Abstract
We assessed several routes of immunization with vaccinia virus (VACV) in protecting mice against ectromelia virus (ECTV). By a wide margin, skin scarification provided the greatest protection. Humoral immunity and resident-memory T cells notwithstanding, several approaches revealed that circulating, memory CD8+ T cells primed via scarification were functionally superior and conferred enhanced virus control. Immunization via the epithelial route warrants further investigation, as it may also provide enhanced defense against other infectious agents.
TEXT
Analogous to the protection of humans against smallpox, vaccinia virus (VACV) protects mice against ectromelia virus (ECTV; “mousepox”) (1–4). In this study, we examined whether the route of immunization with VACV (strain Western Reserve; 1 × 106 PFU dose) influences the generation of protective immunity in BALB/c mice (females, 6 to 8 weeks old). The following vaccination routes were chosen: skin scarification (s.s.), intraperitoneal (i.p.) inoculation, or subcutaneous (s.c.) injection.
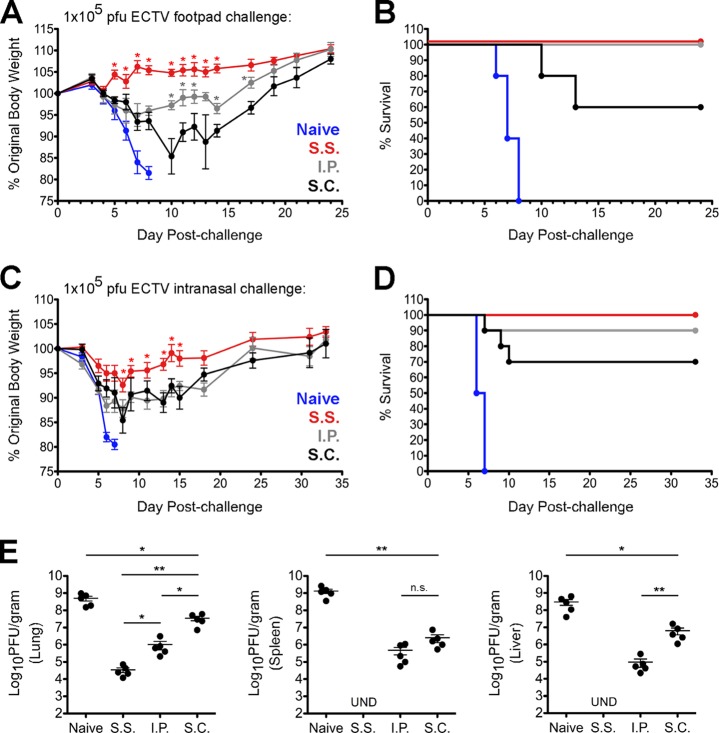
Recent studies have shown that epithelial infection elicits skin resident-memory T cells (TRM) that are highly effective at controlling a homologous, cutaneous virus challenge (5–7). Consequently, we hypothesized that the s.s. vaccination group (s.s. mice) would most effectively control a dermal (i.e., footpad [f.p.]) ECTV (Moscow strain) challenge given 30 days postimmunization. In contrast to the other groups, s.s. mice showed no signs of morbidity (Fig. 1A) or mortality (Fig. 1B) following a footpad infection with high-dose ECTV (1 × 105 PFU).
FIG 1.
Scarification of VACV elicits optimal control of ECTV regardless of challenge route. (A and B) Groups of naive or vaccinated mice (5 per group) were challenged with 1 × 105 PFU of ECTV in the left hind footpad and subsequently monitored for weight loss (A) and mortality (B). (C and D) Groups of naive or vaccinated mice (10 per group) were challenged with 1 × 105 PFU of ECTV via the i.n. route and subsequently monitored for weight loss (C) and mortality (D). (E) Separate cohorts of vaccinated mice (5 per group) were infected with 1 × 105 PFU of ECTV via the i.n. route. On day 7 postchallenge, the indicated organs were isolated and levels of ECTV were quantified using standard plaque assays. These data are representative of two independent experiments. UND, undetectable. *, P value < 0.05; **, P value < 0.01. Statistical analysis was performed using GraphPad Prism. Error bars represent the mean and standard error of the mean. (A to E) For i.p. and s.c. injections, the virus inoculum was given in a total volume of 100 μl of 1× PBS. Scarification was performed at the base of the tail using a 27-gauge needle and a 10-μl drop of VACV in 1× PBS.
To determine whether s.s. protects against ECTV infection via a heterologous route, we challenged groups of vaccinated mice (on day 30 postimmunization) via the intranasal (i.n.) route with the same dose of ECTV as described above. Although all groups experienced signs of morbidity, weight loss was significantly less severe in the s.s. group (Fig. 1C). All s.s. mice survived the i.n. challenge, but 10% of i.p. mice and 30% of s.c. mice did not (Fig. 1D). Notably, the s.s. group displayed the lowest virus titers in multiple organs at day 7 postchallenge (Fig. 1E). Additionally, none of the s.s. mice developed pock lesions, whereas some surviving animals in the i.p. and s.c. groups developed lesions on the tail or limbs (data not shown).
In general, s.s. mice were the only group of vaccinated animals in our study that failed to develop pock lesions, regardless of the route of ECTV challenge. These observations are consistent with previous reports on monkeypox infection of nonhuman primates (8–10) in which no pock lesions were observed on animals inoculated with Dryvax (Wyeth) smallpox vaccine administered by scarification. However, lesions did materialize in the context of other vaccination protocols, such as intramuscular (i.m.) injection of modified vaccinia virus Ankara (MVA) (8), that did not employ an epithelial route. Therefore, it is plausible that skin TRM, which are generated by scarification but not i.m. injection, help to prevent the appearance of lesions, which occur as a consequence of virus replication in the skin (11).
To explore the protective mechanisms provided by s.s. immunization, we assessed adaptive immune responses within each group. First, we measured VACV-specific antibody levels in each vaccination group at day 30 postimmunization. As shown in Fig. 2, vaccination via the i.p. route resulted in the greatest level of circulating antibody. Interestingly, it has been previously concluded that antibody is the sole correlate of protective immunity against secondary poxvirus challenge (10, 12–14). Given this precedent, we were surprised to observe that s.s. mice had significantly lower levels of circulating antibodies than i.p. mice. This apparent divergence from past studies (10, 12, 13) may be due to differences in dose or route of challenge. For example, it is possible that antibodies by themselves are sufficient after low-dose challenge with ECTV (12, 13), but T-cell responses become more critical as the amount of challenge inoculum increases.
FIG 2.
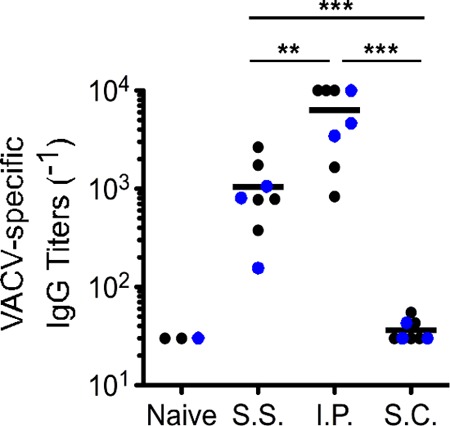
Immunization via the i.p. route yields the highest levels of circulating antipoxvirus antibodies. Plasma was isolated by retro-orbital bleeding from mice that had been immunized with VACV 30 days earlier via the indicated routes. Levels of circulating antibodies were quantified using plates coated with VACV at 1 × 106 PFU per well. Antibody titers were determined by calculating the 50% effective concentration (EC50) using nonlinear regression in GraphPad Prism (version 5.0a). Values calculated above or below the dilution range were set to 1/30 and 1/10,000, respectively. **, P value < 0.01; ***, P value < 0.001. The solid black lines represent the mean value within each group. Statistical analysis was performed using GraphPad Prism. Each data point represents one individual mouse. Black data points are from one individual experiment, and blue data points are from a second independent trial.
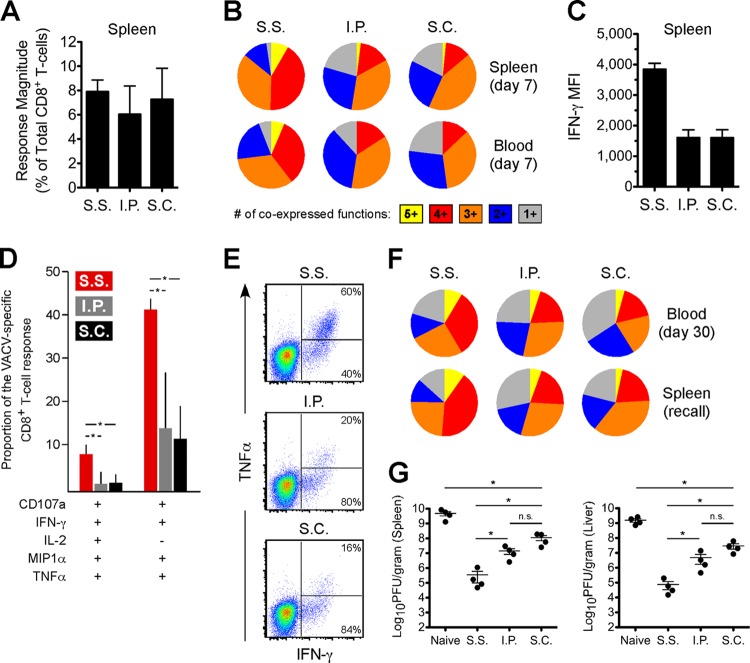
To evaluate poxvirus-specific CD8+ T-cell (TCD8+) responses, we used a pool of previously identified VACV class I epitopes (15) and measured five functional parameters (CD107a, gamma interferon [IFN-γ], interleukin 2 [IL-2], MIP1α, and tumor necrosis factor alpha [TNF-α]) by using intracellular cytokine staining (ICS) assays. These ICS assays were carried out as previously described (16, 17), and anti-CD107a was included at the start of all stimulations to measure levels of degranulation (18). Our gating strategy and Boolean analysis were similar to those employed previously (16).
At day 7 postimmunization, we identified VACV-specific TCD8+ in the spleen and blood of all groups. Despite similar magnitudes of total response between groups (Fig. 3A), VACV-specific TCD8+ from s.s. mice displayed an enhanced polyfunctional profile (Fig. 3B) and higher IFN-γ expression on a per-cell basis (Fig. 3C). The two permutations that contributed most to the observed differences were cells positive for all five functions and those positive for all measured parameters except IL-2 (Fig. 3D). There was also significantly higher coexpression of TNF-α with IFN-γ among VACV-specific TCD8+ from s.s. mice in both the spleen and blood (Fig. 3E). The higher multifunctional nature of TCD8+ from s.s. mice was maintained into the memory phase (at day 30) and also during a secondary recall response (Fig. 3F).
FIG 3.
Scarification of VACV primes highly functional TCD8+ with enhanced antiviral capacity. (A) Response magnitudes, on a percentage basis, to the MHC class I peptide pool (final concentration of each peptide, 2 μg/ml) at day 7 in the spleen (5 per group) was calculated by summing across all functional combinations. Note that the total numbers of virus-specific TCD8+ per spleen were similar across the groups as well. (B) TCD8+ responses from the spleen (5 per group) and blood (5 per group) at day 7 were divided into the relative coexpression of all parameters and grouped according to the degree of coexpression. (C) The median fluorescence intensity (MFI) of IFN-γ expression was calculated among day 7 TCD8+ responses in the spleen. (D) TCD8+ responses at day 7 in the spleen were divided into the relative contribution of each functional combination to the response as a whole. The two permutations that contributed most prominently to the higher polyfunctional response among s.s. TCD8+ are shown. Error bars represent the standard deviation. *, P value < 0.05. (E) Flow cytometric plots showing TNF-α versus IFN-γ are shown for representative TCD8+ responses in the spleen. Percentages denote the proportion of IFN-γ+ cells that are positive or negative for TNF-α. (F) The functional profiles are shown for memory TCD8+ responses in the blood (5 per group) or during a secondary recall response in the spleen 5 days (5 per group; day 35 after initial immunization) following f.p. challenge with 1 × 105 PFU of ECTV. (A to F) The data are representative of three independent experiments. (G) Bulk splenic TCD8+ were negatively selected from mice (n = 5, pooled) that had been vaccinated 30 days earlier and then transferred into naive animals. A total of 5 × 106 TCD8+ in 100 μl of sterile 1× PBS were transferred via lateral tail vein injection. Following f.p. ECTV challenge 1 day posttransfer, the indicated organs were isolated at day 7 and viral load was quantified using plaque assays. The data are representative of two independent experiments. *, P value < 0.05. Statistical analysis was performed using GraphPad Prism. Error bars represent the mean and standard error of the mean.
Prior work has demonstrated a correlation between control of HIV replication and the presence of polyfunctional TCD8+ (17, 19–21). Since TCD8+ primed by scarification displayed higher functionality, we hypothesized that TCD8+ from s.s. mice would confer greater control of ECTV in vivo. To test this, we purified total splenic TCD8+ from vaccinated mice (n = 5, pooled), transferred 5 × 106 cells (22) into naive mice, and subsequently infected them with ECTV (3 × 104 PFU; f.p. route). Stimulation of cells using the major histocompatibility complex (MHC) class I peptide pool revealed that equivalent numbers of VACV-specific TCD8+ were transferred across all groups (data not shown). As shown in Fig. 3G, mice that received TCD8+ primed by s.s. achieved the highest degree of control over ECTV, demonstrating a direct relationship between virus control and the functional capacity of antiviral TCD8+.
The ability of s.s. vaccination to generate skin TRM cells has been of recent interest. As expected, we found in this study that mice vaccinated by scarification of VACV, which induces poxvirus-specific TRM cells in the skin (5, 7, 23), most effectively controlled a challenge dose of ECTV given via cutaneous inoculation. However, perhaps less predictably, s.s. mice also demonstrated a better outcome following heterologous i.n. challenge and most effectively controlled ECTV in the lungs. Therefore, it appears that circulating T-cell responses elicited by epithelial infection deserve consideration in addition to skin-resident populations. Since this study employed a somewhat virulent strain of VACV, future work will determine if these results hold true in the context of attenuated vaccine strains, such as MVA or Lister.
In summary, we found that in comparison with other routes, epithelial inoculation generated circulating TCD8+ with superior ability to secrete multiple cytokines and better control over ECTV replication in vivo. Importantly, antibody levels alone did not dictate the degree of protection or correlate with full virus control. Instead, it appears that both antibodies and TCD8+ cooperate to bring about optimal control of a high-dose ECTV challenge. These findings point toward the importance of investigating the protective effects of scarification in the context of other pathogens.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grant U19-AI083008. This study includes work carried out within the Kimmel Cancer Center Flow Cytometry Facility, which is supported in part by NCI Cancer Center support grant P30 CA56036. MHC class I peptides for TCD8+ stimulation were obtained through the NIH Biodefense and Emerging Infections Research Resources Repository (H-2 Peptide Arrays, Epitopes of Vaccinia Virus Proteins, NR-4058).
Footnotes
Published ahead of print 4 June 2014
REFERENCES
- 1.Burnet FM, Boake WC. 1946. The relationship between the virus of infectious ectromelia of mice and vaccinia virus. J. Immunol. 53:1–13 [PubMed] [Google Scholar]
- 2.Fenner F. 1947. Studies in infectious ectromelia of mice; immunization of mice against ectromelia with living vaccinia virus. Aust. J. Exp. Biol. Med. Sci. 25:257–274 [DOI] [PubMed] [Google Scholar]
- 3.Trentin JJ, Ferrigno MA. 1957. Control of mouse pox (infectious ectromelia) by immunization with vaccinia virus. J. Natl. Cancer Inst. 18:757–767 [PubMed] [Google Scholar]
- 4.Buller RM, Wallace GD. 1985. Reexamination of the efficacy of vaccination against mousepox. Lab. Anim. Sci. 35:473–476 [PubMed] [Google Scholar]
- 5.Liu L, Zhong Q, Tian T, Dubin K, Athale SK, Kupper TS. 2010. Epidermal injury and infection during poxvirus immunization is crucial for the generation of highly protective T cell-mediated immunity. Nat. Med. 16:224–227. 10.1038/nm.2078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gebhardt T, Wakim LM, Eidsmo L, Reading PC, Heath WR, Carbone FR. 2009. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat. Immunol. 10:524–530. 10.1038/ni.1718 [DOI] [PubMed] [Google Scholar]
- 7.Jiang X, Clark RA, Liu L, Wagers AJ, Fuhlbrigge RC, Kupper TS. 2012. Skin infection generates non-migratory memory CD8+ T(RM) cells providing global skin immunity. Nature 483:227–231. 10.1038/nature10851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Earl PL, Americo JL, Wyatt LS, Eller LA, Whitbeck JC, Cohen GH, Eisenberg RJ, Hartmann CJ, Jackson DL, Kulesh DA, Martinez MJ, Miller DM, Mucker EM, Shamblin JD, Zwiers SH, Huggins JW, Jahrling PB, Moss B. 2004. Immunogenicity of a highly attenuated MVA smallpox vaccine and protection against monkeypox. Nature 428:182–185. 10.1038/nature02331 [DOI] [PubMed] [Google Scholar]
- 9.Hooper JW, Thompson E, Wilhelmsen C, Zimmerman M, Ichou MA, Steffen SE, Schmaljohn CS, Schmaljohn AL, Jahrling PB. 2004. Smallpox DNA vaccine protects nonhuman primates against lethal monkeypox. J. Virol. 78:4433–4443. 10.1128/JVI.78.9.4433-4443.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edghill-Smith Y, Golding H, Manischewitz J, King LR, Scott D, Bray M, Nalca A, Hooper JW, Whitehouse CA, Schmitz JE, Reimann KA, Franchini G. 2005. Smallpox vaccine-induced antibodies are necessary and sufficient for protection against monkeypox virus. Nat. Med. 11:740–747. 10.1038/nm1261 [DOI] [PubMed] [Google Scholar]
- 11.Esteban DJ, Buller RM. 2005. Ectromelia virus: the causative agent of mousepox. J. Gen. Virol. 86:2645–2659. 10.1099/vir.0.81090-0 [DOI] [PubMed] [Google Scholar]
- 12.Panchanathan V, Chaudhri G, Karupiah G. 2006. Protective immunity against secondary poxvirus infection is dependent on antibody but not on CD4 or CD8 T-cell function. J. Virol. 80:6333–6338. 10.1128/JVI.00115-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Panchanathan V, Chaudhri G, Karupiah G. 2010. Antiviral protection following immunization correlates with humoral but not cell-mediated immunity. Immunol. Cell Biol. 88:461–467. 10.1038/icb.2009.110 [DOI] [PubMed] [Google Scholar]
- 14.Panchanathan V, Chaudhri G, Karupiah G. 2008. Correlates of protective immunity in poxvirus infection: where does antibody stand? Immunol. Cell Biol. 86:80–86. 10.1038/sj.icb.7100118 [DOI] [PubMed] [Google Scholar]
- 15.Tscharke DC, Woo WP, Sakala IG, Sidney J, Sette A, Moss DJ, Bennink JR, Karupiah G, Yewdell JW. 2006. Poxvirus CD8+ T-cell determinants and cross-reactivity in BALB/c mice. J. Virol. 80:6318–6323. 10.1128/JVI.00427-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hersperger AR, Siciliano NA, Eisenlohr LC. 2012. Comparable polyfunctionality of ectromelia virus- and vaccinia virus-specific murine T cells despite markedly different in vivo replication and pathogenicity. J. Virol. 86:7298–7309. 10.1128/JVI.00038-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hersperger AR, Pereyra F, Nason M, Demers K, Sheth P, Shin LY, Kovacs CM, Rodriguez B, Sieg SF, Teixeira-Johnson L, Gudonis D, Goepfert PA, Lederman MM, Frank I, Makedonas G, Kaul R, Walker BD, Betts MR. 2010. Perforin expression directly ex vivo by HIV-specific CD8 T-cells is a correlate of HIV elite control. PLoS Pathog. 6:e1000917. 10.1371/journal.ppat.1000917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Betts MR, Brenchley JM, Price DA, De Rosa SC, Douek DC, Roederer M, Koup RA. 2003. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J. Immunol. Methods 281:65–78. 10.1016/S0022-1759(03)00265-5 [DOI] [PubMed] [Google Scholar]
- 19.Betts MR, Nason MC, West SM, De Rosa SC, Migueles SA, Abraham J, Lederman MM, Benito JM, Goepfert PA, Connors M, Roederer M, Koup RA. 2006. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood 107:4781–4789. 10.1182/blood-2005-12-4818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seder RA, Darrah PA, Roederer M. 2008. T-cell quality in memory and protection: implications for vaccine design. Nat. Rev. Immunol. 8:247–258. 10.1038/nri2274 [DOI] [PubMed] [Google Scholar]
- 21.Makedonas G, Betts MR. 2006. Polyfunctional analysis of human t cell responses: importance in vaccine immunogenicity and natural infection. Springer Semin. Immunopathol. 28:209–219. 10.1007/s00281-006-0025-4 [DOI] [PubMed] [Google Scholar]
- 22.Xu RH, Fang M, Klein-Szanto A, Sigal LJ. 2007. Memory CD8+ T cells are gatekeepers of the lymph node draining the site of viral infection. Proc. Natl. Acad. Sci. U. S. A. 104:10992–10997. 10.1073/pnas.0701822104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu L, Fuhlbrigge RC, Karibian K, Tian T, Kupper TS. 2006. Dynamic programming of CD8+ T cell trafficking after live viral immunization. Immunity 25:511–520. 10.1016/j.immuni.2006.06.019 [DOI] [PubMed] [Google Scholar]