ABSTRACT
The migration of pathogen-specific T cells into nonlymphoid tissues, such as the lung, is critical to control peripheral infections. Use of in vivo intravascular labeling of leukocytes has allowed for improved discrimination between cells located in the blood from cells present within peripheral tissues, such as the lung. This is particularly important in the lung, which is comprised of an intricate network of blood vessels that harbors a large proportion of the total blood volume at any given time. Recent work has demonstrated that >80% of antigen-specific effector CD8 T cells remain in the pulmonary vasculature following an intratracheal infection with a systemic viral pathogen. However, it remains unclear what proportion of effector CD8 T cells are located within lung tissue following a localized respiratory viral infection. We confirm that most effector and memory CD8 T cells are found in the vasculature after an intranasal infection with the systemic pathogens lymphocytic choriomeningitis virus (LCMV) or vaccinia virus (VACV). In contrast, following pulmonary viral infections with either respiratory syncytial virus (RSV) or influenza A virus (IAV), 80 to 90% of the antigen-specific effector CD8 T cells were located within lung tissue. Similarly, the majority of antigen-specific CD4 T cells were present within lung tissue during a pulmonary viral infection. Furthermore, a greater proportion of gamma interferon-positive (IFN-γ+) effector CD8 and CD4 T cells were located within lung tissue following a localized respiratory viral infection. Our results indicate that T cells exhibit significantly altered distribution patterns dependent upon the tissue tropism of the infection.
IMPORTANCE The migration of T cells to nonlymphoid sites, such as the lung, is critical to mediate clearance of viral infections. The highly vascularized lung holds up to 40% of blood, and thus, the T cell response may be a reflection of lymphocytes localized to the pulmonary vasculature instead of lung tissue. We examined the localization of T cell responses within the lung following either a localized or systemic viral infection. We demonstrate that following intranasal infection with a systemic pathogen, most T cells are localized to the pulmonary vasculature. In contrast, T cells are primarily localized to lung tissue following a respiratory viral infection. Our results demonstrate vast differences in the localization of T cell responses within the lung parenchyma between pathogens that can replicate locally versus systemically and that intravascular antibody labeling can be utilized to assess the localization patterns of T cell responses in nonlymphoid organs.
INTRODUCTION
An intricate network of blood vessels is associated with the bronchial tree and alveolar sacs of the lung (1). The vascular network is necessary for respiratory function as well as for the trafficking of leukocytes into the lung during infection (2). Leukocytes that remain in the vasculature can be detected by intravenous (i.v.) administration of a specific antibody prior to perfusion and tissue isolation (3–5). Recent work has shown that 80 to 95% of T cell receptor (TCR) transgenic effector and memory CD8 T cells are confined to the pulmonary vasculature following an intratracheal lymphocytic choriomeningitis virus (LCMV) infection despite extensive lung perfusion (4). Importantly, LCMV disseminates systemically even after an intratracheal or intranasal (i.n.) infection. Therefore, it remains unclear what proportion of T cells isolated from a perfused lung are within the lung tissue versus the pulmonary vasculature following an intranasal inoculation with a virus causing a localized respiratory infection.
CD8 T cells play a critical role in mediating clearance of acute localized respiratory viral infections such as those caused by respiratory syncytial virus (RSV) and influenza A virus (IAV) (6, 7). Due to the critical role CD8 T cell responses play in mediating the clearance of respiratory viral infections, we sought to determine whether the majority of endogenous virus-specific CD8 T cells were located within lung tissue following a localized pulmonary viral infection. In contrast to systemic infections, our results demonstrate that 80 to 90% of endogenous virus-specific effector and memory CD8 T cells are located within lung tissue following a localized pulmonary viral infection. Furthermore, using cytokine reporter mice, we show that gamma interferon-positive (IFN-γ+) endogenous effector CD8 T cells are highly enriched within lung tissue following a respiratory viral infection compared to a systemic infection. These data indicate that the tissue tropism of a virus significantly impacts the localization pattern of virus-specific effector and memory CD8 T cells.
MATERIALS AND METHODS
Mice, adoptive transfers, and infection.
C57BL/6NCr mice (6 to 8 weeks old) were obtained from the National Cancer Institute (Frederick, MD). For adoptive transfers, 1 × 103 naive P14 CD90.1/CD90.2 cells were injected i.v. 1 day prior to infection with one of the following: (i) 2 × 105 PFU LCMV Armstrong intraperitoneally (i.p.); (ii) 5 × 105 PFU LCMV Armstrong i.n.; or (iii) 230 50% tissue culture infective units (TCIU50) of IAV strain A/Puerto Rico/8/1934 (PR8) expressing GP33-41 (i.e., amino acids 33 to 41 from LCMV glycoprotein) (IAV-GP33) (i.n.). BALB/cAnNCr mice (6 to 8 weeks old) were infected i.n. with 5 × 105 PFU LCMV Armstrong, 5 × 103 PFU vaccinia virus (VACV) strain Western Reserve, 110 to 150 TCIU50 IAV PR8 or 1.6 × 106 RSV A2. Ifng/CD90.1 knock-in mice (8) obtained from Stanley Perlman (University of Iowa) with permission from Casey Weaver (University of Alabama at Birmingham) were infected i.n. with either 5 × 105 PFU LCMV Armstrong or 110 TCIU50 IAV PR8. All experimental procedures involving mice were approved by the University of Iowa Animal Care and Use Committee.
Intravascular antibody labeling and tissue processing.
One microgram of CD90.2-PE (CD90.2 labeled with phycoerythrin [PE]) (clone 53-2.1) antibody was injected via the tail vein i.v. 3 min prior to euthanasia, and cells from various tissues were subsequently prepared as previously described (4).
Tetramer and antibody staining.
Cells were incubated with a single tetramer, either LCMV GP33-41 or IAV NP366-374 (i.e., amino acids 366 to 374 from nucleoprotein) for C57BL/6 mice or LCMV NP118-126, VACV F226-34, IAV HA518-526 (i.e., amino acids 518 to 526 from hemagglutinin), or RSV M282-90 (i.e., amino acids 82 to 90 from M2) for BALB/c mice (obtained from the NIH Tetramer Facility) and subsequently stained for extracellular expression of CD90.2 (clone 53-2.1), CD8 (clone 53-6.7), CD4 (clone RM4-5), CD11a (clone M17/4), and CD49d (clone R1-2) and intracellular expression of Foxp3 (clone FJK-16S; all antibodies obtained from BioLegend, San Diego, CA) as previously described (9).
Plaque assay.
Lungs were harvested on day 4 postinfection (p.i.). Whole lungs were homogenized, and supernatants were subsequently collected and flash-frozen. For plaque assay, supernatants were thawed and plated on BSC40 cells for VACV-infected lungs, MDCK cells for IAV-infected lungs, or Vero cells for either LCMV- or RSV-infected lungs as previously described (10–12). Briefly, samples were diluted in serum-free medium and plated on the appropriate cell line with rocking for 1 to 1.5 h. For LCMV and RSV, cells were subsequently overlaid with a 1:1 ratio of serum-containing medium and 1% agar. On day 3 for LCMV or day 5 for RSV, the plaques were stained with a 1:1 ratio of medium and 1% agar containing 0.01% neutral red. The plaques were visually quantified 24 h later. For IAV plaque assays, infected MDCK cells were overlaid with a 1:1 ratio of serum-containing medium and 1.6% agar. On day 3 p.i., the agar plug was removed, and the cells were fixed with 70% ethanol. The IAV-infected MDCK cells were subsequently stained with crystal violet and rinsed with warm water, and plaques were immediately counted. For VACV plaque assays, serum-containing medium was added to the VACV-infected BSC40 cells. On day 2 p.i., the infected cells were fixed with 7% formaldehyde, stained with crystal violet, and rinsed with warm water. VACV plaques were subsequently counted for virus quantification.
Statistical analysis.
Data were compiled, and statistical analysis was calculated by performing a one-way analysis of variance (ANOVA) with a Tukey's posttest for more than two groups or a Student's unpaired t test for two groups in Prism software (GraphPad Software, San Diego, CA). Values that were significantly different are indicated as follows: *, P < 0.05; **, P < 0.01; ***, P < 0.001.
RESULTS
Virus-specific CD8 T cells preferentially localize to the lung following RSV infection.
Intravascular staining has been utilized in several recent studies to discriminate between leukocytes in lung tissue versus pulmonary vasculature and has been previously shown not to stain perivascular cells (3–5). To determine the proportion of endogenous antigen-specific CD8 T cells that have entered lung tissue following a localized pulmonary viral infection, RSV-infected mice were injected intravenously with an anti-CD90.2 antibody (Fig. 1A) to specifically label all T cells in the peripheral blood. In addition, T cells localized within either secondary lymphoid tissues or bronchoalveolar lavage (BAL) fluid were protected from intravascular labeling with antibody (Fig. 1A). Antibody specific to CD90.2 was chosen to avoid any potential issues with the known downregulation of CD8α on murine CD8 T cells following antigen stimulation (13). The frequency of total CD8 T cells not labeled with antibody, representing cells in lung tissue, increased following RSV infection, peaking at day 8 p.i. (Fig. 1B). Similarly, the frequency of unlabeled CD11ahi CD8lo T cells, used to define antigen-specific cells as previously described (13, 14), and M282-90 tetramer-positive CD8 T cells also peaked in lung tissue at day 8 p.i. The frequency of total CD8 T cells that remained unlabeled in the lung decreased significantly by day 30 p.i., consistent with contraction of the T cell response. However, the frequency of unlabeled antigen-specific CD8 T cells located within lung tissue remained relatively stable and similar to the peak of the response. Overall, approximately 85% of tetramer-positive CD8 T cells in the perfused lung remained in the lung tissue for up to 30 days following RSV infection. Furthermore, >95% of antigen-specific CD8 T cells that expressed either one of the tissue-resident markers CD69 and CD103 (15–17) were located within lung tissue at day 30 p.i. (Fig. 1D). However, only 40% or 50% of antigen-specific CD8 T cells within lung tissue expressed either CD69 or CD103, respectively, 30 days following RSV infection (Fig. 1E). In contrast to the antigen-specific cells, the frequency of unlabeled CD11alo CD8hi naive T cells remained relatively constant, suggesting that naive T cells do not preferentially enter the lung following infection.
FIG 1.
Preferential recruitment of antigen-specific CD8 T cells to the lung following pulmonary infection. (A) Representative dot plots of labeled T cells following i.v. anti-CD90.2 antibody administration. The numbers in the graphs are the frequencies of unlabeled CD8 T cells on day 8 after RSV infection. PBL, peripheral blood leukocytes; dLN, draining lymph nodes; BAL, bronchoalveolar lavage fluid. (B and C) Frequencies of unlabeled CD8 T cell (B) or CD4 T cell (C) populations at multiple time points following RSV infection of BALB/c mice (n = 8). (D) Concatenated dot plots of the frequency of unlabeled CD69+ or CD103+ M282-90 tetramer-positive (Tet+) CD8 T cells at day 30 p.i. (E) Frequency of unlabeled M282-90 tetramer-positive CD8 T cells that are either CD69+ or CD103+ at day 30 p.i. Combined results are shown from two independent experiments.
Similar to CD8 T cells, conventional CD4 T cells shared the same localization pattern within the lung parenchyma following RSV infection (Fig. 1C). The frequency of CD4 T cells that were unlabeled within lung tissue increased following infection, peaking at day 8 p.i., but decreased over time in correlation with contraction of the T cell response. However, approximately 70% of antigen-experienced CD11ahi CD49d+ CD4 T cells (18) remained localized to lung tissue up to 30 days following RSV infection, whereas the frequency of unlabeled naive CD11alo CD49d− CD4 T cells was unchanged. Regulatory Foxp3+ CD4 T cells exhibited a pattern of localization within lung tissue that was similar to that of antigen-experienced CD4 T cells, peaking at day 8 p.i. and decreasing over time. This suggests that regulatory CD4 T cells localize within lung tissue coincident with the effector T cell response to suppress the adaptive immune response.
Increased frequency of effector T cells in lung tissue following pulmonary infections.
To determine whether a localized pulmonary infection would induce the preferential migration of effector CD8 T cells into lung tissue compared to a systemic infection, BALB/c mice were infected i.n. with IAV or RSV, both of which cause infections that are localized to the lung, or with LCMV or VACV, both of which cause systemic infections. On day 8 following either IAV or RSV infection, there was a significant (P < 0.01) increase in the frequency of unlabeled CD8 T cells located in lung tissue compared to intranasal infection with the systemic pathogens LCMV and VACV (Fig. 2A). In addition, there was a significantly (P < 0.01) increased frequency of unlabeled antigen-specific tetramer-positive CD8 T cells in lung tissue on day 8 following IAV and RSV infection than after either LCMV or VACV infection (Fig. 2B). Similarly, following either IAV or RSV infection at day 8 p.i., there was a significantly (P < 0.01) increased frequency of antigen-specific CD11ahi CD8lo T cells that were unlabeled in lung tissue compared to either LCMV or VACV (Fig. 2C). However, there was no difference in the frequency of unlabeled CD11alo CD8hi naive T cells in lung tissue following any infection (Fig. 2D). These data indicate that antigen-specific CD8 T cells preferentially enter lung tissue and are not simply trapped within the pulmonary vasculature following a viral infection restricted primarily to the respiratory tract.
FIG 2.
Increased lung localization of antigen-specific CD8 T cells following a restricted pulmonary infection. On day 8 p.i., cells were isolated from the lung following i.v. labeling in BALB/c mice. (A to D) Frequencies of unlabeled total (A), tetramer-positive (B), CD11ahi CD8lo (C), and CD11alo CD8hi (D) T cells following LCMV, VACV, IAV, and RSV infection. A one-way ANOVA with a Tukey's posttest was performed. Values that are significantly different (**, P < 0.01) are indicated by bars and two asterisks. Data are combined from two independent experiments (n = 8, where n is the number of mice from both experiments combined).
The distribution of CD4 T cells following systemic and localized pulmonary infections in BALB/c mice was also assessed by intravascular labeling. Recent work by Turner et al. indicates that tissue-resident memory CD4 T cells primarily reside at locations determined by the initial site of infection and demonstrates that the majority of memory CD4 T cells are localized within lung tissue following IAV infection (19). We find that similar to CD8 T cells, conventional Foxp3−, regulatory Foxp3+, and antigen-specific CD11ahi CD49d+ CD4 T cells were preferentially localized within lung tissue following either IAV or RSV infection compared to either LCMV or VACV infection at day 8 p.i. (Fig. 3A to C). Similar to CD11alo CD8hi naive T cells, there was no difference in the frequency of unlabeled CD11alo CD49d− CD4 T cells between infections (Fig. 3D), indicating that naive CD4 T cells were not preferentially recruited to the site of infection. These data suggest that similar to antigen-specific CD8 T cells, activated CD4 T cells preferentially migrate into the lung following a localized pulmonary infection. Importantly, these differences in CD4 and CD8 T cell migration between localized and systemic viral respiratory infection were not due to deficient virus replication in lungs (Fig. 4). Following i.n. infection, lung viral titers were similar for LCMV, VACV, IAV, and RSV (Fig. 4). Furthermore, there was increased virus in LCMV- and VACV-infected lungs following i.n. infections compared to i.p. infections.
FIG 3.

CD4 T cells are preferentially localized to the lung following a local pulmonary infection. Lymphocytes were isolated from the lung on day 8 p.i. following i.v. labeling in BALB/c mice. (A to D) Frequencies of unlabeled conventional Foxp3− (A), regulatory Foxp3+ (B), CD11ahi CD49d+ (C), and CD11alo CD49d− CD4 (D) T cells following LCMV, VACV, IAV, and RSV infection. A one-way ANOVA with a Tukey's posttest was performed. Values that are significantly different are indicated by bars and asterisks as follows: *, P < 0.05; **, P < 0.01; ***, P < 0.001. Combined results from two independent experiments are shown (n = 8).
FIG 4.

Virus replication in the respiratory tract following localized or systemic infections. Viral titers were quantified in the lungs at day 4 following either LCMV, VACV, IAV, or RSV infection by plaque assay. LCMV and VACV were administered i.p. and i.n. Combined results from two independent experiments are shown (n = 7 or 8).
To determine whether monoclonal TCR transgenic CD8 T cells exhibit localization patterns similar to those of endogenous polyclonal T cells, we adoptively transferred 1 × 103 LCMV GP33-specific P14 CD8 T cells into naive C57BL/6 mice and challenged them with LCMV i.p., LCMV i.n., or recombinant IAV-GP33 i.n. On day 8 p.i., a significantly (P < 0.001) higher frequency of P14 CD8 T cells were located within lung tissue following an IAV-GP33 infection compared to either i.p. or i.n. LCMV infections (Fig. 5A). Furthermore, using the LCMV clone 13 strain, which has an increased capacity to disseminate compared to LCMV Armstrong (20, 21), a similar localization pattern of P14 cells in the lung was observed following i.n. infection (Fig. 5B).
FIG 5.
Increased frequency of antigen-specific CD8 T cells confined to lung tissue following localized respiratory viral infection. P14 CD8 T cells were adoptively transferred i.v. into C57BL/6 mice and infected the following day with LCMV Armstrong i.p. or i.n. or IAV-GP33 i.n. (A) Concatenated dot plots depicting the frequency of CD90.2 unlabeled CD8 T cells of P14 cells in the lung at day 8 p.i. The numbers in the graphs represent the mean percentages of CD90.2 P14 cells that are i.v. label negative ± standard errors of the means (SEM) for the groups. (B) Frequency of unlabeled P14 cells on day 8 in the lung following infection with either LCMV Armstrong (given i.n.) or clone 13 (CL13) (given i.n. or i.p.). (C and D) Frequencies of unlabeled endogenous CD8 T cells (C) and GP33-41 tetramer-positive endogenous CD8 T cells (D). Values that are significantly different (P < 0.001) from the values for the other experimental groups are indicated by three asterisks. Combined results from two independent experiments are shown (n = 8).
When assessing the endogenous T cell response in the recipient mice, a higher frequency of total CD8 T cells was localized to lung tissue following challenge with IAV-GP33 compared to either route of LCMV infection (Fig. 5C), similar to the results observed for BALB/c mice. Furthermore, a significantly higher proportion of endogenous GP33-41 tetramer-specific CD8 T cells were located within lung tissue following a localized IAV-GP33 infection (Fig. 5D). Taken together, our data demonstrate that the localization pattern of antigen-specific T cells is governed by both the route of infection and tissue tropism of the pathogen.
The majority of IFN-γ+ effector CD8 T cells are located within the lung following a respiratory virus infection.
Our data thus far have indicated that there is an increased frequency of antigen-specific T cells within the lung tissue following a localized pulmonary infection. However, we further sought to determine whether there were functional differences between CD8 T cells recruited into the lung compared to cells in the peripheral blood following a localized respiratory tract infection versus a systemic infection. To address this question, we infected IFN-γ reporter mice that express CD90.1 following induction of Ifng gene transcription (8) with either LCMV or IAV i.n. Following infection with either virus, a large portion of tetramer-positive CD8 T cells were also CD90.1+, indicating that these cells had recently produced IFN-γ (Fig. 6A). To compare CD90.1 expression in the different infections, values were normalized by determining the ratio of the percentage of CD90.1+ unlabeled tetramer-positive CD8 T cells to the percentage of CD90.1+ labeled tetramer-positive CD8 T cells. There was an approximately 2-fold increase in CD90.1/IFN-γ+, tetramer-positive CD8 T cells (P < 0.001) localized to lung tissue following IAV infection compared to LCMV infection (Fig. 6B). Furthermore, the geometric mean fluorescence intensity (gMFI) of CD90.1 expression was significantly (P < 0.01) increased in the unlabeled tetramer-positive CD8 T cells following IAV infection than in either the labeled IAV-specific CD8 T cells or LCMV-specific CD8 T cells (Fig. 6C). Similar to CD8 T cells, there was a significantly increased proportion of antigen-experienced CD4 T cells (P < 0.01) localized to lung tissue following a localized IAV infection compared to LCMV infection (Fig. 6D). Furthermore, the antigen-experienced CD4 T cells within the lung tissue had a higher gMFI of CD90.1 compared to cells in the pulmonary vasculature following IAV infection and not LCMV infection (Fig. 6E). These data demonstrate that IFN-γ+ effector CD8 and CD4 T cells are enriched within lung tissue following a pulmonary viral infection compared to a systemic viral infection.
FIG 6.
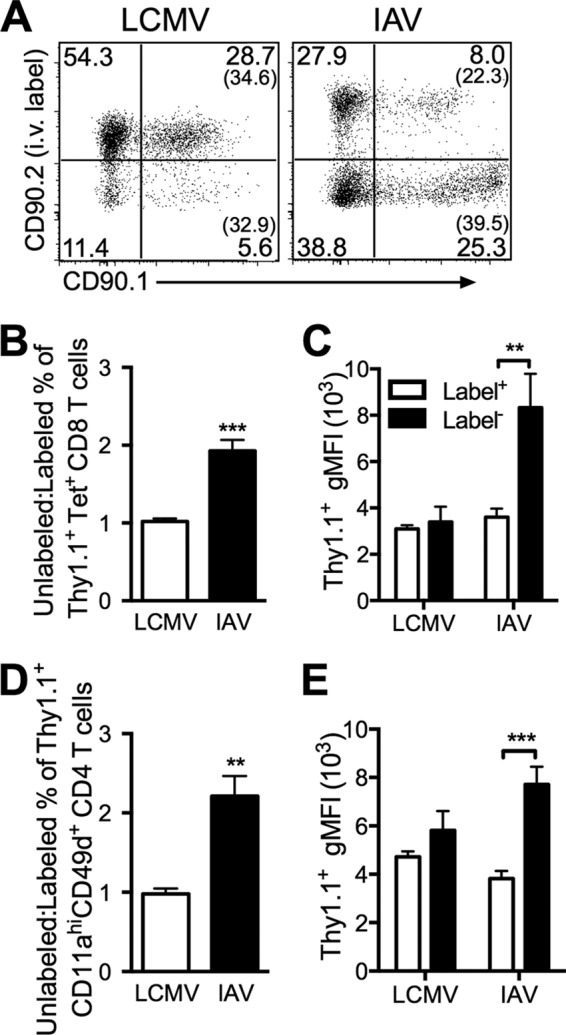
Increased proportion of virus-specific CD8 T cells produce IFN-γ in lung tissue following a localized pulmonary infection. Ifng/CD90.1 knock-in mice were infected with either LCMV or IAV i.n. and harvested 8 days later. (A) Representative dot plots of intravascular staining for CD90.2 and CD90.1. The numbers in parentheses in the graphs represent the frequencies of CD90.1+ (IFN-γ+) CD8 T cells of the total unlabeled or labeled tetramer-positive CD8 T cell populations. (B and D) Ratio of the percentage of unlabeled CD90.1+ T cells to the percentage of labeled CD90.1+ T cells for tetramer-positive CD8 T cells (B) and CD11ahi CD49d+ CD4 T cells (D). (C and E) Geometric MFI (gMFI) for CD90.1+ staining in the labeled and unlabeled tetramer-positive CD8 T cell populations (C) and CD11ahi CD49d+ CD4 T cells (E). A Student's unpaired t test (B and D) or a two-way ANOVA with a Bonferroni's posttest (C and E) was performed (n = 5). Values that are significantly different are indicated by bars and asterisks as follows: **, P < 0.01; ***, P < 0.001. Combined results from two independent experiments are shown.
DISCUSSION
Previous work has demonstrated that 80 to 95% of effector and memory TCR transgenic CD8 T cells are confined to the pulmonary vasculature following an intratracheal challenge with the systemic pathogen LCMV (4). Similarly, we demonstrate following an intranasal infection with the systemic pathogen LCMV or VACV that the majority of CD8 T cells remain in the pulmonary vasculature of the lung. In contrast, challenge with either RSV or IAV, both of which replicate only in the respiratory tract, induce the preferential migration of antigen-specific effector CD8 T cells into lung tissue. This is important, as CD8 T cells play a critical role in mediating clearance of respiratory viral infections such as those caused by RSV and IAV (6, 7, 22, 23). In addition, we find that the majority of IFN-γ+ effector CD8 T cells are located within the lung following a localized respiratory infection compared to a systemic infection. This suggests that there is a greater proportion of fully differentiated CD8 T cells capable of exerting effector functions located within lung tissue following a localized respiratory infection where these cells play a critical role in mediating viral clearance.
We also observed that the frequency of RSV-specific CD8 T cells located within lung tissue remains relatively high (∼85%) through day 30 p.i. Expression of CD69 and CD103 has been used to identify tissue-resident memory CD8 T cells that have been shown to form stable long-lasting populations in the lung tissue following respiratory viral infections (24–27). Up to 40% or 50% of the antigen-specific CD8 T cells expressed either CD69 or CD103, respectively, and >95% of these cells were unlabeled, suggesting that they are tissue-resident memory T cells. The role of tissue-resident memory T cells has not been extensively examined in the lung environment following RSV infection. However, studies have indicated that tissue-resident memory T cells correlate with local protective immunity during either Sendai virus or influenza virus infection (28, 29). Thus, it is likely that the tissue-resident memory CD8 T cells following RSV infection contribute to protection against secondary infections. In addition, this unique subset of memory cells has been characterized and shown to contribute to local immunity in the skin, brain, vaginal tract, and intestinal epithelium (17, 30–33).
Similar results were observed for the CD4 T cell response. The majority of antigen-experienced effector and memory CD4 T cells were located within lung tissue following localized respiratory infections caused by either RSV or IAV compared to a significantly reduced frequency following infection with systemic pathogens. These results are in agreement with recent work by Turner et al. which shows that most of the effector CD4 T cells are located within lung tissue following IAV infection (19). In addition, lung niches permit the formation and maintenance of IAV-specific, tissue-resident memory CD4 T cells. We also find that following RSV infection, the majority of antigen-experienced CD4 T cells remain in lung tissue for up to 30 days.
Our findings indicate the distinct localization patterns of effector T cells within lung tissue following systemic versus localized respiratory viral infections. Our results highlight the need to accurately assess the total number of antigen-specific T cells within lung tissue following a pulmonary infection with a pathogen capable of replicating systemically. In contrast, the majority of antigen-specific T cells are found within the lung tissue following infection with a pathogen that replicates primarily in the respiratory tract. This lessens the need to take measures to discriminate the location of antigen-specific T cells following a localized respiratory viral infection. Overall, our study indicates that i.v. labeling of T cells using a CD90-specific antibody is a straightforward method to distinguish both CD4 and CD8 T cells in peripheral blood from cells in the lung and that virus-specific CD8 T cells rapidly enter lung tissue following a localized respiratory viral infection.
ACKNOWLEDGMENTS
We thank Kevin Legge and John Harty for critically reviewing the manuscript.
Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under award numbers R56AI106776 (to S.M.V.) and T32AI007485 (to C.J.K. and K.A.W.).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
We declare that we have no financial conflicts of interest.
Footnotes
Published ahead of print 4 June 2014
REFERENCES
- 1.Hislop AA. 2002. Airway and blood vessel interaction during lung development. J. Anat. 201:325–334. 10.1046/j.1469-7580.2002.00097.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hogg JC, Doerschuk CM. 1995. Leukocyte traffic in the lung. Annu. Rev. Physiol. 57:97–114. 10.1146/annurev.ph.57.030195.000525 [DOI] [PubMed] [Google Scholar]
- 3.Teijaro JR, Turner D, Pham Q, Wherry EJ, Lefrancois L, Farber DL. 2011. Tissue-retentive lung memory CD4 T cells mediate optimal protection to respiratory virus infection. J. Immunol. 187:5510–5514. 10.4049/jimmunol.1102243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson KG, Sung H, Skon CN, Lefrancois L, Deisinger A, Vezys V, Masopust D. 2012. Intravascular staining redefines lung CD8 T cell responses. J. Immunol. 189:2702–2706. 10.4049/jimmunol.1201682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galkina E, Thatte J, Dabak V, Williams MB, Ley K, Braciale TJ. 2005. Preferential migration of effector CD8+ T cells into the interstitium of the normal lung. J. Clin. Invest. 115:3473–3483. 10.1172/JCI24482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cannon MJ, Stott EJ, Taylor G, Askonas BA. 1987. Clearance of persistent respiratory syncytial virus infections in immunodeficient mice following transfer of primed T cells. Immunology 62:133–138 [PMC free article] [PubMed] [Google Scholar]
- 7.Taylor PM, Askonas BA. 1986. Influenza nucleoprotein-specific cytotoxic T-cell clones are protective in vivo. Immunology 58:417–420 [PMC free article] [PubMed] [Google Scholar]
- 8.Harrington LE, Janowski KM, Oliver JR, Zajac AJ, Weaver CT. 2008. Memory CD4 T cells emerge from effector T-cell progenitors. Nature 452:356–360. 10.1038/nature06672 [DOI] [PubMed] [Google Scholar]
- 9.Weiss KA, Christiaansen AF, Fulton RB, Meyerholz DK, Varga SM. 2011. Multiple CD4+ T cell subsets produce immunomodulatory IL-10 during respiratory syncytial virus infection. J. Immunol. 187:3145–3154. 10.4049/jimmunol.1100764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olson MR, Varga SM. 2007. CD8 T cells inhibit respiratory syncytial virus (RSV) vaccine-enhanced disease. J. Immunol. 179:5415–5424. 10.4049/jimmunol.179.8.5415 [DOI] [PubMed] [Google Scholar]
- 11.Szretter KJ, Balish AL, Katz JM. 2006. Influenza: propagation, quantification, and storage. Curr. Protoc. Microbiol. Chapter 15:Unit 15G.1. 10.1002/0471729256.mc15g01s3 [DOI] [PubMed] [Google Scholar]
- 12.Olson MR, McDermott DS, Varga SM. 2012. The initial draining lymph node primes the bulk of the CD8 T cell response and influences memory T cell trafficking after a systemic viral infection. PLoS Pathog. 8:e1003054. 10.1371/journal.ppat.1003054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rai D, Pham NL, Harty JT, Badovinac VP. 2009. Tracking the total CD8 T cell response to infection reveals substantial discordance in magnitude and kinetics between inbred and outbred hosts. J. Immunol. 183:7672–7681. 10.4049/jimmunol.0902874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Radziewicz H, Ibegbu CC, Fernandez ML, Workowski KA, Obideen K, Wehbi M, Hanson HL, Steinberg JP, Masopust D, Wherry EJ, Altman JD, Rouse BT, Freeman GJ, Ahmed R, Grakoui A. 2007. Liver-infiltrating lymphocytes in chronic human hepatitis C virus infection display an exhausted phenotype with high levels of PD-1 and low levels of CD127 expression. J. Virol. 81:2545–2553. 10.1128/JVI.02021-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee YT, Suarez-Ramirez JE, Wu T, Redman JM, Bouchard K, Hadley GA, Cauley LS. 2011. Environmental and antigen receptor-derived signals support sustained surveillance of the lungs by pathogen-specific cytotoxic T lymphocytes. J. Virol. 85:4085–4094. 10.1128/JVI.02493-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mizukawa Y, Yamazaki Y, Shiohara T. 2008. In vivo dynamics of intraepidermal CD8+ T cells and CD4+ T cells during the evolution of fixed drug eruption. Br. J. Dermatol. 158:1230–1238. 10.1111/j.1365-2133.2008.08516.x [DOI] [PubMed] [Google Scholar]
- 17.Gebhardt T, Wakim LM, Eidsmo L, Reading PC, Heath WR, Carbone FR. 2009. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat. Immunol. 10:524–530. 10.1038/ni.1718 [DOI] [PubMed] [Google Scholar]
- 18.McDermott DS, Varga SM. 2011. Quantifying antigen-specific CD4 T cells during a viral infection: CD4 T cell responses are larger than we think. J. Immunol. 187:5568–5576. 10.4049/jimmunol.1102104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Turner DL, Bickham KL, Thome JJ, Kim CY, D'Ovidio F, Wherry EJ, Farber DL. 2014. Lung niches for the generation and maintenance of tissue-resident memory T cells. Mucosal Immunol. 7:501–510. 10.1038/mi.2013.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matloubian M, Kolhekar SR, Somasundaram T, Ahmed R. 1993. Molecular determinants of macrophage tropism and viral persistence: importance of single amino acid changes in the polymerase and glycoprotein of lymphocytic choriomeningitis virus. J. Virol. 67:7340–7349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahmed R, Salmi A, Butler LD, Chiller JM, Oldstone MB. 1984. Selection of genetic variants of lymphocytic choriomeningitis virus in spleens of persistently infected mice. Role in suppression of cytotoxic T lymphocyte response and viral persistence. J. Exp. Med. 160:521–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taylor G, Stott EJ, Hayle AJ. 1985. Cytotoxic lymphocytes in the lungs of mice infected with respiratory syncytial virus. J. Gen. Virol. 66:2533–2538. 10.1099/0022-1317-66-12-2533 [DOI] [PubMed] [Google Scholar]
- 23.Flynn KJ, Belz GT, Altman JD, Ahmed R, Woodland DL, Doherty PC. 1998. Virus-specific CD8+ T cells in primary and secondary influenza pneumonia. Immunity 8:683–691. 10.1016/S1074-7613(00)80573-7 [DOI] [PubMed] [Google Scholar]
- 24.Wu T, Hu Y, Lee YT, Bouchard KR, Benechet A, Khanna K, Cauley LS. 2014. Lung-resident memory CD8 T cells (TRM) are indispensable for optimal cross-protection against pulmonary virus infection. J. Leukoc. Biol. 95:215–224. 10.1189/jlb.0313180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marshall DR, Turner SJ, Belz GT, Wingo S, Andreansky S, Sangster MY, Riberdy JM, Liu T, Tan M, Doherty PC. 2001. Measuring the diaspora for virus-specific CD8+ T cells. Proc. Natl. Acad. Sci. U. S. A. 98:6313–6318. 10.1073/pnas.101132698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hogan RJ, Usherwood EJ, Zhong W, Roberts AA, Dutton RW, Harmsen AG, Woodland DL. 2001. Activated antigen-specific CD8+ T cells persist in the lungs following recovery from respiratory virus infections. J. Immunol. 166:1813–1822. 10.4049/jimmunol.166.3.1813 [DOI] [PubMed] [Google Scholar]
- 27.Wakim LM, Gupta N, Mintern JD, Villadangos JA. 2013. Enhanced survival of lung tissue-resident memory CD8+ T cells during infection with influenza virus due to selective expression of IFITM3. Nat. Immunol. 14:238–245. 10.1038/ni.2525 [DOI] [PubMed] [Google Scholar]
- 28.Hogan RJ, Zhong W, Usherwood EJ, Cookenham T, Roberts AD, Woodland DL. 2001. Protection from respiratory virus infections can be mediated by antigen-specific CD4+ T cells that persist in the lungs. J. Exp. Med. 193:981–986. 10.1084/jem.193.8.981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ray SJ, Franki SN, Pierce RH, Dimitrova S, Koteliansky V, Sprague AG, Doherty PC, de Fougerolles AR, Topham DJ. 2004. The collagen binding α1β1 integrin VLA-1 regulates CD8 T cell-mediated immune protection against heterologous influenza infection. Immunity 20:167–179. 10.1016/S1074-7613(04)00021-4 [DOI] [PubMed] [Google Scholar]
- 30.Cuburu N, Graham BS, Buck CB, Kines RC, Pang YY, Day PM, Lowy DR, Schiller JT. 2012. Intravaginal immunization with HPV vectors induces tissue-resident CD8+ T cell responses. J. Clin. Invest. 122:4606–4620. 10.1172/JCI63287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wakim LM, Woodward-Davis A, Bevan MJ. 2010. Memory T cells persisting within the brain after local infection show functional adaptations to their tissue of residence. Proc. Natl. Acad. Sci. U. S. A. 107:17872–17879. 10.1073/pnas.1010201107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Masopust D, Choo D, Vezys V, Wherry EJ, Duraiswamy J, Akondy R, Wang J, Casey KA, Barber DL, Kawamura KS, Fraser KA, Webby RJ, Brinkmann V, Butcher EC, Newell KA, Ahmed R. 2010. Dynamic T cell migration program provides resident memory within intestinal epithelium. J. Exp. Med. 207:553–564. 10.1084/jem.20090858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mackay LK, Stock AT, Ma JZ, Jones CM, Kent SJ, Mueller SN, Heath WR, Carbone FR, Gebhardt T. 2012. Long-lived epithelial immunity by tissue-resident memory T (TRM) cells in the absence of persisting local antigen presentation. Proc. Natl. Acad. Sci. U. S. A. 109:7037–7042. 10.1073/pnas.1202288109 [DOI] [PMC free article] [PubMed] [Google Scholar]





