ABSTRACT
Immunization with virus-like particles (VLPs) containing the Newcastle disease virus (NDV) core proteins, NP and M, and two chimera proteins (F/F and H/G) containing the respiratory syncytial virus (RSV) F- and G-protein ectodomains fused to the transmembrane and cytoplasmic domains of NDV F and HN proteins, respectively, stimulated durable RSV-neutralizing antibodies, F-protein-specific long-lived, bone marrow-associated plasma cells (LLPCs), and B cell memory, in striking contrast to RSV infection, which did not (M. R. Schmidt, L. W. McGinnes, S. A. Kenward, K. N. Willems, R. T. Woodland, and T. G. Morrison, J. Virol. 86:11654–11662, 2012). Here we report the characterization of a VLP with an RSV F-protein ectodomain fused to the NDV F-protein heptad repeat 2 (HR2), transmembrane, and cytoplasmic domain sequences, creating a chimera with two tandem HR2 domains, one from the RSV F protein and the other from the NDV F-protein ectodomain (F/HR2F). The F/HR2F chimera protein was efficiently assembled into VLPs along with the H/G chimera protein. This VLP (VLP-H/G+F/HR2F) stimulated anti-F-protein and anti-G-protein IgG, durable RSV-neutralizing antibodies, and anti-RSV F-protein-secreting LLPCs. However, the subtypes of anti-F-protein IgG induced were different from those elicited by VLPs containing the F/F chimera (VLP-H/G+F/F). Most importantly, VLP-H/G+F/HR2F did not induce RSV F-protein-specific B cell memory, as shown by the adoptive transfer of B cells from immunized animals to immunodeficient animals. The VLP did, however, induce B cell memory specific to the RSV G protein. Thus, the form of the F protein has a direct role in inducing anti-F-protein B cell memory.
IMPORTANCE The development of vaccines for respiratory syncytial virus (RSV) is hampered by a lack of a clear understanding of the requirements for eliciting protective as well as durable human immune responses to virus antigens. The results of this study indicate that the form of the RSV F protein has a direct and significant impact on the type of anti-F-protein IgG antibodies induced and the generation of F-protein-specific memory. Identification of the conformation of the RSV F protein that most effectively stimulates not only LLPCs and but also memory B cells will be important in the future development of RSV vaccines.
INTRODUCTION
Human respiratory syncytial virus (RSV) is the single most important cause of acute viral respiratory disease in infants and young children (1, 2). Elderly and immunocompromised populations are also at risk for serious RSV disease, accounting for approximately 10,000 deaths per year among individuals greater than 64 years of age and 14,000 to 60,000 hospitalizations per year (3–5). In addition, RSV infections result in high mortality rates in stem cell transplant patients (6) and in populations with cardiopulmonary diseases (7). Despite the significance of RSV disease in several different populations, there are no vaccines available.
Many vaccine candidates have been characterized in preclinical and clinical studies over 5 decades. These candidates have failed due to three interrelated problems. The first is safety, an issue that has dominated RSV vaccine development for years. An early vaccine candidate, a formalin-inactivated preparation of purified virus (FI-RSV), not only failed to protect infants from infection but also unexpectedly resulted in enhanced, life-threatening respiratory disease (ERD) upon subsequent infection with RSV (reviewed in references 8 to 11). The mechanisms responsible for this unusual response to a classically prepared vaccine are not completely understood even after decades of research using animal models.
A second problem in RSV vaccine development is a lack of understanding of the requirements for the generation of protective immunity to RSV infection in humans. Many vaccine candidates are reported to be protective in animal models and, while stimulating antibody responses in humans, have failed to stimulate significant levels of protection in human trials (reviewed in reference 12). While there are likely many reasons for these observations, one important but unresolved issue is the most effective form of the RSV F protein for stimulating protective, neutralizing antibodies in humans. The paramyxovirus F protein is folded into a metastable conformation and upon fusion activation refolds through a series of conformational intermediates into the postfusion conformation, which is structurally very different from the prefusion form (13–19). It is logical to assume that antibodies stimulated by the prefusion form of F protein would be most effective at virus neutralization, and there is evidence for this conclusion (20, 21). However, others have suggested that the postfusion form also elicits protective, neutralizing antibody responses (22). Thus, it remains to be established which form of the F protein is the best antigen for stimulating effective human neutralizing antibodies.
A third very important problem is a lack of understanding of the requirements in both human and murine systems for the induction of long-lived humoral and memory immune responses to RSV, a topic that has not received a great deal of attention. One of the hallmarks of RSV infection is the observation that humans can experience repeated infection caused by the same virus serogroup multiple times over several years or even within the same season (12, 23). The reasons for the failure of RSV infection to protect against subsequent infection are not clear, but the inadequate memory response to RSV natural infection illustrates a major problem that must be overcome to control RSV disease. Indeed, most RSV vaccine candidates have failed to stimulate long-term protective responses in human trials (12, 23), illustrating the lack of knowledge of the immune mechanisms required to generate protective long-term anti-RSV immune responses.
As demonstrated by Schmidt et al. (24), we reproduced in a murine system the transient protective responses and the lack of memory responses to RSV intranasal infection typically seen in humans (23). To define the mechanisms that underlie these inadequate immune responses and to gain insights into the role of protein conformation in effective immune responses, we have developed a platform for the presentation of different conformational forms of RSV F and G proteins by incorporating these proteins into virus-like particles (VLPs) (25, 26). These particles are based on the core proteins, M and NP, of Newcastle disease virus (NDV) (26–28). We incorporated the ectodomains of the RSV F and G proteins into these VLPs by creating chimera proteins composed of the ectodomains of the RSV glycoproteins fused to the transmembrane (TM) and cytoplasmic (CT) domains of the NDV F and HN proteins, respectively (26). We showed that immunization of mice with these VLPs (VLP-H/G+F/F) elicited antibody responses to both F and G proteins, durable RSV-neutralizing antibodies, F-protein-specific long-lived, bone marrow-associated plasma cells (LLPCs), and anti-RSV F-protein memory responses, whereas RSV infection did not (24). Here we show that an alternative form of the VLP-associated RSV F protein failed to stimulate memory responses. This result underscores the importance of the form of the F protein in stimulating not only efficacious antibody responses but also memory responses.
MATERIALS AND METHODS
Cells, virus, and plasmids.
ELL-0 cells (avian fibroblasts), Vero cells, HEp-2 cells, and COS-7 cells were obtained from the American Type Culture Collection. ELL-0 cells were maintained in Eagle's minimal essential medium (EMEM; Gibco) supplemented with 10% fetal calf serum (FCS). COS-7 cells were grown in Dulbecco modified Eagle medium (DMEM) supplemented with penicillin-streptomycin (Pen-Strep) and 10% fetal calf serum. Vero cells and HEp-2 cells were grown in DMEM supplemented with penicillin, streptomycin, and 5% or 10% fetal calf serum, respectively. The RSV A2 strain was obtained from R. Finberg.
The cDNAs encoding the NDV NP and M protein have been previously described (28). The construction of genes encoding the chimera proteins F/F and H/G, which contain the sequences encoding the ectodomains of the RSV F and G proteins fused to the sequences encoding the transmembrane and cytoplasmic domains of the NDV F and HN proteins, respectively, have been previously described (26). The expression and incorporation of these chimera proteins into VLPs have been previously reported (25, 26).
The F/HR2F chimera protein contains sequences encoding the entire ectodomain of the RSV F protein fused to sequences encoding the NDV F-protein heptad repeat 2 (HR2), TM, and CT domains. The RSV sequences were generated by PCR of pCAGGS-RSV F-protein DNA using GGTTATTGTGCTGTCGACTCATTTTGGC as the forward primer and CCAGCATTTACGTTACCTAATAATTCATCGG as the reverse primer. The NDV sequences were generated by PCR of pCAGGS-NDV F-protein DNA using CTTGGGAACGTCAACAAC as the forward primer and CTTGGGAACGTCAACAAC as the reverse primer. PCR products containing RSV sequences were digested with XhoI and ScaI, while PCR products containing NDV F-protein sequences were digested with ScaI and HindIII. The two PCR products were then ligated to the XhoI- and HindIII-digested pCAGGS vector. The RSV F and NDV F HR2 domains were fused such that the two HR2 helices were in register (Fig. 1). The resulting plasmid was sequenced in its entirety to verify the gene junctions and to ensure that no additional changes were introduced during the PCRs.
FIG 1.
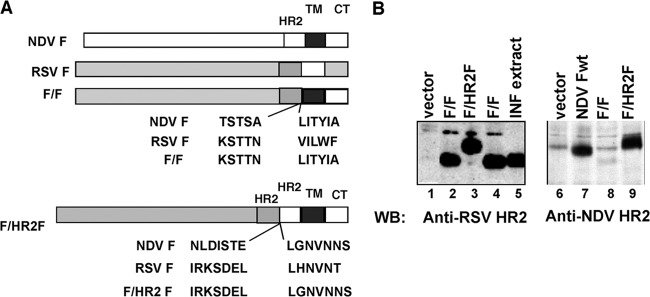
Construction and expression of chimeric F proteins. (A) (Top) Construction of the previously described F/F chimera protein as well as the sequences at the fusion junction of the chimera protein (26); (bottom) construction of the F/HR2F protein as well as the sequences at the fusion junction of the chimera protein. The HR2 domains of the RSV F protein and the NDV F protein were fused in register to maintain the helical structure. (B) The surface expression of chimera F proteins in avian cells is shown. The surfaces of avian cells transfected with cDNAs encoding F/F or F/HR2F chimera proteins were biotinylated, the cells were lysed, and the biotin-labeled molecules were precipitated with NeutrAvidin. Precipitated proteins were separated by SDS-polyacrylamide gel electrophoresis in the presence of reducing agent, and separated proteins were detected by Western blotting (WB) with anti-RSV HR2 peptide antibodies (lanes 1 to 5) or anti-NDV HR2 peptide antibodies (lanes 6 to 9). INF, infected.
Polyacrylamide gel electrophoresis, silver staining, and Western analysis.
Proteins in extracts, virus, or VLPs were resolved on 8% SDS-polyacrylamide gels (bis-Tris gels; Invitrogen). Silver staining of proteins in the polyacrylamide gels was accomplished as recommended by the manufacturer (Pierce). Quantification of the NP and M, F/F, F/HR2F, and H/G proteins in the polyacrylamide gels was accomplished with stained gels, as previously described (26). For Western analysis, proteins in the polyacrylamide gels were transferred to polyvinylidene difluoride membranes (PerkinElmer) using dry transfer (iBlot; Invitrogen). Proteins were detected in the blots (29) using the antibodies noted in the appropriate figure legends.
Antibodies.
Polyclonal goat anti-RSV antibody (Biodesign) was used for enzyme-linked immunosorbent assay (ELISA) and immunofluorescence. RSV F-protein monoclonal antibody (MAb) clone 131-2A (Chemicon) was used in plaque assays. MAb 1112 (a generous gift of J. Beeler) (30) and MAb anti-5C4 (a generous gift of B. Graham) (19) were used for fluorescence-activated cell sorting (FACS) analysis of transfected cells and binding to VLPs. Anti-RSV F-protein HR2 antibody is a polyclonal antibody specific to the HR2 domain of the RSV F protein (26). Anti-NDV F-protein HR2 antibody is a polyclonal antibody specific to the HR2 domain of the NDV F protein (31). Secondary antibodies against goat, mouse, and rabbit IgG were purchased from Sigma. Rabbit anti-goat antibody coupled to Alexa Fluor 568 and goat anti-mouse antibody coupled to Alexa Fluor 488 were obtained from Invitrogen. Antibodies for FACS analysis of murine spleen cells were anti-CD19 allophycocyanin (Biolegend) and anti-B220 peridinin chlorophyll protein (BD Pharmingen).
Biotinylation and immunofluorescence of cell surfaces.
Biotinylation of surface-expressed RSV F chimera proteins was accomplished as previously described (32, 33). Cell surface-biotinylated molecules were precipitated from cell lysates using NeutrAvidin-agarose (32, 33). Precipitated F proteins were detected by Western analysis using anti-RSV F HR2 or anti-NDV F HR2 antibodies.
Surface-expressed chimera proteins were also visualized by immunofluorescence or detected by flow cytometry. For microscopic visualization of transfected cells, avian or COS-7 cells, growing on coverslips and transfected with cDNAs encoding F chimera proteins or F and G chimera proteins, were fixed with 2% formaldehyde and then incubated with goat anti-RSV antibody followed by antigoat antibody coupled to Alexa Fluor 568, as previously described (33, 34). Images were acquired with a Leica DM IRE2 inverted fluorescence microscope with phase objectives.
Detection and quantification of chimera proteins were also accomplished by flow cytometry. Avian cells transfected with pCAGGS-F/F or pCAGGS-F/HR2F were washed in phosphate-buffered saline (PBS), removed from the plates with cell dissociation buffer (Sigma), resuspended and washed in FACS buffer (Hanks balanced salt solution; Gibco), and filtered through a 100-μm-pore-size nylon mesh. Cells were then incubated with mouse monoclonal antibody for 30 min on ice, washed twice in FACS buffer, and incubated with sheep anti-mouse IgG coupled to Alexa Fluor 488 for 30 min on ice in the dark. After three washes in FACS buffer, cells were resuspended in FACS buffer containing propidium iodide (PI; 10 μg/ml). Flow cytometry was accomplished with a MACSQuant analyzer flow cytometer (Miltenyi Biotec), and data were analyzed using FlowJo software with gating on PI-negative, fluorescein isothiocyanate-positive cells.
VLP preparation, purification, and characterization.
For the preparation of VLPs to be used as immunogens (VLP-H/G+F/F, VLP-H/G+F/HR2F), ELL-0 cells growing in T-150 flasks were transfected with cDNAs encoding the NDV M protein and NP and the H/G and F/F chimera proteins, as previously described (25, 26), or cDNAs encoding the H/G and F/HR2F chimera proteins. At 24 h posttransfection, heparin was added to the cells at a final concentration of 10 μg/ml (25, 26) to inhibit rebinding of released VLPs to cells. At 48, 72, and 96 h posttransfection, cell supernatants were collected and VLPs were purified by sequential pelleting and sucrose gradient fractionation, as previously described (25, 26). The concentrations of proteins in the purified VLPs were determined by the use of silver-stained polyacrylamide gels and by Western analysis using marker proteins for standard curves (26).
Preparation of RSV and FI-RSV, RSV plaque assays, and antibody neutralization.
RSV was grown in HEp-2 cells (25, 26), and RSV plaque assays were accomplished on Vero cells (25, 26). FI-RSV was prepared as previously described (25).
Antibody neutralization assays have been previously described (25, 26). The neutralization titer was defined as the log2 value of the reciprocal of the dilution of serum that reduced the virus titer by 60%.
Animals, animal immunization, and RSV challenge.
Four-week-old BALB/c mice from The Jackson Laboratory or Taconic Laboratories were housed (in groups of five mice each) under pathogen-free conditions in microisolator cages at the University of Massachusetts Medical Center animal quarters. BALB/c rag1−/− mice (generously provided by Ann Rothstein, University of Massachusetts Medical School) and TCRβδ−/− mice (generously provided by Eva Szomolanyi-Tsuda, University of Massachusetts Medical School) were generated from breeding pairs housed under pathogen-free conditions and received acidified (HCl; pH 2.8 to 3.2) water containing trimethoprim-sulfamethoxazole (Goldline Laboratories) ad libitum for seven consecutive days every other week (35).
Protocols requiring open cages were accomplished in biosafety cabinets. BALB/c mice were immunized by intramuscular (i.m.) inoculation of 10 or 30 μg total VLP protein in 0.05 ml of TNE (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 5 mM EDTA) containing 10% sucrose. For infection or challenge of BALB/c or rag1−/− mice with RSV, the animals were lightly anesthetized with isoflurane and then infected by intranasal (i.n.) inoculation of RSV (titers are indicated in the appropriate figure legends). All animal procedures and infections were performed in accordance with the University of Massachusetts Medical School IACUC- and IBC-approved protocols.
Detection of virus in lung tissue.
Four days after RSV challenge (36), the mice were sacrificed by CO2 asphyxiation. The lungs were removed aseptically, and each lobe was placed separately in 0.5 ml of 30% sucrose in PBS and frozen on dry ice. The lungs were stored at −80°C. Upon thawing, the lungs were weighed and then homogenized in the storage buffer using a Dounce homogenizer (Kontes). The homogenate was centrifuged at 12,000 rpm for 15 min, and the virus titer in the supernatant was determined by plaque assay as described above.
ELISA protocols.
For determination of serum antibody titers, blood was obtained from immunized animals by tail vein nicks and centrifuged in BD Microtainer serum separator tubes to remove blood cells. For ELISA, microtiter wells were coated with the F or G protein as previously described (24, 26). Briefly, the G-protein target antigen was that present in extracts from 293T cells transfected with pCAGGS-G. Dilutions of the transfected cell extract used as the target were adjusted so that the amounts of G protein were comparable from experiment to experiment, as determined by Western blotting. The F-protein target used for ELISAs was purified recombinant F protein (generous gift of Novavax, Inc.). Each well contained 25 ng F protein. ELISAs were performed as previously described (26). The titers of anti-G-protein antibodies were defined as the reciprocal of the serum dilution that gave an optical density (OD) 3-fold over the background OD (26). Titers for anti-F-protein antibodies were defined as the reciprocal of the serum dilution that gave an OD of 0.2, since the background values for this target antigen were zero (26).
For antibody binding to VLPs, microtiter wells were coated with different concentrations of monoclonal antibodies in 50 μl PBS for 24 to 30 h at 4°C. The wells were then incubated in 100 μl PBS–1% bovine serum albumin (BSA) for 16 h at 4°C. After washing the wells three times with PBS, 1 μl VLPs (1 ng total protein) in 50 μl PBS–1% BSA was added to each well and the plate was incubated for 2 h at room temperature. After six washes in PBS, goat anti-RSV was added to each well in 50 μl of PBS–1% BSA and the plate was incubated for 1.5 to 2 h at room temperature. After six washes in PBS, rabbit anti-goat antibody coupled to horseradish peroxidase (HRP) was added in 50 μl–1% BSA and the mixture was incubated for 1.5 h at room temperature. Bound HRP was detected by adding 50 μl 3,3′5,5′-tetramethylbenzidine (TMB; Sigma) and incubating for 5 to 20 min at room temperature until a blue color developed. The reaction was stopped with 50 μl 1 N sulfuric acid. The color was read in a SpectraMax Plus plate reader (Molecular Devices) using SoftMax Pro software.
Pulmonary histology of RSV-infected mice.
The mice were anesthetized with isoflurane and exsanguinated after the right caudal artery was severed. The lungs were then fixed via infusion of 4% formalin through the trachea, removed, immersed in 4% formalin for 24 h, embedded in paraffin, sectioned, and stained with hematoxylin-eosin (H&E) by the University of Massachusetts Diabetes Endocrinology Research grant-supported Histology Core Facility. Four sections per mouse were obtained, and sections from each mouse were scored blindly for the degree of inflammation of blood vessels and airways on a scale of from 0 to 3, as previously described (25, 37).
Purification of splenic B cells and adoptive transfer.
Splenocytes from disrupted spleens were washed, and red blood cells were lysed with Gey's solution (Sigma, Inc.), washed, resuspended at 50 × 106/ml in anti-Thy1.2 (MAb J1J10), and incubated on ice for 45 min. Cells were then washed and treated with rabbit complement (Pel-Freeze H2) for 45 min at 37°C (35). FACS analysis using anti-CD19 demonstrated that the B cells were approximately 90% pure. Adoptive transfer of purified B cells to rag1−/− mice (12 × 106 cells/mouse) was accomplished by periorbital inoculation.
ELISpot assay of spleen and bone marrow cells.
Splenocytes prepared from disrupted spleens were filtered through Nytex mesh (mesh size, 100 to 120 μm) and washed in balanced salt solution (BSS) containing 5% fetal calf serum or 0.3% BSA, and red blood cells were lysed in Gey's solution. Washed cells, resuspended in BSS, were counted and resuspended at a concentration of 10 × 106 to 20 × 106/ml of live cells in complete medium (RPMI) containing 10% serum, Pen-Strep, glutamine, and β-mercaptoethanol (βME; 5 × 10−5 M). B cell numbers were determined by FACS analysis using anti-CD19 and anti-B220 antibodies. Bone marrow cells were flushed from the femurs using a 25-gauge needle and BSS buffer, filtered, washed, counted, and resuspended at 2 × 109/ml.
For enzyme-linked immunosorbent spot (ELISpot) assays, ELISpot plates (Millipore) were coated overnight with purified F protein (25 ng/well in PBS). The wells were washed eight times with water, drained, and incubated for 1 h in compete medium (RPMI containing 10% serum, Pen-Strep, glutamine, and βME at 5 × 10−5 M). Fourfold serial dilutions of spleen cells were added, in triplicate, to precoated wells, and the plates were incubated at 37°C for 6 h. The plates were washed eight times with water and blocked overnight in PBS containing 1% BSA or 3% fetal calf serum. The wells were incubated with biotinylated anti-mouse IgG (1/2,000 dilution; Southern) for 1 h at room temperature, followed by eight water washes. The wells were then incubated for 1 h at room temperature with streptavidin-alkaline phosphatase (1/4,000 dilution; Southern) diluted in PBS–1% BSA, washed, and then developed with 5-bromo-4-chloro-3-indolylphosphate–nitroblue tetrazolium (BCIP/NBT; 1 tablet/10 ml autoclaved Millipore water) until purple spots appeared. The spots were counted using a CTL Immunospot S5 analyzer.
Statistical analysis.
Statistical analyses (Student t test) of the data were accomplished using GraphPad Prism software.
RESULTS
Expression and incorporation of RSV F/HR2F chimera protein into VLPs.
Current models of paramyxovirus fusion include the proposal that the heptad repeat 2 (HR2 or HRB) domains in the prefusion paramyxovirus F protein form a homotrimer that disassociates upon fusion activation (17). To determine if altering the structure of our previously described RSV-NDV F chimera protein, F/F (diagramed in Fig. 1A, top, and in references 24 and 26), would affect the immune responses to the F protein, we extended the ectodomain of the F/F chimera protein by inserting the NDV HR2 domain between the RSV HR2 domain and the NDV F TM domain with the goal of altering the stability of the prefusion form of F protein. This insertion created the F/HR2F chimera protein with two tandem HR2 domains, diagramed in Fig. 1A, bottom. The F/HR2F protein, which included insertion of 34 additional amino acids from the NDV F protein as well as a glycosylation addition site, was expressed in cells (not shown) and on cell surfaces (Fig. 1B) at levels comparable to those of our previously characterized F/F chimera protein (compare lanes 2 and 4 with lane 3, Fig. 1B). That the F/HR2F protein contains the HR2 domains of both RSV F and NDV F proteins is shown by its reactivity to both anti-RSV HR2 peptide antibody (Fig. 1B, lane 3) and anti-NDV HR2 peptide antibody (Fig. 1B, lane 9), while the F/F chimera protein binds only the RSV anti-HR2 peptide antibody (Fig. 1B, lanes 2 and 4), consistent with its amino acid sequence.
To identify possible differences in the abilities of the F/F and F/HR2F proteins to direct membrane fusion, the proteins were expressed in avian cells or in COS-7 cells in the absence or presence of the H/G chimera (Fig. 2). When expressed in avian cells, the cells in which the VLPs were made, neither F chimera protein directed syncytium formation either in the absence or in the presence of the H/G protein (Fig. 2A to D). This result indicates that any expressed prefusion F protein should not be activated during VLP assembly. In contrast, when expressed in COS-7 cells, the F/HR2F protein directed syncytium formation even in the absence of H/G, while the F/F protein did not (Fig. 2G and E, respectively). The F/F protein directed the formation of small syncytia in the presence of H/G (Fig. 2F), and expression of H/G with F/HR2F enhanced syncytium formation (Fig. 2H). Thus, the F/HR2F protein mediates membrane fusion more readily than the F/F protein, indicating structural differences in the two chimera proteins.
FIG 2.
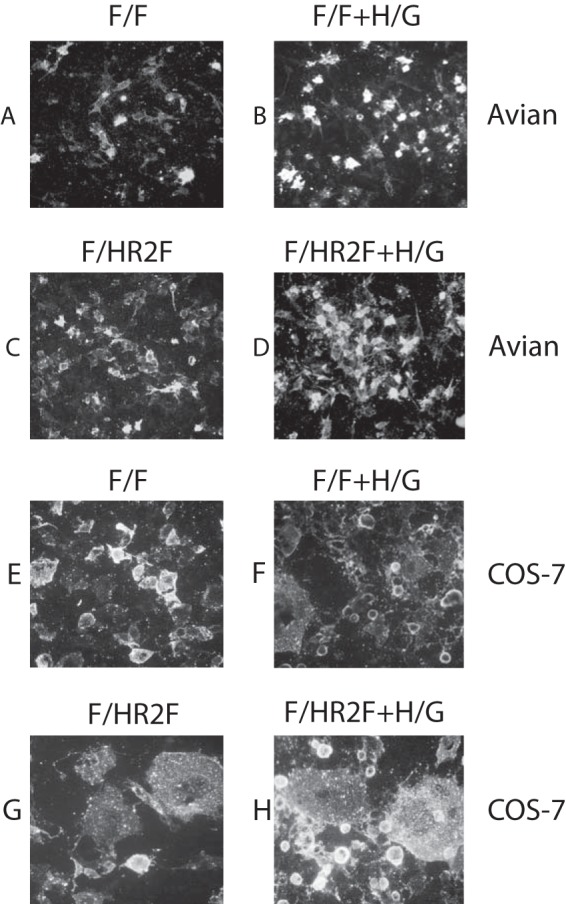
Syncytium formation in different cell types. Avian cells or COS-7 cells were transfected with cDNAs encoding the F/F chimera protein (A, B, E, F) or the F/HR2F chimera protein (C, D, G, H) in the absence (A, C, E, G) or presence (B, D, F, H) of cDNA encoding the H/G chimera. At 48 h posttransfection, cells were fixed with paraformaldehyde and then stained with goat anti-RSV polyclonal antibody, and binding of antibody was detected with rabbit anti-goat antibody coupled to Alexa Fluor 568. Images were opened in Adobe Photoshop software using identical settings for all images.
The F/HR2F chimera protein was incorporated into VLPs as efficiently as the F/F chimera protein. Figure 3 shows the F/F and F/HR2F protein content of VLPs released from avian cells when coexpressed with the NDV M protein and NP as well as a chimera protein containing the ectodomain sequences of the RSV G protein (H/G) that we have previously described (25). The incorporation of the G-protein chimera protein into VLPs was unaffected by coexpression with either the F/F or the F/HR2F protein, as indicated by our finding that the levels of H/G protein in three separate sets of VLPs, normalized to the chimera F-protein content, were indistinguishable (P = 0.83).
FIG 3.
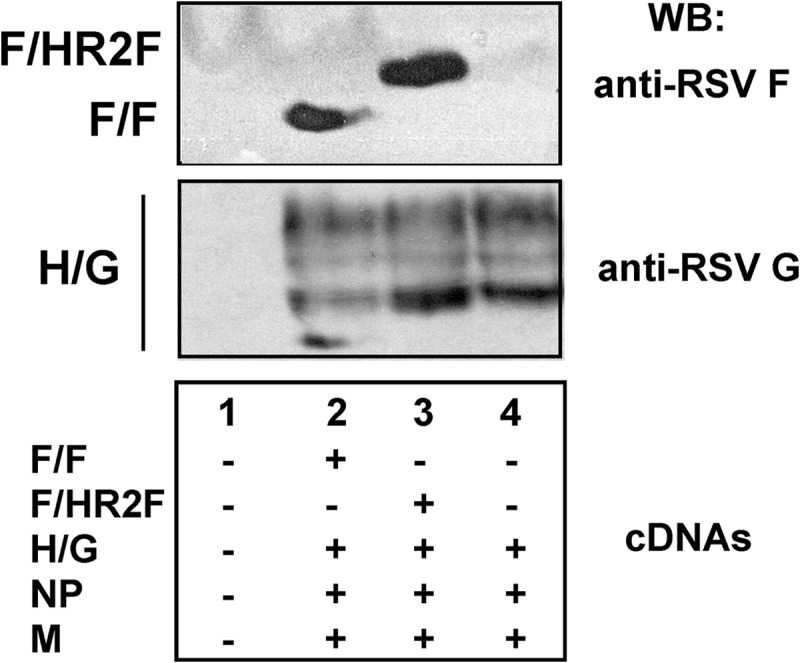
Incorporation of chimera proteins into VLPs. Avian cells were transfected with empty vector or combinations of cDNAs encoding the genes indicated at the bottom. Equivalent amounts of DNA were used for all cDNAs. VLPs, harvested from 48 to 96 h after transfection, were purified as described in Materials and Methods, and proteins present in the purified particles were separated on SDS-polyacrylamide gels in the presence of reducing agent. The F-protein chimera proteins F/F and F/HR2F were detected with anti-RSV F-protein HR2 peptide antibody, while the H/G chimera protein was detected with anti-RSV antibody, which detects only G protein and not F protein on Western blots (26), in contrast to F protein detection by this antibody by immunofluorescence, as in Fig. 2.
To assess the presence of prefusion F protein in expressed F/F and F/HR2F chimeras, two approaches were used, and both approaches utilized a monoclonal antibody, 5C4. This antibody has been shown to detect antigenic site ϕ, which is present only in the prefusion form of the RSV F protein (19, 21). The relative amounts of site ϕ on avian cell surfaces after transfection with pCAGGS-F/F or pCAGGS-F/HR2F were first determined by flow cytometry. To control for variations in transfection efficiency from experiment to experiment, 5C4 binding was normalized to binding by MAb 1112, an antibody specific for site I (site B) (30). Binding of MAb 1112 to cells expressing F/F or F/HR2F was reproducibly identical in parallel samples in single experiments (not shown); thus, its binding serves to normalize the binding of 5C4 from experiment to experiment. As shown in Fig. 4A, the binding of 5C4 relative to that of MAb 1112 is the same for F/F and F/HR2F, suggesting that site ϕ is equally available in both chimera proteins.
FIG 4.
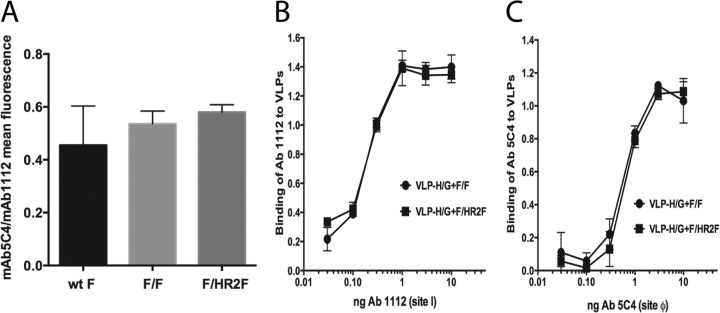
Antigenic site ϕ in chimera proteins. (A) Cells transfected with pCAGGS-F/F, pCAGGS-F/HR2F, or the pCAGGS-F wild type (wt) for 48 h were analyzed by flow cytometry for binding to anti-5C4 and to anti-1112 MAbs, as described in Materials and Methods. The graph shows the ratios of the mean intensity of fluorescence for binding of the two antibodies, 5C4 and 1112. (B and C) VLP-HG+F/F and VLP-H/G+F/HR2F binding to increasing amounts of MAb 1112 (B) and MAb 5C4 (C).
To determine if F/F or F/HR2F assembled into VLPs retained site ϕ, binding of both VLPs to increasing amounts of MAb 5C4 (Fig. 4C) was assessed in parallel with VLP binding to MAb 1112 (Fig. 4B). Clearly, F chimera proteins in both VLPs contain site ϕ.
Anti-RSV F- and G-protein antibody responses to NDV VLPs containing the F/HR2F chimera.
To characterize the immune responses to F/HR2F, we immunized mice with VLPs assembled with both the F/HR2F chimera and the H/G chimera (VLP-H/G+F/HR2F). Groups of five mice were immunized with two different concentrations of purified VLP-H/G+F/HR2F by intramuscular (i.m.) inoculation to mimic standard immunization. As positive controls, another group of mice was infected with RSV by intranasal inoculation to mimic natural infection. Negative controls were sham-vaccinated mice inoculated i.m. with buffer. Sera were collected from these animals from 20 to 430 days postimmunization. Anti-RSV F-protein IgG antibody titers (Fig. 5A) as well as anti-RSV G-protein IgG titers (Fig. 5B) in the sera of mice at each time point after a single immunization were determined by ELISA. To compare the antibody levels to those stimulated by VLP-H/G+F/F previously reported (26), representative ELISA titers of antibody to this VLP determined in parallel are also included.
FIG 5.
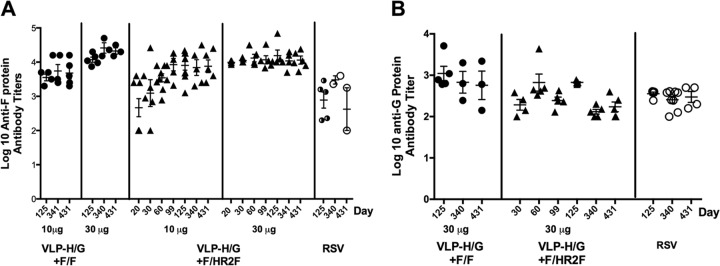
Comparisons of levels of serum antibodies in mice immunized with VLP-H/G+F/F and VLP-H/G+F/HR2F. Groups of five mice were immunized with 10 μg or 30 μg total VLP-H/G+F/HR2F. The amounts of the F and G chimera proteins in 10 μg of VLPs were 0.7 and 0.8 μg, respectively, while the amounts of the F and G chimera proteins in 30 μg of VLPs were 2.1 and 2.4 μg, respectively. Another group of five mice was infected with RSV (2.3 × 106 PFU/mouse). Serum antibodies specific to the F protein (A) or G protein (B) were determined with time after immunization. The full time course of serum responses to 10 and 30 μg of VLP-H/G+F/F has been previously reported (26), and results for representative sera are shown here for comparison to the responses to VLP-H/G+F/HR2F.
Similar to the results that we have previously reported for VLP-H/G+F/F (26), the anti-RSV F- and G-protein antibody titers obtained by stimulation with VLP-H/G+F/HR2F were robust and remained relatively constant for the entire course of the experiment. The titers were very similar to those obtained with VLP-H/G+F/F immunization. These results are consistent with VLP stimulation of long-lived antibody responses in the absence of added adjuvants.
Protective responses to VLP-H/G+F/HR2F.
The protective immune responses to VLP-H/G+F/HR2F were assessed by determining the neutralization titers in sera from immunized animals and by determining the protection of immunized mice from RSV replication in the lungs upon live virus challenge. To assess the neutralizing antibody responses, sera obtained from mice at each time point described in Fig. 5 were pooled, and neutralization titers were determined in an in vitro plaque reduction assay as described in Materials and Methods. Figure 6A shows the neutralization titers with time after immunization with both concentrations of VLPs. Neutralizing antibody titers increased until 50 to 60 days postimmunization and remained relatively constant. These titers were similar to those that we have previously reported in the sera of mice immunized with VLP-H/G+F/F (26). In addition, as we have previously noted, the neutralization titers in the sera of mice infected i.n. with RSV peaked early and then declined significantly with time, although total IgG antibody titers were relatively constant (26).
FIG 6.
Protective immune responses in VLP-H/G+F/HR2F-immunized animals. (A) Neutralization titers in sera pooled from five mice immunized with either 10 μg or 30 μg VLP-H/G+F/HR2F and harvested at different times after a single immunization were determined in a plaque reduction assay. The titers in sera from animals infected with RSV as described in the legend to Fig. 5 are shown for comparison. (B) Protection of VLP-H/G+F/HR2F-immunized mice from an RSV challenge is shown. Groups of five mice were immunized with VLP-H/G+F/HR2F (2.4 μg F protein per mouse) or infected with RSV (7 × 105 PFU/mouse). Two other groups of mice were not immunized. At day 52 postimmunization or postinfection, mice were challenged with RSV (2.3 × 106 PFU/mouse), and at 4 days postchallenge, the lungs were harvested and virus titers were determined by plaque assay. As a negative control, one set of mice was not immunized or challenged.
To assess protection from RSV challenge, mice immunized with VLP-H/G+F/HR2F were challenged with RSV at 50 days postimmunization. Figure 6B shows the virus titers obtained from lungs harvested at 4 days postchallenge with RSV. Clearly, immunization with VLP-H/G+F/HR2F protected mice challenged with RSV from RSV replication in the lungs, a result identical to the results obtained with VLP-H/G+F/F (26).
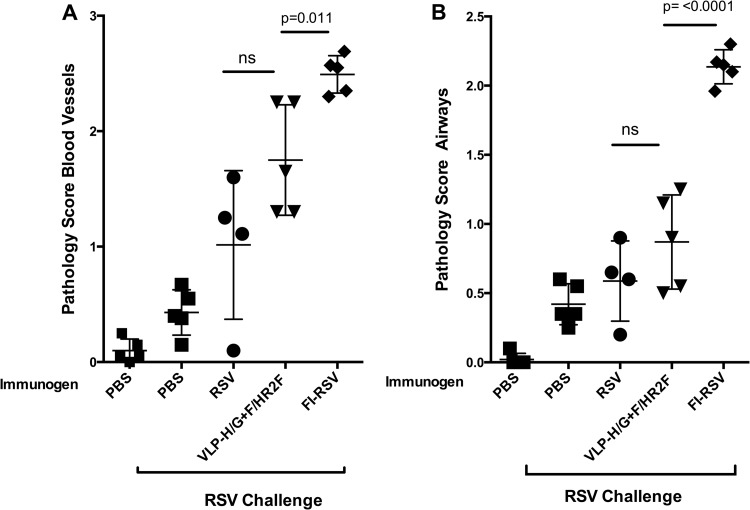
Lung pathology after RSV challenge.
We have previously reported that RSV challenge of VLP-H/G+F/F-immunized mice did not result in a lung pathology typical of that seen after RSV challenge of mice immunized with formalin-inactivated RSV (FI-RSV) (26). To determine if similar results are obtained after RSV challenge of VLP-H/G+F/HR2F-immunized mice, groups of five mice were immunized with VLP-H/G+F/HR2F, RSV, or FI-RSV or sham vaccinated. Mice were challenged with RSV, and lung sections were assessed for pathology (25, 26) by scoring for inflammation around blood vessels and airways, as shown in Fig. 7A and B, respectively. While immunization with FI-RSV recapitulated the previously reported abnormal histology of the lung sections, the lungs of VLP-H/G+F/HR2F-immunized mice had scores that were statistically significantly lower than the scores for the lungs of FI-RSV-immunized mice. The differences between RSV challenges of RSV-infected or VLP-immunized mice were not statistically significant. However, two of the five VLP-immunized mice showed inflammation around blood vessels somewhat enhanced over that observed for mice immunized with RSV.
FIG 7.
Scores of inflammation of lungs after RSV challenge of immunized mice. Four groups of five mice were immunized with RSV i.n. (7 × 105 PFU/mouse), 30 μg VLP-H/G+F/HR2F, or FI-RSV (equivalent of 7 × 106 PFU/mouse). Another two groups of five mice received PBS. At day 39, the animals were boosted with VLPs (10 μg VLPs) or infected with RSV (7 × 105 PFU/mouse), FI-RSV (equivalent of 7 × 106 PFU/mouse), or PBS. On day 47, four groups of mice, indicated at the bottom, were challenged with RSV i.n. (7 × 105 PFU/mouse), and their lungs were harvested 6 days later. Tissue sections stained with H&E from each mouse were scored for inflammation on a scale of from 0 to 3, as described in Materials and Methods. (A) Scores for blood vessels; (B) scores for airways. Bars indicate the groups compared for statistical analyses using GraphPad Prism software (Student t test), with significant P values shown above the bars. ns, not significant. Differences in the scores between RSV-infected/RSV-challenged mice and VLP immunized mice were not significant.
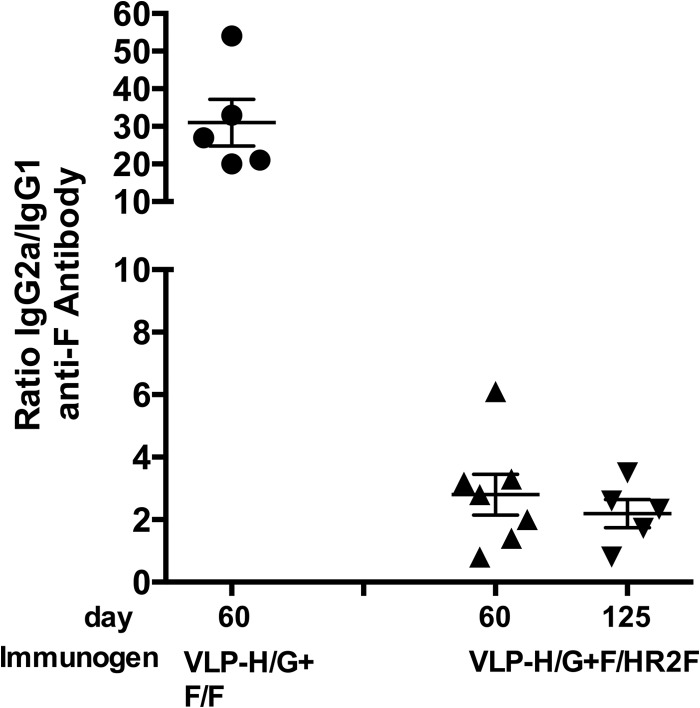
Subtypes of IgG antibodies stimulated by VLP-H/G+F/HR2F.
The enhanced, life-threatening respiratory disease (ERD) observed after RSV challenge of mice immunized with FI-RSV or UV-irradiated RSV has been associated with TH2-biased immune responses (reviewed in references 8 and 38). The ratio of IgG subtypes during infection or immunization has been used as an indicator of TH1- or TH2-biased immune responses (for example, see references 39 and 40). Because there was a suggestion of somewhat enhanced inflammation after RSV challenge of VLP-H/G+F/HR2F-immunized mice, we characterized the IgG2a/IgG1 ratios in sera from immunized mice. Figure 8 shows that while the anti-F-protein antibody responses to VLP-H/G+F/F were primarily IgG2a, with the IgG2a/IgG1 ratios being approximately 30, as we have previously reported (26), anti-F-protein antibody responses to VLP-H/G+F/HR2F were significantly different, with the IgG2a/IgG1 ratios being approximately 2. This result suggests that the anti-F-protein immune responses stimulated by the two VLPs are different in character.
FIG 8.
Ratio of IgG2a to IgG1 in sera from VLP-immunized animals. Groups of five mice were immunized with 30 μg of VLP-H/G+F/F or VLP-H/G+F/HR2F as described in the legend to Fig. 5. The amounts of IgG1 and IgG2a (in ng/ml) in each serum sample were calculated on the basis of standard curves of purified murine IgG2a or IgG1. Each assay was done at least two times. The graph shows the ratios of IgG2a to IgG1 for each mouse at 60 days (VLP-H/G+F/F) or 60 and 125 days (VLP-H/G+F/HR2F) postimmunization. Differences between the ratios in the sera of VLP-H/G+F/F-immunized mice and the sera of VLP-H/G+F/HR2F-immunized mice at 60 or 125 days were significant, with P values of 0.0003 and 0.0017, respectively.
Memory responses to VLP-H/G+F/HR2F.
To characterize further the immune responses generated by VLP-H/G+F/HR2F, we compared the induction of memory responses to the two VLPs. We have previously reported that VLP-H/G+F/F stimulated memory responses as well as F-protein-specific long-lived, bone marrow-associated plasma cells (LLPCs) (24). The existence of significant serum anti-F- and anti-G-protein antibody titers at 430 days after immunization with VLP-H/G+F/HR2F is evidence for the stimulation of LLPCs by this VLP. Indeed, the bone marrow of animals immunized with VLP-H/G+F/HR2F contained cells secreting anti-F-protein antibody (Fig. 9), while the bone marrow of animals infected with RSV did not.
FIG 9.

Anti-F-protein-specific LLPCs. Groups of mice were immunized with 10 μg/mouse of VLP-H/G+F/HR2F protein, infected with RSV (2.25 × 106 PFU/mouse), or sham immunized with PBS. After 430 days, the mice were infected with RSV i.n., and the mice were sacrificed 4 days after infection. Bone marrow cells were prepared as described in Materials and Methods, and the number of cells secreting anti-F-protein antibodies per 106 cells was determined by ELISpot assay analysis.
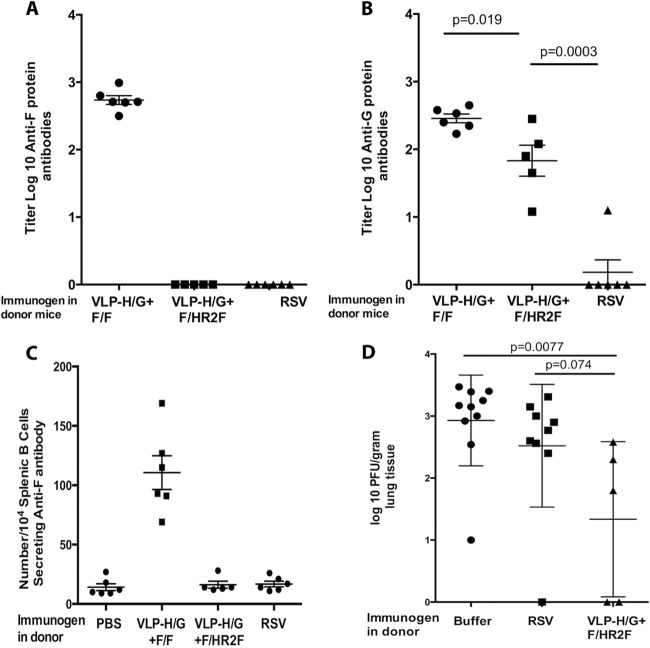
To evaluate the generation of memory B cells specific for RSV F and G proteins after VLP-H/G+F/HR2F immunization, we performed an adoptive transfer experiment with semipurified B cells, which were transferred from immunized mice into immunodeficient rag1−/− mice, and assessed the anti-RSV immune responses in the recipient mice, comparing them with our previously reported responses in recipient mice after B cell transfer from VLP-H/G+F/F-immunized mice (24). Groups of mice immunized with either 30 μg of VLP-H/G+F/HR2F i.m., infectious RSV i.n., or buffer i.m. were sacrificed at 430 days after immunization, and B cell-enriched splenic populations were injected into syngeneic, immunodeficient, BALB/c rag1−/− mice. After 6 days, the recipient mice were challenged with an intranasal inoculation of RSV. Four days after challenge, the sera, lungs, and spleens were harvested from the recipient mice for determination of the serum levels of anti-F-protein and anti-G-protein antibodies, anti-F-protein antibody-secreting cells in the spleens, and virus titers in the lungs.
Figure 10A and B show the titers of antibodies to the RSV F protein and G protein, respectively, in the sera of the recipient mice. In contrast to our previous finding (24) showing that the recipients of spleen cells from VLP-H/G+F/F-immunized mice had serum anti-F-protein antibodies (illustrated in Fig. 10A), the recipients of B cells from VLP-H/G+F/HR2F-immunized mice had no detectable anti-F-protein antibodies in their sera (Fig. 10A), while they did have anti-G-protein antibody titers (Fig. 10B). Indeed, mice receiving B cells from VLP-H/G+F/HR2F-immunized mice also had no detectable anti-F-protein antibody-secreting spleen cells (Fig. 10C). Recipient mice were, however, partially protected from RSV replication in their lungs after RSV challenge (Fig. 10D). This partial protection may be due to the memory immune responses to the RSV G protein.
FIG 10.
Adoptive transfer of B cells from VLP-H/G+F/HR2F-immunized mice. Groups of five mice were immunized with 30 μg VLP-H/G+F/HR2F, infected with RSV, or sham vaccinated with buffer. At 430 days postimmunization, B cells were harvested and partially purified as described in Materials and Methods. Purified B cells were transferred to five or six rag1−/− mice (106 cells/mouse). After 6 days, the recipient mice were challenged with RSV (7.5 × 105 PFU/mouse). At 4 days postchallenge, the mice were sacrificed and serum, spleens, and lungs were harvested. (A and B) The titers of serum antibodies to RSV F (A) and to RSV G (B) in recipient mice were determined by ELISA, as described in the legend to Fig. 5, with means and standard deviations indicated. The titers in serum from mice receiving B cells from VLP-H/G+F/F-immunized mice, as previously reported (24), are shown for comparison. (C) Spleen cells from recipient mice were prepared for ELISpot assay analysis as described in Materials and Methods. The number of anti-F-protein antibody-secreting cells per 104 splenic B cells in each mouse in each group is shown, and means and standard deviations are indicated. The titers of these cells in mice receiving B cells from VLP-H/G+F/F-immunized mice, as previously reported (24), are shown for comparison. (D) The titers of RSV in the lungs of RSV-challenged B cell recipient mice were determined as described in Materials and Methods, and means and standard deviations are indicated. Titers in different lobes of the lungs were determined for some mice, and the data for each lobe are reported. The bars at the top of panels B and D indicate the results of statistical analysis for the groups compared using GraphPad Prism software (Student t test), with P values shown above the bars.
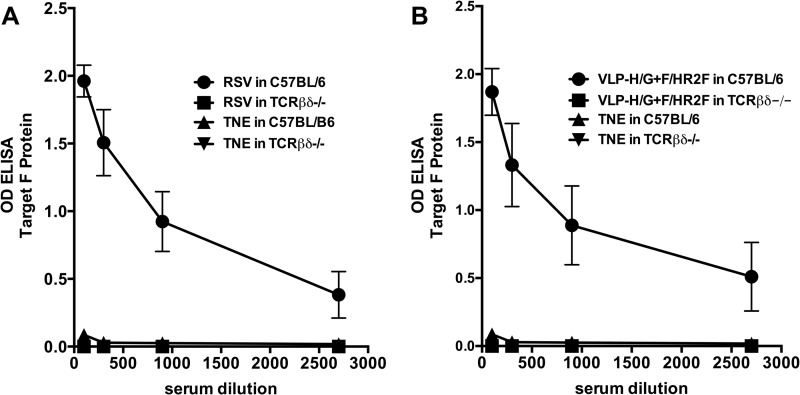
T cell dependence of anti-F-protein antibody responses.
That VLP-H/G+F/HR2F did not stimulate memory responses, as defined by the adoptive transfer experiments, but did stimulate LLPCs is puzzling, in light of the predominance of evidence that LLPCs and memory B cells are both products of germinal center (GC) reactions (41, 42). However, it has been reported that T cell-independent (TI) antigens may induce LLPCs in the absence of the formation of germinal centers (43). Thus, it was possible that the anti-F-protein antibodies detected at 430 days after immunization with VLP-H/G+F/HR2F were induced though a TI response to this form of the F protein. To determine if anti-F-protein antibody responses to VLP-H/G+F/HR2F were TI, a group of T cell-deficient C57BL/6 TCRβδ-knockout mice and a control group of wild-type C57BL/6 mice were immunized with a single dose of VLP-H/G+F/HR2F. Equivalent groups were infected with RSV to determine if virus-associated F protein could function as a TI antigen. Sham-vaccinated mice were also included. Anti-F-protein antibody responses were measured by ELISA at 36 days postimmunization. Figure 11 shows that anti-F-protein antibodies were readily detected in control, wild-type mice, but no anti-F-protein antibodies were detected in VLP-H/G+F/HR2F-immunized T cell-deficient mice even at low serum dilutions (Fig. 11B). These results are consistent with the conclusion that the response to the F chimera protein in these VLPs is T cell dependent (TD). Similarly, virus infection did not result in any anti-F-protein TI immune responses (Fig. 11A). The results suggest that the failure of VLP-H/G+F/HR2F to stimulate memory responses while stimulating long-lived, anti-F-protein antibody-secreting cells cannot be due to the T cell independence of this RSV F antigen. Rather, the form of the RSV F protein has a significant role in stimulating TD memory responses to RSV F protein.
FIG 11.
T cell dependence of anti-F-protein antibody responses to VLP-H/G+F/HR2F. C57BL/6 or C57BL/6 TCRβδ−/− (TCRβδ−/−) mice (five mice per group) were immunized i.m. with 30 μg total VLP-H/G+F/HR2F protein, sham vaccinated with buffer (TNE), or infected i.n. with RSV (2 × 106 PFU/mouse). After 36 days, sera from mice in each group were pooled and anti-F-protein antibodies in the pooled sera were measured by ELISA, as described in Materials and Methods. The ODs at 450 nm of the different serum dilutions are shown. (A) Results obtained after RSV infection; (B) results obtained after VLP immunization. Each point is the mean of three determinations, with the standard deviations indicated.
DISCUSSION
RSV vaccine development has been hampered by poorly defined requirements for the induction of protective, long-lived immune responses to RSV. In contrast to the majority of viral infections, humans experience repeated RSV infections caused by the same virus serogroup (12, 23) for reasons that are poorly understood. Indeed, we reported that a single intranasal infection of mice with RSV stimulated only transient neutralizing antibodies, no bone marrow-associated long-lived plasma cells, and no memory B cells, results that parallel the responses to human infections. Pulendran and Ahmed (44) have noted that a successful RSV vaccine, in contrast to most vaccines, must stimulate better immune responses than natural infection. To date, the analyses of the induction of long-term protective responses to vaccine candidates have not been the primary focus of RSV vaccine development.
We have previously reported that the ectodomain of the RSV F protein anchored in a VLP membrane stimulated durable neutralizing serum antibodies, LLPCs secreting anti-F-protein antibodies, and anti-F-protein B cell memory, all essential elements of long-term protective immunity. Here we report that these properties of immune responses to a slightly modified version of the VLP-associated RSV F protein ectodomain are quite distinct. In the new version of the RSV F protein described here, F/HR2F, we fused the HR2 (or HRB) domain of the RSV F protein to the HR2 domain of the NDV F protein, creating an ectodomain with two HR2 domains. Available crystal structures of prefusion paramyxovirus F proteins show the HR2 domain in a trimeric stalk connecting a globular head with the transmembrane domain (17, 19). While this trimer structure may be due to or at least stabilized by a trimerization sequence fused to the carboxyl terminus of the F-protein ectodomain for cell secretion and crystallization, current models of paramyxovirus fusion posit that, upon activation of fusion, the HR2 trimer disassociates, facilitating the refolding of the F protein into the postfusion form with the HR2 and HR1 (HRA) domains, forming a six-stranded, coiled coil (17). Thus, it was possible that the inclusion of two tandem HR2 domains might affect the conversion of the prefusion form to the postfusion form of the F protein and, therefore, influence immune responses to the F protein. To explore differences in the F/HR2F and F/F proteins, we assessed the biological activities of the two proteins in both avian cells and COS-7 cells. First, neither protein directed fusion in avian cells either in the presence or in the absence of the H/G chimera protein. Thus, any prefusion form of the proteins delivered to cell surfaces would not likely be activated during VLP assembly. In contrast, the insertion of the NDV HR2 domain resulted in syncytium formation in COS-7 cells both in the presence and in the absence of the H/G chimera protein, while the F/F protein directed syncytium formation only in the presence of H/G, and the syncytia were smaller than those resulting from F/HR2F expression. These results may be interpreted in several ways. It is possible that a higher percentage of the F/HR2F protein, delivered to the cell surface and into VLPs, may be in a prefusion form, whereas the F/F protein is not. However, the finding that both proteins contain equivalent levels of antigenic site ϕ is not consistent with this explanation. It is more likely that the prefusion F/HR2F is more readily activated than the prefusion F/F. The results do suggest that the conformation or stability of the F/F and F/HR2F proteins, or both, is different.
Insertion of the NDV HR2 domain sequences into the F/F chimera protein had no effect on the expression or assembly of the chimera protein into VLPs. This VLP-associated modified F protein, F/HR2F, also stimulated in mice titers of total anti-F IgG comparable to those stimulated by the VLP-associated F/F chimera protein. Furthermore, the neutralization titers in the sera of mice immunized with VLP-H/G+F/HR2F were similar to those in the sera of mice immunized with VLP-H/G+F/F (6 to 7 log2 units versus 5.5 to 6 log2 units, respectively). In addition, this F/HR2F-containing VLP stimulated anti-F-protein antibody-secreting bone marrow-associated plasma cells, as did the F/F-containing VLPs. However, there was a dramatic difference in the types of anti-F-protein IgG antibodies stimulated by the two VLPs. While VLPs containing F/F stimulated primarily IgG2a antibodies with IgG2a/IgG1 ratios of approximately 30 after a single immunization, VLPs containing F/HR2F stimulated antibodies with a ratio of approximately 2, very similar to the IgG subtype profile induced by RSV infection (26). However, the most dramatic difference between the responses to the F/F- and F/HR2F-containing VLPs was in their differential ability to induce F-protein-specific memory B cell responses. Immunization with the VLPs expressing F/HR2F failed to induce anti-F-protein memory B cells. These VLPs did, however, stimulate anti-G-protein memory responses, indicating a selective antigen-specific memory defect. Thus, the conformation of the F protein can directly dictate the generation of protein-specific memory B cells and the character of the antibody response.
A key step in the generation of LLPCs and memory B cells is the formation of germinal centers (GCs). GCs are specialized microenvironments in spleen and lymph nodes (LNs) that contain B cells undergoing class switch recombination, somatic hypermutation, and affinity maturation. The signals that direct the partitioning of GC B cells into LLPCs or memory B cell pools are not well understood (42), but the predominance of evidence indicates that LLPCs in bone marrow are derived from GC reactions, as are memory B cells, raising the question of why F/HR2F fails to stimulate B cell memory, whereas LLPC production is unimpaired. There are studies suggesting that LLPCs can also be derived from B cells stimulated by TI antigens that have not entered GCs (43). These cells may be isotype switched but have not undergone somatic hypermutation (45). Thus, it is possible that LLPCs from F/HR2F VLP-immunized animals were not derived from GCs, whereas those from F/F VLP-immunized animals were derived from GCs. We directly addressed the possibility that the VLP-associated F/HR2F functioned as a TI antigen by assessing the F-protein antibody responses to VLP-H/G+F/HR2F in mice with T cell receptor mutations that rendered them T cell deficient. True TI antigens readily stimulate immune responses in these animals. However, no anti-F-protein antibodies were generated in these mice, indicating that F/HR2F is a T cell-dependent (TD) antigen.
There are several possible explanations to account for the differential stimulation of F-protein-specific memory B cells by VLPs containing different forms of the RSV F-protein ectodomain. We assayed for memory generation by adoptively transferring semipurified B cells from immunized animals into immunodeficient rag1−/− mice and then assessed the anti-F-protein antibody responses in the recipient mice upon restimulation by RSV infection. One possible explanation for the failure to detect F/HR2F-stimulated anti-F-protein memory in this way is that reactivation of memory B cells in the recipient mice may be more T cell dependent than that induced by VLPs containing F/F. In our adoptive transfer experiments, we transferred B cells that were approximately 90% pure; thus, the numbers of T cells transferred were very limited. The requirement for T cell help to reactivate B memory cells specific for particle immunogens (as opposed to soluble antigens) is controversial. It has been shown that virus-specific memory B cells generated by cytomegalovirus infection, tick-borne encephalitis virus infection, or a particulate complex of ovalbumin and alum can be reactivated in the absence of cognate or bystander T cells upon a second exposure to the particulate antigens (46–48). These results led MacLeod et al. to suggest that the requirements for T cells in the reactivation of memory B cells vary with the form of the antigen (49). Given this consideration, it is possible that the F/HR2F-containing VLPs do induce memory B cells but their reactivation requires significantly more T cell help than reactivation of cells induced by F/F-containing VLPs.
Another potential explanation for the differential induction of memory responses is suggested by Kasturi et al. (50). These investigators immunized mice with nanoparticles coupled to influenza virus hemagglutinin (HA) or with HA nanoparticles assembled with a Toll-like receptor 4 (TLR4) or TLR7 agonist, or both, and showed that the TLR agonists dramatically increased the numbers and durability of GCs as well as the numbers of anti-HA-secreting LN cells. Furthermore, they showed that inclusion of both TLR agonists dramatically increased the number and durability of GCs and antibody-secreting LN cells over the number and durability observed with one TLR agonist. These observations suggest the possibility that the different VLPs vary in the efficiency of stimulation of innate immune responses ultimately required for memory generation. Indeed, it has been reported that native RSV F protein is a TLR4 agonist (51). However, differences in TLR signaling cannot fully explain the lack of F-protein-specific memory to F/HR2F since the same VLP stimulated memory to the RSV G protein. Differences due to innate immune engagement should be manifested in differences in both F- and G-protein memory responses.
Another possible explanation to account for differences in memory generation by different VLPs may be due to differences in the interactions of VLPs with antigen-presenting cells, such as dendritic cells, a possibility suggested by the different biological activities of the F/F and F/HR2F proteins. Such differential interactions could result in differences in the nature or efficiency of antigen presentation and, consequently, T cell activation (52) and induction of B cell memory.
These possibilities to account for the failure of the F/HR2F protein to induce B cell memory are under investigation, as are the conformational differences between F/F and F/HR2F proteins. Our results do demonstrate that the form of the RSV F protein in a VLP has a significant role in the nature of the immune responses to this protein. Identification of the conformation of the RSV F protein that most effectively stimulates both LLPCs and memory B cells will be important in the future development of RSV vaccines.
ACKNOWLEDGMENTS
This study was supported by grants from the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (AI093791 to T.G.M., AI041054 and AI084800 to R.T.W. and M.R.S., and AI057319 to A. Rothman). Core resources supported by Diabetes Research Center grant DK32520 were also used.
We thank Ann Rothstein and Eva Szomolanyi-Tsuda for mutant mice. We thank Barney Graham, NIH, NIAID, for 5C4 antibody and Judy Beeler, FDA, for MAb 1112.
Footnotes
Published ahead of print 25 June 2014
REFERENCES
- 1.Karron RA. 2008. Respiratory syncytial virus and parainfluenza virus vaccines, p 1283 In Plotkin SA, Orenstein WA, Offit PA. (ed), Vaccines, 5th ed. Saunders-Elsevier, Philadelphia, PA [Google Scholar]
- 2.Nair H, Nokes DJ, Gessner BD, Dherani M, Madhi SA, Singleton RJ, O'Brien KL, Roca A, Wright PF, Bruce N, Chandran A, Theodoratou E, Sutanto A, Sedyaningsih ER, Ngama M, Munywoki PK, Kartasasmita C, Simoes EAF, Rudan I, Weber MW, Campbell H. 2010. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet 375:1545–1555. 10.1016/S0140-6736(10)60206-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Han LL, Alexander JP, Anderson LJ. 1999. Respiratory syncytial virus pneumonia among the elderly: an assessment of disease burden. J. Infect. Dis. 179:25–30. 10.1086/314567 [DOI] [PubMed] [Google Scholar]
- 4.Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE. 2005. Respiratory syncytial virus infection in elderly and high-risk adults. N. Engl. J. Med. 352:1749–1759. 10.1056/NEJMoa043951 [DOI] [PubMed] [Google Scholar]
- 5.Falsey AR, Walsh EE. 2000. Respiratory syncytial virus infection in adults. Clin. Microbiol. Rev. 13:371–384. 10.1128/CMR.13.3.371-384.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raboni SM, Nogueira MB, Tsuchiya LR, Takahashi GA, Pereira LA, Pasquini R, Siqueira MM. 2003. Respiratory tract viral infections in bone marrow transplant patients. Transplantation 76:142–146. 10.1097/01.TP.0000072012.26176.58 [DOI] [PubMed] [Google Scholar]
- 7.Walsh EE, Falsey AR, Hennessey PA. 1999. Respiratory syncytial and other virus infections in persons with chronic cardiopulmonary disease. Am. J. Respir. Crit. Care Med. 160:791–795. 10.1164/ajrccm.160.3.9901004 [DOI] [PubMed] [Google Scholar]
- 8.Collins PL, Crowe JE. 2007. Respiratory syncytial virus and metapneumovirus, 5th ed, vol 2 Lippincott Williams & Wilkins, Philadelphia, PA [Google Scholar]
- 9.van Drunen Littel-van den Hurk S, Mapletoft JW, Arsic N, Kovacs-Nolan J. 2007. Immunopathology of RSV infection: prospects for developing vaccines without this complication. Rev. Med. Virol. 17:5–34. 10.1002/rmv.518 [DOI] [PubMed] [Google Scholar]
- 10.Openshaw PJ, Culley FJ, Olszewska W. 2002. Immunopathogenesis of vaccine-enhanced RSV disease. Vaccine 20(Suppl 1):S27–S31. 10.1016/S0264-410X(01)00301-2 [DOI] [PubMed] [Google Scholar]
- 11.Openshaw PJ, Tregoning JS. 2005. Immune responses and disease enhancement during respiratory syncytial virus infection. Clin. Microbiol. Rev. 18:541–555. 10.1128/CMR.18.3.541-555.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Power UF. 2008. Respiratory syncytial virus (RSV) vaccines—two steps back for one leap forward. J. Clin. Virol. 41:38–44. 10.1016/j.jcv.2007.10.024 [DOI] [PubMed] [Google Scholar]
- 13.Jardetzky TS, Lamb RA. 2004. A class act. Nature 427:307–308. 10.1038/427307a [DOI] [PubMed] [Google Scholar]
- 14.Smith EC, Popa A, Chang A, Masante C, Dutch RE. 2009. Viral entry mechanisms: the increasing diversity of paramyxovirus entry. FEBS J. 276:7217–7227. 10.1111/j.1742-4658.2009.07401.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lamb RA, Parks GD. 2007. Paramyxoviridae: the viruses and their replication, p 1450–1496 In Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Strauss SE. (ed), Fields virology, 5th ed, vol 1 Lippincott Williams & Wilkins, Philadelphia, PA [Google Scholar]
- 16.Yin H-S, Paterson RG, Wen X, Lamb RA, Jardetzky TS. 2005. Structure of the uncleaved ectodomain of the paramyxovirus (hPIV3) fusion protein. Proc. Natl. Acad. Sci. U. S. A. 102:9288–9293. 10.1073/pnas.0503989102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yin H-S, Wen X, Paterson RG, Lamb RA, Jardetzky TS. 2006. Structure of the parainfluenza virus 5 F protein in its metastable, prefusion conformation. Nature 439:38–44. 10.1038/nature04322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McLellan JS, Yang Y, Graham BS, Kwong PD. 2011. Structure of respiratory syncytial virus fusion glycoprotein in the postfusion conformation reveals preservation of neutralizing epitopes. J. Virol. 85:7788–7796. 10.1128/JVI.00555-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McLellan JS, Chen M, Leung S, Graepel KW, Du X, Yang Y, Zhou T, Baxa U, Yasuda E, Beaumont T, Kumar A, Modjarrad K, Zheng Z, Zhao M, Xia N, Kwong PD, Graham BS. 2013. Structure of RSV fusion glycoprotein trimer bound to a prefusion-specific neutralizing antibody. Science 340:1113–1117. 10.1126/science.1234914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Magro M, Mas V, Chappell K, Vazquez M, Cano O, Luque D, Terron MC, Melero JA, Palomo C. 2012. Neutralizing antibodies against the preactive form of respiratory syncytial virus fusion protein offer unique possibilities for clinical intervention. Proc. Natl. Acad. Sci. U. S. A. 109:3089–3094. 10.1073/pnas.1115941109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McLellan JS, Chen M, Joyce MG, Sastry M, Stewart-Jones GBE, Yang Y, Zhang B, Chen L, Srivatsan S, Zheng A, Zhou T, Graepel KW, Kumar A, Moin S, Boyington JC, Chuang G-Y, Soto C, Baxa U, Bakker AQ, Spits H, Beaumont T, Zheng Z, Xia N, Ko S-Y, Todd J-P, Rao S, Graham BS, Kwong PD. 2013. Structure-based design of a fusion glycoprotein vaccine for respiratory syncytial virus. Science 342:592–598. 10.1126/science.1243283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith G, Raghunandan R, Wu Y, Liu Y, Massare M, Nathan M, Zhou B, Lu H, Boddapati S, Li J, Flyer D, Glenn G. 2012. Respiratory syncytial virus fusion glycoprotein expressed in insect cells form protein nanoparticles that induce protective immunity in cotton rats. PLoS One 7:e50852. 10.1371/journal.pone.0050852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hall CB. 2001. Respiratory syncytial virus and parainfluenza virus. N. Engl. J. Med. 344:1917–1928. 10.1056/NEJM200106213442507 [DOI] [PubMed] [Google Scholar]
- 24.Schmidt MR, McGinnes LW, Kenward SA, Willems KN, Woodland RT, Morrison TG. 2012. Long term and memory immune responses in mice against Newcastle disease virus-like particles containing respiratory syncytial virus glycoprotein ectodomains. J. Virol. 86:11654–11662. 10.1128/JVI.01510-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murawski MR, McGinnes LW, Finberg RW, Kurt-Jones EA, Massare M, Smith G, Heaton PM, Fraire A, Morrison TG. 2010. Newcastle disease virus-like particles containing respiratory syncytial virus G protein induced protection in BALB/c mice with no evidence of immunopathology. J. Virol. 84:1110–1123. 10.1128/JVI.01709-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McGinnes LW, Gravel KA, Finberg RW, Kurt-Jones EA, Massare MJ, Smith G, Schmidt MR, Morrison TG. 2011. Assembly and immunological properties of Newcastle disease virus-like particles containing the respiratory syncytial virus F and G proteins. J. Virol. 85:366–377. 10.1128/JVI.01861-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McGinnes LW, Pantua H, Laliberte JP, Gravel KA, Jain S, Morrison TG. 2010. Assembly and biological and immunological properties of Newcastle disease virus-like particles. J. Virol. 84:4513–4523. 10.1128/JVI.01931-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pantua HD, McGinnes LW, Peeples ME, Morrison TG. 2006. Requirements for the assembly and release of Newcastle disease virus-like particles. J. Virol. 80:11062–11073. 10.1128/JVI.00726-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laliberte JP, McGinnes LW, Peeples ME, Morrison TG. 2006. Integrity of membrane lipid rafts is necessary for the ordered assembly and release of infectious Newcastle disease virus particles. J. Virol. 80:10652–10662. 10.1128/JVI.01183-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beeler JA, van Wyke Coelingh K. 1989. Neutralization epitopes of the F glycoprotein of respiratory syncytial virus: effect of mutation upon fusion function. J. Virol. 63:2941–2950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dolganiuc V, McGinnes L, Luna EJ, Morrison TG. 2003. Role of the cytoplasmic domain of the Newcastle disease virus fusion protein in association with lipid rafts. J. Virol. 77:12968–12979. 10.1128/JVI.77.24.12968-12979.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jain S, McGinnes LW, Morrison TG. 2007. Thiol/disulfide exchange is required for membrane fusion directed by the Newcastle disease virus fusion protein. J. Virol. 81:2328–2339. 10.1128/JVI.01940-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jain S, McGinnes LW, Morrison TG. 2008. Overexpression of thiol/disulfide isomerases enhances membrane fusion directed by Newcastle disease virus fusion protein. J. Virol. 82:12039–12048. 10.1128/JVI.01406-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laliberte JP, McGinnes LW, Morrison TG. 2007. Incorporation of functional HN-F glycoprotein containing complexes into Newcastle disease virus is dependent on cholesterol and membrane lipid raft integrity. J. Virol. 81:10636–10648. 10.1128/JVI.01119-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schmidt MR, Appel MC, Giassi LJ, Greiner DL, Shultz LD, Woodland RT. 2008. Human BLyS facilitates engraftment of human PBL derived B cells in immunodeficient mice. PLoS One 3:e3192. 10.1371/journal.pone.0003192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murawski MR, Bowen GN, Cerny AM, Anderson LJ, Haynes LM, Tripp RA, Kurt-Jones EA, Finberg RW. 2009. Respiratory syncytial virus activates innate immunity through Toll-like receptor 2. J. Virol. 83:1492–1500. 10.1128/JVI.00671-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mok H, Lee S, Utley TJ, Shepherd BE, Polosukhin VV, Collier ML, Davis NL, Johnson RE, Crowe JE. 2007. Venezuelan equine encephalitis virus replicon particles encoding respiratory syncytial virus surface glycoproteins induce protective mucosal responses in mice and cotton rats. J. Virol. 81:13710–13722. 10.1128/JVI.01351-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Collins PL, Graham BS. 2008. Viral and host factors in human respiratory syncytial virus pathogenesis. J. Virol. 82:2040–2055. 10.1128/JVI.01625-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Delgado MF, Coviello S, Monsalvo AC, Melendi GA, Hernandez JZ, Batalle JP, Diaz L, Trento A, Chang H-Y, Mitzner W, Ravetch J, Melero JA, Irusta PM, Polack FP. 2009. Lack of antibody affinity maturation due to poor Toll-like receptor stimulation leads to enhanced respiratory syncytial virus disease. Nat. Med. 15:34–41. 10.1038/nm.1894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Markine-Goriaynoff D, Coutelier J-P. 2002. Increased efficacy of the immunoglobulin G2a subclass in antibody-mediated protection against lactate dehydrogenase-elevating virus-induced polioencephalomyelitis revealed with switch mutants. J. Virol. 76:432–435. 10.1128/JVI.76.1.432-435.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shlomchik MJ, Weisel F. 2012. Germinal centers. Immunol. Rev. 247:5–10. 10.1111/j.1600-065X.2012.01125.x [DOI] [PubMed] [Google Scholar]
- 42.Shlomchik MJ, Weisel F. 2012. Germinal center selection and the development of memory B and plasma cells. Immunol. Rev. 247:52–63. 10.1111/j.1600-065X.2012.01124.x [DOI] [PubMed] [Google Scholar]
- 43.Bortnick A, Chernova I, Quinn WJ, Mugnier M, Cancro MP, Allman D. 2012. Long-lived bone marrow plasma cells are induced early in response to T cell-independent or T cell-dependent antigens. J. Immunol. 188:5389–5396. 10.4049/jimmunol.1102808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pulendran B, Ahmed R. 2011. Immunological mechanisms of vaccination. Nat. Immunol. 12:509–517. 10.1038/ni.2039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kaji T, Ishige A, Hikida M, Taka J, Hijikata A, Kubo M, Nagashima T, Takahashi Y, Kurosaki T, Okada M, Ohara O, Rajewsky K, Takemori T. 2012. Distinct cellular pathways select germline-encoded and somatically mutated antibodies into immunological memory. J. Exp. Med. 209:2079–2097. 10.1084/jem.20120127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hebeis BJ, Klenovsek K, Rohwer P, Ritter U, Schneider A, Mach M, Winkler TH. 2004. Activation of virus-specific memory B Cells in the absence of T cell help. J. Exp. Med. 199:593–602. 10.1084/jem.20030091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Klenovsek K, Weisel F, Schneider A, Appelt U, Jonjic S, Messerle M, Bradel-Tretheway B, Winkler TH, Mach M. 2007. Protection from CMV infection in immunodeficient hosts by adoptive transfer of memory B cells. Blood 110:3472–3479. 10.1182/blood-2007-06-095414 [DOI] [PubMed] [Google Scholar]
- 48.Leclerc C, Sedlik C, Lo-Man R, Charlot B, Rojas M, Dériaud E. 1995. Stimulation of a memory B cell response does not require primed helper T cells. Eur. J. Immunol. 25:2533–2538. 10.1002/eji.1830250919 [DOI] [PubMed] [Google Scholar]
- 49.MacLeod MKL, Clambey ET, Kappler JW, Marrack P. 2009. CD4 memory T cells: what are they and what can they do? Semin. Immunol. 21:53–61. 10.1016/j.smim.2009.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kasturi SP, Skountzou I, Albrecht RA, Koutsonanos D, Hua T, Nakaya HI, Ravindran R, Stewart S, Alam M, Kwissa M, Villinger F, Murthy N, Steel J, Jacob J, Hogan RJ, Garcia-Sastre A, Compans R, Pulendran B. 2011. Programming the magnitude and persistence of antibody responses with innate immunity. Nature 470:543–547. 10.1038/nature09737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kurt-Jones EA, Popova L, Kwinn L, Haynes LM, Jones LP, Tripp RA, Walsh EE, Freeman MW, Golenbock DT, Anderson LJ, Finberg RW. 2000. Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nat. Immunol. 1:398–401. 10.1038/80833 [DOI] [PubMed] [Google Scholar]
- 52.Ballesteros-Tato A, Randall TD. 2014. Priming of T follicular helper cells by dendritic cells. Immunol. Cell Biol. 92:22–27. 10.1038/icb.2013.62 [DOI] [PMC free article] [PubMed] [Google Scholar]