ABSTRACT
Understanding the cytokine/chemokine networks in CD4+ and CD8+ T cells during the acute phase of infection is crucial to design therapies for the control of early human immunodeficiency virus (HIV)/simian immunodeficiency virus (SIV) replication. Here, we measured early changes in CD4+ and CD8+ T cells in the peripheral blood (PB), bone marrow (BM), and axillary lymph node (ALN) tissue of rhesus macaques infected with SIVMAC251. At 21 days after infection, all tissues showed a statistically significant loss of CD4+ T cells along with immune activation of CD8+ T cells in PB and ALN tissue. Twenty-eight different cytokines/chemokines were quantified in either anti-CD3/28 antibody- or staphylococcal enterotoxin B-stimulated single-positive CD4+ and CD8+ T cells. PB CD4+ T cells produced predominantly interleukin-2 (IL-2), whereas CD4+ and CD8+ T-cell subsets in tissues produced β-chemokines both before and 21 days after SIV infection. Tissues generally exhibited massive upregulation of many cytokines/chemokines following infection, possibly in an attempt to mitigate the loss of CD4+ T cells. There was no evidence of a T-helper 1 (TH1)-to-TH2 shift in CD4+ T cells or a T-cytotoxic 1 (TC1)-to-TC2 cytokine shift in CD8+ T cells in PB, BM, and ALN T-cell subsets during the acute phase of SIV infection. Despite the upregulation of several important effector cytokines/chemokines (IL-2, IL-12, IL-17, gamma interferon, granulocyte-macrophage colony-stimulating factor) by CD4+ and CD8+ T cells, upregulation of β-chemokines (CCL2 and CCL22), basic fibroblast growth factor (FGF-basic), hepatocyte growth factor (HGF), and migration inhibition factor (MIF) may provide a poor prognosis either by inducing increased virus replication or by other unknown mechanisms. Therefore, drugs targeting β-chemokines (CCL2 and CCL22), FGF-basic, HGF, or MIF might be important for developing effective vaccines and therapeutics against HIV.
IMPORTANCE Human immunodeficiency virus (HIV)/simian immunodeficiency virus (SIV) infection results in early depletion of CD4+ T cells and dysregulation of protective immune responses. Therefore, understanding the cytokine/chemokine networks in CD4+ and CD8+ T cells in different tissues during the acute phase of infection is crucial to the design of therapies for the control of early viral replication. Here, we measured early changes in CD4+ and CD8+ T cells in peripheral blood (PB), bone marrow (BM), and axillary lymph node (ALN) tissue of rhesus macaques infected with SIVMAC251. There was no evidence of a T-helper 1 (TH1)-to-TH2 shift in CD4+ T cells or a T-cytotoxic 1 (TC1)-to-TC2 cytokine shift in CD8+ T cells in PB, BM, and ALN T-cell subsets during the acute phase of SIV infection. Despite the upregulation of several important effector cytokines/chemokines by CD4+ and CD8+ T cells, upregulation of β-chemokines, fibroblast growth factor-basic, hepatocyte growth factor, and migration inhibition factor may provide a poor prognosis.
INTRODUCTION
Human immunodeficiency virus type 1 (HIV-1) infection causes a progressive impairment of the immune system characterized by massive CD4+ T-cell depletion and sustained immune activation and inflammation. Antiretroviral therapy (ART) has reduced AIDS morbidity and mortality drastically and averted an estimated 4.2 million deaths in low- and middle-income countries (1). Currently, with more effective treatment, people living with HIV have a nearly normal life expectancy. However, HIV infection causes marked immune activation and inflammation that are not completely corrected even with ART and control of viral replication. Individuals on successful ART have persistent T-cell activation, an increased incidence of cardiovascular disease, neurologic disease, and other comorbidities associated with chronic macrophage activation and inflammation (2, 3). Chronic and deleterious immune activation is a hallmark of HIV/simian immunodeficiency virus (SIV) infection. Our recent data suggest that pathogenic SIVMAC251 infection induces higher expression of several cytokines/chemokines in plasma, as well as in intestinal single-positive (SP) CD4+ and CD8+ T cells (4, 5). Early loss of intestinal CD4+ T cells because of SIVMAC251 infection was associated with downregulation of multiple T-helper 1 (TH1) and TH2 cytokines/chemokines, whereas increased production of multiple cytokines such as interleukin-17 (IL-17), gamma interferon (IFN-γ), CCL4, and granulocyte-macrophage colony-stimulating factor (GM-CSF) in CD8+ T cells was indicative of a functional immune response. Additionally, the increased production of macrophage migration inhibition factor (MIF) and basic fibroblast growth factor (FGF-basic) observed in HIV/SIV infection was thought to be linked with increased virus replication and disease progression (5–8). Therefore, understanding cytokine/chemokine networks during HIV/SIV infection is important for the development of effective vaccines and therapeutics.
Reports on TH1 and TH2 responses in either PB mononuclear cells (PBMCs) or lymph node (LN) mononuclear cells are conflicting with respect to the cytokine profiles in chronic HIV infection (9–12). Mice infected with murine leukemia virus (MuLV) constitutively produce TH1 and TH2 cytokines during the first week of infection. Selective depletion of CD4+ T-cell subsets during chronic MuLV infection induced increased expression of TH2 cytokines (13). Our study found no evidence of a mucosal TH1-to-TH2 or T-cytotoxic 1 (TC1)-to-TC2 cytokine profile shift during the acute phase of SIV infection (5). Limited data exist on how cytokine/chemokine profiles in different T-cell subsets of lymphoid tissues and bone marrow (BM) lymphocytes are affected during the acute phase of HIV infection, despite the fact that peripheral LN and BM are considered major virus reservoirs for HIV/SIV infection (14–17).
Therefore, this study was conducted to compare the profiles of 28 different cytokines/chemokines in either anti-CD3/28 antibody- or staphylococcal enterotoxin B (SEB)-stimulated SP CD4+ and CD8+ T cells sorted from PB, BM, and axillary LN (ALN) mononuclear cells from rhesus macaques (RMs) before and 21 days after infection with SIVMAC251 to determine changes in cytokine/chemokine networks in these tissues.
MATERIALS AND METHODS
Animal sampling and virus inoculation.
Nine adult female Indian RMs (Macaca mulatta) initially negative for SIV, HIV-2, type D retrovirus, and simian T-cell leukemia virus type 1 infections were treated with depot-medroxyprogesterone acetate (Depo-Provera; 30 mg intramuscularly) and 4 weeks later inoculated with 500 50% tissue culture infective doses (TCID50) of SIVMAC251 intravaginally at the Tulane National Primate Research Center (TNPRC). Sodium heparin-anticoagulated PB, ALN, and femoral BM biopsy samples were collected before and 21 days after SIV infection. The 21-day time point was chosen because it is the nadir of CD4+ T-cell depletion in the gut and when evidence of macrophage infection in lymphoid tissues becomes evident (2, 18, 19). All sample collections were performed under the supervision of TNPRC veterinarians in accordance with the standards incorporated in the Guide for the Care and Use of Laboratory Animals (20) and with the approval of the Tulane Institutional Animal Care and Use Committee. All veterinary procedures were performed on sedated animals.
Quantitation of plasma viral RNA.
SIV RNA was detected in plasma samples by quantitative reverse transcription (RT)-PCR at the Wisconsin National Primate Research Center as previously reported (21, 22). The lower limit of detection of the RT-PCR assay was 60 SIV RNA copies/ml of plasma.
Lymphocyte isolation.
Lymphocytes were isolated from PB by density gradient centrifugation (Lymphocyte Separation Medium; Cellgro) as previously described (23–26). BM cells were filtered, lysed with ACK solution (BioWhittaker), and washed with phosphate-buffered saline (PBS) (26). For lymphocyte isolation from ALNs, tissues were minced by gently pressing them through 100-μm cell strainers and washed with PBS (25, 27). All cells were suspended in RPMI 1640 medium containing 10% fetal bovine serum (RPMI-10). All lymphocytes were more than 90% viable, as determined by the trypan blue dye exclusion method.
Immunofluorescent staining and flow cytometry analysis.
For flow cytometry staining, cells were adjusted to 106/100 μl and 100-μl volumes of cells were incubated with appropriately diluted concentrations of antibodies for 30 min at room temperature. Stained cells were washed once with PBS and fixed with 1× BD stabilizing fixative buffer (BD Biosciences, San Jose, CA). Cells were kept protected from light at 4°C, and acquisition was performed on a Becton, Dickinson LSRFortessa within 24 h of staining as described previously (5, 25, 26, 28, 29). Cells were first labeled with LIVE/DEAD stain (Invitrogen, Life Technologies) and then surface stained with anti-CD3 (SP34.2; BD Biosciences), anti-CD4 (L200; BD Biosciences), anti-CD8 (3B5; Invitrogen), anti-programmed cell death 1 (anti-PD-1) (J105; eBioscience), and anti-CD38 (OKT10; NIH Nonhuman Primate Reagent Resource) monoclonal antibodies (MAbs). At least 20,000 events were collected from each sample by lymphocyte gating, and the data were analyzed with FlowJo software, version 9.7.5 (TreeStar, Ashland, OR).
Cell sorting and culturing in vitro.
Five RMs were selected for cell sorting and cytokine/chemokine analysis after in vitro stimulation. SP CD4+ and CD8+ T cells were sorted from PB, BM, and ALN mononuclear cells (3.5 × 107 to 6.0 × 107) with anti-CD4 or anti-CD8 nonhuman primate magnetic cell separation microbeads (Miltenyi Biotec) for magnetically activated cell sorting as reported earlier (5). The use of anti-CD4 or anti-CD8 microbeads depletes the mononuclear cell population of double-positive (DP) CD4+ CD8+ cells. Between 2.3 × 106 and 11.0 × 106 sorted cells of each population were obtained and found to be 89 to 99% pure by a flow cytometry assay with anti-CD3, -CD4, and -CD8 MAbs. Sorted SP CD4+ and CD8+ T cells (5 × 105 sorted cells/well) were stimulated with either anti-CD3/28 MAbs (CD3, 6G12 obtained from NIH Nonhuman primate reagent resource and used at 10 μg/ml; CD28, CD28.2 obtained from BD Biosciences and used at 1 μg/ml) or SEB (used at 2 μg/ml; Toxin Technologies) in RPMI-10 at 37°C in the presence of 5% CO2 along with the appropriate medium control. For anti-CD3/28 MAb stimulation, plates were first coated overnight at 4°C with anti-CD3 MAbs diluted in PBS. Purified anti-CD28 MAbs were added to the cell culture at the time of cell stimulation. Cell culture supernatants were collected 72 h after stimulation. The concentrations of cytokines/chemokines in the cell culture supernatants were quantified with a Cytokine Monkey Magnetic 28-Plex panel (Invitrogen, Life Technologies) by following the manufacturer's instructions. The 28 analytes detected by this panel are epidermal growth factor (EGF), eotaxin (CCL11), FGF-basic, granulocyte colony-stimulating factor (G-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF), hepatocyte growth factor (HGF), interleukin-1β (IL-1β), IL-2, IL-4, IL-5, IL-6, IL-8 (CXCL8), IL-12, IL-15, IL-17, IL-RA, IFN-γ, IFN-γ-inducible protein 10 (IP-10; CXCL10), interferon-inducible T-cell alpha chemoattractant (I-TAC; CXCL11), macrophage-derived chemokine (MDC; CCL22), macrophage inflammatory protein 1α (MIP)-1α (CCL3), MIP-1β (CCL4), macrophage migration inhibition factor (MIF), monocyte chemotactic protein 1 (MCP-1; CCL2), monokine induced by IFN-γ (MIG; CXCL9), regulated on activation normal T cell expressed and secreted (RANTES; CCL5), tumor necrosis factor alpha (TNF-α), and vascular endothelial growth factor (VEGF). The concentration of each cytokine/chemokine was determined from a standard curve derived from either a human or a rhesus recombinant protein and therefore represents an estimate of the cytokine/chemokine concentration in each sample. All of the MAbs used in this assay are cross-reactive with RM cytokines/chemokines, as reported earlier (5, 30–34). The multiplex plate was read with a Bio-Plex 200 suspension array Luminex system (Bio-Rad) as reported previously (5, 35).
Data presentation and statistical analysis.
Graphical presentation and statistical analysis of the data were performed with GraphPad Prism (version 5.0f; GraphPad). A two-tailed paired t test (α = 0.05) was used to determine the statistical significance of differences between samples from the preinfection period and 21 days after SIV infection (postinfection). Fold changes were used to categorize the direction of changes in the expression of either anti-CD3/28 MAb or SEB-stimulated cultures (5). Upregulation or downregulation was determined as a more-than-1.5-fold increase or decrease in the concentration of either anti-CD3/28 MAb- or SEB-stimulated cultures compared to the preinfection concentration. Cytokines/chemokines with less-than-1.5-fold changes in concentration in either direction in anti-CD3/28 MAb- or SEB-stimulated cultures compared to the preinfection concentration were determined to have been maintained following infection.
RESULTS
Plasma viral loads.
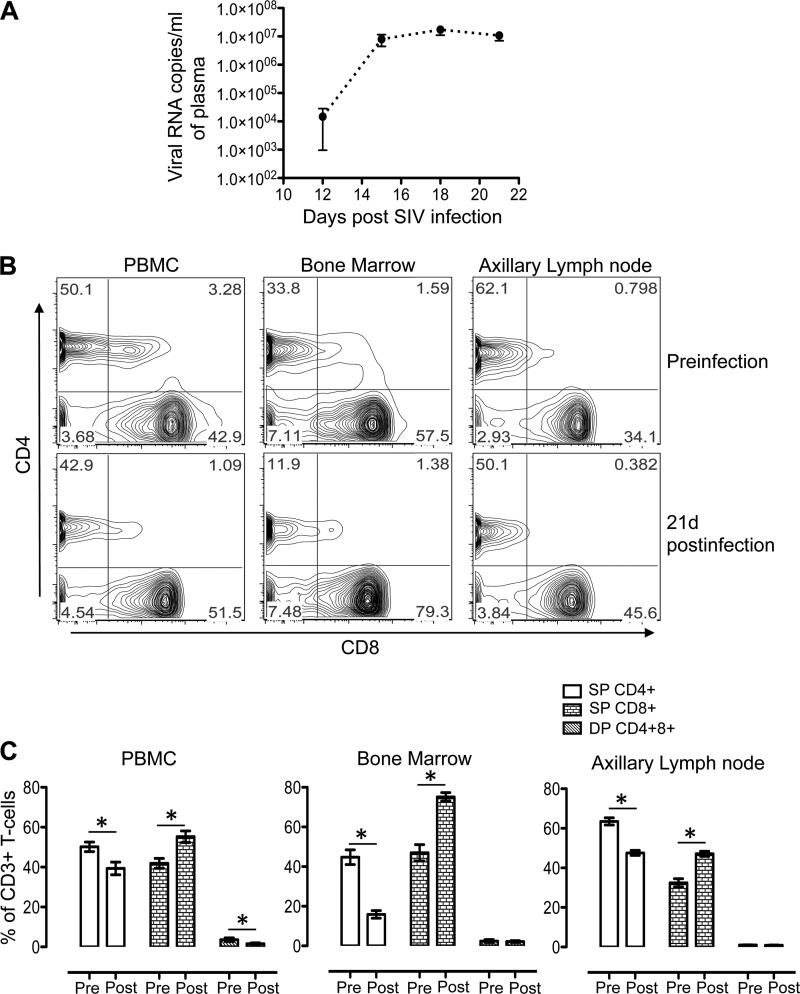
All nine SIV-infected RMs had high plasma viral loads (log10 5.95 to log10 7.43 viral RNA copies/ml of plasma) 21 days after SIV infection, where a mean peak viral load of 107 RNA copies/ml was attained at 18 days after SIV infection (Fig. 1A).
FIG 1.
(A) Mean (± standard errors) plasma viral loads in macaques during the acute phase of infection with SIVMAC251, as determined by RT-PCR (n = 9). (B) Representative contour plots showing SP CD4+ or CD8+ and DP CD4+ CD8+ T-cell populations in PB, BM, and ALN mononuclear cells before and 21 days after SIVMAC251 infection. (C) Mean percentages (± standard errors) of SP CD4+ or CD8+ and DP CD4+ CD8+ T cells of SIV-infected macaques before infection (Pre) and 21 days after infection (Post) (n = 9). Plots were generated by gating CD3+ T cells. Asterisks indicate statistically significant differences from the preinfection levels in the respective cell populations (P < 0.05).
Changes in the frequencies of CD4+ and CD8+ T cells in PB, BM, and ALN tissues.
All nine RMs experienced lymphopenia at the onset of viremia. In all three of the tissue types, the percentages of CD4+ T cells decreased significantly at 21 days after SIV infection (mean values of 50.2% [day 0] versus 39.3% [postinfection], 44.7% [day 0] versus 15.9% [postinfection], and 63.5% [day 0] versus 47.6% [postinfection] for PB, BM, and ALN mononuclear cells, respectively) compared to the preinfection levels (Fig. 1B and C). Conversely, significantly increased percentages of CD8+ T cells were observed at 21 days after SIV infection (mean values of 41.9% [day 0] versus 55.3% [postinfection], 47.0% [day 0] versus 75.2% [postinfection], and 32.4% [day 0] versus 47.1% [postinfection] for PB, BM, and ALN mononuclear cells, respectively (Fig. 1B and C). A significant reduction in the percentages of DP CD4+ CD8+ T cells (3.6% preinfection versus 1.6% postinfection) was observed only in PB mononuclear cells.
Decreased PD-1 expression in CD4+ T cells and increased CD38 expression in CD8+ T cells were evident in PB and ALN mononuclear cells.
Surface expression of PD-1 and CD38 was measured by flow cytometry assay in all of the macaques before and 21 days after SIV infection, along with isotype controls (Fig. 2A and B; see Fig. S1 in the supplemental material). PD-1, a member of the CD28 family, is an immune inhibitory receptor that is expressed on the surface of activated T cells (36). PD-1 interacts with PD-L1 and PD-L2 ligands and thereby restrains T-cell function in nonlymphoid and lymphoid organs, respectively. Increased expression of PD-1 in antigen-specific T cells is considered a measure of CD4+ and CD8+ T-cell exhaustion and dysfunction during chronic infection (37–40). In this study, we observed significantly decreased PD-1 expression in SP CD4+ T cells in both PB and ALN mononuclear cells (mean values of 34.9% preinfection versus 21.8% postinfection and 20.8% preinfection versus 9.6% postinfection in PB and ALN mononuclear cells, respectively; Fig. 2A and C). In contrast, there was a slight increase in PD-1 expression in SP CD8+ T cells in BM (mean values, 41.9% preinfection versus 49.3% postinfection) and ALN (mean values, 21.2% preinfection versus 25.1% postinfection) (Fig. 2C). However, the changes in PD-1 expression in CD8+ T cells were not statistically significant.
FIG 2.
Surface expression of PD-1 and CD38 phenotypic markers in SP CD4+ and CD8+ T cells in PB, BM, and ALN mononuclear cells before and 21 days after SIVMAC251 infection. Representative contour plots showing PD-1 expression in SP CD4+ T cells (A) and CD38 expression in SP CD8+ T cells (B) before and 21 days after SIV infection. FSC, forward-angle light scatter. (C) Mean percentages (± standard errors) of T-cell exhaustion (PD-1) and activation (CD38) in SP CD4+ and CD8+ T cells of nine SIV-infected macaques before infection (Pre) and 21 days after infection (Post) are shown. Plots were generated by gating CD3+ T cells. Asterisks indicate statistically significant differences from the preinfection levels in the respective cell populations (P < 0.05).
Surface CD38 expression on different T-cell subsets is also predictive of T-cell activation. Increased CD38 surface expression on CD8+ T cells is considered to be an indicator of immune activation and a strong prognostic marker of disease progression and death in HIV-infected patients (41). We observed significantly increased expression of CD38 on CD8+ T cells in PBMCs (mean values, 6.7% preinfection versus 12.3% postinfection) and ALN (mean values, 9.7% preinfection versus 19.3% postinfection) 21 days after SIV infection, compared to the preinfection levels; however, the upregulation of CD38 expression was not detected in CD4+ T cells in any of the tissues examined (Fig. 2C). No significant change in CD38 expression was detected in BM CD4+ and CD8+ T cells.
Dynamics of individual cytokine/chemokine responses in CD4+ and CD8+ T cells following acute SIV infection.
The concentrations of 24 of 28 cytokines/chemokines showed detectable changes in either anti-CD3/28 MAb- or SEB-stimulated CD4+ and CD8+ T cells (see Fig. 3 to 7). Four cytokines/chemokines that did not induce positive responses in the presence of either anti-CD3/28 MAbs or SEB compared to the medium control were CCL11, G-CSF, IL-4, and IL-15. Only three cytokines/chemokines were present at detectable levels in medium control samples before infection and 21 days after SIV infection (see Fig. S2 in the supplemental material). CCL2 was detected in PB CD4+ T cells, and CCL5 was detected in PB CD4+ and CD8+ T cells; the concentrations of both chemokines were upregulated after SIV infection. CCL2 was also detected in BM SP CD8+ T cells, and its expression was slightly upregulated following infection. However, none of these changes were statistically significant (P > 0.05). However, significant upregulation of MIF expression was detected in SP CD8+ T cells in medium control samples during SIV infection compared to the preinfection time point in all three of the tissue types (P < 0.05). Significant upregulation of MIF expression was also detected in SP CD4+ BM T cells. The responses of most of the cytokines/chemokines detected in either anti-CD3/28 MAb- or SEB-stimulated cultures were similar; however, SEB-stimulated cultures generally had lesser responses than anti-CD3/28 MAb-stimulated cultures. SEB has been used as a T-cell superantigen and provides useful information on T-cell signaling pathways; however, anti-CD3/28 MAb stimulation is considered to be a more physiologically relevant mode of in vivo T-cell activation than SEB (42, 43). Anti-CD3/28 MAbs and SEB stimulate T cells by inducing TCR signaling and by cross-linking of major histocompatibility complex class II (MHCII) and the TCR, respectively (44–46).
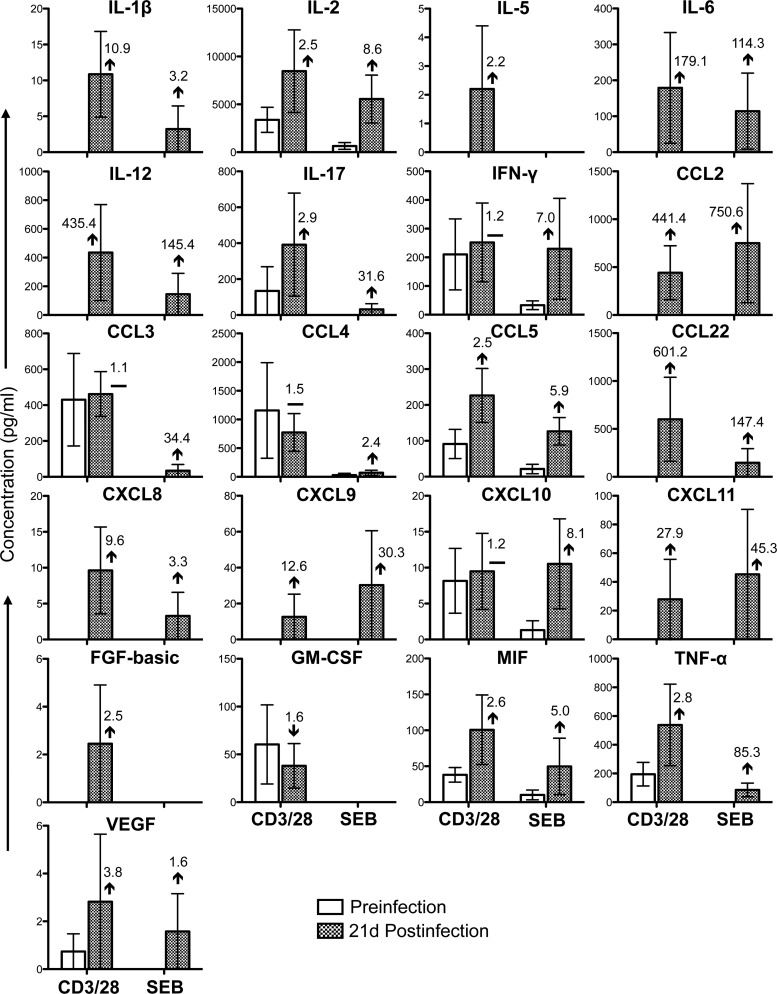
FIG 3.
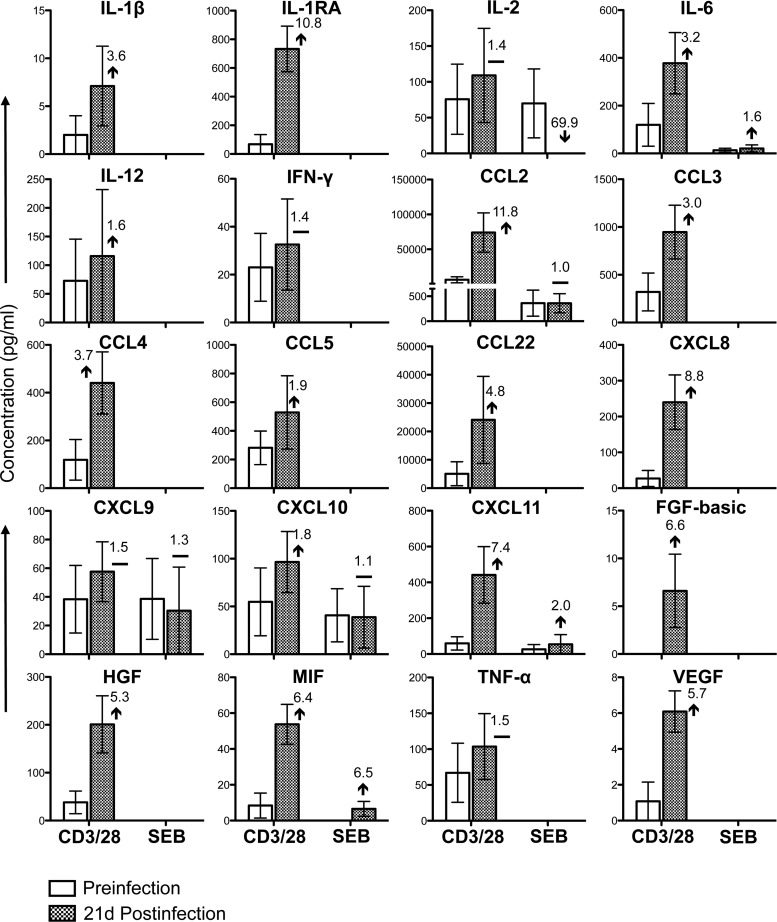
Cytokine/chemokine profiles of PB CD3+ CD4+ T cells during the acute phase of SIVMAC251 infection. Bar graphs show 21 different cytokine/chemokine responses observed before and 21 days after SIV infection in culture supernatants from sorted SP CD4+ T cells stimulated with either anti-CD3/28 MAbs or SEB after subtraction of medium control values (n = 5). The value and arrow or line in each bar for the postinfection time point show the mean fold increase (upward arrow) or decrease (downward arrow) or no change (horizontal line) compared to the preinfection level.
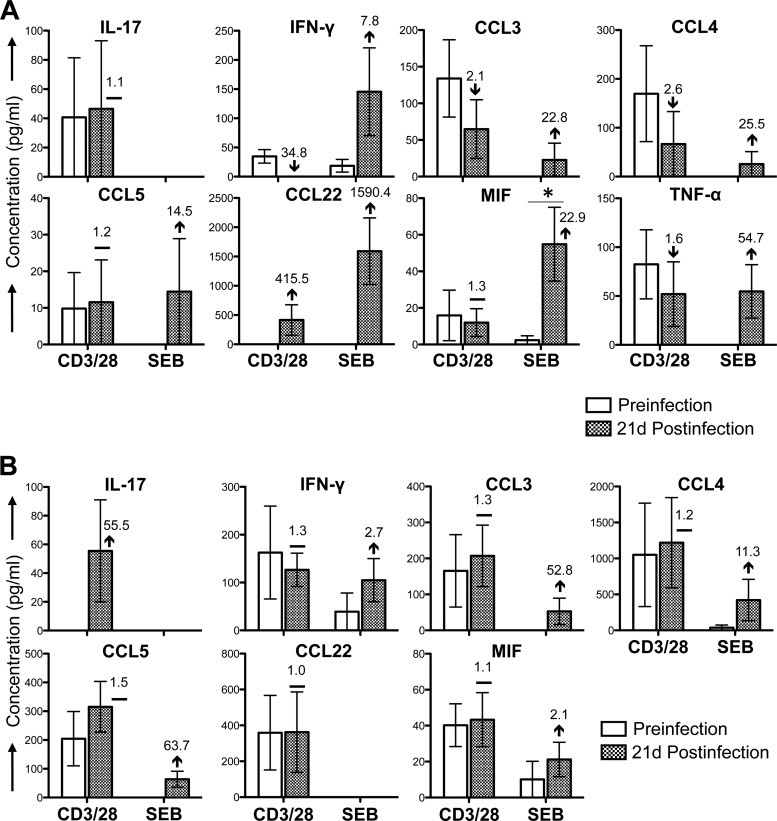
FIG 7.
Cytokine/chemokine profiles of ALN CD3+ CD4+ and CD3+ CD8+ T cells during the acute phase of SIVMAC251 infection. Bar graphs show differences in cytokine/chemokine responses observed before and 21 days after SIV infection in culture supernatants from sorted SP CD4+ T cells (A) and SP CD8+ T cells (B) stimulated with either anti-CD3/28 MAbs or SEB after subtraction of medium control values (n = 5). The value and arrow or line in each bar for the postinfection time point show the mean fold increase (upward arrow) or decrease (downward arrow) or no change (horizontal line) compared to the preinfection level. Asterisks indicate statistically significant differences from preinfection levels in the respective cell populations (P < 0.05).
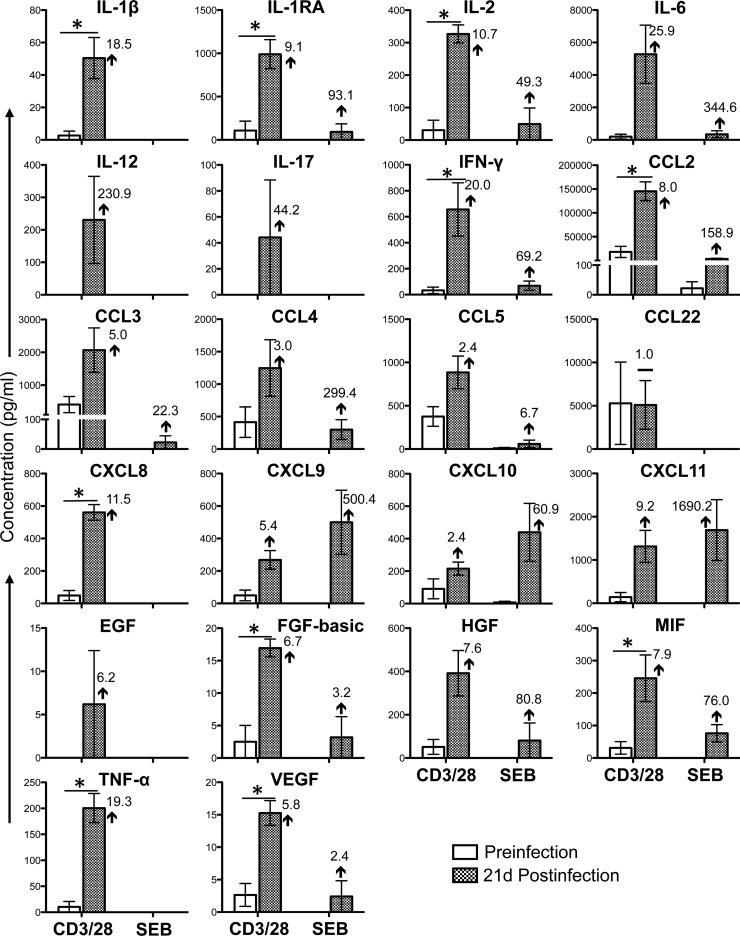
Twenty-one cytokines/chemokines were present at detectable concentrations in anti-CD3/28 MAb-stimulated cultures of SP CD4+ T cells isolated from PB (Fig. 3). Following the acute phase of SIV infection, the concentrations of four of these cytokines/chemokines (IFN-γ, CCL3, CCL4, and CXCL10) were maintained and that of one cytokine, GM-CSF, was downregulated. However, many of the cytokines/chemokines that were at undetectable concentrations prior to infection were upregulated during the acute phase of SIV infection (IL-1β, IL-2, IL-5, IL-6, IL-12, IL-17, CCL2, CCL5, CCL22, CXCL8, CXCL9, CXCL11, FGF-basic, MIF, TNF-α, and VEGF). In SP CD8+ PB T cells stimulated with anti-CD3/28 MAbs, 14 cytokine/chemokine responses were detected (Fig. 4). With the exception of VEGF, the concentrations of all of the cytokines/chemokines detected (IL-1β, IL-6, IL-17, IFN-γ, CCL2, CCL3, CCL4, CCL5, CCL22, CXCL9, GM-CSF, HGF, and MIF) were upregulated postinfection. As in SP CD4+ cells, many of these cytokines/chemokines were detectable only following SIV infection. However, none of the differences between the pre- and postinfection levels were found to be statistically significant (P > 0.05). In SEB-stimulated cultures, 18 cytokines/chemokines in PB CD4+ T cells and 6 cytokines/chemokines in CD8+ T cells were detected (Fig. 3 and 4). Although the patterns in SEB- and anti-CD3/28 MAb-stimulated CD4+ cells were generally similar, increased concentrations of the chemokines CCL2, CXCL9, CXCL10, and CXCL11 were measured in SEB-stimulated cultures possibly because of the mechanism of action of SEB, which involves cross-linking of MHCII and TCR. All of the cytokines/chemokines detected in SEB-stimulated PB CD8+ T cells followed the same patterns as in anti-CD3/28 MAb-stimulated cells and were detected at lower concentrations than in anti-CD3/28 MAb-stimulated cultures, with the exception of MIF, whose responses were downregulated at 21 days postinfection. (Fig. 4). Upregulation of IL-17 and maintenance of IFN-γ in CD4+ T cells were also confirmed by measuring those cytokines in the plasma of three RMs before and 21 days after SIV infection (see Fig. S3 in the supplemental material).
FIG 4.
Cytokine/chemokine profiles of PB CD3+ CD8+ T cells during the acute phase of SIVMAC251 infection. Bar graphs show 14 different cytokine/chemokine responses observed before and 21 days after SIV infection in culture supernatants from sorted SP CD8+ T cells stimulated with either anti-CD3/28 MAbs or SEB after subtraction of medium control values (n = 5). The value and arrow or line in each bar for the postinfection time point show the mean fold increase (upward arrow) or decrease (downward arrow) or no change (horizontal line) compared to the preinfection level. Asterisks indicate statistically significant differences from preinfection levels in the respective cell populations (P < 0.05).
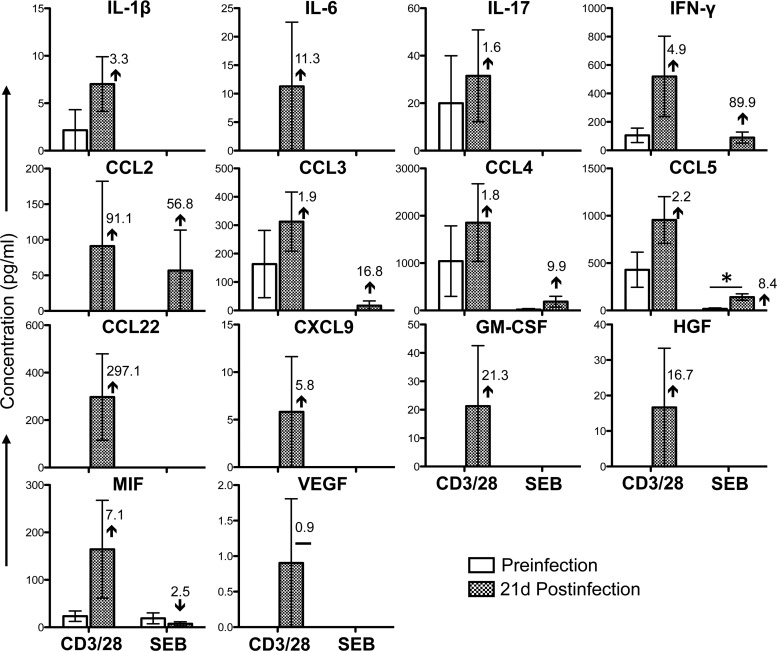
Twenty cytokine/chemokine responses were detectable in anti-CD3/28 MAb-stimulated SP CD4+ BM T cells and were either maintained (IL-2, IFN-γ, CXCL9, and TNF-α) or upregulated (IL-1β, IL-1RA, IL-6, IL-12, CCL2, CCL3, CCL4, CCL5, CCL22, CXCL8, CXCL10, CXCL11, FGF-basic, HGF, MIF, and VEGF) following SIV infection (Fig. 5). Twenty-two cytokine/chemokine responses were detectable in anti-CD3/28 MAb-stimulated SP CD8+ T cells isolated from BM (Fig. 6). With the exception of CCL22, the concentration of which was maintained after SIV infection, all of the other detectable cytokine/chemokine responses were upregulated postinfection (IL-1β, IL-1RA, IL-2, IL-6, IL-12, IL-17, IFN-γ, CCL2, CCL3, CCL4, CCL5, CXCL8, CXCL9, CXCL10, CXCL11, EGF, FGF-basic, HGF, MIF, TNF-α, and VEGF) (Fig. 6). Among these upregulated cytokine/chemokine responses, 10 (IL-1β, IL-1RA, IL-2, IFN-γ, CCL2, CXCL8, FGF-basic, MIF, TNF-α, and VEGF) were statistically significantly different from the preinfection values (P < 0.05). The differences between the cytokine expression profiles of T cells isolated from PB and those from BM, including several PB cytokines/chemokines undetectable in BM (for example, IL-5 and GM-CSF; Fig. 3 to 6), indicate that blood contamination of BM samples was low to minimal. In SEB-stimulated BM CD4+ T cells, only seven cytokines/chemokines were present at detectable concentrations and showed a pattern similar to that of anti-CD3/28 MAb-stimulated cells, except IL-2 and CXCL10, which were either downregulated or maintained after SIV infection (Fig. 5). Fifteen cytokine/chemokine responses were detectable in SEB-stimulated CD8+ T cells, and these were all upregulated at 21 days postinfection in comparison to the levels before infection (Fig. 6). Moreover, SEB stimulation resulted in higher CXCL9, CXCL10, and CXCL11 responses than anti-CD3/28 MAb stimulation (Fig. 6).
FIG 5.
Cytokine/chemokine profiles of BM CD3+ CD4+ T cells during the acute phase of SIVMAC251 infection. Bar graphs show 20 different cytokine/chemokine responses observed before and 21 days after SIV infection in culture supernatants from sorted SP CD4+ T cells stimulated with either anti-CD3/28 MAbs or SEB after subtraction of medium control values (n = 5). The value and arrow or line in each bar for the postinfection time point show the mean fold increase (upward arrow) or decrease (downward arrow) or no change (horizontal line) compared to the preinfection level.
FIG 6.
Cytokine/chemokine profiles of BM CD3+ CD8+ T cells during the acute phase of SIVMAC251 infection. Bar graphs show 22 different cytokine-chemokine responses observed before and 21 days after SIV infection in culture supernatants from sorted SP CD8+ T cells stimulated with either anti-CD3/28 MAbs or SEB after subtraction of medium control values (n = 5). The value and arrow or line in each bar for the postinfection time point show the mean fold increase (upward arrow) or decrease (downward arrow) or no change (horizontal line) compared to the preinfection level. Asterisks indicate statistically significant differences from the preinfection levels in the respective cell populations (P < 0.05).
Cytokine/chemokine responses in T cells isolated from ALN were more limited than those in PB and BM. SP CD4+ T cells isolated from ALN had detectable responses to eight cytokines/chemokines in cultures stimulated with anti-CD3/28 MAbs (Fig. 7A). Most of the responses were downregulated (IFN-γ, CCL3, CCL4, TNF-α) or maintained (IL-17, MIF, CCL5) postinfection. Only CCL22 expression was upregulated, with a mean concentration of 415.5 pg/ml postinfection versus undetectability prior to infection. CD8+ ALN T cells had detectable responses to seven cytokines/chemokines (Fig. 7B), and most of these responses were maintained at 21 days after SIV infection (IFN-γ, CCL3, CCL4, CCL5, CCL22, and MIF). Two out of five macaques had upregulated and detectable CD8+ cell-specific IL-17 responses 21 days after SIV infection (mean, 55.5 pg/ml) compared to undetectable IL-17 responses preinfection (Fig. 7B). However, none of these changes in anti-CD3/28 MAb-stimulated cells were statistically significant (P > 0.05). In ALN tissues, SEB stimulation had a substantially different impact on many cytokine/chemokine responses from that of anti-CD3/28 stimulation. In CD4+ ALN cells, all of the cytokines/chemokines that were detected in CD3/28-stimulated cultures were also detected in SEB-stimulated cultures, except IL-17 (Fig. 7A). The distinct mechanisms of action of anti-CD3/28 MAbs and SEB likely account for the higher concentrations of SEB-induced IFN-γ, CCL5, CCL22, and MIF in ALN SP CD4+ T cells (Fig. 7A). Upregulation of several cytokine/chemokine responses (CCL3, CCL4, and TNF-α for SP CD4+ T cells and IFN-γ, CCL3, CCL4, CCL5, and TNF-α for SP CD8+ T cells) was observed in SEB-stimulated cultures compared to those in anti-CD3/28 MAb-stimulated samples; however, the concentration of each cytokine/chemokine was lower than that in anti-CD3/28 MAb-stimulated samples (Fig. 7).
Cytokine/chemokine concentration changes in different cell populations following acute SIV infection.
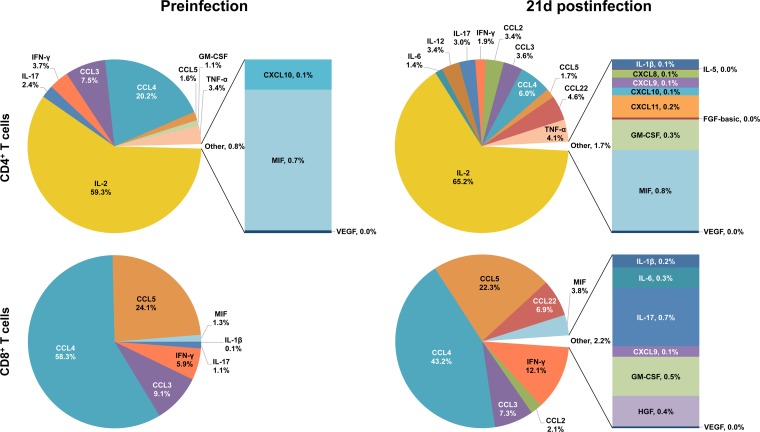
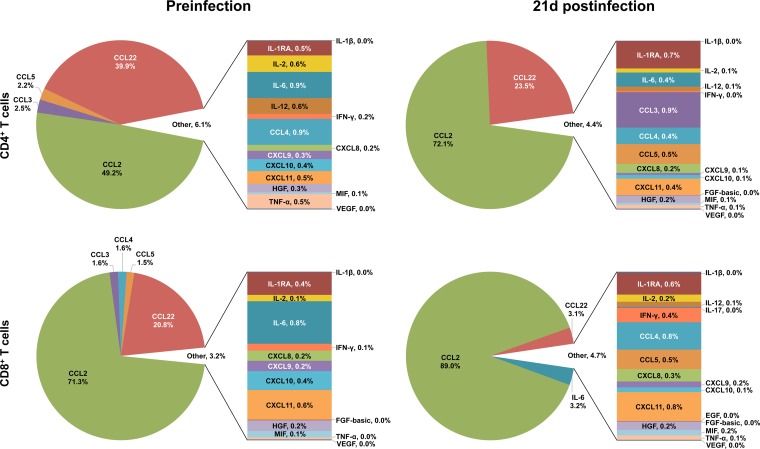
Depictions of the relative contributions of individual cytokine/chemokine concentrations to overall expression before and after SIV infection in each cell and tissue type following anti-CD3/28 MAb stimulation are helpful in elucidating the overall role of cytokine/chemokine networks in SIV infection (see Fig. 8 to 10). In SP CD4+ PB T cells, IL-2 expression accounts for most (59.3%) of the total cytokine/chemokine responses prior to infection and increases during acute infection (65.2%) despite the increased expression of other cytokines/chemokines (Fig. 8). CCL3 and CCL4 are the other major molecules expressed preinfection (combined expression of 27.6%) in CD4+ T cells; however, their expression decreased during infection and accounted for a smaller percentage of activity in the cytokine storm postinfection, where the concentrations of many other/new cytokines/chemokines are upregulated, including those of ILs (IL-6, IL-12, etc.) and other chemokines (CCL2, CCL22, etc.) (Fig. 8). In contrast, the chemokines CCL3, CCL4, and CCL5 account for most of the total cytokine/chemokine responses prior to infection and are maintained as major chemokines after SIV infection in SP CD8+ PB T cells (91.5% preinfection versus 72.8% postinfection; Fig. 8). Additional increases in the CCL2, CCL22, MIF, and IFN-γ responses after SIV infection also modify the cytokine network of the CD8+ T-cell population.
FIG 8.
Relative contributions of cytokine/chemokine expression in PB during the acute phase of SIVMAC251 infection. The pie charts illustrate the relative percent contributions of the cytokines/chemokines based upon their concentrations (pg/ml) in the culture supernatants of sorted SP CD4+ and CD8+ T cells stimulated with anti-CD3/28 MAbs before and 21 days after SIV infection. Medium control values were subtracted from all values before analysis (n = 5). Cytokines/chemokines with contributions of <1% are shown in a bar graph and combined and designated “Other” in the pie chart for clarity. Cytokines/chemokines with overall contributions of 0.01 to 0.04% are represented by 0% in the pie chart.
FIG 10.
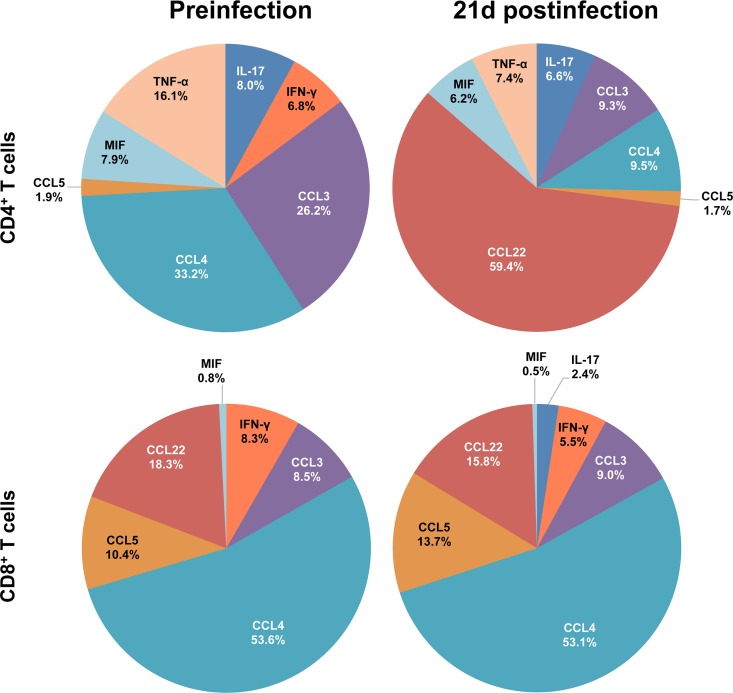
Relative contributions of cytokine/chemokine expression in ALN during the acute phase of SIVMAC251 infection. The pie charts illustrate the relative percent contributions of the cytokines/chemokines based upon their concentrations (pg/ml) in culture supernatants of sorted SP CD4+ and CD8+ T cells stimulated with anti-CD3/28 MAbs before and 21 days after SIV infection. Medium control values were subtracted from all values before analysis (n = 5).
CCL2 and CCL22 account for a large proportion of cytokine/chemokine expression by both CD4+ and CD8+ T cells in BM (Fig. 9). Prior to infection, these two chemokines accounted for 89.1 to 92.1% of the total cytokine/chemokine responses in these cells types. Other β-chemokines (CCL3 and CCL5 in CD4+ T cells and CCL3, CCL4, and CCL5 in CD8+ T cells) were also expressed by BM T cells prior to infection. Overall increased expression of CCL2 was observed in SP CD4+ BM T cells postinfection in comparison to the preinfection level (Fig. 9). The concentrations of many other cytokines/chemokines (CCL4, CCL5, CXCL9, CXCL10, etc.) were upregulated following infection, but their percentages were small (4.4%), in contrast to the overall expression of CCL2 and CCL22 (95.6%) (Fig. 9). In CD8+ T cells, CCL2 accounts for most of the cytokine/chemokine expression (71.3%) compared to CCL22 expression (20.8%) prior to infection. Following SIV infection, the cytokine profile of CD8+ T cells showed a trend similar to that of CD4+ T cells, with upregulation of CCL2 expression (89% postinfection versus 71.3% preinfection; Fig. 9). There was no change in CCL22 expression in BM CD8+ T cells after infection, leading to lower overall expression of CCL22 (3.1%) than at the preinfection time point (20.8%) (Fig. 9). IL-6, which showed a 25.9-fold upregulation in concentration following infection (Fig. 6), accounted for 3.2% of the total cytokine/chemokine responses postinfection (Fig. 9). Despite significant increases in the expression of many other cytokines/chemokines (IL-1β, IL-2, IFN-γ, FGF basic, MIF, etc.) after SIV infection, they accounted for only a very small percentage (4.7%) of the total cytokine/chemokine responses of BM CD8+ T cells.
FIG 9.
Relative contributions of cytokine/chemokine expression in BM during the acute phase of SIVMAC251 infection. The pie charts illustrate the relative percent contributions of the cytokines/chemokines based upon their concentrations (pg/ml) in culture supernatants of sorted SP CD4+ and CD8+ T cells stimulated with anti-CD3/28 MAbs before and 21 days after SIV infection. Medium control values were subtracted from all values before analysis (n = 5). Cytokines/chemokines with contributions of <1% are shown in a bar graph and combined and designated “Other” in the pie chart for clarity. Cytokines/chemokines with overall contributions of 0.01 to 0.04% are represented by 0% in the pie chart.
In ALN SP CD4+ T cells, CCL3 and CCL4 accounted for 59.4% of the cytokine/chemokine expression prior to infection; however, postinfection, CCL22 was found to be the major chemokine upregulated (59.4%; Fig. 10), whereas the expression of both CCL3 and CCL4 was downregulated. The cytokine profile of CD8+ ALN T cells remained largely unchanged at 21 days after SIV infection, except for an increase in the concentration of IL-17, which was undetectable prior to infection (Fig. 10).
DISCUSSION
Cytokines/chemokines are important secretory proteins that mediate cellular interactions and regulate cell growth and secretions. Cytokines/chemokines are deployed early in the initial stage of viral infections and act as an essential component of the host defense. Several studies have shown that early cytokine-mediated immune responses can completely clear a viral infection (47, 48). Most of the damage imposed on virus-infected cells is the result of responses induced by multiple proinflammatory cytokines/chemokines. Interestingly, the regulation of cytokines/chemokines is considered important for initial virus control and most viruses have developed strategies to modulate cytokine signaling. HIV and SIV use several strategies to evade innate and adaptive immune responses. Here, we have measured the production of 28 cytokines/chemokines before and 21 days after SIV infection in PB, BM, and ALN tissues to determine early changes in the activated CD4+ and CD8+ T-cell-mediated cytokine/chemokine responses.
The absence of changes in PD-1 expression in CD8+ T cells in all three of the tissue types suggests that CD8+ T-cell exhaustion was not occurring early in infection. However, the depletion of PD-1-positive CD4+ T cells in PBMCs and ALNs suggests that those PD-1+ cells are activated target cells. Increased CD38 expression in PB and ALN CD8+ T cells also suggests that those cells are highly activated after SIV infection. There are conflicting reports on the changing cytokine responses of PB CD4+ T cells during HIV infection. Several studies found decreased production of IL-2 and IFN-γ (predominately TH1) and increased production of IL-4, IL-5, and IL-10 (predominately TH2) in PB CD4+ T cells following HIV-1 infection, attributing this to a TH1-to-TH2 shift in cytokine responses (9, 10). However, other studies found IL-2 and IL-4 responses to be barely detectable while the expression of both IFN-γ and IL-10 was increased after HIV infection (12). In PB CD4+ T cells, we detected IL-2, IL-5, and IFN-γ but not IL-4 responses (Fig. 3). Despite an increased concentration of IL-5 along with the maintenance of IFN-γ following acute SIV infection, the concentration of IFN-γ was 114.5 times that of IL-5. The IL-2 concentration was also increased following acute infection; however, IL-2 is a growth factor that is not restricted to a single cell subtype (49). In summary, our data do not support a shift from a TH1 to a TH2 response in PB CD4+ T cells at 21 days after SIV infection. Additionally, we did not detect measurable IL-4 and IL-5 responses in CD4+ T cells isolated from BM and ALN and there was no evidence of a TC1-to-TC2 shift of CD8+ T cells in PB, BM, or ALN tissues.
We detected seven proinflammatory cytokines (IL-1β, IL-6, IL-12, IL-17, IFN-γ, MIF, and TNF-α) in both CD4+ and CD8+ T-cell subsets from all three of the tissue types tested. All of the proinflammatory cytokine responses were either maintained or upregulated 21 days after SIV infection in T cells isolated from PB and BM tissues. Several of these cytokines are important for a functional immune response to SIV. IFN-γ, which plays an important role in the inhibition of HIV pathogenesis (50, 51), was upregulated in CD8+ and maintained in CD4+ anti-CD3/28 MAb-stimulated T-cell populations. IL-17 also plays a vital role in the regulation of functional immune responses following infection and was upregulated in both T-cell subsets of PB and BM CD8+ T cells. Increased MIF expression, which has been associated with poor prognoses for individuals infected with HIV (6, 52), was upregulated in medium control cultures from all three of the tissue types examined and also in both T-cell subsets of stimulated PB and BM tissues, as reported for intestinal T-cell subsets earlier (5).
IL-1β has been associated with clinical symptoms and secondary complications of advanced HIV/AIDS (53, 54), as well as enhancement of HIV-1 replication in vitro (55). IL-1RA is stimulated by HIV-1 in vitro (56) and directly inhibits the proinflammatory effect of IL-1β (57, 58), with 100-fold or greater levels of IL-1RA inhibiting the biological effects of IL-1β (59). The ratio of these two cytokines affects the progression of many different infectious diseases, with overproduction of IL-1β and/or underproduction of IL-1RA negatively affecting the prognosis (60). In our study, we found that both before and after SIV infection, IL-1RA levels were higher than IL-1β levels in anti-CD3/28 MAb-stimulated BM T-cell subpopulations. Prior to infection, the level of IL-1RA was 33.8 (CD4+) to 40.0 (CD8+) times as high as that of IL-1β in CD4+ and CD8+ T cells. After SIV infection, the fold difference between the IL-RA and IL-1β responses in CD4+ T cells reached >100-fold, which indicates the role of IL-1RA in blocking the proinflammatory activity of IL-1β. The same was not true of BM CD8+ T cells, where the increase in the IL-1β response (18.5-fold) was significantly greater than that in the IL-1RA response (9.1-fold) after SIV infection, dropping the difference between IL-RA and IL-1β to 19.6-fold. Additionally, in PB, IL-1β was detected in both CD4+ and CD8+ T cells following SIV infection; however, IL-1RA was not, indicating that proinflammatory activity is continuing unabated in these cell types and may be influencing disease progression.
Chemokines are vital in the pathogenesis of HIV infection and play an important role in inflammatory and immune responses by trafficking immune cells. Four α-chemokines (CXCL8, -9, -10, and -11) and five β-chemokines (CCL2, -3, -4, -5, and -22) were detectable in this study. With the exception of PB CD4+ T cells, β-chemokines accounted for most of the total cytokine/chemokine production in all anti-CD3/28 MAb-stimulated cell populations (61.3 to 97.4%). The distribution of these chemokines varies between tissue types, and T-cell subtypes. CCL3, -4, -5, and -22 have been hypothesized to have a role in protection against HIV infection because of competitive inhibition of HIV replication and correlation with resistance or control of infection (61–67). Conversely, CCL2 is a proinflammatory chemokine that supports HIV replication and has been correlated with large viral loads and several secondary complications of HIV infection (68, 69). Additionally, studies have shown that high levels of CCL3, -4, and -5 may also have a detrimental effect on the HIV infection prognosis (70). In LN, a major reservoir of HIV/SIV infection, upregulation of CCL3, -4, and -5 has been shown to occur during chronic pathogenic SIV infection (70); however, in both T-cell subtypes, upregulation of these molecules was not evident during the acute phase of infection when cells were stimulated with anti-CD3/28 MAbs. Additionally, in CD4+ LN T cells, CCL3 and -4 were downregulated following the acute phase of infection. This may indicate that significant upregulation of β-chemokines might be characteristic of chronic SIV infection and cell populations other than CD4+ and CD8+ T cells. In both PB and BM T-cell populations, α-chemokines were present, but at much lower concentrations than β-chemokines, and were upregulated following infection. CXCL9, -10, and -11 had also been shown to stimulate HIV-1 replication in PB T cells and are associated with a poor disease prognosis (71). No α-chemokines were detected in either subpopulation of LN T cells before or after SIV infection, despite reports showing upregulation of mRNAs for these chemokines in LN tissues as early as 10 days after SIV infection (72). This indicates the role of non-CD4+ and CD8+ T cells in the upregulation of α-chemokines.
IL-2 is essential for the growth, differentiation, and proliferation of T cells and is produced as part of a normal functional immune response. IL-2 has been tested as a potential therapeutic strategy to treat HIV, as it increases the production of CD4+ T cells; however, it was found to have no therapeutic benefit, possibly because the CD4+ T cells it induced were not functionally important in aiding immune defenses against HIV infection or proinflammatory effects were negating any positive consequences (73). In CD4+ T cells isolated from PB, IL-2 accounts for most of the cytokine/chemokine expression in anti-CD3/28 MAb-stimulated cells both prior to and 21 days after SIV infection. Despite the increased production of IL-2 by PB CD4+ T cells, no increased activation, as detected by CD38 expression, was observed in PB CD4+ T cells. Interestingly, absence of detectable IL-2 responses and lack of changes in CD38 expression in ALN CD4+ T cells suggest a tissue-specific role for IL-2 in CD4+ T-cell activation. Increased concentrations of growth factors such as FGF-basic, HGF, and VEGF detected in stimulated BM and PB T cells are considered to be associated with a poor prognosis and secondary complications during HIV infection (7, 8, 74, 75). In contrast, the production of GM-CSF, which is part of a functional immune response and has been proposed to have an adjuvant role in HIV vaccine (76), was downregulated in anti-CD3/28 MAb-stimulated PB CD4+ T cells and remained at undetectable concentrations in other tissues.
In conclusion, loss of CD4+ T cells and immune activation of CD8+ T cells were detected in PB and ALN tissues. Despite the loss of CD4+ T cells in BM, no sign of CD4+ T-cell exhaustion or CD8+ T-cell activation was detected, which is suggestive of tissue-specific changes during the acute phase of SIV infection. There was no evidence of a TH1-to-TH2 shift in CD4+ T cells or a TC1-to-TC2 cytokine shift in CD8+ T cells in the PB, BM, and ALN T-cell subsets during the acute phase of SIV infection. β-Chemokines were the major cytokine/chemokine products of all of the T-cell subsets from all three of the tissue types examined, except for CD4+ PB T cells, where IL-2 was the major cytokine produced. Our experimental design does not address the cytokine/chemokine responses of other cell populations or time points beyond the acute phase of SIV infection. Increased CD4+- and CD8+-specific cytokine/chemokine responses in PB and BM, respectively, after SIV infection suggest that different tissue-specific T-cell responses are actively mounting functional cytokine/chemokine responses that are responsible for mitigating some of the impact of the loss of functional TH cells. The only increased production of IL-17 by ALN CD8+ T cells suggested an influx of Tc17 cells during the acute phase of SIV infection that might play an important role in regulating functional immune responses and tissue homeostasis. Despite the upregulation of several important effector cytokine/chemokine responses (IL-2, IL-12, IL-17, IFN-γ, GM-CSF) by CD4+ and CD8+ T cells, the upregulation of β-chemokines (CCL2 and CCL22), FGF-basic, HGF, and MIF may provide a poor prognosis either by inducing increased virus replication or by other unknown mechanisms. Therefore, drugs targeting β-chemokines (CCL2 and CCL22), FGF-basic, HGF, or MIF might be important for developing effective vaccines and therapeutics against HIV.
Supplementary Material
ACKNOWLEDGMENTS
We thank Maury Duplantis, Mary Barnes, and all of the animal care staff of the Division of Veterinary Medicine at the Tulane National Primate Research Center for their technical assistance. We also thank Diganta Pan for help with the tissue processing and staining.
This work was supported by National Institutes of Health grants P20 GM103458-09 and R21 AI080395 (B.P.).
Footnotes
Published ahead of print 11 June 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JVI.00774-14.
REFERENCES
- 1.Doherty M, Ford N, Vitoria M, Weiler G, Hirnschall G. 2013. The 2013 WHO guidelines for antiretroviral therapy: evidence-based recommendations to face new epidemic realities. Curr. Opin. HIV AIDS 8:528–534. 10.1097/COH.0000000000000008 [DOI] [PubMed] [Google Scholar]
- 2.Burdo TH, Lackner A, Williams KC. 2013. Monocyte/macrophages and their role in HIV neuropathogenesis. Immunol. Rev. 254:102–113. 10.1111/imr.12068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vinikoor MJ, Cope A, Gay CL, Ferrari G, McGee KS, Kuruc JD, Lennox JL, Margolis DM, Hicks CB, Eron JJ. 2013. Antiretroviral therapy initiated during acute HIV infection fails to prevent persistent T-cell activation. J. Acquir. Immune Defic. Syndr. 62:505–508. 10.1097/QAI.0b013e318285cd33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu H, Wang X, Morici LA, Pahar B, Veazey RS. 2011. Early divergent host responses in SHIVsf162P3 and SIVmac251 infected macaques correlate with control of viremia. PLoS One 6(3):e17965. 10.1371/journal.pone.0017965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kenway-Lynch CS, Das A, Pan D, Lackner AA, Pahar B. 2013. Dynamics of cytokine/chemokine responses in intestinal CD4+ and CD8+ T cells during acute simian immunodeficiency virus infection. J. Virol. 87:11916–11923. 10.1128/JVI.01750-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Delaloye J, De Bruin IJ, Darling KE, Reymond MK, Sweep FC, Roger T, Calandra T, Cavassini M. 2012. Increased macrophage migration inhibitory factor (MIF) plasma levels in acute HIV-1 infection. Cytokine 60:338–340. 10.1016/j.cyto.2012.07.027 [DOI] [PubMed] [Google Scholar]
- 7.Presta M, Dell'Era P, Mitola S, Moroni E, Ronca R, Rusnati M. 2005. Fibroblast growth factor/fibroblast growth factor receptor system in angiogenesis. Cytokine Growth Factor Rev. 16:159–178. 10.1016/j.cytogfr.2005.01.004 [DOI] [PubMed] [Google Scholar]
- 8.Zanin V, Delbue S, Marcuzzi A, Tavazzi E, Del Savio R, Crovella S, Marchioni E, Ferrante P, Comar M. 2012. Specific protein profile in cerebrospinal fluid from HIV-1-positive cART-treated patients affected by neurological disorders. J. Neurovirol. 18:416–422. 10.1007/s13365-012-0109-y [DOI] [PubMed] [Google Scholar]
- 9.Clerici M, Hakim FT, Venzon DJ, Blatt S, Hendrix CW, Wynn TA, Shearer GM. 1993. Changes in interleukin-2 and interleukin-4 production in asymptomatic, human immunodeficiency virus-seropositive individuals. J. Clin. Invest. 91:759–765. 10.1172/JCI116294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klein SA, Dobmeyer JM, Dobmeyer TS, Pape M, Ottmann OG, Helm EB, Hoelzer D, Rossol R. 1997. Demonstration of the Th1 to Th2 cytokine shift during the course of HIV-1 infection using cytoplasmic cytokine detection on single cell level by flow cytometry. AIDS 11:1111–1118. 10.1097/00002030-199709000-00005 [DOI] [PubMed] [Google Scholar]
- 11.Maggi E, Mazzetti M, Ravina A, Annunziato F, de Carli M, Piccinni MP, Manetti R, Carbonari M, Pesce AM, del Prete G, et al. 1994. Ability of HIV to promote a TH1 to TH0 shift and to replicate preferentially in TH2 and TH0 cells. Science 265:244–248. 10.1126/science.8023142 [DOI] [PubMed] [Google Scholar]
- 12.Graziosi C, Pantaleo G, Gantt KR, Fortin JP, Demarest JF, Cohen OJ, Sekaly RP, Fauci AS. 1994. Lack of evidence for the dichotomy of TH1 and TH2 predominance in HIV-infected individuals. Science 265:248–252. 10.1126/science.8023143 [DOI] [PubMed] [Google Scholar]
- 13.Gazzinelli RT, Makino M, Chattopadhyay SK, Snapper CM, Sher A, Hugin AW, Morse HC., III 1992. CD4+ subset regulation in viral infection. Preferential activation of Th2 cells during progression of retrovirus-induced immunodeficiency in mice. J. Immunol. 148:182–188 [PubMed] [Google Scholar]
- 14.Chakrabarti L, Isola P, Cumont MC, Claessens-Maire MA, Hurtrel M, Montagnier L, Hurtrel B. 1994. Early stages of simian immunodeficiency virus infection in lymph nodes. Evidence for high viral load and successive populations of target cells. Am. J. Pathol. 144:1226–1237 [PMC free article] [PubMed] [Google Scholar]
- 15.Lackner AA, Vogel P, Ramos RA, Kluge JD, Marthas M. 1994. Early events in tissues during infection with pathogenic (SIVmac239) and nonpathogenic (SIVmac1A11) molecular clones of simian immunodeficiency virus. Am. J. Pathol. 145:428–439 [PMC free article] [PubMed] [Google Scholar]
- 16.Pantaleo G, Graziosi C, Demarest JF, Butini L, Montroni M, Fox CH, Orenstein JM, Kotler DP, Fauci AS. 1993. HIV infection is active and progressive in lymphoid tissue during the clinically latent stage of disease. Nature 362:355–358. 10.1038/362355a0 [DOI] [PubMed] [Google Scholar]
- 17.Carter CC, Onafuwa-Nuga A, McNamara LA, Riddell Jt, Bixby D, Savona MR, Collins KL. 2010. HIV-1 infects multipotent progenitor cells causing cell death and establishing latent cellular reservoirs. Nat. Med. 16:446–451. 10.1038/nm.2109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lackner AA, Veazey RS. 2007. Current concepts in AIDS pathogenesis: insights from the SIV/macaque model. Annu. Rev. Med. 58:461–476. 10.1146/annurev.med.58.082405.094316 [DOI] [PubMed] [Google Scholar]
- 19.Burdo TH, Soulas C, Orzechowski K, Button J, Krishnan A, Sugimoto C, Alvarez X, Kuroda MJ, Williams KC. 2010. Increased monocyte turnover from bone marrow correlates with severity of SIV encephalitis and CD163 levels in plasma. PLoS Pathog. 6(4):e1000842. 10.1371/journal.ppat.1000842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Council NR. 2001. Guide for the care and use of laboratory animals, 8th ed. National Academies Press, Washington, DC [Google Scholar]
- 21.Valentine LE, Loffredo JT, Bean AT, Leon EJ, MacNair CE, Beal DR, Piaskowski SM, Klimentidis YC, Lank SM, Wiseman RW, Weinfurter JT, May GE, Rakasz EG, Wilson NA, Friedrich TC, O'Connor DH, Allison DB, Watkins DI. 2009. Infection with “escaped” virus variants impairs control of simian immunodeficiency virus SIVmac239 replication in Mamu-B*08-positive macaques. J. Virol. 83:11514–11527. 10.1128/JVI.01298-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cline AN, Bess JW, Piatak M, Jr, Lifson JD. 2005. Highly sensitive SIV plasma viral load assay: practical considerations, realistic performance expectations, and application to reverse engineering of vaccines for AIDS. J. Med. Primatol. 34:303–312. 10.1111/j.1600-0684.2005.00128.x [DOI] [PubMed] [Google Scholar]
- 23.Pahar B, Li J, Rourke T, Miller CJ, McChesney MB. 2003. Detection of antigen-specific T cell interferon γ expression by ELISPOT and cytokine flow cytometry assays in rhesus macaques. J. Immunol. Methods 282:103–115. 10.1016/j.jim.2003.08.003 [DOI] [PubMed] [Google Scholar]
- 24.Pahar B, Cantu MA, Zhao W, Kuroda MJ, Veazey RS, Montefiori DC, Clements JD, Aye PP, Lackner AA, Lovgren-Bengtsson K, Sestak K. 2006. Single epitope mucosal vaccine delivered via immuno-stimulating complexes induces low level of immunity against simian-HIV. Vaccine 24:6839–6849. 10.1016/j.vaccine.2006.06.050 [DOI] [PubMed] [Google Scholar]
- 25.Pahar B, Lackner AA, Veazey RS. 2006. Intestinal double-positive CD4+ CD8+ T cells are highly activated memory cells with an increased capacity to produce cytokines. Eur. J. Immunol. 36:583–592. 10.1002/eji.200535520 [DOI] [PubMed] [Google Scholar]
- 26.Das A, Xu H, Wang X, Yau CL, Veazey RS, Pahar B. 2011. Double-positive CD21+ CD27+ B cells are highly proliferating memory cells and their distribution differs in mucosal and peripheral tissues. PLoS One 6(1):e16524. 10.1371/journal.pone.0016524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pahar B, Lackner AA, Piatak M, Jr, Lifson JD, Wang X, Das A, Ling B, Montefiori DC, Veazey RS. 2009. Control of viremia and maintenance of intestinal CD4+ memory T cells in SHIV(162P3) infected macaques after pathogenic SIV(MAC251) challenge. Virology 387:273–284. 10.1016/j.virol.2009.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang X, Das A, Lackner AA, Veazey RS, Pahar B. 2008. Intestinal double-positive CD4+ CD8+ T cells of neonatal rhesus macaques are proliferating, activated memory cells and primary targets for SIVMAC251 infection. Blood 112:4981–4990. 10.1182/blood-2008-05-160077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pahar B, Amedee AM, Thomas J, Dufour JP, Zhang P, Nelson S, Veazey RS, Bagby GJ. 2013. Effects of alcohol consumption on antigen-specific cellular and humoral immune responses to SIV in rhesus macaques. J. Acquir. Immune Defic. Syndr. 64:332–341. 10.1097/QAI.0b013e31829f6dca [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Teigler JE, Iampietro MJ, Barouch DH. 2012. Vaccination with adenovirus serotypes 35, 26, and 48 elicits higher levels of innate cytokine responses than adenovirus serotype 5 in rhesus monkeys. J. Virol. 86:9590–9598. 10.1128/JVI.00740-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Didier ES, Sugimoto C, Bowers LC, Khan IA, Kuroda MJ. 2012. Immune correlates of aging in outdoor-housed captive rhesus macaques (Macaca mulatta). Immun. Ageing 9:25. 10.1186/1742-4933-9-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Asquith M, Pasala S, Engelmann F, Haberthur K, Meyer C, Park B, Grant KA, Messaoudi I. 2014. Chronic ethanol consumption modulates growth factor release, mucosal cytokine production, and microRNA expression in nonhuman primates. Alcohol. Clin. Exp. Res. 38:980–993. 10.1111/acer.12325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yin J, Vahey MT, Dai A, Lewis MG, Arango T, Yalley-Ogunro J, Greenhouse J, Mendoza K, Khan A, Sardesai NY, Weiss W, Komisar J, Boyer JD. 2012. Plasmodium inui infection reduces the efficacy of a simian immunodeficiency virus DNA vaccine in a rhesus macaque model through alteration of the vaccine-induced immune response. J. Infect. Dis. 206:523–533. 10.1093/infdis/jis404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goode D, Aravantinou M, Jarl S, Truong R, Derby N, Guerra-Perez N, Kenney J, Blanchard J, Gettie A, Robbiani M, Martinelli E. 2014. Sex hormones selectively impact the endocervical mucosal microenvironment: implications for HIV transmission. PLoS One 9(5):e97767. 10.1371/journal.pone.0097767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramesh G, Benge S, Pahar B, Philipp MT. 2012. A possible role for inflammation in mediating apoptosis of oligodendrocytes as induced by the Lyme disease spirochete Borrelia burgdorferi. J. Neuroinflammation 9:72. 10.1186/1742-2094-9-72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Intlekofer AM, Thompson CB. 2013. At the bench: preclinical rationale for CTLA-4 and PD-1 blockade as cancer immunotherapy. J. Leukoc. Biol. 94:25–39. 10.1189/jlb.1212621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hong JJ, Amancha PK, Rogers K, Ansari AA, Villinger F. 2013. Re-evaluation of PD-1 expression by T cells as a marker for immune exhaustion during SIV infection. PLoS One 8(3):e60186. 10.1371/journal.pone.0060186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakanjako D, Ssewanyana I, Mayanja-Kizza H, Kiragga A, Colebunders R, Manabe YC, Nabatanzi R, Kamya MR, Cao H. 2011. High T-cell immune activation and immune exhaustion among individuals with suboptimal CD4 recovery after 4 years of antiretroviral therapy in an African cohort. BMC Infect. Dis. 11:43. 10.1186/1471-2334-11-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C, Mncube Z, Duraiswamy J, Zhu B, Eichbaum Q, Altfeld M, Wherry EJ, Coovadia HM, Goulder PJ, Klenerman P, Ahmed R, Freeman GJ, Walker BD. 2006. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature 443:350–354. 10.1038/nature05115 [DOI] [PubMed] [Google Scholar]
- 40.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. 2006. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 439:682–687. 10.1038/nature04444 [DOI] [PubMed] [Google Scholar]
- 41.Giorgi JV, Liu Z, Hultin LE, Cumberland WG, Hennessey K, Detels R. 1993. Elevated levels of CD38+ CD8+ T cells in HIV infection add to the prognostic value of low CD4+ T cell levels: results of 6 years of follow-up. The Los Angeles Center, Multicenter AIDS Cohort study. J. Acquir. Immune Defic. Syndr. 6:904–912 [PubMed] [Google Scholar]
- 42.Schwartz RH. 1990. A cell culture model for T lymphocyte clonal anergy. Science 248:1349–1356. 10.1126/science.2113314 [DOI] [PubMed] [Google Scholar]
- 43.Yang LP, Byun DG, Demeure CE, Vezzio N, Delespesse G. 1995. Default development of cloned human naive CD4 T cells into interleukin-4- and interleukin-5-producing effector cells. Eur. J. Immunol. 25:3517–3520. 10.1002/eji.1830251247 [DOI] [PubMed] [Google Scholar]
- 44.Duffy D, Rouilly V, Libri V, Hasan M, Beitz B, David M, Urrutia A, Bisiaux A, Labrie ST, Dubois A, Boneca IG, Delval C, Thomas S, Rogge L, Schmolz M, Quintana-Murci L, Albert ML, Milieu Interieur C. 2014. Functional analysis via standardized whole-blood stimulation systems defines the boundaries of a healthy immune response to complex stimuli. Immunity 40:436–450. 10.1016/j.immuni.2014.03.002 [DOI] [PubMed] [Google Scholar]
- 45.Fleischer B, Schrezenmeier H. 1988. T cell stimulation by staphylococcal enterotoxins. Clonally variable response and requirement for major histocompatibility complex class II molecules on accessory or target cells. J. Exp. Med. 167:1697–1707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smith-Garvin JE, Koretzky GA, Jordan MS. 2009. T cell activation. Annu. Rev. Immunol. 27:591–619. 10.1146/annurev.immunol.021908.132706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McClary H, Koch R, Chisari FV, Guidotti LG. 2000. Relative sensitivity of hepatitis B virus and other hepatotropic viruses to the antiviral effects of cytokines. J. Virol. 74:2255–2264. 10.1128/JVI.74.5.2255-2264.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oldstone MB. 2013. Lessons learned and concepts formed from study of the pathogenesis of the two negative-strand viruses lymphocytic choriomeningitis and influenza. Proc. Natl. Acad. Sci. U. S. A. 110:4180–4183. 10.1073/pnas.1222025110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mosmann TR, Sad S. 1996. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol. Today 17:138–146. 10.1016/0167-5699(96)80606-2 [DOI] [PubMed] [Google Scholar]
- 50.Freel SA, Lamoreaux L, Chattopadhyay PK, Saunders K, Zarkowsky D, Overman RG, Ochsenbauer C, Edmonds TG, Kappes JC, Cunningham CK, Denny TN, Weinhold KJ, Ferrari G, Haynes BF, Koup RA, Graham BS, Roederer M, Tomaras GD. 2010. Phenotypic and functional profile of HIV-inhibitory CD8 T cells elicited by natural infection and heterologous prime/boost vaccination. J. Virol. 84:4998–5006. 10.1128/JVI.00138-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Freel SA, Picking RA, Ferrari G, Ding H, Ochsenbauer C, Kappes JC, Kirchherr JL, Soderberg KA, Weinhold KJ, Cunningham CK, Denny TN, Crump JA, Cohen MS, McMichael AJ, Haynes BF, Tomaras GD. 2012. Initial HIV-1 antigen-specific CD8+ T cells in acute HIV-1 infection inhibit transmitted/founder virus replication. J. Virol. 86:6835–6846. 10.1128/JVI.00437-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Regis EG, Barreto-de-Souza V, Morgado MG, Bozza MT, Leng L, Bucala R, Bou-Habib DC. 2010. Elevated levels of macrophage migration inhibitory factor (MIF) in the plasma of HIV-1-infected patients and in HIV-1-infected cell cultures: a relevant role on viral replication. Virology 399:31–38. 10.1016/j.virol.2009.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dinarello CA. 1991. Interleukin-1 and interleukin-1 antagonism. Blood 77:1627–1652 [PubMed] [Google Scholar]
- 54.Louie S, Cai J, Law R, Lin G, Lunardi-Iskandar Y, Jung B, Masood R, Gill P. 1995. Effects of interleukin-1 and interleukin-1 receptor antagonist in AIDS-Kaposi's sarcoma. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 8:455–460. 10.1097/00042560-199504120-00004 [DOI] [PubMed] [Google Scholar]
- 55.Poli G, Kinter AL, Fauci AS. 1994. Interleukin 1 induces expression of the human immunodeficiency virus alone and in synergy with interleukin 6 in chronically infected U1 cells: inhibition of inductive effects by the interleukin 1 receptor antagonist. Proc. Natl. Acad. Sci. U. S. A. 91:108–112. 10.1073/pnas.91.1.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zavala F, Rimaniol AC, Boussin F, Dormont D, Bach JF, Descamps-Latscha B. 1995. HIV predominantly induces IL-1 receptor antagonist over IL-1 synthesis in human primary monocytes. J. Immunol. 155:2784–2793 [PubMed] [Google Scholar]
- 57.Seckinger P, Lowenthal JW, Williamson K, Dayer JM, Macdonald HR. 1987. A urine inhibitor of interleukin 1 activity that blocks ligand binding. J. Immunol. 139:1546–1549 [PubMed] [Google Scholar]
- 58.Seckinger P, Williamson K, Balavoine JF, Mach B, Mazzei G, Shaw A, Dayer JM. 1987. A urine inhibitor of interleukin 1 activity affects both interleukin 1 alpha and 1 beta but not tumor necrosis factor alpha. J. Immunol. 139:1541–1545 [PubMed] [Google Scholar]
- 59.Arend WP, Welgus HG, Thompson RC, Eisenberg SP. 1990. Biological properties of recombinant human monocyte-derived interleukin 1 receptor antagonist. J. Clin. Invest. 85:1694–1697. 10.1172/JCI114622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Arend WP. 2002. The balance between IL-1 and IL-1Ra in disease. Cytokine Growth Factor Rev. 13:323–340. 10.1016/S1359-6101(02)00020-5 [DOI] [PubMed] [Google Scholar]
- 61.Cocchi F, Devico AL, Garzinodemo A, Arya SK, Gallo RC, Lusso P. 1995. Identification of RANTES, Mip-1-alpha, and Mip-1-beta as the major HIV-suppressive factors produced by Cd8+ T cells. Science 270:1811–1815. 10.1126/science.270.5243.1811 [DOI] [PubMed] [Google Scholar]
- 62.Ullum H, Lepri Alessandro C, Victor J, Aladdin H, Phillips Andrew N, Gerstoft J, Skinhoj P, Pedersen Bente K. 1998. Production of β-chemokines in human immunodeficiency virus (HIV) infection: evidence that high levels of macrophage inflammatory protein-1β are associated with a decreased risk of HIV disease progression. J. Infect. Dis. 177:331–336. 10.1086/514192 [DOI] [PubMed] [Google Scholar]
- 63.Paxton WA, Liu R, Kang S, Wu L, Gingeras TR, Landau NR, Mackay CR, Koup RA. 1998. Reduced HIV-1 infectability of CD4+ lymphocytes from exposed-uninfected individuals: association with low expression of CCR5 and high production of beta-chemokines. Virology 244:66–73. 10.1006/viro.1998.9082 [DOI] [PubMed] [Google Scholar]
- 64.Zagury D, Lachgar A, Chams V, Fall LS, Bernard J, Zagury JF, Bizzini B, Gringeri A, Santagostino E, Rappaport J, Feldman M, O'Brien SJ, Burny A, Gallo RC. 1998. C-C chemokines, pivotal in protection against HIV type 1 infection. Proc. Natl. Acad. Sci. U. S. A. 95:3857–3861. 10.1073/pnas.95.7.3857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lambert JS, Machado ES, Watson DC, Sill AM, Lim JK, Charurat M, Cunha SM, Afonso AO, Oliviera RH, Tanuri A, DeVico AL. 2007. Production of the HIV-suppressive chemokines CCL3/MIP-1alpha and CCL22/MDC is associated with more effective antiretroviral therapy in HIV-infected children. Pediatr. Infect. Dis. J. 26:935–944. 10.1097/INF.0b013e31812714db [DOI] [PubMed] [Google Scholar]
- 66.Alkhatib G, Locati M, Kennedy PE, Murphy PM, Berger EA. 1997. HIV-1 coreceptor activity of CCR5 and its inhibition by chemokines: independence from G protein signaling and importance of coreceptor downmodulation. Virology 234:340–348. 10.1006/viro.1997.8673 [DOI] [PubMed] [Google Scholar]
- 67.Garzino-Demo A, Moss RB, Margolick JB, Cleghorn F, Sill A, Blattner WA, Cocchi F, Carlo DJ, DeVico AL, Gallo RC. 1999. Spontaneous and antigen-induced production of HIV-inhibitory beta-chemokines are associated with AIDS-free status. Proc. Natl. Acad. Sci. U. S. A. 96:11986–11991. 10.1073/pnas.96.21.11986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ansari AW, Bhatnagar N, Dittrich-Breiholz O, Kracht M, Schmidt RE, Heiken H. 2006. Host chemokine (C-C motif) ligand-2 (CCL2) is differentially regulated in HIV type 1 (HIV-1)-infected individuals. Int. Immunol. 18:1443–1451. 10.1093/intimm/dxl078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sozzani S, Introna M, Bernasconi S, Polentarutti N, Cinque P, Poli G, Sica A, Mantovani A. 1997. MCP-1 and CCR2 in HIV infection: regulation of agonist and receptor expression. J. Leukoc. Biol. 62:30–33 [DOI] [PubMed] [Google Scholar]
- 70.LaFranco-Scheuch L, Abel K, Makori N, Rothaeusler K, Miller CJ. 2004. High beta-chemokine expression levels in lymphoid tissues of simian/human immunodeficiency virus 89.6-vaccinated rhesus macaques are associated with uncontrolled replication of simian immunodeficiency virus challenge inoculum. J. Virol. 78:6399–6408. 10.1128/JVI.78.12.6399-6408.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lane BR, King SR, Bock PJ, Strieter RM, Coffey MJ, Markovitz DM. 2003. The C-X-C chemokine IP-10 stimulates HIV-1 replication. Virology 307:122–134. 10.1016/S0042-6822(02)00045-4 [DOI] [PubMed] [Google Scholar]
- 72.Reinhart TA, Fallert BA, Pfeifer ME, Sanghavi S, Capuano S, III, Rajakumar P, Murphey-Corb M, Day R, Fuller CL, Schaefer TM. 2002. Increased expression of the inflammatory chemokine CXC chemokine ligand 9/monokine induced by interferon-gamma in lymphoid tissues of rhesus macaques during simian immunodeficiency virus infection and acquired immunodeficiency syndrome. Blood 99:3119–3128. 10.1182/blood.V99.9.3119 [DOI] [PubMed] [Google Scholar]
- 73.Abrams D, Levy Y, Losso MH, Babiker A, Collins G, Cooper DA, Darbyshire J, Emery S, Fox L, Gordin F, Lane HC, Lundgren JD, Mitsuyasu R, Neaton JD, Phillips A, Routy JP, Tambussi G, Wentworth D. 2009. Interleukin-2 therapy in patients with HIV infection. N. Engl. J. Med. 361:1548–1559. 10.1056/NEJMoa0903175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ascherl G, Sgadari C, Bugarini R, Bogner J, Schatz O, Ensoli B, Sturzl M. 2001. Serum concentrations of fibroblast growth factor 2 are increased in HIV type 1-infected patients and inversely related to survival probability. AIDS Res. Hum. Retroviruses 17:1035–1039. 10.1089/088922201300343717 [DOI] [PubMed] [Google Scholar]
- 75.Maier JA, Mariotti M, Albini A, Comi P, Prat M, Comogilio PM, Soria MR. 1996. Over-expression of hepatocyte growth factor in human Kaposi's sarcoma. Int. J. Cancer 65:168–172. [DOI] [PubMed] [Google Scholar]
- 76.Haddad D, Ramprakash J, Sedegah M, Charoenvit Y, Baumgartner R, Kumar S, Hoffman SL, Weiss WR. 2000. Plasmid vaccine expressing granulocyte-macrophage colony-stimulating factor attracts infiltrates including immature dendritic cells into injected muscles. J. Immunol. 165:3772–3781. 10.4049/jimmunol.165.7.3772 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.