ABSTRACT
We report a novel extraribosomal innate immune function of mammalian ribosomal protein L13a, whereby it acts as an antiviral agent. We found that L13a is released from the 60S ribosomal subunit in response to infection by respiratory syncytial virus (RSV), an RNA virus of the Pneumovirus genus and a serious lung pathogen. Unexpectedly, the growth of RSV was highly enhanced in L13a-knocked-down cells of various lineages as well as in L13a knockout macrophages from mice. In all L13a-deficient cells tested, translation of RSV matrix (M) protein was specifically stimulated, as judged by a greater abundance of M protein and greater association of the M mRNA with polyribosomes, while general translation was unaffected. In silico RNA folding analysis and translational reporter assays revealed a putative hairpin in the 3′untranslated region (UTR) of M mRNA with significant structural similarity to the cellular GAIT (gamma-activated inhibitor of translation) RNA hairpin, previously shown to be responsible for assembling a large, L13a-containing ribonucleoprotein complex that promoted translational silencing in gamma interferon (IFN-γ)-activated myeloid cells. However, RNA-protein interaction studies revealed that this complex, which we named VAIT (respiratory syncytial virus-activated inhibitor of translation) is functionally different from the GAIT complex. VAIT is the first report of an extraribosomal L13a-mediated, IFN-γ-independent innate antiviral complex triggered in response to virus infection. We provide a model in which the VAIT complex strongly hinders RSV replication by inhibiting the translation of the rate-limiting viral M protein, which is a new paradigm in antiviral defense.
IMPORTANCE The innate immune mechanisms of host cells are diverse in nature and act as a broad-spectrum cellular defense against viruses. Here, we report a novel innate immune mechanism functioning against respiratory syncytial virus (RSV), in which the cellular ribosomal protein L13a is released from the large ribosomal subunit soon after infection and inhibits the translation of a specific viral mRNA, namely, that of the matrix protein M. Regarding its mechanism, we show that the recognition of a specific secondary structure in the 3′ untranslated region of the M mRNA leads to translational arrest of the mRNA. We also show that the level of M protein in the infected cell is rate limiting for viral morphogenesis, providing a rationale for L13a to target the M mRNA for suppression of RSV growth. Translational silencing of a viral mRNA by a deployed ribosomal protein is a new paradigm in innate immunity.
INTRODUCTION
Respiratory syncytial virus (RSV) is a clinically important pediatric pathogen with no reliable vaccine or therapy (1, 2). Immunization with denatured virion particles provides little protection and instead triggers an enhanced disease or “immune potentiation” upon subsequent infection, the mechanism of which remains obscure (3). Immunity acquired after natural RSV infection is short-lived and does not provide protection against reinfection (1). The current focus is thus on the host cell's innate immunity, which is a broad-spectrum defense mechanism, operative against a variety of pathogens, including bacteria, viruses, and diverse other foreign entities (4). In innate immunity, nonself molecules of pathogens, known broadly as pathogen-associated molecular patterns (PAMPs), are detected by cognate cellular PAMP recognition receptors (PRRs), which triggers a cascade of signaling pathways that eventually activates defense genes such as interferon (IFN) (5–8). Well-studied PRRs include Toll-like receptors (TLRs), DEAD box RNA helicases such as RIG-I and MDA5, and NOD-like receptors (NLRs) (6, 7), but the molecular nature of the PAMP of RSV is still unclear.
Our previous work identified a ribosomal protein L13a-dependent translational silencing mechanism, responsible for attenuating the expression of a cohort of mRNAs encoding inflammatory proteins directly at the level of translation (9–12). In this mechanism, macrophage or monocytes, upon treatment with IFN-γ, release L13a from the 60S ribosomal subunit (10). The released L13a assembles a large multiprotein complex (13) and binds to a specific RNA hairpin, named gamma-activated inhibitor of translation (GAIT) element (14), in the 3′ untranslated region (3′UTR) of the target proinflammatory mRNAs and silences their translation (12). As a corollary, this mechanism specifically silences GAIT element-containing cellular mRNAs; it is also found exclusively in immunity-related cells such as macrophages, dendritic cells (DCs), and T cells, but not in any other cell types tested, such as fibroblasts or epithelial cells (9), even though the nonimmune cells constitute a large population in higher eukaryotes and serve as hosts for a variety of pathogens (15). Our subsequent studies using macrophage-specific L13a−/− mice confirmed and extended the role of the GAIT mechanism as an endogenous defense against uncontrolled inflammation (16). It is to be mentioned that the ribosomal loss of L13a has no deleterious effect on global translation and cellular growth and that macrophage-specific depletion of L13a has no consequence on animal viability, further underscoring a specific immunological role of the GAIT mechanism (12, 16, 17). To investigate whether RSV could deliberately manipulate the L13-dependent endogenous cellular mechanism to regulate the host's immune response, we first compared RSV growth in normal and L13a-deficient cells. To our surprise, significant enhancement of viral growth was observed in the L13-deficient cells, in immune as well as nonimmune cell types, providing the first indication of a novel role of L13 in cell-intrinsic, innate antiviral defense. More-detailed studies unraveled a direct cis-acting silencing mechanism in which the translation of a single, growth-limiting viral mRNA is silenced by the released nonribosomal L13a. This silencing was mediated by the ribosome-released L13a in the form of an RNA-binding complex assembled on the 3′UTR of viral mRNA. Even though the virally activated translational silencing mechanism shared L13a with the GAIT mechanism, multiple functional and molecular properties established a clear distinction between the two, such that the viral mechanism was given a new name, VAIT (respiratory syncytial virus-activated inhibitor of translation).
MATERIALS AND METHODS
Cell culture and virus growth.
The human alveolar epithelial A549 cell line (ATCC CCL-185; ATCC, Manassas, VA) was cultured in monolayers using standard Eagle's minimal essential medium (MEM) containing l-glutamine, heat-inactivated fetal bovine serum (10%, vol/vol), penicillin (100 U/ml), and streptomycin (100 μg/ml). Bone marrow-derived mouse macrophage cells were obtained as follows. Age-matched C57BL/6 wild-type and macrophage-specific L13a−/− mice were euthanized by CO2 asphyxiation, and single-cell suspensions were made from the bone marrow of femurs, using a 26 gauge (26G) needle and syringe. For differentiation and maturation, the cell populations were grown in L929 cell conditioned medium in the presence of recombinant macrophage colony-stimulating factor (Peprotech) at a concentration of 10 ng/ml for 7 days. The resultant macrophages were >90% pure, as checked by positive staining for CD11b and F4/80, and were replated into fresh dishes for use in RSV infection and related studies.
Wild-type RSV strain Long (serotype A) was grown, and its titer was determined on HEp-2 cell monolayers, using standard conditions as described previously (18, 19). The virus was purified on a sucrose density gradient as described previously (20) and stored frozen in small portions at −80°C. Unless otherwise stated, a multiplicity of infection (MOI) of 3 was used for RSV infection in cell culture. For attached cells (all cells used here other than macrophages), medium was replaced with fresh medium 4 h after addition of RSV to remove unadsorbed virus.
To measure RSV plaquing efficiency, virus-infected macrophages were photographed at a magnification of ×20, and digital pictures were captured. The infected cell foci in a total of 20 fields were counted and photographed, and their diameters were measured by a ruler and corrected for magnification.
The human metapneumovirus (hMPV) strain CAN97-83 and vesicular stomatitis virus (VSV) strain Indiana were grown and their titers determined as described previously (21, 22).
Plasmid constructs.
Luciferase reporter plasmids for transient transfection were constructed by cloning the indicated 3′UTR regions of the RSV M gene mRNA into the unique SpeI and HindIII restriction sites of the firefly luciferase vector described before (12), starting with the viral genome RNA (since the genome has no intron) and using standard reverse transcription (RT)-PCR-based cloning procedures. All expression clones were in the pCAGGS expression vector such that a FLAG tag (DYKDDDDKP) was added to the N terminus of the recombinant protein (23).
The L13a short hairpin RNA (shRNA) clone was constructed by ligating synthetic double-stranded DNA, corresponding to a prevalidated L13a siRNA sequence, into the BglII and HindIII sites of the pSUPER.puro vector (OligoEngine, Seattle, WA) essentially as described before (17, 24). A unique BamHI site was placed in the loop region of the sequence for initial screening of the plasmid clones, which were later confirmed by sequencing (17, 24). One shRNA was designed against the following 3′UTR sequence of L13a: CCAGTTACTATGAGTGAAA. The negative-control (nontargeting) shRNA was similarly constructed, using the siCONTROL sequence shown below. A549 cells were transfected with these plasmids, and stable transfectants were obtained by selection against puromycin (1.5 μg/ml). Loss of L13a was confirmed by immunoblotting using the rabbit antibody described previously (12).
siRNA, transfection, immunoblot, and reporter luciferase assays.
Two small interfering RNAs (siRNAs) against the 3′UTR of M mRNA had the guide (sense) strands 5′CAUCAGUGUGUUAAUUCAU3′ (siRNA 1) and 5′CUCUGUGGUUCAACCAAUC3′ (siRNA 2). The control siRNA contained the guide strand 5′UAAGGCUAUGAAGAGAUAC3′ (siCONTROL nontargeting siRNA; Thermo Fisher Scientific). All siRNAs were transfected as described previously (18). Transient transfections were performed using Lipofectamine 2000 (Life Technologies). Immunoblotting was performed using appropriate antibodies and horseradish peroxidase (HRP)-based chemiluminescence detection system (Licor). Dual-luciferase assays were done as described previously, using a Renilla luciferase plasmid to normalize for transfection efficiency of the firefly luciferase reporter plasmids (23).
Polysomal analysis.
Polysomes were analyzed in 5 to 50% linear sucrose gradients as described previously (12). Gradients were fractionated using an ISCO gradient fractionation system equipped with a UA-6 detector; light RNP fractions, 40S, 60S, 80S, and heavy polysome fractions were monitored by continuous UV absorption profiles at A254. The collected fractions were used to isolate translationally active and inactive pools of mRNAs. Total RNA was isolated from these fractions by extraction with TRIzol (Invitrogen, Carlsbad, CA) and purified by an RNeasy minikit (Qiagen, Valencia, CA). The RNA was used as the template in real-time reverse transcription-PCR quantification with the primers listed in Table 1.
TABLE 1.
Sequences of real-time qPCR primers for RSV gene mRNAsa
| Gene encoding: | Sense | Starting nt no. | Primer sequence (5′ to 3′) | Product size (kb) |
|---|---|---|---|---|
| NS1 | Fwd | 207 | GAATGGCATTGTGTTTGTGC | |
| Rev | 297 | TGGCATTGTTGTGAAATTGG | 91 | |
| NS2 | Fwd | 241 | TGCACAAAGTGGGAAGCAC | |
| Rev | 354 | TGCCAATGCATTCTAAGAACC | 114 | |
| N | Fwd | 771 | TGCAGGGCAAGTGATGTTAC | |
| Rev | 861 | TTCCATTTCTGCTTGCACAC | 91 | |
| P | Fwd | 602 | GGCAAGACTCAGGAATGAGG | |
| Rev | 705 | TCCCTTCCAACAGGTTGTTC | 104 | |
| M | Fwd | 249 | AATGCCCAGCAAATTTACCA | |
| Rev | 341 | GCCTTGATTTCACAGGGTGT | 93 | |
| SH | Fwd | 285 | CCAATCTGATGGCACAAAAC | |
| Rev | 376 | GCTTGCATGGTGAGATGTTG | 92 | |
| G | Fwd | 382 | AAGTCAACCCTGCAATCCAC | |
| Rev | 477 | TTTGTTTTGGCGTTGTTTTG | 96 | |
| F | Fwd | 1242 | TAGGAGCCATTGTGTCATGC | |
| Rev | 1333 | ATCGCACCCGTTAGAAAATG | 92 | |
| M2 | Fwd | 104 | CCCATGCACTGCTTGTAAGA | |
| Rev | 208 | CCAACTCTGCAGCTCCACTT | 105 | |
| L | Fwd | 5732 | TCCTGCTACAGATGCAACCA | |
| Rev | 5830 | ACAGGCAATTCAGCATCACA | 99 | |
| GAPDH | Fwd | 746 | CTGGAAAACCCTGCCAAATA | |
| Rev | 837 | TGCTCAGTTTAGCCCAGGAT | 92 |
The expected PCR product sizes were calculated from the starting nucleotide numbers. Reverse transcription of the positive-sense mRNA was conducted with the reverse (Rev) primers, followed by PCR conducted with both forward (Fwd) and reverse primers.
To detect the proteins released from the ribosomes (see Fig. 3D), the cytoplasmic extract was layered on a 20% sucrose cushion made in the same buffer (10) and centrifuged as above. The top portion of the layer (containing released proteins) and the precipitated ribosome at the bottom were collected and analyzed by immunoblotting with appropriate antibodies.
FIG 3.
Translational repression by L13a requires cis-acting sequences of the M mRNA. (A) Two regions of RSV A serotype M mRNA (left) with potential hairpin structures predicted by Mfold (right) (12, 27). The start and the stop codons are in lowercase; the two hairpin sequences are underlined. (B) The M hairpin region inhibits translation in cis in RSV-infected cells in an L13a-dependent manner. The 243-nt-long sequence near the 3′-end (A) was cloned downstream of the luciferase reporter construct as shown, the DNA was transfected into A549 cells (or A549 L13a knockdown clone 4), and Luc activity was measured with or without RSV infection at 24 h postinfection. Control cells (left) were transfected with Luc plasmid without the M sequence and treated similarly. Significant differences are indicated by the P values. (C) Recombinantly overexpressed M protein alone stimulates RSV growth. A549 cells were transiently transfected with M expression clones with (M-U) or without (M) 3′UTR or with N and P expression clones and also infected with RSV. Total M protein was detected by immunoblotting with a polyclonal antibody against full-length M, and all recombinant proteins, which were FLAG tagged, were detected by monoclonal FLAG antibody. All protein bands are marked with their molecular mass values: 30 kDa (M), 38 kDa (P), and 42 kDa (N). The first lane (—) represents control, untransfected but RSV-infected A549 cells. (D) L13a is rapidly released from ribosomes in RSV-infected epithelial cells, as detected by immunoblotting; control L11 protein was not released. Infections with VSV and hMPV, carried out similarly, did not promote L13a release, even at 24 hpi.
RNA EMSA.
U937 and A549 cells were grown in monolayers, and the A549 cells were infected with RSV at an MOI of 3 for 12 h. The cells were washed three times with phosphate-buffered saline (PBS), resuspended in buffer (50 mM Tris-Cl [pH 8.0], 50 mM NaCl, 1 mM phenylmethylsulfonyl fluoride [PMSF], 1 mM dithiothreitol [DTT]), and lysed by freeze-thaw cycles. The lysate was centrifuged at 10,000 × g for 5 min, and the supernatant was collected and used in an electrophoretic mobility shift assay (EMSA). Synthetic ceruloplasmin GAIT RNA (9) and the RSV M hairpin RNA (named VAIT RNA) were purchased commercially, and their 3′ ends were biotinylated using the Pierce RNA 3′-end biotinylation kit (Thermo Scientific). The labeled GAIT or VAIT RNA (50 fmol) was incubated for 30 min in ice with 5 μg of cellular protein in a 20-μl reaction mixture containing 10 mM HEPES (pH 7.3), 20 mM KCl, 1 mM MgCl2, 1 mM DTT, 5% glycerol, and 10 μg yeast tRNA. In competition experiments, the corresponding unlabeled RNAs (at 10- or 100-fold) were added to the extract 10 min before addition of the labeled probe. RNA-protein complexes were separated by native gel electrophoresis (5% polyacrylamide in 0.5× Tris-borate EDTA [TBE] buffer) at 4°C. Complexes were transferred to a nylon membrane and detected by chemiluminescence using the Pierce Lightshift chemiluminescent RNA EMSA kit (Thermo Scientific).
RESULTS
Loss of L13a promotes better RSV growth in cell culture.
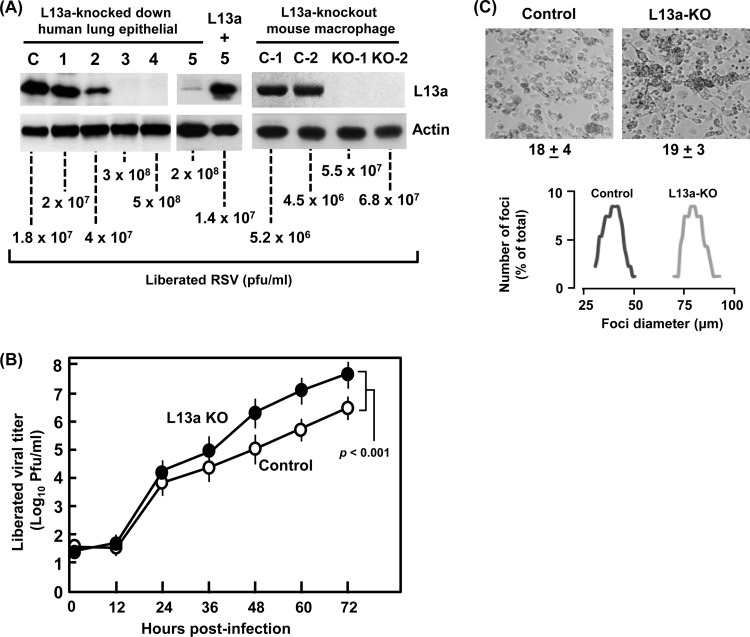
In our first set of studies, we created stable transfectant lung epithelial A549 cell lines, expressing specific shRNA against L13a. Four independent clones were tested for loss of L13a, among which no loss was seen in one clone and around 80% loss was seen in another, whereas two clones showed near-complete loss (Fig. 1A, left panel). These cells were infected with RSV, and viral growth was quantified by plaque assay of the liberated virus in the infected-cell medium at 72 h postinfection (hpi). It was clear that virus replication inversely correlated with the L13a level of the cell, such that fully L13a-deficient cells generated nearly 10 times more infective virus than those containing wild-type levels of L13a (Fig. 1A, left panel). Essentially identical results were also seen in L13a-knocked-down HeLa cells (data not shown). To ascertain that the effect of shRNA was L13a specific and not due to an off-target effect, we created another stable cell line in A549, in which an shRNA against the 3′UTR of L13a was expressed, and the reduction of L13a protein was confirmed; this clone also supported a high yield of RSV (Fig. 1A). We then restored L13a in this cell line by transient transfection of a FLAG-tagged L13a plasmid lacking the 3′UTR, refractory to the shRNA (Fig. 1A). Restoration of L13a indeed caused significant reduction of RSV growth.
FIG 1.
L13 deficiency leads to better virus growth. (A, left) Five independent A549 cell clones with stably integrated anti-L13a shRNA (1 to 5) or control nonspecific shRNA (denoted C) were infected with RSV, and the total proteins of infected cell monolayers at 18 hpi were analyzed for L13a (and control actin) by immunoblotting. Clones 1 to 4 used shRNA against the coding sequence; however, in clone 5 the shRNA was against the 3′UTR, and thus, L13a could be restored by transient transfection with FLAG-L13a plasmid (1 μg DNA per 5 × 105 cells), and virus was added 24 h after transfection. In parallel wells, virus liberated in cell-free medium at 72 hpi was quantified by standard serial dilution and plaque assay on HEp-2 cells. Note the clonal variation of L13 silencing and the inverse correlation between L13a levels and viral titer. (A, right) As described above for the left panel, except that macrophages from two L13a KO mice (L13aflox/floxLysMCre+) (KO-1 and KO-2) and two control mice (L13aflox/flox) (C-1 and C-2) were harvested and used in RSV infection. (B) Infectious virus, liberated in the medium at different times following infection of cultured cells, was plaque assayed on HEp-2 cells. Numbers from the two KO macrophage lines and the two controls were averaged and plotted with error bars and the P value as shown. (C) RSV infection foci formed on control and L13a KO macrophages. We used a low MOI (∼0.5) for better recognition of the foci in these cells. Top, photomicrographs of the infected macrophages. Note that macrophages are relatively round and loosely attached to the plastic surface; thus, in contrast to the large syncytia that are characteristically formed in RSV-infected epithelial cell monolayers, the infected macrophages produce clumps or foci. The average number (with standard error) of recognizable foci in multiple fields of the same area is written below each image and is similar in control (18 ± 4) and KO (19 ± 3) cells. However, note the larger size of the foci in the KO, consistent with reinfection of a greater number of neighboring cells by the larger number of released progeny virus (A and B). Bottom, the diameters of 200 foci were measured in control (dark line) and L13a-KO (gray line) fields, and the percentage of each size category was plotted for those that were above 1%.
We then tested virus growth in L13a knockout (KO) cells. Using the Cre-Lox technology and macrophage-specific lysozyme M gene promoter to drive Cre recombinase expression, we have recently created a macrophage-specific L13 knockout (L13-KO) mouse (16). We isolated the L13a-KO macrophages from these mice and conducted similar RSV growth experiments on these cells in culture. Results (Fig. 1A, right panel) showed that although macrophages were less permissive for RSV replication than lung epithelial cells (25), the KO macrophages from two different animals supported roughly 10- to 12-fold-higher viral replication than those from the control animals. Although the progeny viral yield was affected by L13a, essentially all cells were infected by the input virus (at an MOI of 3), as confirmed by immunostaining with anti-RSV antibody (data not shown). A detailed kinetics analysis of virus production (Fig. 1B) further confirmed that L13a regulates intracellular replication of the virus, ultimately leading to a larger burst size from the KO cell that is 1 order of magnitude higher (P > 0.001 at 72 h) than in control cells.
The overall higher virus growth in L13a-deficient cells was further evidenced by comparing the efficiency of plaque formation in the two kinds of macrophages. An equal number of infectious virus particles (based on determination of PFU on the HEp2 cell monolayer) was added to the wild-type and KO macrophage monolayers (at an MOI of ∼0.5), and the characteristic infection foci containing syncytial clumps were scored at 72 h postinfection in multiple fields. Nearly equal numbers of foci were found in wild-type and KO cells (Fig. 1C, top panel; 18 ± 4 and 19 ± 3, respectively, in the field), suggesting that similar number of cells were infected initially; however, the foci in the L13a KO cells were nearly twice as large (averaging 80 μm versus 40 μm in control; Fig. 1C, bottom panel). Overall, most foci in the control consisted of only two fused cells, whereas in the L13a KO macrophages an average focus involved four cells. Together, these results indicate that higher virus growth in KO cells is due to a more robust and faster intracellular virus replication rather than an effect on the early events of infection such as virion reception or entry. Clearly, in cells of both epithelial and immune origins, L13a effectively functions as an intracellular antiviral host factor against RSV.
L13a is a translational repressor of RSV matrix protein mRNA.
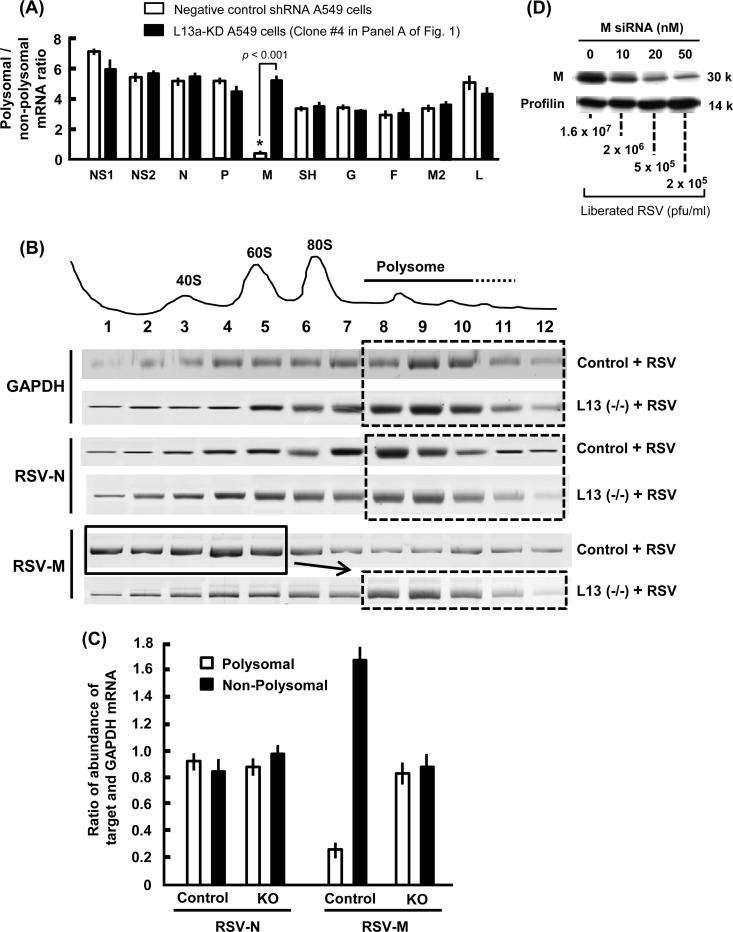
The ratio of polyribosomal and free nonpolyribosomal fractions of a particular mRNA is a quantitative measure of its translational status (12, 26, 27). In an attempt to decipher how the ribosomal protein L13a may moderate virus growth, we measured this ratio for each major viral mRNA in wild-type and L13a-deficient cells. Polysomal and free ribosomal populations were fractionated by sucrose gradient centrifugation, and the amount of each RSV gene mRNA in the fractions was quantified by real-time PCR. In our first set of studies, we used L13-silenced A549 clone 4 (Fig. 1A) and analyzed the ratio of polysomal to nonpolysomal abundance for a quick screening of all 10 major viral mRNAs. The results (Fig. 2A) clearly show that among all viral mRNAs, the matrix (M, also known as M1) mRNA is unique in its poor association with the polysomes in wild-type A549 cells, but this association is significantly improved in the L13a-knocked-down cells, suggesting a specifically improved translation in the absence of L13a.
FIG 2.
L13a deficiency rescues the polysomal association of viral M mRNA. (A) Polysomal association in L13 knockdown A549 cells. Ribosomal fractions isolated from RSV-infected L13a-silenced A549 clone 4 and control cells (Fig. 1A) were subjected to 5 to 25% sucrose gradient centrifugation as described in Materials and Methods (12). Total RNA from each fraction was purified and used as the template for real-time RT-PCR with the gene-specific primers described in Materials and Methods. For each gene, the ratio of polysomal to nonpolysomal mRNA amount was calculated and plotted. Note the uniquely low polysomal abundance of the M mRNA in the control cell and its restoration in L13a-deficient cells, the highly significant difference being indicated by the low P value. Average results from three experiments are presented with standard error bars. (B) Polysomal association profile of selected mRNA in L13a knockout and control mouse macrophages. Bone marrow-derived macrophages were infected with RSV, and polysome fractionation and RT-PCR were performed as described for panel A. Representative agarose gel profiles of the ethidium bromide-stained PCR products are shown. A plot of the A254 values of the fractions (1 through 12) is shown at the top. Note the shift of a large pool of M mRNA from the nonpolysomal (fractions 1 to 5; solid line box) to the polysomal (fractions 8 to 12; dashed line box) region upon loss of L13a, whereas the distributions of two control mRNAs, namely, viral N and cellular GAPDH, were not affected. (C) Quantification of the results shown in panel B. Ratios of target mRNA/GAPDH mRNA in polysomal and nonpolysomal fractions were determined by measuring the intensities of the corresponding bands for two viral mRNAs, L13-regulated M and control N. (D) RNA interference (RNAi)-mediated knockdown of M protein severely inhibits RSV growth. RSV infection was carried out in A549 cells as described for Fig. 1A, except that the virus was added 6 h after transfection with increasing concentrations (0 to 50 nM) of anti-M siRNA1 (see Materials and Methods). Silencing of M was confirmed by immunoblotting, while profilin (control) was unaffected. The virus liberated in the medium at 48 h postinfection was plaque assayed on an HEp-2 cell layer as described for Fig. 1A. Use of anti-M siRNA2 generated essentially identical results (not shown).
We then extended this investigation to L13-KO mouse macrophages and quantified each viral mRNA in the gradient fractions by specific reverse transcription-quantitative PCR (qRT-PCR). These results (Fig. 2B and C) essentially confirmed the previous findings and showed that polysomal association of M was indeed enhanced in the L13a-KO cells, whereas the control viral N and cellular GAPDH (glyceraldehyde-3-phosphate dehydrogenase) mRNAs were equally abundant in polysomal and free ribosomal fractions. Together, these results indicate that L13a may effectively function as a translational inhibitor of RSV M gene mRNA.
RSV M protein is rate limiting for virus growth.
The correlation between increased polysomal association of M mRNA and enhanced RSV growth (Fig. 1 and 2) leads to the hypothesis that M mRNA translation and hence M protein steady-state levels are limiting in the RSV-infected cell. If this is true, then further reduction of M protein levels should reduce virus growth in the wild-type cell. To test these, we transfected A549 cells with increasing amounts of anti-M siRNA so that a range of silencing would be achieved and tested RSV growth in these cells as before. Indeed, greater silencing of M expression led to progressively lower virus production (Fig. 2D). Essentially identical results were obtained with two different siRNAs; therefore, only one set is presented as representative. The negative-control siRNA (used at 50 nM) had no effect on M proteins or viral titer (data not shown). Together, these results suggest that the translational status of the RSV M mRNA is restricted in a normal cell.
The M gene mRNA 3′UTR is essential for L13a-mediated translation repression.
As mentioned previously, in the context of IFN-γ signaling in immune cells, extraribosomal L13a represses translation by binding to the GAIT hairpin in the 3′UTR of the target mRNA (10–13). The start and end sequences of all RSV genes are well defined, and thus the coding as well as the 3′UTR regions of M mRNA are precisely known (28). To determine if the ability of L13a to silence M translation also requires a cis-acting sequence, we subjected the 3′-terminal 250-nucleotide (nt) segment of the M mRNA to the mFold algorithm (29). Interestingly, we found two prospective secondary structures, both of which were hairpins with a short loop and a stem with a bulge in the middle (Fig. 3A). While one of them was in the 3′UTR (M hairpin-2), the other one was upstream, inside the coding sequence (M hairpin-1). Both the putative hairpins showed distant resemblance to the original GAIT hairpin (Fig. 3A, right). There was no major sequence homology between the two hairpin sequences.
For experimental assessment of the role of the hairpin region, we cloned this segment downstream of the luciferase (Luc) coding sequence of our translational reporter plasmid (12) and also cloned the two hairpins individually. Following transient transfection of the plasmid into A549 cells and RSV infection, we observed significantly lower Luc activity from constructs containing the hairpin in the 3′UTR, whereas the hairpin internal to the coding sequence did not inhibit Luc activity (Fig. 3B). Use of the noninhibitory hairpin-1 in tandem with hairpin-2 did not influence the inhibitory activity of hairpin-2. The silencing due to hairpin-2, harbored in the 3′UTR, was relieved in L13a knockdown (KD) A549 cells (Fig. 1A, clone 4). Together, these results lead to the conclusion that L13a is required for the endogenously imposed translational silencing of RS viral M mRNA mediated by the cis-acting hairpin-2 sequence in the 3′UTR.
Note that our M protein knockdown results, presented earlier (Fig. 2C), suggested that intracellular M level is limiting for RSV growth. The discovery of the regulatory 3′UTR of the M mRNA, as described above, allowed us to perform the reciprocal experiment and ask if increasing the amount of M would promote better virus growth. To answer this, we transfected cells with plasmids expressing recombinant FLAG-tagged M, in which the 3′UTR is either present or deleted. The cells were infected with RSV. We then detected the recombinant M protein by anti-FLAG and total M protein by an M-specific polyclonal antibody in immunoblotting. The liberated progeny virus was assayed by plaquing on HEp-2 cells as before. The results (Fig. 3C) show that the M clone that included the 3′UTR (Fig. 3C, lane M-U) did not express M protein well. In stark contrast, the M clone that lacked the 3′UTR (Fig. 3C, lane M) and contained only the coding sequence produced large amounts of M, elevating the total amount of M protein of the infected cell about 5-fold above the virally produced level. Increased M protein level was accompanied by enhanced virus growth, about 30-fold above the normal titer (from 1.1 × 107/ml to 4.4 × 108/ml), which was similar to virus growth in the L13a-deficient cells seen before (3 to 5 × 108/ml [Fig. 1A]). Recombinant expression of two other RSV proteins, namely, the nucleocapsid protein N and the phosphoprotein P, did not boost M synthesis or virus growth, showing that virally produced N and P were not limiting for virus growth and that the stimulatory effect of M was specific.
RSV infection triggers release of L13a from the ribosome.
Finally, we wanted to test whether L13a, in its role to silence M translation, functions as part of the ribosome or as a free protein. Thus, we tested whether L13a is released from ribosomes in the RSV-infected cell, as there is no report of such a release in any viral infection. Cells infected with RSV at an MOI of 3 were harvested at three different postinfection time points. To isolate the ribosomal and nonribosomal fractions, the total extract was subjected to centrifugation through a 20% sucrose cushion, and the released material on top and the sedimented ribosomes at the bottom were analyzed by immunoblotting. Previously, this method was successfully used to identify the release of L13a from the large ribosomal subunit in IFN-γ-activated human monocytes (9, 10). Indeed, a substantial portion of L13a (roughly half) was found to be released as early as 6 h after RSV infection, in comparison to uninfected cells. The release was L13a specific, since L11, another ribosomal large subunit protein, was not released (Fig. 3D). It was also RSV specific, since infection with VSV or hMPV, two other nonsegmented negative-strand RNA viruses, did not induce L13a release. Moreover, the titers of the progeny virus released from wild-type and L13a KD cells were essentially identical (number of VSV progeny, 4 × 1010 ± 1 × 1010/ml; number of hMPV progeny, 8 × 106 ± 1 × 106/ml).
RSV-activated translational suppression exploits a potentially novel, IFN-γ-independent mechanism.
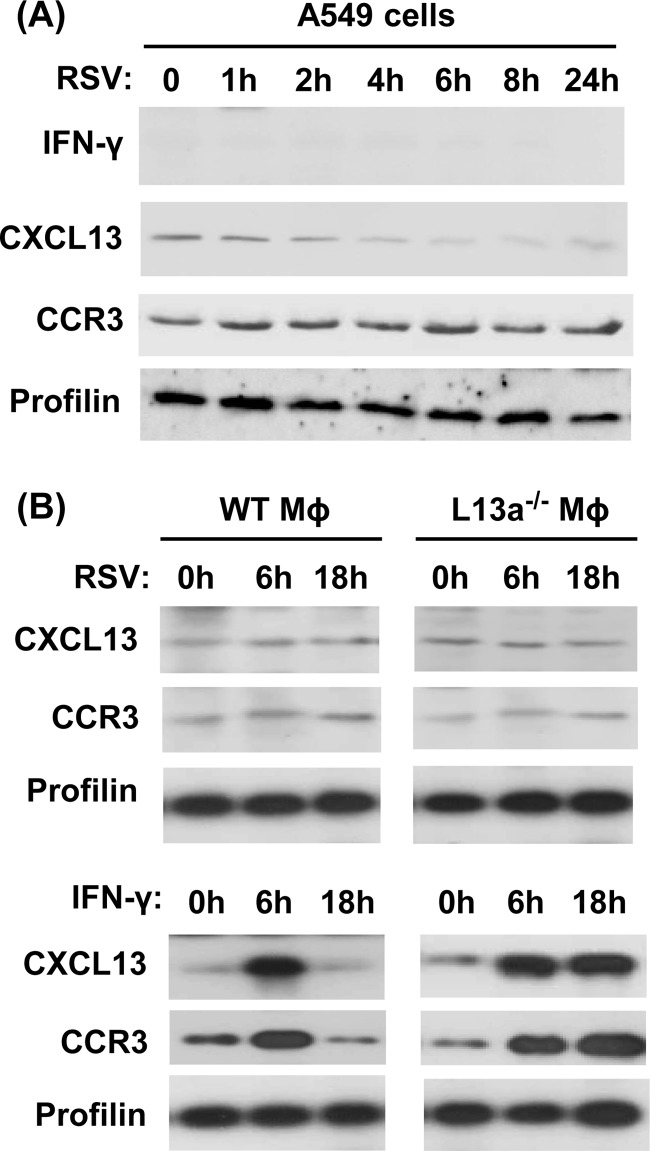
Our finding that RSV-triggered translational silencing of M occurs in nonimmune cells suggested that its mechanism is different from that of GAIT. To further ascertain the uniqueness of the mechanism, we monitored the possible induction of IFN-γ and steady-state levels of two GAIT-regulated molecules, CCR3 and CXCL13 (12), in RSV-infected A549 cells at different time points of postinfection. The results (Fig. 4A) show no detectable IFN-γ and no change in the basal, uninfected levels of CCR3 and CXCL13 at all times of RSV infection in A549 cells. CCR3 and CXCL13 levels also remained unchanged in primary macrophages obtained from both control and macrophage-specific L13a KO mice following RSV infection in cell culture (Fig. 4B). However, our recent studies of an endotoxemia model in macrophage-specific L13 knockout mice show higher translational status of these two mRNAs (16). Consistent with our published findings, our positive control, i.e., IFN-γ-treated wild-type macrophages, showed rapid upregulation of both proteins (at 6 h) followed by shutdown at later times (18 h), whereas sustained increase was seen in L13 KO macrophages, confirming a functional GAIT pathway in these cells (12). In all cells, control profilin was not affected by any treatment. Overall, these results further document an RSV-triggered signaling pathway that is independent of IFN-γ.
FIG 4.
The RSV (VAIT) mechanism functionally differs from that of GAIT. A549 cells (A) or primary macrophages (Mϕ) (B) were either infected with RSV (at an MOI of 3) or treated with IFN-γ (500 units/ml) as shown, and at various times thereafter, total cell lysates were prepared and subjected to immunoblotting to detect the indicated proteins. Note the induction of L13-regulated CXCL13 and CCR3 in IFN-γ-treated macrophages but no such induction upon RSV infection.
RNA-protein interaction assay indicates a novel virus-activated translation-inhibitory complex.
To gain molecular insights into the uniqueness of the viral silencing mechanism, we performed RNA EMSAs. We synthesized two kinds of RNA, namely, the GAIT hairpin and the M hairpin; we also prepared extracts from two kinds of cells, namely, IFN-γ-treated monocytic U937 cells and RSV-infected epithelial A549 cells. EMSA was then conducted in various homologous and heterologous RNA-protein combinations. Our first set of binding reactions were positive controls in which we reproduced the results in published literature (9, 14), indicating that GAIT RNA indeed generates a RNP complex when extracts from 24-h IFN-γ-treated U937 cells were used and that this complex did not occur with extracts made after 8 h of IFN-γ treatment (Fig. 5A, lanes 1 and 2). Moreover, formation of the GAIT RNP was strongly inhibited by L13a antibody but not by control nonimmune IgG (Fig. 5A, lanes 3 and 4). The formation of this GAIT RNP was also competed out by only 10× excess of GAIT RNA (Fig. 5A, lanes 5 and 6) but, interestingly, not by viral M hairpin RNA, which we refer to as VAIT RNA, even when used at 100× excess (Fig. 5A, lanes 7 and 8). In reciprocal experiments, the extract of virus-infected cell extract did not generate any GAIT RNP complex (Fig. 5A, lane 10). These results (Fig. 5A), therefore, revealed a high degree of specificity of interaction between the cis-acting RNA elements and their cognate silencing complexes.
FIG 5.
Specific RNP complexes formed by GAIT (A) and VAIT (B) RNA. EMSA with synthetic biotin-labeled RNA was performed as described in Materials and Methods, using extracts of either RSV-infected A549 cells (lanes 9, 10, and 12 to 18) or U937 cells (lanes 1 to 8 and 19) grown in the presence of IFN-γ (500 units/ml) for 8 h or 24 h, as shown. Where indicated, the extract was preincubated with either rabbit polyclonal L13a antibody (αL13a) (lanes 3 and 13) or control nonimmune immunoglobulin (IgG) (lanes 4 and 14). For competition, the same GAIT or VAIT RNAs, but not labeled with biotin, were used in either 10-fold or 100-fold excess as indicated. In both panels, the positions of the mobility shifted (retarded) RNA band (RNP complex) and the free RNA are marked.
We then performed similar EMSAs using the viral VAIT RNA, which further established the specificity. In these results (Fig. 5B), VAIT RNP specifically formed with infected-A549 cell extract, whereas control, uninfected-cell extract did not. As with GAIT RNP, the VAIT RNP formation was also inhibited by L13 antibody, suggesting that L13a was a key component of both RNPs. The VAIT RNP was competed out by homologous VAIT RNA at only 10× excess, whereas the heterologous GAIT RNA failed to compete even at 100× excess. Finally, in striking contrast with GAIT RNA, the VAIT RNA did not form any RNP with the 24-h IFN-γ-treated monocyte extract.
Together, these results show that even though the viral VAIT sequence, like its cellular counterpart GAIT, uses L13a for translational silencing, the overall silencing RNP assembled by the viral sequence is highly specific and differs from the GAIT complex, likely because it contains specific proteins that are found only in the virus-infected cell.
DISCUSSION
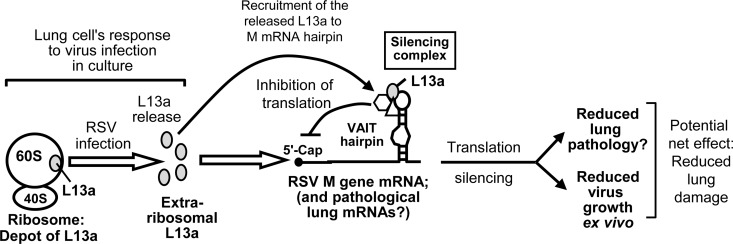
The collective results presented here suggest that the host cell responds to RSV infection by releasing the large ribosomal subunit protein L13a, which then interacts with the VAIT hairpin sequence in the 3′UTR of the matrix protein (M) mRNA to reduce its translation. The ability of this L13a to suppress additional VAIT RNA sequence, delivered by transfection, shows that L13a is in excess over the amount of this RNA present in the RSV-infected cell. As M is a rate-limiting protein for virus growth, the regulation of its synthesis by L13a reduces RSV growth in cultured cell lines and primary macrophages and may also moderate RSV disease pathogenesis. These findings are summarized in a schematic model (Fig. 6).
FIG 6.
Model for regulation of RSV growth by L13a. An unknown but potentially novel signaling mechanism promotes rapid release of L13a from RSV-infected cell ribosomes, and this extraribosomal L13a then silences viral M translation by recruiting an RNP complex at the VAIT hairpin in the 3′UTR (see the text for details). We present this cellular response as an innate immune mechanism that moderates virus growth and possibly the associated pathology of the host.
To our knowledge, this is the first report of a ribosomal protein acting as an antiviral, thus representing a new paradigm in innate immunity. Our results indicate that it is not a broad-spectrum antiviral factor as it is neither activated by nor inhibitory to human metapneumovirus, another member of the Paramyxoviridae family and also a significant pediatric pathogen. RSV is the prototype member of the Pneumovirus genus of this family; the only other known member of this small genus is the pneumonia virus of mice (PVM), which is strictly a mouse virus (30, 31). We have not tested if PVM is regulated by murine L13a; nonetheless, it may be unlikely because the PVM M gene 3′UTR (31) does not generate a fold resembling those of RSV VAIT (data not shown). However, murine and human L13a are nearly identical in sequence, with only 7 nonidentical amino acids of a total of 203, of which 4 are highly conservative replacements. Nevertheless, it is an interesting possibility that the viral target gene and the paired ribosomal protein as its translational repressor will vary among different viruses. In this scenario, a specific rate-limiting protein in each virus may act as a rheostat of viral growth, regulated by a host ribosomal protein. For example, PVM may use the relatively long 3′UTR of its SH or G genes and a ribosomal protein other than L13a.
RNA viruses undergo rapid mutational changes that must be balanced by fitness to selection pressure (32, 33). Our findings lead to the interesting scenario that natural mutational drift in the VAIT sequence may modulate its interaction with L13a, in turn regulating RSV replication. Specifically, one would expect that RSV will attempt to mutate the VAIT sequence to evade L13a-mediated cellular immunity, so long as such mutations do not compromise any essential function(s) of the 3′UTR that are currently unknown. It is to be noted that the secondary structure, rather than the exact sequence of the cellular GAIT element, plays a major role in its function (12), and thus, the same may be true for VAIT. In either case, natural sequence changes in the 3′UTR region of the M mRNA may act as a specific translational regulator of RSV growth and thus have an impact on host-virus interaction. It is also possible that a total inactivation or loss of the VAIT hairpin would result in a hypervirulent virus and that an optimal amount of silencing represents an important strategy to achieve the proper level of viral virulence in the host, explaining the evolutionary preservation of the hairpin. Proper recognition of this hairpin by infected-cell proteins may underlie the stringent specificity of the VAIT RNP assembly over the cellular GAIT RNP that we have observed (Fig. 5).
Comparative modeling with prokaryotic (47) and eukaryotic (34) ribosomes suggests that L13a is located near the surface of a large ribosomal subunit and uses a single Arg at position 68 to anchor with rRNA (35). This structural feature is consistent with its dissociation in response to specific signals (10), of which we now know two, namely, IFN-γ and RSV. Other surface ribosomal proteins may also dissociate under conditions that are yet to be discovered and assemble a silencing complex on specific viral mRNA structures. Such complexes may consist of common subunits as well as specific ones for each viral target gene.
The signaling pathway that triggers L13a dissociation in RSV-infected cells remains to be unraveled and is under investigation. For GAIT, recent results have identified a death-associated protein kinase signaling cascade responsible for L13a phosphorylation (36) in the IFN-γ-treated immune cells. This pathway apparently begins with the activation of DAPK (death-associated protein kinase-1) via an unknown mechanism, which then phosphorylates and activates a second kinase of this family, namely, ZIPK (zipper-interacting protein kinase). ZIPK then phosphorylates L13a, promoting its release from the ribosomes. In the case of RSV, however, the responsible kinase and the signaling mechanism may be substantially different because of the ability of RSV to stimulate L13a release in nonimmune cells, where GAIT is inactive. This provides an opportunity to discover a unique and contrasting pathway of L13a-mediated translational silencing.
In recent years, translational silencing complexes of various kinds, such as the microRNA-assembled RISC (RNA-induced silencing complex), have been found to localize in specific cytoplasmic entities, such as P bodies, stress granules, GW bodies, which are defined by protein markers and functional roles, although considerable overlap among them is known (37–42). Interestingly, several viruses induce stress granules in the infected cells, the exact function of which is not well understood (42). Detailed studies with RSV (43–46) showed the induction of at least two types of cytoplasmic entities, the stress granules (SGs) and the viral inclusion bodies, both of which contained the RNA-binding protein HuR (46), a cardinal regulator of mRNA translation and stability. Interestingly, RSV replication was impaired in a stable epithelial cell line in which the SG response was inhibited by reduced expression of the Ras-GAP SH3 domain-binding protein (G3BP) (46). Use of specific probes showed the presence of viral RNA in both SGs and viral inclusion bodies, suggested that these granules may be important physiological sites of viral transcription and translation. Considering the role of L13a in translational regulation of RSV M mRNA, it is tempting to speculate that the corresponding silencing complex may also localize to one of these granules, or perhaps to a novel one yet to be discovered.
In conclusion, we have offered evidence for a novel paradigm of inducible innate immunity, in which the ribosomal protein L13a is mobilized in response to the infection itself. The response is rapid since it involves release of preassembled L13a, thus ensuring viral inhibition at an early stage of infection. In future studies, it will be important to determine the exact composition and structure of the novel VAIT RNP and how it differs from that of the cellular GAIT, which should eventually shed light on the molecular mechanism of how it specifically inhibits viral translation.
ACKNOWLEDGMENTS
This work was supported by Public Health Service Grants NIH HL79164 (B.M.) and NIH S10 OD010381 (S.B.) and American Heart Association Grant-in-Aid 0855555D (B.M.) and Predoctoral Fellowship Grant 11PRE7660008 (D.P.).
We thank Titus Barik for his help with the statistical analysis of the data.
Footnotes
Published ahead of print 4 June 2014
REFERENCES
- 1.Collins PL, Melero JA. 2011. Progress in understanding and controlling respiratory syncytial virus: still crazy after all these years. Virus Res. 162:80–99. 10.1016/j.virusres.2011.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sommer C, Resch B, Simões EA. 2011. Risk factors for severe respiratory syncytial virus lower respiratory tract infection. Open Microbiol. J. 5:144–154. 10.2174/1874285801105010144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Graham BS. 2011. Biological challenges and technological opportunities for respiratory syncytial virus vaccine development. Immunol. Rev. 239:149–166. 10.1111/j.1600-065X.2010.00972.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barik S. 2013. Respiratory syncytial virus mechanisms to interfere with type 1 interferons. Curr. Top. Microbiol. Immunol. 372:173–191. 10.1007/978-3-642-38919-1_9 [DOI] [PubMed] [Google Scholar]
- 5.Kumar H, Kawai T, Akira S. 2011. Pathogen recognition by the innate immune system. Int. Rev. Immunol. 30:16–34. 10.3109/08830185.2010.529976 [DOI] [PubMed] [Google Scholar]
- 6.de Koning HD, Simon A, Zeeuwen PL, Schalkwijk J. 2012. Pattern recognition receptors in infectious skin diseases. Microbes Infect. 14:881–893. 10.1016/j.micinf.2012.03.004 [DOI] [PubMed] [Google Scholar]
- 7.Lee CC, Avalos AM, Ploegh HL. 2012. Accessory molecules for Toll-like receptors and their function. Nat. Rev. Immunol. 12:168–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arpaia N, Barton GM. 2013. The impact of Toll-like receptors on bacterial virulence strategies. Curr. Opin. Microbiol. 16:17–22. 10.1016/j.mib.2012.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mazumder B, Fox PL. 1999. Delayed translational silencing of ceruloplasmin transcript in gamma interferon-activated U937 monocytic cells: role of the 3′ untranslated region. Mol. Cell. Biol. 19:6898–6905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mazumder B, Sampath P, Seshadri V, Maitra RK, DiCorleto PE, Fox PL. 2003. Regulated release of L13a from the 60S ribosomal subunit as a mechanism of transcript-specific translational control. Cell 115:187–198. 10.1016/S0092-8674(03)00773-6 [DOI] [PubMed] [Google Scholar]
- 11.Mazumder B, Seshadri V, Fox PL. 2003. Translational control by the 3′-UTR: the ends specify the means. Trends Biochem. Sci. 28:91–98. 10.1016/S0968-0004(03)00002-1 [DOI] [PubMed] [Google Scholar]
- 12.Vyas K, Chaudhuri S, Leaman DW, Komar AA, Musiyenko A, Barik S, Mazumder B. 2009. Genome-wide polysome profiling reveals an inflammation-responsive posttranscriptional operon in gamma interferon-activated monocytes. Mol. Cell. Biol. 29:458–470. 10.1128/MCB.00824-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sampath P, Mazumder B, Seshadri V, Gerber CA, Chavatte L, Kinter M, Ting SM, Dignam JD, Kim S, Driscoll DM, Fox PL. 2004. Noncanonical function of glutamyl-prolyl-tRNA synthetase: gene-specific silencing of translation. Cell 119:195–208. 10.1016/j.cell.2004.09.030 [DOI] [PubMed] [Google Scholar]
- 14.Sampath P, Mazumder B, Seshadri V, Fox PL. 2003. Transcript-selective translational silencing by gamma interferon is directed by a novel structural element in the ceruloplasmin mRNA 3′ untranslated region. Mol. Cell. Biol. 23:1509–1519. 10.1128/MCB.23.5.1509-1519.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Villenave R, Shields MD, Power UF. 2013. Respiratory syncytial virus interaction with human airway epithelium. Trends Microbiol. 21:238–244. 10.1016/j.tim.2013.02.004 [DOI] [PubMed] [Google Scholar]
- 16.Poddar D, Basu A, Baldwin WM, III, Kondratov RV, Barik S, Mazumder B. 2013. An extraribosomal function of ribosomal protein L13a in macrophages resolves inflammation. J. Immunol. 190:3600–3612. 10.4049/jimmunol.1201933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chaudhuri S, Vyas K, Kapasi P, Komar AA, Dinman JD, Barik S, Mazumder B. 2007. Human ribosomal protein L13a is dispensable for canonical ribosome function but indispensable for efficient rRNA methylation. RNA 13:2224–2237. 10.1261/rna.694007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bitko V, Barik S. 2001. Phenotypic silencing of cytoplasmic genes using sequence-specific double-stranded short interfering RNA and its application in the reverse genetics of wild type negative-strand RNA viruses. BMC Microbiol. 1:34. 10.1186/1471-2180-1-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bitko V, Shulyayeva O, Mazumder B, Musiyenko A, Ramaswamy M, Look DC, Barik S. 2007. Nonstructural proteins of respiratory syncytial virus suppress premature apoptosis by an NF-kappaB-dependent, interferon-independent mechanism and facilitate virus growth. J. Virol. 81:1786–1795. 10.1128/JVI.01420-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bitko V, Musiyenko A, Shulyayeva O, Barik S. 2005. Inhibition of respiratory viruses by nasally administered siRNA. Nat. Med. 11:50–55. 10.1038/nm1164 [DOI] [PubMed] [Google Scholar]
- 21.Guerrero-Plata A, Baron S, Poast JS, Adegboyega PA, Casola A, Garofalo RP. 2005. Activity and regulation of alpha interferon in respiratory syncytial virus and human metapneumovirus experimental infections. J. Virol. 79:10190–10199. 10.1128/JVI.79.16.10190-10199.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barik S, Banerjee AK. 1991. Cloning and expression of the vesicular stomatitis virus phosphoprotein gene in Escherichia coli: analysis of phosphorylation status versus transcriptional activity. J. Virol. 65:1719–1726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Swedan S, Musiyenko A, Barik S. 2009. Respiratory syncytial virus nonstructural proteins decrease levels of multiple members of the cellular interferon pathways. J. Virol. 83:9682–9693. 10.1128/JVI.00715-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Musiyenko A, Bitko V, Barik S. 2007. RNAi-dependent and -independent antiviral phenotypes of chromosomally integrated shRNA clones: role of VASP in respiratory syncytial virus growth. J. Mol. Med. 85:745–752. 10.1007/s00109-007-0179-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Panuska JR, Cirino NM, Midulla F, Despot JE, McFadden ER, Jr, Huang YT. 1990. Productive infection of isolated human alveolar macrophages by respiratory syncytial virus. J. Clin. Invest. 86:113–119. 10.1172/JCI114672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arava Y, Wang Y, Storey JD, Liu CL, Brown PO, Herschlag D. 2003. Genome-wide analysis of mRNA translation profiles in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. U. S. A. 100:3889–3894. 10.1073/pnas.0635171100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johannes G, Carter MS, Eisen MB, Brown PO, Sarnow P. 1999. Identification of eukaryotic mRNAs that are translated at reduced cap binding complex eIF4F concentrations using a cDNA microarray. Proc. Natl. Acad. Sci. U. S. A. 96:13118–13123. 10.1073/pnas.96.23.13118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fearns R, Peeples ME, Collins PL. 2002. Mapping the transcription and replication promoters of respiratory syncytial virus. J. Virol. 76:1663–1672. 10.1128/JVI.76.4.1663-1672.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zuker M. 2003. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 31:3406–3415. 10.1093/nar/gkg595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brock LG, Karron RA, Krempl CD, Collins PL, Buchholz UJ. 2012. Evaluation of pneumonia virus of mice as a possible human pathogen. J. Virol. 86:5829–5843. 10.1128/JVI.00163-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krempl CD, Lamirande EW, Collins PL. 2005. Complete sequence of the RNA genome of pneumonia virus of mice (PVM). Virus Genes 30:237–249. 10.1007/s11262-004-5631-4 [DOI] [PubMed] [Google Scholar]
- 32.Domingo E, Holland JJ. 1997. RNA virus mutations and fitness for survival. Annu. Rev. Microbiol. 51:151–178. 10.1146/annurev.micro.51.1.151 [DOI] [PubMed] [Google Scholar]
- 33.Novella IS, Quer J, Domingo E, Holland JJ. 1999. Exponential fitness gains of RNA virus populations are limited by bottleneck effects. J. Virol. 73:1668–1671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ben-Shem A, Jenner L, Yusupova G, Yusupov M. 2010. Crystal structure of the eukaryotic ribosome. Science 330:1203–1209. 10.1126/science.1194294 [DOI] [PubMed] [Google Scholar]
- 35.Das P, Basu A, Biswas A, Poddar D, Andrews J, Barik S, Komar AA, Mazumder B. 2013. Insights into the mechanism of ribosomal incorporation of mammalian L13a protein during ribosome biogenesis. Mol. Cell. Biol. 33:2829–2842. 10.1128/MCB.00250-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mukhopadhyay R, Ray PS, Arif A, Brady AK, Kinter M, Fox PL. 2008. DAPK-ZIPK-L13a axis constitutes a negative-feedback module regulating inflammatory gene expression. Mol. Cell 32:371–382. 10.1016/j.molcel.2008.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goodier JL, Zhang L, Vetter MR, Kazazian HH., Jr 2007. LINE-1 ORF1 protein localizes in stress granules with other RNA-binding proteins, including components of RNA interference RNA-induced silencing complex. Mol. Cell. Biol. 27:6469–6483. 10.1128/MCB.00332-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jain S, Parker R. 2013. The discovery and analysis of P bodies. Adv. Exp. Med. Biol. 768:23–43. 10.1007/978-1-4614-5107-5_3 [DOI] [PubMed] [Google Scholar]
- 39.Leung AK, Calabrese JM, Sharp PA. 2006. Quantitative analysis of Argonaute protein reveals microRNA-dependent localization to stress granules. Proc. Natl. Acad. Sci. U. S. A. 103:18125–18130. 10.1073/pnas.0608845103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stoecklin G, Kedersha N. 2013. Relationship of GW/P-bodies with stress granules. Adv. Exp. Med. Biol. 768:197–211. 10.1007/978-1-4614-5107-5_12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yao B, Li S, Chan EK. 2013. Function of GW182 and GW bodies in siRNA and miRNA pathways. Adv. Exp. Med. Biol. 768:71–96. 10.1007/978-1-4614-5107-5_6 [DOI] [PubMed] [Google Scholar]
- 42.White JP, Lloyd RE. 2012. Regulation of stress granules in virus systems. Trends Microbiol. 20:175–183. 10.1016/j.tim.2012.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fricke J, Koo LY, Brown CR, Collins PL. 2013. p38 and OGT sequestration into viral inclusion bodies in cells infected with human respiratory syncytial virus suppresses MK2 activities and stress granule assembly. J. Virol. 87:1333–1347. 10.1128/JVI.02263-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lifland AW, Jung J, Alonas E, Zurla C, Crowe JE, Jr, Santangelo PJ. 2012. Human respiratory syncytial virus nucleoprotein and inclusion bodies antagonize the innate immune response mediated by MDA5 and MAVS. J. Virol. 86:8245–8258. 10.1128/JVI.00215-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hanley LL, McGivern DR, Teng MN, Djang R, Collins PL, Fearns R. 2010. Roles of the respiratory syncytial virus trailer region: effects of mutations on genome production and stress granule formation. Virology 406:241–252. 10.1016/j.virol.2010.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lindquist ME, Lifland AW, Utley TJ, Santangelo PJ, Crowe JE., Jr 2010. Respiratory syncytial virus induces host RNA stress granules to facilitate viral replication. J. Virol. 84:12274–12284. 10.1128/JVI.00260-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ban N, Nissen P, Hansen J, Moore PB, Steitz TA. 2000. The complete atomic structure of the large ribosomal subunit at 2.4 A resolution. Science 289:905–920. 10.1126/science.289.5481.905 [DOI] [PubMed] [Google Scholar]