ABSTRACT
We developed a Moloney mouse leukemia virus (MLV)-based retroviral replicating vector (RRV), Toca 511, which has displayed tumor specificity in resected brain tumor material and blood in clinical trials. Here, we investigated the interaction between Toca 511 and human host cells, and we show that RRVs do not induce type I interferon (IFN) responses in cultured human tumor cells or cultured human primary cells. However, exogenous type I IFN inhibited RRV replication in tumor cells and induced IFN-regulated genes, albeit at a lower level than in primary cells. Unexpectedly, RRVs did not induce IFN-α production upon incubation in vitro with human plasmacytoid dendritic cells (pDCs), whereas lentivirus vector and heat-treated RRVs did. Coincubation of RRVs with heat-treated RRVs or with lentivirus vector suppressed IFN-α production in pDCs, suggesting that native RRV has a dominant inhibitory effect on type I IFN induction. This effect is sensitive to trypsin treatment. In addition, heat treatment inactivated that activity but exposed an immune-stimulatory activity. The immune-stimulating component is sensitive to deglycosidases, trypsin, and phospholipase C treatment. Experiments with retroviral nonreplicating vectors and virus-like particles demonstrated that the immunosuppressive activity is not associated with the amphotropic envelope or the glyco-Gag protein. In summary, our data provide evidence that RRVs do not directly trigger type I IFN responses in IFN-responsive tumor cells. Moreover, RRVs appear to carry a heat-labile component that actively suppresses activation of cellular innate immune responses in pDCs. Inhibition of IFN induction by RRVs and the reduced response to IFN should facilitate tumor-specific infection in vivo.
IMPORTANCE RRVs have a convincing preference for replicating in tumor cells in animal models, and we observed similar preferences in the initial treatment of human glioblastoma patients. This study investigates the basis for the interaction between RRV and human host cells (tumor versus nontumor) in vitro. We found that RRVs do not trigger an IFN-α/β response in tumor cells, but the cells are capable of responding to type I IFNs and of producing them when stimulated with known agonists. Surprisingly, the data show that RRVs can actively inhibit induction of cellular innate immunity and that this inhibitory activity is heat labile and trypsin sensitive and not attributable to the envelope protein. These data partially explain the observed in vivo tumor specificity.
INTRODUCTION
We are developing retroviral replicating vectors (RRVs) as anticancer agents, and we are conducting investigational clinical trials in patients with high-grade glioma (HGG; http://www.clinicaltrial.gov; NCT01156584, NCT01470794, and NCT01985256) with an RRV (Toca 511; vocimagene amiretrorepvec) based on Moloney murine leukemia virus (MLV). This virus has an amphotropic envelope protein and encodes an optimized yeast-derived cytosine deaminase designed to convert 5-fluorocytosine to 5-fluorouracil in infected tumors. In general, a potential limitation for replicating viruses as anticancer agents is attaining sufficient viral spread within the tumor mass before immune clearance of the virus (1). The noncytolytic nature of RRVs may render them less likely to trigger innate immune responses than directly oncolytic viruses, such as those based on adenovirus or vaccinia virus. RRVs appear to be relatively noninflammatory, weak immunogens and, in rodent tumor models, replicate in target tumors without extensive replication elsewhere in healthy tissues (2, 3). This last property has also been demonstrated in dog patients with high-grade gliomas (J. Robbins et al., unpublished data). Furthermore, these results are consistent with published data that reported rapid elimination of detectable virus upon infusing normal monkeys with amphotropic murine replicating retrovirus preparations (4). Initial observations in human clinical trials with Toca 511 also support this model, and such properties are a potential advantage for RRVs as anticancer agents. We previously speculated (2, 5) that this specificity arises out of a combination of the need for replicating cell targets for productive infection by gammaretroviruses (6) and common defects in the cellular innate immune signaling pathways in tumor cells (7). The innate immune response, besides constituting a direct system of immune defense, is thought to be a necessary precursor to adaptive immunity (8). Moreover, viral restriction factors such as APOBEC3G, tetherin, and other host restriction factors (9) are generally downstream effectors induced by the type I interferons (IFNs) which, in turn, are induced by activation of the innate immune signaling pathways through pattern recognition receptors (PRRs). Therefore, we wanted to investigate whether RRVs are truly less inflammatory and have a relatively attenuated ability to stimulate the innate immune system. This could account for the lack of viral clearance from tumors by the adaptive immune response and also the permissive virus replication in these tumors.
The innate immune response is predominately mediated by interaction of pathogen-associated molecular patterns (PAMP) with PRRs present on the cell surface or within the intracellular compartments. PRRs which detect viral components include Toll-like receptors (TLR2, TLR3, TLR4, TLR7, TLR8, and TLR10), RIG-I-like receptors (RIG-1 and MDA5), PKR, DAI, and STING (10, 11). Upon induction of PRRs, IFN-α/β is produced and activates the type I IFN signaling pathway in an autocrine or paracrine fashion, which subsequently leads to activation of an antiviral state in the cells. Defects in type I IFN signaling have been reported in tumor cells, including human glioma cells (12–14), resulting in the tumor cells avoiding cell death and/or the promotion of cell proliferation, thereby providing an optimal niche for viral infection and replication within the cells of such cancers.
In this study, we investigated the interaction between RRV infection and the type I IFN-dependent antiviral response in human tumor cells and normal cells. Our in vitro results showed that, although the replication of RRV is markedly inhibited by exogenous interferon treatment, RRV infection is a less inflammatory event than the response to other viral entities, including lentiviral vectors. Our data provide direct evidence that the amphotropic MLV-based RRV does not directly trigger the type I IFN response in IFN-responsive human glioma tumor-derived cells or cultured nontransformed fibroblast and primary endothelial cells. Moreover, our data provide the first direct evidence that native RRV particles actively suppress innate immunity. We also show that an immune-stimulating component, other than viral RNA, is associated with RRV particles and can be revealed by disrupting the native structure of the particle. From these data, we inferred, by process of elimination, that both the IFN immune-stimulating and immune-suppressive components are likely attributable to the structural elements of the viral Gag-Pol polyprotein.
The inhibition of type I IFN production by native RRVs, combined with the reduced response to type I IFN in cancer cells, is in agreement with our observations of selective replication of amphotropic MLV-based RRVs in tumors without viral elimination (2). Although defects in the tumor cell pattern recognition and downstream type I IFN signaling pathways likely play a role in the host-virus interaction, other factors (apart from the cell target replication requirement) are likely involved in determining tissue or cell type infection in vivo.
MATERIALS AND METHODS
Cell culture.
293T cells were obtained through a materials transfer agreement with the Indiana University Vector Production Facility, and Stanford University deposited the cells with ATCC (SD-3515; lot 2634366). 293GP cells (15), which express high levels of Moloney MLV Gag and Pol, was a kind gift from Theodore Friedmann at UCSD. 293GP/pBA9b cells, which contain an integrated pBA9b provirus, are derived from 239GP cells which were transduced with a glycoprotein G of vesicular stomatitis virus (VSV-G)-pseudotyped MLV vector. Other cell lines were obtained from ATCC: human fibrosarcoma cells (HT1080); human astrocytoma U87-MG cells (HTB-14); human prostate adenocarcinoma PC3 cells (CRL-1435); human prostate carcinoma LN-CaP cells (CRL-1740); human Sup-T1 T lymphocytes (CRL-1942); human fibroblast cell lines WI-38 (CCL-75) and CCD-1070Sk (CRL-2091). Primary human umbilical vein endothelial cells (HUVEC) were obtained from Vec Technologies. 293T, 293GP, 293GP/pBA9b, HT1080 and U87-MG cells were cultured in complete Dulbecco's modified Eagle's medium containing 10% fetal bovine serum (FBS; HyClone), sodium pyruvate, Glutamax, and penicillin-streptomycin and antibiotics (penicillin, 100 IU/ml; streptomycin, 50 μg/ml). LN-CaP and Sup-T1 cells were cultured in complete RPMI medium containing 10% FBS, Glutamax, and penicillin-streptomycin. WI-38 and CCD-1070Sk cells were cultured in minimal essential medium containing 10% FBS, Glutamax and penicillin-streptomycin. Primary HUVEC were cultured in MCDB-131C medium (Vec Technologies).
For experiments in which U87-MG cells were treated with exogenous type I IFN, recombinant IFN-α2a, or recombinant IFN-β (products 11100-1 and 11415-1, respectively; PBL Interferon Source) at 200 U/ml, the treatment was applied to the culture medium during the time of initial infection and at the time of each cell passage (every 3 to 4 days). For experiments in which U87-MG cells were treated with poly(I·C), cells were seeded at 1 × 105 cells per well in a 12-well plate the day before transfection. Cells were then transfected with 10 μg of poly(I·C) (catalog number P0913; Sigma) by using FuGene HD transfection reagent (catalog number 04709691001; Roche). Approximately 8 h posttransfection, supernatant was collected for an enzyme-linked (sandwich) immunosorbent assay (ELISA; PBL Interferon Source) to quantify the amounts of IFN-α and IFN-β.
Production of MLV-based replicating particles, nonreplicating viral particles, virus-like particles (VLPs), and VLP/pBA9b.
Toca 511 and RRV-GFP are Moloney MLV-based RRVs with an amphotropic envelope gene and an encephalomyocarditis virus internal ribosome entry site (IRES)-transgene cassette downstream of the env gene (5). The pBA9b plasmid is an MLV-based retroviral nonreplicating vector (16). Expression vectors encoding MLV Gag-Pol, the amphotropic envelope gene, and the VSV-G gene were utilized. Virus stocks of RRV-GFP and Toca 511 were generated from stable human producer cell lines followed by column purification. Virus stocks of Toca 511 pseudotyped with a VSV-G vector (Toca 511-G), an MLV-based nonreplicating vector pseudotyped with 4070A amphotropic envelope protein (MLV-A), VSV-G (MLV-G), or with 4070A amphotropic and VSV-G envelope protein (MLV-A+G) were produced by transient transfection in 293T cells seeded at 3 × 104 cells per cm2 the day before transfection. Cells were transfected with plasmid DNA (pAC3-yCD2 and CMV–VSV-G for Toca 511-G, CMV-gag-pol, pBA9b, and CMV-ampho for MLV-A, CMV–gag-pol, pBA9b, and CMV–VSV-G for MLV-G, and CMV-gag-pol, pBA9b, CMV-ampho, and CMV–VSV-G for MLV-A+G) 20 h post-cell seeding using the calcium phosphate method. Eighteen hours posttransfection, cells were washed with phosphate-buffered saline (PBS) twice and incubated with fresh complete culture medium. Viral supernatant was collected approximately 42 h posttransfection and filtered through a 0.45-μm filter. Filtered viral supernatant was subjected to benzonase (catalog number E8263; Sigma) treatment at 5 U/ml at 4°C overnight in order to further reduce contamination with transfection-derived plasmid DNA, followed by centrifugation at 38,465 × g for 250 min. For Toca 511-G and MLV-A+G viral particles, high-speed centrifugation purification with a sucrose cushion was performed to remove unincorporated viral glycoproteins.
RRV viral titers were determined as described previously (5). Briefly, vector preparations titers were determined on PC3 cells by single-cycle infection of the vector. The single-cycle infection was guaranteed by azidothymidine treatment 24 h postinfection, followed by quantitative PCR (qPCR) of target cell genomic DNA specific for viral vector DNA 48 h postinfection, to quantify the number of viral DNA copies per cell genome. Integrated viral DNA was expected to be the predominant species measured due to the short half-life of unintegrated MLV DNA after reverse transcription (17). Viral titers, reported in transduction units (TU) per milliliter, were determined by calculation of threshold cycle (CT) values derived from a standard curve ranging from 1 × 105 copies to 1 × 101 copies of plasmid DNA and from a known amount of genomic DNA input, the number of cells, and a dilution of the viral stock per reaction mixture.
VLPs produced in this study were virus-like particles that lacked envelope glycoprotein and viral RNA or virus-like particles that lacked envelope glycoprotein but that were packaged with pBA9b viral RNA (VLP/pBA9b). Supernatant from >95% confluent 293GP cells, which express high levels of MLV gag and pol, or 293GP/pBA9b cells, previously transduced with the VSV-G-pseudotyped MLV vector at a multiplicity of infection (MOI) of 50, was collected and filtered through a 0.45-μm filter followed by centrifugation at 19,500 × g for 30 min at 4°C. All concentrated viral particles were suspended in formulation buffer.
Production of lentiviral vector.
The HIV-1-based lentiviral vector carrying a green fluorescent protein (GFP) gene and pseudotyped with VSV-G (LV-G) was produced by transient transfection in 293T cells as previously described (18). Viral supernatant was collected 36 h posttransfection followed by benzonase treatment at 5 U/ml overnight at 4°C. Subsequently, the viral supernatant was concentrated by high-speed centrifugation, and concentrated virus stock was resuspended in formulation buffer (2). The viral titer was determined by transducing 293T cells with serial 1:10 dilutions from the concentrated virus stock in a total volume of 1 ml. Cells were harvested 48 h postransduction, followed by flow cytometric analysis to determine the percentage of GFP-positive cells. The viral titer is reported as TU/ml and was calculated as follows: (percent GFP-positive cells) × (number of cells seeded) × (dilution factor).
Viral replication monitored by GFP expression.
To monitor viral replication in HT1080, U87-MG, LN-CaP, Sup-T1, and WI-38 cells and primary HUVEC, 2 × 105 cells in T25 flasks were infected with RRV-GFP at an MOI of 0.1. Every 3 to 4 days, a portion of cells was passaged with fresh culture medium for continued monitoring of viral replication, and at the same time another portion of cells was harvested for GFP expression determinations by flow cytometric analysis. Cells harvested for flow cytometric analysis were washed with PBS and centrifuged at 100 × g for 5 min. Cell pellets were resuspended in PBS containing 1% paraformaldehyde. The percentage of GFP-positive cells was determined by flow cytometry using proper gating to exclude GFP-negative cells. The percentage of GFP-positive cells was measured by flow cytometry using the FL1 channel (BD FACSCanto II; BD Biosciences). Viral replication kinetics was determined by plotting the percentage of GFP-positive cells over time.
Viral particle internalization assay.
Comparable amounts of viral particles (Toca 511 and VLPs) used for the pDC bioassay were labeled with carboxyfluorescein diacetate succinimidyl ester (CFDA SE) using the Vybrant CFDA SE cell tracer kit (Molecular Probes). The labeled VLPs were dialyzed against PBS overnight at 4°C and added to pDCs. Intracellular fluorescent-conjugated carboxyfluorescein succinimidyl ester (CFSE) at the indicated time points was detected by flow cytometry using the FL-1 channel.
Deglycosidase, trypsin, and phospholipase C treatments of viral particles.
Toca 511 was treated with deglycosidases according to the manufacturer's protocol (catalog number V493A; Promega), with trypsin at 40 μg/ml (catalog number T1426; Sigma-Aldrich), or with phospholipase C (PLC) at 50 μg/ml (catalog number P8804; Sigma-Aldrich) in a total volume of 50 μl. All reaction mixtures were incubated for 30 min at 37°C. At the end of incubation, 1 ml of PBS was added to each sample, followed by centrifugation at 19,500 × g for 30 min at 4°C. The samples were resuspended in 20 μl PBS for immunoblotting or pDC bioassay.
Determination of relative expression of cellular RNA by qRT-PCR.
RNA was extracted from cells by using the RNeasy kit with DNase I treatment (Qiagen). Reverse transcription (RT) was carried out with 100 ng total RNA and the high-capacity cDNA reverse transcription kit (Applied Biosystems [ABI]). qPCR was performed using the TaqMan Universal PCR master mix, No AmpErase UNG (4324018; ABI), and TaqMan gene expression primers (Hs00169345_m1 for EIF2AK2/PKR, Hs00222415_m1 for APOBEC3G, and Hs99999905_m1 for glyceraldehyde 3-phosphate dehydrogenase; ABI) according to the manufacturer's protocols. The relative expression of each gene was expressed as 2−ΔΔCT.
Immunoblotting.
Viral pellets were resuspended in PBS and 2× loading buffer and subjected to PAGE. Anti-MLV p30, anti-gp70, and anti-VSV-G antibodies were used to detect the expression of MLV capsid protein, envelope protein, and VSV-G, respectively. Incorporation of amphotropic envelope protein and/or VSV-G into MLV-based viral particles was detected by using anti-MLV p30 (CRL-1912; ATCC), anti-gp70 (83A25; material transfer agreement with Leonard Evans at NIH/NIAID), or anti-VSV-G (ab50549; Abcam) antibodies and the corresponding secondary antibodies conjugated to horseradish peroxidase to detect expression of MLV capsid protein (CA), envelope protein, or VSV-G, respectively.
Isolation of plasmacytoid dendritic cells from human PBMCs and quantification of IFN-α.
Buffy coats were obtained from the San Diego Blood Bank. Peripheral blood mononuclear cells (PBMCs) were purified from the blood of healthy human donors by density gradient centrifugation using Ficoll-Paque (MT-25-072-CVRF; Cellgro). Isolation of pDCs was subsequently enriched by negative selection using magnetic beads (130-092-207; Miltenyi Biotec). Cells were maintained in AIM V (12055-091; Invitrogen) in the presence of recombinant human interleukin-3 at 10 ng/ml (203-IL; R&D Systems). The purity of pDCs (>95%) was confirmed by flow cytometric analysis using anti-CD45 and anti-CD303 antibodies (130-080-021 and 130-090-905; Miltenyi Biotec). Cells were incubated with retroviral vectors at an MOI of 1, 10, or 100 or with lentiviral vector as a positive control at an MOI of 20. Approximately 36 h postinfection, supernatant was collected for an ELISA (PBL Interferon Source) to quantify the amount of IFN-α. Synthetic oligonucleotide CpG ODN2395 was obtained from InvivoGen (tlrl-odnc). A final concentration of 1 μM was used to activate TLR9 in pDCs.
RESULTS
Replication kinetics of the RRV expressing GFP in cultured human tumor cells and primary cells.
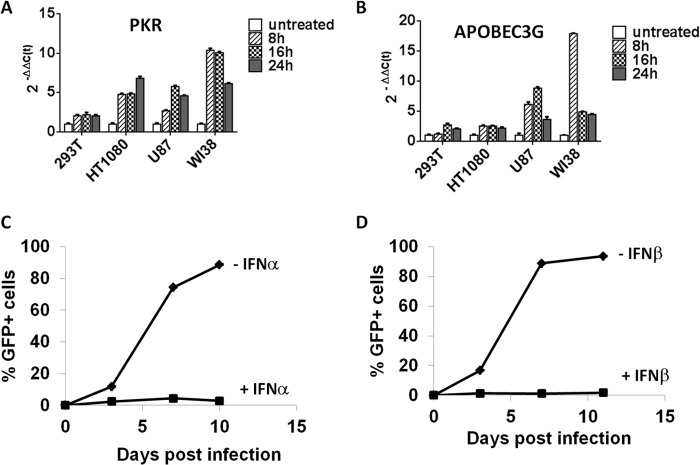
We used an RRV containing an IRES-GFP cassette downstream of env (RRV-GFP) (5) to monitor viral spread over time in infected cultured cells. We first examined the extent of viral replication of RRV-GFP in a panel of established human tumor cell lines, including U87-MG, HT1080, LNCaP, and Sup-T1. In addition, we also examined viral replication of RRV-GFP in nontransformed WI-38 lung fibroblasts and established primary HUVEC. RRV-GFP replicated efficiently in all tumor cells (Fig. 1A to D) and, surprisingly, in primary cells (Fig. 1E and F), which are expected to have intact type I IFN signaling pathways. The time required for the vector to reach maximal infectivity varied, ranging from 6 to 28 days, depending somewhat on the rate of cell proliferation, a major determinant for efficient viral spread and productive infection. The replication kinetics of the RRV-GFP was particularly slow in LNCaP and WI-38 cells, which correlates with their slow cell proliferation rates in culture. Nevertheless, the vector reached 80 to 90% infectivity by day 28 and day 24 postinfection in LNCaP and WI-38 cells, respectively. Together, the data indicated that RRV-GFP replicates in proliferating tumor and primary fibroblast and endothelial cells in vitro.
FIG 1.

Replication kinetics of RRV-GFP in a panel of human tumor and nontransformed cell lines and primary cells. Cells were infected with RRV-GFP at an MOI of 0.1 on day zero and passaged twice weekly. The replication kinetics of each vector was obtained by plotting the percentage of GFP-positive cells versus time.
Replication of the RRV expressing GFP is sensitive to the antiviral response induced by exogenous IFN-α/β.
For simple retroviruses such as MLV-based RRVs, it is known that the selectivity toward tumor cells relies, at least in part, on the proliferation of the tumor cells. Although the rate of viral replication does not always correlate with cell proliferation, proliferation is necessary for virus replication. The type I IFN pathway plays a direct role in regulating antiviral function, but it could also have an antiviral effect that relies solely on its antiproliferative effect on target cells. We attempted to differentiate between these two types of antiviral action to better understand the observed selectivity of RRVs for tumor cells. We examined a panel of human tumor-derived cell lines and nontransformed fibroblast cells (WI-38) and asked whether they were sensitive to the antiviral response by exogenous IFN-α/β. We first compared the basal and IFN-α-induced expression of the genes for two proteins, PKR and APOBEC3G. PKR is an IFN-α/β-responsive gene commonly used for monitoring the activation of the type I IFN pathway, and APOBEC3G has been shown to inhibit replication of retroviruses (19, 20). Cells were treated with exogenous IFN-α (at 200 U/ml) and harvested at 8, 16, and 24 h posttreatment, and gene expression was measured by qRT-PCR. PKR and APOBEC3G gene expression was induced as early as 8 h and was sustained for up to 24 h posttreatment with exogenous IFN-α (Fig. 2A and B). The responsiveness to exogenous IFN-α was highest in nontransformed WI-38 cells, intermediate in HT1080 and U87-MG cells, and lowest in 293T cells. The marginal induction of PKR and APOBEC3G gene expression observed in 293T cells is consistent with data reported by Noser et al., in which the cells showed resistance to IFN-α treatment (21). We next examined whether replication of the RRV-GFP is sensitive to exogenous IFN-α/β treatment. In this experiment, we tested U87-MG cells because the virus replicates most rapidly in this cell line, possibly due to some defects in the type I IFN pathway. As shown in Fig. 2C and D, viral replication was completely inhibited by concomitant addition of IFN-α or IFN-β, with residual GFP expression observed in cells treated with IFN-α. The difference in antiviral function between IFN-α and IFN-β is in agreement with differential activities among subtypes of type I IFN reported previously (22). Taken together, the data indicated that HT-1080 and U87-MG tumor cells respond to exogenous type I IFN-α/β, although the levels of IFN-responsive gene induction appear reduced in these cells compared to that in WI-38 fibroblasts. Further, in U87-MG cells, the IFN-mediated antiviral response resulted in remarkable attenuation of viral replication.
FIG 2.
U87-MG cells are responsive to type I IFN signaling. (A and B) The type I IFN-inducible genes for PKR and APOBEC3G in a panel of established cell lines were induced by exogenous IFN-α at 200 U/ml. Induction of PKR and APOBEC3G was measured by qRT-PCR at 8, 16, and 24 h post-addition of IFN-α. The fold induction was calculated relative to the response in untreated cells, and the relative expression level of each gene was calculated by 2−ΔΔCT. The data shown represent means ± standard deviations (n = 3). (C and D) Replication kinetics of RRV-GFP in U87-MG cells in the presence and absence of IFN-α (C) or IFN-β (D).
Toca 511 RRV does not trigger the IFN-α/β response in U87-MG cells.
The above findings suggested that RRV-GFP upon entry or during its life cycle does not directly trigger IFN-α/β responses in the host cells. To confirm that the RRV-GFP was neither vector nor transgene specific, we assessed the IFN-α/β response to Toca 511 by measuring pathway-specific gene expression in U87-MG cells infected with Toca 511 at an MOI of 10. At 16 h post-Toca 511 infection, or IFN-β treatment, cells were harvested for gene expression analysis by PCR arrays. As expected, several known type I IFN-α/β inducible genes, including Bst2, CXCL10, PKR, IFI and IFITM, ISG, Mx, and OAS, in cells treated with exogenous IFN-β were upregulated by more than 100-fold above those of untreated cells (data not shown). In contrast, there was no global induction of the IFN-α/β-inducible genes observed in cells infected with Toca 511 (data not shown). In addition to the viral replication data, the gene expression PCR array data further support the notion that simple MLV-based RRVs do not directly trigger a type I IFN response in IFN-responsive U87-MG tumor cells.
As the viral genome of RRVs such as Toca 511 consists of two identical single-stranded RNA copies with extensive secondary structures, the nucleic acid component presumably can serve as a potential PAMP motif during its life cycle in the host cells. It is possible that U87-MG cells have a functional TLR pathway but are deficient in IFN-α/β production due to deletions and rearrangement in the IFN gene cluster located at chromosome 9p21-22 (12, 23). To further examine the interaction of TLR and type I IFN signaling pathways and to delineate the mechanism supporting the permissiveness of Toca 511 replication in U87-MG cells, we asked whether U87-MG cells are capable of producing IFN-α/β upon activation of TLR3, which is expressed in most cells and serves as a key sensor for viral double-stranded RNA. U87-MG cells were treated with Toca 511 or with poly(I·C) (an agonist for TLR3, RIG-1, and MDA5) as a positive control. The same experiment was performed with CCD-1070Sk human dermal primary fibroblasts, which have been shown to support the replication of the RRV-GFP (data not shown), as fibroblasts are known to be key IFN-β-producing cells (24). Figure 3A shows that IFN-β production was significantly induced in both cell lines treated with poly(I·C), whereas IFN-β production was undetectable when cells were treated with Toca 511 at 8, 16, and 24 h postinfection, respectively. In contrast, IFN-α expression was not detected in either CCD-1070Sk or U87-MG cells treated with poly(I·C) or Toca 511 (Fig. 3B). The absence of IFN-α expression as a result of secondary induction by IFN-β in CCD-1070Sk and U87-MG cells is likely due to the sample collection before IFN-α production, although in U87-MG cells the absence of IFN-α induction could also have been due to deletion of the IFN-α gene cluster in chromosome 9p21-22, which has been described generally in glioma (12, 23). Altogether, our data indicate that U87-MG supports replication of MLV-based RRV even when both TLR (IFN production) and type I IFN signaling pathways are at least partially functional in these cells.
FIG 3.

Type I IFN production induced by poly(I·C) and Toca 511 in CCD-1070Sk fibroblasts and U87-MG glioma cells. Cells were transfected with poly(I·C) at 10 μg/ml or infected with Toca 511 at an MOI of 10. Culture supernatants from untreated and poly(I·C)- or Toca 511-treated cells were collected 8 h posttreatment for an ELISA to measure the amount of IFN-β (A) and IFN-α (B), respectively. Data shown represent means ± standard deviations (n = 3).
An active immunosuppressive component associated with Toca 511 blocks the IFN-α/β response in human pDCs.
We could not exclude the possibility that RRV may trigger other PRRs, such as TLR7 and TLR9, and that this could occur in vivo in nonmalignant cells within a tumor microenvironment. Therefore, we examined the antiviral response of human primary pDCs to RRV, as these cells are a component of the tumor microenvironment in some tumor types.
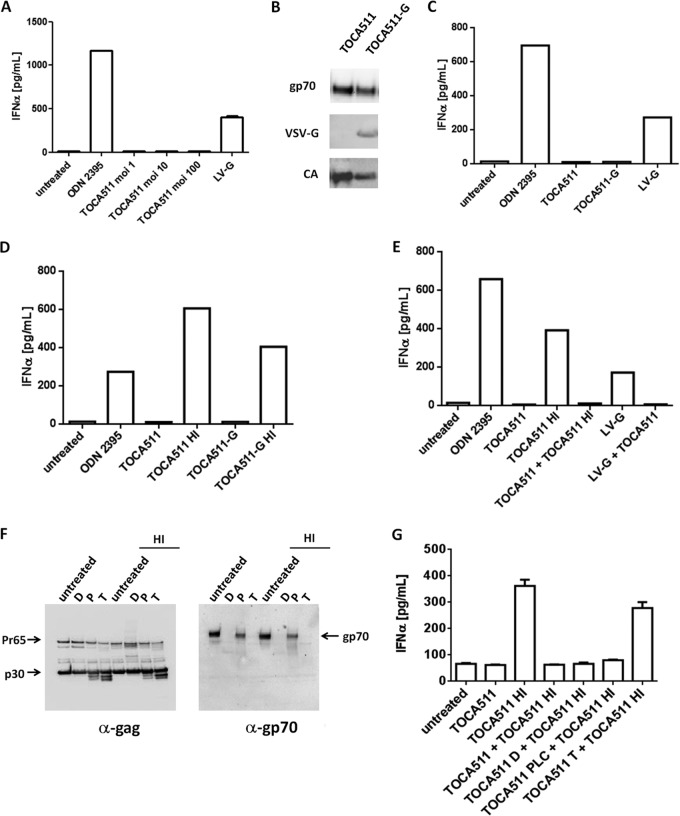
pDCs are a subset of human dendritic cells that differentially express TLR7 and TLR9 (25). As with lentiviral vectors, the duplicates of single-stranded RNA viral genomes encapsulated in the RRV viral particles and unmethylated double-stranded DNA that arises from reverse transcription could potentially serve as PAMP motifs for TLR7 and TLR9, respectively. We examined the sequence of Toca 511 for TLR-activating GU-rich sequences and identified several GU-rich (ranging from 60 to 80%) motifs of 20-nucleotide lengths located in the psi region of the viral genome (Fig. 5). To investigate whether the viral genome of Toca 511 could potentially be a PAMP motif for TLR7 in human pDCs, we isolated pDCs from PBMCs of healthy individuals and exposed the cells to Toca 511 at an MOI of 1, 10, or 100. These cells do not replicate, and therefore viral entry, but not productive infection, is expected. In our experiments, ODN2395 (an agonist for TLR9) and a lentiviral vector pseudotyped with vesicular stomatitis virus glycoprotein, LV-G (an agonist for TLR7), were included as positive controls for induction of IFN-α production in pDCs. Our data showed that Toca 511, even at high MOIs, did not induce IFN-α production in human pDCs, whereas a significant level of IFN-α production was detected in cells treated with ODN2395 or LV-G (Fig. 4A). The production of IFN-α in pDCs treated with LV-G is consistent with data reported by Brown et al. (26). Furthermore, we have performed the pDC experiments using mouse pDC preparations exposed to RRV and LV-G, and the results were essentially identical to those shown here with the human pDC preparations (data not shown).
FIG 5.
Sequence homology of the viral genome between Toca 511 and the pBA9b vector. (A) Diagram of sequence overlap between the viral genome of Toca 511 and the pBA9b vector. (B) Four GU-rich regions were identified in the psi sequence and 5′-gag in Toca 511 (highlighted), and the first 3 regions were present in the pBA9b vector. The underlined ATG indicates the start codon for the Gag polypeptide. The asterisk indicates where the gag sequence ends in the pBA9b vector.
FIG 4.
MLV-based retroviral vector suppression of IFN-α production by human pDCs is sensitive to enzyme treatment. (A) pDCs isolated from healthy individuals were stimulated with ODN2395 or Toca 511 at an MOI of 1, 10, and 100 or lentiviral vector pseudotyped with VSV-G at an MOI of 20. Production of IFN-α was measured 30 h after stimulation. (B) Western blot of Toca 511 and Toca 511 pseudotyped with a VSV-G (Toca 511-G) vector by using antibodies against the amphotropic envelope glycoprotein (gp70), VSV-G protein, and the capsid protein (CA). (C) IFN-α production of pDCs stimulated with Toca 511 or Toca 511-G at an MOI of 20. (D) IFN-α production of pDCs stimulated with Toca 511 or Toca 511-G at an MOI of 20 with or without heat inactivation. (E) Blockade of IFN-α production in pDCs stimulated and coincubated with Toca 511, Toca 511 HI (heat treated), or Toca 511 and LV-G. (F) Immunoblots of heat- and non-heat-treated Toca 511 and enzyme digestion with deglycodiases, phospholipase C, or trypsin. D, deglysidases; P, phospholipase C; T, trypsin. (G) Blockade of IFN-α production in pDCs is removed by coincubation of trypsin-treated and heat-treated Toca 511.
We next explored the possibility that the viral glycoprotein involved in cell entry plays a role in determining the subcellular localization of the viral particle upon entry and its exposure to cellular immune sensors. To this end, we generated Toca 511 with both amphotropic and VSV-G proteins on the viral particles in order to redirect Toca 511 to the endocytic pathway. As shown in Fig. 4B, both amphotropic Env and VSV-G proteins were efficiently incorporated into viral particles, with a slight decrease of the amphotropic Env incorporation when VSV-G was coexpressed. In addition, by qPCR the function of VSV-G in directing viral entry and one-round transduction, resulting in the integration of proviral DNA into the host genome, was confirmed using CHO-K1 cells, which are permissive for VSV-G-pseudotyped RRV but not for amphotropic enveloped RRV (27). We then examined whether Toca 511 pseudotyped with VSV-G, which directs the viral particles preferentially toward the endocytic pathway, could trigger the activation of endosomal TLRs (TLR7/8 or TLR9). Cultured human pDCs were treated with Toca 511 or Toca 511 coenveloped with VSV-G. Consistent with previous data showing that Toca 511 by itself did not stimulate human pDCs to produce IFN-α, Toca 511 coenveloped with VSV-G also did not activate endosomal TLRs in human pDCs (Fig. 4C).
The immunosuppressive component associated with Toca 511 can be abrogated by heat or enzyme treatment to trigger an IFN-α/β response in human pDCs.
One explanation for the finding that Toca 511 does not trigger a type IFN response in human pDCs is that an immunosuppressive component is associated with RRV particles. To investigate whether the classic viral inactivation method by heat (28) would inactivate a suppressive component and allow the induction of IFN-α/β expression, we treated Toca 511 with heat at 56°C for 30 min prior to exposure to pDCs in culture. Our study revealed that in contrast to untreated Toca 511, heat-treated Toca 511 induced marked IFN-α production in pDCs (Fig. 4D). A similar result was obtained when Toca 511 coenveloped with VSV-G was treated with heat (Fig. 4D). To further investigate this, we coincubated untreated Toca 511 with heat-treated Toca 511 or with lentiviral vector at a 1:1 ratio (transduction units) prior to exposure to pDCs. Our data showed that coincubation of Toca 511 with heat-treated Toca 511 or with lentiviral vector pseudotyped with VSV-G resulted in blockade of IFN-α production in human pDCs (Fig. 4E).
To investigate whether the component is sensitive to enzyme treatment that can alter the protein structure of the viral particles, we sought to examine the effects of deglycosidases, PLC, and trypsin on pDC stimulation. As seen in Fig. 4F, the electrophoresis pattern of the Gag polyprotein from heat-treated Toca 511 was comparable to that of the non-heat-treated Toca 511. Coincubation of deglycosidases, PLC, or trypsin with Toca 511 altered the electrophoresis pattern of the Gag polyprotein and/or the envelope protein. For example, both deglycosidase and trypsin treatment resulted in a change in Gag polyprotein intensity and a decrease in the molecular size and/or disappearance of the gp70 band. We treated Toca 511 with deglycosidases, PLC, or trypsin and coincubated it with heat-treated Toca 511 prior to exposure to pDCs in culture. Our data revealed that coincubation of trypsin-treated, but not deglycosidase- or PLC-treated, Toca 511 with heat-treated Toca 511 abrogated the dominant immunosuppressive activity of Toca 511 and partially induced IFN-α production in pDCs (Fig. 4G).
Together, the data indicate that there is an active immunosuppressive component associated with Toca 511 viral particles and that heat or trypsin treatment of Toca 511 viral particles abrogates the immunosuppressive function, which leads to production of IFN-α in pDCs. The trypsin data further strengthen the notion that the immunosuppressive component is a protein component. The data also indicate that productive infection is not required for activation of cellular innate responses in pDCs.
The immunosuppressive component associated with Toca 511 is not due to the presence of the glyco-Gag or the amphotropic envelope glycoprotein.
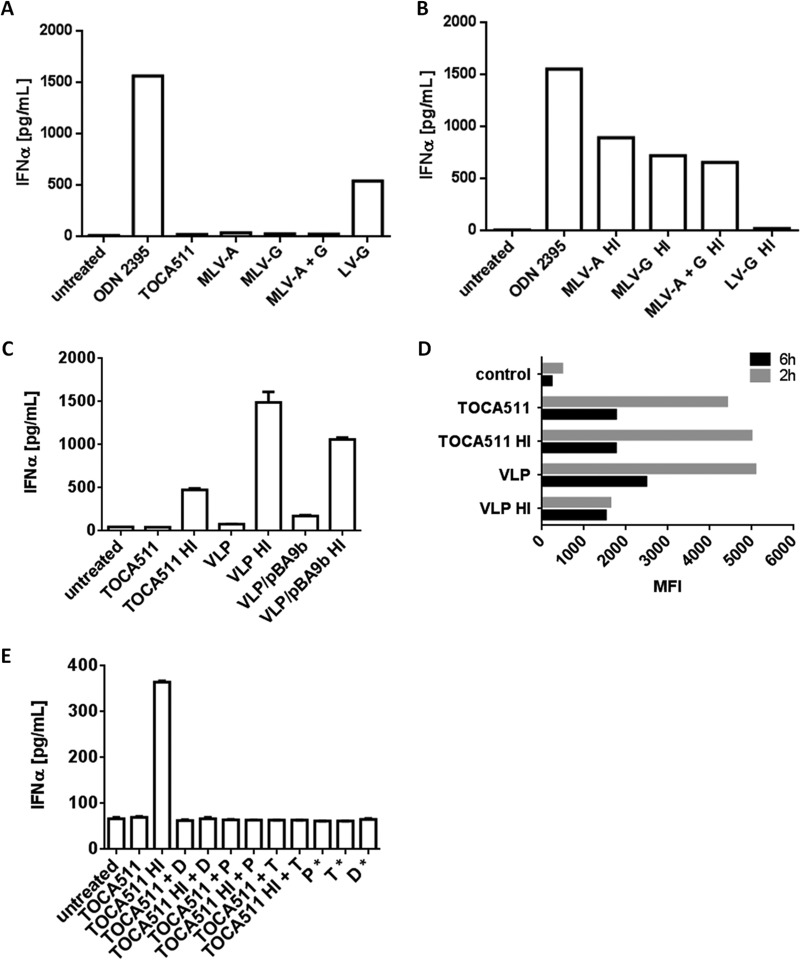
The glyco-Gag (Pr80gag) expressed by retroviruses has shown to be incorporated into viral particles (29, 30) and plays a role in inhibiting the function of APOBEC3 in target cells (31). In addition, an immunosuppressive peptide located in the TM subunit of several MLV envelope proteins has been described (32, 33). To ask where the immunosuppressive component resides in the viral particles, 3 retroviral nonreplicating vectors pseudotyped with the amphotropic (MLV-A), VSV-G glycoprotein (MLV-G), or amphotropic envelope plus VSV-G glycoproteins (MLV-A+G) that do not express glyco-Gag were generated. The viral RNA (pBA9b) used to generate these vectors shares 100% sequence homology with Toca 511 in the psi region, in which several GU-rich sequence motifs are present (Fig. 5). Consistent with previous data, none of the 3 vectors stimulated pDCs to produce IFN-α (Fig. 6A). However, when these vectors were subjected to heat treatment, all 3 vectors stimulated pDCs to produce IFN-α (Fig. 6B).
FIG 6.
Immunosuppressive and immune-stimulating component(s) associated with Toca 511 is independent of the amphotropic envelope glycoprotein and viral nucleic acid. (A) IFN-α production of pDCs stimulated with nonreplicating retroviral vectors pseudotyped with amphotropic envelope protein (MLV-A), VSV-G (MLV-G), or both (MLV-A+G) at an MOI of 20. (B) IFN-α production of pDCs stimulated with heat-treated retroviral nonreplicating vectors pseudotyped with amphotropic envelope protein (MLV-A), VSV-G (MLV-G), or both (MLV-A+G) at an MOI of 20. ODN2395 and lentiviral vector pseudotyped with VSV-G at an MOI of 20 were included as positive controls. (C) Heat-treated VLP and VLP/pBA9b induced IFN-α production in pDCs. (D) CFSE-labeled Toca 511 and VLP were added to pDCs at 37°C to allow for internalization. Cells were harvested at 2 h and 6 h postincubation. The control represents cells incubated with dialyzed dye alone to assess background fluorescence. (E) IFN-α production of pDCs stimulated with heat- or non-heat-treated Toca 511, with and without enzyme digestion. The asterisk indicates coincubation of pDCs with enzyme alone.
To further examine whether the envelope glycoprotein could be part of the immunosuppressive component, we generated nonenveloped VLPs packaged with pBA9b (VLP/pBA9b) or without pBA9b vector (VLPs). Consistent with the observed data, VLPs and VLP/pBA9b did not induce IFN-α production in pDCs, whereas heat-treated VLPs and VLP/pBA9b did (Fig. 6C). Collectively, the data indicated that an immunosuppressive component associated with MLV-based viral particles blocks type I IFN production in pDCs and that the function of the immunosuppressive component associated with viral particles is a dominant active process. In addition, the inability of the MLV-A, MLV-G, and MLV-A+G vectors to stimulate IFN-α production in pDCs in the absence of glyco-Gag suggests that glyco-Gag is not part of the immunosuppressive component. Furthermore, the immunosuppressive function is likely not attributable to the amphotropic enveloped glycoprotein. Finally, the ability of VLPs without pBA9b viral RNA and after heat treatment to induce IFN-α production in pDCs also suggests that there is possibly another PAMP, in addition to the viral RNA, present in the viral particles.
Internalization of Toca 511 and VLPs by pDCs.
The ability of VLPs carrying or lacking the pBA9b viral RNA to induce IFN-α production in pDCs suggests that the PRR may be located on the cell surface as VLPs are not expected to be internalized efficiently in the absence of envelope protein. We examined the capacity of freshly isolated immature pDCs to internalize Toca 511 and VLPs lacking the pBA9b viral RNA by using CFSE-labeled Toca 511 and VLPs. Figure 6D shows that both Toca 511 and VLPs (heat or non-heat treated) were rapidly internalized by pDC, as measured by the mean fluorescent intensity (MFI), suggesting that the internalization process in pDCs is independent of the Pit-2 receptor. Nevertheless, internalization of heat-treated VLPs was always less efficient. Interestingly, a decrease in the MFI was observed over a course of 6 h postincubation with the exception of heat-treated VLPs. One possible explanation is viral protein turnover after internalization. Given that both Toca 511 and VLPs can be internalized by pDCs, the cellular compartment of the unidentified PRR is not limited to the cell surface.
Toca 511-associated nonviral nucleic acid PAMP is sensitive to treatment with deglycosidases, trypsin, or PLC.
To further characterize the unidentified nonviral nucleic acid PAMP associated with Toca 511, we asked whether it is sensitive to enzyme treatment that can alter the protein components, but not the nucleic acid components, of the viral particles. We examined the effects of deglycosidases, PLC, and trypsin on pDC stimulation. As seen in Fig. 6E, the alterations of the viral proteins shown in Fig. 4F were correlated with the loss of its ability to induce IFN-α production in pDCs after heat treatment. In contrast to the immunosuppressive component associated with Toca 511, which is sensitive only to trypsin, the immune-stimulating component associated with Toca 511 viral particles is sensitive to deglycosidases, PLC, and trypsin. All together, the data suggest that both the immune-stimulating and immune-suppressive components are likely structural elements and, therefore, viral protein based, but in the case of the immune-stimulating component, also involve lipid and/or carbohydrate components.
DISCUSSION
In this study, we used human tumor cells, including glioma, fibroblast, and endothelial cells and pDCs that resemble part of the tumor microenvironment to investigate the interplay between MLV-based RRVs and the type I IFN-dependent antiviral response in tumor cells. We found that RRVs are sensitive to exogenous IFN-α and -β and that the lower levels of responsiveness to gene induction observed in HT1080 and U87-MG cells are consistent with the concept that tumor cells are often defective for steps in the type I IFN pathway. In addition, we showed that MLV-based RRVs did not elicit a type I IFN-mediated antiviral response upon viral infection of tumor cells, nontransformed fibroblasts, or primary endothelial cells; exposure of human pDCs to RRV also did not lead to IFN-α production. In addition, we showed that the lack of an IFN-mediated antiviral response was not due to the inability of the cells to produce type I IFN.
Although type I IFNs have been implicated as important factors for antiviral immunity in the early stages of infection with the Friend strain (FV) of MLV (34), recent papers pointed to the role of TLR7 and Myd88 in mediating the antibody response against retrovirus infection in mice and suggested that activation of the innate response via type I IFN may be dispensable in resistant mouse strains due to their robust and long-lasting antibody responses (35, 36). Thus, it is reasonable to speculate that MLV-based retroviral infection may favor a Th2-biased immune response, as previously described for certain strains and isolates of respiratory syncytial virus and measles virus (37). Presumably, humans may function similarly to resistant mouse strains, so that the type I IFN-α/β response is dispensable and that a high baseline level of APOBEC3G in resting immune cells controls the initial viral replication, followed by a robust antibody response (20).
Despite the current understanding of the functional roles of Myd88 and TLR7 in anti-MLV responses and the implication that retroviral RNA interacts with TLR7 in an endosomal compartment post-viral entry, we did not observe activation of TLR7 in pDCs by replicating or nonreplicating vectors with amphotropic, VSV-G, or both envelope glycoproteins unless the viral particles had been heat treated prior to exposure to pDCs. Intriguingly, the ability of VLPs in the absence of the retroviral RNA to induce IFN-α production in pDCs suggests that there is another PAMP, in addition to the retroviral RNA, present in Toca 511. Furthermore, the experiments using MLV-based retroviral nonreplicating vectors pseudotyped with amphotropic and VSV-G envelope glycoproteins as well as VLPs led us to identify an additional immunosuppressive component, other than the amphotropic envelope glycoprotein, that is associated with RRVs. Thus, it is reasonable to conclude that there is an additional PAMP other than viral RNA and an immunosuppressive component associated with MLV-based viral particles. Furthermore, exposure of heat-treated MLV-based viral particles concurrently leads to the exposure of the encrypted PAMP and inactivation of the immunosuppressive component, leading to IFN-α production in pDCs.
It is also important to note that the MLV-based nonreplicating vectors generated in our studies, which were capable of inducing IFN-α production in human pDCs under heat treatment, did not express glyco-Gag. The N-terminal fragment of glyco-gag expressed from an RRV has been shown to be incorporated into MLV viral particles (29, 30) and plays a role in inhibiting the function of APOBEC3 in target cells (31). If this is so, our data suggest that this APOBEC3 inhibition occurs through a mechanism that is distinct from the type I IFN-regulated activity. Furthermore, given that both an HIV-1 vector and VLPs can induce IFN-α production in DCs (presumably mediated via TLR7 and TLR7-independent pathways, respectively), our data showing that Toca 511 can actively inhibit lentiviral vector-induced IFN-α production in pDCs (Fig. 5E) suggest that the inhibition may be through a common downstream component in the TLR7 and TLR7-independent pathways. The mechanism of induction of IFN-α production in human pDCs by heat-treated MLV-based viral particles was not further investigated here.
As retroviral particles made from different cell types are known to incorporate different cellular proteins (38), our data suggest that the immunosuppressive and immune-stimulatory component are likely due to viral constituents. This hypothesis is further supported by the results with vectors produced from a canine producer cell line that had similar properties to vectors produced from human cell lines. Further support for viral constituents being the active components for immune suppression comes from our results showing that coincubation of amphotropic envelope protein-associated microvesicles produced by transient transfection in 293T and heat-treated VLPs lacking the viral RNA did not suppress the induction of IFN-α production in pDCs (data not shown). However, it is possible that different host proteins in viral particles produced from different cells can mediate this blockade. Additional experiments are needed to completely rule out this possibility. The source of the immunosuppressive component associated with viral particles was not further defined in these experiments.
A common hallmark among malignant cells is a reduced responsiveness to IFN due to defects in the type I IFN pathway. Thus, tumor-derived cell lines are thought to be more vulnerable to virus infection. In contrast to VSV (21), RRV-GFP replicates efficiently in a panel of tumor cell lines, including PC3 cells (which we used routinely to determine titers of RRVs), and nontransformed fibroblasts and endothelial cells. In addition, we found that the RRV-GFP did not replicate more efficiently in IFN-α/β-resistant cells than the parental cells in the absence of IFN-α/β (data not shown). Thus, more extreme defects in type I IFN signaling in host cells are not necessary for productive infection with MLV-based RRVs in these in vitro systems, but defects in IFN signaling may play a permissive role for tumors in vivo. Also, it is possible that the active suppression of IFN induction observed in human cells via an unknown mechanism also occurs in the fibroblasts and endothelial cells. Of note, recently discovered cytosolic nucleic acid sensors, including HMGBs, TREX1, cGAS, and DHX9/36, involved in the cellular innate immune response may also play a role in this blockade (39–42).
In summary, we did not observe an induction of a type I IFN response by amphotropic MLV-based RRV in human tumor cells, nontransformed fibroblasts, primary endothelial cells, or pDCs. Our findings corroborate that cell proliferation is an important determinant in supporting RRV replication and, unlike VSV and other oncolytic viruses and lentiviral vectors, RRVs do not induce cellular innate immune activity in cell cultures. Recent findings by Agudo et al. demonstrated that lentiviral vectors can activate the innate response in the liver via a TLR-independent pathway (43). It is still likely that there is an in vivo mechanism of type I IFN induction, other than through the cells studied here, that suppresses viral replication in normal tissue and yet allows for the tumor-specific replication observed in some mouse tumor models and in dogs and humans with gliomas. In fact, recent results from our laboratory in mouse models suggest that the site of the tumor plays a role in whether or not there is in vivo induction of type I IFN to control viral replication (D. Ostertag et al., submitted for publication). In addition, many mechanisms have been described for tumors to create immune privilege, such as downregulation of adaptive immunity, that could contribute to selective replication in tumors (44). Although the limitations of our in vitro system need to be kept in mind, these findings both demonstrate the qualitative difference in interferon responses to RRVs compared to responses to other common viral vectors and are consistent with the observed efficient tumor-specific spread and efficacy of RRVs in orthotopic glioma mouse models.
ACKNOWLEDGMENTS
We thank Hung Fan, John Coffin, Joseph Sodroski, Nicholas A. Boyle, and Alessandro Lobbia for critical readings of the manuscript.
The work was supported by Accelerate Brain Cancer Cure, the American Brain Tumor Association, the Musella Foundation, the National Brain Tumor Society, and Voices Against Brain Cancer.
Footnotes
Published ahead of print 25 June 2014
REFERENCES
- 1.Prestwich RJ, Errington F, Diaz RM, Pandha HS, Harrington KJ, Melcher AA, Vile RG. 2009. The case of oncolytic viruses versus the immune system: waiting on the judgment of Solomon. Hum. Gene Ther. 20:1119–1132. 10.1089/hum.2009.135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ostertag D, Amundson KK, Lopez Espinoza F, Martin B, Buckley T, Galvao da Silva AP, Lin AH, Valenta DT, Perez OD, Ibanez CE, Chen CI, Pettersson PL, Burnett R, Daublebsky V, Hlavaty J, Gunzburg W, Kasahara N, Gruber HE, Jolly DJ, Robbins JM. 2012. Brain tumor eradication and prolonged survival from intratumoral conversion of 5-fluorocytosine to 5-fluorouracil using a nonlytic retroviral replicating vector. Neuro Oncol. 14:145–159. 10.1093/neuonc/nor199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang W, Tai CK, Kershaw AD, Solly SK, Klatzmann D, Kasahara N, Chen TC. 2006. Use of replication-competent retroviral vectors in an immunocompetent intracranial glioma model. Neurosurg. Focus 20:E25. 10.3171/foc.2006.20.4.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cornetta K, Moen RC, Culver K, Morgan RA, McLachlin JR, Sturm S, Selegue J, London W, Blaese RM, Anderson WF. 1990. Amphotropic murine leukemia retrovirus is not an acute pathogen for primates. Hum. Gene Ther. 1:15–30. 10.1089/hum.1990.1.1-15 [DOI] [PubMed] [Google Scholar]
- 5.Perez OD, Logg CR, Hiraoka K, Diago O, Burnett R, Inagaki A, Jolson D, Amundson K, Buckley T, Lohse D, Lin A, Burrascano C, Ibanez C, Kasahara N, Gruber HE, Jolly DJ. 2012. Design and selection of Toca 511 for clinical use: modified retroviral replicating vector with improved stability and gene expression. Mol. Ther. 20:1689–1698. 10.1038/mt.2012.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamashita M, Emerman M. 2006. Retroviral infection of non-dividing cells: old and new perspectives. Virology 344:88–93. 10.1016/j.virol.2005.09.012 [DOI] [PubMed] [Google Scholar]
- 7.Stojdl DF, Lichty BD, ten Oever BR, Paterson JM, Power AT, Knowles S, Marius R, Reynard J, Poliquin L, Atkins H, Brown EG, Durbin RK, Durbin JE, Hiscott J, Bell JC. 2003. VSV strains with defects in their ability to shutdown innate immunity are potent systemic anti-cancer agents. Cancer Cell 4:263–275. 10.1016/S1535-6108(03)00241-1 [DOI] [PubMed] [Google Scholar]
- 8.Janeway CA, Jr, Medzhitov R. 2002. Innate immune recognition. Annu. Rev. Immunol. 20:197–216. 10.1146/annurev.immunol.20.083001.084359 [DOI] [PubMed] [Google Scholar]
- 9.Wolf D, Goff SP. 2008. Host restriction factors blocking retroviral replication. Annu. Rev. Genet. 42:143–163. 10.1146/annurev.genet.42.110807.091704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakhaei P, Hiscott J, Lin R. 2010. STING-ing the antiviral pathway. J. Mol. Cell Biol. 2:110–112. 10.1093/jmcb/mjp048 [DOI] [PubMed] [Google Scholar]
- 11.Randall RE, Goodbourn S. 2008. Interferons and viruses: an interplay between induction, signalling, antiviral responses and virus countermeasures. J. Gen. Virol. 89:1–47. 10.1099/vir.0.83391-0 [DOI] [PubMed] [Google Scholar]
- 12.Cerami E, Demir E, Schultz N, Taylor BS, Sander C. 2010. Automated network analysis identifies core pathways in glioblastoma. PLoS One 5:e8918. 10.1371/journal.pone.0008918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marozin S, Altomonte J, Stadler F, Thasler WE, Schmid RM, Ebert O. 2008. Inhibition of the IFN-beta response in hepatocellular carcinoma by alternative spliced isoform of IFN regulatory factor-3. Mol. Ther. 16:1789–1797. 10.1038/mt.2008.201 [DOI] [PubMed] [Google Scholar]
- 14.Wong LH, Krauer KG, Hatzinisiriou I, Estcourt MJ, Hersey P, Tam ND, Edmondson S, Devenish RJ, Ralph SJ. 1997. Interferon-resistant human melanoma cells are deficient in ISGF3 components, STAT1, STAT2, and p48-ISGF3γ. J. Biol. Chem. 272:28779–28785. 10.1074/jbc.272.45.28779 [DOI] [PubMed] [Google Scholar]
- 15.Burns JC, Friedmann T, Driever W, Burrascano M, Yee JK. 1993. Vesicular stomatitis virus G glycoprotein pseudotyped retroviral vectors: concentration to very high titer and efficient gene transfer into mammalian and nonmammalian cells. Proc. Natl. Acad. Sci. U. S. A. 90:8033–8037. 10.1073/pnas.90.17.8033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sheridan PL, Bodner M, Lynn A, Phuong TK, DePolo NJ, de la Vega DJ, Jr, O'Dea J, Nguyen K, McCormack JE, Driver DA, Townsend K, Ibanez CE, Sajjadi NC, Greengard JS, Moore MD, Respess J, Chang SM, Dubensky TW, Jr, Jolly DJ, Sauter SL. 2000. Generation of retroviral packaging and producer cell lines for large-scale vector production and clinical application: improved safety and high titer. Mol. Ther. 2:262–275. 10.1006/mthe.2000.0123 [DOI] [PubMed] [Google Scholar]
- 17.Andreadis ST, Brott D, Fuller AO, Palsson BO. 1997. Moloney murine leukemia virus-derived retroviral vectors decay intracellularly with a half-life in the range of 5.5 to 7.5 hours. J. Virol. 71:7541–7548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dull T, Zufferey R, Kelly M, Mandel RJ, Nguyen M, Trono D, Naldini L. 1998. A third-generation lentivirus vector with a conditional packaging system. J. Virol. 72:8463–8471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rulli SJ, Jr, Mirro J, Hill SA, Lloyd P, Gorelick RJ, Coffin JM, Derse D, Rein A. 2008. Interactions of murine APOBEC3 and human APOBEC3G with murine leukemia viruses. J. Virol. 82:6566–6575. 10.1128/JVI.01357-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Santiago ML, Montano M, Benitez R, Messer RJ, Yonemoto W, Chesebro B, Hasenkrug KJ, Greene WC. 2008. Apobec3 encodes Rfv3, a gene influencing neutralizing antibody control of retrovirus infection. Science 321:1343–1346. 10.1126/science.1161121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Noser JA, Mael AA, Sakuma R, Ohmine S, Marcato P, Lee PW, Ikeda Y. 2007. The RAS/Raf1/MEK/ERK signaling pathway facilitates VSV-mediated oncolysis: implication for the defective interferon response in cancer cells. Mol. Ther. 15:1531–1536. 10.1038/sj.mt.6300193 [DOI] [PubMed] [Google Scholar]
- 22.da Silva AJ, Brickelmaier M, Majeau GR, Lukashin AV, Peyman J, Whitty A, Hochman PS. 2002. Comparison of gene expression patterns induced by treatment of human umbilical vein endothelial cells with IFN-alpha 2b vs. IFN-beta 1a: understanding the functional relationship between distinct type I interferons that act through a common receptor. J. Interferon Cytokine Res. 22:173–188. 10.1089/107999002753536149 [DOI] [PubMed] [Google Scholar]
- 23.Pomykala HM, Bohlander SK, Broeker PL, Olopade OI, Diaz MO. 1994. Breakpoint junctions of chromosome 9p deletions in two human glioma cell lines. Mol. Cell. Biol. 14:7604–7610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raj NB, Pitha PM. 1981. Analysis of interferon mRNA in human fibroblast cells induced to produce interferon. Proc. Natl. Acad. Sci. U. S. A. 78:7426–7430. 10.1073/pnas.78.12.7426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kadowaki N, Ho S, Antonenko S, Malefyt RW, Kastelein RA, Bazan F, Liu YJ. 2001. Subsets of human dendritic cell precursors express different toll-like receptors and respond to different microbial antigens. J. Exp. Med. 194:863–869. 10.1084/jem.194.6.863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brown BD, Sitia G, Annoni A, Hauben E, Sergi LS, Zingale A, Roncarolo MG, Guidotti LG, Naldini L. 2007. In vivo administration of lentiviral vectors triggers a type I interferon response that restricts hepatocyte gene transfer and promotes vector clearance. Blood 109:2797–2805. 10.1182/blood-2006-10-049312 [DOI] [PubMed] [Google Scholar]
- 27.Abe A, Chen ST, Miyanohara A, Friedmann T. 1998. In vitro cell-free conversion of noninfectious Moloney retrovirus particles to an infectious form by the addition of the vesicular stomatitis virus surrogate envelope G protein. J. Virol. 72:6356–6361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Diebold SS, Kaisho T, Hemmi H, Akira S, Reis e Sousa C. 2004. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science 303:1529–1531. 10.1126/science.1093616 [DOI] [PubMed] [Google Scholar]
- 29.Edwards SA, Fan H. 1979. gag-Related polyproteins of Moloney murine leukemia virus: evidence for independent synthesis of glycosylated and unglycosylated forms. J. Virol. 30:551–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fujisawa R, McAtee FJ, Favara C, Hayes SF, Portis JL. 2001. N-terminal cleavage fragment of glycosylated Gag is incorporated into murine oncornavirus particles. J. Virol. 75:11239–11243. 10.1128/JVI.75.22.11239-11243.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stavrou S, Nitta T, Kotla S, Ha D, Nagashima K, Rein AR, Fan H, Ross SR. 2013. Murine leukemia virus glycosylated Gag blocks apolipoprotein B editing complex 3 and cytosolic sensor access to the reverse transcription complex. Proc. Natl. Acad. Sci. U. S. A. 110:9078–9083. 10.1073/pnas.1217399110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mangeney M, Heidmann T. 1998. Tumor cells expressing a retroviral envelope escape immune rejection in vivo. Proc. Natl. Acad. Sci. U. S. A. 95:14920–14925. 10.1073/pnas.95.25.14920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmidt DM, Sidhu NK, Cianciolo GJ, Snyderman R. 1987. Recombinant hydrophilic region of murine retroviral protein p15E inhibits stimulated T-lymphocyte proliferation. Proc. Natl. Acad. Sci. U. S. A. 84:7290–7294. 10.1073/pnas.84.20.7290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gerlach N, Schimmer S, Weiss S, Kalinke U, Dittmer U. 2006. Effects of type I interferons on Friend retrovirus infection. J. Virol. 80:3438–3444. 10.1128/JVI.80.7.3438-3444.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Browne EP. 2011. Toll-like receptor 7 controls the anti-retroviral germinal center response. PLoS Pathog. 7:e1002293. 10.1371/journal.ppat.1002293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kane M, Case LK, Wang C, Yurkovetskiy L, Dikiy S, Golovkina TV. 2011. Innate immune sensing of retroviral infection via Toll-like receptor 7 occurs upon viral entry. Immunity 35:135–145. 10.1016/j.immuni.2011.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schlender J, Hornung V, Finke S, Gunthner-Biller M, Marozin S, Brzozka K, Moghim S, Endres S, Hartmann G, Conzelmann KK. 2005. Inhibition of toll-like receptor 7- and 9-mediated alpha/beta interferon production in human plasmacytoid dendritic cells by respiratory syncytial virus and measles virus. J. Virol. 79:5507–5515. 10.1128/JVI.79.9.5507-5515.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Santos S, Obukhov Y, Nekhai S, Bukrinsky M, Iordanskiy S. 2012. Virus-producing cells determine the host protein profiles of HIV-1 virion cores. Retrovirology 9:65. 10.1186/1742-4690-9-65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gao D, Wu J, Wu YT, Du F, Aroh C, Yan N, Sun L, Chen ZJ. 2013. Cyclic GMP-AMP synthase is an innate immune sensor of HIV and other retroviruses. Science 341:903–906. 10.1126/science.1240933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim T, Pazhoor S, Bao M, Zhang Z, Hanabuchi S, Facchinetti V, Bover L, Plumas J, Chaperot L, Qin J, Liu YJ. 2010. Aspartate-glutamate-alanine-histidine box motif (DEAH)/RNA helicase A helicases sense microbial DNA in human plasmacytoid dendritic cells. Proc. Natl. Acad. Sci. U. S. A. 107:15181–15186. 10.1073/pnas.1006539107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yan N, Regalado-Magdos AD, Stiggelbout B, Lee-Kirsch MA, Lieberman J. 2010. The cytosolic exonuclease TREX1 inhibits the innate immune response to human immunodeficiency virus type 1. Nat. Immunol. 11:1005–1013. 10.1038/ni.1941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yanai H, Ban T, Wang Z, Choi MK, Kawamura T, Negishi H, Nakasato M, Lu Y, Hangai S, Koshiba R, Savitsky D, Ronfani L, Akira S, Bianchi ME, Honda K, Tamura T, Kodama T, Taniguchi T. 2009. HMGB proteins function as universal sentinels for nucleic-acid-mediated innate immune responses. Nature 462:99–103. 10.1038/nature08512 [DOI] [PubMed] [Google Scholar]
- 43.Agudo J, Ruzo A, Kitur K, Sachidanandam R, Blander JM, Brown BD. 2012. A TLR and non-TLR mediated innate response to lentiviruses restricts hepatocyte entry and can be ameliorated by pharmacological blockade. Mol. Ther. 20:2257–2267. 10.1038/mt.2012.150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shanker A, Marincola FM. 2011. Cooperativity of adaptive and innate immunity: implications for cancer therapy. Cancer Immunol. Immunother. 60:1061–1074. 10.1007/s00262-011-1053-z [DOI] [PMC free article] [PubMed] [Google Scholar]