ABSTRACT
Respiratory syncytial virus (RSV) infection is the number one cause of bronchiolitis in infants, yet no vaccines are available because of a lack of knowledge of the infant immune system. Using a neonatal mouse model, we previously revealed that mice initially infected with RSV as neonates develop Th2-biased immunopathophysiologies during reinfection, and we demonstrated a role for enhanced interleukin-4 receptor α (IL-4Rα) expression on T helper cells in these responses. Here we show that RSV infection in neonates induced limited type I interferon (IFN) and plasmacytoid dendritic cell (pDC) responses. IFN alpha (IFN-α) treatment or adoptive transfer of adult pDCs capable of inducing IFN-α prior to neonatal RSV infection decreased Th2-biased immunopathogenesis during reinfection. A reduced viral load and downregulation of IL-4Rα on Th2 cells were observed in IFN-α-treated neonatal mice, suggesting dual mechanisms of action.
IMPORTANCE Respiratory syncytial virus (RSV) is the most significant cause of lower respiratory tract infection in infancy worldwide. Despite the dire need, we have failed to produce efficacious RSV vaccines or therapeutics. Part of the reason for this failure is our lack of understanding of how RSV interacts with the infant immune system to suppress the development of protective immunity. In the study described in the present paper, we used a neonatal mouse model, which more closely mimics human infants, to study the role of the innate immune system, particularly type I interferons (IFNs) and plasmacytoid dendritic cells (pDCs), in the pathogenesis of RSV infection. RSV infection in neonates induced limited type I IFN and pDC responses. IFN-α treatment or adoptive transfer of adult pDCs capable of producing IFN-α prior to neonatal RSV infection decreased Th2-biased immunopathogenesis during reinfection. These data suggest that IFN-α is a promising target for future RSV vaccine design.
INTRODUCTION
Respiratory syncytial virus (RSV) infection causes a significant global medical and economic burden. It is the number one cause of bronchiolitis in infants, and every child is infected by RSV by the age of 2 years. Moreover, a significant number of infants who acquire severe disease from RSV infection develop asthma that persists into adulthood (1). The annual medical cost due to RSV infection in young children is estimated to exceed $600 million (2). Insufficient knowledge of infant immunity was responsible for the failure of an RSV vaccine brought to clinical trials in the 1960s (3). This lack of knowledge, combined with the early use of adult animal models to understand a disease in infants, is the main reason that we lack an RSV vaccine today.
Our and other laboratories have therefore developed a neonatal mouse model that better mimics RSV disease in infants (4–9). In this model, neonatal mice (<7 days old) are infected with RSV and various immunological parameters are measured. Notably, RSV-infected neonates mount limited Th1 (CD3+, CD4+, interferon gamma-positive [IFN-γ+], interleukin-4-negative [IL-4−]) responses compared to the responses of their adult counterparts (4). This observation is in line with the findings from postmortem histological examinations of human infants who died of severe RSV infections (10). Furthermore, when reinfected, mice initially infected as neonates respond with larger numbers of Th2 (CD3+, CD4+, IFN-γ-negative [IFN-γ−], IL-4-positive [IL-4+]) (4, 6, 8, 9) and multifunctional Th (CD3+, CD4+, IFN-γ+, IL-4+) cells (4), while the number of Th1 cells upon reinfection is equivalent to that in mice initially infected as adults. This biased Th2 response observed upon reinfection is associated with enhanced airway hyperreactivity (AHR), airway and lung eosinophilia, and mucus hyperproduction (4, 6, 8, 9), all of which are consistent with the observations for infants from the 1960s clinical trials who died from community-acquired RSV infection (3).
The neonatal mouse model of RSV infection has proven to be an invaluable model to study and understand RSV pathogenesis in infants (5). Using this model, we have recently demonstrated that interleukin 4 receptor α (IL-4Rα) on Th cells plays a pathogenic role in RSV reinfection-associated immunopathophysiology (4). We showed that neonatal Th cells (i.e., Th1 and Th2 cells) express higher levels of IL-4Rα than adult Th cells. When Il4ra is specifically deleted from Th cells, all parameters of immunopathophysiology associated with RSV reinfection are ablated or substantially decreased in mice initially infected as neonates. Along with the discovery of a role for IL-4Rα, other groups have demonstrated that IL-13, a ligand for IL-4Rα, also plays a pathogenic role during reinfection in this neonatal mouse model (9). Human genetic analysis, which demonstrates that certain IL-4Rα polymorphisms (11) and IL-13/IL-4 haplotypes (12) are associated with severe RSV infection, further corroborates these neonatal mouse data.
Though it is clear that the adaptive arm of the immune system and its key molecules play vital roles in the immunopathogenesis of RSV disease, adaptive immune responses are first instructed by the innate response. Data generated from human primary cells and adult animal models shed light on the innate immune responses to RSV. In particular, several laboratories have demonstrated that type I IFNs (mainly IFN-α and IFN-β) and their major producers, plasmacytoid dendritic cells (pDCs), are important in protection from RSV disease. It has been reported that RSV stimulates pDCs isolated from human peripheral blood mononuclear cells (13, 14) or murine bone marrow-derived pDCs (15, 16) to produce type I IFNs ex vivo. Furthermore, several publications have demonstrated that RSV infection of adult mice induces pulmonary type I IFNs in vivo (17, 18) and that intranasal administration of recombinant IFN-α significantly reduces the lung viral load, inflammation, weight loss, and clinical illness scores of RSV-infected adult mice (19). It has also been demonstrated that RSV infection induces recruitment of pDCs to the lungs of adult mice and the depletion of pDCs results in an increased pulmonary viral load and immunopathology (17).
Despite the apparent roles of type I IFNs and pDCs in determining RSV morbidity, there are few studies investigating type I IFNs and pDCs in infants. Data demonstrate that pDCs are recruited to the mucosal compartment of the airways and are readily detectable in nasal washes from children infected with RSV (20); however, there are very low levels of type I IFNs in nasal washes from RSV-infected infants (21). Recent data from Stuart Turvey's group support these findings and demonstrate that pDCs isolated from the cord blood of healthy term infants are unable to mount robust type I IFN responses upon RSV infection ex vivo, unlike pDCs from healthy adults, suggesting that human infant pDCs are immature and/or less responsive at birth (22).
The present study is the first attempt, to our knowledge, to characterize the role of type I IFN and pDC responses in immunopathogenesis during RSV reinfection using a neonatal mouse model. Our data demonstrate that neonatal mice mounted limited type I IFN and pDC responses during primary RSV infection. Treatment of neonatal mice with IFN-α or adoptive transfer of adult pDCs prior to RSV infection significantly reduced the Th2-biased immunopathophysiology, strongly suggesting that limited type I IFN and pDC responses during infancy permit the development of a Th2-biased immunopathophysiology in response to RSV infection.
MATERIALS AND METHODS
Mice.
BALB/c mice were purchased from Harlan Laboratories (Indianapolis, IN) and maintained in specific-pathogen-free facilities at the Louisiana State University Health Sciences Center (LSUHSC; New Orleans, LA) and the University of Tennessee Health Science Center (UTHSC; Memphis, TN). Breeders were time mated, and pups born on the same date were used for experiments. A set of pups was allowed to mature to 6 to 8 weeks of age and were then used as adult controls or the source of pDCs for adoptive transfer studies.
All animal protocols were prepared in accordance with the Guide for the Care and Use of Laboratory Animals (23) and approved by the Institutional Animal Care and Use Committee at LSUHSC or UTHSC.
IFN-α treatment.
Mouse recombinant IFN-αA was purchased from PBL Interferon Source (Piscataway, NJ). At 16 h prior to RSV infection, 2 × 103 units (the dose extrapolated from the dosage used in adult mice [17]) of IFN-α was instilled intranasally into pups in 10 μl of phosphate-buffered saline (PBS) containing 0.1% bovine serum albumin (BSA). Control pups (pups treated with vehicle, here abbreviated NS) received 10 μl of PBS containing 0.1% BSA.
Purification of pDCs or CD11c+ cells and adoptive transfer.
pDCs were purified from adult spleens using OptiPrep gradient medium (Sigma-Aldrich, St. Louis, MO) and a negative-selection kit from Miltenyi Biotec Inc. (Auburn, CA). Briefly, a single-cell suspension of spleen cells was prepared using a gentleMACS Octo dissociator and a spleen dissociation kit. Spleen cells were then suspended in Hanks balanced salt solution containing 15% OptiPrep density gradient medium and overlaid onto 11.5% OptiPrep and centrifuged. Low-density cells mostly consisting of dendritic cells were collected and subjected to further purification for pDCs using the negative-selection kit per the manufacturer's instruction. The purity of the pDCs isolated was verified by flow cytometry and was above 85%. For adoptive transfer studies, pDCs were purified as described above and suspended in 10 μl serum-free medium (SFM; SFM4MegaVir; HyClone, Logan, UT), and 3 × 104 pDCs were intranasally instilled into pups at 16 h prior to RSV infection. Control pups received 10 μl SFM.
CD11c+ cells were purified from adult spleens for adoptive transfer. Adult splenocytes were isolated as described above, and CD11c+ cells were selected using a positive-selection kit from Stemcell Technologies (Vancouver, British Columbia, Canada). The purity of isolation was above 90%. CD11c+ cells were suspended in 10 μl SFM, and 3 × 104 cells were instilled intranasally into pups at 16 h prior to RSV infection. Control pups received 10 μl SFM.
RSV infection.
Human RSV strain A2 (Advanced Biotechnologies Inc., Columbia, MD) was propagated in and harvested from Vero cells (ATCC, Manassas, VA) cultured in SFM and stored at −80°C until use. Five-day-old pups or 6- to 8-week-old adults were infected intranasally with RSV in SFM at a dose of 2 × 105 50% tissue culture infectious dose (TCID50) per gram of body weight. Control mice received SFM.
Assessment of pulmonary viral load.
At 4 days after the initial RSV infection, lungs were isolated, weighed, and frozen until analysis by either plaque assay (24) or real-time reverse transcription-PCR (RT-PCR) methods (25). For plaque assays, supernatants from centrifuged lung homogenates were added onto Vero cells and overlaid with 0.3% agarose (MP Biochemicals, Santa Ana, CA). Cells were incubated for 3 days, fixed with 1% formaldehyde, and stained with 0.5% neutral red (Sigma-Aldrich). The plaques were counted, and viral loads were expressed as the number of PFU per gram of lung tissue.
For real-time PCR, RNA was isolated from frozen lungs using an RNeasy plus minispace kit (Qiagen, Valencia, CA) per the manufacturer's instructions. Real-time RT-PCR was then performed using a SuperScript III Platinum one-step quantitative RT-PCR (qRT-PCR) kit from Life Technologies (Carlsbad, CA). The primers (25) used for the reaction were NS1 forward primer (5′-CACAACAATGCCAGTGCTACAA-3′) and NS1 reverse primer (5′-TTAGACCATTAGGTTGAGAGCAATGT-3′).
Cell staining and flow cytometry.
To measure pDC levels in the lung, single cells were isolated and labeled with a fixable viability dye and antibodies to CD11c (N418) and PDCA-1 (eBio129c). Stained cells were then assayed on a Canto II flow cytometer (BD Biosciences, Franklin Lakes, NJ), and flow data were analyzed and plotted with FlowJo software (version 7.6.5 for Windows; Tree Star, Ashland, OR). Single live cells were gated before any further analysis. All buffers, viability dyes, and antibodies were purchased from eBiosciences unless otherwise stated.
To determine the CD4+ T cell populations in the lung, single cells were isolated as described above and stimulated for 5 h in RPMI 1640 (HyClone) containing 10% heat-inactivated fetal bovine serum (FBS; Life Technologies), 100 U/ml penicillin (HyClone), 100 mg/ml streptomycin (HyClone), 5 ng/ml phorbol-12-myristate-13-acetate (PMA; Sigma-Aldrich), and 500 ng/ml ionomycin (Sigma-Aldrich) in the presence of a protein transport inhibitor (1 μl/106 cells; GolgiPlug; BD Biosciences, Franklin Lakes, NJ). After stimulation, the cells were stained with a fixable viability dye, fixed, permeabilized, and labeled with antibodies to CD3 (17A2), CD4 (RM4-4; Biolegend), IFN-γ (XMG1.2), and IL-4 (BVD6-24G2). Flow data were then acquired, analyzed, and plotted as described above.
Pulmonary function test.
Airway resistance to methacholine (MeCh; Sigma-Aldrich) challenge was measured using a Scireq FlexiVent system (Montreal, QC, Canada). Briefly, mice were anesthetized, tracheotomized, and ventilated, and the resistance of the airway was measured and calculated. Raw data were normalized by subtracting the individual baseline values obtained with 0 mg/ml MeCh, and these data were plotted as normalized resistance.
Lung histopathology.
Lungs were retroperfused to remove blood and then gently inflated and fixed with zinc formalin (Thermo Fisher Scientific). After fixation, tissue sections were prepared following standard procedures. Some slides were stained with hematoxylin-eosin (H&E) to identify cellular infiltrates. In particular, the eosinophils in the peribronchial area of the lung specimens were quantified and expressed as the number of eosinophils per surface area of the lung parenchyma. Other slides were stained with periodic acid-Schiff (PAS) to quantify mucus production in the airway epithelial cells. Images of randomly selected airways were acquired and analyzed with ImageJ software, and mucus expression was expressed as the number of mucus-positive cells per surface area of airway epithelial cells.
BALF cellularity.
Bronchoalveolar lavage fluid (BALF) was isolated in 1 ml of PBS containing 2% heat-inactivated FBS. BALF cells were counted, centrifuged onto slides, stained with stain from a Hema-3 staining kit (Thermo Fisher Scientific, Waltham, MA), and differentiated by two unbiased observers using standard morphological criteria. Supernatants were collected, snap-frozen, and stored at −80°C for cytokine analysis.
Cytokine measurement.
Real-time PCR was performed to measure the kinetics of IFN-α expression in the lung. Total lung RNA was isolated using a Quick-RNA MiniPrep kit from Zymo Research (Irvine, CA), and then real-time RT-PCR was performed using a SuperScript III Platinum one-step qRT-PCR kit (Life Technologies). The primers used for the reaction were IFN-α4 forward primer (5′-TGATGAGCTACTACTGGTCAGC-3′) and IFN-α4 reverse primer (5′-GATCTCTTAGCACAAGGATGGC-3′).
To measure the protein levels of type I IFNs in the lung, total lung proteins were isolated using the T-PER tissue protein extraction reagent and quantified using the bicinchoninic acid protein assay reagent (Thermo Fisher Scientific). The levels of IFN-α and IFN-β were then measured using enzyme-linked immunosorbent assay (ELISA) kits per the manufacturer's instructions (PBL Interferon Source). The limit of detection was 12.5 pg/ml for IFN-α and 15.6 pg/ml for IFN-β. All data presented are above the limit of detection.
The levels of other cytokines in the BALF, including IFN-γ, IL-12 (p40), IL-4, and IL-13, were measured using a Milliplex mouse cytokine/chemokine assay kit (Millipore Corporation, Billerica, MA) on a Bio-Plex system (Bio-Rad Laboratories, Hercules, CA). Data outside the sensitivity level of detection were excluded.
Statistics.
Data were plotted as means ± standard errors of the means (SEMs) and analyzed using Prism (version 6) software (GraphPad Software, La Jolla, CA). Student's t test was used for all mouse studies except for the pulmonary function test, data from which were analyzed by two-way analysis of variance (ANOVA) and Bonferroni post hoc tests. Each figure represents the results of one experiment, and every experiment was repeated at least twice. Differences were considered significant if P was <0.05.
RESULTS
RSV infection induced limited type I IFN and pDC responses in neonatal mouse lungs.
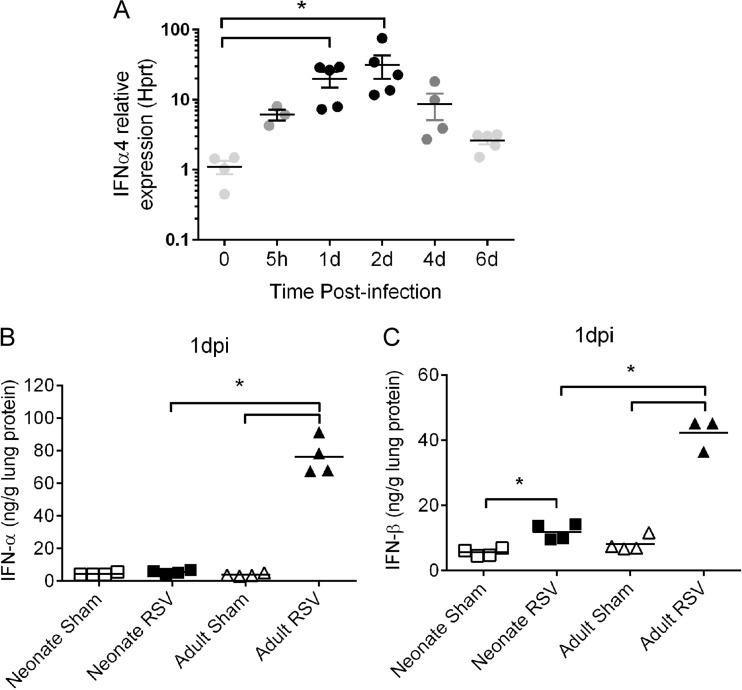
Type I IFNs are important antiviral cytokines induced during viral infections. We measured the amount of type I IFNs in the lung at various time points after RSV infection. Neonates were infected with RSV, and lungs were isolated at 5 h postinfection and 1, 2, 4, and 6 days postinfection (dpi). The expression of IFN-α was measured at these time points using real-time RT-PCR. Although it has been reported that RSV A2 and clinical strains are capable of inhibiting type I IFN expression in human cells ex vivo (26), we observed induction of IFN-α in the lungs of RSV-infected mice as early as 5 h postinfection and a peak in IFN-α at 1 and 2 dpi (Fig. 1A). We further measured the protein levels of type I IFNs, including both IFN-α and IFN-β, in the lung homogenates of RSV-infected neonatal mice versus those in adult mice. The level of IFN-β was slightly, but significantly, increased at 1 dpi in neonates (Fig. 1C; RSV-infected neonate versus sham-infected neonate, 11.84 ± 1.22 versus 5.57 ± 0.57 ng/g lung protein), whereas the level of IFN-α was not significantly different from that in sham-infected neonates (Fig. 1B; RSV-infected neonate versus sham-infected neonate, 5.51 ± 0.51 versus 4.35 ± 0.39 ng/g lung protein). More importantly, adults infected with RSV induced significantly larger amounts of IFN-α (Fig. 1B; RSV-infected adult versus sham-infected adult, 76.16 ± 5.59 versus 3.77 ± 0.45 ng/g lung protein) and IFN-β (Fig. 1C; RSV-infected adult versus sham-infected adult, 42.25 ± 2.92 versus 8.14 ± 1.15 ng/g lung protein) than sham-infected adults at 1 dpi. The levels of both IFN-α and IFN-β in RSV-infected adults were significantly higher than those in RSV-infected neonates.
FIG 1.
RSV infection induced limited type I IFN responses in neonates. (A) Kinetics of IFN-α in neonates. Five-day-old neonates were infected with RSV, and the expression of IFNa4 was measured at various time points postinfection via real-time RT-PCR. The expression of IFN-α relative to that of the Hprt housekeeping gene is shown. Data are for 3 to 5 mice. (B and C) To compare the protein levels of type I IFNs, neonates or adults were infected with RSV or medium, and the concentrations of IFN-α and IFN-β in lung homogenates at 1 dpi were measured using ELISA. (B) IFN-α production in neonates and adults. (C) IFN-β production in neonates and adults. Data are for 3 to 4 mice. Individual data are shown as the mean ± SEM. *, P < 0.05.
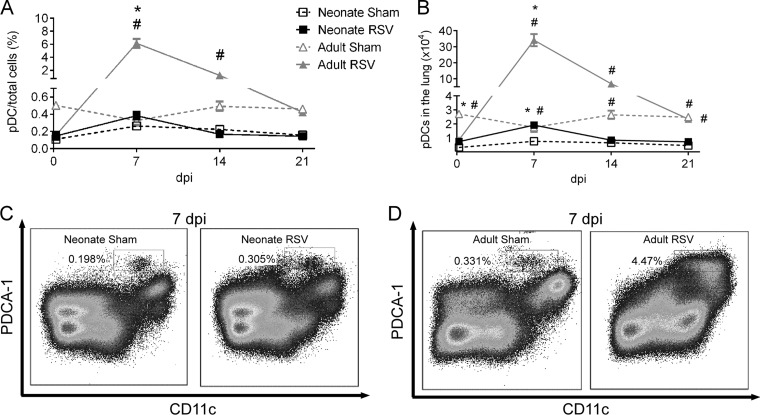
Numerous reports have shown that pDCs are a major source of type I IFNs during viral infections (27, 28); we therefore compared the kinetics of pDCs during RSV infection in neonatal versus adult mice. Neonates or adults were infected with RSV, and the number of pDCs in the lung was measured at 5 h postinfection and 7, 14, and 21 dpi using flow cytometry (Fig. 2). RSV infection induced the recruitment of pDCs to the lung, with levels peaking at 7 dpi and declining to baseline levels by 21 dpi in both adults and neonates (Fig. 2A and B). The frequency of pDCs was substantially greater at 7 dpi in RSV-infected adults than sham-infected adults (Fig. 2D; 6.16% ± 0.68% versus 0.33% ± 0.06%), whereas there was only a slight, but significant, increase in the frequency of pDCs from RSV-infected neonates compared to the frequency of pDCs from sham-infected neonates (Fig. 2C; 0.38% ± 0.05% versus 0.26% ± 0.01%). Additionally, the frequency of pDCs was significantly greater at 7 dpi in adults infected with RSV (6.16% ± 0.68%) than neonates infected with RSV (0.38% ± 0.05%). This observation is consistent with the data for type I IFN presented above, and both results indicated that RSV infection in neonates induced limited type I IFN and pDC responses in the lung.
FIG 2.
RSV infection induced limited recruitment of pDCs to neonatal lungs. Five-day-old pups or adults were infected with RSV or sham infected, and the numbers of pDCs in the lungs at various time points were measured using flow cytometry. (A) Kinetics of pulmonary pDCs expressed as the percentage of total lung cells; (B) kinetics of pDCs expressed as total numbers of pDCs in the lungs; (C) representative flow plots for pDCs (CD11c+, PDCA-1 positive) in neonates at 7 dpi; (D) representative flow plots for pDCs in adults at 7 dpi. Data are for 4 mice. *, P < 0.05 for RSV-infected versus sham-infected mice; #, P < 0.05 for adults versus neonates. Data are presented as the mean ± SEM.
IFN-α treatment prior to neonatal RSV infection mitigated AHR and lung pathology during reinfection.
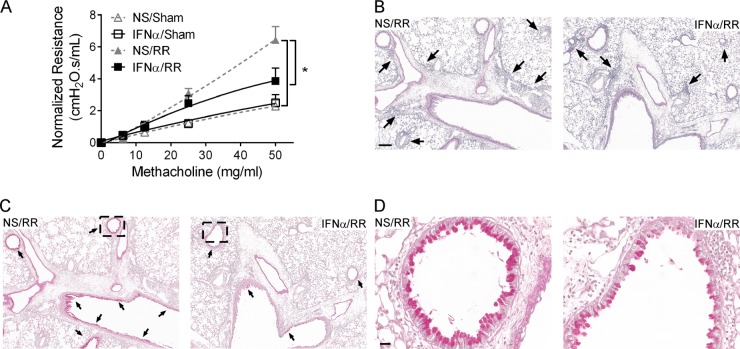
Both our laboratories (4) and other groups (8, 9) have shown that mice initially infected as neonates exhibit exacerbated AHR and lung pathology during RSV reinfection compared to the AHR and lung pathology exhibited by mice initially infected as adults; we therefore monitored lung function and pathology following reinfection in mice that were treated with IFN-α prior to neonatal RSV infection. Four-day-old pups were treated intranasally with 2 × 103 U (the dose was extrapolated from the dosage used in adult mice [17]) of IFN-α or vehicle and then infected with RSV or serum-free medium at 16 h posttreatment. These mice were then reinfected with RSV at 4 weeks after primary infection, and AHR and lung pathology were assessed at 6 days after reinfection (Fig. 3). Pulmonary viral load data from other groups have shown that RSV infects and replicates in the lung following reinfection (9).
FIG 3.
IFN-α treatment prior to neonatal RSV infection attenuated AHR, inflammation, and mucus hyperproduction during RSV reinfection. Four-day-old pups were treated with IFN-α or vehicle. These mice were infected 16 h later, allowed to mature to adults, and reinfected with RSV at 4 weeks after initial infection. Airway hyperactivity and lung pathology were assessed at 6 days after reinfection. (A) Airway resistance to methacholine. Data are for 5 to 6 mice. *, P < 0.05. (B) Lung tissue stained with H&E. Arrows, inflammatory cells. Magnification, ×100. Bar = 200 μm. (C) Lung tissue stained with PAS. Magenta staining indicates mucus. Arrows, mucus-positive regions. Magnification, ×100. (D) Enlarged images of selected regions in panel C. Magnification, ×400. Bar = 50 μm. NS/sham, mice treated with vehicle and mock infected; IFN-α/sham, mice treated with IFN-α and mock infected; NS/RR, mice treated with vehicle, infected as neonates, and reinfected with RSV as adults; IFN-α/RR, mice treated with IFN-α, infected as neonates, and reinfected with RSV as adults. Data are presented as the mean ± SEM.
As expected, the airway resistance to methacholine in mice initially infected as neonates (mice treated with vehicle, infected as neonates, and reinfected with RSV as adults [NS/RR mice]) was significantly greater than that in sham-infected mice (mice treated with vehicle and mock infected [NS/sham mice]) (Fig. 3A; 6.44 ± 0.83 versus 2.29 ± 0.38 cm H2O · s/ml). IFN-α treatment prior to neonatal RSV infection substantially decreased AHR (for mice treated with IFN-α, infected as neonates, and reinfected with RSV as adults [IFN-α/RR mice] versus NS/RR mice, 3.86 ± 0.81 versus 6.44 ± 0.83 cm H2O · s/ml), while there was no significant difference in AHR between mice treated with IFN-α prior to RSV infection and mice treated with vehicle prior to sham infection (IFN-α/RR mice versus NS/sham mice, 3.86 ± 0.81 versus 2.29 ± 0.38 cm H2O · s/ml). No difference in airway resistance was observed between sham-infected mice treated with IFN-α (IFN-α/sham mice) and vehicle (NS/sham mice) (2.50 ± 0.51 versus 2.29 ± 0.38 cm H2O · s/ml).
Consistent with the lung function data, lung pathology demonstrated that RSV reinfection induced moderate peribronchial and perivascular inflammation in the lung, which was reduced with IFN-α treatment prior to RSV infection (Fig. 3B). In particular, a striking decrease in eosinophils was observed around the airways of mice treated with IFN-α compared to that in control mice (IFN-α/RR versus NS/RR mice, 1,095 ± 185 versus 66 ± 31 eosinophils/surface area of lung parenchyma). In addition, significantly fewer mucus-producing cells, indicated by positive PAS staining, were detected in the lungs of mice treated with IFN-α than in the lungs of control mice (Fig. 3C and D; IFN-α/RR versus NS/RR mice, 0.40 ± 0.02 versus 0.26 ± 0.03 mucus-positive cells/surface area of airway). Little to no inflammation or mucus production was observed in mice treated with IFN-α or vehicle and sham infected (data not shown).
These data demonstrate a prophylactic effect of IFN-α on RSV infection in neonates that included significantly reduced AHR, pulmonary inflammation, and mucus hyperproduction during RSV reinfection.
IFN-α treatment prior to neonatal RSV infection mitigated Th2-biased responses during reinfection.
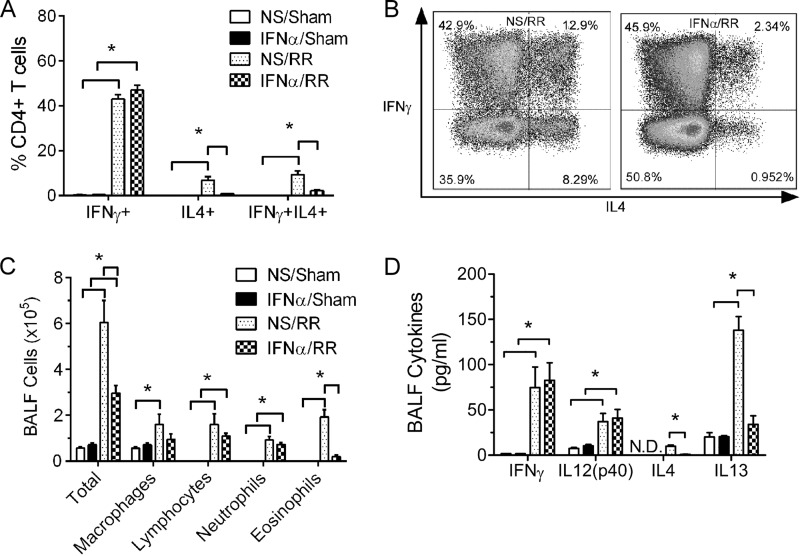
Cellular immunological analysis has previously revealed that the AHR and lung pathology in mice initially infected as neonates are associated with biased pulmonary Th2 responses (4). We therefore measured various parameters of the Th cell immune response in the lung, including pulmonary Th cell subsets, the cellularity of bronchoalveolar lavage fluid (BALF), and cytokine levels in BALF (Fig. 4).
FIG 4.
IFN-α treatment prior to neonatal RSV infection mitigated the Th2-biased responses during reinfection. Four-day-old pups were treated with IFN-α or vehicle. These mice were infected 16 h later, allowed to mature to adults, and reinfected with RSV at 4 weeks after initial infection. Pulmonary T helper cell profiles and BALF cellularity and cytokine levels were measured at 6 dpi. (A) Pulmonary Th profile. Data are for 4 to 5 mice. *, P < 0.05. (B) Representative flow plot of Th cells from mice treated with IFN-α or vehicle and infected with RSV. (C) BALF cellularity. Data are for 5 to 6 mice. *, P < 0.05. (D) BALF cytokine levels. Data are for 3 to 5 mice. *, P < 0.05. NS/sham, mice treated with vehicle and mock infected; IFN-α/sham, mice treated with IFN-α and mock infected; NS/RR, mice treated with vehicle, infected as neonates, and reinfected with RSV as adults; IFN-α/RR, mice treated with IFN-α, infected as neonates, and reinfected with RSV as adults. Data are presented as the mean ± SEM.
Mice were treated and infected as described above, and lung cells were isolated at 6 days after reinfection and stained for surface markers (CD3 and CD4) and intracellular cytokines (IFN-γ and IL-4). We have previously shown that mice initially infected as neonates induce considerably more Th2 cells in the lung during reinfection than mice initially infected as adults, while the level of Th1 cells in the lung remains similar (hence, the response is Th2 biased) (4). Here, we confirmed that RSV reinfection of mice initially infected as neonates (NS/RR) induced high levels of Th1 cells (CD3+, CD4+, IFN-γ+, IL-4−) and moderate levels of Th2 cells (CD3+, CD4+, IFN-γ−, IL-4+) and multifunctional Th cells (CD3+, CD4+, IFN-γ+, IL-4+) in the lung (Fig. 4A). IFN-α treatment prior to neonatal RSV infection substantially reduced the level of Th2 cells (for IFN-α/RR versus NS/RR mice, 0.84% ± 0.16% versus 6.80% ± 1.66%) and multifunctional cells (2.15% ± 0.42% versus 9.39% ± 1.67%), while it maintained the level of Th1 cells (46.96% ± 2.14% versus 42.94% ± 2.04%) (Fig. 4A and B).
As described in our previous publications (4, 6), RSV reinfection increased the levels of inflammatory cells in the BALF, including alveolar macrophages, lymphocytes, neutrophils, and eosinophils (Fig. 4C). Consistent with the reduced pulmonary inflammation (Fig. 3B), IFN-α treatment prior to neonatal RSV infection significantly decreased the total amount of inflammatory cells in the BALF during reinfection compared to that in mice treated with vehicle (for IFN-α/RR versus NS/RR mice, 2.96 × 105 ± 0.34 × 105 versus 6.04 × 105 ± 0.96 × 105 cells/ml). This decrease in total inflammatory cells was mainly due to the decrease in eosinophils (0.20 × 105 ± 0.07 × 105 versus 1.92 × 105 ± 0.32 × 105 cells/ml).
In line with the BALF cellularity and pulmonary Th profile, IFN-α treatment prior to neonatal RSV infection significantly reduced Th2 cytokine levels in BALF during reinfection, including both IL-4 and IL-13 (Fig. 4D; for IFN-α/RR versus NS/RR mice, 0.98 ± 0.12 versus 9.88 ± 1.42 pg/ml for IL-4 and 34.10 ± 9.47 versus 137.93 ± 15.1 pg/ml for IL-13). No difference in Th1 cytokines, including both IFN-γ and IL-12, was observed between IFN-α- and vehicle-treated mice (82.79 ± 19.31 versus 74.70 ± 22.76 pg/ml for IFN-γ and 40.98 ± 9.53 versus 37.00 ± 9.12 pg/ml for IL-12).
Collectively, the pulmonary Th profile, BALF cellularity, and cytokine levels described above demonstrate that IFN-α treatment prior to neonatal RSV infection decreased Th2-biased responses and reduced RSV pathogenesis upon reinfection.
Adoptive transfer of purified adult pDCs prior to neonatal RSV infection induced pulmonary IFN-α expression and alleviated RSV reinfection-associated immunopathophysiology.
As demonstrated above, RSV infection induced limited pDC and type I IFN responses in the neonatal lung. Additionally, IFN-α treatment prior to neonatal RSV infection reduced the typical Th2 immunopathophysiology during reinfection. Because pDCs are the major source of type I IFNs (28), we hypothesized that adoptive transfer of adult pDCs prior to neonatal RSV infection would also reduce the Th2 immunopathophysiology during reinfection. Four-day-old pups received 3 × 104 adult pDCs instilled intranasally. The number of pDCs instilled was roughly the number of pulmonary pDCs observed in adult naive mice. Because it is extraordinarily difficult to isolate this number of pDCs from neonates, control pups received vehicle (serum-free medium [SFM]). Pups were infected with RSV at 16 h after adoptive transfer and reinfected at 4 weeks after initial infection. Various endpoints were measured at 6 days after reinfection, including AHR, BALF cellularity, and the pulmonary Th profile (Fig. 5). A set of pups was used to measure IFN-α expression in the lung at 1 dpi.
FIG 5.
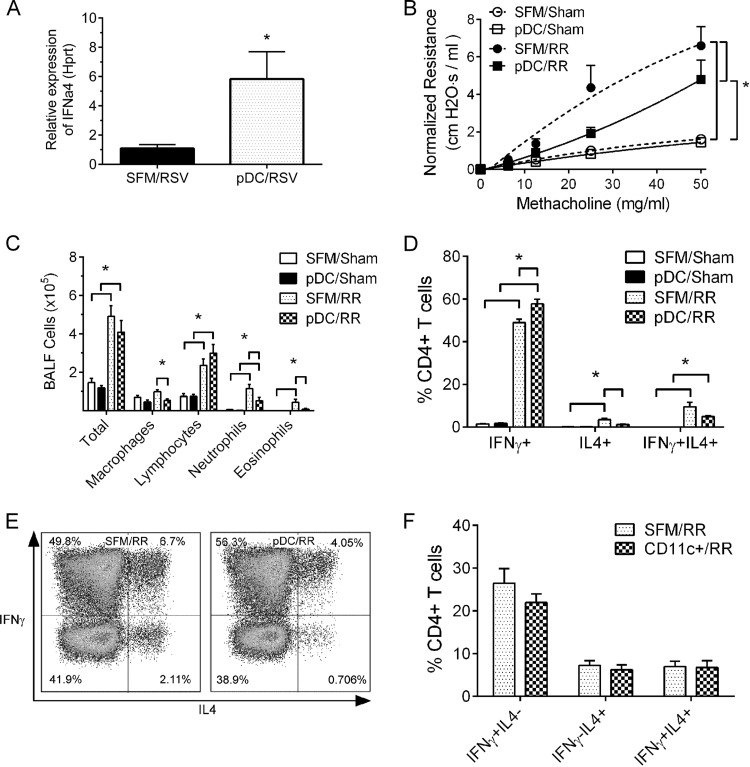
Adoptive transfer of adult pDCs prior to neonatal RSV infection induced pulmonary IFN-α expression and attenuated the Th2-biased immunopathologies during RSV reinfection. Four-day-old pups received adult pDCs (A to E), CD11c+ cells (F), or serum-free medium (SFM) (A to F) at 16 h before initial RSV infection. After 4 weeks, these mice were reinfected with RSV, and airway resistance, BALF cellularity, and Th cell profiles were measured at 6 days after reinfection. (A) Relative IFN-α expression in the lung homogenates at 1 dpi. Data are for 5 mice. *, P < 0.05. (B) Airway resistance to methacholine. Data are for 4 to 5 mice. *, P < 0.05. (C) BALF cellularity. Data are for 4 to 5 mice. *, P < 0.05. (D) Pulmonary Th profile. Data are for 4 to 6 mice. *, P < 0.05. (E) Representative flow plot of the data in panel D. (F) Pulmonary Th profile in mice receiving CD11c+ cells or SFM. Data are for 5 mice. SFM/RSV, pups receiving SFM and infected with RSV; pDC/RSV, pups receiving adult pDCs and infected with RSV; SFM/sham, mice receiving SFM and mock infected; pDC/sham, mice receiving pDCs and mock infected; SFM/RR, mice receiving SFM, infected as neonates, and reinfected with RSV as adults; pDC/RR, mice receiving pDCs, infected as neonates, and reinfected with RSV as adults; CD11c+/RR, mice receiving CD11c+ cells, infected as neonates, and reinfected with RSV as adults. Data are presented as the mean ± SEM.
Because pDCs are the major source of type I IFNs during viral infections, we measured the expression of IFN-α in lung homogenates at 1 dpi in pups receiving adult pDCs or vehicle. As shown in Fig. 5A, pups receiving adult pDCs exhibited a 6-fold induction of IFN-α in the lungs compared to that in the mice receiving vehicle.
RSV reinfection induced AHR in mice initially infected as neonates (Fig. 5B; for mice receiving SFM, infected as neonates, and reinfected with RSV as adults [SFM/RR mice] versus mice receiving SFM and mock infected [SFM/sham mice], 6.60 ± 1.01 versus 1.62 ± 0.15 cm H2O · s/ml). Adoptive transfer of adult pDCs to the lung prior to neonatal RSV infection significantly reduced AHR compared to that in control mice (for mice receiving pDCs, infected as neonates, and reinfected with RSV as adults [pDC/RR mice] versus SFM/RR mice, 4.80 ± 1.03 versus 6.60 ± 1.01 cm H2O · s/ml), and the airway resistance in mice receiving adult pDCs was no different from that in mice receiving vehicle and sham infected (for mice receiving pDCs and mock infected [pDC/sham mice] versus SFM/sham mice, 1.45 ± 0.14 versus 1.62 ± 0.15 cm H2O · s/ml).
Analysis of BALF cellularity demonstrated significant reductions in the number of alveolar macrophages (0.56 × 105 ± 0.09 × 105 versus 0.97 × 105 ± 0.09 × 105 cells/ml), neutrophils (0.51 ± 0.18 versus 1.14 ± 0.22 cells/ml), and, in particular, eosinophils (0.07 ± 0.03 versus 0.43 ± 0.16 cells/ml) during reinfection in the airways of mice receiving adult pDCs and infected with RSV as neonates (pDC/RR mice) compared to the number in control mice receiving vehicle (SFM/RR mice) (Fig. 5C). The total numbers of inflammatory cells and lymphocytes remained comparable between pDC/RR and SFM/RR mice. In addition, mice receiving adult pDCs prior to neonatal RSV infection (pDC/RR mice) recruited fewer Th2 cells (1.31% ± 0.22% versus 3.50% ± 0.69%) and multifunctional Th cells (5.05% ± 0.33% versus 9.54% ± 2.13%) and more Th1 cells (57.78% ± 2.11% versus 49.02% ± 1.59%) than mice receiving vehicle (SFM/RR mice) (Fig. 5D and E).
In order to exclude the possibility that the effects that we observed were due to the adoptive transfer of any type of cells into the neonatal lung, the above-described experiment was repeated with 3 × 104 CD11c+ cells purified from adult spleens, and the Th cell profile during reinfection was analyzed. As shown in Fig. 5F, no difference in any Th cell subset was observed between the mice receiving vehicle and those receiving CD11c+ cells.
Collectively, these data show that adoptive transfer of purified pDCs into the lungs prior to neonatal RSV infection decreased AHR, airway inflammation and eosinophilia, and pulmonary Th2-biased immune responses during RSV reinfection.
IFN-α treatment or adoptive transfer of pDCs prior to neonatal RSV infection reduced the pulmonary viral load during primary infection.
Both IFN-α treatment and adoptive transfer of adult pDCs prior to neonatal RSV infection decreased the Th2-associated immunohistopathology during reinfection. Because IFN-α is an important antiviral cytokine and is produced mainly by pDCs (28), we hypothesized that IFN-α treatment or adoptive transfer of pDCs prior to RSV infection reduces the pulmonary viral load. Four-day-old pups were intranasally instilled with 2 × 103 U of IFN-α, 3 × 104 pDCs, or vehicle. At 16 h after instillation, these mice were infected with RSV and lungs were isolated at 4 dpi for virus quantification using both plaque assays and real-time RT-PCR (Fig. 6).
FIG 6.
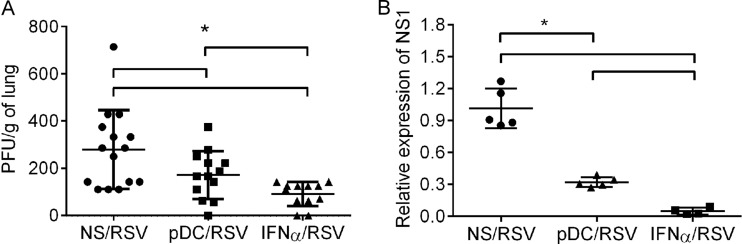
IFN-α treatment or adoptive transfer of pDCs decreased the pulmonary viral load during primary RSV infection. Four-day-old pups were treated with IFN-α or vehicle or received pDCs at 16 h prior to RSV infection. At 4 dpi, lungs were isolated and viral loads were measured in lung homogenates by plaque assay or real-time RT-PCR. (A) Plaque assay. Data are for 12 to 15 mice. *, P < 0.05. (B) Relative expression of the NS1 gene. Threshold cycle values were normalized to those for the Hprt housekeeping gene and then compared to those for NS/RSV mice. Data are for 4 to 5 mice. *, P < 0.05. NS/RSV, mice treated with vehicle and infected with RSV; pDC/RSV, mice receiving pDCs and infected with RSV; IFN-α/RSV, mice treated with IFN-α and infected with RSV. Individual data are shown as the mean ± SEM.
Plaque assays demonstrated that IFN-α treatment considerably reduced the live RSV titer from 280 ± 43 to 91 ± 15 PFU/g of lung (Fig. 6A). Consistent with the plaque assay data, real-time RT-PCR data demonstrated a 96% reduction in the relative expression of the NS1 gene in mice pretreated with IFN-α compared to that in control mice receiving vehicle (Fig. 6B). The adoptive transfer of pDCs prior to RSV infection similarly resulted in a moderate, but significant, reduction in the pulmonary viral load, as evidenced by a decrease in the live RSV titer from 280 ± 43 to 172 ± 28 PFU/g of lung and a 66% reduction in the relative expression of the NS1 gene compared to that in the mice receiving vehicle.
IFN-α treatment prior to neonatal RSV infection reduced the T helper cell response during primary infection.
As mentioned above, we and other groups have shown that the hallmark of the immunopathophysiology during reinfection in mice initially infected as neonates is the biased Th2 responses following reinfection in the adult. Because IFN-α treatment prior to neonatal RSV infection reduced these Th2-biased responses to RSV upon reinfection, we sought to determine if IFN-α treatment also changed the Th profile in the lung during primary infection (Fig. 7). Four-day-old pups were instilled with IFN-α prior to RSV infection. The Th profile was measured in the lung at 6 dpi using flow cytometry.
FIG 7.
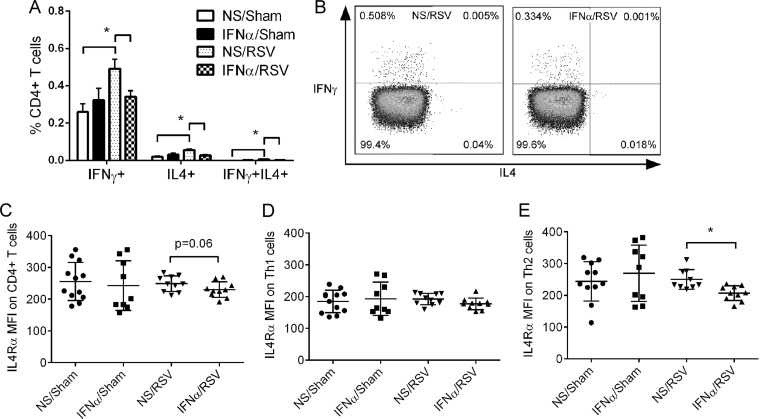
IFN-α treatment reduced Th responses to primary RSV infection in neonates. Four-day-old pups were treated with IFN-α or vehicle at 16 h prior to RSV infection. The pulmonary T cell profile or IL-4Rα expression on Th cells was measured at 6 dpi or 2 dpi, respectively. (A) Pulmonary Th profile at 6 dpi. Data are for 5 to 8 mice. *, P < 0.05. Data are presented as the mean ± SEM. (B) Representative flow plots of the Th profile. (C) Relative expression of IL-4Rα on CD4+ T cells. (D) Relative expression of IL-4Rα on Th1 cells. (E) Relative expression of IL-4Rα on Th2 cells. Data are for 9 to 12 mice. *, P < 0.05. MFI, mean fluorescence intensity; NS/sham, mice treated with vehicle and mock infected; IFN-α/sham, mice treated with IFN-α and mock infected; NS/RSV, mice treated with vehicle and infected with RSV; IFN-α/RSV, mice treated with IFN-α and infected with RSV. Individual data are shown as the mean ± SEM.
Neonatal RSV infection induced the recruitment of Th1 cells, Th2 cells, and multifunctional Th cells in the lungs (Fig. 7A and B). Treatment with IFN-α prior to RSV infection reduced all of these Th cell subsets. Th1 cells declined from 0.49% ± 0.05% to 0.34% ± 0.032%, Th2 cells decreased from 0.057% ± 0.006% to 0.027% ± 0.005%, and multifunctional Th cells decreased from 0.006% ± 0.001% to 0.001% ± 0.0003% of CD4+ T cells. In fact, the levels of these three Th cell subsets decreased to homeostatic baseline levels, as observed in the sham-infected mice (NS/sham or IFN-α/sham mice), despite the fact that live virus was detected in the lungs of IFN-α-treated and RSV-infected mice (Fig. 6).
Importantly, we have recently shown that IL-4Rα expression on T helper cells plays a central pathogenic role in the Th2-biased responses of mice initially infected as neonates (4); we therefore measured the level of IL-4Rα on Th cells during initial RSV infection. Mice were treated with IFN-α prior to RSV infection, and the IL-4Rα levels on pulmonary CD4+ T cells were measured at 2 dpi, as previously published (4). Consistent with our previous findings (4), RSV infection in neonates did not change the expression of IL-4Rα on CD4+ T, Th1, or Th2 cells (Fig. 7C to E). Treatment with IFN-α significantly reduced the expression of IL-4Rα on Th2 cells. No significant decrease in expression of IL-4Rα on Th1 cells was observed between IFN-α- and vehicle-treated mice (mice treated with IFN-α and infected with RSV [IFN-α/RSV mice] and mice treated with vehicle and infected with RSV [NS/RSV mice]) or between IFN-α/RSV mice and NS/sham or IFN-α/sham mice.
Collectively, the data presented above demonstrate that IFN-α treatment prior to neonatal RSV infection reduced pulmonary Th cell responses and IL-4Rα expression on Th2 cells, while it decreased the viral load, suggesting dual mechanisms of action.
DISCUSSION
Despite nearly 60 years of intense research on RSV, we still have no efficacious vaccines or therapeutics for this virus. The initial use of adult instead of neonatal animal models and the lack of knowledge of immune responses in infants are partially responsible for the slow progress. In fact, mice initially infected as neonates develop biased Th2 pulmonary immune responses upon reinfection with RSV, whereas mice initially infected as adults develop dominant Th1 responses upon reinfection with RSV. In the present study, we investigated the role of pDCs and type I IFNs in the pathogenesis of RSV using our neonatal mouse model. We showed that primary RSV infection in neonatal mice induced limited pDC and type I IFN responses compared to the responses seen in adult mice. We further demonstrated that intranasal instillation of recombinant IFN-α or the adoptive transfer of adult pDCs to the lungs prior to neonatal RSV infection considerably decreased the immunopathophysiology during RSV reinfection, including reducing AHR, eosinophilia, mucus hyperproduction, and pulmonary Th2 responses. These data suggest that the limited pDC and type I IFN responses in neonates during primary infection permit the Th2-biased immunopathogenesis during RSV reinfection.
Although both prophylactic IFN-α treatment and adoptive transfer of adult pDCs exerted similar immunomodulatory effects during RSV reinfection, there were differences in the extent of the effects between the two treatments that cannot be explained by the differences in the antiviral properties of IFN-α and pDCs (i.e., pDC transfer had less of an effect on viral load than IFN-α treatment). These differences include the following: (i) IFN-α treatment decreased the number of eosinophils and had no effect on other cell types in the BALF during reinfection, whereas adoptive transfer of pDCs decreased the number of eosinophils, monocytes, and neutrophils (Fig. 4C versus 5B); and (ii) IFN-α treatment decreased pulmonary Th2 cells but had no effect on the number of Th1 cells during reinfection, whereas adoptive transfer of pDCs decreased the number of Th2 cells and increased the number of Th1 cells in the lung (Fig. 4A versus 5C). Although these differences were not tested in this study and were outside the scope of this study, they might be due to properties other than the antiviral effect of pDCs. For example, in addition to producing IFN-α upon viral infection, pDCs have been shown to acquire dendrites, upregulate major histocompatibility complex class II and costimulatory molecules, and activate naive T cells (29). Therefore, adoptively transferred adult pDCs might serve not only as a source of IFN-α but also as additional antigen-presenting cells driving the immune response toward the Th1 arm. Interestingly, adoptive transfer of adult pDCs prior to RSV infection in the neonate appeared to be less protective during the initial infection (i.e., the pulmonary viral load was higher) and less beneficial during reinfection (i.e., AHR was increased) than administration of IFN-α. This phenomenon may be because the number of transferred pDCs was insufficient to overcome the neonatal immune microenvironment of the lung.
The mechanisms via which IFN-α treatment mitigated the Th2-biased immunopathologies during RSV reinfection in mice initially infected as neonates appear to be multifactorial. The data presented here clearly demonstrate that administration of IFN-α reduced the pulmonary viral load during primary infection in the neonate and the Th2 bias during reinfection in the adult. The reduction in viral load undoubtedly accounts for some improvement in RSV-mediated disease. Although it is difficult to dissociate the antiviral effects of IFN-α, our previous study clearly demonstrated that enhanced IL-4Rα expression on neonatal Th cells compared to that on adult Th cells plays a significant role in the development of the Th2-biased immunopathologies following RSV reinfection (4). Furthermore, the reduction of IL-4Rα on Th2 cells during primary infection prevents Th2 bias, airway hyperreactivity, mucus hyperproduction, and inflammation upon reinfection without affecting the viral load (6). In line with this observation, the data presented here demonstrate that IFN-α treatment prior to RSV infection reduces the expression of IL-4Rα on Th2 cells (Fig. 7C to E). Because IL-4Rα levels are higher in neonates, where viral loads are lower than those in adults (30), and do not change in response to RSV infection (4), we also propose that the immunomodulatory aspects of IFN-α play a role in the effects observed here. Thus, we believe that both of the mechanisms of IFN-α action outlined above are involved in the observed protective effect.
Although the IFN-α-induced downregulation of IL-4Rα was moderate during primary infection, the outcome on disease following reinfection was substantial (i.e., airway function was improved, and pulmonary inflammation, mucus hyperproduction, and the Th2-biased response were dramatically reduced). In fact, these results were strikingly similar to those that we previously obtained with antisense oligonucleotides to IL-4Rα: the moderate downregulation of IL-4Rα during neonatal RSV infection completely abolished reinfection-associated airway hyperactivity and significantly decreased Th2-biased responses (6). We believe this phenomenon to be due to fine-tuning of IL-4Rα signaling, which results in a rebalancing of the immune response so that Th2 cells no longer skew the immune response. This is evidenced by the primary Th response, in which the ratio of Th1/Th2 is increased in IFN-α-treated mice (for IFN-α/RSV versus NS/RSV mice, 9.230 ± 8.230 versus 5.137 ± 4.137).
In addition to its antiviral effects, IFN-α has also been shown to promote Th1 responses (31) and suppress Th2 responses (32) ex vivo. As expected in our model (Fig. 7A and B), IFN-α treatment prior to neonatal RSV infection significantly decreased the number of pulmonary Th2 cells. Surprisingly, the number of pulmonary Th1 cells was reduced in IFN-α-treated neonates following RSV infection. Most importantly, the Th cell responses to RSV in IFN-α-treated neonatal mice were reduced to the same level as those in sham-infected animals, yet these diminished initial responses remained adequate to promote strong Th1 and substantially reduced Th2 responses upon reinfection. The reason for the decrease in pulmonary Th1 cells following RSV infection in the neonate treated with IFN-α and the strong Th1 response upon reinfection is unclear (Fig. 6). We cannot exclude the possibility that the reduction in the pulmonary viral load is responsible for the decrease in pulmonary Th1 cells in IFN-α-treated, RSV-infected neonates, but our data presented here demonstrating that IFN-α modulates IL-4Rα levels during initial infection and our prior data (6) demonstrating that modulating IL-4Rα levels at the time of initial infection changes immune responses in the neonate suggest that IFN-α also modulates the Th1/Th2 balance. Thus, we believe that our data support two mechanisms of IFN-α activity on reducing RSV-mediated disease: (i) it reduces the viral load, and (ii) it modulates immune responses through IL-4Rα. We feel that our data are instructive for RSV vaccine design and suggest that an adjuvant capable of inducing IFN-α responses would offer an additional benefit due to its ability to modulate the neonatal immune response.
There were early attempts in the late 1980s and early 1990s to test the feasibility of IFN-α as an RSV therapeutic. Unfortunately, the clinical trial showed that daily intramuscular injection of IFN-α into RSV-infected infants for three consecutive days did not change the overall clinical course, the duration of oxygen requirement, or viral shedding, although the treatment did induce a more rapid drop in the clinical score during the first 3 days (33). The difference between these human studies and our data in neonates may be explained by the different strategies that we employed. First, in our model, IFN-α was instilled directly onto the mucosal surface, where RSV infection naturally occurs, whereas in the human studies, IFN-α was administered systemically. Second, in our model, IFN-α was instilled prior to RSV infection, with the hope of preventing Th2-biased responses during reinfection, whereas in the human studies, IFN-α was administered as a therapy after RSV infection. One very important result of this human study is that it demonstrated that the use of IFN-α in infants is safe (34). In fact, RSV-infected infants ranging from 2 to 8 months old received daily maximal doses of 70,000 U of IFN-α per kg of body weight with no toxicity being observed. One of the most intimidating difficulties in RSV vaccine or therapeutic research is that RSV is an infant disease, and any intervention for infants must be applied with considerable forethought and extreme caution. We feel that the safety of IFN-α in human infants and the encouraging results from our studies strongly suggest that IFN-α is a promising target for future RSV vaccine design due to its immunomodulatory property.
In summary, limited type I IFN and pDC responses in neonatal mice permit the development of Th2-biased immunopathogenesis during RSV reinfection. IFN-α treatment or adoptive transfer of adult pDCs prior to neonatal RSV infection reduced the Th2-biased immunopathophysiologies, possibly because of their antiviral properties and because they downregulated IL-4Rα expression on Th2 cells. These observations support using an adjuvant capable of inducing IFN-α in future RSV vaccine strategies.
ACKNOWLEDGMENTS
We thank Stuart Turvey and Nico Marr for insightful discussions and for sharing relevant human data.
This work was supported by U.S. National Institutes of Health grants (R01AI090059, R01ES015050, and P42ES013648) to S.A.C.
Footnotes
Published ahead of print 11 June 2014
REFERENCES
- 1.Sigurs N, Aljassim F, Kjellman B, Robinson PD, Sigurbergsson F, Bjarnason R, Gustafsson PM. 2010. Asthma and allergy patterns over 18 years after severe RSV bronchiolitis in the first year of life. Thorax 65:1045–1052. 10.1136/thx.2009.121582 [DOI] [PubMed] [Google Scholar]
- 2.Paramore LC, Ciuryla V, Ciesla G, Liu L. 2004. Economic impact of respiratory syncytial virus-related illness in the US: an analysis of national databases. Pharmacoeconomics 22:275–284. 10.2165/00019053-200422050-00001 [DOI] [PubMed] [Google Scholar]
- 3.Kim HW, Canchola JG, Brandt CD, Pyles G, Chanock RM, Jensen K, Parrott RH. 1969. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am. J. Epidemiol. 89:422–434 [DOI] [PubMed] [Google Scholar]
- 4.You D, Marr N, Saravia J, Shrestha B, Lee GI, Turvey SE, Brombacher F, Herbert DR, Cormier SA. 2013. IL-4Ralpha on CD4+ T cells plays a pathogenic role in respiratory syncytial virus reinfection in mice infected initially as neonates. J. Leukoc. Biol. 93:933–942. 10.1189/jlb.1012498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cormier SA, You D, Honnegowda S. 2010. The use of a neonatal mouse model to study respiratory syncytial virus infections. Expert Rev. Anti Infect. Ther. 8:1371–1380. 10.1586/eri.10.125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ripple MJ, You D, Honnegowda S, Giaimo JD, Sewell AB, Becnel DM, Cormier SA. 2010. Immunomodulation with IL-4R alpha antisense oligonucleotide prevents respiratory syncytial virus-mediated pulmonary disease. J. Immunol. 185:4804–4811. 10.4049/jimmunol.1000484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.You D, Becnel D, Wang K, Ripple M, Daly M, Cormier SA. 2006. Exposure of neonates to respiratory syncytial virus is critical in determining subsequent airway response in adults. Respir. Res. 7:107. 10.1186/1465-9921-7-107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Culley FJ, Pollott J, Openshaw PJ. 2002. Age at first viral infection determines the pattern of T cell-mediated disease during reinfection in adulthood. J. Exp. Med. 196:1381–1386. 10.1084/jem.20020943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dakhama A, Park JW, Taube C, Joetham A, Balhorn A, Miyahara N, Takeda K, Gelfand EW. 2005. The enhancement or prevention of airway hyperresponsiveness during reinfection with respiratory syncytial virus is critically dependent on the age at first infection and IL-13 production. J. Immunol. 175:1876–1883. 10.4049/jimmunol.175.3.1876 [DOI] [PubMed] [Google Scholar]
- 10.Welliver TP, Garofalo RP, Hosakote Y, Hintz KH, Avendano L, Sanchez K, Velozo L, Jafri H, Chavez-Bueno S, Ogra PL, McKinney L, Reed JL, Welliver RC. 2007. Severe human lower respiratory tract illness caused by respiratory syncytial virus and influenza virus is characterized by the absence of pulmonary cytotoxic lymphocyte responses. J. Infect. Dis. 195:1126–1136. 10.1086/512615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoebee B, Rietveld E, Bont L, Oosten M, Hodemaekers HM, Nagelkerke NJ, Neijens HJ, Kimpen JL, Kimman TG. 2003. Association of severe respiratory syncytial virus bronchiolitis with interleukin-4 and interleukin-4 receptor alpha polymorphisms. J. Infect. Dis. 187:2–11. 10.1086/345859 [DOI] [PubMed] [Google Scholar]
- 12.Puthothu B, Krueger M, Forster J, Heinzmann A. 2006. Association between severe respiratory syncytial virus infection and IL13/IL4 haplotypes. J. Infect. Dis. 193:438–441. 10.1086/499316 [DOI] [PubMed] [Google Scholar]
- 13.Hornung V, Schlender J, Guenthner-Biller M, Rothenfusser S, Endres S, Conzelmann KK, Hartmann G. 2004. Replication-dependent potent IFN-alpha induction in human plasmacytoid dendritic cells by a single-stranded RNA virus. J. Immunol. 173:5935–5943. 10.4049/jimmunol.173.10.5935 [DOI] [PubMed] [Google Scholar]
- 14.Johnson TR, Johnson CN, Corbett KS, Edwards GC, Graham BS. 2011. Primary human mDC1, mDC2, and pDC dendritic cells are differentially infected and activated by respiratory syncytial virus. PLoS One 6:e16458. 10.1371/journal.pone.0016458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boogaard I, van Oosten M, van Rijt LS, Muskens F, Kimman TG, Lambrecht BN, Buisman AM. 2007. Respiratory syncytial virus differentially activates murine myeloid and plasmacytoid dendritic cells. Immunology 122:65–72. 10.1111/j.1365-2567.2007.02613.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smit JJ, Lindell DM, Boon L, Kool M, Lambrecht BN, Lukacs NW. 2008. The balance between plasmacytoid DC versus conventional DC determines pulmonary immunity to virus infections. PLoS One 3:e1720. 10.1371/journal.pone.0001720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smit JJ, Rudd BD, Lukacs NW. 2006. Plasmacytoid dendritic cells inhibit pulmonary immunopathology and promote clearance of respiratory syncytial virus. J. Exp. Med. 203:1153–1159. 10.1084/jem.20052359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jewell NA, Vaghefi N, Mertz SE, Akter P, Peebles RS, Jr, Bakaletz LO, Durbin RK, Flano E, Durbin JE. 2007. Differential type I interferon induction by respiratory syncytial virus and influenza A virus in vivo. J. Virol. 81:9790–9800. 10.1128/JVI.00530-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guerrero-Plata A, Baron S, Poast JS, Adegboyega PA, Casola A, Garofalo RP. 2005. Activity and regulation of alpha interferon in respiratory syncytial virus and human metapneumovirus experimental infections. J. Virol. 79:10190–10199. 10.1128/JVI.79.16.10190-10199.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gill MA, Palucka AK, Barton T, Ghaffar F, Jafri H, Banchereau J, Ramilo O. 2005. Mobilization of plasmacytoid and myeloid dendritic cells to mucosal sites in children with respiratory syncytial virus and other viral respiratory infections. J. Infect. Dis. 191:1105–1115. 10.1086/428589 [DOI] [PubMed] [Google Scholar]
- 21.McIntosh K. 1978. Interferon in nasal secretions from infants with viral respiratory tract infections. J. Pediatr. 93:33–36. 10.1016/S0022-3476(78)80595-2 [DOI] [PubMed] [Google Scholar]
- 22.Marr N, Wang TI, Kam SH, Hu YS, Sharma AA, Lam A, Markowski J, Solimano A, Lavoie PM, Turvey SE. 2014. Attenuation of respiratory syncytial virus-induced and RIG-I-dependent type I IFN responses in human neonates and very young children. J. Immunol. 192:948–957. 10.4049/jimmunol.1302007 [DOI] [PubMed] [Google Scholar]
- 23.National Research Council. 2011. Guide for the care and use of laboratory animals, 8th ed. National Academies Press, Washington, DC [Google Scholar]
- 24.McKimm-Breschkin JL. 2004. A simplified plaque assay for respiratory syncytial virus—direct visualization of plaques without immunostaining. J. Virol. Methods 120:113–117. 10.1016/j.jviromet.2004.02.020 [DOI] [PubMed] [Google Scholar]
- 25.Boukhvalova MS, Yim KC, Prince GA, Blanco JC. 2010. Methods for monitoring dynamics of pulmonary RSV replication by viral culture and by real-time reverse transcription-PCR in vivo: detection of abortive viral replication. Curr. Protoc. Cell Biol. Chapter 26:Unit 26.6. 10.1002/0471143030.cb2606s46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spann KM, Tran KC, Chi B, Rabin RL, Collins PL. 2004. Suppression of the induction of alpha, beta, and lambda interferons by the NS1 and NS2 proteins of human respiratory syncytial virus in human epithelial cells and macrophages. J. Virol. 78:4363–4369. 10.1128/JVI.78.8.4363-4369.2004 (Erratum, 78:6705, http://dx.doi.org/10.1128/JVI.78.12.6705.2004.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zucchini N, Bessou G, Robbins SH, Chasson L, Raper A, Crocker PR, Dalod M. 2008. Individual plasmacytoid dendritic cells are major contributors to the production of multiple innate cytokines in an organ-specific manner during viral infection. Int. Immunol. 20:45–56. 10.1093/intimm/dxm119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Asselin-Paturel C, Boonstra A, Dalod M, Durand I, Yessaad N, Dezutter-Dambuyant C, Vicari A, O'Garra A, Biron C, Briere F, Trinchieri G. 2001. Mouse type I IFN-producing cells are immature APCs with plasmacytoid morphology. Nat. Immunol. 2:1144–1150. 10.1038/ni736 [DOI] [PubMed] [Google Scholar]
- 29.Villadangos JA, Young L. 2008. Antigen-presentation properties of plasmacytoid dendritic cells. Immunity 29:352–361. 10.1016/j.immuni.2008.09.002 [DOI] [PubMed] [Google Scholar]
- 30.Ruckwardt TJ, Malloy AM, Gostick E, Price DA, Dash P, McClaren JL, Thomas PG, Graham BS. 2011. Neonatal CD8 T-cell hierarchy is distinct from adults and is influenced by intrinsic T cell properties in respiratory syncytial virus infected mice. PLoS Pathog. 7:e1002377. 10.1371/journal.ppat.1002377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brinkmann V, Geiger T, Alkan S, Heusser CH. 1993. Interferon alpha increases the frequency of interferon gamma-producing human CD4+ T cells. J. Exp. Med. 178:1655–1663. 10.1084/jem.178.5.1655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pene J, Rousset F, Briere F, Chretien I, Bonnefoy JY, Spits H, Yokota T, Arai N, Arai K, Banchereau J. 1988. IgE production by normal human lymphocytes is induced by interleukin 4 and suppressed by interferons gamma and alpha and prostaglandin E2. Proc. Natl. Acad. Sci. U. S. A. 85:6880–6884. 10.1073/pnas.85.18.6880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sung RY, Yin J, Oppenheimer SJ, Tam JS, Lau J. 1993. Treatment of respiratory syncytial virus infection with recombinant interferon alfa-2a. Arch. Dis. Child. 69:440–442. 10.1136/adc.69.4.440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Portnoy J, Hicks R, Pacheco F, Olson L. 1988. Pilot study of recombinant interferon alpha-2a for treatment of infants with bronchiolitis induced by respiratory syncytial virus. Antimicrob. Agents Chemother. 32:589–591. 10.1128/AAC.32.4.589 [DOI] [PMC free article] [PubMed] [Google Scholar]