Abstract
Chlorophyll accumulation and photosynthetic gene activation are two hallmarks of greening process in etiolated maize leaves in response to light signals. However, very little is known about the relevant signal transduction pathways mediating these essential processes that lead to photosynthetic competence. It is shown here that a potent and specific protein phosphatase 1 (PP1) and PP2A inhibitor, okadaic acid, efficiently blocks chlorophyll accumulation induced by light in etiolated maize leaves. In addition, the light-inducible expression of two photosynthetic fusion genes can be specifically suppressed by the structurally unrelated PP1 and PP2A inhibitors, okadaic acid and calyculin A, using a sensitive and physiological maize protoplast transient assay. The specificity and effective concentration of the inhibitors in vivo and in vitro strongly suggest that PP1 is required for transmitting light signals. Intriguingly, several partial cDNAs encoding novel as well as conserved PP1 can be identified in maize leaves using the polymerase chain reaction. Studies of chimeric promoters indicate that PP1 activity is essential for the interaction of multiple regulatory elements. Although PP1 and PP2A have been implicated in the suppression of gene activity in yeast and animals, the present data indicate that PP1 appears to be essential for light-dependent gene activation in plants.
Full text
PDF
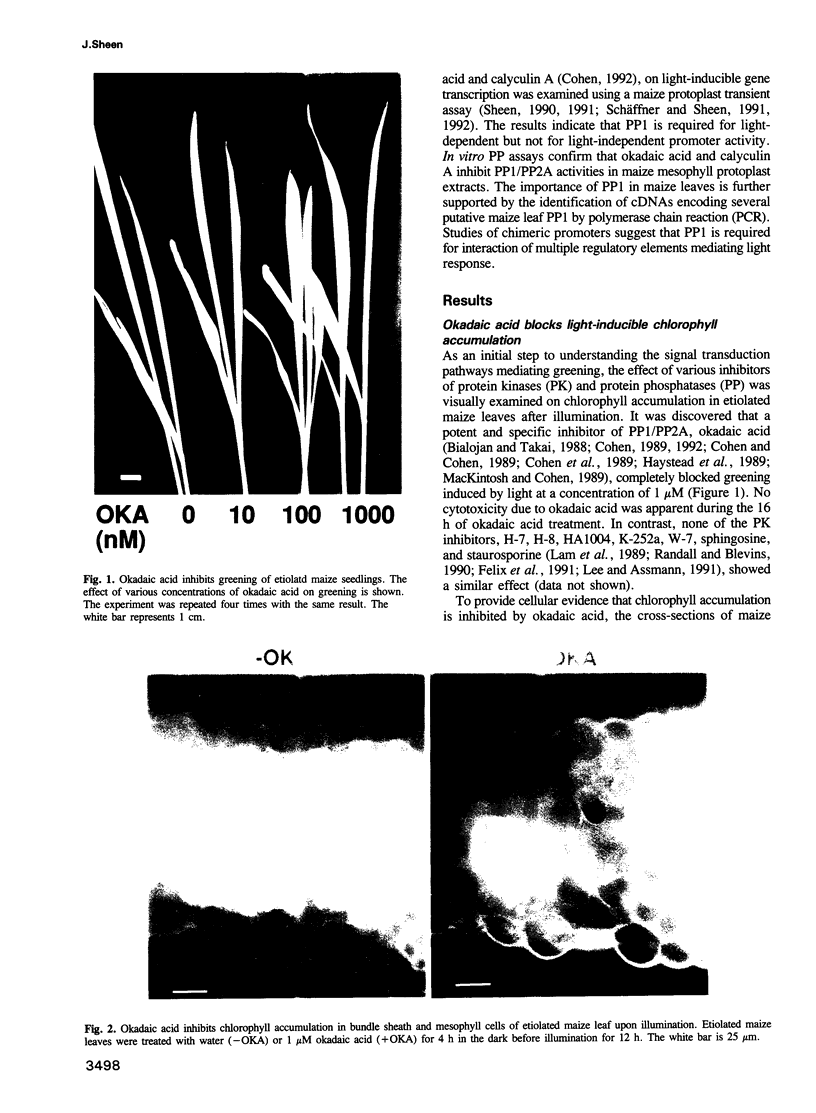
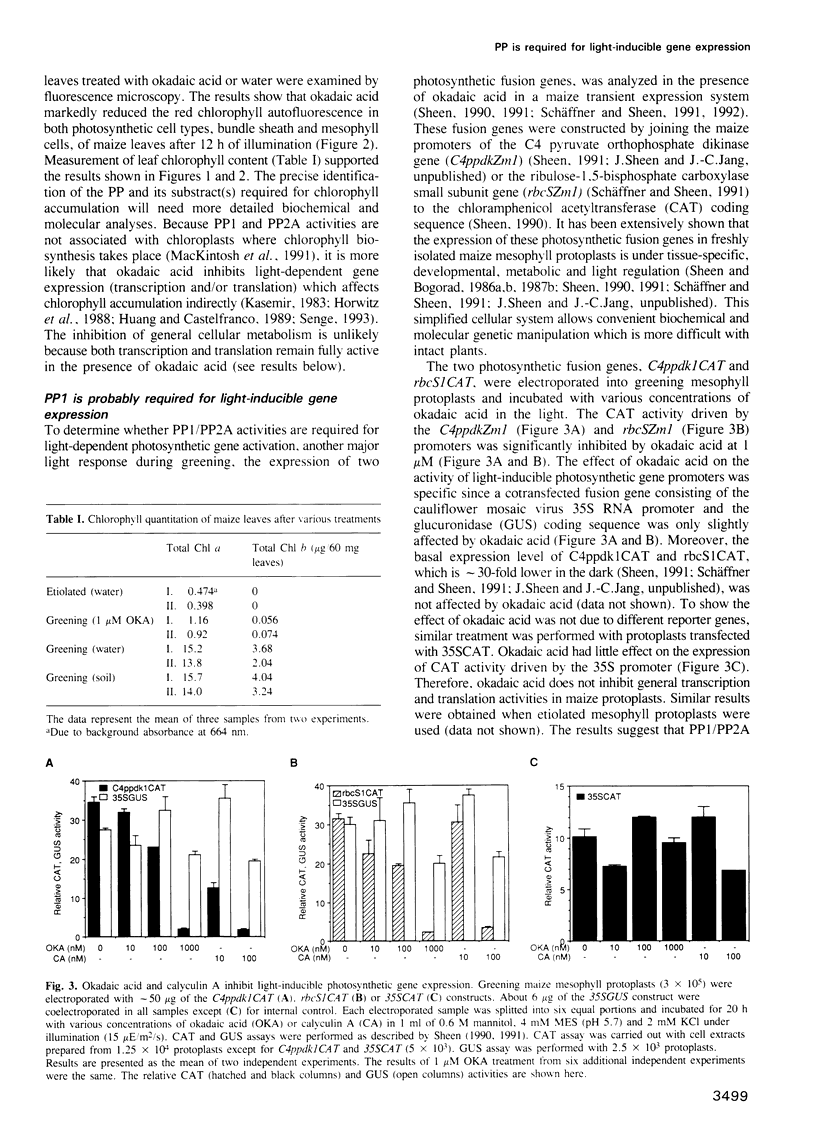






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arndt K. T., Styles C. A., Fink G. R. A suppressor of a HIS4 transcriptional defect encodes a protein with homology to the catalytic subunit of protein phosphatases. Cell. 1989 Feb 24;56(4):527–537. doi: 10.1016/0092-8674(89)90576-x. [DOI] [PubMed] [Google Scholar]
- Axton J. M., Dombrádi V., Cohen P. T., Glover D. M. One of the protein phosphatase 1 isoenzymes in Drosophila is essential for mitosis. Cell. 1990 Oct 5;63(1):33–46. doi: 10.1016/0092-8674(90)90286-n. [DOI] [PubMed] [Google Scholar]
- Bialojan C., Takai A. Inhibitory effect of a marine-sponge toxin, okadaic acid, on protein phosphatases. Specificity and kinetics. Biochem J. 1988 Nov 15;256(1):283–290. doi: 10.1042/bj2560283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budde R. J., Randall D. D. Light as a signal influencing the phosphorylation status of plant proteins. Plant Physiol. 1990 Dec;94(4):1501–1504. doi: 10.1104/pp.94.4.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzby J. S., Yamada T., Tobin E. M. A light-regulated DNA-binding activity interacts with a conserved region of a Lemna gibba rbcS promoter. Plant Cell. 1990 Aug;2(8):805–814. doi: 10.1105/tpc.2.8.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey M. Mechanistic advances in eukaryotic gene activation. Curr Opin Cell Biol. 1991 Jun;3(3):452–460. doi: 10.1016/0955-0674(91)90073-8. [DOI] [PubMed] [Google Scholar]
- Chory J. Light signals in leaf and chloroplast development: photoreceptors and downstream responses in search of a transduction pathway. New Biol. 1991 Jun;3(6):538–548. [PubMed] [Google Scholar]
- Cohen P. T., Brewis N. D., Hughes V., Mann D. J. Protein serine/threonine phosphatases; an expanding family. FEBS Lett. 1990 Aug 1;268(2):355–359. doi: 10.1016/0014-5793(90)81285-v. [DOI] [PubMed] [Google Scholar]
- Cohen P., Cohen P. T. Protein phosphatases come of age. J Biol Chem. 1989 Dec 25;264(36):21435–21438. [PubMed] [Google Scholar]
- Cohen P., Holmes C. F., Tsukitani Y. Okadaic acid: a new probe for the study of cellular regulation. Trends Biochem Sci. 1990 Mar;15(3):98–102. doi: 10.1016/0968-0004(90)90192-e. [DOI] [PubMed] [Google Scholar]
- Cohen P., Schelling D. L., Stark M. J. Remarkable similarities between yeast and mammalian protein phosphatases. FEBS Lett. 1989 Jul 3;250(2):601–606. doi: 10.1016/0014-5793(89)80804-x. [DOI] [PubMed] [Google Scholar]
- Cohen P. Signal integration at the level of protein kinases, protein phosphatases and their substrates. Trends Biochem Sci. 1992 Oct;17(10):408–413. doi: 10.1016/0968-0004(92)90010-7. [DOI] [PubMed] [Google Scholar]
- Cohen P. The structure and regulation of protein phosphatases. Annu Rev Biochem. 1989;58:453–508. doi: 10.1146/annurev.bi.58.070189.002321. [DOI] [PubMed] [Google Scholar]
- Datta N., Cashmore A. R. Binding of a pea nuclear protein to promoters of certain photoregulated genes is modulated by phosphorylation. Plant Cell. 1989 Nov;1(11):1069–1077. doi: 10.1105/tpc.1.11.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehesh K., Bruce W. B., Quail P. H. A trans-acting factor that binds to a GT-motif in a phytochrome gene promoter. Science. 1990 Dec 7;250(4986):1397–1399. doi: 10.1126/science.2255908. [DOI] [PubMed] [Google Scholar]
- Deng X. W., Caspar T., Quail P. H. cop1: a regulatory locus involved in light-controlled development and gene expression in Arabidopsis. Genes Dev. 1991 Jul;5(7):1172–1182. doi: 10.1101/gad.5.7.1172. [DOI] [PubMed] [Google Scholar]
- Deng X. W., Matsui M., Wei N., Wagner D., Chu A. M., Feldmann K. A., Quail P. H. COP1, an Arabidopsis regulatory gene, encodes a protein with both a zinc-binding motif and a G beta homologous domain. Cell. 1992 Nov 27;71(5):791–801. doi: 10.1016/0092-8674(92)90555-q. [DOI] [PubMed] [Google Scholar]
- Dominov J. A., Stenzler L., Lee S., Schwarz J. J., Leisner S., Howell S. H. Cytokinins and auxins control the expression of a gene in Nicotiana plumbaginifolia cells by feedback regulation. Plant Cell. 1992 Apr;4(4):451–461. doi: 10.1105/tpc.4.4.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman A. M., Blumenthal D. K., Krebs E. G. Protein serine/threonine kinases. Annu Rev Biochem. 1987;56:567–613. doi: 10.1146/annurev.bi.56.070187.003031. [DOI] [PubMed] [Google Scholar]
- Felix G., Grosskopf D. G., Regenass M., Boller T. Rapid changes of protein phosphorylation are involved in transduction of the elicitor signal in plant cells. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8831–8834. doi: 10.1073/pnas.88.19.8831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frances S., White M. J., Edgerton M. D., Jones A. M., Elliott R. C., Thompson W. F. Initial characterization of a pea mutant with light-independent photomorphogenesis. Plant Cell. 1992 Dec;4(12):1519–1530. doi: 10.1105/tpc.4.12.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmartin P. M., Memelink J., Hiratsuka K., Kay S. A., Chua N. H. Characterization of a gene encoding a DNA binding protein with specificity for a light-responsive element. Plant Cell. 1992 Jul;4(7):839–849. doi: 10.1105/tpc.4.7.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmartin P. M., Sarokin L., Memelink J., Chua N. H. Molecular light switches for plant genes. Plant Cell. 1990 May;2(5):369–378. doi: 10.1105/tpc.2.5.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruissem W. Chloroplast gene expression: how plants turn their plastids on. Cell. 1989 Jan 27;56(2):161–170. doi: 10.1016/0092-8674(89)90889-1. [DOI] [PubMed] [Google Scholar]
- Hagiwara M., Alberts A., Brindle P., Meinkoth J., Feramisco J., Deng T., Karin M., Shenolikar S., Montminy M. Transcriptional attenuation following cAMP induction requires PP-1-mediated dephosphorylation of CREB. Cell. 1992 Jul 10;70(1):105–113. doi: 10.1016/0092-8674(92)90537-m. [DOI] [PubMed] [Google Scholar]
- Haystead T. A., Sim A. T., Carling D., Honnor R. C., Tsukitani Y., Cohen P., Hardie D. G. Effects of the tumour promoter okadaic acid on intracellular protein phosphorylation and metabolism. Nature. 1989 Jan 5;337(6202):78–81. doi: 10.1038/337078a0. [DOI] [PubMed] [Google Scholar]
- Horwitz B. A., Thompson W. F., Briggs W. R. Phytochrome Regulation of Greening in Pisum: Chlorophyll Accumulation and Abundance of mRNA for the Light-Harvesting Chlorophyll a/b Binding Proteins. Plant Physiol. 1988 Jan;86(1):299–305. doi: 10.1104/pp.86.1.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L., Castelfranco P. A. Regulation of 5-aminolevulinic Acid synthesis in developing chloroplasts : I. Effect of light/dark treatments in vivo and in organello. Plant Physiol. 1989 Jul;90(3):996–1002. doi: 10.1104/pp.90.3.996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T., Karin M. The regulation of transcription by phosphorylation. Cell. 1992 Aug 7;70(3):375–387. doi: 10.1016/0092-8674(92)90162-6. [DOI] [PubMed] [Google Scholar]
- Juszczak R. J., Russell J. H. Inhibition of cytotoxic T lymphocyte-mediated lysis and cellular proliferation by isoquinoline sulfonamide protein kinase inhibitors. Evidence for the involvement of protein kinase C in lymphocyte function. J Biol Chem. 1989 Jan 15;264(2):810–815. [PubMed] [Google Scholar]
- Karlin-Neumann G. A., Brusslan J. A., Tobin E. M. Phytochrome control of the tms2 gene in transgenic Arabidopsis: a strategy for selecting mutants in the signal transduction pathway. Plant Cell. 1991 Jun;3(6):573–582. doi: 10.1105/tpc.3.6.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katagiri F., Chua N. H. Plant transcription factors: present knowledge and future challenges. Trends Genet. 1992 Jan;8(1):22–27. doi: 10.1016/0168-9525(92)90020-5. [DOI] [PubMed] [Google Scholar]
- Kennelly P. J., Krebs E. G. Consensus sequences as substrate specificity determinants for protein kinases and protein phosphatases. J Biol Chem. 1991 Aug 25;266(24):15555–15558. [PubMed] [Google Scholar]
- Kessler D. S., Levy D. E. Protein kinase activity required for an early step in interferon-alpha signaling. J Biol Chem. 1991 Dec 5;266(34):23471–23476. [PubMed] [Google Scholar]
- Kim S. J., Kim K. Y., Tapscott S. J., Winokur T. S., Park K., Fujiki H., Weintraub H., Roberts A. B. Inhibition of protein phosphatases blocks myogenesis by first altering MyoD binding activity. J Biol Chem. 1992 Jul 25;267(21):15140–15145. [PubMed] [Google Scholar]
- Klimczak L. J., Schindler U., Cashmore A. R. DNA binding activity of the Arabidopsis G-box binding factor GBF1 is stimulated by phosphorylation by casein kinase II from broccoli. Plant Cell. 1992 Jan;4(1):87–98. doi: 10.1105/tpc.4.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam E., Benedyk M., Chua N. H. Characterization of phytochrome-regulated gene expression in a photoautotrophic cell suspension: possible role for calmodulin. Mol Cell Biol. 1989 Nov;9(11):4819–4823. doi: 10.1128/mcb.9.11.4819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y., Assmann S. M. Diacylglycerols induce both ion pumping in patch-clamped guard-cell protoplasts and opening of intact stomata. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2127–2131. doi: 10.1073/pnas.88.6.2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liscum E., Hangarter R. P. Arabidopsis Mutants Lacking Blue Light-Dependent Inhibition of Hypocotyl Elongation. Plant Cell. 1991 Jul;3(7):685–694. doi: 10.1105/tpc.3.7.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKintosh C., Coggins J., Cohen P. Plant protein phosphatases. Subcellular distribution, detection of protein phosphatase 2C and identification of protein phosphatase 2A as the major quinate dehydrogenase phosphatase. Biochem J. 1991 Feb 1;273(Pt 3):733–738. doi: 10.1042/bj2730733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKintosh C., Cohen P. Identification of high levels of type 1 and type 2A protein phosphatases in higher plants. Biochem J. 1989 Aug 15;262(1):335–339. doi: 10.1042/bj2620335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKintosh R. W., Haycox G., Hardie D. G., Cohen P. T. Identification by molecular cloning of two cDNA sequences from the plant Brassica napus which are very similar to mammalian protein phosphatases-1 and -2A. FEBS Lett. 1990 Dec 10;276(1-2):156–160. doi: 10.1016/0014-5793(90)80531-m. [DOI] [PubMed] [Google Scholar]
- Manzara T., Carrasco P., Gruissem W. Developmental and organ-specific changes in promoter DNA-protein interactions in the tomato rbcS gene family. Plant Cell. 1991 Dec;3(12):1305–1316. doi: 10.1105/tpc.3.12.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran R. Formulae for determination of chlorophyllous pigments extracted with n,n-dimethylformamide. Plant Physiol. 1982 Jun;69(6):1376–1381. doi: 10.1104/pp.69.6.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima K., Wall R. Interleukin-6 signals activating junB and TIS11 gene transcription in a B-cell hybridoma. Mol Cell Biol. 1991 Mar;11(3):1409–1418. doi: 10.1128/mcb.11.3.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitschke K., Fleig U., Schell J., Palme K. Complementation of the cs dis2-11 cell cycle mutant of Schizosaccharomyces pombe by a protein phosphatase from Arabidopsis thaliana. EMBO J. 1992 Apr;11(4):1327–1333. doi: 10.1002/j.1460-2075.1992.tb05177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perisic O., Lam E. A tobacco DNA binding protein that interacts with a light-responsive box II element. Plant Cell. 1992 Jul;4(7):831–838. doi: 10.1105/tpc.4.7.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quail P. H. Phytochrome: a light-activated molecular switch that regulates plant gene expression. Annu Rev Genet. 1991;25:389–409. doi: 10.1146/annurev.ge.25.120191.002133. [DOI] [PubMed] [Google Scholar]
- Rodermel S. R., Bogorad L. Maize plastid photogenes: mapping and photoregulation of transcript levels during light-induced development. J Cell Biol. 1985 Feb;100(2):463–476. doi: 10.1083/jcb.100.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rundle S. J., Nasrallah J. B. Molecular characterization of type 1 serine/threonine phosphatases from Brassica oleracea. Plant Mol Biol. 1992 Nov;20(3):367–375. doi: 10.1007/BF00040596. [DOI] [PubMed] [Google Scholar]
- Sarokin L. P., Chua N. H. Binding sites for two novel phosphoproteins, 3AF5 and 3AF3, are required for rbcS-3A expression. Plant Cell. 1992 Apr;4(4):473–483. doi: 10.1105/tpc.4.4.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler U., Cashmore A. R. Photoregulated gene expression may involve ubiquitous DNA binding proteins. EMBO J. 1990 Nov;9(11):3415–3427. doi: 10.1002/j.1460-2075.1990.tb07549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler U., Menkens A. E., Beckmann H., Ecker J. R., Cashmore A. R. Heterodimerization between light-regulated and ubiquitously expressed Arabidopsis GBF bZIP proteins. EMBO J. 1992 Apr;11(4):1261–1273. doi: 10.1002/j.1460-2075.1992.tb05170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber S. L. Immunophilin-sensitive protein phosphatase action in cell signaling pathways. Cell. 1992 Aug 7;70(3):365–368. doi: 10.1016/0092-8674(92)90158-9. [DOI] [PubMed] [Google Scholar]
- Schäffner A. R., Sheen J. Maize C4 photosynthesis involves differential regulation of phosphoenolpyruvate carboxylase genes. Plant J. 1992 Mar;2(2):221–232. doi: 10.1046/j.1365-313x.1992.t01-44-00999.x. [DOI] [PubMed] [Google Scholar]
- Schäffner A. R., Sheen J. Maize rbcS promoter activity depends on sequence elements not found in dicot rbcS promoters. Plant Cell. 1991 Sep;3(9):997–1012. doi: 10.1105/tpc.3.9.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schönthal A., Alberts A. S., Frost J. A., Feramisco J. R. Differential regulation of jun family gene expression by the tumor promoter okadaic acid. New Biol. 1991 Oct;3(10):977–986. [PubMed] [Google Scholar]
- Schüle R., Muller M., Kaltschmidt C., Renkawitz R. Many transcription factors interact synergistically with steroid receptors. Science. 1988 Dec 9;242(4884):1418–1420. doi: 10.1126/science.3201230. [DOI] [PubMed] [Google Scholar]
- Sheen J. Y., Bogorad L. Differential expression of C4 pathway genes in mesophyll and bundle sheath cells of greening maize leaves. J Biol Chem. 1987 Aug 25;262(24):11726–11730. [PubMed] [Google Scholar]
- Sheen J. Y., Bogorad L. Differential expression of six light-harvesting chlorophyll a/b binding protein genes in maize leaf cell types. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7811–7815. doi: 10.1073/pnas.83.20.7811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheen J. Y., Bogorad L. Expression of the ribulose-1,5-bisphosphate carboxylase large subunit gene and three small subunit genes in two cell types of maize leaves. EMBO J. 1986 Dec 20;5(13):3417–3422. doi: 10.1002/j.1460-2075.1986.tb04663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheen J. Metabolic repression of transcription in higher plants. Plant Cell. 1990 Oct;2(10):1027–1038. doi: 10.1105/tpc.2.10.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheen J. Molecular mechanisms underlying the differential expression of maize pyruvate, orthophosphate dikinase genes. Plant Cell. 1991 Mar;3(3):225–245. doi: 10.1105/tpc.3.3.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegl G., MacKintosh C., Stitt M. Sucrose-phosphate synthase is dephosphorylated by protein phosphatase 2A in spinach leaves. Evidence from the effects of okadaic acid and microcystin. FEBS Lett. 1990 Sep 17;270(1-2):198–202. doi: 10.1016/0014-5793(90)81267-r. [DOI] [PubMed] [Google Scholar]
- Smith R. D., Walker J. C. Expression of multiple type 1 phosphoprotein phosphatases in Arabidopsis thaliana. Plant Mol Biol. 1993 Jan;21(2):307–316. doi: 10.1007/BF00019946. [DOI] [PubMed] [Google Scholar]
- Smith R. D., Walker J. C. Isolation and expression of a maize type 1 protein phosphatase. Plant Physiol. 1991 Oct;97(2):677–683. doi: 10.1104/pp.97.2.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L., Doxsee R. A., Harel E., Tobin E. M. CA-1, a novel phosphoprotein, interacts with the promoter of the cab140 gene in Arabidopsis and is undetectable in det1 mutant seedlings. Plant Cell. 1993 Jan;5(1):109–121. doi: 10.1105/tpc.5.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trewavas A., Gilroy S. Signal transduction in plant cells. Trends Genet. 1991 Nov-Dec;7(11-12):356–361. doi: 10.1016/0168-9525(91)90255-o. [DOI] [PubMed] [Google Scholar]
- Wang W. D., Gralla J. D. Differential ability of proximal and remote element pairs to cooperate in activating RNA polymerase II transcription. Mol Cell Biol. 1991 Sep;11(9):4561–4571. doi: 10.1128/mcb.11.9.4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisshaar B., Armstrong G. A., Block A., da Costa e Silva O., Hahlbrock K. Light-inducible and constitutively expressed DNA-binding proteins recognizing a plant promoter element with functional relevance in light responsiveness. EMBO J. 1991 Jul;10(7):1777–1786. doi: 10.1002/j.1460-2075.1991.tb07702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]







