ABSTRACT
Mucosal epithelial cell surface galactosylceramide (Galcer) has been postulated to be a receptor for HIV-1 envelope (Env) interactions with mucosal epithelial cells. Disruption of the HIV-1 Env interaction with such alternate receptors could be one strategy to prevent HIV-1 entry through the mucosal barrier. To study antibody modulation of HIV-1 Env-Galcer interactions, we used Galcer-containing liposomes to assess whether natural- and vaccine-induced monoclonal antibodies can block HIV-1 Env binding to Galcer. HIV-1 Env gp140 proteins bound to Galcer liposomes with Kds (dissociation constants) in the nanomolar range. Several HIV-1 ALVAC/AIDSVAX vaccinee-derived monoclonal antibodies (MAbs) specific for the gp120 first constant (C1) region blocked Galcer binding of a transmitted/founder HIV-1 Env gp140. Among the C1-specific MAbs that showed Galcer blocking, the antibody-dependent cellular cytotoxicity-mediating CH38 IgG and its natural IgA isotype were the most potent blocking antibodies. C1-specific IgG monoclonal antibodies that blocked Env binding to Galcer induced upregulation of the gp120 CD4-inducible (CD4i) epitope bound by MAb 17B, demonstrating that a conformational change in gp120 may be required for Galcer blocking. However, the MAb 17B itself did not block Env-Galcer binding, suggesting that the C1 antibody-induced gp120 conformational changes resulted in alteration in a Galcer binding site distant from the CD4i 17B MAb binding site.
IMPORTANCE Galactosyl ceramide, a glycosphingolipid, has been postulated to be a receptor for the HIV-1 envelope glycoprotein (Env) interaction with mucosal epithelial cells. Here, we have mimicked this interaction by using an artificial membrane containing synthetic Galcer and recombinant HIV-1 Env proteins to identify antibodies that would block the HIV-1 Env-Galcer interaction. Our study revealed that a class of vaccine-induced human antibodies potently blocks HIV-1 Env-Galcer binding by perturbing the HIV-1 Env conformation.
INTRODUCTION
Understanding the interaction of HIV-1 virions with epithelial cells at mucosal sites of HIV-1 entry is critical for investigating any modulatory effect of vaccine-induced antibodies on such interactions and to design strategies for prevention of HIV-1 transmission (1–3). Entry of HIV-1 through both columnar and squamous epithelial surfaces allows virions to reach tissue depths and potentially infect target T cells and macrophages (4). Virions have been suggested to be trancytosed through epithelial cells via endocytic vesicles as well as migration through epithelial cell layers by movement through mucosal epithelial cell intercellular spaces (5, 6). Previous studies showed that CD4-independent infection of epithelial or neural cells by certain strains of HIV-1 can occur, suggesting that HIV-1 can employ alternate receptors for mucosal entry (7–10). The interaction of HIV-1 with such alternate receptors can be adhesive in nature, like those described for cell-cell transmission (11, 12), and therefore could potentially facilitate the mucosal entry of HIV-1. Thus, any mode of disruption of viral adhesion to epithelial cells could be a strategy to hinder mucosal entry of HIV-1.
One proposed alternate receptor for HIV-1, the glycosphingolipid galactosyl ceramide (Galcer) is expressed on the surface of colonic epithelial cells (13–15) and vaginal epithelium (16, 17), as well as primary mammary epithelial cells (18). Antibodies against Galcer have been reported to inhibit epithelial cell–HIV-1 virion binding (7, 8, 19), and previous biochemical studies showed that the HIV-1 envelope (Env) gp120 interacts with Galcer, supporting the view that Galcer might play the role of an alternate receptor for HIV-1 (7–10, 20–24). Enhanced expression of Galcer on an endocervical epithelial cell line, following infection with Chlamydia trachomatis, increased binding of HIV-1 and levels of virus in cocultures (25), and endosomal uptake of HIV-1 by Galcer-expressing mammary epithelial cells has been reported to facilitate infection of CD4 T cells (18).
While the other HIV-1 vaccine efficacy trials were unsuccessful in demonstrating efficacy (26–33), the RV144 ALVAC/AIDSVAX trial showed 31.2% efficacy (34). Subsequent immune correlate analysis revealed that antibodies targeting the HIV-1 envelope (Env) glycoprotein gp120 variable regions 1 and 2 (V1/V2) were correlated inversely with infection risk, whereas Env-specific plasma IgA antibodies were directly correlated with infection risk (35). Further studies raised the hypothesis that the observed protection in the RV144 trial could partially have been due to antibody-dependent cellular cytotoxicity (ADCC)-mediating antibodies (35); the majority of those isolated from vaccine recipients mapped to epitopes within the gp120 first constant (C1) region (36). The gp120 C1-specific IgA antibodies derived from RV144 vaccinee plasma blocked the binding and the ADCC-mediating abilities of anti-gp120 IgG antibodies, providing a rationale for the direct correlation observed between infection risk and high plasma Env-specific IgA (37). Furthermore, HIV-1 antibodies isolated from RV144 vaccinees were shown to capture infectious virions (38). However, the abilities of these antibodies to inhibit HIV-1 interactions with mucosal surfaces (2, 3) have not been tested. Therefore, we were interested in determining whether RV144 vaccinee-derived antibodies block HIV-1 Env binding to Galcer and, subsequently, whether such antibodies are effective in hindering mucosal entry of HIV-1.
In this report, we have used Galcer-containing liposomes and HIV-1 Env gp140 proteins as mimics of epithelial cells and HIV-1 virions/HIV-1-infected cells, respectively, to examine the HIV-1 Env-Galcer interaction and the ability of several natural and ALVAC/AIDSVAX vaccine-induced HIV-1 Env antibodies to inhibit this interaction. While earlier studies implicated the third variable region (V3) loop (39) and the gp41 membrane-proximal external region (MPER) in Galcer binding (40, 41), our results showed that many natural neutralizing and nonneutralizing antibodies that target gp120 and gp41 regions did not block the Env-Galcer interaction. Remarkably, the ALVAC/AIDSVAX vaccine-induced antibodies targeting the gp120 first constant region (C1) blocked clade C transmitted/founder (T/F) virus gp140-Galcer interactions with varied potency. In particular, the ADCC-mediating CH38 IgG and its IgA2 isotype (36) antibodies were the potent blockers of a T/F virus gp140-Galcer interactions. Furthermore, our results demonstrated that the Galcer-blocking potency of the gp120 C1-specific antibodies derived from ALVAC/AIDSVAX vaccinees directly correlated with their ability to induce conformational changes on the Env that exposed the CD4-inducible (CD4i) epitope. The CD4i-binding MAb 17B did not block T/F virus gp140-Galcer binding either before or after CD4 triggering gp140, nor did the C1-binding and CD4i-upregulating MAb A32. These data indicate that the ALVAC/AIDSVAX vaccine regimen induced a novel class of gp120 C1 region-binding antibodies that altered the HIV-1 Env conformation and disrupted Env-Galcer interactions. These antibodies can be tested in passive protection trials against simian-human immunodeficiency virus (SHIV) challenge in nonhuman primates to determine any protective potential of gp120-Galcer-blocking antibodies (42).
MATERIALS AND METHODS
Lipids.
Lyophilized powder of d-galactosyl-β-1,1′ N-octanoyl-d-erythro-sphingosine (Galcer) and chloroform stock of 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) were purchased from Avanti Polar Lipids (Alabaster, AL).
HIV-1 Env proteins.
The consensus, chronic, and T/F-type Env gp140 proteins were produced as recombinants as described previously (43).
Monoclonal antibodies.
The mouse monoclonal antibody antigalactocerebroside (anti-Galcer MAb) (44) was obtained from Millipore. The 2F5, 4E10, and 2G12 MAbs were purchased from Polymun Scientific (Vienna, Austria). The PG9 MAb was a gift from D. Burton (Scripps Research Institute, La Jolla, CA), and VRC01 MAb was a gift from J. Mascola (National Institutes of Health, Vaccine Research Center, Bethesda, MD). The b12 MAb was procured from Quality Biological (Gaithersburg, MD). The gp41 MPER-specific mouse monoclonal antibody 13H11 (45) was produced as a recombinant as described previously (46). The HIV-1 Env IgG antibodies from RV144 vaccinees were generated as described earlier (36). The CH38 IgA2 and 7B2 IgA2 antibodies were made as recombinants by using protocols published earlier (37). HIV-1 nonreactive human plasma IgA was obtained from Lee Biosolutions (St. Louis, MO). The Synagis MAb (Palivizumab), a humanized MAb directed against the F protein of respiratory syncytial virus (RSV) (47), was purchased from MedImmune (Gaithersburg, MD) and was used as a negative control in some of the experiments.
Liposome preparations.
The POPC liposomes and Galcer liposomes were made via an extrusion technique. Appropriate volumes of stock solutions of POPC (for making POPC liposomes) or Galcer (dissolved in chloroform–methanol [70:30, vol/vol]) and POPC (in chloroform) were mixed together in a 1:1 molar ratio. This composition was chosen based on earlier reports that the apical membranes of epithelial cells contained 37 ± 9.1 mol% glycosphingolipids (15) and that Env binding to Galcer liposomes reached a maximum at a Galcer content above 55 mol% (22). The lipid mixture was dried in a stream of gaseous nitrogen, and any residual solvent was removed by drying the lipid film under vacuum overnight. The dried lipid mixture was hydrated with an appropriate volume of phosphate-buffered saline (PBS; pH 7.4) and incubated at 60°C for 45 min. The hydrated mixture was then sonicated in a bath sonicator (Misonix 3000) and extruded through 0.4-μm and 0.1-μm polycarbonate membranes in a miniextruder obtained from Avanti Polar Lipids.
Biolayer interferometry assay.
Galcer binding of HIV-1 gp140 proteins was assayed by using the biolayer interferometry (BLI) technique and a ForteBio OctetRed 96 instrument with aminopropylsilane (APS) biosensors. Briefly, the Galcer and POPC liposomes were loaded onto APS biosensors by dipping them into sample plate wells containing 250 μM liposomes for 10 min. As a control, an APS sensor was dipped into a well containing PBS buffer. The blank and liposome-loaded APS sensors were washed in PBS for 1 min. In order to minimize nonspecific interactions of HIV gp140 proteins with the blank and liposome-loaded APS sensors, they were coated with bovine serum albumin (BSA) by dipping sensors into wells containing 0.1% BSA for 5 min, followed by a wash with PBS for 1 min. The interaction of HIV gp140 proteins with Galcer liposomes was measured by monitoring the wavelength shift (in nanometers). After a 2-min dip in PBS to obtain the baseline signal, the blank and liposome-loaded APS sensors were dipped into wells containing gp140 proteins at 0.71 μM (or at the indicated concentration) for 30 min, followed by a 10-min wash in PBS. The signal from the blank APS sensor was subtracted to obtain signal specific to Galcer and POPC liposome binding. For blocking JRFL-gp140 binding of Galcer by a precoated anti-Galcer MAb (see Fig. 1F, below), Galcer liposomes were loaded onto three sensors. One of the Galcer liposome sensors was dipped into a well with PBS buffer (control), and the remaining two were dipped into anti-Galcer MAb (150 μg/ml) for 30 min to determine the association with the anti-Galcer MAb. The PBS-treated Galcer liposome sensor and an anti-Galcer precoated Galcer liposome sensor were dipped into a well containing JRFL gp140 (50 μg/ml) for 30 min to monitor Env binding to Galcer. As a control, another anti-Galcer-precoated Galcer liposome sensor was dipped into PBS. Subsequently, all these sensors were dipped into PBS to follow dissociation for 30 min. Blank APS sensors were used in parallel to subtract binding due to nonspecific interactions.
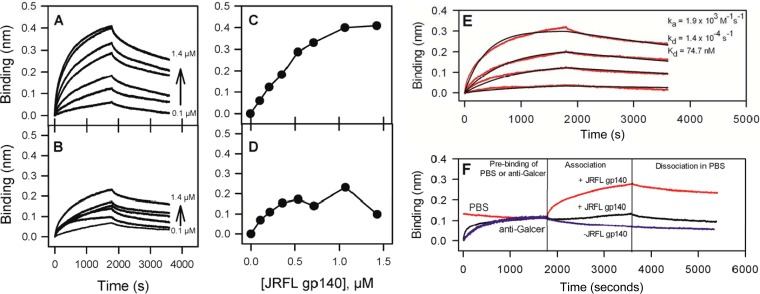
FIG 1.
HIV-1 Env JRFL gp140 binds to Galcer liposomes, and an anti-galactocerebroside antibody blocks this interaction. (A and B) The time courses of binding of JRFL gp140 at various concentrations to Galcer (A) and POPC (B) liposomes. The background binding of JRFL gp140 to blank sensors was subtracted to obtain the specific binding levels shown. (C and D) The last 25 s of the association phase (shown in panels A and B) were averaged and plotted to compare Galcer liposome binding (C) and POPC liposome binding (D) as a function of concentration of JRFLgp140. (E) Galcer-specific binding (after POPC binding was subtracted) time courses of JRFL gp140 at concentrations of 0.36 to 1.43 μM (red lines) were globally fitted (black lines) by using a 1:1 binding model that yielded ka and kd and derived Kd values. (F) Time courses of prebinding of anti-Galcer MAb at 150 μg/ml (black line) or PBS (red line) followed by JRFL gp140 (0.71 μM) binding to Galcer liposomes and the subsequent dissociation phases are shown. The PBS-treated Galcer liposome binding curve was normalized to the anti-Galcer binding endpoint. Signal from a blank sensor treated similarly was subtracted from the experimental to obtain the specific binding signal shown here.
For the Galcer-blocking assay, Galcer-liposome binding of 1086.C gp140 at 50 μg/ml was monitored for 30 min, and dissociation was monitored for 1 h. In parallel, Galcer-liposome binding of 1086.C gp140 (50 μg/ml) incubated with a 3 M excess of antibodies was monitored. The Galcer-liposome-binding responses (after subtracting the signal found with blank sensors) at the end of a 1-h dissociation phase were averaged (over a 20-s window). The percent Galcer blocking was calculated using the following equation: [(A − B)/A] × 100%, where A is the Galcer binding response of 1086.C gp140 and B is the Galcer binding response of 1086.C gp140 in the presence of a 3 M excess of the antibody of interest.
The binding of 1086.C gp140 to CH38 IgG and IgA2 antibodies pairs was measured by coupling the antibodies to amine-reactive sensors (AR2G sensors) as per the manufacturer's instruction. The antibody-coupled AR2G sensors were dipped into wells containing 1086.C gp140 at various concentrations and subsequently in PBS buffer for monitoring association and dissociation, respectively. CH65 IgG (a broadly neutralizing influenza virus antibody [48]) antibody-immobilized AR2G sensors were used as blank sensors so that nonspecific binding signal could be subtracted. The experiments were performed in triplicate. Titration curves were fit to a Langmuir 1:1 binding model using ForteBio Data Analysis software.
SPR assay.
All surface plasmon resonance (SPR) assays were performed using a Biacore 3000 instrument at 25°C, and data analyses were done using BIAEvaluation 4.1 software. The 17B MAb upregulation assay was performed using a CM5 chip immobilized with 17B MAb (6,000 to 7,000 resonance units [RU]) by a standard amine-coupling procedure in three flow cells. The fourth flow cell was immobilized with 6,000 RU of Synagis (47) and used as a negative control surface to determine responses due to nonspecific interactions to be subtracted as background. The T/F HIV Env 1086.C gp140 (40 μg/ml) was set up to flow over the antibody surfaces at a 20-μl/minute flow rate for 2 min. Dissociation was followed for 500 s after the injection of Env protein was complete. In order to measure 17B upregulation, 1086.C gp140 (40 μg/ml) was mixed with the antibodies (100 μg/ml) listed in Table 1, below, and injected over antibody surfaces. The binding data were processed to obtain the specific binding response by subtracting the binding response on the RSV-specific MAb Synagis surface. The specific binding responses from three 17B surfaces were averaged and are presented in Table 1. The percentage of 17B upregulation was calculated using the following equation: [(D − C)/C] × 100%, where C and D are the 17B-binding responses of 1086.C gp140 in the absence and in the presence of antibody, respectively.
TABLE 1.
1086.C gp140 binding to CD4i epitope-specific MAb 17B in the absence or presence of various C1-specific antibodies and control antibodiesa
| Antibody | 17B binding response (RU) | % upregulation |
|---|---|---|
| None | 357 ± 37 | 0.0 |
| Synagis | 363 ± 38 | 1.8 |
| A32 | 636 ± 40 | 78.3 |
| CH29 | 386 ± 24 | 8.1 |
| CH38 | 756 ± 44 | 111.9 |
| CH40 | 361 ± 39 | 1.2 |
| CH51 | 408 ± 44 | 14.4 |
| CH52 | 430 ± 48 | 20.5 |
| CH54 | 474 ± 51 | 32.9 |
| CH77 | 358 ± 24 | 0.3 |
| CH78 | 292 ± 37 | −17.9 |
| CH81 | 433 ± 47 | 21.5 |
| CH89 | 477 ± 53 | 33.7 |
| CH90 | 358 ± 38 | 0.5 |
| CH91 | 633 ± 65 | 77.5 |
| CH92 | 362 ± 38 | 1.6 |
| CH94 | 382 ± 26 | 7.2 |
| CH29 IgA2 | 369 ± 22 | 3.6 |
| CH38 IgA2 | 673 ± 41 | 88.7 |
The 17B binding experiment was performed, and the percent upregulation was calculated as described in Materials and Methods. An average of three measurements of 17B binding and the estimated standard deviations are shown.
ITC.
All isothermal titration calorimetry (ITC) measurements were performed using a MicroCal iTC200 instrument (GE Healthcare). Prior to titration, the antibodies and 1086.C gp140 protein were extensively dialyzed against PBS buffer (pH 7.4). The MAbs CH38 IgG and CH38 IgA2 were placed in the syringe at 19.4 μM and injected (2.5 μl per injection) into a cell containing 1086.C gp140 at 1.9 μM for a duration of 5 s with 180-s spacing between injections. Reference power was kept at 6 μCal. Experiments were performed in duplicate at 25°C. Using the Origin 7 program, the titration data were analyzed by fitting the integrated titration peaks with a single-site binding model. The determined stoichiometry (N), association constant (K) of the reaction, reaction enthalpy (ΔH), and the derived entropy change (ΔS) are displayed in Fig. 6C and D, below, along with the titration data.
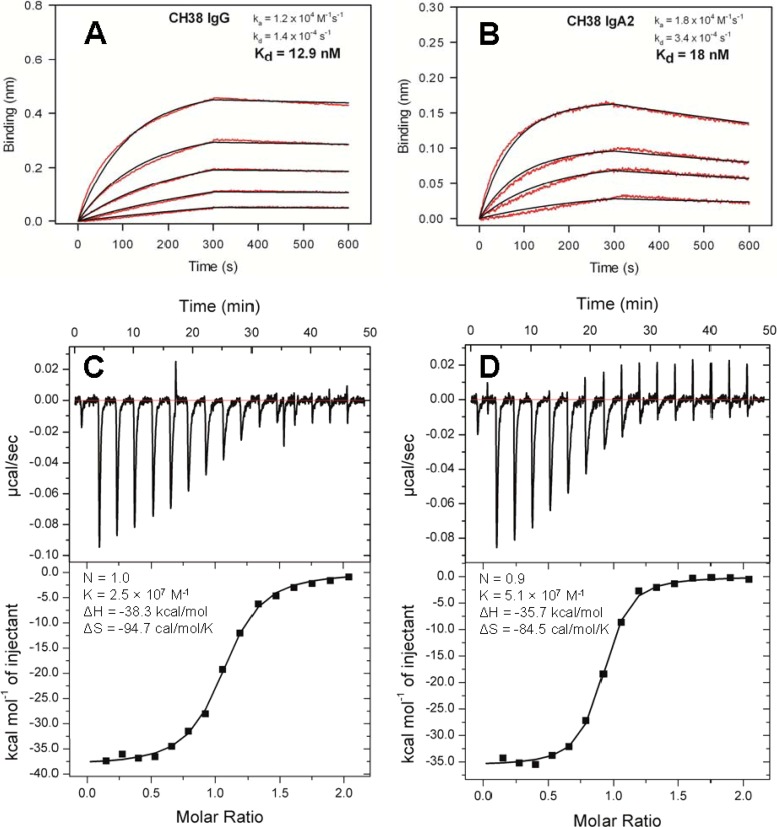
FIG 6.
Kinetics and thermodynamics of CH38 IgG-IgA2 antibody pair interaction with 1086.C gp140. (A and B) 1086.C gp140 binding titration time courses (red lines) for the CH38 IgG (A) and CH38 IgA2 (B) antibodies at 45 to 357 nM and 89 nM to 357 nM, respectively. The time courses were globally fitted by using a 1:1 binding model, which yielded ka and kd and the derived Kd values. The black lines are the best fit of the time courses. Titrations were carried out in duplicate. Representative data with fits are shown. (C and D) Representative raw data and isotherms of ITC measurements of the interaction of CH38 IgG (C) and CH38 IgA2 (D).
Statistical analysis.
A Spearman correlation analysis was used to determine the nonparametric statistical dependence between 1086.C gp140-binding and Galcer-blocking abilities. The apparent outliers were not included in this analysis.
Binding to Env on the surface of HIV-1 infected CD4+ cells.
Primary CD4+ T cells were isolated from an HIV-1-seronegative donor, activated, and infected with an infectious molecular clone that encodes the HIV-1 subtype C Env from isolate 1086.C (GenBank accession number ACS67968) in an isogenic backbone that contains the Renilla luciferase reporter gene and all viral open reading frames (49). Cell activation and infection were conducted as previously described (50). Mock-infected and HIV-1 1086.C-infected cells were incubated with the RSV-specific negative control Palivizumab or with MAb CH38 at 1 μg/ml for 2 h at 37°C. Primary Ab binding was detected by secondary labeling with fluorescein isothiocyanate (FITC)-conjugated goat anti-human IgG (KPL Inc., Gaithersburg, MD). Live and HIV-1-infected cells were identified by staining with a viability dye and analyzed for intracellular expression of p24 by using standard methods.
ADCC assays.
ADCC activity was determined in a luciferase-based assay as previously described (51). Briefly, CEM.NKRCCR5 cells (from Alexandra Trkola; NIH AIDS Reagent Program, Division of AIDS, NIAID, NIH) infected with the HIV-1 1086.C infectious molecular clone were used as targets. NK effector cells were isolated by negative selection with magnetic beads (Miltenyi Biotec GmbH, Germany) from cryopreserved peripheral blood mononuclear cells (PBMC) collected from a healthy HIV-1-seronegative donor. The NK cells were added to the HIV-1-infected target cells at a ratio of 5:1. Serial dilutions of Palivizumab and CH38 (concentration range, 40 to 0.4 μg/ml) were added to wells of a 96-well plate containing the targets and effector cells. Plasma from an HIV-1-seronegative donor and an HIV-1-seropositive donor were used as negative and positive controls, respectively, at final dilutions of 1:1,000. The assay plates were incubated for 6 h at 37°C in 5% CO2. ADCC activity (percent killing) was calculated from the change in luciferase activity resulting from the loss of intact target cells in wells containing effector cells, target cells, and Ab compared to control wells containing target cells and effector cells alone.
RESULTS
HIV-1 Env JRFL gp140 binds to Galcer liposomes.
Since the ectodomain of HIV-1 envelope glycoprotein gp160 consists of both the gp120 and gp41 portion, we reasoned that gp140 proteins (i.e., gp160 without the transmembrane domain and cytoplasmic tail) would be an appropriate mimic and we used them in examining their binding to Galcer liposomes that optimally present Galcer in the correct orientation (see Fig. S1 in the supplemental material). We first tested the binding of JRFL gp140, an HIV-1 Env protein from a chronically infected individual. Figure 1A and B display the time courses of binding and dissociation of JRFL gp140 at various concentrations to Galcer liposomes and to Galcer-free POPC liposomes, respectively. The near-steady-state binding level for the JRFL gp140 interaction with Galcer liposomes was markedly higher than its binding to the POPC liposomes (Fig. 1C and D). The low-avidity binding of gp140 to POPC liposomes reached saturation at a relatively lower concentration of Env protein. This lower level of binding of gp140 to POPC liposomes is due to nonspecific binding of gp140 to lipids, as it has been reported before that gp41 segments, such as the fusion peptide-proximal region, immunodominant loop, and membrane-proximal region, interact with membranes of different lipid compositions (52, 53). Analysis of kinetics of JRFL gp140 binding to Galcer liposomes (Fig. 1E) yielded an apparent affinity (Kd) value of 74.7 nM, with an association rate constant (ka) of 1.9 × 103 M−1 s−1 and a dissociation rate constant (kd) of 1.4 × 10−4 s−1.
Next, to validate whether the binding of JRFL gp140 to Galcer liposomes was via an interaction with Galcer, we precoated the Galcer liposomes with anti-Galcer MAb (44) and then tested the binding of JRFL gp140. Figure 1F shows the binding profiles of JRFL gp140 binding to Galcer liposomes immobilized on sensors that were first dipped in either PBS buffer or in anti-Galcer MAb. Galcer liposome binding of JRFL gp140 was completely blocked by the pretreatment of Galcer liposomes with anti-Galcer MAb, thus demonstrating that the binding of JRFL gp140 to Galcer liposomes is indeed via the Galcer interaction. In order to understand whether the gp140 interaction with Galcer liposomes involves only the galactose head group, we tested the binding of gp140 protein to Galcer liposomes in the absence and in the presence of various concentrations of galactose. No blocking by galactose was detectable (see Fig. S2 in the supplemental material), suggesting the importance of linkage of galactose to the ceramide moiety for the gp140-Galcer liposome interaction.
Galcer binding of chronic, consensus, and transmitted/founder virus envelope gp140s.
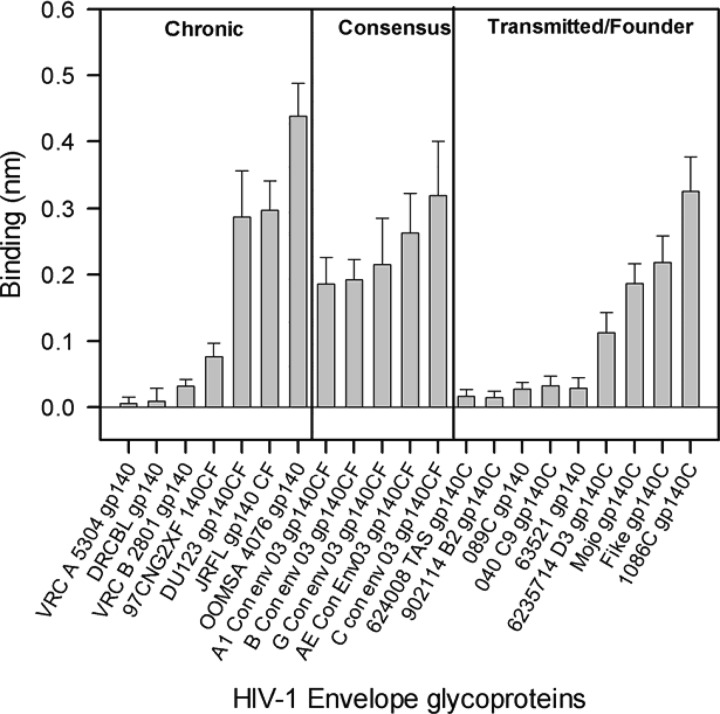
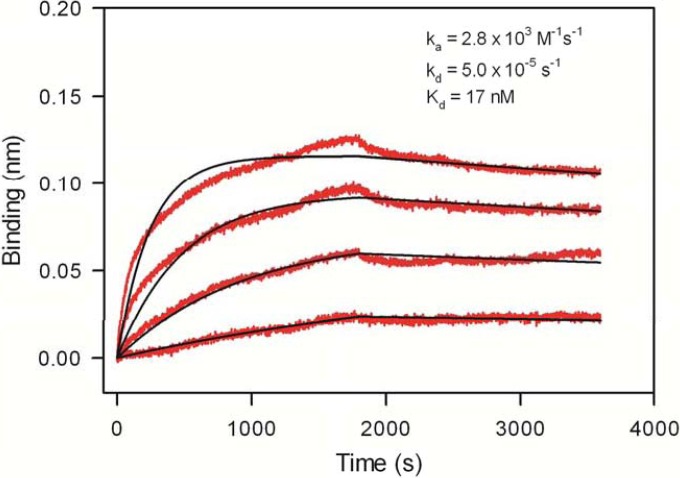
Since new infections from a transmitting chronic HIV+ individual via the mucosal route is established typically by a single variant, the transmitted/founder (T/F) virus, there is considerable interest in understanding the differential behavior of T/F viruses (54–56). In order to investigate whether T/F virus Env proteins possess a Galcer-binding ability different from that of chronic virus Env proteins and gp140 proteins that have consensus sequences, we carried out a study of Galcer liposome binding of a large panel of Env proteins. The Galcer liposome binding of this large panel of Env proteins is summarized in Fig. 2; Env proteins in each group bound to Galcer liposomes. All of the tested consensus gp140 proteins bound to Galcer, and there was no clear distinction between the ability of chronic and T/F virus Env gp140 proteins to bind to Galcer. There were no significant clade differences in Galcer binding of Env gp140 proteins that we studied here (see Table S1in the supplemental material). The majority of the chronic virus Env gp140s we have tested (4/7) showed strong binding to Galcer liposomes while others showed relatively weaker binding. Galcer liposome binding varied among the T/F virus Env proteins, with 4/9 showing strong binding. Among the T/F virus Env we tested, the clade C.1086 gp140 bound to Galcer with the highest affinity (Fig. 3), with a Kd of 17 nM (Fig. 3). Taken together, these data indicated that many HIV-1 Env gp140 proteins bind Galcer liposomes and there was no significant difference between Galcer binding of chronic and T/F virus Env.
FIG 2.
Comparison of Galcer liposome binding of various HIV-1 Env glycoproteins. Galcer liposome binding of gp140 proteins is shown for a group of chronic HIV-1 Env proteins, consensus sequences, and transmitted/founder HIV-1 Env. The binding values displayed are averages of the last 20 s of the 30-min association phase. The binding data and the error bars are from two separate measurements. JRFL gp140 was included in every experiment, and its Galcer binding was used as a reference to normalize data obtained from the different experiments.
FIG 3.
Kinetics of Galcer-specific binding of a transmitted/founder HIV-1 Env protein. Galcer-specific binding (with POPC binding subtracted) time courses are shown for T/F HIV-1 Env 1086.C gp140 (red lines) at concentrations of 0.1 to 1.43 μM. The time courses were globally fitted (black lines) by using a 1:1 binding model, yielding ka and kd and the derived Kd values. Representative data of two independent experiments are presented.
Ability of HIV-1 Env IgG antibodies to block Galcer binding of a transmitted/founder virus Env.
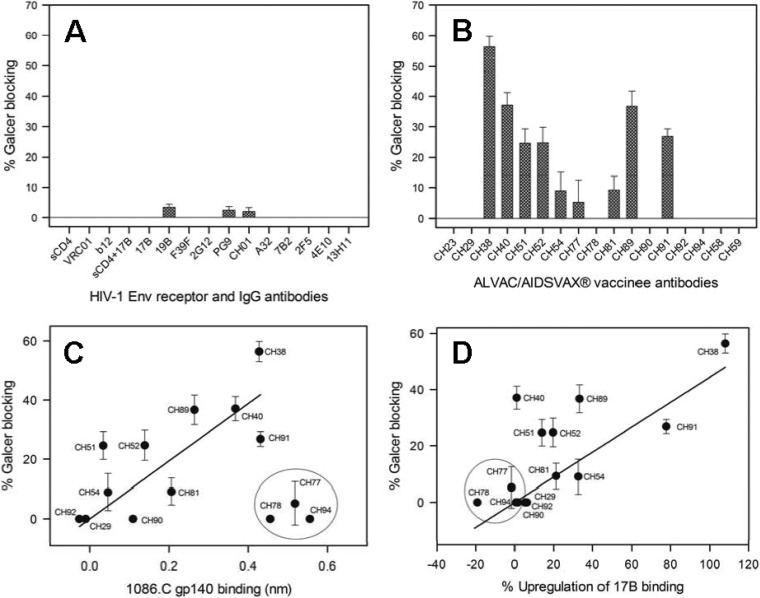
One of our major goals was to apply this assay for identification of HIV-1 Env antibodies that block the T/F virus Env-Galcer interaction with high potency. To accomplish this, we chose a clade C T/F virus Env, 1086.C gp140, that showed the highest affinity Galcer binding, and we tested the ability of various HIV-1 Env antibodies to block this interaction. Figure 4A shows the percent blocking of Galcer binding of 1086.C gp140 by soluble CD4 (sCD4) and several monoclonal antibodies targeting gp120 and gp41 regions. Neither sCD4 nor the CD4i MAb 17B with and without CD4 triggering of 1086.C gp140 blocked its Galcer binding. Other tested MAbs that target different gp120 regions also did not block Galcer binding of 1086.C gp140 (Fig. 4A). The gp41 MAb 7B2, along with the MPER broadly neutralizing antibodies 2F5 and 4E10, also did not block (Fig. 4A). Next, we screened several RV135/144 vaccinee-derived MAbs that target the gp120 C1 region (blockable by A32 MAb [36], which recognizes a discontinuous epitope from the C1 and C4 regions [57]), V3 region, and V2 region (Fig. 4B). The two V2-specific MAbs CH58 and CH59 did not show any Galcer blocking ability. The V3-specific tier 1 neutralizing MAb CH23 (36) also did not block Galcer binding of 1086.C gp140. However, the C1 region-specific CH38 IgG showed the strongest blocking (∼60%) among all the vaccinee antibodies tested (Fig. 4B). Several other C1 region-specific antibodies blocked the Galcer binding of 1086.C gp140 with relatively weak or moderate efficiency (5 to 35%). Interestingly, a positive correlation (Spearman correlation coefficient [ρ] of 0.8349; P = 0.0014) between the strength of A32 MAb blockable C1-specific antibody (36) binding to Env and the blocking of Env-Galcer binding was observed (Fig. 4C). Thus, CH29 and CH92 MAbs that did not bind 1086.C gp140 showed no Galcer blocking ability. On the other hand, the strongly binding 1086.C gp140 MAbs CH40, CH91, and CH38 exhibited high Galcer blocking. However, an outlier group of 3 antibodies (CH77, CH78, and CH94) bound to Env gp140 strongly but did not block Galcer binding. Taken together, these data showed that while Galcer-blocking ability was restricted to A32 MAb-blockable C1-specific vaccinee-derived MAbs, not all high-affinity C1-specific vaccinee-derived MAbs blocked Galcer binding of 1086.C gp140.
FIG 4.
The majority of HIV-1 Env antibodies do not block Galcer binding of a T/F virus Env, but a few RV135/144 vaccinee-derived antibodies do partially block Galcer binding. (A) Comparison of the percent blocking of Galcer liposome binding of 1086.C gp140 by sCD4, gp120 CD4 binding site-specific MAbs VRC01 and b12, CD4i epitope binding MAb 17B with and without sCD4, gp120 V3 loop-specific MAbs 19B and F39F, glycan-specific gp120 MAb 2G12, glycan-dependent gp120 V1V2 MAbs PG9 and CH01, gp120 C1 region-specific MAb A32, gp41 immunodominant loop-specific MAb 7B2 and gp41, and membrane-proximal external region-specific MAbs 2F5, 4E10, and 13H11. (B) The ability of blocking Galcer binding of 1086C gp140 by RV135/144 vaccinee antibodies specific to the gp120 V3 region (CH23), gp120 C1 region (CH29 to CH94), and specific to the gp120 V1V2 region (CH58 and CH59). The percent blocking data and the error bars shown are from two separate measurements. (C) The correlation between blocking of Galcer binding and 1086.C gp140 binding is shown for RV135/144 C1 IgG antibodies. The solid line is the linear fit of the data excluding the outliers circled. Statistical analysis that was performed excluding the outliers (n = 11) yielded a Spearman correlation coefficient of 0.8349 and a P value of 0.0014. (D) The percent blocking of Galcer binding 1086.C gp140 is correlated with the percent upregulation of 17B binding of 1086.C gp140 by the RV135/144 C1 IgG antibodies. The solid line is a linear fit of all data. Statistical analysis was performed and included all data (n = 14) and resulted in a Spearman correlation coefficient of 0.6496 and a P value of 0.0119. Exclusion of the circled antibodies in the statistical analysis (n = 11) resulted in a Spearman correlation coefficient of 0.5597 and a P value of 0.0734.
Bonsignori et al. reported that the RV135/144 vaccinee-derived C1-specific MAbs target distinct but overlapping epitopes that are A32 blockable (36). The observation that some A32 MAb-blockable C1-specific antibodies (CH77, CH78, and CH94) strongly bound to 1086.C gp140 (Fig. 4C, group of outliers) but did not block Galcer binding suggests that either the antibodies bind to distinct sites on C1 and/or have differential effects upon Env binding. One explanation of such an effect would be that C1 antibodies that induce conformational changes in gp120 might alter Env reactivity with Galcer. Since a previously described C1-specific MAb, A32, induced a conformational change in gp120 that resulted in the exposure of the coreceptor binding site (CD4i epitopes) (58), we tested whether the Galcer-blocking and nonblocking C1 antibodies would differentially upregulate the CD4i epitope upon binding to 1086.C gp140. For this purpose, we monitored the binding of 17B, a MAb specific for the coreceptor binding site that becomes exposed upon CD4 binding of Env (59), to 1086.C gp140 alone and in the presence of Galcer blocking or nonblocking C1 antibodies (Table 1). As previously observed for other Env proteins (58), 17B MAb binding of 1086.C gp140 was enhanced when the Env was bound by A32 MAb. Interestingly, several gp120 C1-specific RV135/144 vaccinee antibodies also upregulated 17B MAb binding of 1086.C gp140 (Table 1). However, there were a few antibodies that did not upregulate 17B binding. These results suggested that the gp120 C1-specific RV135/144 vaccinee antibodies are of two types: one type that induces rearrangement of Env, resulting in exposure of the CD4i epitope, and another type that binds to the overlapping C1 region but does not induce this gp120 conformational rearrangement.
However, we found that the differential 17B upregulation behavior of gp120 C1-specific RV135/144 vaccinee antibodies correlated with their ability to block Galcer binding of 1086.C gp140 (Fig. 4D). Remarkably, the three gp120 C1 antibodies (CH77, CH78, and CH94) that failed to block Galcer binding (Fig. 4C, outliers) did not upregulate 17B binding of 1086C gp140 (Fig. 4D, circled). The CH29 and CH92 MAbs did not bind 1086C gp140 (Fig. 4C) and therefore showed no upregulation of 17B binding. There was a linear correlation between 17B upregulation of 1086.C gp140 by RV135/144 C1 antibodies and their ability to block the 1086.C gp140-Galcer interaction (ρ = 0.6496; P = 0.0119). One exception was CH40, which exhibited moderate Galcer blocking but did not upregulate 17B binding. Thus, these results showed that the RV135/144 vaccinee-derived C1 antibodies that bound strongly to 1086.C gp140 and blocked Galcer binding of 1086.C gp140 also induced gp120 conformational changes associated with upregulation of 17B binding.
Ability of monomeric IgA2 antibodies to block Galcer binding of a transmitted/founder virus Env.
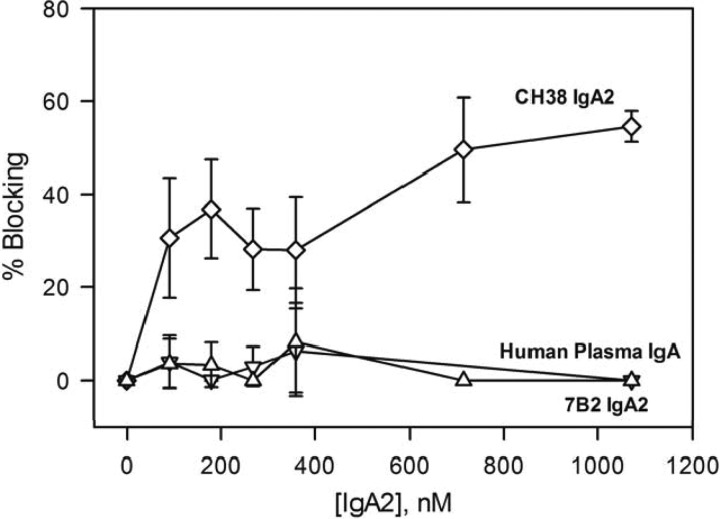
Since the RV144 vaccinee-derived CH38 MAb was originally an IgA isotype (36), we tested additional monomeric IgA2 Env antibodies for their ability to block Galcer binding of 1086.C gp140. As observed with the CH38 IgG isotype previously (36), CH38 IgA2 also blocked A32 MAb binding to Env (see Fig. S3 in the supplemental material). The concentration dependence of blocking of Galcer binding of 1086.C gp140 by CH38 IgA2 along with gp41 immunodominant region-specific 7B2 IgA2 and human plasma IgA nonreactive for HIV-1 (as a negative control) is presented in Fig. 5. The negative-control IgA did not block Galcer binding of 1086.C gp140. As observed with its IgG isotype, 7B2 IgA2 did not block the binding of 1086.C gp140 to Galcer liposomes. Like its IgG isotype, CH38 IgA2 also blocked 1086.C gp140 binding to Galcer (60% at saturation).
FIG 5.
Selected monomeric IgA2 gp120 antibodies block Galcer binding of a T/F virus Env gp140. The percent blocking of Galcer liposome binding of 1086.C gp140 (50 μg/ml) is shown as a function of concentration of monomeric IgA 2 antibodies CH38 (diamonds), 7B2 (inverted triangles), and human plasma IgA nonreactive for HIV-1, used as a negative control (triangles). The percent blocking data and the error bars shown are from two separate measurements.
Since both IgG and IgA2 isotypes of CH38 blocked Galcer binding of 1086.C gp140 with approximately equal potency, we asked whether the binding affinities of this antibody pair toward 1086.C gp140 were similar. Figure 6A and B shows the binding interaction of 1086.C gp140 with CH38 IgG and CH38 IgA2. Global fitting of the binding curves revealed that there was no significant difference in 1086.C gp140 binding affinities. We also measured the thermodynamic parameters of CH38 IgG and IgA2 isotype binding to 1086.C gp140 by performing ITC. The ITC study indicated that both the IgG and IgA2 isotypes of CH38 bound with similar enthalpy and entropy changes (Fig. 6C and D). The stoichiometry and affinity of 1086.C gp140 binding were also similar for CH38 IgG and IgA2 isoforms, even though the ITC-determined affinities were weaker than the BLI-determined Kd values. Thus, CH38 IgG and IgA2 isotypes have similar 1086.C gp140 binding affinities and thermodynamic profiles and block Galcer binding of 1086.C gp140 with an equal efficiency.
Recognition of HIV-1-infected T cell surface Env and ADCC mediation by CH38 MAb.
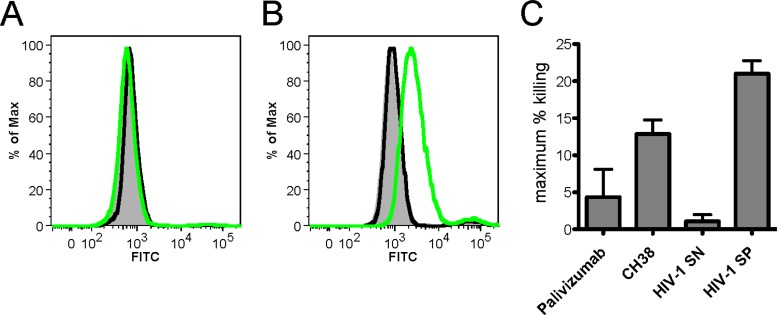
We next asked whether CH38 IgG, the potent Galcer-blocking antibody identified above, would recognize Env presented on 1086.C HIV-1 virions and on CD4+ 1086.C HIV-1-infected T cells. In virus capture assays, the CH38 IgG did not capture 1086.C virions (see Fig. S4 in the supplemental material). In contrast, CH38 IgG bound well to 1086.C HIV-1-infected CD4+ T cells but not to mock-infected cells (Fig. 7A and B). The CH38 IgG also weakly mediated ADCC of 1086.C HIV-1-infected CD4+ T cells (Fig. 7C). These data indicate that CH38 IgG is capable of recognizing Env on the 1086.C virus-infected CD4+ T cells but not on the 1086.C virions, suggesting that any effect of CH38 IgG inhibiting Galcer-gp120 binding in vivo would necessarily be targeted to gp120 on virus-infected cells interacting with epithelial cell Galcer.
FIG 7.
CH38 binds the surface of HIV-1-infected cells and mediates ADCC. (A and B) Activated primary human CD4+ T cells were mock infected (A) or infected with HIV-1 1086.c infectious molecular clone (IMC) (B) for 3 days. The cells were incubated with no MAbs (filled gray), negative-control MAb Palivizumab (black lines), or CH38 (green lines) for 2 h at 37°C to allow binding. Both Palivizumab and CH38 were used at 1 μg/ml. The cells were stained with a viability dye and anti-p24 to identify viable infected cells, and MAb binding was detected by secondary staining with FITC-conjugated goat anti-human IgG. Representative histograms are shown. (C) Peak ADCC activity of CH38 in the 6-h luciferase cell killing assay with HIV-1 1086.c IMC-infected CEM.NKRCCR5 target cells and NK cells isolated from a healthy donor. Palivizumab and plasma from an HIV-1-seronegative (HIV-1 SN) donor were included as negative controls, and plasma from an HIV-1-seropositive donor (HIV-1 SP) was included as a positive control.
In summary, we have shown that Galcer binding to Env gp140 is blocked by select IgG antibodies that target the gp120 C1 epitope and that also induce CD4i MAb binding. Among the C1-specific MAbs that blocked Galcer, both the IgG and IgA2 isotypes of CH38 MAb strongly induced 17B MAb binding and gave the strongest blocking of T/F Env gp140 to Galcer. These data suggest that the blocking of Galcer is allosterically mediated by C1-specific MAbs that induce the CD4i epitope.
DISCUSSION
The mechanism of transmission of cell-free HIV-1 viruses and HIV-1-infected cells across the genital epithelia remains unclear, although earlier studies demonstrated the ability of one or more strains of HIV-1 to infect CD4-negative cell lines (7, 9, 19). The ability of HIV-1 gp120 to interact with Galcer supports the view that Galcer might play the role of an alternate receptor for HIV-1 (7–10, 20–24). With Galcer present in the apical membrane of the epithelial cells lining the mucosal surfaces, including colonic (10) and vaginal and mammary epithelium (16–18), the HIV-1 Env-Galcer interaction could play an important role in establishing initial adhesion of HIV-1 virions/infected cells. Thus, if Galcer plays a role in HIV-1 transmission, we hypothesized that antibodies that block Galcer-Env interactions could potentially be protective at the mucosal site of HIV-1 entry. Only a few studies have attempted to develop agents or identify Env antibodies that inhibit the Env-Galcer interaction (39, 60). In this study, we used a biolayer interferometry assay to detect HIV-1 Env glycoproteins that bound to Galcer liposomes, screened several antibodies against HIV-1 Env glycoprotein, and identified gp120 C1-specific antibodies that blocked Galcer binding of a T/F HIV-1 Env glycoprotein.
We examined the time-resolved binding of HIV-1 Env gp140 with Galcer presented in a physiologically relevant bilayer form. Previous studies used Galcer in multilamellar liposomes (22) and planar mono- or bilayer forms (20, 21, 24) to detect an Env-Galcer interaction and did not provide time-resolved binding data. In our assay, the Galcer binding on-rate was on the order of 103 M−1 s−1 and the off-rate was on the order of 10−4 to 10−5 s−1 with Kd values in nanomolar range (17 to 75 nM). These rate constant-derived Kd values are consistent with those from earlier studies that used different techniques to determine the rgp120-Galcer interaction quantitatively (8, 20, 21).
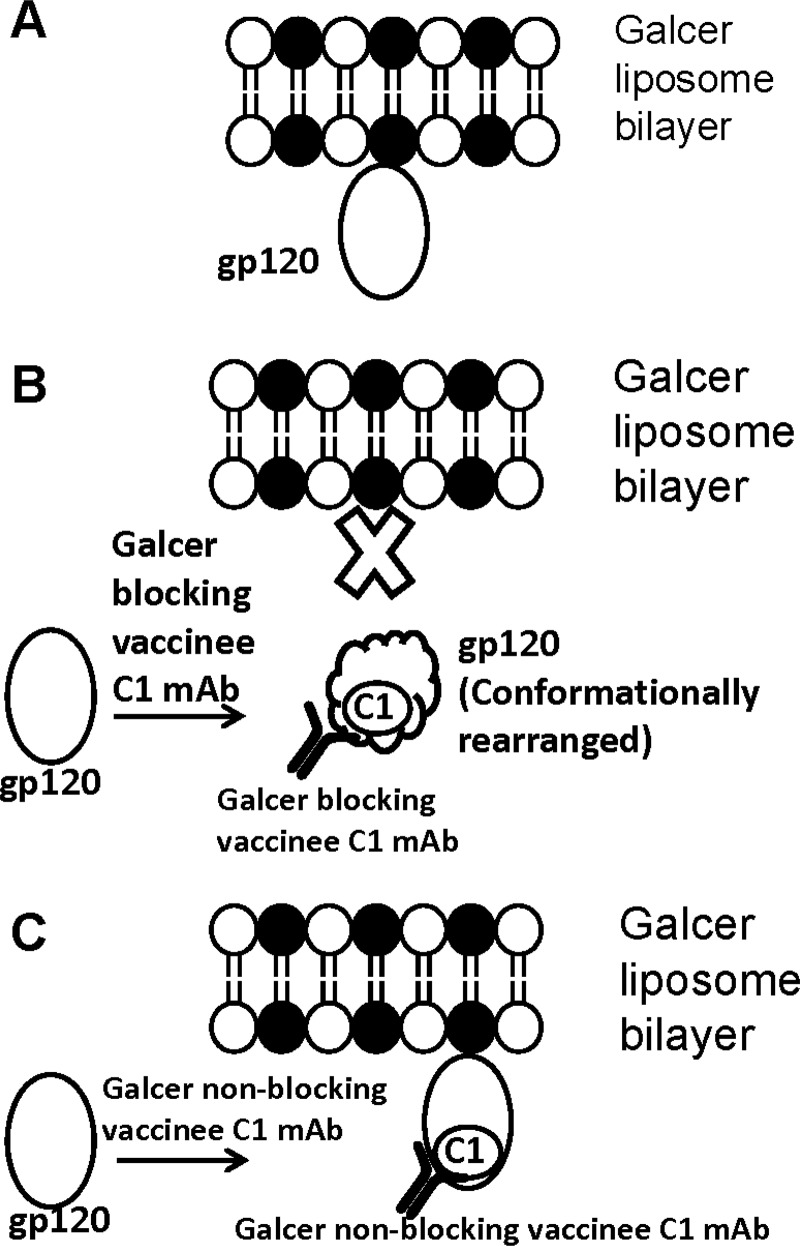
Earlier studies that used a high-performance liquid chromatography assay and recombinant gp120 monolayer binding assay with gp120 and synthetic peptides derived from gp120 suggested that the Galcer binding site is in or proximal to the V3 loop (24, 39). In our studies, with the V3 MAbs 19B and F39F and Env 1086.C gp140, we did not observe any blocking of Galcer-Env interactions. Nor did we observe Galcer blocking by V1/V2 MAbs or the CD4i MAb 17B, with or without CD4 triggering of gp120. Instead, Galcer blocking was observed only for antibodies specific for the gp120 C1 region, suggesting that the Galcer binding site could involve the gp120 C1 region. However, not all C1-specific MAbs showed blocking of Galcer. Interestingly, we found a positive correlation between Galcer blocking and the extent of CD4i upregulation by C1-specific antibodies. These results indicated two important features about the binding properties of C1-specific antibodies. First, select C1 MAbs can induce CD4i epitopes, similar to that of the previously described CD4i-inducible MAb A32. Second, upon binding to gp120, C1-specific antibodies can differentially alter Env reactivity. Thus, based on the ability to upregulate CD4i and to block Galcer binding, we have found three different classes of C1-specific MAbs (Fig. 8). The first class is represented by Galcer-blocking C1-specific MAbs that alter Env reactivity such that the CD4i epitope is exposed while the Galcer binding site is simultaneously masked/disrupted (Fig. 8B). A second class of C1-specific antibodies includes the Galcer nonblocking MAbs, those that neither induce CD4i nor mask the Galcer binding site (Fig. 8C). A32 MAb, the third class of antibody, is an exception in that it upregulates the CD4i epitope but does not block Galcer. Conversely, CH40 can block Galcer without inducing the CD4i epitope. Taken together, these data suggest that conformational changes outside of the CD4i epitope are required for Galcer blocking. Recently, emerging data revealed that some RV144 vaccinee-derived C1-specific antibodies synergize the ADCC activity of V2 antibodies by upregulating the V2 epitope (61). Thus, while each class of C1 MAbs can differentially affect Env gp120, our data suggest that Galcer blocking is mediated not through steric effects upon binding to gp120 C1 but rather by an allosteric effect on the Galcer binding site, and such an effect can be associated with induction of the CD4i epitope. However, to date, there is no evidence to indicate that vaccine-induced Galcer-blocking antibodies contributed to reduction of acquisition of HIV-1 in the RV144 trial.
FIG 8.
Schematic diagram depicting the allosteric masking of the Galcer binding site by some gp120 C1-specific antibodies derived from RV135/144 vaccinees. (A) A pictorial representation of the binding of HIV-1 Env to the Galcer liposome bilayer is shown. In the Galcer liposome bilayer depiction, filled and open black circles with tails represent Galcer and POPC molecules, respectively. For simplisticity, only gp120 is shown as an ellipse. (B) A few RV135/144 vaccinee-derived C1 MAbs bind gp120, induce a conformational change (gp120 is shown shaped like a cloud), and block Galcer binding. (C) Some RV135/144 vaccinee-derived C1 MAbs bind gp120 but do not induce a conformational change and hence do not interfere in Galcer binding of Env.
Earlier studies have shown that cell-associated simian immunodeficiency virus establishes infection of rhesus macques upon vaginal exposure (62, 63), and transmission occurs from HIV-1-infected cells to uninfected CD4+ T cells (11, 64). Our results showed that the Galcer-blocking CH38 IgG bound well to the 1086.C virus-infected cells even though it failed to capture the cell-free 1086.C virions. These results suggest that by binding the Env expressed on the virus-infected cells, CH38 could potentially inhibit the interaction of virus-infected cells and epithelial cell Galcer. In the immune correlate analysis of RV144, high levels of plasma IgA antibodies against HIV-1 Env correlated with increased infection risk (35), suggesting that a certain class of plasma IgA competes with IgG for binding to Env and could block effector function (37). The Galcer binding assay we have described here is aimed at mimicking the interactions of HIV-1 virions or HIV-1-infected cells with epithelial cells at the mucosal surfaces, and in this regard we have identified the blocking ability of an originally isolated IgA antibody from vaccinees. Thus, certain IgA antibodies could have divergent functions, depending on the form of IgA (i.e., monomeric, secretory, dimeric) and the location (systemic versus mucosal). Thus, while IgA antibodies cannot mediate ADCC via NK cells, they can block transcytosis of HIV-1 virions and CD4+ T cell infection by potentially hindering HIV-1 Env binding to Galcer (40, 65, 66).
In summary, we have identified a class of C1-specific vaccine-induced IgG and IgA2 antibodies that block the HIV-1 Env-Galcer interaction, and such antibodies could be potential candidates for blocking the binding of virus-infected cells to epithelial cells in vivo. Further studies, such as testing the effect of Galcer-blocking antibodies on inhibition of epithelial cell binding of HIV-1-infected cells, cell-to-cell transmission of HIV-1, and infection of rhesus macaques by SHIV-infected cells in a passive protection trial, would help for understanding whether Galcer-Env interactions play a role in HIV-1 transmission.
Supplementary Material
ACKNOWLEDGMENTS
This research was conducted as part of the Collaboration for AIDS Vaccine Discovery (CAVD) with support from the Bill & Melinda Gates Foundation to B.F.H. (OPP1033098 and OPP1040758) and from the Center for HIV/AIDS Vaccine Immunology (CHAVI; AI067854) and the Center for HIV/AIDS Vaccine Immunogen Discovery (CHAVI-ID; UM1AI100645-01) from the NIH, NIAID.
The views expressed in this article are those of the authors and should not be construed as official or as representing the views of the Department of Defense or the Department of the Army.
Footnotes
Published ahead of print 11 June 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JVI.01031-14.
REFERENCES
- 1.Haynes BF, Shattock RJ. 2008. Critical issues in mucosal immunity for HIV-1 vaccine development. J. Allergy Clin. Immunol. 122:3–9. 10.1016/j.jaci.2008.03.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hope TJ. 2011. Moving ahead an HIV vaccine: to neutralize or not, a key HIV vaccine question. Nat. Med. 17:1195–1197. 10.1038/nm.2528 [DOI] [PubMed] [Google Scholar]
- 3.McElrath MJ, Haynes BF. 2010. Induction of immunity to human immunodeficiency virus type-1 by vaccination. Immunity 33:542–554. 10.1016/j.immuni.2010.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carias AM, McCoombe S, McRaven M, Anderson M, Galloway N, Vandergrift N, Fought AJ, Lurain J, Duplantis M, Veazey RS, Hope TJ. 2013. Defining the interaction of HIV-1 with the mucosal barriers of the female reproductive tract. J. Virol. 87:11388–11400. 10.1128/JVI.01377-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shukair SA, Allen SA, Cianci GC, Stieh DJ, Anderson MR, Baig SM, Gioia CJ, Spongberg EJ, Kauffman SM, McRaven MD, Lakougna HY, Hammond C, Kiser PF, Hope TJ. 2013. Human cervicovaginal mucus contains an activity that hinders HIV-1 movement. Mucosal Immunol. 6:427–434. 10.1038/mi.2012.87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hladik F, McElrath MJ. 2008. Setting the stage: host invasion by HIV. Nat. Rev. Immunol. 8:447–457. 10.1038/nri2302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhat S, Spitalnik SL, Gonzalez-Scarano F, Silberberg DH. 1991. Galactosyl ceramide or a derivative is an essential component of the neural receptor for human immunodeficiency virus type 1 envelope glycoprotein gp120. Proc. Natl. Acad. Sci. U. S. A. 88:7131–7134. 10.1073/pnas.88.16.7131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harouse JM, Bhat S, Spitalnik SL, Laughlin M, Stefano K, Silberberg DH, Gonzalez-Scarano F. 1991. Inhibition of entry of HIV-1 in neural cell lines by antibodies against galactosyl ceramide. Science 253:320–323. 10.1126/science.1857969 [DOI] [PubMed] [Google Scholar]
- 9.Harouse JM, Kunsch C, Hartle HT, Laughlin MA, Hoxie JA, Wigdahl B, Gonzalez-Scarano F. 1989. CD4-independent infection of human neural cells by human immunodeficiency virus type 1. J. Virol. 63:2527–2533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yahi N, Baghdiguian S, Moreau H, Fantini J. 1992. Galactosyl ceramide (or a closely related molecule) is the receptor for human immunodeficiency virus type 1 on human colon epithelial HT29 cells. J. Virol. 66:4848–4854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen P, Hubner W, Spinelli MA, Chen BK. 2007. Predominant mode of human immunodeficiency virus transfer between T cells is mediated by sustained Env-dependent neutralization-resistant virological synapses. J. Virol. 81:12582–12595. 10.1128/JVI.00381-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dale BM, Alvarez RA, Chen BK. 2013. Mechanisms of enhanced HIV spread through T-cell virological synapses. Immunol. Rev. 251:113–124. 10.1111/imr.12022 [DOI] [PubMed] [Google Scholar]
- 13.Holgersson J, Jovall PA, Breimer ME. 1991. Glycosphingolipids of human large intestine: detailed structural characterization with special reference to blood group compounds and bacterial receptor structures. J. Biochem. 110:120–131 [DOI] [PubMed] [Google Scholar]
- 14.Raff MC, Mirsky R, Fields KL, Lisak RP, Dorfman SH, Silberberg DH, Gregson NA, Leibowitz S, Kennedy MC. 1978. Galactocerebroside is a specific cell-surface antigenic marker for oligodendrocytes in culture. Nature 274:813–816 [PubMed] [Google Scholar]
- 15.Simons K, van Meer G. 1988. Lipid sorting in epithelial cells. Biochemistry 27:6197–6202. 10.1021/bi00417a001 [DOI] [PubMed] [Google Scholar]
- 16.Bobardt MD, Chatterji U, Selvarajah S, Van der Schueren B, David G, Kahn B, Gallay PA. 2007. Cell-free human immunodeficiency virus type 1 transcytosis through primary genital epithelial cells. J. Virol. 81:395–405. 10.1128/JVI.01303-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yeaman GR, Asin S, Weldon S, Demian DJ, Collins JE, Gonzalez JL, Wira CR, Fanger MW, Howell AL. 2004. Chemokine receptor expression in the human ectocervix: implications for infection by the human immunodeficiency virus-type I. Immunology 113:524–533. 10.1111/j.1365-2567.2004.01990.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dorosko SM, Connor RI. 2010. Primary human mammary epithelial cells endocytose HIV-1 and facilitate viral infection of CD4+ T lymphocytes. J. Virol. 84:10533–10542. 10.1128/JVI.01263-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fantini J, Cook DG, Nathanson N, Spitalnik SL, Gonzalez-Scarano F. 1993. Infection of colonic epithelial cell lines by type 1 human immunodeficiency virus is associated with cell surface expression of galactosylceramide, a potential alternative gp120 receptor. Proc. Natl. Acad. Sci. U. S. A. 90:2700–2704. 10.1073/pnas.90.7.2700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Conboy JC, McReynolds KD, Gervay-Hague J, Saavedra SS. 2000. gp120 binds cooperatively to several biologically relevant glycosphingolipids: quantitative measurements at equilibrium by total internal reflection fluorescence microscopy. Angew. Chem. Int. Ed. Engl. 39:2882–2884. [DOI] [PubMed] [Google Scholar]
- 21.Conboy JC, McReynolds KD, Gervay-Hague J, Saavedra SS. 2002. Quantitative measurements of recombinant HIV surface glycoprotein 120 binding to several glycosphingolipids expressed in planar supported lipid bilayers. J. Am. Chem. Soc. 124:968–977. 10.1021/ja011225s [DOI] [PubMed] [Google Scholar]
- 22.Long D, Berson JF, Cook DG, Doms RW. 1994. Characterization of human immunodeficiency virus type 1 gp120 binding to liposomes containing galactosylceramide. J. Virol. 68:5890–5898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McAlarney T, Apostolski S, Lederman S, Latov N. 1994. Characteristics of HIV-1 gp120 glycoprotein binding to glycolipids. J. Neurosci. Res. 37:453–460. 10.1002/jnr.490370404 [DOI] [PubMed] [Google Scholar]
- 24.Nehete PN, Vela EM, Hossain MM, Sarkar AK, Yahi N, Fantini J, Sastry KJ. 2002. A post-CD4-binding step involving interaction of the V3 region of viral gp120 with host cell surface glycosphingolipids is common to entry and infection by diverse HIV-1 strains. Antiviral Res. 56:233–251. 10.1016/S0166-3542(02)00130-4 [DOI] [PubMed] [Google Scholar]
- 25.Schust DJ, Ibana JA, Buckner LR, Ficarra M, Sugimoto J, Amedee AM, Quayle AJ. 2012. Potential mechanisms for increased HIV-1 transmission across the endocervical epithelium during C. trachomatis infection. Curr. HIV Res. 10:218–227. 10.2174/157016212800618093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buchbinder SP, Mehrotra DV, Duerr A, Fitzgerald DW, Mogg R, Li D, Gilbert PB, Lama JR, Marmor M, Del Rio C, McElrath MJ, Casimiro DR, Gottesdiener KM, Chodakewitz JA, Corey L, Robertson MN. 2008. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet 372:1881–1893. 10.1016/S0140-6736(08)61591-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duerr A, Huang Y, Buchbinder S, Coombs RW, Sanchez J, del Rio C, Casapia M, Santiago S, Gilbert P, Corey L, Robertson MN. 2012. Extended follow-up confirms early vaccine-enhanced risk of HIV acquisition and demonstrates waning effect over time among participants in a randomized trial of recombinant adenovirus HIV vaccine (Step Study). J. Infect. Dis. 206:258–266. 10.1093/infdis/jis342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Flynn NM, Forthal DN, Harro CD, Judson FN, Mayer KH, Para MF. 2005. Placebo-controlled phase 3 trial of a recombinant glycoprotein 120 vaccine to prevent HIV-1 infection. J. Infect. Dis. 191:654–665. 10.1086/428404 [DOI] [PubMed] [Google Scholar]
- 29.Gilbert PB, Ackers ML, Berman PW, Francis DP, Popovic V, Hu DJ, Heyward WL, Sinangil F, Shepherd BE, Gurwith M. 2005. HIV-1 virologic and immunologic progression and initiation of antiretroviral therapy among HIV-1-infected subjects in a trial of the efficacy of recombinant glycoprotein 120 vaccine. J. Infect. Dis. 192:974–983. 10.1086/432734 [DOI] [PubMed] [Google Scholar]
- 30.Gray GE, Allen M, Moodie Z, Churchyard G, Bekker LG, Nchabeleng M, Mlisana K, Metch B, de Bruyn G, Latka MH, Roux S, Mathebula M, Naicker N, Ducar C, Carter DK, Puren A, Eaton N, McElrath MJ, Robertson M, Corey L, Kublin JG. 2011. Safety and efficacy of the HVTN 503/Phambili study of a clade-B-based HIV-1 vaccine in South Africa: a double-blind, randomised, placebo-controlled test-of-concept phase 2b study. Lancet Infect. Dis. 11:507–515. 10.1016/S1473-3099(11)70098-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hammer SM, Sobieszczyk ME, Janes H, Karuna ST, Mulligan MJ, Grove D, Koblin BA, Buchbinder SP, Keefer MC, Tomaras GD, Frahm N, Hural J, Anude C, Graham BS, Enama ME, Adams E, Dejesus E, Novak RM, Frank I, Bentley C, Ramirez S, Fu R, Koup RA, Mascola JR, Nabel GJ, Montefiori DC, Kublin J, McElrath MJ, Corey L, Gilbert PB. 2013. Efficacy trial of a DNA/rAd5 HIV-1 preventive vaccine. N. Engl. J. Med. 369:2083–2092. 10.1056/NEJMoa1310566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McElrath MJ, De Rosa SC, Moodie Z, Dubey S, Kierstead L, Janes H, Defawe OD, Carter DK, Hural J, Akondy R, Buchbinder SP, Robertson MN, Mehrotra DV, Self SG, Corey L, Shiver JW, Casimiro DR. 2008. HIV-1 vaccine-induced immunity in the test-of-concept Step Study: a case-cohort analysis. Lancet 372:1894–1905. 10.1016/S0140-6736(08)61592-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pitisuttithum P, Gilbert P, Gurwith M, Heyward W, Martin M, van Griensven F, Hu D, Tappero JW, Choopanya K. 2006. Randomized, double-blind, placebo-controlled efficacy trial of a bivalent recombinant glycoprotein 120 HIV-1 vaccine among injection drug users in Bangkok, Thailand. J. Infect. Dis. 194:1661–1671. 10.1086/508748 [DOI] [PubMed] [Google Scholar]
- 34.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, Premsri N, Namwat C, de Souza M, Adams E, Benenson M, Gurunathan S, Tartaglia J, McNeil JG, Francis DP, Stablein D, Birx DL, Chunsuttiwat S, Khamboonruang C, Thongcharoen P, Robb ML, Michael NL, Kunasol P, Kim JH. 2009. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N. Engl. J. Med. 361:2209–2220. 10.1056/NEJMoa0908492 [DOI] [PubMed] [Google Scholar]
- 35.Haynes BF, Gilbert PB, McElrath MJ, Zolla-Pazner S, Tomaras GD, Alam SM, Evans DT, Montefiori DC, Karnasuta C, Sutthent R, Liao HX, DeVico AL, Lewis GK, Williams C, Pinter A, Fong Y, Janes H, DeCamp A, Huang Y, Rao M, Billings E, Karasavvas N, Robb ML, Ngauy V, de Souza MS, Paris R, Ferrari G, Bailer RT, Soderberg KA, Andrews C, Berman PW, Frahm N, De Rosa SC, Alpert MD, Yates NL, Shen X, Koup RA, Pitisuttithum P, Kaewkungwal J, Nitayaphan S, Rerks-Ngarm S, Michael NL, Kim JH. 2012. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N. Engl. J. Med. 366:1275–1286. 10.1056/NEJMoa1113425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bonsignori M, Pollara J, Moody MA, Alpert MD, Chen X, Hwang KK, Gilbert PB, Huang Y, Gurley TC, Kozink DM, Marshall DJ, Whitesides JF, Tsao CY, Kaewkungwal J, Nitayaphan S, Pitisuttithum P, Rerks-Ngarm S, Kim JH, Michael NL, Tomaras GD, Montefiori DC, Lewis GK, DeVico A, Evans DT, Ferrari G, Liao HX, Haynes BF. 2012. Antibody-dependent cellular cytotoxicity-mediating antibodies from an HIV-1 vaccine efficacy trial target multiple epitopes and preferentially use the VH1 gene family. J. Virol. 86:11521–11532. 10.1128/JVI.01023-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tomaras GD, Ferrari G, Shen X, Alam SM, Liao HX, Pollara J, Bonsignori M, Moody MA, Fong Y, Chen X, Poling B, Nicholson CO, Zhang R, Lu X, Parks R, Kaewkungwal J, Nitayaphan S, Pitisuttithum P, Rerks-Ngarm S, Gilbert PB, Kim JH, Michael NL, Montefiori DC, Haynes BF. 2013. Vaccine-induced plasma IgA specific for the C1 region of the HIV-1 envelope blocks binding and effector function of IgG. Proc. Natl. Acad. Sci. U. S. A. 110:9019–9024. 10.1073/pnas.1301456110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu P, Yates NL, Shen X, Bonsignori M, Moody MA, Liao HX, Fong Y, Alam SM, Overman RG, Denny T, Ferrari G, Ochsenbauer C, Kappes JC, Polonis VR, Pitisuttithum P, Kaewkungwal J, Nitayaphan S, Rerks-Ngarm S, Montefiori DC, Gilbert P, Michael NL, Kim JH, Haynes BF, Tomaras GD. 2013. Infectious virion capture by HIV-1 gp120-specific IgG from RV144 vaccinees. J. Virol. 87:7828–7836. 10.1128/JVI.02737-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cook DG, Fantini J, Spitalnik SL, Gonzalez-Scarano F. 1994. Binding of human immunodeficiency virus type I (HIV-1) gp120 to galactosylceramide (GalCer): relationship to the V3 loop. Virology 201:206–214. 10.1006/viro.1994.1287 [DOI] [PubMed] [Google Scholar]
- 40.Alfsen A, Iniguez P, Bouguyon E, Bomsel M. 2001. Secretory IgA specific for a conserved epitope on gp41 envelope glycoprotein inhibits epithelial transcytosis of HIV-1. J. Immunol. 166:6257–6265. 10.4049/jimmunol.166.10.6257 [DOI] [PubMed] [Google Scholar]
- 41.Yu H, Alfsen A, Tudor D, Bomsel M. 2008. The binding of HIV-1 gp41 membrane proximal domain to its mucosal receptor, galactosyl ceramide, is structure-dependent. Cell Calcium 43:73–82. 10.1016/j.ceca.2007.04.011 [DOI] [PubMed] [Google Scholar]
- 42.Burton DR, Hessell AJ, Keele BF, Klasse PJ, Ketas TA, Moldt B, Dunlop DC, Poignard P, Doyle LA, Cavacini L, Veazey RS, Moore JP. 2011. Limited or no protection by weakly or nonneutralizing antibodies against vaginal SHIV challenge of macaques compared with a strongly neutralizing antibody. Proc. Natl. Acad. Sci. U. S. A. 108:11181–11186. 10.1073/pnas.1103012108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liao HX, Tsao CY, Alam SM, Muldoon M, Vandergrift N, Ma BJ, Lu X, Sutherland LL, Scearce RM, Bowman C, Parks R, Chen H, Blinn JH, Lapedes A, Watson S, Xia SM, Foulger A, Hahn BH, Shaw GM, Swanstrom R, Montefiori DC, Gao F, Haynes BF, Korber B. 2013. Antigenicity and immunogenicity of transmitted/founder, consensus, and chronic envelope glycoproteins of human immunodeficiency virus type 1. J. Virol. 87:4185–4201. 10.1128/JVI.02297-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ranscht B, Clapshaw PA, Price J, Noble M, Seifert W. 1982. Development of oligodendrocytes and Schwann cells studied with a monoclonal antibody against galactocerebroside. Proc. Natl. Acad. Sci. U. S. A. 79:2709–2713. 10.1073/pnas.79.8.2709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alam SM, Scearce RM, Parks RJ, Plonk K, Plonk SG, Sutherland LL, Gorny MK, Zolla-Pazner S, Vanleeuwen S, Moody MA, Xia SM, Montefiori DC, Tomaras GD, Weinhold KJ, Karim SA, Hicks CB, Liao HX, Robinson J, Shaw GM, Haynes BF. 2008. Human immunodeficiency virus type 1 gp41 antibodies that mask membrane proximal region epitopes: antibody binding kinetics, induction, and potential for regulation in acute infection. J. Virol. 82:115–125. 10.1128/JVI.00927-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nicely NI, Dennison SM, Spicer L, Scearce RM, Kelsoe G, Ueda Y, Chen H, Liao HX, Alam SM, Haynes BF. 2010. Crystal structure of a non-neutralizing antibody to the HIV-1 gp41 membrane-proximal external region. Nat. Struct. Mol. Biol. 17:1492–1494. 10.1038/nsmb.1944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Johnson S, Oliver C, Prince GA, Hemming VG, Pfarr DS, Wang SC, Dormitzer M, O'Grady J, Koenig S, Tamura JK, Woods R, Bansal G, Couchenour D, Tsao E, Hall WC, Young JF. 1997. Development of a humanized monoclonal antibody (MEDI-493) with potent in vitro and in vivo activity against respiratory syncytial virus. J. Infect. Dis. 176:1215–1224. 10.1086/514115 [DOI] [PubMed] [Google Scholar]
- 48.Whittle JR, Zhang R, Khurana S, King LR, Manischewitz J, Golding H, Dormitzer PR, Haynes BF, Walter EB, Moody MA, Kepler TB, Liao HX, Harrison SC. 2011. Broadly neutralizing human antibody that recognizes the receptor-binding pocket of influenza virus hemagglutinin. Proc. Natl. Acad. Sci. U. S. A. 108:14216–14221. 10.1073/pnas.1111497108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Edmonds TG, Ding H, Yuan X, Wei Q, Smith KS, Conway JA, Wieczorek L, Brown B, Polonis V, West JT, Montefiori DC, Kappes JC, Ochsenbauer C. 2010. Replication competent molecular clones of HIV-1 expressing Renilla luciferase facilitate the analysis of antibody inhibition in PBMC. Virology 408:1–13. 10.1016/j.virol.2010.08.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ferrari G, Pollara J, Kozink D, Harms T, Drinker M, Freel S, Moody MA, Alam SM, Tomaras GD, Ochsenbauer C, Kappes JC, Shaw GM, Hoxie JA, Robinson JE, Haynes BF. 2011. An HIV-1 gp120 envelope human monoclonal antibody that recognizes a C1 conformational epitope mediates potent antibody-dependent cellular cytotoxicity (ADCC) activity and defines a common ADCC epitope in human HIV-1 serum. J. Virol. 85:7029–7036. 10.1128/JVI.00171-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liao HX, Bonsignori M, Alam SM, McLellan JS, Tomaras GD, Moody MA, Kozink DM, Hwang KK, Chen X, Tsao CY, Liu P, Lu X, Parks RJ, Montefiori DC, Ferrari G, Pollara J, Rao M, Peachman KK, Santra S, Letvin NL, Karasavvas N, Yang ZY, Dai K, Pancera M, Gorman J, Wiehe K, Nicely NI, Rerks-Ngarm S, Nitayaphan S, Kaewkungwal J, Pitisuttithum P, Tartaglia J, Sinangil F, Kim JH, Michael NL, Kepler TB, Kwong PD, Mascola JR, Nabel GJ, Pinter A, Zolla-Pazner S, Haynes BF. 2013. Vaccine induction of antibodies against a structurally heterogeneous site of immune pressure within HIV-1 envelope protein variable regions 1 and 2. Immunity 38:176–186. 10.1016/j.immuni.2012.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moreno MR, Giudici M, Villalain J. 2006. The membranotropic regions of the endo and ecto domains of HIV gp41 envelope glycoprotein. Biochim. Biophys. Acta 1758:111–123. 10.1016/j.bbamem.2006.01.007 [DOI] [PubMed] [Google Scholar]
- 53.Moreno MR, Pascual R, Villalain J. 2004. Identification of membrane-active regions of the HIV-1 envelope glycoprotein gp41 using a 15-mer gp41-peptide scan. Biochim. Biophys. Acta 1661:97–105. 10.1016/j.bbamem.2003.12.003 [DOI] [PubMed] [Google Scholar]
- 54.Nawaz F, Cicala C, Van Ryk D, Block KE, Jelicic K, McNally JP, Ogundare O, Pascuccio M, Patel N, Wei D, Fauci AS, Arthos J. 2011. The genotype of early-transmitting HIV gp120s promotes α4β7-reactivity, revealing α4β7+/CD4+ T cells as key targets in mucosal transmission. PLoS Pathog. 7:e1001301. 10.1371/journal.ppat.1001301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Parrish NF, Wilen CB, Banks LB, Iyer SS, Pfaff JM, Salazar-Gonzalez JF, Salazar MG, Decker JM, Parrish EH, Berg A, Hopper J, Hora B, Kumar A, Mahlokozera T, Yuan S, Coleman C, Vermeulen M, Ding H, Ochsenbauer C, Tilton JC, Permar SR, Kappes JC, Betts MR, Busch MP, Gao F, Montefiori D, Haynes BF, Shaw GM, Hahn BH, Doms RW. 2012. Transmitted/founder and chronic subtype C HIV-1 use CD4 and CCR5 receptors with equal efficiency and are not inhibited by blocking the integrin alpha4beta7. PLoS Pathog. 8:e1002686. 10.1371/journal.ppat.1002686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Parrish NF, Gao F, Li H, Giorgi EE, Barbian HJ, Parrish EH, Zajic L, Iyer SS, Decker JM, Kumar A, Hora B, Berg A, Cai F, Hopper J, Denny TN, Ding H, Ochsenbauer C, Kappes JC, Galimidi RP, West AP, Jr, Bjorkman PJ, Wilen CB, Doms RW, O'Brien M, Bhardwaj N, Borrow P, Haynes BF, Muldoon M, Theiler JP, Korber B, Shaw GM, Hahn BH. 2013. Phenotypic properties of transmitted founder HIV-1. Proc. Natl. Acad. Sci. U. S. A. 110:6626–6633. 10.1073/pnas.1304288110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moore JP, Sodroski J. 1996. Antibody cross-competition analysis of the human immunodeficiency virus type 1 gp120 exterior envelope glycoprotein. J. Virol. 70:1863–1872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wyatt R, Moore J, Accola M, Desjardin E, Robinson J, Sodroski J. 1995. Involvement of the V1/V2 variable loop structure in the exposure of human immunodeficiency virus type 1 gp120 epitopes induced by receptor binding. J. Virol. 69:5723–5733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kwong PD, Wyatt R, Robinson J, Sweet RW, Sodroski J, Hendrickson WA. 1998. Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature 393:648–659. 10.1038/31405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Garg H, Francella N, Tony KA, Augustine LA, Barchi JJ, Jr, Fantini J, Puri A, Mootoo DR, Blumenthal R. 2008. Glycoside analogs of beta-galactosylceramide, a novel class of small molecule antiviral agents that inhibit HIV-1 entry. Antiviral Res. 80:54–61. 10.1016/j.antiviral.2008.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pollara J, Bonsignori M, Moody MA, Liu P, Alam SM, Hwang KK, Gurley TC, Kozink DM, Armand LC, Marshall DJ, Whitesides JF, Kaewkungwal J, Nitayaphan S, Pitisuttithum P, Rerks-Ngarm S, Robb ML, O'Connell RJ, Kim JH, Michael NL, Montefiori DC, Tomaras GD, Liao HX, Haynes BF, Ferrari G. 2014. HIV-1 vaccine-induced C1 and V2 Env-specific antibodies synergize for increased antiviral activities. J. Virol. 88:7715–7726. 10.1128/JVI.00156-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kaizu M, Weiler AM, Weisgrau KL, Vielhuber KA, May G, Piaskowski SM, Furlott J, Maness NJ, Friedrich TC, Loffredo JT, Usborne A, Rakasz EG. 2006. Repeated intravaginal inoculation with cell-associated simian immunodeficiency virus results in persistent infection of nonhuman primates. J. Infect. Dis. 194:912–916. 10.1086/507308 [DOI] [PubMed] [Google Scholar]
- 63.Salle B, Brochard P, Bourry O, Mannioui A, Andrieu T, Prevot S, Dejucq-Rainsford N, Dereuddre-Bosquet N, Le Grand R. 2010. Infection of macaques after vaginal exposure to cell-associated simian immunodeficiency virus. J. Infect. Dis. 202:337–344. 10.1086/653619 [DOI] [PubMed] [Google Scholar]
- 64.Martin N, Welsch S, Jolly C, Briggs JA, Vaux D, Sattentau QJ. 2010. Virological synapse-mediated spread of human immunodeficiency virus type 1 between T cells is sensitive to entry inhibition. J. Virol. 84:3516–3527. 10.1128/JVI.02651-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bomsel M, Heyman M, Hocini H, Lagaye S, Belec L, Dupont C, Desgranges C. 1998. Intracellular neutralization of HIV transcytosis across tight epithelial barriers by anti-HIV envelope protein dIgA or IgM. Immunity 9:277–287. 10.1016/S1074-7613(00)80610-X [DOI] [PubMed] [Google Scholar]
- 66.Magerus-Chatinet A, Yu H, Garcia S, Ducloux E, Terris B, Bomsel M. 2007. Galactosyl ceramide expressed on dendritic cells can mediate HIV-1 transfer from monocyte derived dendritic cells to autologous T cells. Virology 362:67–74. 10.1016/j.virol.2006.11.035 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.