respectively.
Animal model of corneal infection.
All animal studies were approved by the Harvard Medical Area Institutional Animal Care and Use Committee and adhered to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Scratch-injured eyes of anesthetized mice of various backgrounds, 6 to 8 weeks old, were challenged in vivo with wild-type (WT) or Δica S. aureus strains using the method described for inducing Pseudomonas aeruginosa infections (19). For evaluation of antibody-mediated prophylactic protection preceding infection, 200 μl of rabbit antibody to 9GlcNH2-TT or control NRS was injected i.p. 24 and 4 h prior to infection of the eyes. For evaluation of the therapeutic efficacy of antibody, 200 μl of antibody to 9GlcNH2-TT or NRS was injected i.p. 8, 24, and 32 h after initiation of infection. For evaluation of the therapeutic efficacy of the human MAbs, 200 μg of MAb F598 to PNAG or 200 μg of an irrelevant human IgG MAb was injected i.p. 4, 24, and 32 h after initiation of infection. For evaluation of the therapeutic efficacy of topical application of MAb F598 to PNAG, 10 μg of MAb or control human IgG was applied to the infected eyes in a 5-μl volume 4, 24, and 32 h postinfection. For all experiments, bacterial levels and corneal pathology were determined at 48 h postinfection. Each experiment depicted in the Results is indicative of a single experiment with replication and reproducibility shown by using multiple strains of S. aureus for each experimental condition.
Corneal pathology was recorded using a scale of 0 to 4: 0, eye macroscopically identical to the uninfected contralateral control eye; 1, faint opacity partially covering the pupil; 2, dense opacity covering the pupil; 3, dense opacity covering the entire anterior segment; 4, perforation of the cornea, phthisis bulbi (shrinkage of the globe after inflammatory disease). The eyes were evaluated independently by two trained individuals with no knowledge at the time of evaluation of either the S. aureus strain or the treatment given to the mice. When the scores were discrepant between the evaluators (<3% of evaluations), the final score was the lower, more conservative value.
Immunohistochemistry.
Sections of infected corneas were prepared and stained with hematoxylin and eosin (H&E) by the Harvard Rodent Pathology Core. Additional sections were deparaffinized using EZDewax, hydrated in water, and blocked with 1% bovine serum albumin (BSA)-PBS for 4 h. Several different antibodies were used for immunohistochemistry, including anti-human IgG directly conjugated to AF488 (green); goat anti-mouse C3 polyclonal antibody (Gene Tex GT X77263), followed by a donkey anti-goat IgG antibody conjugated to AF568 (red); and a rat anti-mouse Ly6G antibody to mouse PMNs directly conjugated to AF647 (blue). Sections were stained by application of the antibodies for 4 h at room temperature followed by washing with PBS and visualization by confocal scanning laser microcopy as described previously (11).
Statistical analysis.
For pairwise comparisons of bacterial tissue levels, CFU/cornea were log-transformed for analysis by t tests. For nonparametric and noncontinuous data, the Mann-Whitney U test was used. For multiple comparisons using t tests, Bonferroni's correction to the P value was used. One-sided significance (P) values were determined for comparisons between mice receiving control and those receiving PNAG-specific antibody, since, based on prior findings and sensible expectations, our null hypothesis before experimentation was that the PNAG-specific antisera and MAbs would not lead to higher bacterial levels and corneal pathology. Additionally, if animals receiving the immune sera or MAbs had higher corneal pathology or tissue burdens, we would have attributed that difference to chance and called the difference “not statistically significant.”
For multigroup analysis of some nonparametric data, the Kruskal-Wallis analysis of variance (ANOVA) followed by Dunn's procedure for pairwise comparisons was performed. Multigroup comparisons were two-sided. The PRISM statistical software (GraphPad) was used. P values of <0.05 were considered significant.
RESULTS
Each experimental result is indicative of a single experiment with replication and reproducibility shown by using two or more strains of S. aureus for most experimental conditions or having control groups of WT mice treated with control or PNAG-specific MAbs in most of the experiments investigating the roles of different host factors in mediating adaptive immunity to S. aureus.
Prophylactic and therapeutic efficacy of polyclonal antibody to PNAG in murine ulcerative keratitis.
To initially establish the efficacy of antibody-mediated protection from S. aureus corneal infection in scratch-injured mouse eyes, we i.p. injected either NRS or polyclonal antibody to PNAG, representing the type of immune response to be engendered in actively immunized individuals, 24 and 4 h prior to infection with S. aureus strain 10833, 15981, or LAC. After 48 h, the time of maximal pathology and greatest corneal bacterial burdens, we compared the pathology scores and bacterial levels in the corneal tissues. Intraperitoneal injection of antibody to PNAG reduced the corneal pathology (see Fig. S1A in the supplemental material) as well as the number of CFU/cornea (see Fig. S1B) in scratch-injured mouse eyes for all three S. aureus isolates. Similarly, therapeutic i.p. administration of antibodies to PNAG 8, 24, and 32 h postinfection with S. aureus reduced the consequences of staphylococcal keratitis by diminishing the corneal pathology and bacterial levels in the corneal tissues (see Fig. S1C and D).
Therapeutic efficacy of monoclonal antibody to PNAG in Staphylococcus aureus ulcerative keratitis.
A fully human IgG1 MAb to PNAG has been developed (17) and undergone phase I safety and pharmacokinetic analysis in healthy humans with no significant adverse events noted (20). Following intravenous (i.v.) infusion into humans, the anti-PNAG MAb exhibited a long serum half-life of 655 h (∼27 days), a low systemic clearance of 0.0716 ml/h/kg, a low volume of distribution of 62.0 ml/kg, and a high level of functional, opsonic antibody maintained out to 50 days post-i.v. injection (20). Such a product is a strong candidate for i.v. administration to humans with suspected or diagnosed keratitis caused by PNAG-producing organisms, particularly if MAb administration, due to a long half-life, could reduce the frequent administration of antibiotics needed to treat microbial infections (21). We thus evaluated the therapeutic efficacy of the MAb to PNAG in the scratch-injured corneal infection model by injecting it i.p. 8, 24, and 32 h after S. aureus infection. We found that the i.p.-injected anti-PNAG MAb reduced pathology and bacterial counts in the corneal tissues for three S. aureus strains compared with the control MAb (Fig. 1A and B), indicating that systemic administration can deliver sufficient MAb to the cornea to promote bacterial killing and limit inflammatory damage.
FIG 1.
Effect of systemic (i.p.) or topical therapeutic administration of MAb to PNAG on corneal disease and infection with S. aureus in A/J mice. At 8, 24, and 32 h postinfection, 200 μg of control IgG MAb (◻) or MAb to PNAG (△) in 200 μl was injected i.p. (A and B), or 10 μg in 5 μl was applied topically 4, 24, and 32 h postinfection (C and D), and pathology scores (A and C) and numbers of CFU/cornea (B and D) were determined 48 h postinfection. Symbols represent individual animals; bars represent the medians. P values were determined by nonparametric t tests.
Another approach in using the MAb for therapeutic efficacy is to apply small amounts topically to the eye. We thus evaluated the efficacy of applying 5 μl containing 10 μg of the MAb to PNAG or control IgG1 at 4, 24, and 32 h postinfection and found that this treatment significantly lowered the pathology scores and the number of CFU in the corneas of mice infected with four different S. aureus strains (Fig. 1C and D).
Specificity of the protective effect of the MAb to PNAG against S. aureus corneal infection.
Loss of PNAG synthesis by deletion of the ica locus encoding the PNAG biosynthetic proteins in strain 10833 resulted in reduced corneal pathology and bacterial burdens compared to those induced by the same strain producing PNAG after restoration of an intact chromosomal ica locus (see Fig. S2 in the supplemental material). We found for additional Δica strains that there was sufficient residual infection to allow evaluation of the specificity of the protection by the MAb to PNAG using additional PNAG-negative isogenic strains of S. aureus. Comparing the effects of the MAb to PNAG on corneal infections caused by the WT and those caused by Δica mutants of S. aureus strains MN8, Newman, and LAC by topically administering the MAb 4, 24, and 32 h post-corneal infection resulted in the expected reductions in pathology and bacterial burdens in mice challenged with the WT strains but no protective effect against any of the Δica mutants (see Fig. S3 in the supplemental material). Of note, comparing the pathology and bacterial burdens between the wild type and Δica mutants in the two mouse groups given the control IgG1 (essentially nonimmune) also showed reduced virulence of these PNAG-deficient strains, comparable to the results shown in Fig. S2 in the supplemental material with strain 10833.
Need for PMN emigration into the cornea for protective efficacy.
In vitro, S. aureus and other PNAG-producing organisms are killed by the MAb to PNAG in a PMN-dependent opsonization assay (11, 17). To ascertain if this immune effector was also manifest in vivo, we tested the protective efficacy of topical, therapeutic administration of the MAb to PNAG in WT and ICAM-1-deficient C57BL/6 mice (22). Lack of ICAM-1 led to loss of the protective efficacy of the MAb against S. aureus strains 15981 and LAC (Fig. 2), indicative of a need for PMN as a cofactor for MAb-mediated protection. Of note, the ICAM-1-deficient mice given either the control MAb or MAb to PNAG had significantly higher bacterial burdens than did the WT mice given the control MAb, indicative of a key role of ICAM-1-dependent PMN recruitment in innate immune resistance to S. aureus keratitis, but there was no significant difference in the corneal pathologies between these two groups. This outcome is likely reflective of the role of PMN influx in the inflammatory damage to the cornea mediated by infection, wherein the lack of ICAM-1 and limitation of PMN influx result in lower corneal pathology in spite of higher bacterial levels (23–25). While this result suggests that limiting pathology by inhibiting PMN influx might be beneficial, the increased bacterial burdens and likelihood of dissemination to extraocular tissues (25) in the face of PMN deficiencies likely preclude such an approach. An analogous situation was found in humans, wherein inhibition of neutrophil influx into the lungs of cystic fibrosis (CF) patients infected with P. aeruginosa by an experimental anti-inflammatory drug, BIIL 284 (26), resulted in early termination of the clinical trial due to worse outcomes in treated patients (27).
FIG 2.
Effect of loss of ICAM-1 on efficacy of topical, therapeutic administration of MAb to PNAG. At 4, 24, and 32 h postinfection with S. aureus strain 15981 (A and B) or LAC (C and D), 10 μg of control IgG MAb (◻) or MAb to PNAG (△) in 5 μl was applied to eyes of wild-type (WT) C57BL/6 or ICAM-1 knockout (KO) mice, and pathology scores (A and C) and numbers of CFU/cornea (B and D) were determined 48 h postinfection. Symbols represent individual animals; bars represent the medians. P values were determined by t tests for the effect of the MAb to PNAG in WT mice and by ANOVA followed by Sidak's multiple-comparison test, which yielded the indicated P value comparing WT mice given control MAb to both groups of ICAM-1 KO mice. Overall ANOVA P values for all pairwise comparisons of significance were ≤0.005. NS, not significant.
Requirement for innate T cell factors in immunity to S. aureus corneal infections.
It has become increasingly apparent that endogenous innate immune factors can also be important components for efficacy of acquired immune effectors such as antibodies (28, 29), notably as cofactors in vaccine-induced immunity in the lung (30–32). We therefore evaluated the role of T cells and the IL-17/IL-22 pathways in antibody-mediated reductions in corneal pathology and bacterial levels in S. aureus-infected eyes.
When the MAb to PNAG was applied topically to the eyes of RAG1 KO mice, deficient in all functional T cells, there was no reduction in pathology score or bacterial burdens for either S. aureus strain 15981 or S. aureus strain 15981 LAC compared to mice given the control IgG1 MAb, whereas WT C57BL/6 mice had the expected protective effect from MAb treatment (Fig. 3A to D). Depletion of CD4+ T cells reduced the levels of these cells in the spleen from 20.5% ± 2.0% (standard deviation [SD]) to 0.05 ± 0.01% (SD) and also removed the ability of the MAb to PNAG to reduce corneal pathology or bacterial burdens (Fig. 3E to H). Depletion of CD8+ T cells reduced their levels in the spleen from 9.7% ± 2% (SD) to 0.6% ± 0.3% (SD), but these mice were still able to decrease levels of S. aureus LAC in the cornea as well as the pathological consequences when treated with the MAb to PNAG (see Fig. S4 in the supplemental material). Notably, when comparing pathology scores and bacterial burdens between mice given the control MAb (essentially nonimmune) and with intact T cell function and RAG1 KO or CD4-depleted animals, we saw a difference only in the bacterial levels in RAG1 KO mice infected with S. aureus LAC (Fig. 3D), suggesting that T cell deficiency does not routinely enhance susceptibility to or consequences of S. aureus eye infections in nonimmune animals.
FIG 3.
Effect of loss of all T cells and CD4+ T cells on efficacy of topical, therapeutic administration of MAb to PNAG. (A to D) At 4, 24, and 32 h postinfection with S. aureus strain 15981 (A and B) or LAC (C and D), 10 μg of control IgG MAb (◻) or MAb to PNAG (△) in 5 μl was applied to eyes of WT C57BL/6 or RAG1 KO mice, and pathology scores (A and C) and numbers of CFU/cornea (B and D) were determined 48 h postinfection. (E to H) Twenty-four hours prior to infection with S. aureus strain 15981 (E and F) or LAC (G and H), A/J mice were injected i.p. with 500 μg of either control IgG or rat-anti mouse CD4 (x axis labels). At 4, 24, and 32 h postinfection, 10 μg of control IgG MAb (◻) or MAb to PNAG (△) in 5 μl was applied to eyes of WT C57BL/6 mice, and pathology scores (E and G) and numbers of CFU/cornea (F and H) were determined 48 h postinfection. Symbols represent individual animals; bars represent the medians. (A to D) P values were determined by t tests. (E to H) P values were determined by t tests with Bonferroni's correction for multiple comparisons.
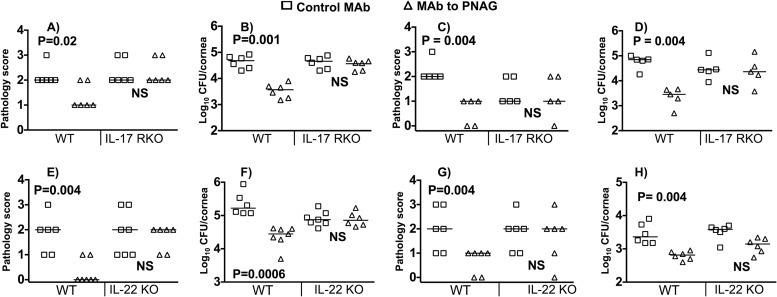
IL-17, a cytokine primarily produced by Th17 cells and more recently shown to be produced by PMNs in the eye during Aspergillus infection (33), is known for mediating innate inflammatory responses in ocular tissues during both experimental mouse and natural human infections with viral, bacterial, and fungal pathogens (33–35). However, these studies did not address the role of IL-17 in facilitating the efficacy of adaptive immunity, where it can have a variable effect depending on the organism, immune effector, and tissue being infected. Therefore, we evaluated the impact of loss of signaling mediated by the IL-17 receptor on the protective effect of the MAb to PNAG in S. aureus keratitis. In IL-17R KO mice (Fig. 4A to D), the MAb lost its ability to protect against S. aureus keratitis caused by either strain 15981 (Fig. 4A and B) or strain LAC (Fig. 4C and D). Of note, the differences in corneal pathology scores between WT and IL-17R KO mice given control MAb were not significantly different, suggesting that for S. aureus, unlike fungal and viral pathogens, loss of responsiveness to IL-17 does not compromise ocular innate immunity (33–35).
FIG 4.
Effect of loss of IL-17 and IL-22 responsiveness on efficacy of topical, therapeutic administration of MAb to PNAG. At 4, 24, and 32 h postinfection with S. aureus strain 15981 (A, B, E, and F) or LAC (C, D, G, and H), 10 μg of control IgG MAb (◻) or MAb to PNAG (△) in 5 μl was applied to eyes of WT C57BL/6, IL-17 receptor knockout (RKO), or IL-22 KO mice, and pathology scores (A, C, E, and G) and numbers of CFU/cornea (B, D, F, and H) were determined 48 h postinfection. Symbols represent individual animals; bars represent the medians. P values at the top of each figure were determined by nonparametric t tests.
The cytokine IL-22 is a member of the IL-10 family of immune mediators that is produced by a multitude of T cells but has effects primarily upon tissue-specific cells such as epithelial cells and hepatocytes (36). It has been reported to play a role in corneal healing after an abrasion (37) and P. aeruginosa keratitis (38), but its role in facilitating antibody-mediated immunity in the anterior eye has not been explored. We found that IL-22 KO mice could not utilize the anti-PNAG MAb to promote S. aureus clearance from the abraded cornea (Fig. 4E to H). However, IL-22 KO mice treated with control MAb did not appear to be any more susceptible to S. aureus infection than were WT mice given control MAb, indicating that the loss of IL-22 does not impact innate immunity to this pathogen.
Effect of microbiome on antibody-mediated immunity in the eye.
Germfree mice are well known to lack fully mature systemic immune systems (39), so we tested the effect of MAb-mediated protection in these animals. Germfree C57BL/6 mice given the MAb to PNAG could not reduce bacterial levels or corneal pathology scores in comparison to mice given the control MAb when challenged with either S. aureus LAC or S. aureus 15981 (Fig. 5A to D). Germfree mice exposed to conventional mice for 3 weeks gained the ability to clear S. aureus when provided with the MAb to PNAG (Fig. 5E to H).
FIG 5.
Loss of protective efficacy in germfree mice that is restored following acquisition of normal microbial flora. Topical administration of MAb to PNAG 4, 24, and 32 h postinfection with S. aureus 15981 (A and B) or LAC (C and D) failed to reduce corneal pathology scores (A and C) or bacterial burdens (B and D) in germfree C57BL/6 mice. Conversion of germfree mice to normal microbial flora via 3 weeks of cohousing with WT C57BL/6 mice induced protective efficacy of topically applied MAb to PNAG after challenge with S. aureus 15981 (E and F) or LAC (G and H), comparable to that seen in the WT mice used in the cohousing groups. Symbols represent individual animals; bars represent the medians. P values were determined by nonparametric t tests.
Histologic analysis of murine corneas during S. aureus infection.
Analysis of the histology of corneas in uninfected and MAb-treated germfree and conventional wild-type mice infected with S. aureus LAC showed a normal corneal appearance in uninfected animals as visualized by H&E staining (Fig. 6A). In conventional, wild-type mice treated with control MAb, there was a pronounced inflammatory infiltrate that was maximal at 48 h (Fig. 6B), whereas S. aureus-infected WT mice treated with the anti-PNAG MAb had only a small presence of inflammatory cells at all time points, as visualized by H&E staining (Fig. 6C). These results are a further indication of the protective nature of the MAb. A large proportion of the inflammatory cells in control mice stained positive for the LY6 marker on PMNs (Fig. 6D). In contrast, germfree mice given either control MAb or anti-PNAG MAb had little inflammatory cell infiltration visualized by H&E staining (Fig. 6E and F), indicating that in the absence of a microbiome-matured immune system, these mice were unable to recruit the inflammatory cells needed for control of S. aureus infection by MAb to PNAG. There were virtually no LY6+ cells detectable in the corneas of S. aureus-infected germfree mice regardless of the MAb administered. However, in germfree mice given the MAb to PNAG, there was detectable human IgG associated with deposition of C3 onto bacterial cells (Fig. 6F) but only minimal detection of control MAb or C3 binding in mice immunized with this control (Fig. 6E). Thus, in spite of effective antibody binding and C3 deposition on infecting microbial cells, microbiome-mediated immune cell maturation is essential for effective antibody-mediated host resistance to corneal infection.
FIG 6.
Histological appearance, inflammatory cell infiltration, and binding of C3 and anti-PNAG MAb to S. aureus cells in corneas of wild-type and germfree mice. (A to C) Uninfected corneas (A) appeared comparable between WT and germfree mice, indicating no major effect from lack of a microbiota on the basic appearance of murine corneal tissues. Wild-type mice (B) given the control MAb and infected with S. aureus strain LAC showed a heavy corneal inflammatory cell at 48 h postinfection, whereas wild-type mice given the anti-PNAG MAb (C) had much lower levels of inflammatory cells 48 h postinfection. (D) Expression of LY6 antigen on inflammatory cells in eyes of control MAb or anti-PNAG-treated mice. (E and F) In contrast, infected germfree mice treated with either control MAb (E) or anti-PNAG MAb (F) showed little evidence of inflammatory cell infiltration at 48 h (anti-LY6), indicating that the cellular components of immunity needed to clear S. aureus cells were not recruited to the corneas of these mice. In germfree mice treated with the control MAb, low levels of C3 deposition (red) or of binding of the control MAb to bacterial cells (green) were seen, whereas in germfree mice treated with the anti-PNAG MAb, clear evidence of antibody bound to infecting S. aureus cells (green) and C3 deposition (red) was seen. Bars, 10 μm.
DISCUSSION
Understanding the immune factors needed to protect the eye against infections, which are a leading cause of blindness in the world, is critical for development of vaccines and immunotherapies. The experimental outcomes reported here indicate that immunity to S. aureus corneal keratitis mediated by a human MAb to PNAG, an antigenic target present on many bacterial and fungal organisms causing blindness (11), requires endogenous cytokine production, microbiome-matured CD4+ T cells, and effective PMN recruitment to the eye. Critically important, PNAG-specific immunity was manifest without obvious residual inflammatory damage that could cloud the cornea or otherwise compromise vision, since a main concern of immunotherapies for eye diseases is that the inflammation needed to promote pathogen clearance will still cause significant tissue damage and thus be counterproductive.
The experimental results highlight important contributions as well as differences in various host immunologic factors that are part of both innate and adaptive immunity in the immunologically privileged eye. PMN recruitment via ICAM-1 and innate immune effectors activated by IL-17 and IL-22 were key contributors to the efficacy of PNAG-specific antibody-mediated immunity to S. aureus. However, only ICAM-1 deficiency was associated with decreased clearance of S. aureus from the cornea compared to that in WT mice given control MAb. Studies analyzing the role of IL-17 in innate immunity to other eye pathogens generally show a critical role for this cytokine (33–35). Of interest, in the eye, PMNs can also be sources of IL-17, at least in the presence of infection by the fungal pathogen Aspergillus (33), where it acts in an autocrine fashion to promote fungal killing via induction of reactive oxygen species.
The lack of requirement for IL-17 in innate immunity to S. aureus ocular infection is similar to what was previously found with P. aeruginosa lung infection, where loss of IL-17 did not impact innate immune clearance in nonimmune animals but IL-17 was needed for protection from infection after active vaccination (30). The findings here with S. aureus are, however, distinct from those with P. aeruginosa corneal infection, where loss of IL-17 activity actually reduced the consequences of P. aeruginosa corneal infection in nonimmune mice (40). For further perspective, IL-17 production by γδ T cells has been shown to be important for resistance to S. aureus surgical-site infections (41, 42), but neutralization of IL-17A in BALB/c mice did not affect the response to a primary S. aureus dermonecrotic skin infection (43). In these studies by Montgomery et al. (43), immunity to a second S. aureus skin infection in BALB/c mice, as manifested by reduced lesion sizes, could be mediated by antibody transferred from BALB/c mice after primary infection. However, a contribution of IL-17-dependent T cell immunity was also found in that transfer of immune T cells from BALB/c mice following a primary infection to B-cell-deficient μMt BALB/c mice also resulted in reduced lesion size, which was lost after treatment with antibody to IL-17A. Of note, C57BL/6 mice did not react to a primary S. aureus skin infection with a response that reduced lesion size following secondary infection (43). Here, we found that antibody to PNAG protected both A/J and C57BL/6 mice from S. aureus corneal infections. The basis for the different requirements for IL-17 in innate immunity between S. aureus and other pathogens or in different settings of S. aureus infection is unclear, but the findings presented here highlight how reliance on host cellular immune effectors can be highly variable depending on the pathogen or site of infection.
Both IL-17 and IL-22 mediate a panoply of responses from cells expressing receptors for these cytokines, and a major source of both of these cytokines is the lymphoid-tissue inducer-like cells with the surface marker phenotype CD4+ CD3− NK1.1− CD11b− Gr1− CD11c− B220− (44). Notably, these cells are present in RAG KO mice and produce IL-17 and IL-22 in response to zymosan injection (44), but the lack of effectiveness of antibody in the RAG KO mice indicates that the cytokines are not sufficient by themselves to mediate antibody-based protective immunity in the eye. Additionally, we have previously shown that IL-17R KO mice are also deficient in ICAM-1, which we previously associated with the improved outcome from P. aeruginosa keratitis due to decreased corneal pathology without impact on bacterial clearance (40). However, in order for antibody-mediated immunity to be effective against bacterial pathogens such as P. aeruginosa and S. aureus, PMN-mediated opsonic killing is required (45, 46). Overall, there is a clear interplay between factors such as IL-17 and IL-22 that modulate PMN recruitment into the cornea and also impact CD4+ T cells to effectively coordinate adaptive immunity in this immune-privileged site.
The lack of protective efficacy of the MAb to PNAG in RAG KO and CD4-depleted mice demonstrated the need for mature CD4+ T cells along with cytokines produced by these cells for effective antibody-mediated clearance of S. aureus from the eye. This was also true for resistance to a secondary S. aureus skin infection following a primary infection to induce immunity (43). It is not clear if the T cells entering the ocular tissues were activated by a specific, T cell receptor-mediated recognition of staphylococcal antigens acquired by natural exposure to microbes or were providing additional components of innate immunity independent of antigen recognition needed for the MAb to PNAG to provide protective immunity to S. aureus keratitis.
Germfree mice were unable to recruit LY6+ inflammatory cells to the infected cornea, rendering the MAb therapy ineffective, as it was in ICAM-1 KO mice. Studies have indicated a potential resident ocular microbial flora in the conjunctiva and eyelid (47), but whether these are long-term colonizers or are transiently present by continual contamination from the skin followed by clearance is not established. The healthy corneal ocular surface itself does not appear to have any resident microbial cells. Nonetheless, it is becoming increasingly clear that microbiome-matured immune effectors are critical for orchestrating effective acquired immunity to many pathogens in different tissues. The lack of antibody-mediated protective efficacy in germfree mice suggests that there is likely an S. aureus-specific natural T cell response needed for ocular immunity. But, as the microbiota are also needed for full immune system maturation (48, 49), we cannot exclude a possible non-antigen-specific T cell response as a contributing factor to antibody-mediated ocular immunity. Indeed, discriminating between these two mechanisms of immune activation in a germfree mouse system is very difficult, as the natural microbiota drives both innate and adaptive T cell immune maturation. Notably, the results here are comparable to those of Joshi et al. (50), who immunized severe combined immunodeficient (SCID) mice with antibody to the staphylococcal iron surface determinant B (IsdB) and found no protection against lethal S. aureus challenge, whereas WT mice were highly protected. Protection was not obtained in IL-23-deficient mice and was associated with IL-17 production. While an IsdB vaccine showed no efficacy in a large vaccine trial in humans undergoing cardiothoracic surgery (51), the animal results did validate the need for complex innate and adaptive components of the immune system to work along with antibody to clear bacterial infections.
The findings reported here on effective immunity to S. aureus in the eye could have broad-based implications for human health and prevention of blindness, inasmuch as PNAG production is commonly detected among many pathogens that cause eye infections (11), supporting clinical testing of active or passive immunotherapy for prophylactic or therapeutic interventions against many serious ocular infections. The human IgG1 MAb to PNAG has undergone successful phase I safety and pharmacokinetic analysis in healthy volunteers (20), and vaccine production is in progress for human testing. However, if and when evaluations of efficacy against ocular pathogens, or pathogens causing infections in any tissue, are undertaken, it might be important to establish that natural immunity in humans engenders the needed cofactors for vaccine-induced or passively administered antibodies to be effective in pathogen clearance with minimal tissue pathology. Traditional antibody-based vaccine approaches to surface antigens have focused mostly on showing an effector function such as opsonic or bactericidal killing mediated by the antibodies, which, while critical, may not be assessing the entire landscape of factors needed for in vivo immunity. Incorporating studies of cellular and cytokine responses might add to the predictive efficacy of preclinical analysis of vaccine candidates and also point to a need to evaluate these responses in humans during clinical development of immunotherapies in order to maximize the chances for success of new anti-infective vaccines and antibodies.
Supplementary Material
ACKNOWLEDGMENTS
We thank the Network on Antibiotic Resistance in S. aureus (NARSA) for providing staphylococcal strain LAC through the NARSA program, supported under National Institute of Allergy and Infectious Diseases/National Institutes of Health (NIH) contract HHSN272200700055C. We thank the Brigham and Women's Hospital, Brigham Research Institute antibody core facility, for provision of antibody to CD8.
This work was supported by National Heart, Lung and Blood Institute grant R01 HL092515 (to G.P.P.) and National Eye Institute grant R01 EY016144 (to G.B.P.). Germfree mice were provided by the Center for Clinical and Translational Metagenomics at Brigham & Women's Hospital, with support from the Harvard Digestive Diseases Center (grant P30 DK034854).
Footnotes
Published ahead of print 9 June 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.01951-14.
REFERENCES
- 1.Hu VH, Holland MJ, Burton MJ. 2013. Trachoma: protective and pathogenic ocular immune responses to Chlamydia trachomatis. PLoS Negl. Trop. Dis. 7:e2020. 10.1371/journal.pntd.0002020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Babayan SA, Allen JE, Taylor DW. 2012. Future prospects and challenges of vaccines against filariasis. Parasite Immunol. 34:243–253. 10.1111/j.1365-3024.2011.01350.x. [DOI] [PubMed] [Google Scholar]
- 3.Nadig S, Velusamy N, Lalitha P, Kar S, Sharma S, Arakere G. 2012. Staphylococcus aureus eye infections in two Indian hospitals: emergence of ST772 as a major clone. Clin. Ophthalmol. 6:165–173. 10.2147/OPTH.S23878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stapleton F, Keay L, Edwards K, Holden B. 2013. The epidemiology of microbial keratitis with silicone hydrogel contact lenses. Eye Contact Lens 39:79–85. 10.1097/ICL.0b013e3182713919. [DOI] [PubMed] [Google Scholar]
- 5.Vajpayee RB, Sharma N, Sinha R, Agarwal T, Singhvi A. 2007. Infectious keratitis following keratoplasty. Surv. Ophthalmol. 52:1–12. 10.1016/j.survophthal.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 6.Tewari A, Sood N, Vegad MM, Mehta DC. 2012. Epidemiological and microbiological profile of infective keratitis in Ahmedabad. Indian J. Ophthalmol. 60:267–272. 10.4103/0301-4738.98702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hsueh PR, Teng LJ, Chen WH, Pan HJ, Chen ML, Chang SC, Luh KT, Lin FY. 2004. Increasing prevalence of methicillin-resistant Staphylococcus aureus causing nosocomial infections at a university hospital in Taiwan from 1986 to 2001. Antimicrob. Agents Chemother. 48:1361–1364. 10.1128/AAC.48.4.1361-1364.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alexandrakis G, Alfonso EC, Miller D. 2000. Shifting trends in bacterial keratitis in south Florida and emerging resistance to fluoroquinolones. Ophthalmology 107:1497–1502. 10.1016/S0161-6420(00)00179-2. [DOI] [PubMed] [Google Scholar]
- 9.Bourcier T, Thomas F, Borderie V, Chaumeil C, Laroche L. 2003. Bacterial keratitis: predisposing factors, clinical and microbiological review of 300 cases. Br. J. Ophthalmol. 87:834–838. 10.1136/bjo.87.7.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McKenney D, Pouliot KL, Wang Y, Murthy V, Ulrich M, Doring G, Lee JC, Goldmann DA, Pier GB. 1999. Broadly protective vaccine for Staphylococcus aureus based on an in vivo-expressed antigen. Science 284:1523–1527. 10.1126/science.284.5419.1523. [DOI] [PubMed] [Google Scholar]
- 11.Cywes-Bentley C, Skurnik D, Zaidi T, Roux D, Deoliveira RB, Garrett WS, Lu X, O'Malley J, Kinzel K, Zaidi T, Rey A, Perrin C, Fichorova RN, Kayatani AK, Maira-Litran T, Gening ML, Tsvetkov YE, Nifantiev NE, Bakaletz LO, Pelton SI, Golenbock DT, Pier GB. 2013. Antibody to a conserved antigenic target is protective against diverse prokaryotic and eukaryotic pathogens. Proc. Natl. Acad. Sci. U. S. A. 110:E2209–E2218. 10.1073/pnas.1303573110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kropec A, Maira-Litran T, Jefferson KK, Grout M, Cramton SE, Gotz F, Goldmann DA, Pier GB. 2005. Poly-N-acetylglucosamine production in Staphylococcus aureus is essential for virulence in murine models of systemic infection. Infect. Immun. 73:6868–6876. 10.1128/IAI.73.10.6868-6876.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cerca N, Jefferson KK, Maira-Litran T, Pier DB, Kelly-Quintos C, Goldmann DA, Azeredo J, Pier GB. 2007. Molecular basis for preferential protective efficacy of antibodies directed to the poorly acetylated form of staphylococcal poly-N-acetyl-beta-(1-6)-glucosamine. Infect. Immun. 75:3406–3413. 10.1128/IAI.00078-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.von Kockritz-Blickwede M, Rohde M, Oehmcke S, Miller LS, Cheung AL, Herwald H, Foster S, Medina E. 2008. Immunological mechanisms underlying the genetic predisposition to severe Staphylococcus aureus infection in the mouse model. Am. J. Pathol. 173:1657–1668. 10.2353/ajpath.2008.080337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bullard DC, Hu X, Schoeb TR, Collins RG, Beaudet AL, Barnum SR. 2007. Intercellular adhesion molecule-1 expression is required on multiple cell types for the development of experimental autoimmune encephalomyelitis. J. Immunol. 178:851–857. 10.4049/jimmunol.178.2.851. [DOI] [PubMed] [Google Scholar]
- 16.Gening ML, Maira-Litran T, Kropec A, Skurnik D, Grout M, Tsvetkov YE, Nifantiev NE, Pier GB. 2010. Synthetic β-(1->6)-linked N-acetylated and nonacetylated oligoglucosamines used to produce conjugate vaccines for bacterial pathogens. Infect. Immun. 78:764–772. 10.1128/IAI.01093-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kelly-Quintos C, Cavacini LA, Posner MR, Goldmann D, Pier GB. 2006. Characterization of the opsonic and protective activity against Staphylococcus aureus of fully human monoclonal antibodies specific for the bacterial surface polysaccharide poly-N-acetylglucosamine. Infect. Immun. 74:2742–2750. 10.1128/IAI.74.5.2742-2750.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pier GB, Boyer D, Preston M, Coleman FT, Llosa N, Mueschenborn-Koglin S, Theilacker C, Goldenberg H, Uchin J, Priebe GP, Grout M, Posner M, Cavacini L. 2004. Human monoclonal antibodies to Pseudomonas aeruginosa alginate that protect against infection by both mucoid and nonmucoid strains. J. Immunol. 173:5671–5678. 10.4049/jimmunol.173.9.5671. [DOI] [PubMed] [Google Scholar]
- 19.Preston MJ, Fleiszig SM, Zaidi TS, Goldberg JB, Shortridge VD, Vasil ML, Pier GB. 1995. Rapid and sensitive method for evaluating Pseudomonas aeruginosa virulence factors during corneal infections in mice. Infect. Immun. 63:3497–3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vlock D, Lee JC, Kropec-Huebner A, Pier GB. 2010. Pre-clinical and initial phase I evaluations of a fully human monoclonal antibody directed against the PNAG surface polysaccharide on Staphylococcus aureus, abstr. G1-1654/329. Abstr. 50th Intersci. Conf. Antimicrob. Agents Chemother. [Google Scholar]
- 21.Chuang CC, Hsiao CH, Tan HY, Ma DH, Lin KK, Chang CJ, Huang YC. 2012. Staphylococcus aureus ocular infection: methicillin-resistance, clinical features, and antibiotic susceptibilities. PLoS One 8:e42437. 10.1371/journal.pone.0042437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu H, Gonzalo JA, St Pierre Y, Williams IR, Kupper TS, Cotran RS, Springer TA, Gutierrez-Ramos JC. 1994. Leukocytosis and resistance to septic shock in intercellular adhesion molecule 1-deficient mice. J. Exp. Med. 180:95–109. 10.1084/jem.180.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chusid MJ, Nelson DB, Meyer LA. 1986. The role of the polymorphonuclear leukocyte in the induction of corneal edema. Invest. Ophthalmol. Vis. Sci. 27:1466–1469. [PubMed] [Google Scholar]
- 24.Hazlett LD. 2005. Role of innate and adaptive immunity in the pathogenesis of keratitis. Ocul. Immunol. Inflamm. 13:133–138. 10.1080/09273940490912362. [DOI] [PubMed] [Google Scholar]
- 25.Zaidi TS, Zaidi T, Pier GB. 2010. Role of neutrophils, MyD88-mediated neutrophil recruitment and complement in antibody-mediated defense against Pseudomonas aeruginosa keratitis. Invest. Ophthalmol. Vis. Sci. 51:2085–2093. 10.1167/iovs.09-4139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Doring G, Bragonzi A, Paroni M, Akturk FF, Cigana C, Schmidt A, Gilpin D, Heyder S, Born T, Smaczny C, Kohlhaufl M, Wagner TO, Loebinger MR, Bilton D, Tunney MM, Elborn JS, Pier GB, Konstan MW, Ulrich M. 2014. BIIL 284 reduces neutrophil numbers but increases P. aeruginosa bacteremia and inflammation in mouse lungs. J. Cyst. Fibros. 13:156–163. 10.1016/j.jcf.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Konstan MW, Doring G, Heltshe SL, Lands LC, Hilliard KA, Koker P, Bhattacharya S, Staab A, Hamilton A, Investigators and Coordinators of BI Trial 543.45 2014. A randomized double blind, placebo controlled phase 2 trial of BIIL 284 BS (an LTB4 receptor antagonist) for the treatment of lung disease in children and adults with cystic fibrosis. J. Cyst. Fibros. 13:148–155. 10.1016/j.jcf.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deng Q, Sun M, Yang K, Zhu M, Chen K, Yuan J, Wu M, Huang X. 2013. MRP8/14 enhances corneal susceptibility to Pseudomonas aeruginosa infection by amplifying inflammatory responses. Invest. Ophthalmol. Vis. Sci. 54:1227–1234. 10.1167/iovs.12-10172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tullos NA, Thompson HW, Taylor SD, Sanders M, Norcross EW, Tolo I, Moore Q, Marquart ME. 2013. Modulation of immune signaling, bacterial clearance, and corneal integrity by Toll-like receptors during Streptococcus pneumoniae keratitis. Curr. Eye Res. 38:1036–1048. 10.3109/02713683.2013.804094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Priebe GP, Walsh RL, Cederroth TA, Kamei A, Coutinho-Sledge YS, Goldberg JB, Pier GB. 2008. IL-17 is a critical component of vaccine-induced protection against lung infection by lipopolysaccharide-heterologous strains of Pseudomonas aeruginosa. J. Immunol. 181:4965–4975. 10.4049/jimmunol.181.7.4965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu W, Huang J, Duan B, Traficante DC, Hong H, Risech M, Lory S, Priebe GP. 2012. Th17-stimulating protein vaccines confer protection against Pseudomonas aeruginosa pneumonia. Am. J. Respir. Crit. Care Med. 186:420–427. 10.1164/rccm.201202-0182OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thakur A, Kyd J, Xue M, Willcox MD, Cripps A. 2001. Effector mechanisms of protection against Pseudomonas aeruginosa keratitis in immunized rats. Infect. Immun. 69:3295–3304. 10.1128/IAI.69.5.3295-3304.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taylor PR, Roy S, Leal SM, Jr, Sun Y, Howell SJ, Cobb BA, Li X, Pearlman E. 2014. Activation of neutrophils by autocrine IL-17A-IL-17RC interactions during fungal infection is regulated by IL-6, IL-23, RORγT and dectin-2. Nat. Immunol. 15:143–151. 10.1038/ni.2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang H, Li H, Li Y, Zou Y, Dong X, Song W, Jia C, Li S, Xi H, Liu D, Wang Y. 2013. IL-17 plays a central role in initiating experimental Candida albicans infection in mouse corneas. Eur. J. Immunol. 43:2671–2682. 10.1002/eji.201242891. [DOI] [PubMed] [Google Scholar]
- 35.Taylor PR, Leal SM, Jr, Sun Y, Pearlman E. 2014. Aspergillus and Fusarium corneal infections are regulated by Th17 cells and IL-17-producing neutrophils. J. Immunol. 192:3319–3327. 10.4049/jimmunol.1302235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zenewicz LA, Flavell RA. 2011. Recent advances in IL-22 biology. Int. Immunol. 23:159–163. 10.1093/intimm/dxr001. [DOI] [PubMed] [Google Scholar]
- 37.Li Z, Burns AR, Miller SB, Smith CW. 2011. CCL20, γδ T cells, and IL-22 in corneal epithelial healing. FASEB J. 25:2659–2668. 10.1096/fj.11-184804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hazlett L D, Jiang X, McClellan SA. 16 April 2014. IL-10 function, regulation, and in bacterial keratitis. J. Ocul. Pharmacol. Ther. 10.1089/jop.2014.0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hooper LV, Littman DR, Macpherson AJ. 2012. Interactions between the microbiota and the immune system. Science 336:1268–1273. 10.1126/science.1223490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zaidi TS, Zaidi T, Pier GB, Priebe GP. 2012. Topical neutralization of interleukin-17 during experimental Pseudomonas aeruginosa corneal infection promotes bacterial clearance and reduces pathology. Infect. Immun. 80:3706–3712. 10.1128/IAI.00249-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maher BM, Mulcahy ME, Murphy AG, Wilk M, O'Keeffe KM, Geoghegan JA, Lavelle EC, McLoughlin RM. 2013. Nlrp-3-driven interleukin 17 production by γδ T cells controls infection outcomes during Staphylococcus aureus surgical site infection. Infect. Immun. 81:4478–4489. 10.1128/IAI.01026-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murphy AG, O'Keeffe KM, Lalor SJ, Maher BM, Mills KH, McLoughlin RM. 2014. Staphylococcus aureus infection of mice expands a population of memory γδ T cells that are protective against subsequent infection. J. Immunol. 192:3697–3708. 10.4049/jimmunol.1303420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Montgomery CP, Daniels M, Zhao F, Alegre ML, Chong AS, Daum RS. 2014. Protective immunity against recurrent Staphylococcus aureus skin infection requires antibody and IL-17A. Infect. Immun. 82:2125–2134. 10.1128/IAI.01491-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Takatori H, Kanno Y, Watford WT, Tato CM, Weiss G, Ivanov II, Littman DR, O'Shea JJ. 2009. Lymphoid tissue inducer-like cells are an innate source of IL-17 and IL-22. J. Exp. Med. 206:35–41. 10.1084/jem.20072713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maira-Litran T, Kropec A, Goldmann DA, Pier GB. 2005. Comparative opsonic and protective activities of Staphylococcus aureus conjugate vaccines containing native or deacetylated staphylococcal poly-N-acetyl-beta-(1-6)-glucosamine. Infect. Immun. 73:6752–6762. 10.1128/IAI.73.10.6752-6762.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cerca N, Maira-Litran T, Jefferson KK, Grout M, Goldmann DA, Pier GB. 2007. Protection against Escherichia coli infection by antibody to the Staphylococcus aureus poly-N-acetylglucosamine surface polysaccharide. Proc. Natl. Acad. Sci. U. S. A. 104:7528–7533. 10.1073/pnas.0700630104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Willcox MD. 2013. Characterization of the normal microbiota of the ocular surface. Exp. Eye Res. 117:99–105. 10.1016/j.exer.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 48.Avci FY, Kasper DL. 2010. How bacterial carbohydrates influence the adaptive immune system. Annu. Rev. Immunol. 28:107–130. 10.1146/annurev-immunol-030409-101159. [DOI] [PubMed] [Google Scholar]
- 49.Mazmanian SK, Liu CH, Tzianabos AO, Kasper DL. 2005. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell 122:107–118. 10.1016/j.cell.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 50.Joshi A, Pancari G, Cope L, Bowman EP, Cua D, Proctor RA, McNeely T. 2012. Immunization with Staphylococcus aureus iron regulated surface determinant B (IsdB) confers protection via Th17/IL17 pathway in a murine sepsis model. Hum. Vaccin. Immunother. 8:336–346. 10.4161/hv.18946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fowler VG, Allen KB, Moreira ED, Moustafa M, Isgro F, Boucher HW, Corey GR, Carmeli Y, Betts R, Hartzel JS, Chan IS, McNeely TB, Kartsonis NA, Guris D, Onorato MT, Smugar SS, DiNubile MJ, Sobanjo-ter Meulen A. 2013. Effect of an investigational vaccine for preventing Staphylococcus aureus infections after cardiothoracic surgery: a randomized trial. JAMA 309:1368–1378. 10.1001/jama.2013.3010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.