Abstract
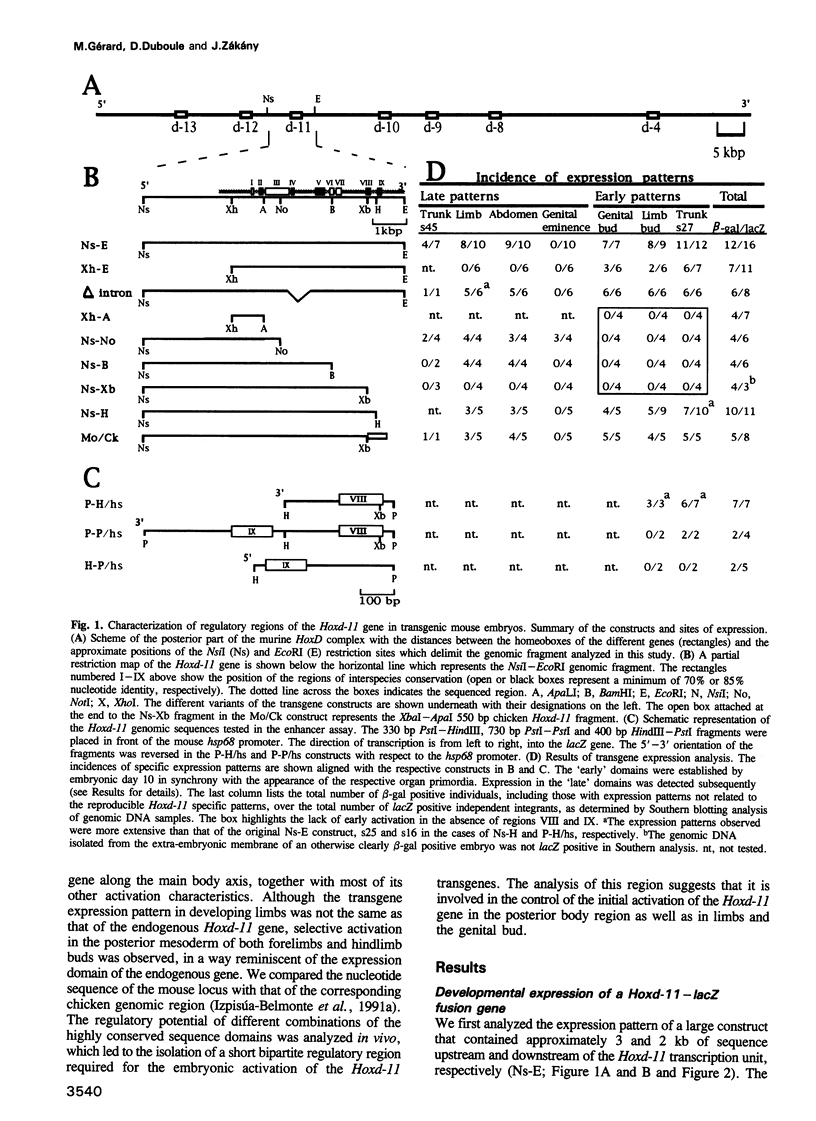
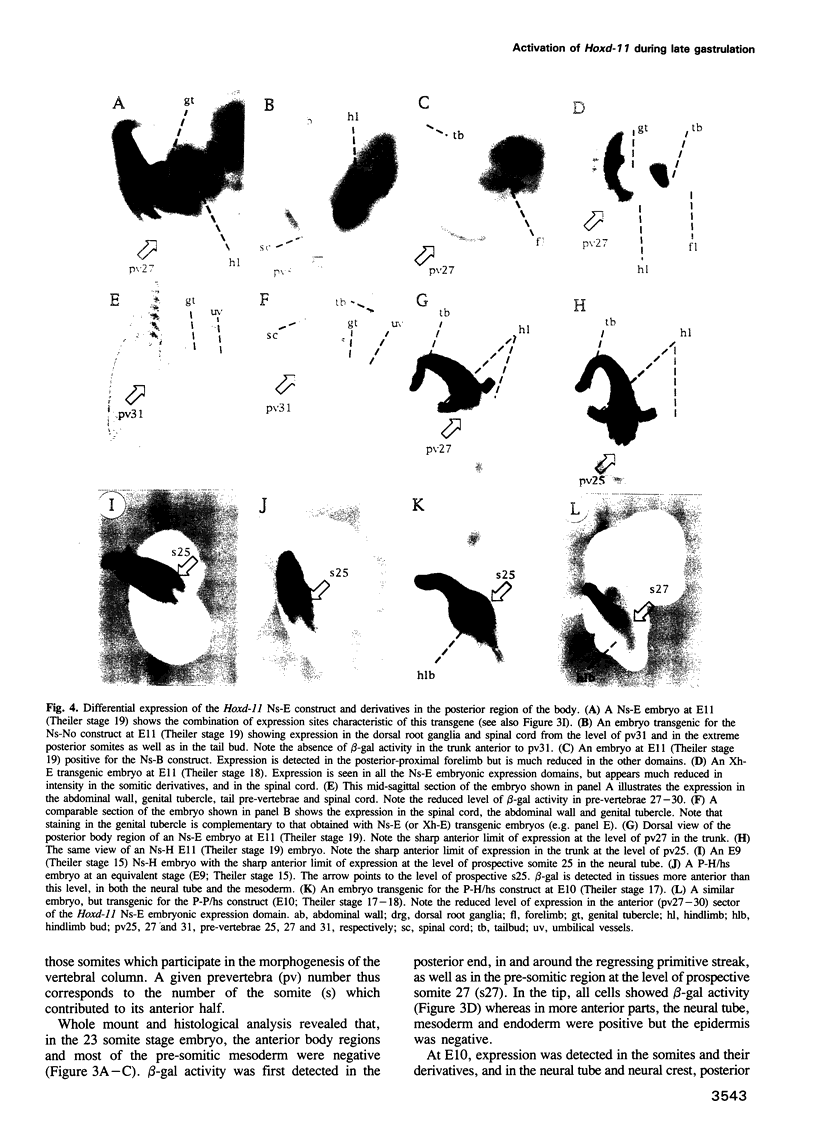
We have used reporter gene constructs to study the cis regulation of the Hoxd-11 gene (previously Hox-4.6) in transgenic mice. We identified a 5 kb regulatory unit, which was able to reproduce important aspects of the initial activation of the gene along the major body axis. The comparison of the nucleotide sequence of this DNA fragment with the corresponding avian genomic region revealed the presence of seven highly homologous stretches of DNA outside the protein coding regions. In particular, the 3' flanking region contained two such domains that are required to mediate the embryonic activation. A chimeric construct containing the two short homologous regions from the chicken gene could replace the complete murine fragment thus demonstrating that the conserved domains carry the main regulatory elements involved in this activation. The first half of this bipartite regulatory region has enhancer activity when tested with a heterologous promoter, while the second half is required to restrict the enhancer activity to the proper expression domain. These results suggest that stage- and tissue-specific cooperation between regulatory elements is required to control properly the activity of the Hoxd-11 promoter.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bieberich C. J., Utset M. F., Awgulewitsch A., Ruddle F. H. Evidence for positive and negative regulation of the Hox-3.1 gene. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8462–8466. doi: 10.1073/pnas.87.21.8462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisaka O., Capecchi M. R. Regionally restricted developmental defects resulting from targeted disruption of the mouse homeobox gene hox-1.5. Nature. 1991 Apr 11;350(6318):473–479. doi: 10.1038/350473a0. [DOI] [PubMed] [Google Scholar]
- Chisaka O., Musci T. S., Capecchi M. R. Developmental defects of the ear, cranial nerves and hindbrain resulting from targeted disruption of the mouse homeobox gene Hox-1.6. Nature. 1992 Feb 6;355(6360):516–520. doi: 10.1038/355516a0. [DOI] [PubMed] [Google Scholar]
- Dollé P., Izpisúa-Belmonte J. C., Boncinelli E., Duboule D. The Hox-4.8 gene is localized at the 5' extremity of the Hox-4 complex and is expressed in the most posterior parts of the body during development. Mech Dev. 1991 Dec;36(1-2):3–13. doi: 10.1016/0925-4773(91)90067-g. [DOI] [PubMed] [Google Scholar]
- Dollé P., Izpisúa-Belmonte J. C., Brown J. M., Tickle C., Duboule D. HOX-4 genes and the morphogenesis of mammalian genitalia. Genes Dev. 1991 Oct;5(10):1767–1767. doi: 10.1101/gad.5.10.1767. [DOI] [PubMed] [Google Scholar]
- Dollé P., Izpisúa-Belmonte J. C., Falkenstein H., Renucci A., Duboule D. Coordinate expression of the murine Hox-5 complex homoeobox-containing genes during limb pattern formation. Nature. 1989 Dec 14;342(6251):767–772. doi: 10.1038/342767a0. [DOI] [PubMed] [Google Scholar]
- Duboule D., Dollé P. The structural and functional organization of the murine HOX gene family resembles that of Drosophila homeotic genes. EMBO J. 1989 May;8(5):1497–1505. doi: 10.1002/j.1460-2075.1989.tb03534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duboule D. The vertebrate limb: a model system to study the Hox/HOM gene network during development and evolution. Bioessays. 1992 Jun;14(6):375–384. doi: 10.1002/bies.950140606. [DOI] [PubMed] [Google Scholar]
- Durand B., Saunders M., Leroy P., Leid M., Chambon P. All-trans and 9-cis retinoic acid induction of CRABPII transcription is mediated by RAR-RXR heterodimers bound to DR1 and DR2 repeated motifs. Cell. 1992 Oct 2;71(1):73–85. doi: 10.1016/0092-8674(92)90267-g. [DOI] [PubMed] [Google Scholar]
- Graham A., Papalopulu N., Krumlauf R. The murine and Drosophila homeobox gene complexes have common features of organization and expression. Cell. 1989 May 5;57(3):367–378. doi: 10.1016/0092-8674(89)90912-4. [DOI] [PubMed] [Google Scholar]
- Green S., Issemann I., Sheer E. A versatile in vivo and in vitro eukaryotic expression vector for protein engineering. Nucleic Acids Res. 1988 Jan 11;16(1):369–369. doi: 10.1093/nar/16.1.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izpisùa-Belmonte J. C., Dollé P., Renucci A., Zappavigna V., Falkenstein H., Duboule D. Primary structure and embryonic expression pattern of the mouse Hox-4.3 homeobox gene. Development. 1990 Nov;110(3):733–745. [PubMed] [Google Scholar]
- Izpisúa-Belmonte J. C., Duboule D. Homeobox genes and pattern formation in the vertebrate limb. Dev Biol. 1992 Jul;152(1):26–36. doi: 10.1016/0012-1606(92)90153-8. [DOI] [PubMed] [Google Scholar]
- Izpisúa-Belmonte J. C., Falkenstein H., Dollé P., Renucci A., Duboule D. Murine genes related to the Drosophila AbdB homeotic genes are sequentially expressed during development of the posterior part of the body. EMBO J. 1991 Aug;10(8):2279–2289. doi: 10.1002/j.1460-2075.1991.tb07764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izpisúa-Belmonte J. C., Tickle C., Dollé P., Wolpert L., Duboule D. Expression of the homeobox Hox-4 genes and the specification of position in chick wing development. Nature. 1991 Apr 18;350(6319):585–589. doi: 10.1038/350585a0. [DOI] [PubMed] [Google Scholar]
- Jegalian B. G., De Robertis E. M. Homeotic transformations in the mouse induced by overexpression of a human Hox3.3 transgene. Cell. 1992 Dec 11;71(6):901–910. doi: 10.1016/0092-8674(92)90387-r. [DOI] [PubMed] [Google Scholar]
- Kessel M., Balling R., Gruss P. Variations of cervical vertebrae after expression of a Hox-1.1 transgene in mice. Cell. 1990 Apr 20;61(2):301–308. doi: 10.1016/0092-8674(90)90810-2. [DOI] [PubMed] [Google Scholar]
- Lathe R., Vilotte J. L., Clark A. J. Plasmid and bacteriophage vectors for excision of intact inserts. Gene. 1987;57(2-3):193–201. doi: 10.1016/0378-1119(87)90122-3. [DOI] [PubMed] [Google Scholar]
- Le Mouellic H., Lallemand Y., Brûlet P. Homeosis in the mouse induced by a null mutation in the Hox-3.1 gene. Cell. 1992 Apr 17;69(2):251–264. doi: 10.1016/0092-8674(92)90406-3. [DOI] [PubMed] [Google Scholar]
- Lufkin T., Dierich A., LeMeur M., Mark M., Chambon P. Disruption of the Hox-1.6 homeobox gene results in defects in a region corresponding to its rostral domain of expression. Cell. 1991 Sep 20;66(6):1105–1119. doi: 10.1016/0092-8674(91)90034-v. [DOI] [PubMed] [Google Scholar]
- Lufkin T., Mark M., Hart C. P., Dollé P., LeMeur M., Chambon P. Homeotic transformation of the occipital bones of the skull by ectopic expression of a homeobox gene. Nature. 1992 Oct 29;359(6398):835–841. doi: 10.1038/359835a0. [DOI] [PubMed] [Google Scholar]
- Marshall H., Nonchev S., Sham M. H., Muchamore I., Lumsden A., Krumlauf R. Retinoic acid alters hindbrain Hox code and induces transformation of rhombomeres 2/3 into a 4/5 identity. Nature. 1992 Dec 24;360(6406):737–741. doi: 10.1038/360737a0. [DOI] [PubMed] [Google Scholar]
- McGinnis W., Krumlauf R. Homeobox genes and axial patterning. Cell. 1992 Jan 24;68(2):283–302. doi: 10.1016/0092-8674(92)90471-n. [DOI] [PubMed] [Google Scholar]
- Morgan B. A., Izpisúa-Belmonte J. C., Duboule D., Tabin C. J. Targeted misexpression of Hox-4.6 in the avian limb bud causes apparent homeotic transformations. Nature. 1992 Jul 16;358(6383):236–239. doi: 10.1038/358236a0. [DOI] [PubMed] [Google Scholar]
- Pollock R. A., Jay G., Bieberich C. J. Altering the boundaries of Hox3.1 expression: evidence for antipodal gene regulation. Cell. 1992 Dec 11;71(6):911–923. doi: 10.1016/0092-8674(92)90388-s. [DOI] [PubMed] [Google Scholar]
- Püschel A. W., Balling R., Gruss P. Separate elements cause lineage restriction and specify boundaries of Hox-1.1 expression. Development. 1991 May;112(1):279–287. doi: 10.1242/dev.112.1.279. [DOI] [PubMed] [Google Scholar]
- Renucci A., Zappavigna V., Zàkàny J., Izpisúa-Belmonte J. C., Bürki K., Duboule D. Comparison of mouse and human HOX-4 complexes defines conserved sequences involved in the regulation of Hox-4.4. EMBO J. 1992 Apr;11(4):1459–1468. doi: 10.1002/j.1460-2075.1992.tb05190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogina B., Coelho C. N., Kosher R. A., Upholt W. B. The pattern of expression of the chicken homolog of HOX1I in the developing limb suggests a possible role in the ectodermal inhibition of chondrogenesis. Dev Dyn. 1992 Jan;193(1):92–101. doi: 10.1002/aja.1001930112. [DOI] [PubMed] [Google Scholar]
- Ruberte E., Dolle P., Chambon P., Morriss-Kay G. Retinoic acid receptors and cellular retinoid binding proteins. II. Their differential pattern of transcription during early morphogenesis in mouse embryos. Development. 1991 Jan;111(1):45–60. doi: 10.1242/dev.111.1.45. [DOI] [PubMed] [Google Scholar]
- Scott M. P. Vertebrate homeobox gene nomenclature. Cell. 1992 Nov 13;71(4):551–553. doi: 10.1016/0092-8674(92)90588-4. [DOI] [PubMed] [Google Scholar]
- Sham M. H., Hunt P., Nonchev S., Papalopulu N., Graham A., Boncinelli E., Krumlauf R. Analysis of the murine Hox-2.7 gene: conserved alternative transcripts with differential distributions in the nervous system and the potential for shared regulatory regions. EMBO J. 1992 May;11(5):1825–1836. doi: 10.1002/j.1460-2075.1992.tb05234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam P. P., Beddington R. S. The formation of mesodermal tissues in the mouse embryo during gastrulation and early organogenesis. Development. 1987 Jan;99(1):109–126. doi: 10.1242/dev.99.1.109. [DOI] [PubMed] [Google Scholar]
- Umesono K., Giguere V., Glass C. K., Rosenfeld M. G., Evans R. M. Retinoic acid and thyroid hormone induce gene expression through a common responsive element. Nature. 1988 Nov 17;336(6196):262–265. doi: 10.1038/336262a0. [DOI] [PubMed] [Google Scholar]
- Whiting J., Marshall H., Cook M., Krumlauf R., Rigby P. W., Stott D., Allemann R. K. Multiple spatially specific enhancers are required to reconstruct the pattern of Hox-2.6 gene expression. Genes Dev. 1991 Nov;5(11):2048–2059. doi: 10.1101/gad.5.11.2048. [DOI] [PubMed] [Google Scholar]
- Yokouchi Y., Sasaki H., Kuroiwa A. Homeobox gene expression correlated with the bifurcation process of limb cartilage development. Nature. 1991 Oct 3;353(6343):443–445. doi: 10.1038/353443a0. [DOI] [PubMed] [Google Scholar]
- Yu V. C., Delsert C., Andersen B., Holloway J. M., Devary O. V., När A. M., Kim S. Y., Boutin J. M., Glass C. K., Rosenfeld M. G. RXR beta: a coregulator that enhances binding of retinoic acid, thyroid hormone, and vitamin D receptors to their cognate response elements. Cell. 1991 Dec 20;67(6):1251–1266. doi: 10.1016/0092-8674(91)90301-e. [DOI] [PubMed] [Google Scholar]
- Zakany J., Tuggle C. K., Patel M. D., Nguyen-Huu M. C. Spatial regulation of homeobox gene fusions in the embryonic central nervous system of transgenic mice. Neuron. 1988 Oct;1(8):679–691. doi: 10.1016/0896-6273(88)90167-5. [DOI] [PubMed] [Google Scholar]