ABSTRACT
Human immunodeficiency virus type 1 (HIV-1) exploits dendritic cells (DCs) to promote its transmission to T cells. We recently reported that the capture of HIV-1 by mature dendritic cells (MDCs) is mediated by an interaction between the glycosphingolipid (GSL) GM3 on virus particles and CD169/Siglec-1 on MDCs. Since HIV-1 preferentially buds from GSL-enriched lipid microdomains on the plasma membrane, we hypothesized that the virus assembly and budding site determines the ability of HIV-1 to interact with MDCs. In support of this hypothesis, mutations in the N-terminal basic domain (29/31KE) or deletion of the membrane-targeting domain of the HIV-1 matrix (MA) protein that altered the virus assembly and budding site to CD63+/Lamp-1-positive intracellular compartments resulted in lower levels of virion incorporation of GM3 and attenuation of virus capture by MDCs. Furthermore, MDC-mediated capture and transmission of MA mutant viruses to T cells were decreased, suggesting that HIV-1 acquires GSLs via budding from the plasma membrane to access the MDC-dependent trans infection pathway. Interestingly, MDC-mediated capture of Nipah and Hendra virus (recently emerged zoonotic paramyxoviruses) M (matrix) protein-derived virus-like particles that bud from GSL-enriched plasma membrane microdomains was also dependent on interactions between virion-incorporated GSLs and CD169. Moreover, capture and transfer of Nipah virus envelope glycoprotein-pseudotyped lentivirus particles by MDCs were severely attenuated upon depletion of GSLs from virus particles. These results suggest that GSL incorporation into virions is critical for the interaction of diverse enveloped RNA viruses with DCs and that the GSL-CD169 recognition nexus might be a conserved viral mechanism of parasitization of DC functions for systemic virus dissemination.
IMPORTANCE Dendritic cells (DCs) can capture HIV-1 particles and transfer captured virus particles to T cells without establishing productive infection in DCs, a mechanism of HIV-1 trans infection. We have recently identified CD169-mediated recognition of GM3, a host-derived glycosphingolipid (GSL) incorporated into the virus particle membrane, as the receptor and ligand for the DC-HIV trans infection pathway. In this study, we have identified the matrix (MA) domain of Gag to be the viral determinant that governs incorporation of GM3 into HIV-1 particles, a previously unappreciated function of the HIV-1 MA. In addition, we demonstrate that the GSL-CD169-dependent trans infection pathway is also utilized as a dissemination mechanism by henipaviruses. GSL incorporation in henipaviruses was also dependent on the viral capsid (M) protein-directed assembly and budding from GSL-enriched lipid microdomains. These findings provide evidence of a conserved mechanism of retrovirus and henipavirus parasitization of cell-to-cell recognition pathways for systemic virus dissemination.
INTRODUCTION
Human immunodeficiency virus type 1 (HIV-1) transmission worldwide mainly occurs after sexual intercourse and requires initiation of infection in the genital mucosa (1). The precise mechanisms by which HIV-1 is transmitted across the mucosal barrier, establishes productive infection in the genital mucosa, and then spreads systemically remain unclear. In addition to CD4+ T cells (2), dendritic cells (DCs) are one of the first cell types encountering HIV-1 or simian immunodeficiency virus (SIV) in the genital mucosa (3–5; reviewed in references 1 and 6) and are thought to play key roles in establishing virus infection in the genital mucosa. In addition to sentinel functions in peripheral mucosal tissues, DCs are located in the paracortical regions of draining lymphatic tissues lining the sinuses and are uniquely positioned to capture lymph-borne pathogens and to initiate adaptive immune responses. Subversion of DC-CD4+ T cell immunological synapses by HIV-1 might allow efficient virus dissemination in the lymphatic tissues. One such subversion mechanism involves DC-mediated HIV-1 transmission to CD4+ T cells without DCs themselves being productively infected, a process of HIV-1 trans infection (7, 8).
Though HIV-1 binding by DCs has long been thought to be exclusively dependent on gp120 interactions with C-type lectin receptors, such as DC-SIGN, mannose receptor, and dendritic cell immunoreceptor (9, 10), and heparan sulfate proteoglycans (11), HIV-1 capture by DCs can also occur in a gp120-independent manner (12, 13), and interestingly, this gp120-independent mechanism of HIV-1 capture is upregulated upon DC maturation with stimuli that induce type I interferon (IFN) signaling (14). Recently, we and others have identified CD169 (Siglec-1) to be the receptor on DCs which captures HIV-1 particles in a gp120-independent, GM3-dependent manner (14–17). Furthermore, CD169 was shown to be predominantly responsible for mature DC (MDC)-mediated HIV-1 trans infection (14, 16). CD169 is a member of the sialic acid binding immunoglobulin superfamily of lectins expressed on myeloid cells, and its expression is induced by type I IFN (14, 18). CD169 binds specifically to α2-3-linked terminal sialic acids, which are found in proteins and glycosphingolipids (GSLs), including gangliosides, such as GM3 (19).
Assembly and budding of HIV-1 particles have been shown to occur predominantly from morphologically distinct liquid-ordered cholesterol and GSL-enriched plasma membrane microdomains, such as lipid rafts and tetraspanin-enriched microdomains (reviewed in reference 20). Assembly of HIV-1 particles within lipid microdomains involves multiple steps, all of which are mediated by the viral Gag protein, which is sufficient for the assembly and release of virus particles. A stable HIV-1 Gag lipid raft-membrane association is accomplished by a bipartite motif that includes a fatty acid myristate, added cotranslationally to the N terminus of Gag, and the first 31 amino acids of the HIV-1 matrix (MA) protein, which form a highly basic patch on the surface of the protein (21–25), and allows specific contacts with the inner leaflet of the phosphatidylinositol (PI) 4,5-bisphosphate [PI(4,5)P2]-enriched plasma membrane (reviewed in reference 26). Mutations or deletions that either abrogate myristoylation or alter the positively charged nature of the N-terminal domain of the MA protein or, alternatively, disruption or redistribution of the intracellular levels of PI(4,5)P2 can lead to aberrant targeting of Gag to the cytoplasm and/or mislocalize HIV-1 assembly and budding to intracellular membranes, including the multivesicular endosomal body (MVB), the Golgi apparatus, and the endoplasmic reticulum (ER) (22, 27–31). In addition to lipid raft targeting, incorporation of HIV-1 gp120 in the virus particle is also governed by specific interactions between the MA protein and the cytoplasmic tail (CT) of gp41 (32–34). Hence, Gag-dependent HIV-1 assembly and budding from plasma membrane lipid microdomains ensure a unique lipid and protein composition in the viral membrane (35–37).
Recent lipidomic analyses have revealed that unique cellular lipids such as cholesterol, sphingomyelin, and phospholipids with saturated side chains are enriched in HIV-1 and murine leukemia virus (MLV) particles (35, 36, 38). Furthermore, these studies have also highlighted selective enrichment of glucosylceramide and other complex GSLs, including GM3, in the lipid envelopes of the retrovirus particles budding from the plasma membrane (36). Interestingly, our recent findings suggest that MLV trans infection by murine CD169 is dependent on GSLs (14). Besides retroviruses, lipid rafts have also been implicated in the assembly of many other enveloped viruses, including members of Filoviridae (Ebola virus [EBOV] and Marburg virus) (39, 40), Paramyxoviridae (respiratory syncytial virus, Newcastle disease virus, and measles virus) (41–45), and Orthomyxoviridae (influenza virus) (46–49). Furthermore, the ability of the HIV-1 Gag protein to bud in the absence of other viral proteins is a property also shared by the matrix (M) proteins of some of the nonsegmented negative-strand RNA viruses, including Marburg virus and EBOV VP40 proteins and Nipah virus (NiV) M protein (50–54).
Morphogenesis of virus particles within lipid rafts imbues HIV-1 particles with unique cellular lipid and protein compositions (36) that can hypothetically impact the ability of virus particles to interact with target cells. Since the composition of the HIV-1 lipid envelope is determined by the precise site of virus assembly and budding (36) and GSLs, including GM3, are highly enriched in the plasma membrane compared to their levels in other intracellular membranes (55), we wanted to determine the effect of an altered site of viral exit on the ability of HIV-1 particles to interact with mature DCs. In the study described in this report, we compared wild-type HIV-1 particles and those that contain deletions in the MA domain of Gag or selective mutations within the N-terminal positively charged domain of MA that result in virus assembly at the ER or at MVB membranes for the ability to establish mature DC-mediated HIV-1 trans infection of CD4+ T cells. Our data demonstrate that virus-like particles (VLPs) targeted for assembly and budding predominantly from intracellular membranes were deficient for capture by mature DCs. Furthermore, gp120-expressing HIV-1 particles containing mutations in MA were also deficient for capture by mature DCs and attenuated in establishing robust infections in DC-T cell cocultures. Moreover, capture and DC-mediated trans infection of both NiV and Hendra virus (HeV) particles were dependent on the presence of GSLs in the virus particle membranes and the presence of CD169 on mature DCs. These results suggest that incorporation of GSLs in virus particle membranes is critical for the interaction of diverse enveloped RNA viruses with DCs and that the GSL-CD169 recognition nexus might be a conserved mechanism that enveloped viruses use to exploit DCs for systemic virus dissemination.
MATERIALS AND METHODS
Plasmids.
The expression plasmid HIV-1 Gag-eGFP, which expresses a Gag-enhanced green fluorescent protein (eGFP) fusion protein, was obtained from the NIAID AIDS Research and Reference Reagent Program (ARRRP; contributed by Marilyn D. Resh and George Pavlakis). The HIV-1 Gag-mCherry expression plasmid, which expresses a red fluorescent Gag-mCherry fusion protein, has been described previously (13). The MA-deficient plasmid ΔMA15-99-Gag-eGFP (which carries the MA protein with an in-frame deletion of amino acids 15 to 99) and the MA mutant plasmid 29/31KE-Gag-eGFP (which carries the 29/31KE point mutations in the N-terminal basic domain) are isogenic to HIV-1 Gag-eGFP, except for the presence of the indicated in-frame deletion or point mutations in the MA-coding region. Briefly, the HIV-1 Gag-eGFP expression plasmid was digested with ClaI and HindIII restriction enzymes to remove the intervening sequence, the ends were blunted with DNA polymerase (Klenow fragment), and the blunt-ended plasmid was ligated to generate an in-frame deletion that removes 84 amino acids from the matrix domain of Gag (ΔMA15-99-Gag-eGFP). Point mutations in MA (29/31KE) were introduced by site-directed mutagenesis (QuikChange; Agilent Technologies) using the following primers: 5′-GGAAAGAAGAAGTACGAGCTAGAGCACATCGTATGGGCAAGC-3′ and 5′-GCTTGCCCATACGATGTGCTCTAGCTCGTACTTCTTCTTTCC-3′. The HIV Env expression vectors for HxB2 and Lai have been described previously (56, 57). Codon-optimized HeV and NiV M expression plasmids were obtained from Chris Broder. The NiV F and G expression plasmids were obtained from Gary Kobinger. The plasmids expressing Zaire EBOV VP40 and GP were obtained from Elke Mühlberger. HeV M, NiV M, and EBOV VP40 cDNAs were PCR amplified and cloned in frame into the C terminus of eGFP in the expression plasmid pEGFP-C3 (Clontech). HIV Lai-lucΔenv (an Env-deficient HIV Lai provirus molecular clone containing a luciferase gene in place of the Nef open reading frame [ORF]) and HIV NL4-3 have been described previously (58, 59). To create an envelope glycoprotein (GP)-deficient NL4-3 provirus plasmid carrying the intact Nef ORF (NL4-3Δenv), the Env ORF containing deletions in the env sequence of NL4-3.Luc.R−.E− (60) was cloned into the NL4-3 proviral clone. NL4-3 29/31KEΔCT containing lysine-to-glutamate changes at positions 29 and 31 in the Gag ORF and a stop codon at position 712 in the gp160 ORF was a gracious gift of Eric Freed (61). The gp41 cytoplasmic tail deletion mutant NL4-3ΔCT, which contains a stop codon at position 712 in the gp160 ORF, was generated by replacing the env region of NL4-3 with the corresponding region of NL4-3 29/31KEΔCT. The mutant MA ORFs containing either an in-frame truncation (ΔMA15-99) or the 29/31KE mutations were cloned into the NL4-3, NL4-3ΔCT, and NL4-3Δenv proviral clones. All the mutations were confirmed by sequencing. Note that all cloning details are available upon request.
Ethics Statement.
This research has been determined to be exempt by the Institutional Review Board of the Boston University Medical Center since it does not meet the definition of human subject research; all human samples were collected in an anonymous fashion, and no identifiable private information was collected.
Cells and viruses.
Human DCs were derived from CD14+ peripheral blood monocytes, as described previously (14). DCs were matured with ultrapure Escherichia coli K-12 lipopolysaccharide (100 ng/ml; Invivogen) for 2 days prior to use. Primary human CD4+ T cells were positively isolated from CD14-depleted peripheral blood mononuclear cells, using CD4-conjugated magnetic beads and LS magnetically activated cell sorting cell separation columns (Miltenyi Biotech). Positively isolated CD4+ T cells were activated with 2% phytohemagglutinin (Invitrogen) for 2 days, washed, and cultured in interleukin-2 (50 U/ml)-containing RPMI–10% fetal bovine serum medium. Cells of the cell lines Raji (a B cell line), Raji-CD169 (a Raji cell line constitutively expressing CD169), HEK293T (a human kidney epithelial cell line), HeLa, MT4 (a T cell line), TZM-bl, and U373 expressing CD4 and CXCR4 (U373/CD4/CXCR4) (HIV reporter cell lines) have been described previously (14, 17, 62–64). NL4-3, NL4-3ΔCT, NL4-3Δenv, or matrix-deficient NL4-3, NL4-3ΔCT, and NL4-3Δenv viruses were derived from HEK293T cells via calcium phosphate transfection or from HeLa cells via transfection with Lipofectamine 2000 (Invitrogen), as described previously (17). Fluorescent HIV Gag, HeV M-, NiV M-, and EBOV VP40-derived VLPs were generated via transient transfection of HEK293T cells with HIV Gag-eGFP, HIV Gag-mCherry, eGFP-HeV M, eGFP-NiV M, or eGFP-EBOV VP40 expression constructs, and in some cases, the VLPs were generated together with the envelope expression plasmid HIV HxB2 env, NiV F and G, or EBOV GP. To deplete GSLs from virus particles, transfections were performed in the presence of phenyl-2-decanoylamino-3-morpholino-1-propanol (PDMP; 10 μM; Calbiochem), as described previously (12). Luciferase-expressing, single-cycle-of-replication-competent HIV-1 particles pseudotyped with HIV-1 Env, NiV F and G, or EBOV GP were generated from HEK293T cells via cotransfection of HIV Lai-lucΔenv with the HIV Lai, NiV F and G, or EBOV GP expression plasmid, respectively. Infectious viruses or VLP-containing cell supernatants were harvested at 2 days posttransfection, cleared of cell debris by centrifugation (300 × g, 5 min), passed through 0.45-μm-pore-size filters, and stored at −80°C until further use. For some experiments, VLPs in the supernatants were concentrated by ultracentrifugation on a 20% sucrose cushion (at 24,000 rpm and 4°C for 2 h with an SW32Ti rotor [Beckman]). The VLP pellets were resuspended in 140 mM NaCl, 10 mM HEPES buffer, aliquoted, and stored at −80°C. The concentrated VLPs were further purified by velocity gradient centrifugation on an OptiPrep gradient (Axis-Shield), as described previously (35). The visible VLP-containing fraction was collected, concentrated by ultracentrifugation (at 45,000 rpm and 4°C for 40 min with an SW55Ti rotor [Beckman]), resuspended in 140 mM NaCl, 10 mM HEPES buffer, aliquoted, and stored at −80°C. The capsid content of infectious HIV-1 particles or HIV Gag-eGFP or HIV Gag-mCherry VLPs was determined by a p24Gag enzyme-linked immunosorbent assay (ELISA) (12). The capsid or GFP content of HIV Gag-eGFP, HeV M-eGFP NiV M-eGFP, or EBOV VP40-eGFP VLPs was determined by quantitative Western blotting, as described below.
DC capture and transfer assays.
Mature DCs (2 × 105) were incubated with virus (50 ng p24Gag unless noted otherwise) or VLPs (2 ng or increasing amounts of p24Gag) for 2 h at 37°C in complete RPMI medium, washed 4 times with phosphate-buffered saline (PBS), and analyzed for capture using either a p24Gag ELISA or fluorescence-activated cell sorter (FACS) analysis. Virus capture was quantified by measuring the amount of p24Gag associated with lysed MDCs using an in-house p24Gag ELISA described previously (12). All assays were performed with mature DCs derived from a minimum of three independent donors.
Transfer assay.
For transfer of infectious NL4-3 viruses, mature DCs (2 × 105) were incubated with virus (200 ng p24Gag) for 2 h at 37°C in complete RPMI medium, washed 4 times with PBS, and cocultured with MT4 cells at a 1:1 cell ratio in complete RPMI medium containing 1 μM indinavir. In parallel, MT4 cells were spinoculated (65) with cell-free viruses (50 ng p24Gag) at 2,300 rpm with 10 μg/ml Polybrene for 1 h at room temperature, incubated at 37°C for 6 h, washed 3 times with PBS, and cultured in complete RPMI medium and 1 μM indinavir. The cells were washed again on the next day, and the supernatants were harvested at 72 h postinfection. The p24Gag content in the cell-free supernatants was quantified by an ELISA. For a single-round-transfer assays using the pseudotyped HIV Lai-lucΔenv vector, mature DCs (5 × 104) were incubated with virus (10 ng p24Gag) for 2 h at 37°C in complete RPMI medium, washed 4 times with PBS, and cocultured with U373/CD4/CXCR4 cells at a 1:1 cell ratio in complete Dulbecco modified Eagle medium. In parallel, U373/CD4/CXCR4 cells were infected with cell-free virus (10 ng p24Gag). The cells were lysed at 2 days postinfection, and the luciferase activity in the cell lysates was measured as previously described (12). The assays were performed with cells from a minimum of three independent donors.
GSL quantification.
To quantify the incorporation of GM1 in virus particles, VLPs derived from untreated or PDMP-treated HEK293T cells were incubated with 2 μg chorela toxin subunit B (CTxB)-biotin (Sigma) coupled to 25 μl streptavidin-coated 2.8-μm-diameter Dynabeads (Invitrogen) in 1% casein/PBS (Pierce) at room temperature for 1 h with rotation. The virus-bead mixture was washed twice with 0.1% casein/PBS, and the bead-bound virus particles were lysed with SDS-sample buffer. The amount of immunoprecipitated VLPs was measured via quantitative Western blotting, as described below.
The GM3 content in VLPs was quantified as previously described (17), with some modifications. Briefly, Gag-eGFP or MA-deficient Gag-eGFP VLPs (0.5 ng of p24Gag) were adhered onto poly-l-lysine-coated glass coverslips. GM3 was detected using undiluted supernatant from anti-GM3 DH2 hybridoma cells (17), followed by Alexa 594-conjugated goat anti-mouse IgG (10 μg/ml; Invitrogen). As a background control, VLPs were stained with 4 μg/ml mouse IgG3 (eBioscience) in parallel. Slides were analyzed at a magnification of ×63 with oil immersion on a Zeiss fluorescence microscope (Axiovert 200) and then quantified by imaging at least 10 fields per condition (>100 virus particles per field) and analyzing the fluorescence intensity using ImageJ software, as follows. After the background was subtracted, green fluorescent VLPs were identified as particles having signals greater than the mean fluorescent intensity (MFI) plus two times the standard deviation (SD), and the GM3 or background IgG3 fluorescent staining of GFP-VLPs at 605 nm was determined. The background signals (IgG3 staining) were subtracted from the signals obtained with GM3 staining, and the GM3 signals were normalized to those of the wild type Gag-eGFP. The assays were performed with a minimum of two independent VLP preparations.
Quantitative Western blotting.
To detect p24Gag, Pr55Gag, gp120, or GFP, samples were run on SDS-polyacrylamide gels and the proteins were transferred to a nitrocellulose membrane. The p24Gag or Pr55Gag content in virus particles was determined by probing with a rabbit anti-p24 polyclonal antibody (Immunodiagnostics) or a mouse anti-p24 monoclonal antibody (clone 24-2; ARRRP, NIAID), followed by goat anti-rabbit IgG-DyLight 800 (Pierce) or goat anti-mouse IgG DyLight 680 (Pierce) antibody, respectively. The gp120 content of the virus particles was determined by probing with a rabbit anti-gp120 polyclonal antibody (a gift of Nancy Haigwood), and the staining was visualized by goat anti-rabbit IgG-DyLight 800 antibody (Pierce). The GFP content was determined by probing with a goat anti-GFP antibody (Novus), followed by a donkey anti-goat IgG-IRDye 800CW antibody (LI-COR). The membrane was scanned with an Odyssey scanner (LI-COR), and p24Gag/Pr55Gag, gp120, or GFP was quantified using recombinant IIIB p24Gag (ABL), recombinant HIV-1 Bal gp120 (ARRRP, NIAID), or recombinant GFP (Roche) as the standard, respectively.
Cellular imaging analysis.
HEK293T cells, seeded on coverslips in 24-well tissue culture plates, were transfected by calcium phosphate. GM1 was visualized using Alexa 594-conjugated CTxB (10 μg/ml; Invitrogen), and the nucleus was stained with DAPI (4′,6-diamidino-2-phenylindole; Sigma). A series of z-section images was acquired using an Olympus IX70 microscope equipped for DeltaVision deconvolution (Applied Precision). Images were deconvoluted using SoftWoRx software (Applied Precision). For the colocalization study, images of 10 to 19 cells were acquired, and the images were deconvoluted, flattened for maximum intensity, and analyzed for Pearson's coefficient of correlation (R) with ImageJ software.
RESULTS
Mutations in MA alter HIV-1 Gag-eGFP localization.
HIV-1 particles acquire their lipid bilayer as they assemble and bud out from host cell membranes. Hence, the acquisition of gp120-independent DC-binding determinants by HIV-1 particles is presumably dependent on the site from which the virus particle assembles and buds. To determine the molecular constituents necessary for the HIV-1 gp120-independent mechanism of binding to DCs, we have recently developed a novel FACS-based assay that takes advantage of the inherent fluorescence intensity of the Gag-eGFP fusion protein to detect the binding of eGFP-containing HIV-1 Gag VLPs to DCs (13). Expression of the HIV-1 Gag-eGFP fusion protein alone is sufficient for the formation of eGFP-containing fluorescent VLPs that assemble and bud from regions within the cell in a manner indistinguishable from that observed for replication-competent wild-type HIV-1 (66, 67). Using this FACS-based assay, we and others have determined that the GSLs GM1 and GM3 are necessary for HIV-1 to be captured by mature DCs (15, 17) in a CD169-dependent manner (14, 16).
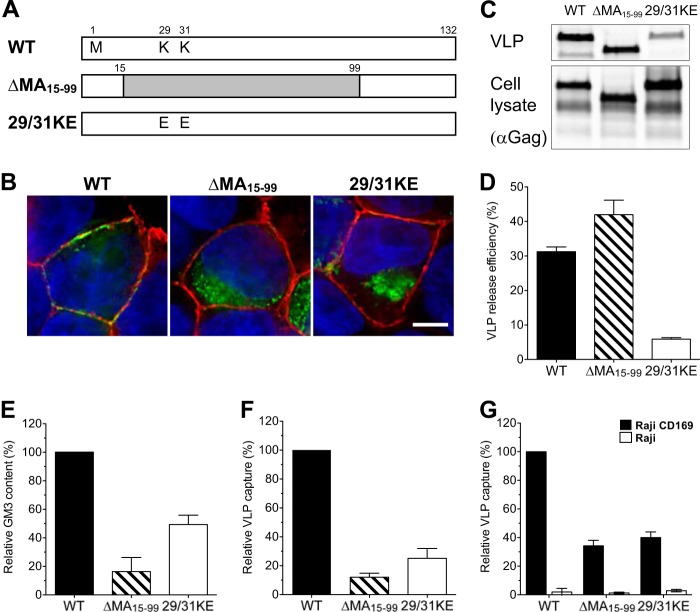
Since HIV-1 assembly and budding occur from GSL-enriched lipid raft plasma membrane microdomains (reviewed in reference 20) and the plasma membrane targeting of the virus assembly machine is dependent on the matrix (MA) domain of Gag that makes specific contacts with the inner leaflet of membranes, we tested the hypothesis that alterations in the site of virus particle assembly and exit, because of mutations in the MA protein, would negatively impact the ability of HIV-1 particles to be captured by DCs. We employed two MA-deficient mutants of HIV-1 Gag-eGFP (Fig. 1A). One construct retained the myristoylation sequence but contained an in-frame deletion in the matrix domain (ΔMA15-99-Gag-eGFP) that removed all of the predicted membrane-targeting domains and is predicted to bud indiscriminately from both intracellular and plasma membranes (28, 31, 68). The mutant 29/31KE-Gag-eGFP contains two lysine-to-glutamate changes at positions 29 and 31 in the Gag ORF (Fig. 1A). Both lysine residues are known to play a role in the proper targeting of Gag to the plasma membrane (29, 30, 61). Since those mutations have not yet been tested in the context of VLPs to see if their presence alters the HIV-1 assembly site, we sought to determine if MA mutant VLPs could recapitulate the previously described effect of MA mutations on infectious virus particle assembly (28–30, 61). We transiently transfected HEK293T cells to determine the effects of MA mutations on intracellular Gag localization and on VLP production. As expected, while wild-type Gag-eGFP was colocalized with GM1 (a lipid raft marker) on the plasma membrane, both ΔMA15-99-Gag-eGFP and 29/31KE-Gag-eGFP fluorescent puncta were seen to be associated mostly with intracellular membranes (Fig. 1B). Furthermore, while transfection with the ΔMA15-99-Gag-eGFP mutant resulted in VLP production to levels similar to those observed with Gag-eGFP (Fig. 1C and D), the efficiency of 29/31KE-Gag-eGFP virus particle release was greatly reduced (particle release was 4-fold less efficient than wild-type Gag-eGFP particle release) (Fig. 1C and D), findings similar to those previously observed with infectious proviral clones (61).
FIG 1.
MA mutant Gag-eGFP VLPs bud from intracellular membranes, and capture of HIV-1 Gag-eGFP VLPs by mature DCs is MA dependent. (A) Construction of HIV-1 matrix mutants. (B) Localization of eGFP fusion proteins in transfected HEK293T cells stained with CTxB-Alexa 594 for GM1 (red) and with DAPI for the nucleus (blue). Bar, 5 μm. (C) Western blot analysis of VLPs produced from transfected HEK293T cells was performed by probing with an anti-p24Gag monoclonal antibody. (D) Efficiency of VLP release from HEK293T cells. The amount of p24Gag in transfected HEK293T cell lysates and supernatants was quantified, and VLP release efficiencies were calculated [release efficiency = amount of p24Gag in the supernatant/(amount of p24Gag in the cell lysates + amount of p24Gag in the supernatant)]. The data shown are the means of triplicate experiments ± SDs. WT, wild-type Gag-eGFP; Δ15-99, ΔMA15-99-eGFP; 29/31KE, 29/31KE-eGFP. (E) The amount of VLP-associated GM3 was quantified by immunofluorescence staining. The value was normalized to that of wild-type Gag-eGFP. The experiment was performed on at least two independent VLP preparations, and the data are reported as the means ± SEMs of a representative experiment. (F) Mature DCs were challenged with VLPs and analyzed for GFP-positive cells by FACS. The percentage of GFP-positive DCs was normalized to that of wild-type Gag-eGFP VLPs. The results represent the means ± SEMs from four independent experiments performed on DCs from four different donors. (G) Raji-CD169 cells or Raji cells were challenged with VLPs, washed, and analyzed for GFP-positive cells. The value was normalized to that of wild-type Gag-eGFP capture by Raji-CD169 cells. The experiment was performed in triplicate at least two independent times, and the data from a representative experiment are shown as the means ± SDs.
HIV-1 particles have been demonstrated to exit through GSL-enriched lipid microdomains (reviewed in reference 20). GM3 is one of the major gangliosides in cell membranes, and we and others have reported that GM3 is the determinant for DC-mediated capture of HIV-1 (15, 17). Furthermore, GM3 incorporation has been shown to be critical for CD169-dependent HIV-1 capture by mature DCs (14, 16). Since the intracellular membrane distribution of lipids is not homogeneous (69) and the plasma membrane is enriched for GSL expression compared to the level of enrichment in intracellular membranes (55), virus particles that bud predominantly from intracellular membranes as opposed to the plasma membrane may incorporate different amounts of GSLs, such as GM3. To determine the GM3 levels in MA mutant Gag-eGFP VLPs, VLPs were derived from HEK293T cells transfected with either the wild-type HIV-1 Gag-eGFP, ΔMA15-99-Gag-eGFP, or 29/31KE-Gag-eGFP plasmid. Purified VLPs were analyzed for GM3 incorporation by an immunofluorescence assay (17). Interestingly, ΔMA15-99-Gag-eGFP or 29/31KE-Gag-eGFP virus-like particles incorporated significantly lower levels of GM3 than wild-type VLPs (Fig. 1E), suggesting that ΔMA15-99-Gag-eGFP or 29/31KE-Gag-eGFP virus-like particles are released predominantly from GM3-deficient intracellular membranes.
To test the ability of mature DCs to capture MA-deficient VLPs, mature DCs were pulsed in parallel with equal amounts of wild-type HIV-1 Gag-eGFP VLPs and matrix-deficient (ΔMA15-99-Gag-eGFP and 29/31KE-Gag-eGFP) VLPs, and the percentage of eGFP-positive cells was determined by FACS. Interestingly, mature DCs were attenuated in their ability to capture ΔMA15-99-Gag-eGFP and 29/31KE-Gag-eGFP VLPs (Fig. 1F; a greater than 75 to 80% reduction in capture). To examine the hypothesis that reduced capture of the MA-deficient Gag-eGFP VLPs was due to a deficiency in the interaction with CD169, Raji-CD169 cells were incubated with equal amounts of HIV-1 Gag-eGFP, ΔMA15-99-Gag-eGFP, and 29/31KE-Gag-eGFP VLPs, and VLP capture was determined by FACS. Interestingly, the level of capture of GM3-deficient ΔMA15-99-Gag-eGFP and 29/31KE-Gag-eGFP VLPs by Raji/CD169 cells was significantly decreased (∼60%) compared to the level of capture of HIV-1 Gag-eGFP VLPs (Fig. 1G). These results suggest that budding from GSL-enriched HIV-1 MA-determined membrane microdomains at the plasma membrane is critical for the efficient acquisition of DC-binding determinants by HIV-1.
MA mutants are attenuated in DC-mediated HIV-1 trans infection.
We next sought to determine if mutations in the membrane-targeting domain in MA which alter the infectious virus particle assembly site would affect mature DC-mediated capture and subsequent HIV-1 trans infection of CD4+ T cells. Deletion (NL4-3 ΔMA15-99) and point (NL4-3 29/31KE) mutations identical to those described above in VLPs (Fig. 1) were introduced into the MA domain of infectious NL4-3 proviral clones. HeLa cells were transfected with the proviral plasmids, and virus production was assessed by a p24Gag ELISA. The total amount of NL4-3 29/31KE virus particles released into the supernatants was significantly less than that observed upon transfection with either wild-type NL4-3 or NL4-3 ΔMA15-99 virus particles (data not shown), as previously reported (29, 30, 61). While wild-type Gag was colocalized with the tetraspanin CD9, which is known to associate with GM3 (70), at the plasma membrane, ΔMA15-99 Gag and 29/31KE Gag were predominantly found in intracellular compartments to colocalize with the late endosomal marker CD63 and/or a lysosomal marker Lamp-1 (data not shown), similar to previously published findings (29, 61).
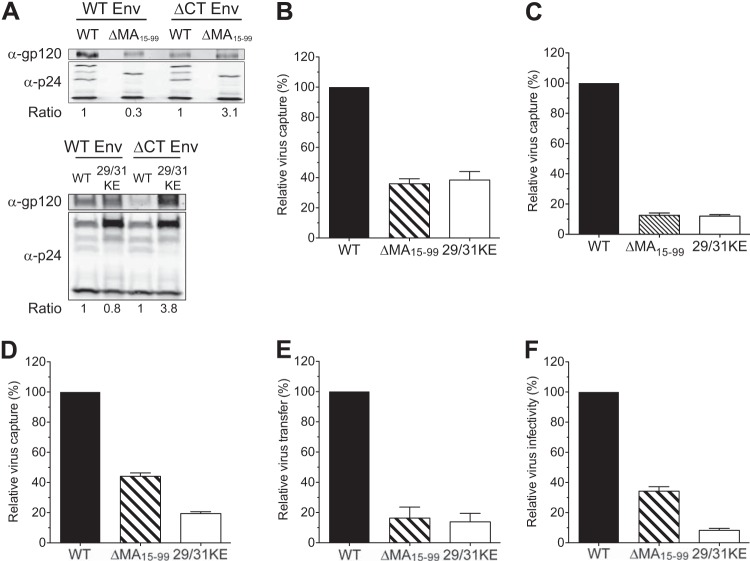
It has long been appreciated that Env incorporation into virus particles is dependent on interactions between the cytoplasmic tail (CT) of gp41 and MA, and previous studies have demonstrated a significant deficit in Env incorporation in viruses with MA mutations (32, 33, 71, 72). We also observed a decrease in gp120 incorporation in NL4-3 ΔMA15-99 and NL4-3 29/31KE virus particles compared to that observed with wild-type NL4-3 (Fig. 2A). To discount the possibility that different levels of Env in virus particles could account for the observed differences in virus capture, we generated MA mutant viruses that do not encode Env (ΔEnv) or encode Env with deletions in the CT of gp41. Similar to previously published results (31, 33, 34), the version of Env with the CT deletion (ΔCT) was efficiently incorporated by MA mutant viruses at levels greater than those observed with wild-type HIV-1 (Fig. 2A). We next tested the ability of mature DCs to capture MA-deficient NL4-3 viruses. Mature DCs were challenged with equal amounts of virus particles, and the amount of cell-associated virus was determined as a quantitative measure of HIV-1 capture (14). Interestingly, capture of MA mutant viruses by mature DCs was dramatically attenuated (an ∼70% decrease in virus capture; Fig. 2B). Similar to the capture of MA mutant viruses encoding wild-type Env by mature DCs, capture of both MA-deficient NL4-3ΔCT and NL4-3ΔEnv viruses was also dramatically attenuated compared to that of the isogenic wild-type Gag-encoding viruses (Fig. 2C and D), suggesting that MA mutant viruses are deficient in accessing the GSL-dependent mechanism of HIV-1 capture by mature DCs. Finally, the extent to which alteration in virus assembly sites can affect DC-mediated HIV-1 trans infection of T cells, mature DCs were pulsed with infectious viruses containing either wild-type Gag or mutations in MA and expressing ΔCT Env and cocultured with MT4 T cells. Note that the MT4 T cell line has previously been shown to support the replication of ΔCT viruses encoding wild-type Gag (73). Although direct (cell-free) infection of MT4 T cells with NL4-3 ΔMA15-99 ΔCT virus resulted in amounts of virus production at day 3 postinfection smaller than those observed upon infection of MT4 T cells with NL4-3ΔCT virus (34.3% ± 2.9% [mean ± standard error of the mean {SEM}] of the amount for NL4-3ΔCT; Fig. 2F), this replication defect was further enhanced upon initiation of infection in MT4 T cells with mature DC-associated NL4-3 ΔMA15-99 ΔCT virus (16.3% ± 7.2% [mean ± SEM] of the amount for NL4-3ΔCT) (Fig. 2E). In contrast, replication of NL4-3 29/31KEΔCT was severely attenuated both in the mature DC–MT4 T cell cocultures and by direct cell-free infection of MT4 T cells (Fig. 2E and F). These results suggest that in addition to the previously described replication defects of MA-deficient viruses in direct cell-free infection of T cells (74), a lack of plasma membrane targeting specificity and decreased assembly and budding from GM3-enriched lipid microdomains further attenuate the replication of MA-deficient HIV-1 in mature DC-T cell cocultures.
FIG 2.
HIV-1 MA mutants are severely attenuated in DC-mediated capture and trans infection of T cells. (A) Quantitative Western blotting of incorporation of wild-type gp120 or gp120 with a cytoplasmic tail deletion in virus particles with wild-type (WT), ΔMA15-99, or 29/31KE matrix proteins. The band intensity was quantified, and the ratio of the amount of gp120 to the total amount of Gag (p24Gag + Pr55Gag) in ΔMA15-99 or 29/31KE virions was normalized to that observed with wild-type Gag containing wild-type Env or ΔCT Env. (B to D) Mature DCs were challenged with NL4-3 viruses containing intact Env (B), NL4-3 viruses containing ΔCT Env (C), or NL4-3 viruses without Env (ΔEnv) (D) and encoding the wild-type, ΔMA15-99, or 29/31KE matrix protein. Cells were washed and lysed for cell-associated p24Gag measurement, and the results were normalized to those observed with viruses encoding wild-type Gag. The data shown are the means ± SEMs of three (B and D) or nine (C) independent experiments with DCs from different donors. (E) Mature DCs were challenged with NL4-3ΔCT viruses encoding the wild-type, ΔMA15-99, or 29/31KE matrix proteins and washed, and DC-to-T cell (MT4) trans infection was quantified. The values were normalized to those for NL4-3ΔCT (wild type). (F) In parallel, cell-free infection of MT4 cells was examined. The data shown are the means ± SEMs of three independent experiments with DCs from three different donors (E) or of four independent experiments performed in triplicate (F).
HeV, NiV, and EBOV VLPs bud out of the GSL-enriched domain.
Budding from lipid microdomains, such as lipid rafts, is a feature that is well conserved among diverse nonsegmented RNA virus families, including members of the Filoviridae (Ebola and Marburg viruses) (39, 40), Paramyxoviridae (respiratory syncytial virus, Newcastle disease virus, and measles virus) (41–45), and Orthomyxoviridae (influenza virus) (46–49). Furthermore, similar to HIV-1 Gag, expression of the M protein of NiV or the VP40 proteins of EBOV and Marburg virus is sufficient for virion morphogenesis (50–52). To date, lipidomic analysis of NiV, HeV, or EBOV has not been performed, and it remains unclear if GSLs are incorporated by these virus particles and whether incorporated GSLs can mediate interactions of viruses with DCs. Hence, we decided to test the hypothesis that VLPs derived from HeV, NiV, and EBOV matrix proteins could also be captured by DCs in a GSL-dependent manner.
eGFP fusion proteins of HeV, NiV, and EBOV matrix proteins (Fig. 3A) were transiently expressed in HEK293T cells, and cell-free supernatants containing VLPs were harvested and purified via pelleting through 20% sucrose cushions. There was robust expression of each of the fusion proteins in transfected HEK293T cells (Fig. 3B), and HeV M VLPs, NiV M VLPs, and EBOV VP40-derived VLPs were efficiently released into the supernatant (Fig. 3C).
FIG 3.
Paramyxovirus and filovirus VLPs bud from GSL-enriched lipid microdomains at the plasma membrane. (A) Constructions of HeV M, NiV M, and EBOV VP40 fusion proteins. (B and C) Western blot analysis of eGFP fusion proteins in transfected HEK293T cell lysates (B) and VLPs in the supernatants (C) in the presence or absence of PDMP. (D) Localization of eGFP fusion proteins in transfected HEK293T cells stained with CTxB for GM1 (red) and with DAPI for the nucleus (blue). The values at the bottom are Pearson's colocalization coefficients of the means ± SDs. (E) Localization of Gag-mCherry and the indicated eGFP fusion proteins in transfected HEK293T cells stained with DAPI (blue). The values at the bottom are Pearson's colocalization coefficients of the means ± SDs. Bar, 10 μm.
Next, we investigated the localization of HeV M-eGFP, NiV M-eGFP, and EBOV VP40-eGFP in transiently transfected HEK293T cells. Fluorescent puncta were found to be associated mostly at the periphery, which is suggestive of filovirus and paramyxovirus particle assembly at the plasma membrane (Fig. 3D), similar to previously published findings (50, 75). Furthermore, GM1 and eGFP fusion proteins of NiV M, HeV M, and EBOV VP40 colocalized at the plasma membrane (Fig. 3D), similar to the site of colocalization observed with HIV-1 Gag, suggesting that NiV M, HeV M, and EBOV VP40 also assembled at GSL-enriched plasma membrane microdomains. To test if the assembly and budding sites of HeV M VLPs, NiV M VLPs, and EBOV VP40-derived VLPs were similar to those of HIV-1 Gag, HEK293T cells were cotransfected with Gag-mCherry and either Gag-eGFP, HeV M-eGFP, NiV M-eGFP, or EBOV VP40-eGFP expression plasmids and were subjected to deconvolution microscopy (Fig. 3E). As expected, Gag-eGFP and Gag-mCherry were almost completely colocalized (R = 0.95 ± 0.02 [mean ± standard deviation]). Furthermore, HeV M-eGFP, NiV M-eGFP, and EBOV VP40-eGFP fluorescence was mostly observed at the plasma membrane and colocalized with Gag-mCherry, though the colocalization efficiency was lower than that observed with eGFP- and mCherry-fused HIV-1 Gag proteins (Fig. 3E), suggesting that EBOV, HeV, and NiV VLPs bud from similar but microscopically distinct plasma membrane microdomains.
To determine if mature DCs could bind paramyxovirus and filovirus VLPs and if the determinants on those VLPs are host cell-derived GSLs, HeV VLPs, NiV VLPs, and EBOV VP40-drived particles were produced from HEK293T cells in the presence of a glucosylceramide synthase inhibitor, PDMP. We have previously demonstrated that PDMP treatment effectively reduces the GSL composition of the virus-producing cells and, hence, reduces the GSL content of the virus particles that bud from PDMP-treated cells (12). Though production of HeV VLPs, NiV VLPs, and EBOV VP40-derived VLPs was unaffected upon PDMP treatment of HEK293T cells (Fig. 3B and C), GSL incorporation into particles was reduced upon production from PDMP-treated cells (Fig. 4A). Mature DCs were challenged with wild-type or GSL-depleted HeV VLPs, NiV VLPs, or EBOV VP40-derived VLPs containing equal amounts of GFP, and the percentage of eGFP-positive cells was quantified by FACS. Interestingly, mature DCs captured HeV VLPs, NiV VLPs, and EBOV VP40-derived VLPs to a similar extent as HIV Gag-eGFP VLPs (Fig. 4B). Furthermore, similar to HIV-1 Gag VLPs, capture of GSL-depleted HeV and NiV VLPs by mature DCs was severely attenuated (>80% inhibition) (Fig. 4B). In contrast, there was a little, if any, reduction in the amount of GSL-deficient EBOV VP40-derived VLPs captured by mature DCs (Fig. 4B). To examine if GSL-dependent capture of HeV and NiV VLPs by mature DCs was mediated by CD169, mature DCs were incubated with VLPs in the presence of neutralizing anti-CD169 antibody. Interestingly, pretreatment of mature DCs with anti-CD169 blocking antibody reduced the amount of HeV and NiV captured by more than 80% (Fig. 4C). To determine if virus particle-associated GSL recognition by CD169 can mediate NiV capture by mature DCs even in the presence of virus envelope glycoproteins, VLPs were pseudotyped with envelope glycoproteins of the requisite virus. It should be noted that cotransfection of EBOV VP40 and GP produces filamentous particles strikingly similar to infectious viruses (39, 51). Capture of NiV F/G-pseudotyped NiV VLPs but not EBOV GP-pseudotyped EBOV VLPs was severely attenuated by GSL depletion (Fig. 4D). These results suggest that, similar to lentivirus particles, paramyxovirus VLPs can also be captured by mature DCs in a GSL- and CD169-dependent manner.
FIG 4.
Capture of Hendra and Nipah virus VLPs by DCs is dependent on GSL-CD169 interaction. (A) VLPs derived from transfected HEK293T cells in the presence or absence of PDMP were immunoprecipitated with CTxB. Immunoprecipitated VLPs were probed for GFP expression via quantitative Western blot analysis. (B) Mature DCs were incubated with VLPs containing equal amounts of GFP produced in the presence or absence of PDMP, washed, and analyzed for GFP-positive cells by flow cytometry. (C) Mature DCs were untreated or incubated with isotype control antibody or anti-CD169 blocking antibody, challenged with VLPs, washed, and analyzed for GFP-positive cells by flow cytometry. (D) Mature DCs were incubated with VLPs pseudotyped with the indicated envelope glycoproteins produced in the presence or absence of PDMP and analyzed for GFP-positive cells by flow cytometry. (E) Mature DCs were challenged with pseudotyped HIV-1 vectors produced in the presence or absence of PDMP and washed, and the amount of cell-associated p24Gag was measured. (F) Mature DCs were incubated with pseudotyped HIV-1 vectors produced in the presence or absence of PDMP, washed, and cocultured with U373/CD4/CXCR4 cells, and the cells were lysed at 2 days postinfection to measure the luciferase activity in lysates. (G) U373/CD4/CXCR4 cells were infected with cell-free pseudotyped HIV-1 vectors produced in the presence or absence of PDMP, and cells were lysed at 2 days postinfection to measure the luciferase activity in cell lysates as a measure of virus infection. The data shown are the means ± SEMs of three independent experiments with DCs from three different donors. NT, not treated; RLU, relative light units.
Receptors for HIV, HeV, or NiV are not abundant on DCs (76, 77). In contrast, there are a multitude of receptors/attachment factors for EBOV on DCs (reviewed in reference 78). Therefore, the receptor density on DCs might determine the dependency on GSLs for DC-mediated enveloped virus capture. To test this hypothesis, HIV-1 particles were pseudotyped with HIV-1 Env, NiV F and G proteins, EBOV GP, or vesicular stomatitis virus G glycoprotein (VSV-G) and examined for DC-mediated capture. HIV-1 particles pseudotyped with HIV-1 Lai Env or NiV F/G were captured by mature DCs in a GSL-dependent manner, since capture of viruses derived from PDMP-treated virus-producing cells was attenuated (Fig. 4E). Interestingly, when HIV-1 was pseudotyped with either EBOV GP or VSV-G, capture by mature DCs was observed in a GSL-independent fashion. Finally, these pseudotyped HIV-1 isolates were examined for trans infection using U373/CD4/CXCR4 cells expressing CD4 and CXCR4 that are susceptible to HIV-1 and NiV infections (63, 79). Interestingly, mature DC-mediated trans infection of U373/CD4/CXCR4 cells of viruses pseudotyped with HIV-1 Lai Env or NiV F/G was GSL dependent, while EBOV GP-pseudotyped HIV-1 was transmitted in a GSL-independent manner (Fig. 4F) and cell-free infection of U373/CD4/CXCR4 cells with HIV-1 vectors pseudotyped with any of the envelope glycoproteins was not dependent on GSLs (Fig. 4G). Though mature DC-mediated trans infection of HIV Env- or NiV F/G-pseudotyped lentivirus particles was dependent on GSLs (Fig. 4F), unlike HIV-1 Env-pseudotyped lentivirus particles, the level of trans infection of NiV F/G-pseudotyped lentivirus particles was not enhanced over that observed upon cell-free infection of U373/CD4/CXCR4 cells. Overall, these results suggest that enveloped viruses whose receptors are poorly expressed on DCs might exploit a GSL- and CD169-dependent pathway for efficient dissemination in vivo.
DISCUSSION
HIV-1 matrix protein plays multiple roles in viral replication (reviewed in references 74 and 80). MA is produced as a Pr55Gag polyprotein and myristoylated at the N terminus. This myristate and a stretch of highly basic amino acids at the N-terminal end of MA play important roles in Gag targeting to the plasma membrane. In addition, interactions of MA with the cytoplasmic tail of gp41, directly or indirectly through a yet to be determined host factor (81), are critical for efficient incorporation of functional Env into budding virus particles (32, 33, 71, 72). Furthermore, recent studies have also suggested that Env trimers on the HIV-1 virion are reorganized to cluster prior to engagement with CD4, a reorganization process triggered by internal core maturation (82). This inside-out signaling is thought to be mediated by MA-gp41 interaction (82). In this study, we describe another important function of MA which to date has been unappreciated. When MA was targeted to the plasma membrane, budding virus particles acquired GM3 from the host membrane, a critical requirement for HIV-1 to access the DC-mediated HIV-1 trans infection pathway. In contrast, when Gag was mistargeted to internal membranes by mutations in the N-terminal basic domain of MA, newly produced HIV-1 particles incorporated lower levels of GM3, and as a consequence, these GM3-deficient particles were severely attenuated for capture by mature DCs and displayed a reduced efficiency of trans infection of T cells.
HIV Pr55Gag is targeted to the inner layer of the plasma membrane via interaction between a highly basic patch on the MA protein and host lipid PI(4,5)P2 (reviewed in reference 26). PI(4,5)P2 is synthesized by type I phosphatidylinositol 4-phosphate 5-kinases and preferentially exists in the inner layer of the plasma membrane (83, 84). Whether PI(4,5)P2 clusters in a particular lipid microdomain and/or influences the lipid composition of the outer layer of the plasma membrane is currently unknown. However, it has been postulated that HIV Gag multimerization at the plasma membrane may alter the structure and/or induce the formation of lipid microdomains (66, 85, 86). It is therefore conceivable that HIV MA protein has evolved to associate with PI(4,5)P2 not only to use it as a Gag assembly platform but also to actively and selectively acquire GSLs, particularly GM3, to exploit DCs for efficient dissemination. The presence of a basic amino acid at position 30 in MA is a signature of most HIV-1 groups, but not SIVcpz and SIVsm, the progenitors of HIV-1 and HIV-2, respectively (87). Interestingly, introduction of a single point mutation in the N-terminal basic domain of MA (M/L30K) was strongly associated with an enhanced replication capacity of SIVcpz in human tonsillar tissue but not in isolated human CD4+ T cells (87, 88), suggesting that the presence of a basic amino acid at position 30 in MA might be involved in facilitating enhanced virus replication under conditions (lymphoid tissues) that favor cell-associated virus transmission (87). It would be interesting to see if the presence of a Met or Leu at position 30 in MA affects the acquisition of GM3 by SIVcpz virions and their subsequent interaction with CD169+ DCs.
Though HIV-1 Gag targeting to the plasma membrane ensures virus assembly and budding primarily at the cell periphery, the plasma membrane is not the most optimal site for virus exit. The ease of detection and neutralization of HIV-1 particles by antibodies is greatly enhanced upon budding from the plasma membrane. Interestingly, in macrophages, HIV-1 particles are released into deep plasma membrane invaginations (89–91), in which HIV-1 is hypothesized to be protected from neutralizing responses (92). However, this type of structure has not been observed in CD4+ T cells. Furthermore, virus particle budding from the plasma membrane could also be a target of cell intrinsic factors, such as BST-2/tetherin. BST-2 is expressed on the plasma membrane upon type I interferon stimulation, and it tethers newly produced virus particles and eventually degrades them via the endosome/lysosome pathway (93). At present, it remains unclear if BST-2 can also restrict virus budding from intracellular membranes. Recent reports have also shown that BST-2/tetherin binding to HIV-1 triggers antiviral immune responses (94–96). We hypothesize that continued selection for the plasma membrane as the preferred site of virus exit, even in the presence of the aforementioned risks, can be explained by our findings in this study. Namely, HIV-1 particles produced from intracellular membranes, while avoiding intrinsic and adaptive immune responses in virus producer cells, are unable to incorporate GSLs and are subsequently unable to exploit DCs as vehicles of systemic dissemination.
In addition to HIV-1, the interaction of two negative-stranded RNA viruses, HeV and NiV, was also dependent on the incorporation of GSLs into virus particle membranes and the expression of CD169 on mature DCs (Fig. 3 and 4). While NiV M has previously been shown to form VLPs when expressed in isolation (52, 97, 98), to our knowledge, this is the first report to indicate that the M proteins of both Nipah and Hendra viruses are associated with plasma membrane lipid rafts to an extent similar to that exhibited by HIV-1 (Fig. 3D) and that expression of HeV M protein alone, like expression of NiV M alone, is also sufficient to form VLPs (Fig. 3C). Though the localization of eGFP fusion proteins of NiV M, HeV M, and EBOV VP40 was observed at GM1-enriched plasma membrane microdomains (Fig. 3D), these were distinct from the HIV Gag-eGFP assembly sites (Fig. 3E). Interestingly, GM1 and GM3 can form distinct micropatches within a single microdomain (99). Thus, it is entirely possible that while GM3 is preferentially incorporated by HIV-1 particles, as reported previously (15, 17), NiV M-, HeV M-, and EBOV VP40-derived VLPs incorporate larger amounts of GM1. As mentioned above, a variety of viruses have utilized GSL-rich lipid microdomains as a platform for virus assembly and budding (reviewed in reference 100). Therefore, it is conceivable that virus incorporation of GSLs for binding CD169 might be a conserved dissemination mechanism of enveloped viruses, although it remains to be determined in the context of infectious viruses.
Virus invasion of a naive host requires the virus to breach the epithelial barrier, which consists of a variety of cells, including DCs. Some viruses, such as influenza virus, have evolved to infect epithelial cells to enter the naive host (101). Other viruses, such as measles virus, have been reported to target and productively infect tissue-resident macrophages, such as alveolar macrophages, and resident basal DCs that extend long plasma membrane protrusions into the extraepithelial space (reviewed in reference 102). Although the exact mode of henipavirus transmission in humans is yet to be defined, Hendra and Nipah viruses can infect primary epithelial cells from the lower respiratory tract in vitro (103). Alternatively, the capture of Hendra and Nipah viruses by lung epithelial resident DCs might also facilitate virus transmission. Moreover, as DCs migrate into lymphoid organs upon pathogen binding and stimulation, it would be possible for DCs to be exploited by viral pathogens as vehicles to systemically spread infection.
Acute virus infections most often result in a robust innate immune response, characterized by the upregulation of interferon-stimulated genes (ISGs) and high levels of type I IFN production. CD169 is a type I IFN-inducible protein (14), and, interestingly, acute pathogenic SIVmac infection in Asian macaques results in the upregulation of CD169 expression (104, 105). Hendra and Nipah virus infection of primary human epithelial cells induces mRNA transcription of type I IFN and ISGs (103), which may trigger ISGs in neighboring uninfected myeloid cells. Thus, inducible expression of CD169 on myeloid cells might be exploited by enveloped viruses, such as henipaviruses and lentiviruses, to systemically disseminate within an infected host.
In contrast to the capture of HIV-1 Gag, HeV M-, and NiV M-derived VLPs, capture of EBOV VP40-derived VLPs by mature DCs occurred in a GSL-independent manner (Fig. 4). Recent estimates suggest that there are over 1,800 GP trimers per Ebola virus virion (108), and there are a number of receptors/attachment factors reported for Ebola and Marburg viruses, including DC-SIGN, which is highly expressed on DCs (109–111; reviewed in reference 78). It is very likely that EBOV VP40-derived VLPs were captured by one of these factors in a GSL-independent fashion. Furthermore, EBOV is able to productively infect DCs in vitro and in vivo (112–116). In fact, we also observed efficient VLP fusion in DCs when EBOV VP40-derived VLPs were pseudotyped with EBOV-GP (data not shown). It is thus conceivable that viruses which can productively infect DCs with a high efficiency do not access the CD169-dependent trans infection pathway. In contrast, receptors for HIV-1 (CD4 antigen and coreceptor, CCR5) and the two defined receptors for Nipah virus, ephrinB2 and ephrinB3, are expressed on DCs at low and undetectable levels, respectively (76, 77, 117). Furthermore, infection of DCs by HIV-1 and NiV has been invariably reported (7, 77, 117, 118). In addition to the paucity of cell surface-expressed receptors, the intracellular environment of DCs can be antagonistic for HIV replication. DCs are equipped with various innate sensors, such as RIG-I, BST-2, and a cryptic sensor detecting newly synthesized viral Gag protein (95, 118, 119), resulting in antiviral responses, including type I interferon secretion. We hypothesize that as a potential mechanism of virus evasion from the DC-intrinsic innate immune responses, HIV-1 and Hendra and Nipah viruses might have evolved to exploit the GSL-CD169 recognition nexus for accessing the DC-mediated trans infection pathway.
In this study, we have shown that interaction of virion-associated GSLs and CD169 is a conserved mechanism among divergent enveloped viruses. One of the chief obstacles for the development of antivirals against RNA viruses is the rapid development of mutations for resistance to antiviral compounds. Since both GSLs and CD169 are host encoded, the GSL-CD169 interaction pathway would not be subject to the development of resistance mutations and should be targeted for the development of therapeutic strategies.
ACKNOWLEDGMENTS
We thank Chris Broder (Uniformed Services University of the Health Sciences) for the generous gifts of the NiV and HeV M expression plasmids, Gary Kobinger (University of Manitoba) for the NiV F and G expression plasmids, Elke Mühlberger (Boston University) for the Ebola Zaire VP40 and GP expression plasmids, Eric Freed (NCI—Frederick) for the NL4-3 29/31KEΔCT proviral clone, Nancy Haigwood (Oregon National Primate Research Institute) for the anti-gp120 antiserum, Sen-Itiroh Hakamori (University of Washington) for the anti-GM3 hybridoma, and Michael Emerman (Fred Hutchinson Cancer Research Center) for the U373/CD4/CXCR4 cell line. The following reagents were obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH: MT4 cells from Douglas Richman, HIV-1 immunoglobulin from NABI and the National Heart, Lung, and Blood Institute (Luiz Barbosa), pGag-eGFP from Marilyn D. Resh, HIV-1 p24 Gag monoclonal antibody (clone 24-2) from Michael Malim, HIV-1 p24 hybridoma (183-H12-5C) from Kathy Wehrly and Bruce Chesebro, pHxB2-env from Kathleen Page and Dan Littman, pNL4-3.Luc.R−.E− from Nathaniel Landau, and the Raji B cell line from Li Wu and Vineet KewalRamani. We thank the BUMC flow cytometry core facility for technical assistance.
This work was supported by NIH grant AI064099 (to S.G.), Research Training in Immunology 5T32AI007309-23 (to C.M.), and the Undergraduate Research Opportunities Program (to H.V.P.).
Footnotes
Published ahead of print 28 May 2014
REFERENCES
- 1.Hladik F, McElrath MJ. 2008. Setting the stage: host invasion by HIV. Nat. Rev. Immunol. 8:447–457. 10.1038/nri2302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li Q, Estes JD, Schlievert PM, Duan L, Brosnahan AJ, Southern PJ, Reilly CS, Peterson ML, Schultz-Darken N, Brunner KG, Nephew KR, Pambuccian S, Lifson JD, Carlis JV, Haase AT. 2009. Glycerol monolaurate prevents mucosal SIV transmission. Nature 458:1034–1038. 10.1038/nature07831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hladik F, Sakchalathorn P, Ballweber L, Lentz G, Fialkow M, Eschenbach D, McElrath MJ. 2007. Initial events in establishing vaginal entry and infection by human immunodeficiency virus type-1. Immunity 26:257–270. 10.1016/j.immuni.2007.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hu J, Gardner MB, Miller CJ. 2000. Simian immunodeficiency virus rapidly penetrates the cervicovaginal mucosa after intravaginal inoculation and infects intraepithelial dendritic cells. J. Virol. 74:6087–6095. 10.1128/JVI.74.13.6087-6095.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spira AI, Marx PA, Patterson BK, Mahoney J, Koup RA, Wolinsky SM, Ho DD. 1996. Cellular targets of infection and route of viral dissemination after an intravaginal inoculation of simian immunodeficiency virus into rhesus macaques. J. Exp. Med. 183:215–225. 10.1084/jem.183.1.215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu L, KewalRamani VN. 2006. Dendritic-cell interactions with HIV: infection and viral dissemination. Nat. Rev. Immunol. 6:859–868. 10.1038/nri1960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cameron PU, Freudenthal PS, Barker JM, Gezelter S, Inaba K, Steinman RM. 1992. Dendritic cells exposed to human immunodeficiency virus type-1 transmit a vigorous cytopathic infection to CD4+ T cells. Science 257:383–387. 10.1126/science.1352913 [DOI] [PubMed] [Google Scholar]
- 8.McDonald D, Wu L, Bohks SM, KewalRamani VN, Unutmaz D, Hope TJ. 2003. Recruitment of HIV and its receptors to dendritic cell-T cell junctions. Science 300:1295–1297. 10.1126/science.1084238 [DOI] [PubMed] [Google Scholar]
- 9.Geijtenbeek TB, Kwon DS, Torensma R, van Vliet SJ, van Duijnhoven GC, Middel J, Cornelissen IL, Nottet HS, KewalRamani VN, Littman DR, Figdor CG, van Kooyk Y. 2000. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell 100:587–597. 10.1016/S0092-8674(00)80694-7 [DOI] [PubMed] [Google Scholar]
- 10.Turville SG, Cameron PU, Handley A, Lin G, Pohlmann S, Doms RW, Cunningham AL. 2002. Diversity of receptors binding HIV on dendritic cell subsets. Nat. Immunol. 3:975–983. 10.1038/ni841 [DOI] [PubMed] [Google Scholar]
- 11.Ugolini S, Mondor I, Sattentau QJ. 1999. HIV-1 attachment: another look. Trends Microbiol. 7:144–149. 10.1016/S0966-842X(99)01474-2 [DOI] [PubMed] [Google Scholar]
- 12.Hatch SC, Archer J, Gummuluru S. 2009. Glycosphingolipid composition of human immunodeficiency virus type 1 (HIV-1) particles is a crucial determinant for dendritic cell-mediated HIV-1 trans-infection. J. Virol. 83:3496–3506. 10.1128/JVI.02249-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Izquierdo-Useros N, Naranjo-Gomez M, Archer J, Hatch SC, Erkizia I, Blanco J, Borras FE, Puertas MC, Connor JH, Fernandez-Figueras MT, Moore L, Clotet B, Gummuluru S, Martinez-Picado J. 2009. Capture and transfer of HIV-1 particles by mature dendritic cells converges with the exosome-dissemination pathway. Blood 113:2732–2741. 10.1182/blood-2008-05-158642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Puryear WB, Akiyama H, Geer SD, Ramirez NP, Yu X, Reinhard BM, Gummuluru S. 2013. Interferon-inducible mechanism of dendritic cell-mediated HIV-1 dissemination is dependent on Siglec-1/CD169. PLoS Pathog. 9:e1003291. 10.1371/journal.ppat.1003291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Izquierdo-Useros N, Lorizate M, Contreras FX, Rodriguez-Plata MT, Glass B, Erkizia I, Prado JG, Casas J, Fabrias G, Krausslich HG, Martinez-Picado J. 2012. Sialyllactose in viral membrane gangliosides is a novel molecular recognition pattern for mature dendritic cell capture of HIV-1. PLoS Biol. 10:e1001315. 10.1371/journal.pbio.1001315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Izquierdo-Useros N, Lorizate M, Puertas MC, Rodriguez-Plata MT, Zangger N, Erikson E, Pino M, Erkizia I, Glass B, Clotet B, Keppler OT, Telenti A, Krausslich HG, Martinez-Picado J. 2012. Siglec-1 is a novel dendritic cell receptor that mediates HIV-1 trans-infection through recognition of viral membrane gangliosides. PLoS Biol. 10:e1001448. 10.1371/journal.pbio.1001448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Puryear WB, Yu X, Ramirez NP, Reinhard BM, Gummuluru S. 2012. HIV-1 incorporation of host-cell-derived glycosphingolipid GM3 allows for capture by mature dendritic cells. Proc. Natl. Acad. Sci. U. S. A. 109:7475–7480. 10.1073/pnas.1201104109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crocker PR, Paulson JC, Varki A. 2007. Siglecs and their roles in the immune system. Nat. Rev. Immunol. 7:255–266. 10.1038/nri2056 [DOI] [PubMed] [Google Scholar]
- 19.Hartnell A, Steel J, Turley H, Jones M, Jackson DG, Crocker PR. 2001. Characterization of human sialoadhesin, a sialic acid binding receptor expressed by resident and inflammatory macrophage populations. Blood 97:288–296. 10.1182/blood.V97.1.288 [DOI] [PubMed] [Google Scholar]
- 20.Ono A. 2010. Relationships between plasma membrane microdomains and HIV-1 assembly. Biol. Cell 102:335–350. 10.1042/BC20090165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lindwasser OW, Resh MD. 2002. Myristoylation as a target for inhibiting HIV assembly: unsaturated fatty acids block viral budding. Proc. Natl. Acad. Sci. U. S. A. 99:13037–13042. 10.1073/pnas.212409999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ono A, Ablan SD, Lockett SJ, Nagashima K, Freed EO. 2004. Phosphatidylinositol (4,5) bisphosphate regulates HIV-1 Gag targeting to the plasma membrane. Proc. Natl. Acad. Sci. U. S. A. 101:14889–14894. 10.1073/pnas.0405596101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ono A, Freed EO. 1999. Binding of human immunodeficiency virus type 1 Gag to membrane: role of the matrix amino terminus. J. Virol. 73:4136–4144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spearman P, Wang JJ, Vander Heyden N, Ratner L. 1994. Identification of human immunodeficiency virus type 1 Gag protein domains essential to membrane binding and particle assembly. J. Virol. 68:3232–3242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou W, Parent LJ, Wills JW, Resh MD. 1994. Identification of a membrane-binding domain within the amino-terminal region of human immunodeficiency virus type 1 Gag protein which interacts with acidic phospholipids. J. Virol. 68:2556–2569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ono A. 2009. HIV-1 assembly at the plasma membrane: Gag trafficking and localization. Future Virol. 4:241–257. 10.2217/fvl.09.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chukkapalli V, Hogue IB, Boyko V, Hu WS, Ono A. 2008. Interaction between the human immunodeficiency virus type 1 Gag matrix domain and phosphatidylinositol-(4,5)-bisphosphate is essential for efficient Gag membrane binding. J. Virol. 82:2405–2417. 10.1128/JVI.01614-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Facke M, Janetzko A, Shoeman RL, Krausslich HG. 1993. A large deletion in the matrix domain of the human immunodeficiency virus gag gene redirects virus particle assembly from the plasma membrane to the endoplasmic reticulum. J. Virol. 67:4972–4980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ono A, Freed EO. 2004. Cell-type-dependent targeting of human immunodeficiency virus type 1 assembly to the plasma membrane and the multivesicular body. J. Virol. 78:1552–1563. 10.1128/JVI.78.3.1552-1563.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ono A, Orenstein JM, Freed EO. 2000. Role of the Gag matrix domain in targeting human immunodeficiency virus type 1 assembly. J. Virol. 74:2855–2866. 10.1128/JVI.74.6.2855-2866.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reil H, Bukovsky AA, Gelderblom HR, Gottlinger HG. 1998. Efficient HIV-1 replication can occur in the absence of the viral matrix protein. EMBO J. 17:2699–2708. 10.1093/emboj/17.9.2699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dorfman T, Mammano F, Haseltine WA, Gottlinger HG. 1994. Role of the matrix protein in the virion association of the human immunodeficiency virus type 1 envelope glycoprotein. J. Virol. 68:1689–1696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Freed EO, Martin MA. 1995. Virion incorporation of envelope glycoproteins with long but not short cytoplasmic tails is blocked by specific, single amino acid substitutions in the human immunodeficiency virus type 1 matrix. J. Virol. 69:1984–1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mammano F, Kondo E, Sodroski J, Bukovsky A, Gottlinger HG. 1995. Rescue of human immunodeficiency virus type 1 matrix protein mutants by envelope glycoproteins with short cytoplasmic domains. J. Virol. 69:3824–3830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brugger B, Glass B, Haberkant P, Leibrecht I, Wieland FT, Krausslich HG. 2006. The HIV lipidome: a raft with an unusual composition. Proc. Natl. Acad. Sci. U. S. A. 103:2641–2646. 10.1073/pnas.0511136103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chan R, Uchil PD, Jin J, Shui G, Ott DE, Mothes W, Wenk MR. 2008. Retroviruses human immunodeficiency virus and murine leukemia virus are enriched in phosphoinositides. J. Virol. 82:11228–11238. 10.1128/JVI.00981-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chazal N, Gerlier D. 2003. Virus entry, assembly, budding, and membrane rafts. Microbiol. Mol. Biol. Rev. 67:226–237. 10.1128/MMBR.67.2.226-237.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aloia RC, Tian H, Jensen FC. 1993. Lipid composition and fluidity of the human immunodeficiency virus envelope and host cell plasma membranes. Proc. Natl. Acad. Sci. U. S. A. 90:5181–5185. 10.1073/pnas.90.11.5181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bavari S, Bosio CM, Wiegand E, Ruthel G, Will AB, Geisbert TW, Hevey M, Schmaljohn C, Schmaljohn A, Aman MJ. 2002. Lipid raft microdomains: a gateway for compartmentalized trafficking of Ebola and Marburg viruses. J. Exp. Med. 195:593–602. 10.1084/jem.20011500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Panchal RG, Ruthel G, Kenny TA, Kallstrom GH, Lane D, Badie SS, Li L, Bavari S, Aman MJ. 2003. In vivo oligomerization and raft localization of Ebola virus protein VP40 during vesicular budding. Proc. Natl. Acad. Sci. U. S. A. 100:15936–15941. 10.1073/pnas.2533915100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Henderson G, Murray J, Yeo RP. 2002. Sorting of the respiratory syncytial virus matrix protein into detergent-resistant structures is dependent on cell-surface expression of the glycoproteins. Virology 300:244–254. 10.1006/viro.2002.1540 [DOI] [PubMed] [Google Scholar]
- 42.Leser GP, Lamb RA. 2005. Influenza virus assembly and budding in raft-derived microdomains: a quantitative analysis of the surface distribution of HA, NA and M2 proteins. Virology 342:215–227. 10.1016/j.virol.2005.09.049 [DOI] [PubMed] [Google Scholar]
- 43.Manie SN, de Breyne S, Vincent S, Gerlier D. 2000. Measles virus structural components are enriched into lipid raft microdomains: a potential cellular location for virus assembly. J. Virol. 74:305–311. 10.1128/JVI.74.1.305-311.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McCurdy LH, Graham BS. 2003. Role of plasma membrane lipid microdomains in respiratory syncytial virus filament formation. J. Virol. 77:1747–1756. 10.1128/JVI.77.3.1747-1756.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vincent S, Gerlier D, Manie SN. 2000. Measles virus assembly within membrane rafts. J. Virol. 74:9911–9915. 10.1128/JVI.74.21.9911-9915.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ali A, Avalos RT, Ponimaskin E, Nayak DP. 2000. Influenza virus assembly: effect of influenza virus glycoproteins on the membrane association of M1 protein. J. Virol. 74:8709–8719. 10.1128/JVI.74.18.8709-8719.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barman S, Nayak DP. 2000. Analysis of the transmembrane domain of influenza virus neuraminidase, a type II transmembrane glycoprotein, for apical sorting and raft association. J. Virol. 74:6538–6545. 10.1128/JVI.74.14.6538-6545.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Scheiffele P, Rietveld A, Wilk T, Simons K. 1999. Influenza viruses select ordered lipid domains during budding from the plasma membrane. J. Biol. Chem. 274:2038–2044. 10.1074/jbc.274.4.2038 [DOI] [PubMed] [Google Scholar]
- 49.Zhang J, Pekosz A, Lamb RA. 2000. Influenza virus assembly and lipid raft microdomains: a role for the cytoplasmic tails of the spike glycoproteins. J. Virol. 74:4634–4644. 10.1128/JVI.74.10.4634-4644.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ciancanelli MJ, Basler CF. 2006. Mutation of YMYL in the Nipah virus matrix protein abrogates budding and alters subcellular localization. J. Virol. 80:12070–12078. 10.1128/JVI.01743-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Noda T, Sagara H, Suzuki E, Takada A, Kida H, Kawaoka Y. 2002. Ebola virus VP40 drives the formation of virus-like filamentous particles along with GP. J. Virol. 76:4855–4865. 10.1128/JVI.76.10.4855-4865.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Patch JR, Crameri G, Wang LF, Eaton BT, Broder CC. 2007. Quantitative analysis of Nipah virus proteins released as virus-like particles reveals central role for the matrix protein. Virol. J. 4:1. 10.1186/1743-422X-4-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Swenson DL, Warfield KL, Kuehl K, Larsen T, Hevey MC, Schmaljohn A, Bavari S, Aman MJ. 2004. Generation of Marburg virus-like particles by co-expression of glycoprotein and matrix protein. FEMS Immunol. Med. Microbiol. 40:27–31. 10.1016/S0928-8244(03)00273-6 [DOI] [PubMed] [Google Scholar]
- 54.Timmins J, Scianimanico S, Schoehn G, Weissenhorn W. 2001. Vesicular release of Ebola virus matrix protein VP40. Virology 283:1–6. 10.1006/viro.2001.0860 [DOI] [PubMed] [Google Scholar]
- 55.Warnock DE, Roberts C, Lutz MS, Blackburn WA, Young WW, Jr, Baenziger JU. 1993. Determination of plasma membrane lipid mass and composition in cultured Chinese hamster ovary cells using high gradient magnetic affinity chromatography. J. Biol. Chem. 268:10145–10153 [PubMed] [Google Scholar]
- 56.Page KA, Landau NR, Littman DR. 1990. Construction and use of a human immunodeficiency virus vector for analysis of virus infectivity. J. Virol. 64:5270–5276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wiley RD, Gummuluru S. 2006. Immature dendritic cell-derived exosomes can mediate HIV-1 trans infection. Proc. Natl. Acad. Sci. U. S. A. 103:738–743. 10.1073/pnas.0507995103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sagar M, Akiyama H, Etemad B, Ramirez N, Freitas I, Gummuluru S. 2012. Transmembrane domain membrane proximal external region but not surface unit-directed broadly neutralizing HIV-1 antibodies can restrict dendritic cell-mediated HIV-1 trans-infection. J. Infect. Dis. 205:1248–1257. 10.1093/infdis/jis183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yamashita M, Emerman M. 2004. Capsid is a dominant determinant of retrovirus infectivity in nondividing cells. J. Virol. 78:5670–5678. 10.1128/JVI.78.11.5670-5678.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Connor RI, Chen BK, Choe S, Landau NR. 1995. Vpr is required for efficient replication of human immunodeficiency virus type-1 in mononuclear phagocytes. Virology 206:935–944. 10.1006/viro.1995.1016 [DOI] [PubMed] [Google Scholar]
- 61.Joshi A, Ablan SD, Soheilian F, Nagashima K, Freed EO. 2009. Evidence that productive human immunodeficiency virus type 1 assembly can occur in an intracellular compartment. J. Virol. 83:5375–5387. 10.1128/JVI.00109-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Harada S, Koyanagi Y, Yamamoto N. 1985. Infection of HTLV-III/LAV in HTLV-I-carrying cells MT-2 and MT-4 and application in a plaque assay. Science 229:563–566. 10.1126/science.2992081 [DOI] [PubMed] [Google Scholar]
- 63.Vodicka MA, Goh WC, Wu LI, Rogel ME, Bartz SR, Schweickart VL, Raport CJ, Emerman M. 1997. Indicator cell lines for detection of primary strains of human and simian immunodeficiency viruses. Virology 233:193–198. 10.1006/viro.1997.8606 [DOI] [PubMed] [Google Scholar]
- 64.Wu L, Martin TD, Carrington M, KewalRamani VN. 2004. Raji B cells, misidentified as THP-1 cells, stimulate DC-SIGN-mediated HIV transmission. Virology 318:17–23. 10.1016/j.virol.2003.09.028 [DOI] [PubMed] [Google Scholar]
- 65.O'Doherty U, Swiggard WJ, Malim MH. 2000. Human immunodeficiency virus type 1 spinoculation enhances infection through virus binding. J. Virol. 74:10074–10080. 10.1128/JVI.74.21.10074-10080.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lindwasser OW, Resh MD. 2001. Multimerization of human immunodeficiency virus type 1 Gag promotes its localization to barges, raft-like membrane microdomains. J. Virol. 75:7913–7924. 10.1128/JVI.75.17.7913-7924.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sandefur S, Smith RM, Varthakavi V, Spearman P. 2000. Mapping and characterization of the N-terminal I domain of human immunodeficiency virus type 1 Pr55(Gag). J. Virol. 74:7238–7249. 10.1128/JVI.74.16.7238-7249.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee PP, Linial ML. 1994. Efficient particle formation can occur if the matrix domain of human immunodeficiency virus type 1 Gag is substituted by a myristylation signal. J. Virol. 68:6644–6654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.van Meer G, Voelker DR, Feigenson GW. 2008. Membrane lipids: where they are and how they behave. Nat. Rev. Mol. Cell Biol. 9:112–124. 10.1038/nrm2330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ono M, Handa K, Sonnino S, Withers DA, Nagai H, Hakomori S. 2001. GM3 ganglioside inhibits CD9-facilitated haptotactic cell motility: coexpression of GM3 and CD9 is essential in the downregulation of tumor cell motility and malignancy. Biochemistry 40:6414–6421. 10.1021/bi0101998 [DOI] [PubMed] [Google Scholar]
- 71.Freed EO, Martin MA. 1996. Domains of the human immunodeficiency virus type 1 matrix and gp41 cytoplasmic tail required for envelope incorporation into virions. J. Virol. 70:341–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yu X, Yuan X, Matsuda Z, Lee TH, Essex M. 1992. The matrix protein of human immunodeficiency virus type 1 is required for incorporation of viral envelope protein into mature virions. J. Virol. 66:4966–4971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Murakami T, Freed EO. 2000. The long cytoplasmic tail of gp41 is required in a cell type-dependent manner for HIV-1 envelope glycoprotein incorporation into virions. Proc. Natl. Acad. Sci. U. S. A. 97:343–348. 10.1073/pnas.97.1.343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bukrinskaya A. 2007. HIV-1 matrix protein: a mysterious regulator of the viral life cycle. Virus Res. 124:1–11. 10.1016/j.virusres.2006.07.001 [DOI] [PubMed] [Google Scholar]
- 75.Geisbert TW, Jahrling PB. 1995. Differentiation of filoviruses by electron microscopy. Virus Res. 39:129–150. 10.1016/0168-1702(95)00080-1 [DOI] [PubMed] [Google Scholar]
- 76.Lee B, Sharron M, Montaner LJ, Weissman D, Doms RW. 1999. Quantification of CD4, CCR5, and CXCR4 levels on lymphocyte subsets, dendritic cells, and differentially conditioned monocyte-derived macrophages. Proc. Natl. Acad. Sci. U. S. A. 96:5215–5220. 10.1073/pnas.96.9.5215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mathieu C, Pohl C, Szecsi J, Trajkovic-Bodennec S, Devergnas S, Raoul H, Cosset FL, Gerlier D, Wild TF, Horvat B. 2011. Nipah virus uses leukocytes for efficient dissemination within a host. J. Virol. 85:7863–7871. 10.1128/JVI.00549-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Martinez O, Leung LW, Basler CF. 2012. The role of antigen-presenting cells in filoviral hemorrhagic fever: gaps in current knowledge. Antiviral Res. 93:416–428. 10.1016/j.antiviral.2012.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bossart KN, Wang LF, Flora MN, Chua KB, Lam SK, Eaton BT, Broder CC. 2002. Membrane fusion tropism and heterotypic functional activities of the Nipah virus and Hendra virus envelope glycoproteins. J. Virol. 76:11186–11198. 10.1128/JVI.76.22.11186-11198.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fiorentini S, Giagulli C, Caccuri F, Magiera AK, Caruso A. 2010. HIV-1 matrix protein p17: a candidate antigen for therapeutic vaccines against AIDS. Pharmacol. Ther. 128:433–444. 10.1016/j.pharmthera.2010.08.005 [DOI] [PubMed] [Google Scholar]
- 81.Checkley MA, Luttge BG, Mercredi PY, Kyere SK, Donlan J, Murakami T, Summers MF, Cocklin S, Freed EO. 2013. Reevaluation of the requirement for TIP47 in human immunodeficiency virus type 1 envelope glycoprotein incorporation. J. Virol. 87:3561–3570. 10.1128/JVI.03299-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chojnacki J, Staudt T, Glass B, Bingen P, Engelhardt J, Anders M, Schneider J, Muller B, Hell SW, Krausslich HG. 2012. Maturation-dependent HIV-1 surface protein redistribution revealed by fluorescence nanoscopy. Science 338:524–528. 10.1126/science.1226359 [DOI] [PubMed] [Google Scholar]
- 83.Downes CP, Gray A, Lucocq JM. 2005. Probing phosphoinositide functions in signaling and membrane trafficking. Trends Cell Biol. 15:259–268. 10.1016/j.tcb.2005.03.008 [DOI] [PubMed] [Google Scholar]
- 84.Roth MG. 2004. Phosphoinositides in constitutive membrane traffic. Physiol. Rev. 84:699–730. 10.1152/physrev.00033.2003 [DOI] [PubMed] [Google Scholar]
- 85.Dalton AK, Ako-Adjei D, Murray PS, Murray D, Vogt VM. 2007. Electrostatic interactions drive membrane association of the human immunodeficiency virus type 1 Gag MA domain. J. Virol. 81:6434–6445. 10.1128/JVI.02757-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ono A, Freed EO. 2001. Plasma membrane rafts play a critical role in HIV-1 assembly and release. Proc. Natl. Acad. Sci. U. S. A. 98:13925–13930. 10.1073/pnas.241320298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wain LV, Bailes E, Bibollet-Ruche F, Decker JM, Keele BF, Van Heuverswyn F, Li Y, Takehisa J, Ngole EM, Shaw GM, Peeters M, Hahn BH, Sharp PM. 2007. Adaptation of HIV-1 to its human host. Mol. Biol. Evol. 24:1853–1860. 10.1093/molbev/msm110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bibollet-Ruche F, Heigele A, Keele BF, Easlick JL, Decker JM, Takehisa J, Learn G, Sharp PM, Hahn BH, Kirchhoff F. 2012. Efficient SIVcpz replication in human lymphoid tissue requires viral matrix protein adaptation. J. Clin. Invest. 122:1644–1652. 10.1172/JCI61429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bennett AE, Narayan K, Shi D, Hartnell LM, Gousset K, He H, Lowekamp BC, Yoo TS, Bliss D, Freed EO, Subramaniam S. 2009. Ion-abrasion scanning electron microscopy reveals surface-connected tubular conduits in HIV-infected macrophages. PLoS Pathog. 5:e1000591. 10.1371/journal.ppat.1000591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jouve M, Sol-Foulon N, Watson S, Schwartz O, Benaroch P. 2007. HIV-1 buds and accumulates in “nonacidic” endosomes of macrophages. Cell Host Microbe 2:85–95. 10.1016/j.chom.2007.06.011 [DOI] [PubMed] [Google Scholar]
- 91.Welsch S, Groot F, Krausslich HG, Keppler OT, Sattentau QJ. 2011. Architecture and regulation of the HIV-1 assembly and holding compartment in macrophages. J. Virol. 85:7922–7927. 10.1128/JVI.00834-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tan J, Sattentau QJ. 2013. The HIV-1-containing macrophage compartment: a perfect cellular niche? Trends Microbiol. 21:405–412. 10.1016/j.tim.2013.05.001 [DOI] [PubMed] [Google Scholar]
- 93.Janvier K, Pelchen-Matthews A, Renaud JB, Caillet M, Marsh M, Berlioz-Torrent C. 2011. The ESCRT-0 component HRS is required for HIV-1 Vpu-mediated BST-2/tetherin down-regulation. PLoS Pathog. 7:e1001265. 10.1371/journal.ppat.1001265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cocka LJ, Bates P. 2012. Identification of alternatively translated tetherin isoforms with differing antiviral and signaling activities. PLoS Pathog. 8:e1002931. 10.1371/journal.ppat.1002931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Galao RP, Le Tortorec A, Pickering S, Kueck T, Neil SJ. 2012. Innate sensing of HIV-1 assembly by tetherin induces NFkappaB-dependent proinflammatory responses. Cell Host Microbe 12:633–644. 10.1016/j.chom.2012.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tokarev A, Suarez M, Kwan W, Fitzpatrick K, Singh R, Guatelli J. 2013. Stimulation of NF-kappaB activity by the HIV restriction factor BST2. J. Virol. 87:2046–2057. 10.1128/JVI.02272-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Patch JR, Han Z, McCarthy SE, Yan L, Wang LF, Harty RN, Broder CC. 2008. The YPLGVG sequence of the Nipah virus matrix protein is required for budding. Virol. J. 5:137. 10.1186/1743-422X-5-137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wolf MC, Wang Y, Freiberg AN, Aguilar HC, Holbrook MR, Lee B. 2009. A catalytically and genetically optimized beta-lactamase-matrix based assay for sensitive, specific, and higher throughput analysis of native henipavirus entry characteristics. Virol. J. 6:119. 10.1186/1743-422X-6-119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fujita A, Cheng J, Hirakawa M, Furukawa K, Kusunoki S, Fujimoto T. 2007. Gangliosides GM1 and GM3 in the living cell membrane form clusters susceptible to cholesterol depletion and chilling. Mol. Biol. Cell 18:2112–2122. 10.1091/mbc.E07-01-0071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Manes S, del Real G, Martinez AC. 2003. Pathogens: raft hijackers. Nat. Rev. Immunol. 3:557–568. 10.1038/nri1129 [DOI] [PubMed] [Google Scholar]
- 101.Sanders CJ, Doherty PC, Thomas PG. 2011. Respiratory epithelial cells in innate immunity to influenza virus infection. Cell Tissue Res. 343:13–21. 10.1007/s00441-010-1043-z [DOI] [PubMed] [Google Scholar]
- 102.de Vries RD, Mesman AW, Geijtenbeek TB, Duprex WP, de Swart RL. 2012. The pathogenesis of measles. Curr. Opin. Virol. 2:248–255. 10.1016/j.coviro.2012.03.005 [DOI] [PubMed] [Google Scholar]
- 103.Escaffre O, Borisevich V, Carmical JR, Prusak D, Prescott J, Feldmann H, Rockx B. 2013. Henipavirus pathogenesis in human respiratory epithelial cells. J. Virol. 87:3284–3294. 10.1128/JVI.02576-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bosinger SE, Sodora DL, Silvestri G. 2011. Generalized immune activation and innate immune responses in simian immunodeficiency virus infection. Curr. Opin. HIV AIDS 6:411–418. 10.1097/COH.0b013e3283499cf6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jaroenpool J, Rogers KA, Pattanapanyasat K, Villinger F, Onlamoon N, Crocker PR, Ansari AA. 2007. Differences in the constitutive and SIV infection induced expression of Siglecs by hematopoietic cells from non-human primates. Cell. Immunol. 250:91–104. 10.1016/j.cellimm.2008.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Reference deleted.
- 107. Reference deleted.
- 108.Booth TF, Rabb MJ, Beniac DR. 2013. How do filovirus filaments bend without breaking? Trends Microbiol. 21:583–593. 10.1016/j.tim.2013.08.001 [DOI] [PubMed] [Google Scholar]
- 109.Alvarez CP, Lasala F, Carrillo J, Muniz O, Corbi AL, Delgado R. 2002. C-type lectins DC-SIGN and L-SIGN mediate cellular entry by Ebola virus in cis and in trans. J. Virol. 76:6841–6844. 10.1128/JVI.76.13.6841-6844.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lin G, Simmons G, Pohlmann S, Baribaud F, Ni H, Leslie GJ, Haggarty BS, Bates P, Weissman D, Hoxie JA, Doms RW. 2003. Differential N-linked glycosylation of human immunodeficiency virus and Ebola virus envelope glycoproteins modulates interactions with DC-SIGN and DC-SIGNR. J. Virol. 77:1337–1346. 10.1128/JVI.77.2.1337-1346.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Marzi A, Moller P, Hanna SL, Harrer T, Eisemann J, Steinkasserer A, Becker S, Baribaud F, Pohlmann S. 2007. Analysis of the interaction of Ebola virus glycoprotein with DC-SIGN (dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin) and its homologue DC-SIGNR. J. Infect. Dis. 196(Suppl 2):S237–S246. 10.1086/520607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Basler CF, Amarasinghe GK. 2009. Evasion of interferon responses by Ebola and Marburg viruses. J. Interferon Cytokine Res. 29:511–520. 10.1089/jir.2009.0076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bosio CM, Aman MJ, Grogan C, Hogan R, Ruthel G, Negley D, Mohamadzadeh M, Bavari S, Schmaljohn A. 2003. Ebola and Marburg viruses replicate in monocyte-derived dendritic cells without inducing the production of cytokines and full maturation. J. Infect. Dis. 188:1630–1638. 10.1086/379199 [DOI] [PubMed] [Google Scholar]
- 114.Geisbert TW, Hensley LE, Larsen T, Young HA, Reed DS, Geisbert JB, Scott DP, Kagan E, Jahrling PB, Davis KJ. 2003. Pathogenesis of Ebola hemorrhagic fever in cynomolgus macaques: evidence that dendritic cells are early and sustained targets of infection. Am. J. Pathol. 163:2347–2370. 10.1016/S0002-9440(10)63591-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mahanty S, Hutchinson K, Agarwal S, McRae M, Rollin PE, Pulendran B. 2003. Cutting edge: impairment of dendritic cells and adaptive immunity by Ebola and Lassa viruses. J. Immunol. 170:2797–2801. 10.4049/jimmunol.170.6.2797 [DOI] [PubMed] [Google Scholar]
- 116.Martinez O, Johnson J, Manicassamy B, Rong L, Olinger GG, Hensley LE, Basler CF. 2010. Zaire Ebola virus entry into human dendritic cells is insensitive to cathepsin L inhibition. Cell. Microbiol. 12:148–157. 10.1111/j.1462-5822.2009.01385.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gupta M, Lo MK, Spiropoulou CF. 2013. Activation and cell death in human dendritic cells infected with Nipah virus. Virology 441:49–56. 10.1016/j.virol.2013.03.004 [DOI] [PubMed] [Google Scholar]
- 118.Manel N, Hogstad B, Wang Y, Levy DE, Unutmaz D, Littman DR. 2010. A cryptic sensor for HIV-1 activates antiviral innate immunity in dendritic cells. Nature 467:214–217. 10.1038/nature09337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Berg RK, Melchjorsen J, Rintahaka J, Diget E, Soby S, Horan KA, Gorelick RJ, Matikainen S, Larsen CS, Ostergaard L, Paludan SR, Mogensen TH. 2012. Genomic HIV RNA induces innate immune responses through RIG-I-dependent sensing of secondary-structured RNA. PLoS One 7:e29291. 10.1371/journal.pone.0029291 [DOI] [PMC free article] [PubMed] [Google Scholar]