ABSTRACT
Noroviruses (NoVs) are the leading cause of nonbacterial acute gastroenteritis worldwide in people of all ages. The P particle is a novel vaccine candidate derived from the protruding (P) domain of the NoV VP1 capsid protein. This study utilized the neonatal gnotobiotic pig model to evaluate the protective efficacies of primary infection, P particles, and virus-like particles (VLPs) against NoV infection and disease and the T cell responses to these treatments. Pigs either were vaccinated intranasally with GII.4/1997 NoV (VA387)-derived P particles or VLPs or were inoculated orally with a GII.4/2006b NoV variant. At postinoculation day (PID) 28, pigs either were euthanized or were challenged with the GII.4/2006b variant and monitored for diarrhea and virus shedding for 7 days. The T cell responses in intestinal and systemic lymphoid tissues were examined. Primary NoV infection provided 83% homologous protection against diarrhea and 49% homologous protection against virus shedding, while the P particle and VLP vaccines provided cross-variant protection (47% and 60%, respectively) against diarrhea. The protection rates against diarrhea are significantly inversely correlated with T cell expansion in the duodenum and are positively correlated with T cell expansion in the ileum and spleen. The P particle vaccine primed for stronger immune responses than VLPs, including significantly higher numbers of activated CD4+ T cells in all tissues, gamma interferon-producing (IFN-γ+) CD8+ T cells in the duodenum, regulatory T cells (Tregs) in the blood, and transforming growth factor β (TGF-β)-producing CD4+ CD25− FoxP3+ Tregs in the spleen postchallenge, indicating that P particles are more immunogenic than VLPs at the same dose. In conclusion, the P particle vaccine is a promising vaccine candidate worthy of further development.
IMPORTANCE The norovirus (NoV) P particle is a vaccine candidate derived from the protruding (P) domain of the NoV VP1 capsid protein. P particles can be easily produced in Escherichia coli at high yields and thus may be more economically viable than the virus-like particle (VLP) vaccine. This study demonstrated, for the first time, the cross-variant protection (46.7%) of the intranasal P particle vaccine against human NoV diarrhea and revealed in detail the intestinal and systemic T cell responses by using the gnotobiotic pig model. The cross-variant protective efficacy of the P particle vaccine was comparable to that of the VLP vaccine in pigs (60%) and to the homologous protective efficacy of the VLP vaccine in humans (47%). NoV is now the leading cause of pediatric dehydrating diarrhea, responsible for approximately 1 million hospital visits for U.S. children and 218,000 deaths in developing countries. The P particle vaccine holds promise for reducing the disease burden and mortality.
INTRODUCTION
Norovirus (NoV), a genus of the family Caliciviridae, is the leading cause of acute nonbacterial gastroenteritis across all age groups, causing approximately half of all gastroenteritis outbreaks worldwide (1, 2). NoV is now the leading cause of acute pediatric gastroenteritis, accounting for approximately 1 million hospital visits for U.S. children (3), an estimated total of 23 million cases in the United States (4), and 1,091,000 inpatient hospitalizations and 218,000 deaths in developing nations (5) annually, although this incidence is expected to be an underestimate, since NoV gastroenteritis often presents as a mild, self-limiting disease. In the United States alone, NoV gastroenteritis is responsible for hospital charges of $284 million annually (6). However, no vaccines or antivirals against NoV are currently available. The predominant circulating NoV strains belong to genogroup II.4 (GII.4), which causes more than 80% of NoV gastroenteritis worldwide (7).
Due to the lack of a cell culture system for the isolation and propagation of human NoV, vaccine development has relied on recombinant NoV capsid proteins, such as virus-like particles (VLPs) and P particles. The capsid of NoV is composed of a single major structural protein of 55 to 60 kDa (VP1) that is divided into shell (S) and protruding (P) domains linked by a short hinge (8). VLPs are formed through the expression of VP1 in a eukaryotic expression system and retain the antigenic structure and histo-blood group antigen (HBGA) receptor binding function (9, 10). P particles form when the P domain is expressed in Escherichia coli (11). Each P particle contains 24 copies of the P domain with a total molecular mass of ∼840 kDa and a diameter of ∼20 nm, an ideal size for an immunogen (11). P particles display HBGA binding patterns similar to those of VLPs and elicit innate, humoral, and cellular immune responses similar to those of VLPs in mice (12). A previous study by Tamminen et al. (13) comparing the immunogenicities of VLPs and P particles in mice suggested that VLPs induce an immune response superior to that induced by P particles. In addition, Tan and Jiang (14) raised concerns that the study of Tamminen et al. utilized P dimers instead of P particles. A later study indicated that P dimers induce weaker immune responses than P particles (12), which may have impacted the results of Tamminen et al. In terms of vaccine production, VLPs require a eukaryotic system, whereas P particles can easily be produced by E. coli at a higher yield than VLPs (15, 16). However, mice are resistant to human NoV infection, so protective efficacy cannot be evaluated. P particles have also been shown to be a useful vaccine platform for dual vaccine development (17, 18). Thus, P particles may be a more economically viable vaccine candidate than VLPs.
The gnotobiotic (Gn) pig model has been used for the study of NoV pathogenesis and vaccines (19–22). Gn pigs have intestinal physiology and immune systems similar to those of humans and are well suited for studies of vaccine-induced immune responses due to the lack of interference from maternal antibodies and extraneous pathogens (19, 20). We recently reported studies of NoV infectivity in the presence or absence of a cholesterol-lowering drug, simvastatin, in a Gn pig challenge model using a large inoculum pool of a human GII.4/2006b NoV variant (22). The median infectious dose (ID50) of the NoV inoculum in Gn pigs at the age of 33 to 34 days was determined. The present study utilizes this well-established Gn pig challenge model to evaluate the immunogenicity and protective efficacy of a GII.4/1997 NoV P particle vaccine candidate and to compare the P particle vaccine with the corresponding VLPs and primary NoV infection.
For nonreplicating vaccines, effective adjuvants and delivery systems are important to the immunogenicity of the vaccine antigens. We used monophosphoryl lipid A (MPL) and chitosan in the P particle vaccine formulation. MPL is a natural substance derived from Salmonella enterica serovar Minnesota and is a potent Toll-like receptor 4 (TLR4) agonist recently approved by the FDA and other regulatory agencies globally. Intranasal (i.n.) administration of hepatitis B virus antigen, tetanus toxoid, or influenza virus antigens with MPL has resulted in increased mucosal and cellular immunity (23). Chitosan is a polysaccharide derived from the partial deacetylation of chitin. The mucoadhesive properties of chitosan increase antigen uptake by mucosal surfaces and reduce clearance by cilia (24). Additionally, chitosan has been shown to shift a biased Th1 response to a balanced Th1/Th2 response (24) and to have adjuvanticity with intranasal HIV (25) and anthrax (26) vaccines. Chitosan and MPL have been used in previous NoV VLP studies (7, 27, 28).
In this study, we evaluated the protective efficacy conferred by P particles, VLPs, or primary NoV infection in Gn pigs challenged with homotypic NoV GII.4. We also examined the total T helper (Th) cell, cytotoxic T lymphocyte (CTL), virus-specific effector/memory T cell, and regulatory T cell (Treg) responses in the intestinal and systemic lymphoid tissues of Gn pigs at challenge and/or postchallenge. Protective immunity, especially among T cells, against NoV infection and diarrhea has not been fully understood. Previous infection studies have indicated that NoV infection provides short-term, homologous protection (29, 30) in humans. Immunity to NoV has been linked to HBGA-blocking antibodies in serum and to CD4+ T cells (28, 31, 32). Effector T cells play an important role in the clearance of NoV infection in humans (33, 34) and mice (35, 36). To our knowledge, this is the first study to compare the protective efficacies induced by P particles, VLPs, and primary NoV infection and to comprehensively examine the T cell responses induced in Gn pigs.
MATERIALS AND METHODS
Virus.
A pool of human stool specimens containing GII.4/2006b variant 092895 (GenBank accession no. KC990829) was collected by Xi Jiang's laboratory at Cincinnati Children's Hospital Medical Center from a child with NoV gastroenteritis in 2008. The specimen pool was processed as we previously described and was used for the virus primary infection and challenge studies (22). The ID50 of the inoculum in Gn pigs is 2.74 × 103 viral RNA copies at the age of 4 to 5 days and 6.43 × 104 viral RNA copies at the age of 33 to 34 days (22). Ten times the ID50 was used for primary infection and challenge. This inoculum dose is consistent with the challenge dose in a previous study in humans (28).
Amino acid sequencing of NoV 092895 VP1.
RNA from the stool was extracted with the QIAamp viral isolation kit (Qiagen), and DNA contamination was eliminated by Turbo DNase (Life Technologies). DNase was subsequently cleaned by using an RNeasy kit (Qiagen). Reverse transcription-PCR (RT-PCR) was performed using oligo(dT) primers and the Maxima H Minus First Strand cDNA synthesis kit (Thermo Scientific). The 1.6-kb VP1 capsid gene was amplified by PCR using MyTaq HS DNA polymerase (Bioline) followed by PCR using PrimeSTAR HS DNA polymerase (TaKaRa) with the following primers: cog2F (5′-CARGARBCNATGTTYAGRTGGATGAG-3′) (37) and JV24 reverse (5′-TTATAATGCACGTCTACGCCC-3′) (38). The amplified fragment was sequenced, ligated into pBlueScript II SK(+), and cloned into NEB10-beta chemically competent E. coli cells (New England BioLabs). The VP1 sequence (GenBank accession no. KC990829) was obtained by sequencing of the resultant recombinants, performed by the Virginia Bioinformatics Institute (Virginia Tech, Blacksburg, VA).
Vaccine preparation.
The P particles and VLPs were both derived from GII.4 strain VA387 (1997 Farmington Hills variant) as described previously (11, 39) and were sterilized with short-wave UV light for 30 min. Synthetic MPL (Avanti Polar Lipids, Inc.) was dissolved in 0.5% triethanolamine (TeOH) and was heated at 65°C for 5 min. MPL-TeOH was sonicated in a bath sonicator, and the pH was adjusted to 7.0. Chitosan (NovaMatrix) was dissolved in water for inoculation (Life Technologies) and was filter sterilized (pore size, 0.2 μm). Vaccines contained 100 μg of P particles or VLPs, 5 mg chitosan, 50 μg MPL, and TNC buffer (40) to a final volume of 1 ml. The sterility of all solutions was monitored by culture on blood agar plates and fluid thioglycolate medium. The endotoxin levels of the P particles were determined by the ToxinSensor Chromogenic LAL endotoxin assay (GenScript) to be 0.8 endotoxin unit (EU)/ml, which is below the maximum recommended level for a recombinant subunit vaccine (41).
Treatment and inoculation of Gn pigs.
Near-term Large White cross pigs were derived via hysterectomy and were maintained in germfree isolator units as described previously (42). Pigs were confirmed to be A+ or H+ prior to inoculation, and sterility was monitored as described previously (22). Pigs (both male and female) were randomly divided into four groups. Each group was composed of at least 6 pigs that came from at least 3 different litters (3 experimental replicates). Pigs in the P particle or VLP group were inoculated intranasally with 3 doses of the vaccine using mucosal atomization devices (MADs) (LMA North America), at postpartum day (PPD) 5 (postinoculation day [PID] 0), PID 10, and PID 21. Pigs in the NoV primary oral infection (NoVPO) group were orally inoculated with 10 ID50s of NoV (2.74 × 104 viral RNA copies) at PPD 5. Control pigs received a diluent or adjuvants only. Pigs were given 4 ml of 200 mM sodium bicarbonate 10 min prior to oral inoculation to reduce gastric acidity. A subset of pigs in each group was orally challenged with 10 ID50s of NoV (6.43 × 105 viral RNA copies) at PID 28 (postchallenge day [PCD] 0) and was monitored daily for clinical signs and virus shedding until PCD 7. All pigs were euthanized at PID 28 or PID 35 (PCD 7) for the isolation of mononuclear cells (MNCs) from the duodenum (20 cm), ileum (20 cm), spleen (whole organ), and blood (70 ml) as described previously (43). All animal experimental procedures were conducted in accordance with protocols approved by the Institutional Animal Care and Use Committee at Virginia Tech.
Detection of NoV shedding and assessment of diarrhea.
Rectal swabs were collected daily following NoV primary infection and challenge for assessment of diarrhea and virus shedding. Diarrhea was scored based on our previously used scaling system (22). Virus shedding was monitored by conventional RT-PCR and TaqMan real-time RT-PCR as described previously (22).
Flow cytometry analysis of total CD3+ CD4+ (Th) cells, total CD3+ CD8+ cells (CTLs), and IFN-γ-producing CD4+ and CD8+ T cells.
Flow cytometry was used to determine the numbers of total CD4+ and CD8+ T cells and of NoV-specific gamma interferon (IFN-γ)-producing CD4+ and CD8+ T cells in the intestinal (duodenum, ileum) and systemic (spleen) tissues and blood of Gn pigs. Cells were stimulated in vitro for 17 h and were stained as described previously (44, 45). The P particles from VA387 (6 μg/ml for the duodenum, ileum, and peripheral blood lymphocytes [PBL] and 12 μg/ml for the spleen; the concentrations were optimized in pilot studies) were added to the MNC cultures as a stimulating antigen. The total number of T cells in each subset was calculated by multiplication of the frequency of the specific subset among lymphocytes, the frequency of lymphocytes among MNCs, and the total number of MNCs isolated per tissue. The data are presented as mean numbers per tissue. Total numbers of IFN-γ+-producing T cells are presented as adjusted mean numbers. Adjusted numbers were derived by subtracting the total number of mock-stimulated MNCs from the total number of P-particle-stimulated MNCs. Isotype-matched irrelevant antibodies were used to establish positive and negative gates of Th cells, CTLs, and IFN-γ-producing T cells. At least 100,000 cells were collected on a BD FACSAria flow cytometer (BD Biosciences) and were analyzed using FlowJo software, version 7.6.4 (TreeStar, Inc.).
Flow cytometry analysis of activated nonregulatory (FoxP3−) and IL-10- and TGF-β-producing Treg (FoxP3+) cells.
MNCs were stained on the day of isolation for activated nonregulatory T cell and Treg analysis as described previously (44). The activated nonregulatory T cells were identified as CD25+ FoxP3− T cells. Tregs were identified as CD4+ CD25− FoxP3+ and CD4+ CD25+ FoxP3+ T cells. The numbers of interleukin 10 (IL-10)- and transforming growth factor β (TGF-β)-producing Tregs (presented as means) were calculated by multiplying the frequencies of IL-10+ and TGF-β+ Tregs among lymphocytes, the frequency of lymphocytes among MNCs, and the total number of MNCs isolated per tissue. All numbers are presented after subtraction of background numbers. Isotype-matched irrelevant antibodies were used to establish positive and negative gates for Tregs and cytokine-producing Tregs.
Statistical analysis.
One-way analysis of variance (ANOVA) with the general linear model (GLM), followed by Duncan's multiple-range test, was used to compare the mean durations of diarrhea and shedding. Fisher's exact test was performed to compare the percentages of pigs with diarrhea and virus shedding. The Kruskal-Wallis rank sum test was used to compare the areas under the curves (AUCs) for diarrhea and the AUCs for viral shedding, as well as the numbers of T cell subsets. For all these tests, statistical significance was assessed at a P value of <0.05. Spearman's rank correlation coefficient was used to evaluate correlations between T cell subsets and protection rates. All correlations were evaluated at a P value of <0.0001. All statistical analyses were performed using SAS Program 9.3 (SAS Institute, Cary, NC, USA).
Nucleotide sequence accession number.
The complete VP1 sequence of NoV GII.4/092895 was deposited in the GenBank database under the accession number KC990829 in August 2013.
RESULTS
The VP1 sequences of VA387 and 092895 have 93.5% homology.
The VP1 sequence was determined for 092895. Sequence alignment indicates that 092895 is a GII.4/2006b variant (data not shown). The amino acid sequences for VA387 VP1 (GenBank accession no. AY038600 and AAK84679) and 092895 VP1 (GenBank accession no. KC990829) have 93.5% homology. Amino acid alignment showed that 33 differences were present, including 21 substitutions in the P2 domain (Table 1). There were 4 differences in epitope A, 1 in epitope B, 1 in epitope C, 3 in epitope D (including 1 deletion), and 3 in epitope E (Table 2).
TABLE 1.
Amino acid differences in the S, P1-1, P2, and P1-2 domains between NoVs VA387 and 092895
| Domain | No. of amino acid differences: |
No. of insertions/deletions | |||
|---|---|---|---|---|---|
| Total | Within one group | Between different groups | That potentially influence protein folding | ||
| S | 3 | 2 | 1 | ||
| P1-1 | 2 | 1 | 1 | ||
| P2 | 21 | 9 | 10 | 1 | 1 |
| P1-2 | 7 | 4 | 2 | 1 | |
TABLE 2.
Amino acid differences in epitopes A to E of NoVs VA387 and 092895
| Strain | Amino acid residuea at the following epitope and position: |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A |
B |
C |
D |
E |
||||||||||||
| 294 | 296 | 297 | 298 | 368 | 372 | 333 | 382 | 340 | 376 | 393 | 394 | 395 | 407 | 412 | 413 | |
| 092895 | A | S | R | N | A | E | V | K | G | Q | S | T | T | S | N | V |
| VA387 | A | S | H | D | T | N | M | K | E | Q | N | N | N | T | G | |
Residues in VA387 that are different from the corresponding residue in 092895 are shown in boldface.
The P particle and VLP vaccines provided similar protection rates, which were lower than that of primary NoV infection, against a homotypic NoV challenge.
The protective efficacy for each group was evaluated following GII.4/2006b NoV challenge. Clinical signs and NoV shedding were monitored daily postchallenge (Table 3). Primary NoV infection (NoVPO) significantly reduced the occurrence of diarrhea (protection rate, 82.9%). The P particle and VLP vaccines reduced the occurrence of diarrhea at similar rates (protection rates, 46.7% and 60.0%, respectively). NoVPO and VLPs shortened the mean duration of diarrhea slightly (by 1.5 and 1.1 days, respectively) from that for control pigs, while P particles reduced the mean area under the curve (AUC) of diarrhea slightly, but these differences were not statistically significant. Although only one NoVPO pig developed diarrhea, several NoVPO pigs had a diarrhea score of 1.5 for 4 to 6 days following challenge.
TABLE 3.
Clinical signs and protective efficacy in previously infected or vaccinated Gn pigs after challenge with GII.4 2006b NoVa
| Group | n | Diarrheab |
Virus shedding |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean % of pigs with diarrhea (no. of pigs with diarrhea/total no.)c | Mean no. of days with diarrhead (SEM) | Mean AUC from PCD 1 to PCD 7 (SEM) | Fold reduction in AUC | Rate of protection against diarrhea (%)e | Mean % of pigs shedding virus (no. of pigs shedding/total no.) | Mean no. of days with sheddingd (SEM) | Mean AUC from PCD 1 to PCD 7 (SEM) | Fold reduction in AUC | Rate of protection against shedding (%)e | ||
| NoVPO | 7 | 14 (1/7) B | 0.3 (0.3) | 6.7 (0.6) | 1.0 | 82.9 | 43 (3/7) | 2.9 (1.4) | 20,775 (57,893) | −5.6 | 48.6 |
| 100 μg P particles + MPL-chitosan (i.n.) | 9 | 44 (4/9) AB | 1.3 (0.6) | 5.4 (0.8) | −1.2 | 46.7 | 89 (8/9) | 1.8 (0.4) | 38,216 (10,568) | −3.1 | 0.0 |
| 100 μg VLPs + MPL-chitosan (i.n.) | 6 | 33 (2/6) AB | 0.7 (0.4) | 6.2 (1.0) | −1.1 | 60.0 | 100 (6/6) | 2.0 (0.4) | 96,428 (25,627) | −1.2 | 0.0 |
| Diluent 5 (oral) or MPL-chitosan (i.n.) | 6 | 83 (5/6) A | 1.8 (0.6) | 6.7 (1.0) | 1.0 | NA | 83 (5/6) | 2.0 (0.7) | 116,960 (78,189) | 1.0 | NA |
Gn pigs were challenged with the human NoV GII.4 2006b variant 092895 at the age of 33 to 34 days. Rectal swabs were collected daily after challenge to determine the occurrence of diarrhea and virus shedding by conventional and real-time RT-PCR. Virus shedding was also detected in intestinal contents.
Fecal scoring system: 0, solid; 1, pasty; 2, semiliquid; 3, liquid. Pigs with scores of 2 or higher were considered diarrheic.
Values in the same column followed by different letters differ significantly (P, <0.05 by Fisher's exact test); there is no significant difference between values followed by the same letter.
From PCD 0 to PCD 7.
Calculated as [1 − (percentage of immunized pigs in each group with diarrhea or shedding/percentage of control pigs with diarrhea or shedding)] × 100. NA, not applicable.
Primary NoV infection provided substantial protection not only against diarrhea but also against homologous-virus reinfection, as evidenced by a reduced percentage of shedding (protection rate, 48.6%). NoV primary infection and P particles also decreased the mean AUC of virus shedding (by 5.6- and 3-fold, respectively), although these differences were not statistically significant. VLPs did not have an effect on virus shedding.
NoV challenge increased the numbers of Th cells and CTLs in the duodena of naïve (control) pigs.
To comprehensively evaluate T cell responses induced by oral NoV inoculation (mimicking primary natural infection) versus intranasal vaccination, we examined total Th cells, CTLs, activated nonregulatory CD25+ FoxP3− T cells, virus-specific IFN-γ-producing effector/memory T cells, CD4+ CD25− FoxP3+ or CD4+ CD25+ FoxP3+ Tregs, and IL-10- or TGF-β-producing Tregs in intestinal and systemic lymphoid tissues. The total Th cells and CTLs were identified using flow cytometry by gating lymphocytes and coexpression of CD3 with CD4 (Th cells) or CD8 (CTLs) (Fig. 1A), and the total numbers of cells isolated from each tissue are presented (Fig. 1B). Since the P particles and VLPs produced similar protection rates in Gn pigs and similar dendritic cell and T cell responses and cytokine production patterns in mice (12), and since the P particle vaccine is targeted for further development in our future studies, we focused on comparing T cell responses induced by the P particle vaccine to those induced by NoV infection and those for controls in Gn pigs.
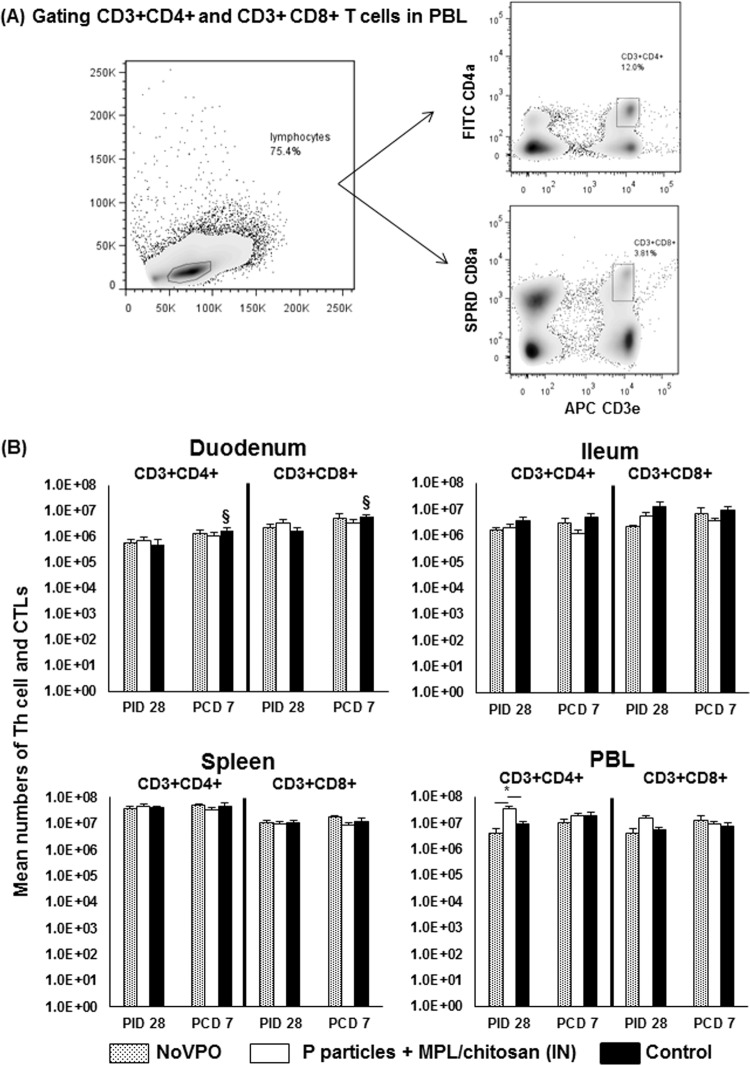
FIG 1.
Th cells and CTLs induced by NoV infection or vaccination pre- and postchallenge. (A) Representative dot plots of frequencies of CD3+ CD4+ and CD3+ CD8+ T cells among lymphocytes from PBL of mock-vaccinated pigs postchallenge. FITC, fluorescein isothiocyanate; SPRD, Spectral Red; APC, allophycocyanin. (B) Mean total numbers plus standard errors of the means (n, 6 to 10) of CD3+ CD4+ and CD3+ CD8+ T cells in intestinal (duodenum, ileum) and systemic (spleen, PBL) tissues pre- and postchallenge. An asterisk above the error bars indicates a significant difference among groups for the same cell type and tissue at the same time point (P, <0.05 by Kruskal-Wallis one-way ANOVA). A section sign indicates that the numbers increased significantly following challenge in the same group. A number sign indicates that the numbers decreased significantly following challenge in the same group. IN, intranasal.
The numbers of total Th cells and CTLs were compared among NoVPO, P-particle-vaccinated, and control pigs pre- and postchallenge. P-particle-vaccinated pigs had significantly higher numbers of Th cells in PBL than NoVPO and control pigs (Fig. 1B) prechallenge. Following challenge, control pigs displayed significant increases in the numbers of Th cells and CTLs in their duodena.
P particles increased the number of activated nonregulatory CD4+ T cells in the circulation prechallenge.
Activated nonregulatory CD4+ and CD8+ T cells were identified by gating CD4+ or CD8+ lymphocytes expressing CD25 but not FoxP3 (Fig. 2A). The data are presented as the mean number of cells per tissue (Fig. 2B). Prechallenge, P-particle-vaccinated pigs had significantly higher numbers of activated CD4+ T cells in PBL than either NoVPO or control pigs (Fig. 2B). This coincides with significantly higher numbers of total Th cells in the PBL of P-particle-vaccinated pigs (Fig. 1B). Additionally, P-particle-vaccinated pigs had significantly higher numbers of activated CD8+ T cells in PBL than NoVPO pigs at PID 28. These data suggest that intranasal administration of P particles with MPL and chitosan adjuvants effectively induced expansion of circulating activated T cells.
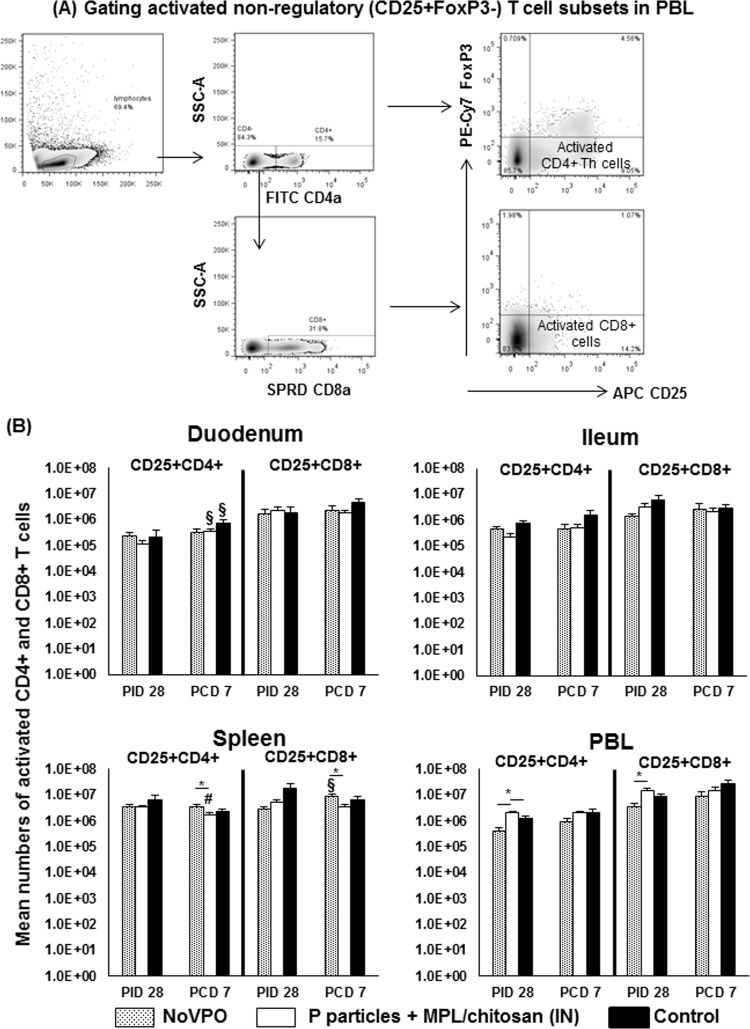
FIG 2.
Activated nonregulatory CD4+ and CD8+ T cells pre- and postchallenge. (A) Representative dot plots of frequencies of CD4+ CD25+ FoxP3− and CD8+ CD25+ FoxP3− activated T cells in PBL from P-particle-vaccinated pigs prechallenge. PE, phycoerythrin. (B) Mean total numbers plus standard errors of the means (n, 6 to 10) of CD4+ CD25+ FoxP3− and CD8+ CD25+ FoxP3− activated T cells prechallenge and postchallenge in intestinal (duodenum, ileum) and systemic (spleen, PBL) tissues. See the legend to Fig. 1 for an explanation of the symbols indicating statistical significance.
The duodenum and spleen are the major effector and memory sites, respectively, for activated T cells postchallenge.
Following challenge, P-particle-vaccinated pigs had significant increases in the number of activated CD4+ T cells in the duodenum, which coincided with a significant decrease in the number of activated CD4+ T cells in the spleen (Fig. 2B). NoV challenge also significantly increased the numbers of activated CD4+ T cells in the duodena of control pigs, reflecting the primary T cell response at the site of viral replication. This increase was confirmed by significant increases in the numbers of total Th cells in the duodena of control pigs (Fig. 1B). NoVPO pigs displayed significant increases in the numbers of activated CD8+ T cells in the spleen, indicating activation of memory CD8+ T cells following challenge at PCD 7. NoVPO pigs had significantly higher numbers of activated CD4+ and CD8+ T cells in the spleen than P-particle-vaccinated pigs. P-particle-vaccinated pigs also had significantly lower numbers of activated CD4+ T cells in the spleen following challenge (Fig. 2B).
NoV-specific IFN-γ-producing T cell responses were low and transient in all groups.
Virus-specific IFN-γ-producing T cells act as effector cells to eliminate virus-infected cells, and their magnitudes in the small intestine are significantly correlated with protective immunity against rotavirus, another enteric virus (45). NoV-specific effector/memory CD4+ and CD8+ T cells from the NoVPO, P-particle-vaccinated, and control groups at PID 28 and at PCD 7 were detected in intestinal and systemic lymphoid tissues by flow cytometry (Fig. 3A). MNCs were stimulated with P particles or with phytohemagglutinin (PHA) or medium only as a positive or background control, respectively. The low numbers of IFN-γ-expressing mock-stimulated MNCs were subtracted from the numbers of P-particle-stimulated MNCs to yield the adjusted virus-specific numbers. The mean numbers of virus-specific IFN-γ+ T cells pre- and postchallenge are shown in Fig. 3B. Neither primary infection nor P particle vaccination significantly altered IFN-γ production by T cells at PID 28 from that for control pigs. These data indicate that primary IFN-γ-producing T cell responses after NoV infection or P particle vaccination are short-term.
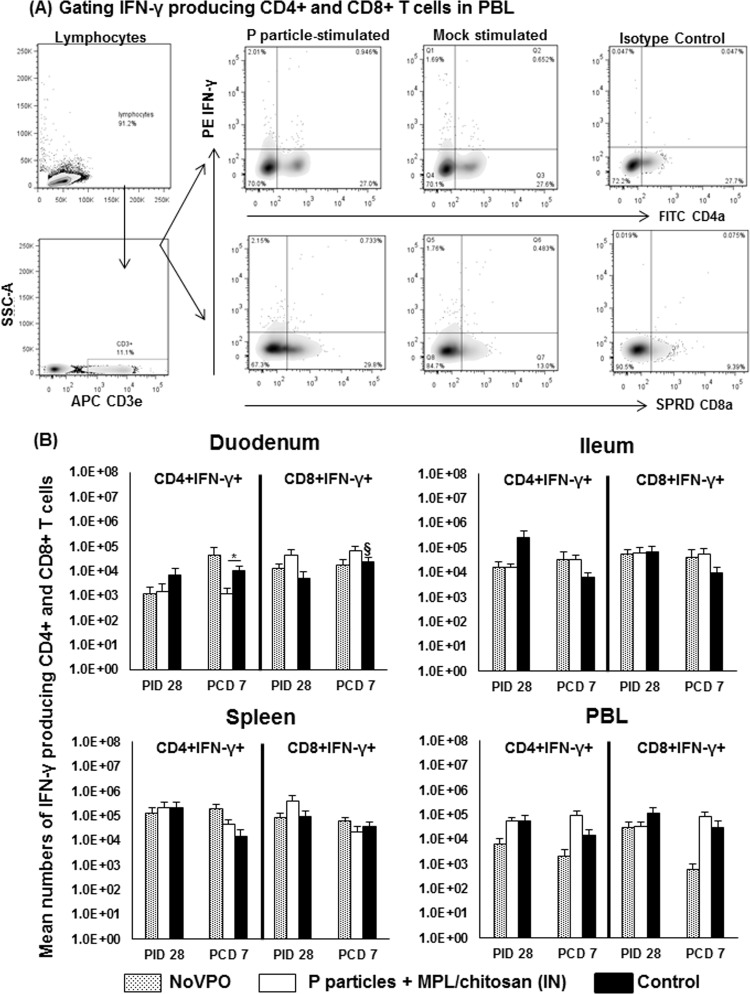
FIG 3.
NoV-specific IFN-γ-producing CD4+ and CD8+ T cell responses pre- and postchallenge. (A) Representative dot plots of frequencies of NoV-specific CD3+ CD4+ IFN-γ+ and CD3+ CD8+ IFN-γ+ effector T cells in PBMCs isolated from the NoVPO group postchallenge and stimulated with P particles (17 h at 37°C). IFN-γ was detected using intracellular staining and flow cytometry. (B) Numbers of IFN-γ-producing CD4+ and CD8+ T cells following subtraction of isotype control and mock-stimulated background numbers. Data presented are mean total numbers plus standard errors of the means (n, 6 to 10) prechallenge and postchallenge in intestinal (duodenum, ileum) and systemic (spleen, PBL) tissues. See the legend to Fig. 1 for an explanation of the symbols indicating statistical significance.
Postchallenge, P-particle-vaccinated pigs had significantly lower numbers of IFN-γ+ CD4+ T cells in the duodenum than control pigs (Fig. 3B). Control pigs displayed significantly higher numbers of IFN-γ+ CD8+ T cells in the duodenum than they had prechallenge, reflecting the development of primary effector T cells at the site of NoV replication. This CD8+ effector T cell expansion is consistent with the expansion of total CTLs in the duodenum (Fig. 1B). The numbers of IFN-γ+ CD4+ and IFN-γ+ CD8+ T cells in the ilea of challenged NoVPO and P-particle-vaccinated pigs were about 5-fold higher (not statistically significant) than those for challenged control pigs at PCD 7 (Fig. 3B).
Lower numbers of CD4+ CD25− FoxP3+ Tregs in the duodenum are associated with increased protective efficacy against NoV challenge.
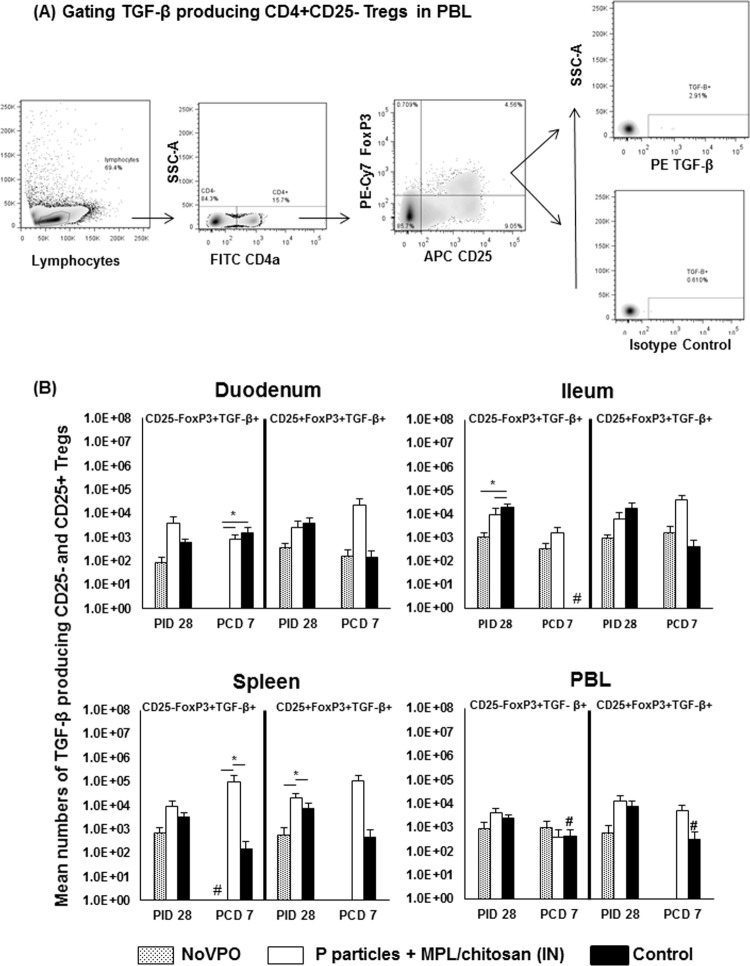
Previous studies suggested that high frequencies of functional CD4+ CD25− Tregs could be an indicator for poor protective immunity against rotavirus (46). In this study, we assessed the relationship between Treg numbers and protective immunity against NoV. Intestinal and systemic Tregs were evaluated using intracellular staining and flow cytometry at PID 28 and PCD 7, when MNCs were isolated (Fig. 4A). Figure 4B shows the total numbers of Tregs in each tissue.
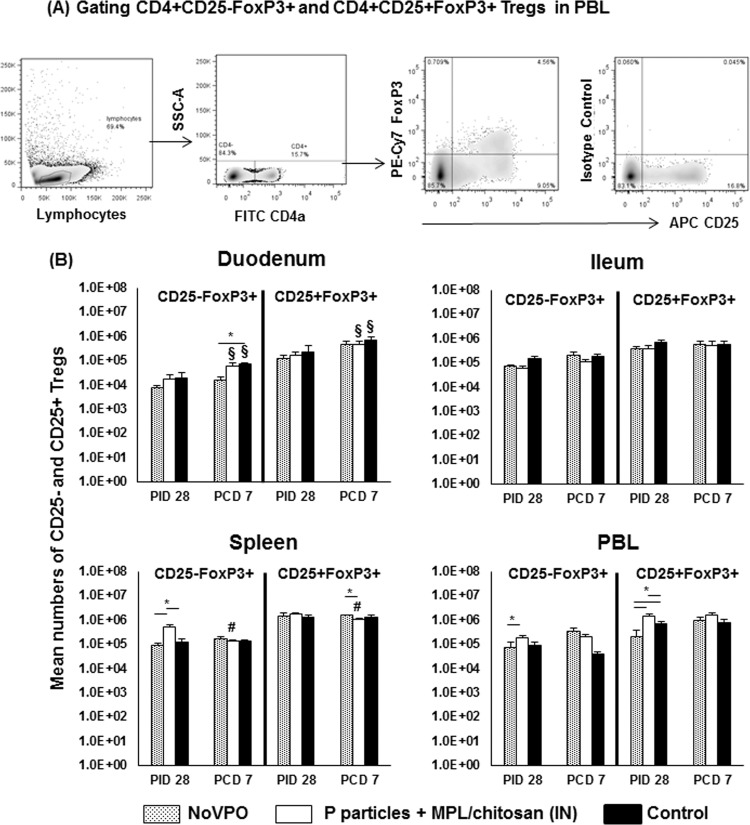
FIG 4.
Treg responses induced by NoV infection or vaccination pre- and postchallenge. (A) Representative dot plots of frequencies of CD4+ CD25− FoxP3+ and CD4+ CD25+ FoxP3+ Tregs from PBL of P-particle-vaccinated pigs prechallenge. (B) Mean total numbers of CD25− and CD25+ Tregs plus standard errors of the means (n, 6 to 10) among total MNCs prechallenge and postchallenge in intestinal (duodenum, ileum) and systemic (spleen, PBL) tissues. See the legend to Fig. 1 for an explanation of the symbols indicating statistical significance.
Prechallenge, there were no significant differences in the intestinal lymphoid tissues among the three groups. NoVPO pigs had significantly lower numbers of CD4+ CD25+ FoxP3+ Tregs in PBL than P-particle-vaccinated and control pigs. However, P-particle-vaccinated pigs had overall higher numbers of CD4+ CD25− FoxP3+ Tregs in the spleen and significantly higher numbers of CD4+ CD25+ FoxP3+ Tregs in PBL than control pigs at PID 28. The increases in the numbers of CD4+ CD25+ FoxP3+ Tregs in P-particle-vaccinated pigs are consistent with the increases in the numbers of total Th cells (Fig. 1B). Additionally, P-particle-vaccinated pigs had significantly higher numbers of CD4+ CD25− FoxP3+ Tregs in the spleen and PBL than NoVPO pigs.
After NoV challenge, NoVPO pigs had significantly lower numbers of CD4+ CD25− FoxP3+ Tregs in the duodenum than controls, corresponding to the highest protection rate conferred by primary NoV infection. P-particle-vaccinated and control pigs had significant increases in both Treg subsets in the duodenum over prechallenge levels, while NoVPO pigs did not. As with activated nonregulatory CD4+ T cells (Fig. 2B), the expansion of both Treg subsets in the duodena of P-particle-vaccinated pigs coincided with their significant reductions in the spleen and with the significantly lower numbers of CD4+ CD25+ FoxP3+ Tregs in the spleens of P-particle-vaccinated pigs than in those of NoVPO pigs postchallenge, at PCD 7 (Fig. 4B).
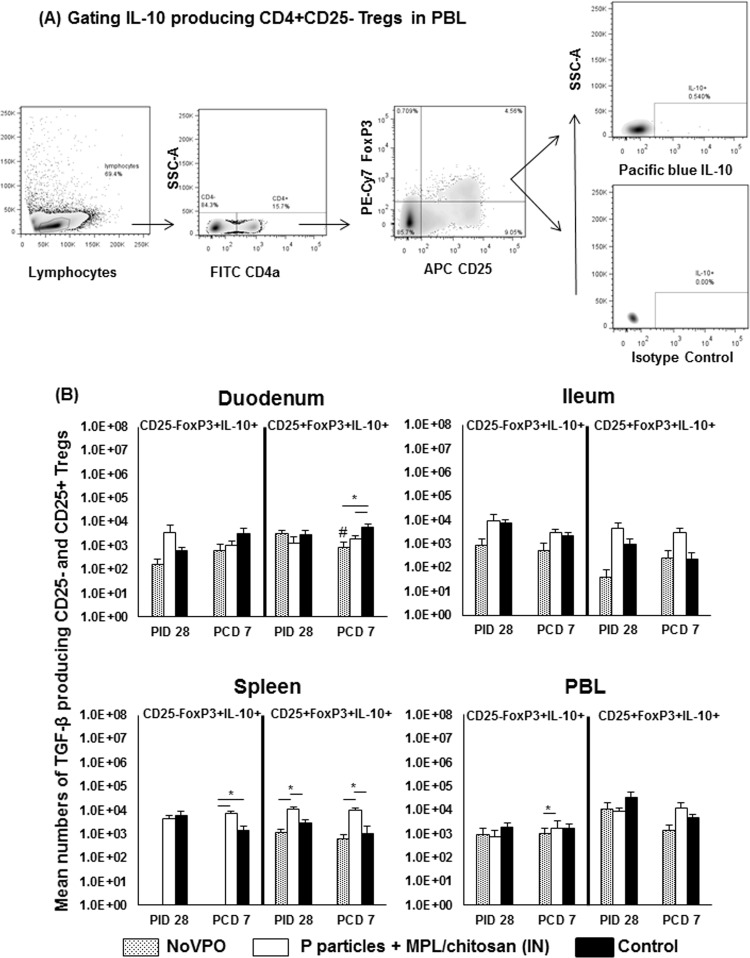
NoVPO and P-particle-vaccinated pigs had reduced levels of TGF-β-producing Tregs in the ileum prechallenge.
The cytokine-producing Treg responses were evaluated by intracellular staining of TGF-β- and IL-10-producing CD25− and CD25+ Tregs (Fig. 5 and 6). Prechallenge, control pigs had significantly higher numbers of TGF-β-producing CD25− Tregs in the ileum than NoVPO and P-particle-vaccinated pigs (Fig. 5B). P-particle-vaccinated pigs had significantly higher numbers of TGF-β-producing CD25+ Tregs in the spleen than NoVPO and control pigs. This pattern was similar to that of IL-10-producing CD25+ Tregs in the spleen (Fig. 6B) prechallenge.
FIG 5.
TGF-β-producing Treg responses induced by NoV infection or vaccination pre- and postchallenge. (A) Representative dot plots of frequencies of cytokine-secreting Tregs from unstimulated MNCs from PBL of P-particle-vaccinated pigs prechallenge. TGF-β production was detected using intracellular staining and flow cytometry. (B) Mean numbers plus standard errors of the means (n, 6 to 10) of cytokine-producing Tregs among Tregs prechallenge and postchallenge. See the legend to Fig. 1 for an explanation of the symbols indicating statistical significance.
FIG 6.
IL-10-producing Treg responses induced by NoV infection or vaccination pre- and postchallenge. (A) Representative dot plots of frequencies of cytokine-secreting Tregs from unstimulated MNCs from PBL of P particle-vaccinated pigs prechallenge. IL-10 production was detected using intracellular staining and flow cytometry. (B) Mean numbers plus standard errors of the means (n, 6 to 10) of cytokine-producing Tregs among Tregs prechallenge (A) and postchallenge. See the legend to Fig. 1 for an explanation of the symbols indicating statistical significance.
After challenge, NoVPO pigs had significantly lower numbers of TGF-β-producing CD25− Tregs in the duodenum than vaccinated pigs and control pigs (Fig. 5B). P-particle-vaccinated pigs had significantly higher numbers of TGF-β-producing CD25− Tregs in the spleen than NoVPO and control pigs. Control pigs had significant reductions in the numbers of TGF-β-producing CD25− Tregs in the ileum and in the numbers of both Treg subsets in PBL. NoVPO pigs had significant reductions in the number of TGF-β-producing CD25− Tregs in the spleen after challenge.
Control pigs, however, had significantly higher numbers of IL-10-producing CD25+ Tregs in the duodenum than NoVPO and vaccinated pigs (Fig. 6B). NoVPO pigs had significantly lower numbers of IL-10-producing CD25− Tregs in the spleen than vaccinated and control pigs. P-particle-vaccinated pigs had significantly higher numbers of IL-10-producing CD25+ Tregs in the spleen than NoVPO and control pigs. Vaccinated pigs also had significantly higher numbers of IL-10-producing CD25− Tregs in PBL than NoVPO pigs. Challenge significantly decreased the number of IL-10-producing CD25− Tregs in the duodena of NoVPO pigs.
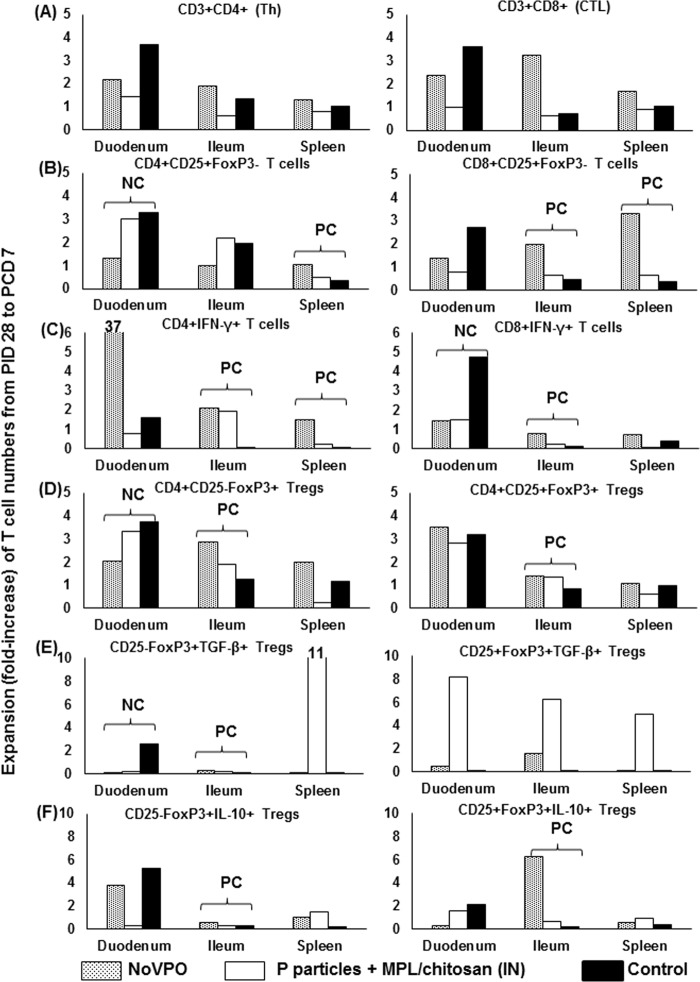
Expansions of T cells in the duodenum were inversely correlated with protection rates, while T cell expansion in the ileum was positively correlated with protection rates.
We calculated the expansion of each type of T cells in each tissue after NoV challenge by dividing postchallenge numbers by prechallenge numbers for each group (NoVPO, P-particle-vaccinated, and control pigs) (Fig. 7). Values of >1 indicate that the numbers of T cell subsets increased following challenge, while values of <1 indicate a decrease. We found that the expansions of T cells (activated CD4+ CD25+ T cells, CD3+ CD8+ IFN-γ+ T cells, CD4+ CD25− FoxP3+ Tregs, and TGF-β+ CD4+ CD25− FoxP3+ Tregs) in the duodenum, which is the site of NoV replication, were all significantly inversely correlated with protection rates against diarrhea (R, −1; P, <0.0001). It should also be noted that CD3+ CD4+ IFN-γ+ T cells in the duodenum expanded 37-fold in NoVPO pigs postchallenge. However, the expansions of T cells in the ileum (including activated CD8+ CD25+ T cells, CD3+ CD4+ IFN-γ+ T cells or CD3+ CD8+ IFN-γ+ T cells, CD4+ CD25+ FoxP3+ Tregs, CD4+ CD25− FoxP3+ Tregs, TGF-β+ CD4+ CD25− FoxP3+ Tregs, IL-10+ CD4+ CD25+ FoxP3+ Tregs, and IL-10+ CD4+ CD25− FoxP3+ Tregs) were all significantly positively correlated with protection rates (R, 1; P, <0.0001). The ileum is the induction site of gut-associated lymphoid tissues. In addition, expansions of T cells (activated CD4+ CD25+ and CD8+ CD25+ T cells, as well as CD3+ CD4+ IFN-γ+ T cells) in the spleen were also significantly positively correlated with protection rates (R, 1; P, <0.0001).
FIG 7.
Expansion of T cell subsets following NoV challenge. Expansion was determined by dividing the postchallenge numbers of each T cell subset by the prechallenge numbers. Shown is the expansion of Th cells and CTLs (A), activated nonregulatory CD25+ FoxP3− T cells (B), IFN-γ-producing T cells (C), CD25− and CD25+ Tregs (D), and TGF-β (E)- and IL-10 (F)-producing Tregs among NoVPO, P-particle-vaccinated, and control pigs. Correlations between T cell expansion and the protection rate against diarrhea were determined with Spearman's rank correlation coefficient (R, +1 or −1; P, <0.0001) in SAS. NC indicates a significant negative correlation with the protection rate against diarrhea (R, −1; P, <0.0001); PC indicates a significant positive correlation with the protection rate against diarrhea (R, 1; P, <0.0001). See Fig. 1 to 6 for the analysis of cell types.
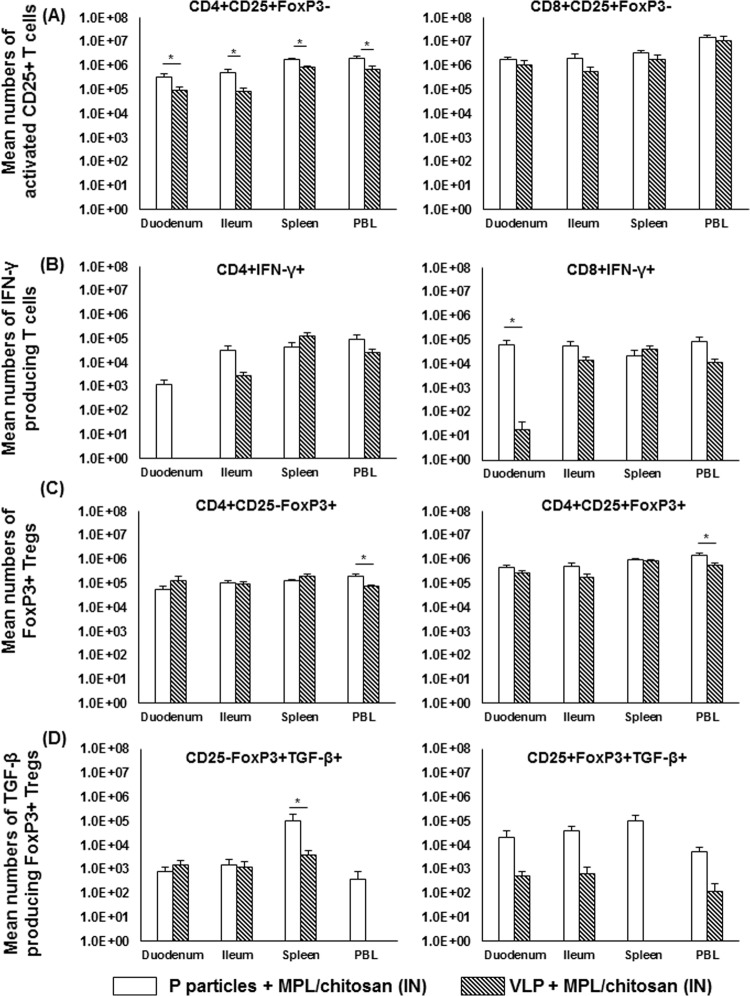
P-particle-vaccinated pigs had stronger effector and regulatory T cell responses than VLP-vaccinated pigs postchallenge.
The T cell responses in P-particle- and VLP-vaccinated pigs postchallenge were compared. The total numbers of activated nonregulatory T cells, IFN-γ-producing T cells, Tregs, and TGF-β-producing Tregs are shown in Fig. 8. P-particle-vaccinated pigs had significantly higher numbers of activated CD4+ CD25+ T cells in all tissues examined (Fig. 8A). These pigs also had significantly higher numbers of CD8+ IFN-γ+ T cells in the duodenum (Fig. 8B). Additionally, P-particle-vaccinated pigs had significantly higher numbers of CD4+ CD25− FoxP3+ and CD4+ CD25+ FoxP3+ Tregs in PBL (Fig. 8C) and significantly higher numbers of TGF-β-producing CD4+ CD25− FoxP3+ Tregs in the spleen (Fig. 8D) than VLP-vaccinated pigs.
FIG 8.
Comparisons of P-particle- and VLP-induced T cell responses following NoV challenge. Shown are total numbers of activated nonregulatory CD25+ FoxP3− T cells (A), IFN-γ-producing T cells (B), FoxP3+ Tregs (C), and TGF-β-producing Tregs (D) among P-particle-vaccinated or VLP-vaccinated pigs postchallenge. Error bars indicate the standard errors of the means. See Fig. 2 to 5 for the analysis of cell types. See the legend to Fig. 1 for an explanation of the symbols indicating statistical significance.
DISCUSSION
Studies of NoV infection and vaccines in humans and mice have focused mainly on evaluating humoral immune responses (13, 27, 28, 34, 47). In this study, we compared the protective efficacies of NoV primary infection, P particles, and VLPs against homotypic GII.4 challenge in Gn pigs. We also evaluated the T cell responses induced by NoV infection and vaccination pre- and/or postchallenge.
Prior NoV GII.4/2006b infection conferred the best protection by significantly reducing the number of pigs with diarrhea (protection rate, 82.9%) and the number of pigs that shed virus (protection rate, 48.6%) following challenge with the same NoV variant (homologous protection). The difference in protection rates between diarrhea and infection are likely the result of asymptomatic infection in 2 out of 7 challenged pigs postchallenge. Asymptomatic NoV infection is common in humans (48). Three intranasal doses of the GII.4/1997 VA387 (Farmington Hills) variant-derived P particle or VLP vaccine provided protection against diarrhea but not against virus shedding in GII.4/2006b-challenged Gn pigs. Previous studies comparing VLPs and P particles in mice (12, 13) have offered conflicting results and have been limited by the inability to infect mice with human NoV. The present study demonstrates that P particles not only are capable of conferring partial protection against human NoV-induced diarrhea in a higher-order animal model but also can provide protection similar to that conferred by VLPs. Further, the protection rates (46.7% and 60.0%, respectively) closely resemble the relative efficacy (47%) of a 2-dose intranasal VLP vaccine in human volunteers challenged with 10 ID50s of homologous Norwalk virus (28), the same challenge dose as that used in this study.
A previous study of a VLP vaccine in Gn pigs reported that oral/intranasal administration of the GII.4 HS66 variant-derived VLPs adjuvanted with ISCOM (immunostimulating complex) or mLT (mutant heat-labile enterotoxin) provided 75% or 100% protection against diarrhea, respectively, and 100% protection against infection following homologous challenge (21). However, only 57% of the challenged control pigs shed virus in the study, indicating that almost half of the reported protection was due to an insufficient viral challenge dose. Thus, the relative protective efficacy of the HS66 VLP-mLT vaccine was similar to the protection rates of the P particles and VLPs that we reported in this study. The Norwalk virus VLP vaccine conferred a protection rate of 26% against virus infection in humans (28), whereas the VA387 VLPs and P particles in our study did not reduce the incidence of virus shedding in Gn pigs, suggesting that higher vaccine doses may be needed. However, these vaccines reduced the diarrhea AUC 1.2- and 3.1-fold, respectively (not statistically significant). The duration of protection conferred by the P particle or VLP vaccine remains to be determined. Previous studies with humans showed that a NoV infection may confer protection for a range of 2 months to 2 years (30) or for as long as 9 years (29) against homologous challenge.
NoV primary infection conferred only partial protection (48.6%) against reinfection upon challenge with 10 ID50s of the same virus inoculum. In contrast, primary infection with human rotavirus conferred 100% protection against reinfection on Gn pigs upon challenge with 105 ID50s of the virulent human rotavirus Wa strain (45). Comprehensive T cell immune responses to NoV infection or P particle or VLP vaccination have not been reported previously for humans or Gn pigs. Limited evidence has suggested that NoV infection in humans induces a primarily Th1 type immune response. NoV-specific IFN-γ was predominantly produced by CD4+ cells in the antigen-stimulated peripheral blood mononuclear cells (PBMCs) of humans infected with Snow Mountain virus (33). Norwalk virus VLPs induced IFN-γ production in the absence of IL-4 in the PBMCs of volunteers (34). Both CD4+ and CD8+ T cells were shown to be crucial for the clearance of NoV infection in mice (35, 36).
In agreement with the disparity in protection rates conferred by primary infections with rotavirus and norovirus, virus-specific IFN-γ-producing T cell responses at challenge and postchallenge in NoVPO pigs were weaker overall than those of rotavirus-infected pigs (20, 45). Thus, virus-specific IFN-γ-producing T cells are not a correlate of protection against NoV challenge, confirming the findings in mice (32). However, NoVPO pigs had a 37-fold increase in IFN-γ-producing CD4+ T cells in the duodenum following challenge, while control pigs experienced a significant increase in IFN-γ-producing CD8+ T cells following challenge (Fig. 3B and 7C). P-particle-vaccinated pigs had a more balanced expansion in CD4+ and CD8+ IFN-γ-producing T cells. These data suggest that CD4+ IFN-γ+ T cells in the duodenum may be important for protection from reinfection. Additionally, these results may indicate the importance of effector CD8+ T cells in the duodenum for the clearance of viral infection. Future studies using CD8 knockout pigs will further elucidate the roles of these cell types in NoV immunity and infection.
We reported previously that rotavirus infection reduced the frequencies and numbers of tissue-residing Tregs and decreased the frequencies of IL-10- and TGF-β-producing CD4+ CD25− FoxP3+ Tregs in the ileum, spleen, and blood at PID 28 (46). The frequencies of IL-10- and TGF-β-producing CD4+ CD25− FoxP3+ Tregs in all sites at PID 28 were significantly inversely correlated with the protection rate against rotavirus diarrhea upon challenge with a virulent rotavirus. In the present study, we determined that the numbers of Tregs in the duodenum at PID 28 are inversely associated with protection rates.
Zhu et al. (32) reported that CD4+ T cells are correlates of immunity in mice following norovirus infection, while IFN-γ+ and CD8+ T cells are not, though these effects may be strain specific (32). It is worth noting that the study examined only immune responses from spleen-derived cells. Our study supports the conclusion of Zhu et al. that IFN-γ and CD8 T cells are not correlates of protection. We were able only to establish an inverse association between duodenal Tregs at PID 28 and the protection rate against diarrhea, not to establish CD8 or CD4 T cells as correlates of protection.
The present study identified correlations between postchallenge expansions of T cell subsets and protection. Interestingly, the correlations are tissue dependent, regardless of the T cell subset. Responses in the effector site, the duodenum, which is also the site of NoV replication, were significantly inversely correlated with protection. However, NoVPO pigs had a 37-fold increase in CD4+ IFN-γ+ T cells in the duodenum, which is consistent with the Th1 responses seen in human volunteers (33). Conversely, significant responses in the memory sites (the ileum and spleen) were positively correlated with protection. These data have multiple implications. First, they illustrate the importance of the selection of tissues for the study of immune responses to NoV infection and vaccines. Second, they suggest that primary NoV infection drives T cell proliferation in the primary infection site, while vaccination or previous infection drives T cell expansion in the spleen or homing to the ileum, and that these T cells are recruited to the effector site upon challenge.
Previous studies comparing the immunogenicities of VLPs and P particles in mice have produced conflicting results (12, 13). Tamminen et al. (13) showed that VLPs induced a balanced Th1/Th2 response and P particles induced a Th2-biased response. Tan and Jiang (14) raised concerns that P dimers were used in that study instead of P particles. Fang et al. (12) found that P particles induced immune responses similar to those induced by VLPs. The present study shows that the P particle vaccine primed for stronger T cell responses overall than VLPs postchallenge in Gn pigs. P particles induced significantly higher numbers of activated CD4+ T cells in all tissues, of IFN-γ+ CD8+ T cells in the duodenum, of Tregs in PBL, and of TGF-β-producing CD4+ CD25− FoxP3+ Tregs in the spleen than VLPs. These results suggest that at the same dose, the P particle vaccine is more immunogenic than the VLP vaccine in a higher-order animal model. Given their similar protection rates and the T cell profiles, P particles are a viable alternative to VLPs as a NoV vaccine candidate.
Another major finding of this study is that the P particle vaccine can provide partial cross protection between two distinct GII.4 variants separated by >9 years. Amino acid sequence analysis suggests antigenic drift in the capsid proteins and immunogenic epitopes of the VA387-derived P particles/VLPs and the challenge strain, 092895 (Tables 1 and 2). The NoV 092895 inoculum in this study is a 2006b variant, while the P particles and VLPs were derived from VA387, a 1997 Farmington Hills variant. These two strains have 93.5% VP1 sequence identity. However, despite this suggested antigenic drift, the major binding pockets remain highly conserved in these two GII.4 variants (49).
NoVs undergo epochal evolution; every 3 to 7 years, a new circulating pandemic strain emerges, replacing previous pandemic strains (50), through mutations in VP1 or replication structures (51) and evasion of herd immunity (52). Previous studies have indicated a lack of protection among different pandemic strains and genogroups (33), including weak heterologous responses (53–55). Vaccine-induced heterologous responses to distinct antigenic NoVs are inconsistent and usually require cocktail vaccines (47, 56, 57). An effective NoV vaccine must induce cross-reactivity with different variants in the same genogroups and any emerging strains without impacting the costs of vaccine production.
In this study, the NoV challenge inoculum was derived from a 2008 isolate of the GII.4/2006b strain, while the P particles and VLPs were derived from a GII.4 Farmington Hills variant isolated in 1997. Accordingly, there have been three antigenic shifts between these two strains (58). Blockade epitope A in the P2 domain is considered to be the immunodominant GII.4 epitope that mutates over time to escape herd immunity and contributes to the epochal evolution of NoV (59). The strains used in this study have four differences among the six epitope A amino acids (Table 2), suggesting that these strains are antigenically distinct in this epitope. This study provides the first indication that a P particle vaccine candidate can confer protection from disease caused by an antigenically different challenge strain. Further, P particle stimulation of MNCs isolated from pigs pre- and postchallenge, including NoVPO pigs and control pigs that had been inoculated only with the challenge NoV GII.4/2006b inoculum, induced IFN-γ production (Fig. 3B). These results indicate that VA387-derived P particles can stimulate the effector/memory T cell response against antigenically distinct strains. There is promise that a GII.4 vaccine candidate produced by current vaccine technologies may provide protection not only against parent strains but also against potentially emerging or reemerging strains.
To summarize, this study has several implications for NoV immunity and vaccine development. First, we have shown that previous infection provides substantial protection from NoV-induced gastroenteritis and partial protection from NoV reinfection. Second, we have shown that P particles are capable of providing partial protection against NoV diarrhea at a rate similar to that for VLPs. Third, we have shown that VA387-derived P particles and VLPs provided cross protection against a different variant of GII.4 NoV, indicating that current NoV vaccine technologies are capable of providing protection against antigenically distinct NoVs. Fourth, we have shown an inverse association between prechallenge duodenal Tregs and protection rates against diarrhea. Fifth, and finally, we have shown that P particles induced stronger T cell responses than VLPs in an animal model that closely resembles the NoV disease in humans. These results indicate that P particles are a viable alternative to VLPs for NoV vaccine development.
In conclusion, primary NoV infection and both vaccine candidates reduced the occurrence of diarrhea, while only primary NoV infection provided partial protection from reinfection. To our knowledge, this is the first study to compare the protective efficacies of VLPs and P particles against NoV infection in a higher-order animal model and also to indicate that cross protection is induced by antigenically distinct NoV vaccines. Additionally, this is the first study to comprehensively evaluate the T cell profiles elicited by NoV primary infection, VLPs, and P particles prior to and/or following homotypic challenge. A study using a computer simulation model has suggested that a NoV vaccine conferring 50% protective efficacy lasting for 12 months could prevent 1.0 million to 2.2 million cases of NoV gastroenteritis and save as much as $2.1 billion in the associated economic burden in the United States (60), including $284 million in hospital costs (6). Based on our findings and these economic considerations, we believe that the P particle vaccine merits further investigation.
ACKNOWLEDGMENTS
We thank Charles Richardson, Ross Taylor, Bryan Steadman, Sheri Klas, and Thomas Foubert [Takeda Vaccines (Montana), Inc.] for providing chitosan, P particles, and VLPs. We also thank Kevin Pelzer, Sherrie Clark-Deener, and Marlice Vonck for veterinarian services; Pete Jobst, Andrea Pulliam, Kim Allen, and Shannon Viers for animal care; and Mariah Weiss for help with sample collection. We thank Melissa Makris for flow cytometry service.
This work was supported by an R01 grant (R01AI089634) from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, to X. Jiang (principal investigator [PI]) and L. Yuan (subcontract PI). E. Giri-Rachman was supported by the Fulbright Visiting Scholar 2012 program.
Footnotes
Published ahead of print 11 June 2014
REFERENCES
- 1.Patel MM, Hall AJ, Vinje J, Parashar UD. 2009. Noroviruses: a comprehensive review. J. Clin. Virol. 44:1–8. 10.1016/j.jcv.2008.10.009 [DOI] [PubMed] [Google Scholar]
- 2.Hall AJ. 2012. Noroviruses: the perfect human pathogens? J. Infect. Dis. 205:1622–1624. 10.1093/infdis/jis251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Payne DC, Vinje J, Szilagyi PG, Edwards KM, Staat MA, Weinberg GA, Hall CB, Chappell J, Bernstein DI, Curns AT, Wikswo M, Shirley SH, Hall AJ, Lopman B, Parashar UD. 2013. Norovirus and medically attended gastroenteritis in U.S. children. N. Engl. J. Med. 368:1121–1130. 10.1056/NEJMsa1206589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mead PS, Slutsker L, Dietz V, McCaig LF, Bresee JS, Shapiro C, Griffin PM, Tauxe RV. 1999. Food-related illness and death in the United States. Emerg. Infect. Dis. 5:607–625. 10.3201/eid0505.990502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patel MM, Widdowson MA, Glass RI, Akazawa K, Vinje J, Parashar UD. 2008. Systematic literature review of role of noroviruses in sporadic gastroenteritis. Emerg. Infect. Dis. 14:1224–1231. 10.3201/eid1408.071114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gastanaduy PA, Hall AJ, Curns AT, Parashar UD, Lopman BA. 2013. Burden of norovirus gastroenteritis in the ambulatory setting—United States, 2001–2009. J. Infect. Dis. 207:1058–1065. 10.1093/infdis/jis942 [DOI] [PubMed] [Google Scholar]
- 7.El-Kamary SS, Pasetti MF, Mendelman PM, Frey SE, Bernstein DI, Treanor JJ, Ferreira J, Chen WH, Sublett R, Richardson C, Bargatze RF, Sztein MB, Tacket CO. 2010. Adjuvanted intranasal Norwalk virus-like particle vaccine elicits antibodies and antibody-secreting cells that express homing receptors for mucosal and peripheral lymphoid tissues. J. Infect. Dis. 202:1649–1658. 10.1086/657087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiang X, Wang M, Wang K, Estes MK. 1993. Sequence and genomic organization of Norwalk virus. Virology 195:51–61. 10.1006/viro.1993.1345 [DOI] [PubMed] [Google Scholar]
- 9.Harrington PR, Vinje J, Moe CL, Baric RS. 2004. Norovirus capture with histo-blood group antigens reveals novel virus-ligand interactions. J. Virol. 78:3035–3045. 10.1128/JVI.78.6.3035-3045.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marionneau S, Ruvoen N, Le Moullac-Vaidye B, Clement M, Cailleau-Thomas A, Ruiz-Palacois G, Huang P, Jiang X, Le Pendu J. 2002. Norwalk virus binds to histo-blood group antigens present on gastroduodenal epithelial cells of secretor individuals. Gastroenterology 122:1967–1977. 10.1053/gast.2002.33661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tan M, Jiang X. 2005. The P domain of norovirus capsid protein forms a subviral particle that binds to histo-blood group antigen receptors. J. Virol. 79:14017–14030. 10.1128/JVI.79.22.14017-14030.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fang H, Tan M, Xia M, Wang L, Jiang X. 2013. Norovirus P particle efficiently elicits innate, humoral and cellular immunity. PLoS One 8:e63269. 10.1371/journal.pone.0063269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tamminen K, Huhti L, Koho T, Lappalainen S, Hytonen VP, Vesikari T, Blazevic V. 2012. A comparison of immunogenicity of norovirus GII-4 virus-like particles and P-particles. Immunology 135:89–99. 10.1111/j.1365-2567.2011.03516.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tan M, Jiang X. 2012. The formation of P particle increased immunogenicity of norovirus P protein. Immunology 136:28–29. 10.1111/j.1365-2567.2012.03555.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tan M, Fang P, Chachiyo T, Xia M, Huang P, Fang Z, Jiang W, Jiang X. 2008. Noroviral P particle: structure, function and applications in virus-host interaction. Virology 382:115–123. 10.1016/j.virol.2008.08.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tan M, Hegde RS, Jiang X. 2004. The P domain of norovirus capsid protein forms dimer and binds to histo-blood group antigen receptors. J. Virol. 78:6233–6242. 10.1128/JVI.78.12.6233-6242.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tan M, Huang P, Xia M, Fang PA, Zhong W, McNeal M, Wei C, Jiang W, Jiang X. 2011. Norovirus P particle, a novel platform for vaccine development and antibody production. J. Virol. 85:753–764. 10.1128/JVI.01835-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tan M, Jiang X. 2012. Norovirus P particle: a subviral nanoparticle for vaccine development against norovirus, rotavirus and influenza virus. Nanomedicine (Lond.) 7:889–897. 10.2217/nnm.12.62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheetham S, Souza M, Meulia T, Grimes S, Han MG, Saif LJ. 2006. Pathogenesis of a genogroup II human norovirus in gnotobiotic pigs. J. Virol. 80:10372–10381. 10.1128/JVI.00809-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Souza M, Cheetham SM, Azevedo MS, Costantini V, Saif LJ. 2007. Cytokine and antibody responses in gnotobiotic pigs after infection with human norovirus genogroup II.4 (HS66 strain). J. Virol. 81:9183–9192. 10.1128/JVI.00558-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Souza M, Costantini V, Azevedo MS, Saif LJ. 2007. A human norovirus-like particle vaccine adjuvanted with ISCOM or mLT induces cytokine and antibody responses and protection to the homologous GII.4 human norovirus in a gnotobiotic pig disease model. Vaccine 25:8448–8459. 10.1016/j.vaccine.2007.09.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bui T, Kocher J, Li Y, Wen K, Li G, Liu F, Yang X, Leroith T, Tan M, Xia M, Zhong W, Jiang X, Yuan L. 2013. Median infectious dose of human norovirus GII.4 in gnotobiotic pigs is decreased by simvastatin treatment and increased by age. J. Gen. Virol. 94(Part 9):2005–2016. 10.1099/vir.0.054080-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baldridge JR, Yorgensen Y, Ward JR, Ulrich JT. 2000. Monophosphoryl lipid A enhances mucosal and systemic immunity to vaccine antigens following intranasal administration. Vaccine 18:2416–2425. 10.1016/S0264-410X(99)00572-1 [DOI] [PubMed] [Google Scholar]
- 24.Illum L, Jabbal-Gill I, Hinchcliffe M, Fisher AN, Davis SS. 2001. Chitosan as a novel nasal delivery system for vaccines. Adv. Drug Deliv. Rev. 51:81–96. 10.1016/S0169-409X(01)00171-5 [DOI] [PubMed] [Google Scholar]
- 25.Arias MA, Van Roey GA, Tregoning JS, Moutaftsi M, Coler RN, Windish HP, Reed SG, Carter D, Shattock RJ. 2012. Glucopyranosyl lipid adjuvant (GLA), a synthetic TLR4 agonist, promotes potent systemic and mucosal responses to intranasal immunization with HIVgp140. PLoS One 7:e41144. 10.1371/journal.pone.0041144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wimer-Mackin S, Hinchcliffe M, Petrie CR, Warwood SJ, Tino WT, Williams MS, Stenz JP, Cheff A, Richardson C. 2006. An intranasal vaccine targeting both the Bacillus anthracis toxin and bacterium provides protection against aerosol spore challenge in rabbits. Vaccine 24:3953–3963. 10.1016/j.vaccine.2006.02.024 [DOI] [PubMed] [Google Scholar]
- 27.Ramirez K, Wahid R, Richardson C, Bargatze RF, El-Kamary SS, Sztein MB, Pasetti MF. 2012. Intranasal vaccination with an adjuvanted Norwalk virus-like particle vaccine elicits antigen-specific B memory responses in human adult volunteers. Clin. Immunol. (Orlando, Fla.) 144:98–108. 10.1016/j.clim.2012.05.006 [DOI] [PubMed] [Google Scholar]
- 28.Atmar RL, Bernstein DI, Harro CD, Al-Ibrahim MS, Chen WH, Ferreira J, Estes MK, Graham DY, Opekun AR, Richardson C, Mendelman PM. 2011. Norovirus vaccine against experimental human Norwalk virus illness. N. Engl. J. Med. 365:2178–2187. 10.1056/NEJMoa1101245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simmons K, Gambhir M, Leon J, Lopman B. 2013. Duration of immunity to norovirus gastroenteritis. Emerg. Infect. Dis. 19:1260–1267. 10.3201/eid1908.130472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parrino TA, Schreiber DS, Trier JS, Kapikian AZ, Blacklow NR. 1977. Clinical immunity in acute gastroenteritis caused by Norwalk agent. N. Engl. J. Med. 297:86–89. 10.1056/NEJM197707142970204 [DOI] [PubMed] [Google Scholar]
- 31.Reeck A, Kavanagh O, Estes MK, Opekun AR, Gilger MA, Graham DY, Atmar RL. 2010. Serological correlate of protection against norovirus-induced gastroenteritis. J. Infect. Dis. 202:1212–1218. 10.1086/656364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu S, Regev D, Watanabe M, Hickman D, Moussatche N, Jesus DM, Kahan SM, Napthine S, Brierley I, Hunter RN, III, Devabhaktuni D, Jones MK, Karst SM. 2013. Identification of immune and viral correlates of norovirus protective immunity through comparative study of intra-cluster norovirus strains. PLoS Pathog. 9:e1003592. 10.1371/journal.ppat.1003592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lindesmith L, Moe C, Lependu J, Frelinger JA, Treanor J, Baric RS. 2005. Cellular and humoral immunity following Snow Mountain virus challenge. J. Virol. 79:2900–2909. 10.1128/JVI.79.5.2900-2909.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tacket CO, Sztein MB, Losonsky GA, Wasserman SS, Estes MK. 2003. Humoral, mucosal, and cellular immune responses to oral Norwalk virus-like particles in volunteers. Clin. Immunol. (Orlando, Fla.) 108:241–247. 10.1016/S1521-6616(03)00120-7 [DOI] [PubMed] [Google Scholar]
- 35.Chachu KA, LoBue AD, Strong DW, Baric RS, Virgin HW. 2008. Immune mechanisms responsible for vaccination against and clearance of mucosal and lymphatic norovirus infection. PLoS Pathog. 4:e1000236. 10.1371/journal.ppat.1000236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tomov VT, Osborne LC, Dolfi DV, Sonnenberg GF, Monticelli LA, Mansfield K, Virgin HW, Artis D, Wherry EJ. 2013. Persistent enteric murine norovirus infection is associated with functionally suboptimal virus-specific CD8 T cell responses. J. Virol. 87:7015–7031. 10.1128/JVI.03389-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kageyama T, Kojima S, Shinohara M, Uchida K, Fukushi S, Hoshino FB, Takeda N, Katayama K. 2003. Broadly reactive and highly sensitive assay for Norwalk-like viruses based on real-time quantitative reverse transcription-PCR. J. Clin. Microbiol. 41:1548–1557. 10.1128/JCM.41.4.1548-1557.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Buesa J, Collado B, Lopez-Andujar P, Abu-Mallouh R, Rodriguez Diaz J, Garcia Diaz A, Prat J, Guix S, Llovet T, Prats G, Bosch A. 2002. Molecular epidemiology of caliciviruses causing outbreaks and sporadic cases of acute gastroenteritis in Spain. J. Clin. Microbiol. 40:2854–2859. 10.1128/JCM.40.8.2854-2859.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jiang X, Wang M, Graham DY, Estes MK. 1992. Expression, self-assembly, and antigenicity of the Norwalk virus capsid protein. J. Virol. 66:6527–6532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arnold M, Patton JT, McDonald SM. 2009. Culturing, storage, and quantification of rotaviruses. Curr. Protoc. Microbiol. Chapter 15:Unit 15C.3. 10.1002/9780471729259.mc15c03s15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brito LA, Singh M. 2011. Acceptable levels of endotoxin in vaccine formulations during preclinical research. J. Pharm. Sci. 100:34–37. 10.1002/jps.22267 [DOI] [PubMed] [Google Scholar]
- 42.Meyer RC, Bohl EH, Kohler EM. 1964. Procurement and maintenance of germ-free seine for microbiological investigations. Appl. Microbiol. 12:295–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yuan L, Ward LA, Rosen BI, To TL, Saif LJ. 1996. Systematic and intestinal antibody-secreting cell responses and correlates of protective immunity to human rotavirus in a gnotobiotic pig model of disease. J. Virol. 70:3075–3083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wen K, Li G, Bui T, Liu F, Li Y, Kocher J, Lin L, Yang X, Yuan L. 2012. High dose and low dose Lactobacillus acidophilus exerted differential immune modulating effects on T cell immune responses induced by an oral human rotavirus vaccine in gnotobiotic pigs. Vaccine 30:1198–1207. 10.1016/j.vaccine.2011.11.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yuan L, Wen K, Azevedo MS, Gonzalez AM, Zhang W, Saif LJ. 2008. Virus-specific intestinal IFN-γ producing T cell responses induced by human rotavirus infection and vaccines are correlated with protection against rotavirus diarrhea in gnotobiotic pigs. Vaccine 26:3322–3331. 10.1016/j.vaccine.2008.03.085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wen K, Li G, Yang X, Bui T, Bai M, Liu F, Kocher J, Yuan L. 2012. CD4+ CD25− FoxP3+ regulatory cells are the predominant responding regulatory T cells after human rotavirus infection or vaccination in gnotobiotic pigs. Immunology 137:160–171. 10.1111/j.1365-2567.2012.03617.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Parra GI, Bok K, Taylor R, Haynes JR, Sosnovtsev SV, Richardson C, Green KY. 2012. Immunogenicity and specificity of norovirus consensus GII.4 virus-like particles in monovalent and bivalent vaccine formulations. Vaccine 30:3580–3586. 10.1016/j.vaccine.2012.03.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McCabe-Sellers BJ, Beattie SE. 2004. Food safety: emerging trends in foodborne illness surveillance and prevention. J. Am. Diet Assoc. 104:1708–1717. 10.1016/j.jada.2004.08.028 [DOI] [PubMed] [Google Scholar]
- 49.Yang Y, Xia M, Tan M, Huang P, Zhong W, Pang XL, Lee BE, Meller J, Wang T, Jiang X. 2010. Genetic and phenotypic characterization of GII-4 noroviruses that circulated during 1987 to 2008. J. Virol. 84:9595–9607. 10.1128/JVI.02614-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Debbink K, Lindesmith LC, Donaldson EF, Baric RS. 2012. Norovirus immunity and the great escape. PLoS Pathog. 8:e1002921. 10.1371/journal.ppat.1002921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Siebenga JJ, Vennema H, Renckens B, de Bruin E, van der Veer B, Siezen RJ, Koopmans M. 2007. Epochal evolution of GGII.4 norovirus capsid proteins from 1995 to 2006. J. Virol. 81:9932–9941. 10.1128/JVI.00674-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Debbink K, Lindesmith LC, Donaldson EF, Costantini V, Beltramello M, Corti D, Swanstrom J, Lanzavecchia A, Vinje J, Baric RS. 2013. Emergence of new pandemic GII.4 Sydney norovirus strain correlates with escape from herd immunity. J. Infect. Dis. 208:1877–1887. 10.1093/infdis/jit370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Iritani N, Seto T, Hattori H, Natori K, Takeda N, Kubo H, Yamano T, Ayata M, Ogura H, Seto Y. 2007. Humoral immune responses against norovirus infections of children. J. Med. Virol. 79:1187–1193. 10.1002/jmv.20897 [DOI] [PubMed] [Google Scholar]
- 54.Rockx B, Baric RS, de Grijs I, Duizer E, Koopmans MP. 2005. Characterization of the homo- and heterotypic immune responses after natural norovirus infection. J. Med. Virol. 77:439–446. 10.1002/jmv.20473 [DOI] [PubMed] [Google Scholar]
- 55.Farkas T, Thornton SA, Wilton N, Zhong W, Altaye M, Jiang X. 2003. Homologous versus heterologous immune responses to Norwalk-like viruses among crew members after acute gastroenteritis outbreaks on 2 US Navy vessels. J. Infect. Dis. 187:187–193. 10.1086/367809 [DOI] [PubMed] [Google Scholar]
- 56.LoBue AD, Lindesmith L, Yount B, Harrington PR, Thompson JM, Johnston RE, Moe CL, Baric RS. 2006. Multivalent norovirus vaccines induce strong mucosal and systemic blocking antibodies against multiple strains. Vaccine 24:5220–5234. 10.1016/j.vaccine.2006.03.080 [DOI] [PubMed] [Google Scholar]
- 57.LoBue AD, Thompson JM, Lindesmith L, Johnston RE, Baric RS. 2009. Alphavirus-adjuvanted norovirus-like particle vaccines: heterologous, humoral, and mucosal immune responses protect against murine norovirus challenge. J. Virol. 83:3212–3227. 10.1128/JVI.01650-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lindesmith LC, Donaldson EF, Lobue AD, Cannon JL, Zheng DP, Vinje J, Baric RS. 2008. Mechanisms of GII.4 norovirus persistence in human populations. PLoS Med. 5:e31. 10.1371/journal.pmed.0050031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Debbink K, Donaldson EF, Lindesmith LC, Baric RS. 2012. Genetic mapping of a highly variable norovirus GII.4 blockade epitope: potential role in escape from human herd immunity. J. Virol. 86:1214–1226. 10.1128/JVI.06189-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bartsch SM, Lopman BA, Hall AJ, Parashar UD, Lee BY. 2012. The potential economic value of a human norovirus vaccine for the United States. Vaccine 30:7097–7104. 10.1016/j.vaccine.2012.09.040 [DOI] [PMC free article] [PubMed] [Google Scholar]