ABSTRACT
Bats are known to harbor emerging RNA viruses. Recent studies have used high-throughput sequencing technology to identify various virus species, including DNA viruses that are harbored by bats; however, little is known about the nature of these potentially novel viruses. Here, we report the characterization of a novel herpesvirus isolated from an Indonesian pteropodid bat. The virus, tentatively named fruit bat alphaherpesvirus 1 (FBAHV1), has a double-stranded DNA genome of 149,459 bp. The phylogenetic analyses suggested that FBAHV1 is phylogenetically grouped with simplexviruses within the subfamily Alphaherpesvirinae. Inoculation of FBAHV1 into laboratory mice caused a lethal infection. Virus infection was observed in lung, liver, and brain tissue. Serological and PCR screening revealed that fruit bats infected with FBAHV1 or its related virus are widely distributed in Indonesia. The identification of FBAHV1 makes a considerable contribution to our understanding of simplexviruses associated with bats.
IMPORTANCE Bats are known to harbor emerging viruses, such as lyssaviruses, henipaviruses, severe acute respiratory syndrome-like coronaviruses, and filoviruses. Although alphaherpesviruses are disseminated in humans and other animals, there is little information about their distribution in bats. Here, we isolated a previously unknown alphaherpesvirus from an Indonesian fruit bat. Genome sequence analysis suggested that the virus is a member of the genus Simplexvirus within the subfamily Alphaherpesvirinae, which also includes common human viruses, such as herpes simplex virus 1 and herpes simplex virus 2. FBAHV1 is the first bat-derived alphaherpesvirus whose complete genome has been sequenced.
INTRODUCTION
Bats belonging to the order Chiroptera are some of the most abundant and widely distributed mammals in the world. Several species of bats harbor highly pathogenic zoonotic viruses, including lyssaviruses and henipaviruses, and are thought to be a reservoir for severe acute respiratory syndrome coronaviruses and filoviruses (1, 2). These viruses are categorized as RNA viruses. Nipah virus is a member of the genus Henipavirus and can be transmitted from free-ranging bats to humans or other mammalian animals, resulting in high morbidity and mortality (3). Thus, bats are thought to be a source of emerging infectious diseases and may harbor as yet unidentified zoonotic viruses. Previous studies used sensitive PCR/reverse transcription-PCR methods, which utilize consensus-degenerate primers based on genomic sequence regions that are conserved between different virus families or subfamilies, to detect novel viral nucleotide sequences in bat tissues (4–11). In addition, genomic sequences from mammalian viruses belonging to the families Adenoviridae, Herpesviridae, Poxviridae, Papillomaviridae, Retroviridae, Circoviridae, Rhabdoviridae, Astroviridae, Flaviviridae, Coronaviridae, and Picornaviridae and the subfamily Parvovirinae have been detected by high-throughput (next-generation) sequencing techniques (12–15).
Many laboratories have used cell culture techniques in an attempt to isolate viruses from bat tissues, sera, and urine; however, it is much more difficult to isolate novel viruses from biological samples than it is to detect viral genome sequences. This is due to a lack of established protocols for virus isolation, the low viral titer in the samples, poor conditions during transportation of samples from the field, and the often small amount of sample available. Nevertheless, recent studies report the successful isolation of novel viruses from bats (16–23), thereby facilitating the classification of viral species and the investigation of their virological properties.
Herpesviruses are DNA viruses that are highly disseminated in humans and other mammals, birds, reptiles, amphibians, and fish and shellfish species. The International Committee on Taxonomy of Viruses has classified more than 100 species within the order Herpesvirales, which comprises three families, three subfamilies, and 19 genera. A typical herpesvirus is composed of an envelope (diameter, 120 to 260 nm), which surrounds an icosahedral cage called a capsid that itself contains a relatively large double-stranded DNA genome (length, 120 to 250 kbp). The subfamilies Alphaherpesvirinae, Betaherpesvirinae, and Gammaherpesvirinae are classified on the basis of their genomic architectures, sequence similarities, and biological properties, including their cell tropism, reproductive cycle, and cytopathogenicity.
To date, six herpesviruses have been isolated from bats. Four of these are alphaherpesviruses, which were isolated from fruit bats (Eidolon dupreanum, Eidolon helvum, and Pteropus lylei), insectivorous bats (Lonchophylla thomasi), and an unidentified bat (24). The remaining two are betaherpesviruses, which were isolated from insectivorous bats (Miniopterus fuliginosus [25] and Miniopterus schreibersii [26]). In addition, studies using high-throughput sequencing and PCR with consensus-degenerate primers have identified partial sequences derived from betaherpesviruses and gammaherpesviruses in the fruit bat (Rousettus aegyptiacus) (27); Pteropus giganteus (28); and the insectivorous bats Eptesicus serotinus, Myotis myotis, Myotis nattereri, Nyctalus noctula, Pipistrellus nathusii, Pipistrellus pipistrellus, and Plecotus auritus (29), Hipposideros diadema (30), Eptesicus fuscus (14), and Rhinolophus ferrumequinum, Tylonycteris robustula, and Myotis ricketti (13). However, the complete genome sequences of these viruses, except for the betaherpesvirus isolated from Minopterus schreibersii, remain to be determined. Also, little is known about the epidemiology of herpesviruses in bats.
Here, we report the characterization of fruit bat alphaherpesvirus 1 (FBAHV1), a novel alphaherpesvirus isolated from an Indonesian pteropodid bat. We determined the complete genome sequence of FBAHV1 and identified it as a possible member of the genus Simplexvirus. FBAHV1 infected different cultured cell lines in vitro and laboratory mice in vivo. Moreover, we detected a high incidence of infection in different bat species captured throughout Indonesia. The results of this study further our knowledge of the biology and epidemiology of bat herpesviruses.
MATERIALS AND METHODS
Ethics statement.
All experiments involving animals in Indonesia were performed in accordance with the ethical guidelines of the Animal Care and Use Committee of the Animal Teaching Hospital, Bogor Agricultural University, which were based on the Guide for the Care and Use of Laboratory Animals, 7th and 8th editions (31, 32), the Guideline on the Care and Use of Animals for Scientific Purposes (33), and other related international guidelines. The protocol was approved by the Animal Care and Use Committee of the Veterinary Teaching Hospital, Bogor Agricultural University (permit number 05-2010 RSHP-IPB).
All of the animal experiments in Japan were performed in accordance with the National University Corporation, Hokkaido University Regulations on Animal Experimentation, which were based on the Law for the Humane Treatment and Management of Animals (law no. 105, 1973), the Fundamental Guidelines for Proper Conduct of Animal Experiment and Related Activities in Academic Research Institutions (notice no. 71 of the Ministry of Education, Culture, Sports, Science and Technology, 2006), Standards Relating to the Care and Management of Laboratory Animals and Relief of Pain (notice no. 88 of the Ministry of Environment, 2006), and other related regulations. The protocol was approved by the Institutional Animal Care and Use Committee of the National University Corporation, Hokkaido University (permit number 12-0071).
Sample information.
Collection and exportation of samples from wild fruit bats were performed with the permission of the Directorate General of Livestock and Animal Health Services, Ministry of Agriculture, Republic of Indonesia. Fruit bats were captured in the following areas: Panjalu, West Java Province (February 2010); Lima Puluh Kota, West Sumatra Province (February 2011); Popayato, Gorontalo Province (February 2011); Paguyaman, Gorontalo Province (February 2011 and February 2012); Surabaya, East Java Province (May 2012); and Magelang, Central Java Province (May 2012). Species were identified according to morphological characteristics and nucleotide sequence analysis of the mitochondrial 16S rRNA and cytochrome b genes as previously described (11). This information is summarized in Table 2.
TABLE 2.
Sample information and PCR, ELISA, and NT results
| Species | Collection location (district) | Date (yr) collected | No. of samples positive/no. of samples tested |
||
|---|---|---|---|---|---|
| PCR | ELISA | NT | |||
| Pteropus vampyrus | Panjalu | 2010 | 2/26 | 13/15 | 12/14 |
| Lima Puluh Kota | 2011 | 0/20 | 8/10 | 5/5 | |
| Surabaya | 2012 | 1/3 | 1/3 | 2/3 | |
| Magelang | 2012 | 2/20 | 5/19 | 9/18 | |
| Pteropus sp.a | Popayato | 2011 | 0/4 | 3/4 | 3/4 |
| Paguyaman | 2011 | 6/23 | 10/17 | 9/16 | |
| Paguyaman | 2012 | 0/2 | 1/2 | 1/2 | |
| Acerodon celebensis | Paguyaman | 2012 | 1/18 | 2/18 | 5/18 |
| Dobsonia sp.b | Paguyaman | 2012 | 0/17 | 1/16 | 0/16 |
| Total | 12/133 | 44/104 | 46/96 | ||
Genetically closely related to Pteropus hypomelanus.
Genetically closely related to Dobsonia moluccensis.
Cell culture and virus isolation.
Fruit bat kidney T1 (FBKT1) cells, established from the kidney of a Pteropus dasymallus yayeyamae bat (17), were a kind gift from Ken Maeda (Yamaguchi University, Yamaguchi, Japan). Vero and SK-N-SH cells were maintained in Eagle's minimum essential medium (MEM) supplemented with 10% fetal bovine serum (FBS). HepG2 cells were maintained in MEM supplemented with 10% FBS and 0.1 mM nonessential amino acids. A549 and HeLa cells were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% FBS. IMR-32 cells were maintained in DMEM supplemented with 10% FBS and 0.1 mM nonessential amino acids. FBKT1 and Huh-7 cells were maintained in RPMI 1640 medium supplemented with 10% FBS. CHO-K1 cells were maintained in F12-K medium supplemented with 10% FBS.
Virus was isolated in the biosafety level 3 facilities at the Research Center for Zoonosis Control, Hokkaido University. Spleen tissue was homogenized in MEM (10%, wt/vol), followed by centrifugation at 1,000 × g for 5 min. The supernatant was used to inoculate Vero and FBKT1 cells grown in six-well culture plates (catalog no. 3516; Corning, Corning, NY). The culture plates were rocked for 1 h at 37°C in a 5% CO2 incubator. The cells were then refed with fresh MEM containing 2% FBS and 2% antibiotic-antimycotic solution (Life Technologies, Carlsbad, CA). FBAHV1 was propagated in Vero cells. The culture supernatant was centrifuged at 28,000 × g for 3 h. The virus pellet was then resuspended in phosphate-buffered saline (PBS) and titrated in a Vero cell-based plaque assay. The median (50%) tissue culture infective dose (TCID50) of the virus stock was determined using Vero cells.
Transmission electron microscopy.
For negative-stain electron microscopy, a concentrated FBAHV1 suspension was deposited on a nickel grid coated with polyvinyl formal (Nissin EM, Tokyo, Japan) and stained with 2% phosphotungstic acid. Samples were observed under a transmission electron microscope (H-7650; Hitachi High-Technologies, Tokyo, Japan).
Complete genome sequencing and analysis.
Viral DNA was extracted from a concentrated FBAHV1 suspension using phenol-chloroform-isoamyl alcohol (25:24:1) and processed for emulsion PCR and pyrosequencing on a GS Junior sequencer (Roche, Branford, CT) according to the manufacturer's instructions. The generated sequence reads were then assembled by de novo assembly using CLC Genomics Workbench software (version 6.0.1; CLC Bio, Aarhus, Denmark). The remaining gaps were amplified by PCR and then closed by iterative conventional Sanger sequencing. To determine the genome termini, viral DNA samples were blunt ended with T4 DNA polymerase and then digested with NotI. The DNA fragment was cloned into the pBluescript II SK(+) plasmid vector and then sequenced. Genetyx software (version 10; Genetyx, Tokyo, Japan) was used to search for predicted open reading frames (ORFs) with a minimum length of 240 bp. Each ORF was then analyzed using a translated BLAST search (BLASTX).
Phylogenetic analysis.
The MEGA (version 5) program was used to construct multiple-sequence alignments on the basis of the amino acid sequences (34). Bayesian phylogenetic analysis was performed using MrBayes software, version 3.2.2 (35), with the WAG amino acid substitution model. The phylogenetic tree was visualized with FigTree software, version 1.4. The viruses and accession numbers for the sequences used in the analysis are listed in Table S1 in the supplemental material.
Indirect immunofluorescence assay.
Cells were infected with FBAHV1 or mock infected for 16 h at a multiplicity of infection (MOI) of 0.1. After fixing with PBS containing 4% paraformaldehyde and permeabilization with PBS containing 0.5% Triton X-100, the cells were stained with an anti-herpes simplex virus 1 (anti-HSV-1) antibody (B0114; Dako, Glostrup, Denmark) at 4°C overnight, followed by an Alexa Fluor 488-conjugated anti-rabbit IgG (Life Technologies) at room temperature for 30 min. B0114 antibody was derived from the serum of a rabbit immunized with HSV-1-infected whole rabbit cornea cells and is cross-reactive with other alphaherpesvirus antigens (36). Mock-infected cells were used as a negative control.
TaqMan real-time PCR assay.
Cells were infected with FBAHV1 at an MOI of 0.5 for 1 h, and DNA was extracted with DNAzol (Molecular Research Center, Cincinnati, OH) at 1, 8, 24, and 48 h postinfection (p.i.). Real-time PCR was performed using a CFX96 real-time PCR detection system (Bio-Rad, Hercules, CA) in a 20-μl total volume containing Brilliant III Ultra-Fast quantitative PCR master mix (Agilent Technologies, Santa Clara, CA), 600 nM each primer, 300 nM TaqMan probe (Life Technologies), and 1 μl of DNA. The sequences of the primers and TaqMan probe were as follows: forward primer, 5′-GGGCCGTGATTTTGTTTGTC-3′; reverse primer, 5′-CCGCCAAGGCATATTTGC-3′; TaqMan probe, 5′-FAM-TAGTGGGCCTCCATGGG-MGB-3′, where FAM is 6-carboxyfluorescein. The 18S rRNA gene was detected and quantified using a TaqMan rRNA control reagent (Life Technologies) and used as an endogenous control for normalization.
Experimental infection of mice.
Four-week-old female BALB/c mice (Japan SLC, Shizuoka, Japan) were infected intranasally with 105 PFU of FBAHV1. On days 1, 3, 5, and 6 p.i., the infected mice were euthanized while they were under deep anesthesia by isoflurane inhalation, and tissue samples (brain, lung, liver, spleen, and kidney) were harvested. DNA was extracted from each tissue sample with DNAzol and then purified by treatment with phenol-chloroform-isoamyl alcohol (25:24:1). Quantification of the FBAHV1 genome in tissue samples was performed by real-time PCR as described above. For histopathological analysis, the remaining tissue samples were fixed in 10% phosphate-buffered formalin, embedded in paraffin, sectioned (4-μm-thick slices), and stained with hematoxylin-eosin. For immunohistochemical analysis, sections were blocked with Block Ace reagent (DS Pharma Biomedical, Osaka, Japan) and incubated with an anti-HSV-1 antibody (B0114; Dako), which cross-reacts with FBAHV1. Specific binding was detected with Envision horseradish peroxidase-labeled anti-rabbit IgG polymer (Dako) and visualized using a Histofine diaminobenzidine substrate kit (Nichirei Biosciences, Tokyo, Japan). For survival analysis, eight female BALB/c mice (4 weeks old; Japan SLC, Shizuoka, Japan) were anesthetized with isoflurane and inoculated intranasally with 105 PFU of FBAHV1. Another four mice were mock infected. Survival was monitored daily for 12 days. Throughout the experiments, the mice were weighed and observed daily. Mice that were reluctant to move, were recumbent, or lost more than 20% of their body weight relative to their maximum weight were euthanized by isoflurane inhalation.
PCR screening.
DNA was extracted from the spleens of fruit bats using DNAzol (Molecular Research Center) and screened for FBAHV1 by PCR with Platinum Taq DNA polymerase (Life Technologies) according to the manufacturer's instructions. The cycling protocol comprised 2 min of incubation at 94°C, followed by 45 cycles each of 94°C for 30 s, 58°C for 30 s, and 72°C for 40 s and a final extension at 72°C for 5 min. The primers used to target the UL19 gene were as follows: 5′-TGACAACAGCTTCTTCCTCGCTAAG-3′ and 5′-AACAAGCCTAGGGGCATTACAAAGG-3′. The PCR products were directly sequenced in both directions.
ELISA.
FBAHV1- or mock-infected Vero cells were lysed in lysis buffer (100 mM Tris-HCl [pH 7.4], 150 mM NaCl, 1 mM EDTA [pH 8.0], 1 mM EGTA, 1% Triton X-100, 0.5% sodium deoxycholate, 1 mM phenylmethylsulfonyl fluoride, Complete protease inhibitor cocktail [Roche]) and used as a source of antigen in the enzyme-linked immunosorbent assay (ELISA). Briefly, Maxisorb ELISA plates (Thermo Fisher Scientific, Waltham, MA) were coated with 100 μl of cell lysate (1 μg/well) at 4°C overnight. The plates were blocked with PBS containing 5% skimmed milk at 37°C for 2 h. Fruit bat sera were heat inactivated at 56°C for 30 min, diluted 1:100 in PBS containing 5% skimmed milk, and added to the ELISA plates. The plates were incubated at room temperature for 30 min. Bound antibodies were detected with horseradish peroxidase-labeled anti-bat IgG (Bethyl Laboratories, Montgomery, TX) and visualized using the tetramethylbenzidine Super Sensitive One Component horseradish peroxidase microwell substrate (SurModics, Eden Prairie, MN). Final optical density (OD) values were calculated by subtracting the mean OD for the mock-infected antigen-coated wells from that for the corresponding FBAHV1-infected antigen-coated wells. Assays were performed in duplicate, and mean values were used for data analysis.
Virus neutralization test.
Heat-inactivated bat sera were serially diluted (duplicate 2-fold serial dilutions, 1:4 to 1:2,048) in 50 μl of MEM containing 2% FBS and 2% antibiotic-antimycotic solution in 96-well culture plates (Corning). An equal volume of MEM containing 2% FBS, 2% antibiotic-antimycotic solution, and 100 TCID50s FBAHV1 was then added to each well, and the plates were incubated for 2 h at 37°C in a 5% CO2 incubator. A Vero cell suspension (100 μl; 2 × 105 cells/ml) was then added to each well, and the plates were incubated at 37°C for a further 5 days. The plates were then fixed with 10% phosphate-buffered formalin, stained with crystal violet, and examined for evidence of a cytopathic effect (CPE). The neutralization titer was defined as the reciprocal of the highest serum dilution that inhibited the CPE by 50%.
Nucleotide sequence accession number.
The complete FBAHV1 genome sequence was deposited in the DDBJ/EMBL/GenBank databases under accession no. AB825953.
RESULTS
Isolation of a novel herpesvirus from the spleens of fruit bats.
As a part of the surveillance activities aimed at identifying potential pathogens in fruit bats, we inoculated fruit bat kidney T1 (FBKT1) cells and African green monkey kidney (Vero) cells with spleen homogenates derived from fruit bats captured at four locations in Indonesia. FBKT1 cells and Vero cells inoculated with a spleen homogenate derived from a single pteropodid bat (Pteropus sp.) captured in February 2011 in Paguyaman showed evidence of cytopathic effects (CPEs): the cells adopted a rounded morphology at days 3 (FBKT1 cells) and 6 (Vero cells) postinoculation (Fig. 1a and b). This pteropodid bat was similar to Pteropus hypomelanus in terms of the level of sequence homology between the 16S rRNA (96%) and cytochrome b (95%) genes (DDBJ/EMBL/GenBank accession numbers AF069537 and AB062472, respectively); therefore, we considered these two species to be closely related. Culture supernatant harvested from the Vero cell monolayer was ultracentrifuged, and the resulting pellet was subjected to further analysis. Total RNA was extracted from the pellet and analyzed by emulsion PCR and pyrosequencing. BLAST analysis identified several nucleotide sequences that showed some homology with the genomes of HSV-1 and herpes simplex virus 2 (HSV-2). Negative-stain electron microscopy identified virus particles (100 to 120 nm) that appeared to have enveloped capsids (Fig. 1c); the morphology of these particles was typical of herpesviruses (37). Thus, we tentatively named this novel herpesvirus fruit bat alphaherpesvirus 1 (FBAHV1).
FIG 1.
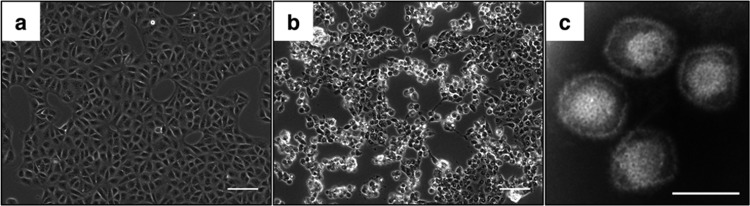
Isolation of FBAHV1 from a fruit bat. Vero cells exposed to a spleen homogenate show a rounded morphology, indicating a cytopathic effect. Vero cells were mock inoculated (a) or inoculated with a spleen homogenate (b). (c) Negative-stain electron micrograph showing enveloped capsids. Bars, 100 μm (a and b) and 100 nm (c).
Genome analysis of FBAHV1.
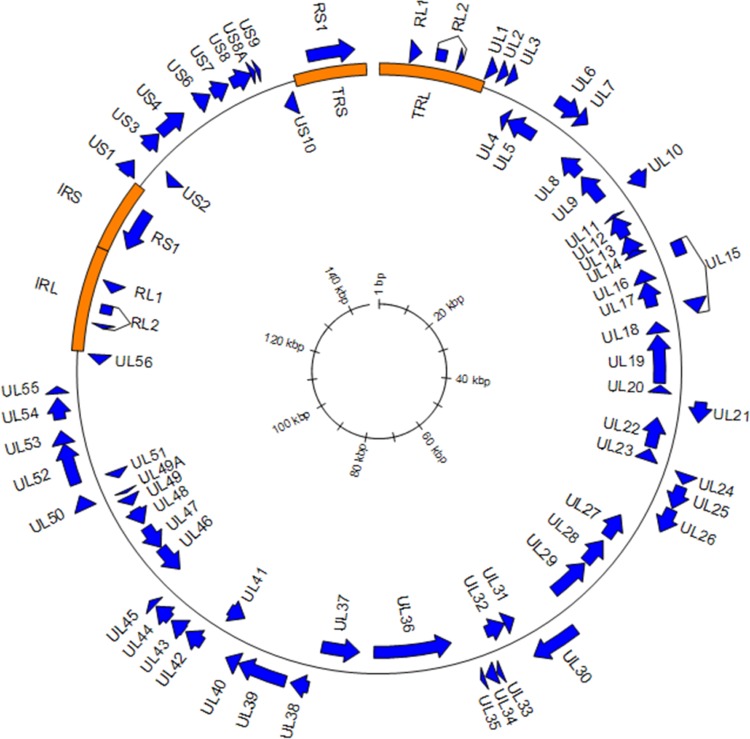
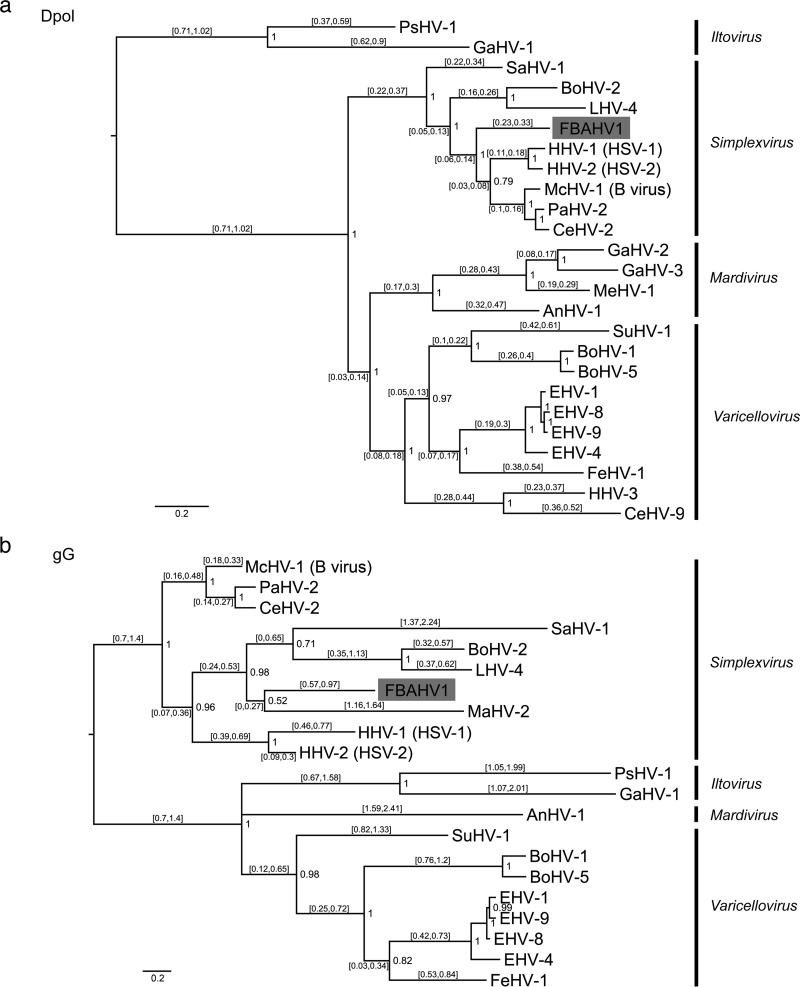
To determine the complete genome sequence of FBAHV1, we extracted DNA from the concentrated virus suspension and processed it for pyrosequencing. We obtained 122,696 reads with an average length of 431 bp. The gaps between the contigs were filled by primer walking. The genome of FBAHV1 is 149,459 bp in length, and it has a GC content of 61% (Fig. 2). The genome sequence has been deposited in the DDBJ/EMBL/GenBank databases (under accession number AB825953). The genome structure comprised unique long (UL) and unique short (US) sequences, each of which was flanked by long inverted repeat (RL) and short inverted repeat (RS) sequences. At least 67 open reading frames (ORFs) showing homology with HSV-1 genes were identified within the UL and US regions, while no homologues of HSV-1 genes US5, US10, US11, and US12 were identified. In addition, RL1, RL2, and RS1, which encode the HSV-1 homologues ICP4, ICP34.5, and ICP0, respectively, were mapped to the RL and RS regions. Among these ORFs, the deduced amino acid sequences of FBAHV1 UL15, UL19, and UL29 showed high sequence identity (>80%) to those of HSV-1 UL15, UL19, and UL29, respectively (Table 1). Bayesian phylogenetic trees were constructed on the basis of the deduced full-length amino acid sequences of viral DNA polymerase (encoded by the UL30 gene) and glycoprotein G (encoded by the US4 gene) (Fig. 3a and b). FBAHV1 clustered with the genus Simplexvirus within the subfamily Alphaherpesvirinae, which belongs to the family Herpesviridae. A previous study also reported the identification of a partial nucleotide sequence (465 bp) of UL30 from alphaherpesviruses isolated from fruit bats in Africa, Cambodia, and Brazil (24). Phylogenetic analysis based on the deduced amino acid sequence of the gene product derived from the partial UL30 sequences isolated from these alphaherpesviruses and from FBAHV1 showed that FBAHV1 is related to CSG248, a virus that was originally isolated from a throat swab taken from Pteropus lylei in Cambodia (see Fig. S1 in the supplemental material). Vero cells, which were used for FBAHV1 isolation, were identified by PCR to be negative for the UL30 gene.
FIG 2.
Map of the FBAHV1 genome. ORFs showing homology to those in HSV-1 (GenBank accession no. NC_001806) are indicated by blue arrows. Long and short inverted repeats are indicated by orange rectangles. The total length of the genome is 149,459 bp. The scale is shown in the center. The introns in the RL2 and UL15 genes are inferred.
TABLE 1.
Comparison of FBAHV1 genes with HSV-1 homologues
| Gene name | Location (nucleotides) | Strand | Size (no. of amino acids) | Product or predicted function | Amino acid identity of HSV-1 homologuea (%) |
|---|---|---|---|---|---|
| RL1 | 2328–3206 | + | 292 | ICP34.5 | 39.0 |
| RL2 | ICP0 | ||||
| Exon 1 | 4288–5061 | + | 257 | 34.8 | |
| Exon 2 | 5995–6330 | + | 111 | 51.0 | |
| UL1 | 8267–9016 | + | 249 | Glycoprotein L | 37.2 |
| UL2 | 9177–9839 | + | 220 | Uracil-DNA glycosylase | 70.0 |
| UL3 | 9923–10591 | + | 222 | Nuclear nonstructural protein | 55.2 |
| UL4 | 10634–11275 | − | 213 | Nuclear nonstructural protein | 49.5 |
| UL5 | 11307–13919 | − | 870 | Helicase-primase helicase subunit | 79.2 |
| UL6 | 13918–15963 | + | 681 | Capsid portal protein | 65.0 |
| UL7 | 15914–16801 | + | 295 | Tegument protein | 60.0 |
| UL8 | 16916–18922 | − | 668 | Helicase-primase subunit | 47.6 |
| UL9 | 19274–21829 | − | 851 | DNA replication origin-binding helicase | 77.9 |
| UL10 | 21786–23147 | + | 453 | Glycoprotein M | 66.1 |
| UL11 | 23482–23739 | − | 85 | Myristylated tegument protein | 44.7 |
| UL12 | 23664–25643 | − | 659 | DNase | 51.0 |
| UL13 | 25646–27193 | − | 515 | Tegument serine/threonine protein kinase | 67.2 |
| UL14 | 26953–27465 | − | 170 | Tegument protein | 61.2 |
| UL15 | DNA packaging terminase subunit 1 | ||||
| Exon 1 | 27680–28705 | + | 342 | 81.9 | |
| Exon 2 | 32097–33272 | + | 391 | 83.6 | |
| UL16 | 28752–29846 | − | 364 | Tegument protein | 57.2 |
| UL17 | 29860–32037 | − | 725 | Putative tegument protein | 59.0 |
| UL18 | 33372–34328 | − | 318 | Capsid triplex subunit 2; VP23 | 76.7 |
| UL19 | 34471–38616 | − | 1381 | Major capsid protein; VP5 | 83.3 |
| UL20 | 38784–39446 | − | 220 | Membrane protein | 64.5 |
| UL21 | 39896–41461 | + | 521 | Tegument protein | 62.7 |
| UL22 | 41550–44126 | − | 858 | Glycoprotein H | 49.7 |
| UL23 | 44292–45281 | − | 329 | Thymidine kinase | 48.2 |
| UL24 | 45361–46152 | + | 263 | Membrane-associated nuclear protein | 58.2 |
| UL25 | 46348–48096 | + | 582 | Capsid protein | 78.2 |
| UL26 | 48177–50063 | + | 628 | Capsid maturation protease | 57.0 |
| UL27 | 50432–52651 | − | 739 | Glycoprotein B | 71.9 |
| UL28 | 53104–55464 | − | 786 | DNA packaging terminase subunit 2 | 72.0 |
| UL29 | 55602–59192 | − | 1196 | DNA-binding protein; ICP8 | 82.1 |
| UL30 | 59620–63327 | + | 1235 | DNA polymerase catalytic subunit; Dpol | 75.3 |
| UL31 | 63254–64186 | − | 310 | Nuclear egress lamina protein | 76.1 |
| UL32 | 64179–65930 | − | 583 | DNA packaging protein | 73.4 |
| UL33 | 65929–66318 | + | 129 | DNA packaging protein | 64.3 |
| UL34 | 66381–67211 | + | 276 | Nuclear egress membrane protein | 57.2 |
| UL35 | 67296–67625 | + | 109 | Small capsid protein; VP26 | 61.1 |
| UL36 | 69012–75671 | − | 2219 | Large tegument protein; VP1-VP2 | 59.2 |
| UL37 | 76894–80151 | − | 1085 | Tegument phosphoprotein | 63.7 |
| UL38 | 80515–81903 | + | 462 | Capsid triplex subunit 1; VP19C | 58.7 |
| UL39 | 82257–85988 | + | 1243 | Ribonucleotide reductase subunit 1; ICP6 | 53.6 |
| UL40 | 86027–87031 | + | 334 | Ribonucleotide reductase subunit 2 | 76.6 |
| UL41 | 87161–88654 | − | 497 | Tegument host shutoff protein | 67.8 |
| UL42 | 89072–90538 | + | 488 | DNA polymerase processivity subunit | 43.4 |
| UL43 | 90696–91874 | + | 392 | Membrane protein | 23.1 |
| UL44 | 92052–93431 | + | 459 | Glycoprotein C | 41.5 |
| UL45 | 93545–94057 | + | 170 | Membrane protein | 43.5 |
| UL46 | 94136–96538 | − | 800 | Tegument protein; VP11-VP12 | 47.4 |
| UL47 | 96601–98727 | − | 708 | Tegument protein; VP13-VP14 | 56.9 |
| UL48 | 99084–100532 | − | 482 | Multifunctional tegument protein; VP16 | 64.7 |
| UL49 | 100927–101796 | − | 289 | Tegument protein; VP22 | 35.1 |
| UL49A | 102114–102359 | − | 81 | Glycoprotein N | 24.7 |
| UL50 | 102372–103436 | + | 354 | Deoxyuridine triphosphatase | 40.5 |
| UL51 | 103553–104242 | − | 229 | Tegument protein | 68.0 |
| UL52 | 104317–107457 | + | 1046 | Helicase-primase primase subunit | 64.7 |
| UL53 | 107427–108434 | + | 335 | Glycoprotein K | 66.3 |
| UL54 | 109273–110898 | + | 541 | Multifunctional regulatory protein; ICP27 | 50.0 |
| UL55 | 111194–111745 | + | 183 | Nuclear protein | 62.8 |
| UL56 | 113410–114273 | − | 287 | Membrane protein | 34.2 |
| RL2 | ICP0 | ||||
| Exon 1 | 116505–116840 | − | 111 | 51.0 | |
| Exon 2 | 117774–118547 | − | 257 | 34.8 | |
| RL1 | 119629–120507 | − | 292 | ICP34.5 | 39.0 |
| RS1 | 123634–127218 | − | 1194 | ICP4 | 57.6 |
| US1 | 128647–129882 | + | 412 | Regulatory protein ICP22 | 39.3 |
| US2 | 130010–130912 | − | 300 | Virion protein | 60.0 |
| US3 | 131149–132546 | + | 465 | Serine/threonine protein kinase | 59.7 |
| US4 | 132640–134952 | + | 770 | Glycoprotein G | 21.2 |
| US6 | 136139–137314 | + | 391 | Glycoprotein D | 58.5 |
| US7 | 137478–138869 | + | 463 | Glycoprotein I | 39.1 |
| US8 | 139089–140747 | + | 552 | Glycoprotein E | 40.0 |
| US8A | 140689–141060 | + | 123 | Membrane protein | 35.0 |
| US9 | 141146–141451 | + | 101 | Membrane protein | 37.1 |
| RS1 | 145076–148660 | + | 1194 | ICP4 | 57.6 |
Reference sequences of HSV-1 ORFs were obtained from the DDBJ/EMBL/GenBank databases (accession no. NC_001806).
FIG 3.
Phylogenetic analysis of FBAHV1. Bayesian phylogenetic trees were constructed on the basis of the full-length amino acid sequences of viral DNA polymerase (Dpol), which is encoded by UL30 (a), and glycoprotein G (gG), which is encoded by US4 (b), from the subfamily Alphaherpesvirinae. The WAG amino acid substitution model was used. Bayesian posterior probabilities are indicated at each tree root. The branch labels represent the 95% highest posterior density interval of the substitution rate. The scale bar represents a distance of 0.2 substitution per site. FBAHV1 is shaded gray. The sequence of the DNA polymerase ORF of macropodid herpesvirus 2 was not determined. The glycoprotein G ORF was absent from the genomes of human herpesvirus 3, cercopithecine herpesvirus 9, gallid herpesvirus 2, gallid herpesvirus 3, and meleagrid herpesvirus 1. The accession numbers for the sequences used in this analysis are shown in Table S1 in the supplemental material. The definitions of the abbreviated virus names are as follows: anatid herpesvirus 1 (AnHV-1), bovine herpesvirus 1 (BoHV-1), bovine herpesvirus 2 (BoHV-2), bovine herpesvirus 5 (BoHV-5), cercopithecine herpesvirus 2 (CeHV-2), cercopithecine herpesvirus 9 (CeHV-9), equid herpesvirus 1 (EHV-1), equid herpesvirus 4 (EHV-4), equid herpesvirus 8 (EHV-8), equid herpesvirus 9 (EHV-9), felid herpesvirus 1 (FeHV-1), gallid herpesvirus 1 (GaHV-1), gallid herpesvirus 2 (GaHV-2), gallid herpesvirus 3 (GaHV-3), human herpesvirus 1 (HHV-1), human herpesvirus 2 (HHV-2), human herpesvirus 3 (HHV-3), leporid herpesvirus 4 (LHV-4), macacine herpesvirus 1 (McHV-1), macropodid herpesvirus 2 (MaHV-2), meleagrid herpesvirus 1 (MeHV-1), papiine herpesvirus 2 (PaHV-2), psittacid herpesvirus 1 (PsHV-1), saimiriine herpesvirus 1 (SaHV-1), and suid herpesvirus 1 (SuHV-1).
Cell tropism of FBAHV1.
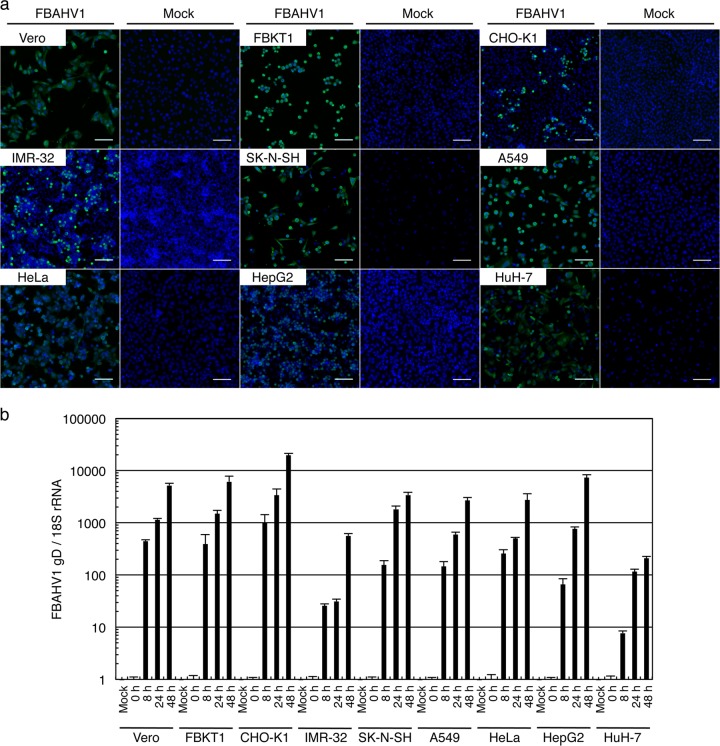
We next examined the susceptibility of several cultured cell lines to FBAHV1 infection. In addition to the Vero and FBKT1 cells that were originally used to isolate the virus, six human cell lines were infected with FBAHV1 at a multiplicity of infection (MOI) of 0.1: human neuroblastoma (IMR-32 and SK-N-SH) cells, human lung carcinoma (A549) cells, human cervical carcinoma (HeLa) cells, human hepatoma (Huh-7) cells, and human hepatoblastoma (HepG2) cells. We also examined CHO-K1 cells, which are resistant to infection by viruses belonging to the genus Simplexvirus (including HSV-1 [38] and macacine herpesvirus 1 [39]). Indirect immunofluorescence analysis using an anti-HSV-1 antibody (B0114) which cross-reacts with other alphaherpesvirus antigens (36) showed that FBAHV1 infected all of the cell lines tested; however, there was no evidence of syncytium formation. Immunofluorescence staining of uninfected cells with the anti-HSV-1 antibody did not show positivity (Fig. 4a). Real-time PCR confirmed viral replication at 8 h p.i. (Fig. 4b). Surprisingly, CHO-K1 cells supported virus replication as efficiently as the other cell lines.
FIG 4.
FBAHV1 infection of different cell types. (a) Each cell line was infected with FBAHV1 (left) or mock infected (right) for 16 h at an MOI of 0.1. After fixation, FBAHV1 antigens (green) were visualized by staining with an anti-HSV-1 polyclonal antibody, followed by Alexa Fluor 488-conjugated anti-rabbit IgG. Cell nuclei (blue) were stained with Hoechst 33342. All cell lines tested were susceptible to FBAHV1 infection. Bars, 100 μm. (b) DNA was extracted from FBAHV1-infected cells (MOI = 0.5) at the indicated times p.i. FBAHV1 genomic DNA was quantified by real-time PCR, and the amount was normalized to that of 18S rRNA. All cell lines tested supported virus replication. Data are expressed as the mean ± standard error of the mean (SEM) from triplicate reactions.
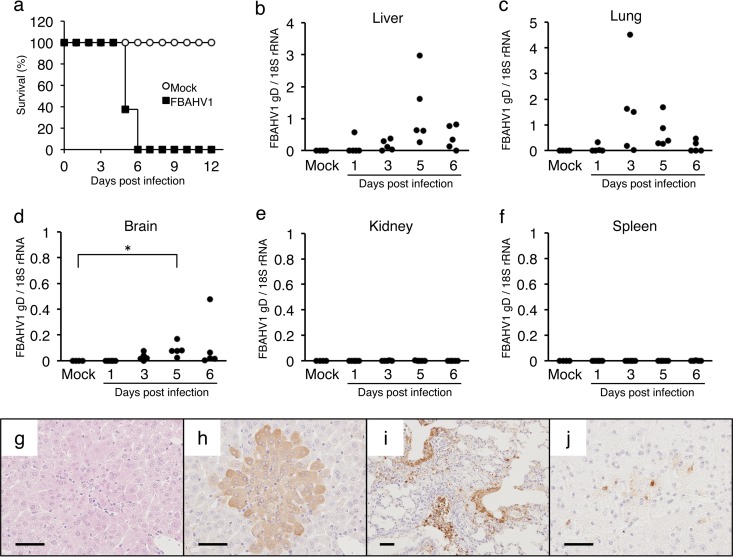
Experimental infection of laboratory mice with FBAHV1.
Laboratory mice are susceptible to infection by HSV-1 and HSV-2 and are used as a model for in vivo studies (40, 41). Therefore, we next examined whether mice were susceptible to infection by FBAHV1. BALB/c mice infected intranasally with 105 PFU of FBAHV1 reached an ethical endpoint within 6 days (Fig. 5a). Gross examination revealed multiple white foci in the livers and extensive red to red-black discoloration in the lungs. The amount of the FBAHV1 genome in the tissues was quantified by real-time PCR. A relatively large amount of FBAHV1 was detected in the liver at 5 days p.i. (Fig. 5b) and in the lungs at 3 days p.i. (Fig. 5c). In addition, increasing amounts of the FBAHV1 genome were detected in the brain (Fig. 5d). However, no or a few copies of the FBAHV1 genome were detected in the kidneys (Fig. 5e) or spleen (Fig. 5f). Histological analysis revealed multifocal hepatic necrosis. Many of the hepatocytes within these necrotic foci contained eosinophilic intranuclear inclusion bodies, which are typical of herpesvirus infections (Fig. 5g). Immunohistochemical staining with an anti-HSV-1 antibody revealed FBAHV1 antigens in hepatocytes within the liver (Fig. 5h), the bronchiolar epithelium in the lungs (Fig. 5i), and neurons and glia within the medulla oblongata (Fig. 5j).
FIG 5.
Experimental infection of laboratory mice with FBAHV1. (a) BALB/c mice were either infected intranasally with 105 PFU of FBAHV1 (n = 8) or mock infected (n = 4). Survival was monitored daily for 12 days. (b to f) Tissues were harvested from five FBAHV1-infected mice at the indicated time points. The amount of the FBAHV1 genome in tissues was determined by real-time PCR and normalized to the amount of 18S rRNA. Four mock-infected mice were included in the analysis as negative controls. *, P < 0.05. (g) Hematoxylin-eosin staining of liver tissue sections from mice at 5 days postinfection with FBAHV1. (h to j) Immunohistochemical staining of liver (h), lung (i), and brain (j) tissues from mice at 5 days postinfection with FBAHV1. FBAHV1 antigens (brown) were detected by staining with an anti-HSV-1 polyclonal antibody. Bars, 50 μm.
Epidemiological screening of Indonesian fruit bats for FBAHV1.
Finally, we used molecular and serological approaches to examine the prevalence of FBAHV1 infection in Indonesian fruit bats. FBAHV1 was isolated from a pteropodid bat (Pteropus sp.) captured in Paguyaman on Sulawesi Island. We also examined samples from fruit bats captured in Panjalu, Surabaya, and Magelang (all on Java Island), Lima Puluh Kota (Sumatra Island), and Popayato (Sulawesi Island) (Table 2).
DNA was extracted from 133 spleen tissue samples taken from 133 fruit bats. PCR was used to screen for the UL19 gene using FBAHV1-specific primers. As shown in Table 1, FBAHV1 and HSV-1 UL19 shared high sequence identity (83.3%), suggesting that the region may be conserved among potential variants of FBAHV1. The results showed that 12/133 (9%) fruit bats harbored sequences that were 99% identical to the sequence of FBAHV1. No variant of FBAHV1 was identified in this assay. These PCR-positive cases belonged to the species Pteropus vampyrus (from Panjalu, Surabaya, and Magelang) and Acerodon celebensis (from Paguyaman) and a Pteropus sp. (from Paguyaman) (Table 2). The last species is related to Pteropus hypomelanus.
Serum samples were obtained from 104 of the 133 fruit bats. An enzyme-linked immunosorbent assay (ELISA) which incorporated lysates of FBAHV1-infected Vero cells as a source of antigen was performed to detect anti-FBAHV1 antibodies in the serum samples. Because we were not able to use fruit bat serum that was negative for FBAHV1 as a control for this assay, we set the arbitrary cutoff value for the optical density (OD) at 0.23. This was based on a value of 3 standard deviations above the mean OD obtained for 50 serum samples that tested negative in a virus neutralization test (NT). We detected anti-FBAHV1 antibodies in 44/104 (42%) serum samples (Table 2). We then performed virus neutralization tests using Vero cells. Of the 104 serum samples collected, 96 were suitable for analysis in the virus NT (8 were not suitable, as they were either cytotoxic or of insufficient volume). FBAHV1-neutralizing antibodies were identified in 46/96 (48%) samples at titers ranging from 4 to 256 (Table 2; see also Fig. S2 in the supplemental material). Seropositive individuals were identified in all fruit bat species tested. High neutralizing antibody titers (>100) were obtained from two Pteropus spp. (see Fig. S2 in the supplemental material).
DISCUSSION
Herpesviruses are common animal pathogens, but little is known about the herpesviruses harbored by different species of bats. Here, we report the characterization of a novel alphaherpesvirus which was isolated from a Pteropus sp., a species of bat that is closely related to Pteropus hypomelanus. We determined the complete genome sequence of the virus and revealed its phylogenetic relationship to existing alphaherpesviruses. We also detected viral genomic DNA and antiviral antibodies in three and four bat species, respectively; these bats were captured at six different locations in Indonesia, and all belonged to the family Pteropodidae. Thus, we named this herpesvirus FBAHV1.
Experimental infection of laboratory mice with FBAHV1 resulted in gross lesions in the lungs and liver and, eventually, death. Simplexviruses are neurotropic. Consistent with this, FBAHV1 infection was also observed in the brain tissues of mice. In the present study, tissue samples obtained from fruit bats were examined macroscopically by veterinary pathologists; however, there were no signs of serious infection, such as massive liver damage or pneumonia. In addition, the virus showed a high seroprevalence in bats. Therefore, these results suggest that natural infection of fruit bats by FBAHV1 may not be lethal. However, further studies are necessary to assess the pathogenic potential of FBAHV1 in both bats and foreign hosts.
Fruit bats that were seropositive for antibodies to FBAHV1 were identified from four species of the family Pteropodidae in different areas of Indonesia, although we must consider the possibility that this seropositivity may be due to immunological cross-reactivity between FBAHV1 and other, as yet unidentified, alphaherpesviruses. However, the result of PCR screening supports the suggestion that FBAHV1 or the viruses closely related to FBAHV1 are distributed over a wide geographical area in Indonesia. Because pteropodid bats are a source of meat for people in North Sulawesi (42), further seroepidemiological studies of FBAHV1 in humans will help elucidate the presence of human cases of FBAHV1 infection.
The phylogenetic analysis showed that FBAHV1 branched from an ancestor of primate simplex viruses (Fig. 3) to form a distinct lineage together with other alphaherpesviruses previously identified in fruit bats (see Fig. S1 in the supplemental material). This lineage is distant from that of parixa virus, which was isolated from an insectivorous bat, Lonchophylla thomasi (see Fig. S1 in the supplemental material). The GenBank database contains 22 complete reference genome sequences for alphaherpesviruses isolated from humans, nonhuman primates, cows, horses, pigs, cats, marmosets, chickens, ducks, turkeys, and parrots. FBAHV1 is the first alphaherpesvirus to be isolated from bats and for which the complete genome sequence has been determined. The data presented in this paper will make a considerable contribution to our understanding of the evolution of herpesviruses.
Supplementary Material
ACKNOWLEDGMENTS
We thank Ken Maeda (Yamaguchi University, Yamaguchi, Japan) for providing the FBKT1 cells.
This study was supported by the Japan Initiative for Global Research Network of Infectious Diseases (J-GRID) from the Ministry of Education, Culture, Sports, Science, and Technology (MEXT), Japan.
Footnotes
Published ahead of print 18 June 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JVI.01277-14.
REFERENCES
- 1.Wong S, Lau S, Woo P, Yuen KY. 2007. Bats as a continuing source of emerging infections in humans. Rev. Med. Virol. 17:67–91. 10.1002/rmv.520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Calisher CH, Childs JE, Field HE, Holmes KV, Schountz T. 2006. Bats: important reservoir hosts of emerging viruses. Clin. Microbiol. Rev. 19:531–545. 10.1128/CMR.00017-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ksiazek TG, Rota PA, Rollin PE. 2011. A review of Nipah and Hendra viruses with an historical aside. Virus Res. 162:173–183. 10.1016/j.virusres.2011.09.026 [DOI] [PubMed] [Google Scholar]
- 4.Drexler JF, Corman VM, Müller MA, Maganga GD, Vallo P, Binger T, Gloza-Rausch F, Rasche A, Yordanov S, Seebens A, Oppong S, Adu Sarkodie Y, Pongombo C, Lukashev AN, Schmidt-Chanasit J, Stöcker A, Carneiro AJ, Erbar S, Maisner A, Fronhoffs F, Buettner R, Kalko EK, Kruppa T, Franke CR, Kallies R, Yandoko ER, Herrler G, Reusken C, Hassanin A, Krüger DH, Matthee S, Ulrich RG, Leroy EM, Drosten C. 2012. Bats host major mammalian paramyxoviruses. Nat. Commun. 3:796. 10.1038/ncomms1796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lau SK, Woo PC, Lai KK, Huang Y, Yip CC, Shek CT, Lee P, Lam CS, Chan KH, Yuen KY. 2011. Complete genome analysis of three novel picornaviruses from diverse bat species. J. Virol. 85:8819–8828. 10.1128/JVI.02364-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Negredo A, Palacios G, Vázquez-Morón S, González F, Dopazo H, Molero F, Juste J, Quetglas J, Savji N, de la Cruz Martínez M, Herrera JE, Pizarro M, Hutchison SK, Echevarría JE, Lipkin WI, Tenorio A. 2011. Discovery of an ebolavirus-like filovirus in Europe. PLoS Pathog. 7:e1002304. 10.1371/journal.ppat.1002304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drexler JF, Seelen A, Corman VM, Fumie Tateno A, Cottontail V, Melim Zerbinati R, Gloza-Rausch F, Klose SM, Adu-Sarkodie Y, Oppong SK, Kalko EK, Osterman A, Rasche A, Adam A, Müller MA, Ulrich RG, Leroy EM, Lukashev AN, Drosten C. 2012. Bats worldwide carry hepatitis E virus-related viruses that form a putative novel genus within the family Hepeviridae. J. Virol. 86:9134–9147. 10.1128/JVI.00800-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tong S, Li Y, Rivailler P, Conrardy C, Castillo DA, Chen LM, Recuenco S, Ellison JA, Davis CT, York IA, Turmelle AS, Moran D, Rogers S, Shi M, Tao Y, Weil MR, Tang K, Rowe LA, Sammons S, Xu X, Frace M, Lindblade KA, Cox NJ, Anderson LJ, Rupprecht CE, Donis RO. 2012. A distinct lineage of influenza A virus from bats. Proc. Natl. Acad. Sci. U. S. A. 109:4269–4274. 10.1073/pnas.1116200109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tao Y, Shi M, Conrardy C, Kuzmin IV, Recuenco S, Agwanda B, Alvarez DA, Ellison JA, Gilbert AT, Moran D, Niezgoda M, Lindblade KA, Holmes EC, Breiman RF, Rupprecht CE, Tong S. 2013. Discovery of diverse polyomaviruses in bats and the evolutionary history of the Polyomaviridae. J. Gen. Virol. 94:738–748. 10.1099/vir.0.047928-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fagrouch Z, Sarwari R, Lavergne A, Delaval M, de Thoisy B, Lacoste V, Verschoor EJ. 2012. Novel polyomaviruses in South American bats and their relationship to other members of the family Polyomaviridae. J. Gen. Virol. 93:2652–2657. 10.1099/vir.0.044149-0 [DOI] [PubMed] [Google Scholar]
- 11.Sasaki M, Setiyono A, Handharyani E, Rahmadani I, Taha S, Adiani S, Subangkit M, Sawa H, Nakamura I, Kimura T. 2012. Molecular detection of a novel paramyxovirus in fruit bats from Indonesia. Virol. J. 9:240. 10.1186/1743-422X-9-240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ge X, Li Y, Yang X, Zhang H, Zhou P, Zhang Y, Shi Z. 2012. Metagenomic analysis of viruses from bat fecal samples reveals many novel viruses in insectivorous bats in China. J. Virol. 86:4620–4630. 10.1128/JVI.06671-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu Z, Ren X, Yang L, Hu Y, Yang J, He G, Zhang J, Dong J, Sun L, Du J, Liu L, Xue Y, Wang J, Yang F, Zhang S, Jin Q. 2012. Virome analysis for identification of novel mammalian viruses in bat species from Chinese provinces. J. Virol. 86:10999–11012. 10.1128/JVI.01394-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Donaldson EF, Haskew AN, Gates JE, Huynh J, Moore CJ, Frieman MB. 2010. Metagenomic analysis of the viromes of three North American bat species: viral diversity among different bat species that share a common habitat. J. Virol. 84:13004–13018. 10.1128/JVI.01255-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li L, Victoria JG, Wang C, Jones M, Fellers GM, Kunz TH, Delwart E. 2010. Bat guano virome: predominance of dietary viruses from insects and plants plus novel mammalian viruses. J. Virol. 84:6955–6965. 10.1128/JVI.00501-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baker KS, Todd S, Marsh GA, Crameri G, Barr J, Kamins AO, Peel AJ, Yu M, Hayman DT, Nadjm B, Mtove G, Amos B, Reyburn H, Nyarko E, Suu-Ire R, Murcia PR, Cunningham AA, Wood JL, Wang LF. 2013. Novel, potentially zoonotic paramyxoviruses from the African straw-colored fruit bat Eidolon helvum. J. Virol. 87:1348–1358. 10.1128/JVI.01202-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maeda K, Hondo E, Terakawa J, Kiso Y, Nakaichi N, Endoh D, Sakai K, Morikawa S, Mizutani T. 2008. Isolation of novel adenovirus from fruit bat (Pteropus dasymallus yayeyamae). Emerg. Infect. Dis. 14:347–349. 10.3201/eid1402.070932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Y, Zhang S, Zhao J, Zhang F, Hu R. 2013. Isolation of Irkut virus from a Murina leucogaster bat in China. PLoS Negl. Trop. Dis. 7:e2097. 10.1371/journal.pntd.0002097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kohl C, Lesnik R, Brinkmann A, Ebinger A, Radonić A, Nitsche A, Mühldorfer K, Wibbelt G, Kurth A. 2012. Isolation and characterization of three mammalian orthoreoviruses from European bats. PLoS One 7:e43106. 10.1371/journal.pone.0043106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raut CG, Yadav PD, Towner JS, Amman BR, Erickson BR, Cannon DL, Sivaram A, Basu A, Nichol ST, Mishra AC, Mourya DT. 2012. Isolation of a novel adenovirus from Rousettus leschenaultii bats from India. Intervirology 55:488–490. 10.1159/000337026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Freuling CM, Beer M, Conraths FJ, Finke S, Hoffmann B, Keller B, Kliemt J, Mettenleiter TC, Mühlbach E, Teifke JP, Wohlsein P, Müller T. 2011. Novel lyssavirus in Natterer's bat, Germany. Emerg. Infect. Dis. 17:1519–1522. 10.3201/eid1708.110201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuzmin IV, Mayer AE, Niezgoda M, Markotter W, Agwanda B, Breiman RF, Rupprecht CE. 2010. Shimoni bat virus, a new representative of the Lyssavirus genus. Virus Res. 149:197–210. 10.1016/j.virusres.2010.01.018 [DOI] [PubMed] [Google Scholar]
- 23.Marsh GA, de Jong C, Barr JA, Tachedjian M, Smith C, Middleton D, Yu M, Todd S, Foord AJ, Haring V, Payne J, Robinson R, Broz I, Crameri G, Field HE, Wang LF. 2012. Cedar virus: a novel henipavirus isolated from Australian bats. PLoS Pathog. 8:e1002836. 10.1371/journal.ppat.1002836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Razafindratsimandresy R, Jeanmaire EM, Counor D, Vasconcelos PF, Sall AA, Reynes JM. 2009. Partial molecular characterization of alphaherpesviruses isolated from tropical bats. J. Gen. Virol. 90:44–47. 10.1099/vir.0.006825-0 [DOI] [PubMed] [Google Scholar]
- 25.Watanabe S, Maeda K, Suzuki K, Ueda N, Iha K, Taniguchi S, Shimoda H, Kato K, Yoshikawa Y, Morikawa S, Kurane I, Akashi H, Mizutani T. 2010. Novel betaherpesvirus in bats. Emerg. Infect. Dis. 16:986–988. 10.3201/eid1606.091567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang H, Todd S, Tachedjian M, Barr JA, Luo M, Yu M, Marsh GA, Crameri G, Wang LF. 2012. A novel bat herpesvirus encodes homologues of major histocompatibility complex classes I and II, C-type lectin, and a unique family of immune-related genes. J. Virol. 86:8014–8030. 10.1128/JVI.00723-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jánoska M, Vidovszky M, Molnár V, Liptovszky M, Harrach B, Benko M. 2011. Novel adenoviruses and herpesviruses detected in bats. Vet. J. 189:118–121. 10.1016/j.tvjl.2010.06.020 [DOI] [PubMed] [Google Scholar]
- 28.Anthony SJ, Epstein JH, Murray KA, Navarrete-Macias I, Zambrana-Torrelio CM, Solovyov A, Ojeda-Flores R, Arrigo NC, Islam A, Ali Khan S, Hosseini P, Bogich TL, Olival KJ, Sanchez-Leon MD, Karesh WB, Goldstein T, Luby SP, Morse SS, Mazet JA, Daszak P, Lipkin WI. 2013. A strategy to estimate unknown viral diversity in mammals. mBio 4(5):e00598-13. 10.1128/mBio.00598-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wibbelt G, Kurth A, Yasmum N, Bannert M, Nagel S, Nitsche A, Ehlers B. 2007. Discovery of herpesviruses in bats. J. Gen. Virol. 88:2651–2655. 10.1099/vir.0.83045-0 [DOI] [PubMed] [Google Scholar]
- 30.Watanabe S, Ueda N, Iha K, Masangkay JS, Fujii H, Alviola P, Mizutani T, Maeda K, Yamane D, Walid A, Kato K, Kyuwa S, Tohya Y, Yoshikawa Y, Akashi H. 2009. Detection of a new bat gammaherpesvirus in the Philippines. Virus Genes 39:90–93. 10.1007/s11262-009-0368-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.National Research Council. 1996. Guide for the care and use of laboratory animals, 7th ed. National Academies Press, Washington, DC [Google Scholar]
- 32.National Research Council. 2011. Guide for the care and use of laboratory animals, 8th ed. National Academies Press, Washington, DC [Google Scholar]
- 33.National Advisory Committee for Laboratory Animal Research. 2004. Guideline on the care and use of animals for scientific purposes. National Advisory Committee for Laboratory Animal Research, Singapore [Google Scholar]
- 34.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28:2731–2739. 10.1093/molbev/msr121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP. 2012. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 61:539–542. 10.1093/sysbio/sys029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bailey CC, Miller AD. 2012. Ulcerative cheilitis in a rhesus macaque. Vet. Pathol. 49:412–415. 10.1177/0300985811400443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pellett PE, Roizman B. 2007. The family Herpesviridae: a brief introduction, p 2479–2499 InKnipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE. (ed), Fields virology, 5th ed, vol 2 Lippincott Williams & Wilkins, Philadelphia, PA [Google Scholar]
- 38.Montgomery R, Warner M, Lum B, Spear P. 1996. Herpes simplex virus-1 entry into cells mediated by a novel member of the TNF/NGF receptor family. Cell 87:427–436. 10.1016/S0092-8674(00)81363-X [DOI] [PubMed] [Google Scholar]
- 39.Fan Q, Amen M, Harden M, Severini A, Griffiths A, Longnecker R. 2012. Herpes B virus utilizes human nectin-1 but not HVEM or PILRα for cell-cell fusion and virus entry. J. Virol. 86:4468–4476. 10.1128/JVI.00041-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nixon B, Stefanidou M, Mesquita PM, Fakioglu E, Segarra T, Rohan L, Halford W, Palmer KE, Herold BC. 2013. Griffithsin protects mice from genital herpes by preventing cell-to-cell spread. J. Virol. 87:6257–6269. 10.1128/JVI.00012-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shivkumar M, Milho R, May JS, Nicoll MP, Efstathiou S, Stevenson PG. 2013. Herpes simplex virus 1 targets the murine olfactory neuroepithelium for host entry. J. Virol. 87:10477–10488. 10.1128/JVI.01748-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee RJ, Gorog AJ, Dwiyahreni A, Siwu S, Riley J, Alexander H, Paoli GD, Ramono W. 2005. Wildlife trade and implications for law enforcement in Indonesia: a case study from North Sulawesi. Biol. Conserv. 123:477–488. 10.1016/j.biocon.2005.01.009 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.