ABSTRACT
It remains a challenge to develop a successful human immunodeficiency virus (HIV) vaccine that is capable of preventing infection. Here, we utilized the benefits of CD40L, a costimulatory molecule that can stimulate both dendritic cells (DCs) and B cells, as an adjuvant for our simian immunodeficiency virus (SIV) DNA vaccine in rhesus macaques. We coexpressed the CD40L with our DNA/SIV vaccine such that the CD40L is anchored on the membrane of SIV virus-like particle (VLP). These CD40L containing SIV VLPs showed enhanced activation of DCs in vitro. We then tested the potential of DNA/SIV-CD40L vaccine to adjuvant the DNA prime of a DNA/modified vaccinia virus Ankara (MVA) vaccine in rhesus macaques. Our results demonstrated that the CD40L adjuvant enhanced the functional quality of anti-Env antibody response and breadth of anti-SIV CD8 and CD4 T cell responses, significantly delayed the acquisition of heterologous mucosal SIV infection, and improved viral control. Notably, the CD40L adjuvant enhanced the control of viral replication in the gut at the site of challenge that was associated with lower mucosal CD8 immune activation, one of the strong predictors of disease progression. Collectively, our results highlight the benefits of CD40L adjuvant for enhancing antiviral humoral and cellular immunity, leading to enhanced protection against a pathogenic SIV. A single adjuvant that enhances both humoral and cellular immunity is rare and thus underlines the importance and practicality of CD40L as an adjuvant for vaccines against infectious diseases, including HIV-1.
IMPORTANCE Despite many advances in the field of AIDS research, an effective AIDS vaccine that can prevent infection remains elusive. CD40L is a key stimulator of dendritic cells and B cells and can therefore enhance T cell and antibody responses, but its overly potent nature can lead to adverse effects unless used in small doses. In order to modulate local expression of CD40L at relatively lower levels, we expressed CD40L in a membrane-bound form, along with SIV antigens, in a nucleic acid (DNA) vector. We tested the immunogenicity and efficacy of the CD40L-adjuvanted vaccine in macaques using a heterologous mucosal SIV infection. The CD40L-adjuvanted vaccine enhanced the functional quality of anti-Env antibody response and breadth of anti-SIV T cell responses and improved protection. These results demonstrate that VLP-membrane-bound CD40L serves as a novel adjuvant for an HIV vaccine.
INTRODUCTION
Novel vaccine approaches that elicit strong humoral and cellular immunity with high functional quality will aid HIV vaccine development. Here, we utilized the benefits of CD40L, a costimulatory molecule belonging to the tumor necrosis factor superfamily (TNFSF), that can stimulate both dendritic cells (DCs) and B cells for enhancing T cell and antibody (Ab) responses (1). CD40L activates DCs and enhances the priming of the cytotoxic CD8 T cell response (2–4). CD40L also enhances the survival and differentiation of activated B cells, leading to increased germinal center (GC) formation, immunoglobulin isotype switching, antibody somatic affinity maturation, and the generation of long-lived plasma cells (5). These immunostimulatory functions of CD40L have made it an attractive vaccine adjuvant (6–12).
Despite the intense interest in CD40L as a key activator of immune responses, there are currently no clinically accepted means for applying CD40L in vivo. Several studies have used agonistic anti-CD40 antibodies to activate CD40-bearing cells to enhance CD8 T-cell responses (3, 4) and antitumor responses (13–15). Agonistic anti-CD40 antibodies also act as a general vaccine adjuvant (16) and synergize with Toll-like receptor agonists to augment vaccine-induced CD8 T-cell responses (17). However, these antibodies induce splenomegaly and shock-like symptoms unless used in small amounts in a single administration (15, 16), which raises concerns about using systemic agonistic anti-CD40 antibodies in humans. Modulated local expression of CD40L at relatively lower levels by DNA or replication-defective viral vaccines could provide a safe alternative to overly potent systemic stimulation by agonistic anti-CD40 Ab or high doses of CD40L protein.
TNFSF proteins are produced as type II trimeric membrane proteins but are proteolytically cleaved from the cell surface to form soluble trimers. There is growing evidence demonstrating the need for multimerization of the trimeric ligands for their activation/adjuvant potential (18–20). Multimerization can be achieved by the membrane-bound form (upon ligation with CD40) or the soluble form fused with multimerization domains of proteins from C1q superfamily molecules (21, 22) and collectin superfamily molecules (23–27) that spontaneously multimerize into molecular structures with extended, trimeric, collagen-like arms. Indeed, a recent study demonstrated that HIV-1 Gag DNA vaccine expressing the 4-trimer form of CD40L (extracellular domain of mouse CD40L fused to the multimerization domain of pulmonary surfactant protein D) is more immunogenic than the soluble 1-trimer form in mice (28).
Many studies have used CD40L DNA expressing the membrane-bound form of CD40L to adjuvant DNA vaccines (6–12). In these studies, the adjuvant effects were strongly associated with the form of antigen. Adjuvant effects were consistently observed if the antigen was located in the cytoplasm (6, 10) or on the cell membrane (7, 8). However, adjuvant effects were not observed if the antigen was secreted (28–30). This becomes a limitation for HIV vaccines expressing virus-like particles (VLPs) and secreted forms of Env (important for high levels of Ab production). To circumvent this problem, we designed our CD40L-adjuvanted DNA/SIV vaccine to coexpress macaque CD40L with the native trimeric form of SIV Env on the membrane of the transfected cell/VLP to promote multimerization of the ligand that is critical for its adjuvant activity (28). In the present study, we evaluated the adjuvant potential of CD40L in the DNA prime of a DNA/MVA SIV vaccine. Our results show that CD40L enhances both humoral and cellular immunity and that these responses are associated with protection against a heterologous mucosal SIVE660 challenge.
MATERIALS AND METHODS
Immunizations and challenge.
Young adult Indian rhesus macaques from the Yerkes breeding colony were cared for under guidelines established by the Animal Welfare Act and the National Institutes of Health (NIH; Bethesda, MD) Guide for the Care and Use of Laboratory Animals using protocols approved by the Emory University (Atlanta, GA) Institutional Animal Care and Use Committee. Macaques were typed for the Mamu-A*01, Mamu-B*08, and Mamu-B*17 alleles as described previously (31–33). Of the 35 macaques, eight were vaccinated with a DNA/MVA SIV vaccine, 12 (six Mamu-A*01 positive, six Mamu-A*01 negative) were vaccinated with the DNA/MVA SIV vaccine with CD40L in the DNA, and 15 were unvaccinated controls. The DNA and rMVA immunizations were delivered intramuscularly in phosphate-buffered saline (PBS) using a hypodermic needle in the outer thigh. The DNA immunogen expressed SIV239 Gag-Pol, Env, Tat, and Rev. The DNA immunogen was constructed by replacing the EcoRI-NheI fragment of the SHIV DNA construct (34) containing HIV-1 89.6 tat, rev, and env genes with an EcoRI-NheI fragment containing SIV tat, rev, and env. Two MVA recombinants, one expressing SIV239 Gag-Pol (35) and the other expressing SIV239 Env (36), were premixed and used for immunizations. Two DNA inoculations were given at weeks 0 and 8, and two rMVA boosters were given at weeks 16 and 24. The DNA was delivered at 3 mg/dose, and the rMVA was delivered at 108 PFU/dose. At 20 to 24 weeks after the final rMVA booster, animals were challenged with weekly doses of SIVE660 intrarectally using a pediatric feeding tube 15 to 20 cm into the rectum. Vanessa Hirsch at the NIH provided the challenge stock (Hirsch-2000 stock).
Collection and processing of rectal secretions, biopsy specimens, and blood.
Rectal secretions were collected with and eluted from Weck-Cel sponges as previously described (34, 37). Peripheral blood mononuclear cells (PBMC) were isolated from whole blood according to standard procedures as described previously (38). Lymphocytes from pinch biopsy specimens from the rectum were obtained as described previously (39). Briefly, 10 to 20 pinch biopsy specimens were collected in complete RPMI 1640 and washed twice with ice-cold Hanks balanced salt solution. Biopsy specimens were digested with 200 U of collagenase IV (Worthington, Lakewood, NJ) and DNase I (Roche, Indianapolis, IN)/ml, passed through decreasing sizes of needles (16-, 18-, and 20-gauge, five to six times with each needle), and filtered through a 100-μm-pore-size filter. Cells were washed twice with RPMI and resuspended in complete RPMI for analysis.
T cell responses.
Intracellular cytokine production was assessed as previously described with a few modifications (2). Briefly, 2 million PBMC were stimulated in 200 μl of RPMI with 10% fetal bovine serum (FBS) in a 5-ml polypropylene tube. SIV-specific stimulations were conducted using a single pool of 125 SIV239 Gag peptides and two pools of 225 SIV239 Env peptides (NIH AIDS Research and Reference Reagent Program, Germantown, MD). All peptides were 15-mers overlapping by 11. Staphylococcal enterotoxin B was used as a positive control at 1 μg/ml. Stimulations were performed in the presence of anti-CD28 and anti-CD49d Abs (1 μg/ml; BD Pharmingen, San Diego, CA). For all stimulations, cells were incubated at 37°C in the presence of 5% CO2 for 6 h. Brefeldin A (10 μg/ml) and GolgiStop (1 μg/ml; BD Pharmingen, San Diego, CA) were added for the last 4 h of incubation. At the end of stimulation, cells were washed once with PBS containing 2% FBS, surface stained with anti-human CD4-PerCP (clone L200; BD Pharmingen), and anti-human CD8-AmCyan (clone SK1; BD Biosciences, San Jose, CA), fixed with Cytofix/Cytoperm (BD Pharmingen), and permeabilized with 1× Permwash (BD Pharmingen). The cells were then stained using a mixture of Abs containing anti-human CD3-Pacific blue (clone SP34-2; BD Pharmingen), anti-human gamma interferon (IFN-γ) Alexa 700 (clone B27; BD Pharmingen), anti-human interleukin-2 (IL-2)–allophycocyanin (clone MQ1-17H12; BD Pharmingen), and anti-human tumor necrosis factor alpha- phycoerythrin-Cy7 (TNF-α–PE–Cy7; clone Mab11; eBioscience, San Diego, CA), washed twice with Permwash and once with 2% FBS in PBS, and resuspended in 1% formalin in PBS. Approximately 500,000 lymphocytes were acquired on the LSRII (BD Immunocytometry Systems, San Jose, CA) and analyzed by using FlowJo software (Tree Star, Ashland, OR). Lymphocytes were identified based on their scatter pattern, and CD3+ CD8− CD4+ cells were considered CD4 T cells, and CD3+ CD8+ CD4− cells were considered CD8 T cells. These CD4 or CD8 T cells were then gated for cytokine-positive cells. Responses that were >0.01% of the respective total CD4 or CD8 T cells and twice the background were considered positive.
T cells were subjected to tetramer staining and typing for the presence of CD4 and CD8 T cells. This was done using a mixture of the following Abs and Gag-CM9 tetramer conjugated to allophycocyanin: anti-human CD3-Alexa Fluor 700 (clone SP34-2; BD Pharmingen), anti-human CD4-PerCP (clone L200; BD Pharmingen), anti-human CD8-AmCyan (clone SK1; BD Biosciences), anti-human CD28-PE-Cy7 (clone CD28.2; Beckman Coulter, Brea, CA), and anti-human CD95-Pacific blue (clone DX2; Invitrogen, Carlsbad, CA). The levels of CD4 T cells in intestinal biopsy specimens are presented as a percentage of total CD3+ T cells.
Measurement of binding Ab responses.
SIV Env-specific binding Abs were measured with enzyme-linked immunosorbent assay (ELISA) using tissue culture-produced SIV Env, captured on a concanavalin A (ConA)-coated plate as described previously (34). Briefly, ELISA plates (Costar; Corning Life Sciences, Lowell, MA) were coated with ConA (25 μg/ml) overnight at 4°C. The plates were washed and incubated with 100 μl of Triton X-100-disrupted undiluted 239 VLP supernatant (generated by transient transfection of 293T cells with the earlier-described SIV239 DNA vaccine expressing Gag, Pol, and Env) or with SIVE660 grown in rhesus PBMC for 1 h. Plates were washed and blocked for 1 h (PBS-Tween with 4% whey and 5% dry milk). Test sera was added to duplicate wells in serial 3-fold dilutions and incubated for 1 h. Plates were then washed, and bound Ab was detected using peroxidase-conjugated anti-monkey IgG (Accurate Chemical and Scientific, Westbury, NY) and tetramethylbenzidine substrate (KPL, Gaithersburg, MD). Reactions were stopped with 100 μl of 2 N H2SO4. Each plate included a standard curve generated using goat anti-monkey IgG and rhesus macaque IgG (both from Accurate Chemical and Scientific Corp.) as described previously (34). Standard curves were fitted, and sample concentrations were interpolated as micrograms of Ab per milliliter of serum using SOFTmax 2.3 software (Molecular Devices, Sunnyvale, CA). The concentrations of IgG are relative to our standard curve and not absolute values.
An NaSCN displacement ELISA modeled after that described previously (40) was used for determining avidity. This assay was conducted using parallel titrations of test sera in our standard ELISA. After the binding of the test sera, the parallel titrations were treated for 10 min at room temperature with PBS or 1.5 M NaSCN (prepared fresh in PBS). The relative levels of bound Ab were then determined using a standard ELISA procedure (see above). The avidity index was calculated by dividing the dilution of the serum that gave an optical density (OD) of 0.5 with NaSCN treatment by the dilution of the serum that gave an OD of 0.5 without NaSCN treatment and multiplying by 100. Each assay included one plate with a standard serum with known avidity. Interassay variation in the avidity index for the standard serum was ±3 for an index of 27.
Measurements for total IgA, anti-SIV Env IgA, or anti-SIV Gag and Pol IgA or IgG were done by ELISA using microtiter plates coated, respectively, with 100 μl of a 0.5-μg/ml concentration of goat anti-monkey IgA (Rockland, Gilbertsville, PA), 1 μg/ml of SIVmac251 rgp130 (Immunodiagnostics, Woburn, MA), or 1:400 diluted SIVmac251 viral lysate (Advanced Biotechnologies, Columbia, MD), which lacks detectable envelope protein at this dilution. These ELISAs and the serum standards have been described previously (34). Plates were developed by consecutive treatments with biotinylated goat anti-monkey IgA (Alpha Diagnostics, San Antonio, TX) or biotinylated goat anti-human IgG (Southern Biotech, Birmingham, AL), avidin-peroxidase, tetramethylbenzidine, and 2 N H2SO4. For rectal secretions, the concentration of anti-env or anti-gag,pol IgA was divided by the total IgA concentration to obtain specific IgA activity. Samples were considered IgA Ab-positive if the Env- or Gag- and Pol- specific IgA activity was greater than or equal to 0.145 or 0.224, respectively. These cutoffs represent the mean specific activity + 3 standard deviations previously established for rectal secretions from naive macaques.
Measurement of neutralizing antibody.
SIV-specific neutralization was measured as a function of reductions in luciferase reporter gene expression after a single round of infection in TZM-bl cells as described previously (41). TZM-bl cells were obtained from the NIH AIDS Research and Reference Reagent Program as contributed by John Kappes and Xiaoyun Wu.
Quantitation of SIV RNA plasma load.
The SIV copy number was determined using a quantitative real-time PCR as previously described (2). All specimens were extracted and amplified in duplicate, with the mean results reported. For viral load determinations in gut, total RNA was extracted from about 1 million cells obtained from gut biopsy specimens and used for quantitative real-time PCR analyses.
Statistical analysis.
A Wilcoxon Mann-Whitney U test was used to compare the Ab responses, T cell responses, and viral RNA levels between DNA and DNA-CD40L groups. The P values were given before correcting for any multiple comparisons. A Pearson product moment correlation method was used for correlation analysis when the data met parametric assumptions. The Spearman's rank correlation method was used for nonparametric data correlations (indicated as rs values on the graphs of various figures of this article). A two-sided P value of <0.05 was considered significant. Statistical analyses were performed using TIBCO Spotfire S+ 8.1 (TIBCO, Somerville, MA).
RESULTS
DNA/SIV vaccine expressing membrane-bound CD40L on SIV VLPs.
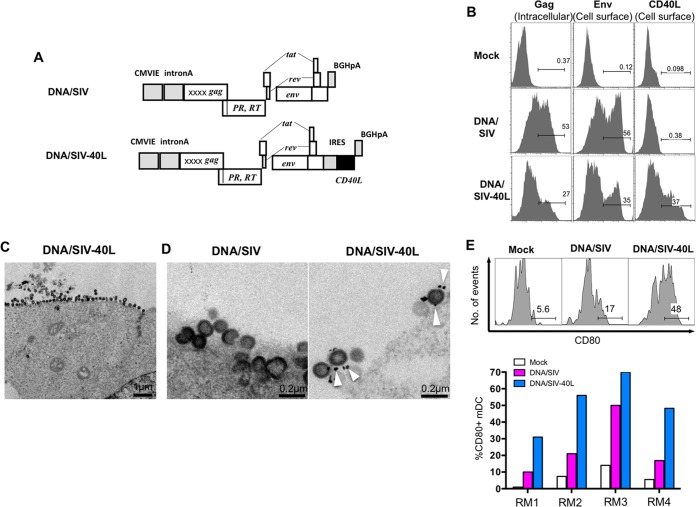
In order to express CD40L in cis along with SIV antigens, we developed the DNA/SIV-40L vaccine by inserting the membrane bound form of a rhesus macaque CD40L gene downstream of the Env gene of our DNA/SIV plasmid (42) that expresses SIV239 Gag-Pol, Env, Tat, and Rev (Fig. 1A). Flow cytometric analyses showed that the SIV Env and CD40L were expressed on the transfected cell membrane and SIV Gag was expressed intracellularly (Fig. 1B). The electron microscopic analyses of vaccine DNAs showed that the transfected cells produced VLPs, and the budding virions in the DNA/SIV-40L transfected cells displayed CD40L (Fig. 1C and D). To determine the biological activity of CD40L, we stimulated PBMC from four rhesus macaques with supernatants obtained from 293T cells that were mock transfected (negative control), DNA/SIV transfected (VLP only control), or DNA/SIV-CD40L transfected (VLP with CD40L) and measured the activation of DCs by testing for surface expression of CD80 (Fig. 1E). Both DNA/SIV and DNA/SIV-40L showed activation of DCs. However, supernatants from DNA/SIV-40L-transfected cells showed a stronger activation of DCs compared to supernatants obtained from DNA/SIV-transfected cells, suggesting that both VLP and CD40L are biologically active in vitro.
FIG 1.
Design and expression of DNA vaccines. (A) Design of DNA vaccines without CD40L (DNA/SIV) and with CD40L (DNA/SIV-40L). The DNA/SIV immunogen expresses SIV239 Gag, Env, protease (PR), reverse transcriptase (RT), rev, and tat, using the cytomegalovirus (CMV) intermediate-early promoter with intron A and stabilized by a bovine growth hormone (BGH) polyadenylation sequence. The DNA/SIV-40L vaccine comprises the same structure as the DNA/SIV vaccine but includes the macaque CD40L sequence as a fusion to an internal ribosome entry site (IRES) inserted downstream of Env. X, safety mutations. (B) Expression of Gag, Env, or CD40L by flow cytometry in 293T cells transfected with DNA/SIV or DNA/SIV-40L plasmids. (C) Electron micrograph of virus-like particles (VLPs) budding from a transfected 293T cell. (D) Immunogold staining for CD40L on VLPs from cells transfected with DNA/SIV or DNA/SIV-40L plasmids. White arrows pointing to dark spots indicate immunogold staining for CD40L. (E) VLPs containing CD40L upregulate CD80 on myeloid DCs (mDC; HLA-DR+, CD11c+, CD123−, CD3−, CD20−, and CD14−). Tissue culture supernatants from 293T cells transfected with DNA/SIV or DNA/SIV-40L plasmids were used for stimulations and cells were stained after 12 h of stimulation. Longer periods (24 to 48 h) of stimulation resulted in significant spontaneous death of DCs, so data for 12 h is shown. RM1 to RM4 represent four rhesus macaques. Mock, supernatants from Lipofectamine-only cultures.
Design of the macaque trial.
Two groups of Indian rhesus macaques (all negative for Mamu-B*08 and -B*17 alleles) were inoculated intramuscularly at weeks 0 and 8 with 3 mg of DNA/SIV (n = 8) or DNA/SIV-40L (n = 12) and then boosted with MVA/SIV at weeks 16 and 24. The MVA immunogen expressed SIV239 Gag, protease (PR), reverse transcriptase (RT), and Env. The DNA/SIV group is referred to here as the “DM” group, and the DNA/SIV-CD40L group is referred to as the “D40LM” group. Six macaques in the CD40L group were positive for Mamu-A*01 allele. A group of 15 SIV-naive macaques served as the control group. Of these 15, 9 were Mamu-A*01 negative, and 6 were Mamu-A*01 positive. All macaques were negative for the Mamu-B*08 and -B*17 haplotypes. Twelve weekly moderate dose intrarectal challenges of SIVE660 (91% related in Gag and 83% related in Env to the SIV239 immunogens) were initiated at 22 to 24 weeks after the final MVA inoculation. Within the CD40L group, all immune analyses except SIV-specific CD8 T cells, were comparable between Mamu-A*01-positive and negative animals and thus we presented the data as a single group. For convenience, we distinguished Mamu-A*01-positive animals (triangles) from the Mamu-A*01-negative animals (circles) with a different symbol in the figures. The immunizations for the DM and D40LM groups were conducted in parallel. Some of the results for the DM animals and the nine Mamu-A*01-negative unvaccinated control animals were described previously (43).
CD40L enhances the magnitude and functional quality of humoral immunity.
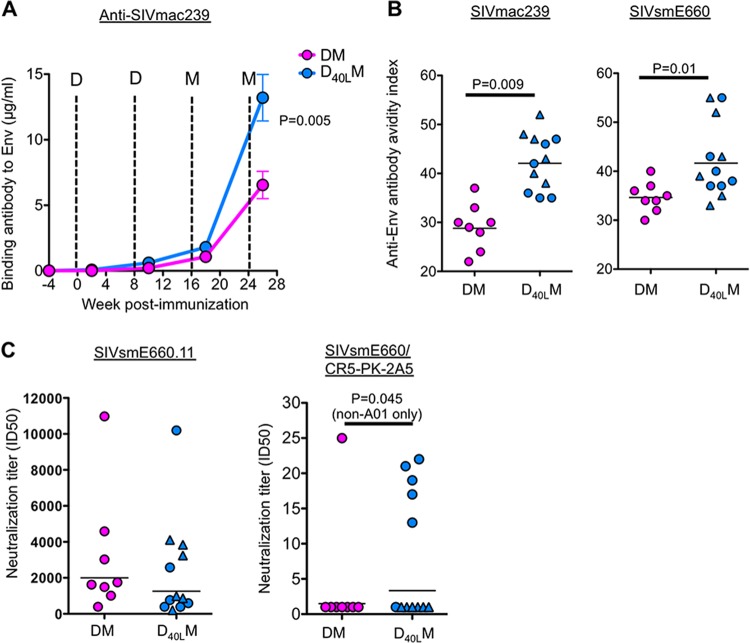
After the two DNA primes, the titer of SIV239 Env-specific binding antibody in serum was low, close to our limit of detection (Fig. 2A). These titers were boosted significantly after the two MVA immunizations. At 2 weeks after the first MVA boost, we saw low but measurable levels of anti-SIV239 Env-specific antibodies in both DM and D40LM groups (Fig. 2A). These binding antibody titers were boosted another 5- to 10-fold by the second MVA, during which the D40LM group displayed 2-fold-higher anti-SIV239 Env antibodies (estimated 13 μg/ml) than in the DM group (estimated 6.5 μg/ml) (P = 0.005). Similarly, the D40LM group tended toward higher anti-SIVE660 Env binding antibodies than the DM group at the same time point (P = 0.057; data not shown).
FIG 2.
Anti-SIV antibody responses postvaccination. (A) Levels of binding antibody against vaccine immunogen SIVmac239 Env postvaccination. (B) Avidity index for full-length Env captured from Triton X-100-disrupted VLPs SIVmac239 or SIVE660 elicited Env-specific IgG at 2 weeks after a second MVA boost. (C) Neutralization titers to tier 1 (SIVE660.11) and tier 2 (SIVE660/CR5-PK-2A5) pseudoviruses. Titers were determined at 2 weeks after a second MVA boost. D, DNA vaccine; M, MVA vaccine.
We checked whether the CD40L-adjuvant improved the functional quality of anti-Env antibody responses because CD40L-CD40 interactions promote germinal center formation and antibody affinity maturation. Using a 1.5 M sodium thiocyanate displacement assay, we measured the avidity of binding antibody specific for SIV239 and SIVE660 Envs. The addition of CD40L enhanced the avidity of SIV239 Env-specific antibody, which was significantly higher in the D40LM group than in the DM group (Fig. 2B). Similarly, the avidity of SIVE660 Env-specific antibody was higher in the D40LM group compared to DM group (Fig. 2B). We also measured the neutralization potential of anti-Env antibody against tier 1 (easy to neutralize) and tier 2 (harder to neutralize) SIVE660 pseudovirus isolates. Both vaccines elicited strong neutralization titers against the tier 1 isolate, and there was no difference between the two groups (Fig. 2C). However, we found that while most animals in the DM group (7 of 8) failed to generate detectable levels of neutralizing antibody titers against the tier 2 isolate (Fig. 2C), in the CD40L group, 5 of 6 Mamu-A*01-negative animals developed measurable levels of neutralizing antibody titers against the tier 2 isolate (Fig. 2C). None of the Mamu-A*01-positive animals (6 of 6) in the CD40L group elicited measurable levels of neutralizing antibody titers against the tier 2 isolate.
CD40L adjuvant enhances the breadth of SIV-specific CD8 T cell immunity.
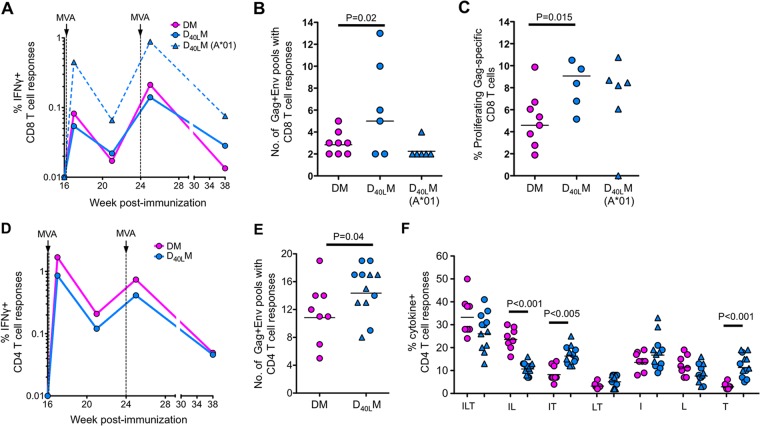
To study the adjuvant effects of CD40L on elicited CD8 and CD4 T cell response, we measured the magnitude, breadth, and cytokine coexpression profiles of SIV Gag- and Env-specific T cells in blood. Despite seeing no differences in the magnitude of SIV-specific CD8 T cell responses, we saw an enhancement of CD8 T cell breadth and in vitro proliferative capacity (Fig. 3).
FIG 3.
Anti-SIV T cell responses postvaccination. (A) Magnitude of IFN-γ+ CD8 T cell responses. (B) Breadth of anti-SIV CD8 T cell responses. Breadth is measured using 13 Gag peptide pools and 11 Env peptide pools. These pools consist of a total of 125 Gag peptides and 225 Env peptides. (C) Percent proliferating Gag-specific CD8 T cells at 1 week after the second MVA, as measured by CFSE dilution. (D) Magnitude of IFN-γ+ CD4 T cell responses. (E) Breadth of anti-SIV CD4 T cell responses. (F) Boolean analysis of anti-SIV CD4 T cell responses at 1 week after the first MVA. Capital letters indicate the coexpression patterns for IFN-γ (I), IL-2 (L), and/or TNF-α (T).
Since Mamu-A*01-positive animals were present only in the CD40L group and due to the presence of immunodominant Gag CM9 epitope, we compared CD8 T cell responses primarily between Mamu-A*01-negative animals to evaluate the adjuvant activity. As observed from our previous trials (38, 44, 45), the frequency of SIV (Gag+Env)-specific CD8 or CD4 T cell responses remained below our detection limit after two DNA vaccinations (data not shown). However, strong SIV-specific CD8 T cell responses were observed in both groups after two MVA boosts and were not significantly different between the two groups (Fig. 3A). A geometric mean frequency of ∼0.08% SIV-specific IFN-γ-producing CD8 T cells was observed in the blood after the first MVA boost, and these responses were boosted by ∼2.6-fold following the second MVA boost. We measured the breadth of SIV-specific CD8 T cell responses using 13 Gag and 11 Env pools, each containing 10 to 20 peptides. These analyses revealed a higher breadth of CD8 T cell responses in the Mamu-A*01-negative animals from the D40LM group (Fig. 3B). The breadth of SIV-specific CD8 T cell responses in the D40LM group (mean of five pools) was ∼1.6-fold higher than in the DM group (mean of three pools) (Fig. 3B). The breadth of CD8 T cell responses in the Mamu-A*01-positive animals of the CD40L group was low (mean of 2.3 pools), presumably due to the presence of the immunodominant Gag CM9 epitope strongly restricting the presentation of other CD8 epitopes.
To understand the proliferative capacity of memory (1 week after the second MVA boost) CD8 T cells in vitro after antigen stimulation, we conducted a carboxyfluorescein diacetate succinimidyl ester (CFSE)-based proliferation assay using SIV Gag peptide pool as stimulation as described previously (42). We found that the CD8 T cells in the D40LM group proliferated better than CD8 T cells in the DM group, suggesting that memory CD8 T cells in the adjuvanted group may have better proliferative capacity (Fig. 3C).
CD40L adjuvant changes the functional quality and enhances the breadth of SIV-specific CD4 T cell immunity.
Similar to the CD8 T cell response, the CD40L adjuvant did not increase the magnitude of SIV-specific CD4 T cell responses but enhanced the breadth (Fig. 3). Strong SIV (Gag+Env)-specific IFN-γ+ or TNF-α+ CD4 T cell responses were observed in both groups at 1 week after the first MVA boost, and these responses tended to be lower after the second MVA boost (Fig. 3D). At the peak response (one week after the first MVA), the magnitude of IFN-γ+ CD4 T cell responses was marginally higher in the DM group (Fig. 3D). This difference was not observed for the magnitude of TNF-α+ CD4 T cell responses (data not shown). However, the breadth of SIV-specific IFN-γ+ CD4 T cell response was significantly higher in the CD40L group (mean of 14.8 pools) than in the DM group (mean of 11.7 pools) (Fig. 3E).
Using a Boolean analysis, we compared the groups to assess for qualitative differences in CD4 T cells that produced IFN-γ, TNF-α, and/or IL-2 cytokines (Fig. 3F). Both groups did not differ in the proportion of triple cytokine-producing cells. However, within the double- and single-cytokine-producing populations, the response in the CD40L group was biased toward IFN-γ+ TNF-α+ double-positive cells and TNF-α single-positive cells, and the response in the DM group was biased toward IFN-γ+ IL-2+ double-positive cells (Fig. 3F). These results demonstrated that CD40L changes the quality of SIV-specific CD4 T cell response by biasing toward higher TNF-α expression, a phenotype that was seen for vaccinia virus-specific CD4 T cells in humans vaccinated with smallpox vaccine (31).
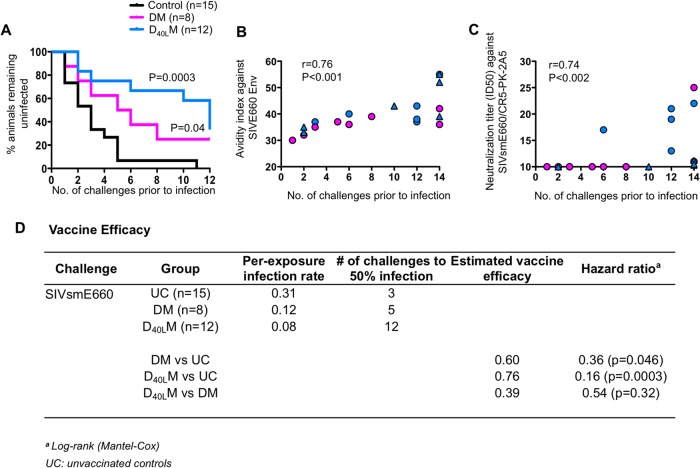
CD40L adjuvant delays acquisition of SIV infection.
At between 20 and 24 weeks after the last MVA boost, we subjected all vaccinated macaques to 12 weekly intrarectal challenges with SIVE660 at 5,000 50% tissue culture infective doses to test the level of protection conferred by these vaccines. Nine Mamu-A*01-negative and six Mamu-A*01-positive unvaccinated macaques served as the unvaccinated control group (Fig. 4). This challenge dose consistently infected 30% of the unvaccinated macaques after the first exposure and resulted in 100% infection of unvaccinated animals by five challenges (32–34, 43). At the end of 12 challenges, 25% of the DM animals and 33% of the D40LM animals remained uninfected (Fig. 4A). However, higher number of challenges was required to infect CD40L-adjuvanted animals. It took just three challenges for 50% infection in the unvaccinated group and five challenges for 50% infection in the DM group (Fig. 4A and D). Impressively, it took 12 challenges for 50% infection in the CD40L group for both non-Mamu-A*01 and Mamu-A*01 animals. By challenge 11, 6 of 8 (75%) macaques in the DM group were infected, whereas only 5 of 12 (42%) macaques in the CD40L group were infected. However, at challenge 12, three macaques became SIV+ in the CD40L group. Overall, the level of protection against acquisition of SIV infection observed in vaccinated macaques was statistically significant against the unvaccinated group for both DM and CD40L groups. The CD40L group showed the lowest per-challenge infection rate of 0.08 compared to the DM (0.12) and unvaccinated (0.31) groups (Fig. 4D). The protection was not significantly different between the two vaccine groups, presumably due to small group sizes. These data demonstrate that CD40L enhances protection from acquisition of heterologous mucosal SIV infection.
FIG 4.
Kaplan-Meier's survival curve analysis postchallenge. (A) Survival curve of DM, DCD40LM, and control groups. (B) Correlation between avidity index of vaccine-elicited SIVE660 Env-specific IgG at 2 weeks after the second MVA boost and the number of challenges to productive infection. (C) Correlation analysis between the neutralization titer against tier 2 SIVE660/CR5-PK-2A5 virus at 2 weeks after the second MVA boost and the number of challenges to productive infection. Uninfected animals are shown at challenge 14. (D) Vaccine efficacy against SIVE660 challenge.
We next looked for correlates of protection against acquisition of SIV infection. Impressively, the enhanced protection from acquisition of SIV infection was strongly associated with the avidity of binding antibody against the challenge virus Env (SIVE660) (Fig. 4B) and neutralization titers against tier-2 E660 isolate (Fig. 4C). The avidity of vaccine-elicited binding antibody against the immunogen Env (SIVmac239) did not correlate with enhanced protection (data not shown). The protection was not associated with a specific TRIM5α genotype (data not shown). We also did not find a significant correlation between prevention of infection and vaccine-elicited SIV239-specific CD8 and CD4 T cell responses (magnitude, breadth, and polyfunctionality) or with the SIV239 or E660 Env-specific binding antibody titers (data not shown). These results demonstrate that the avidity of challenge virus-specific binding antibody serves as a correlate for protection against acquisition of SIVE660 infection and that the inclusion of CD40L adjuvant enhances protection by enhancing the functional quality of anti-Env antibody.
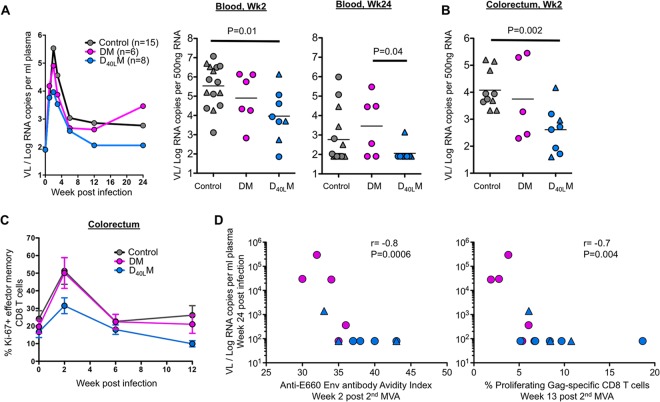
CD40L adjuvant blunts viral load and enhances postinfection viral control.
We next examined the plasma viral load in macaques that were productively infected after challenge (Fig. 5). The plasma viral load in the unvaccinated animals generally peaked at 1 week after the first positive detection at a geometric mean of 3.4 × 105 copies/ml of plasma. These levels contracted to 5.9 × 102 copies during the set point phase (postinfection week 24). The viral load at week 2 postinfection was significantly lower in the D40LM group than in the control group, whereas there was no difference for the D40LM group compared to the DM group (Fig. 5A). However, at 24 weeks postinfection, the plasma viral load in all (except one) of the D40LM animals was below the level of detection, and these levels were significantly lower compared than in the DM animals. Consistent with the week 2 plasma viral load, viral RNA levels in the gut were also lower in the D40LM animals compared to unvaccinated controls (Fig. 5B). In addition, the CD40L-adjuvanted animals showed a marked decrease in CD8 T cell immune activation in the gut (as measured by proliferating CD28− CD95+ memory CD8) compared to DM and unvaccinated controls at 2 weeks postinfection (P = 0.01) (Fig. 5C). Interestingly, animals with higher avidity of antibody against the challenge virus Env postvaccination (2 weeks after the second MVA) and with higher proliferating SIV-specific memory CD8 T cells prechallenge (13 weeks after the second MVA) showed lower viral load during set point, suggesting that antibody avidity and antiviral CD8 T cells contributed to viral control (Fig. 5D).
FIG 5.
Postinfection viral load, immune activation, and correlations. (A) Plasma viral load of non-A01+ and A01+ infected animals. (B) Viral RNA copies per 500 ng of total RNA in the colorectum at 2 weeks postinfection. (C) CD8 T cell immune activation in the gut (as measured by Ki67 expression on CD28− CD95+ effector memory CD8). (D) Correlation between viral load at week 24 postinfection and the avidity index against the SIVE660 at 2 weeks after the second MVA or the percentage of proliferating Gag-specific CD8 T cells at week 13 after the second MVA.
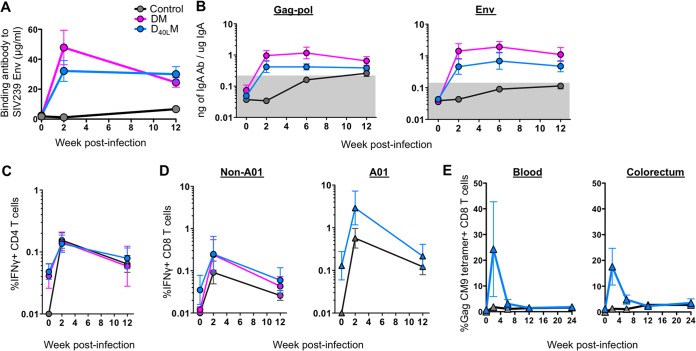
At 2 weeks postinfection in the vaccinated groups, anamnestic anti-SIV IgG responses were observed in blood (Fig. 6A), and previously undetectable anti-SIV IgA was observed in mucosal secretions (Fig. 6B). Anti-SIV IgA responses were first detected for the control group at 6 weeks postinfection. Anamnestic expansions of SIV specific CD4 and CD8 T cells were also observed postinfection (Fig. 6C and D). These responses were generally higher in the vaccinated animals compared to unvaccinated controls. However, no significant differences were observed between the two vaccinated groups. An expansion of Gag-CM9 tetramer-positive T cells was also observed in the blood and gut postinfection in infected Mamu-A*01+ animals (Fig. 6E). None of the measured postinfection responses correlated with viral control.
FIG 6.
Anamnestic expansion of anti-SIV immunity postinfection in infected animals. (A) Titer of SIVmac239 Env-specific antibodies in serum. The mean is shown for each group. (B) Levels of Gag-Pol- or Env-specific IgA specific activity in rectal secretions. The geometric mean is shown for each group. (C) Levels of IFN-γ+ CD4 T cells in the blood. The geometric mean is shown for each group. (D) Levels of IFN-γ+ CD8 T cells in non-A01 and A01 animals. The geometric mean is shown for each group. (E) Frequency of Gag CM9 tetramer-positive CD8 T cells in the blood and colorectum. The mean is shown for each group. The legend for panels B to E is shown in panel A. The error bars indicate the standard errors.
DISCUSSION
It remains a challenge to develop a successful HIV vaccine that is capable of preventing infection. We tested the ability of CD40L to adjuvant the humoral and cellular immunity primed by a DNA vaccine prior to a MVA boost. Our results demonstrated that the CD40L adjuvant enhanced the functional quality of anti-Env antibody response and the breadth of anti-SIV CD8 and CD4 T cell responses, significantly delayed the acquisition of heterologous mucosal SIV infection, and improved the control of acute and set point viremia. Notably, the CD40L adjuvant enhanced the control of viral replication in the gut at the site of challenge and was associated with lower mucosal CD8 immune activation, one of the strong predictors of disease progression (35). Collectively, our results highlight the adjuvant activity for both humoral and cellular immunity of CD40L coexpressed with the DNA prime of a DNA/MVA vaccine. To our knowledge, a single adjuvant that enhances both humoral and cellular immunity is rare and thus underlines the importance and practicality of CD40L as an adjuvant for vaccines against many infectious diseases, including HIV-1 infection.
The mechanisms for protection against SIV acquisition are not completely clear. In our study, we found that antibody avidity strongly stands out as a parameter that significantly correlated with protection, further supporting findings from previous studies (36, 37, 43). Alternative mechanisms for protection could include neutralizing anti-SIV antibodies (against tier 2 virus), particularly in animals that had low avidity anti-SIV antibodies. The mechanisms by which CD40L enhances antibody avidity are not completely clear. The results from our ongoing work suggest that CD40L expressed on VLPs can enhance CD4 helper T cell responses favoring germinal center formation and avidity maturation of Ab responses. In the present study, CD40L-induced SIV-specific CD4 T cells produced lower levels of IFN-γ while producing similar levels of TNF-α. T follicular helper cells generally produce lower levels of IFN-γ while producing similar levels of TNF-α compared to Th1 cells. Unfortunately, we did not measure the production of IL-21, a cytokine made preferentially by T follicular helper cells. Another possible mechanism is that CD40L and Env on the VLPs could directly engage CD40 and Env-specific BCRs on antigen-specific B cells, influencing their proliferative capacity, maturation, and survival.
An important finding from our study is that the CD40L adjuvant enhanced the breadth of SIV-specific CD8 and CD4 T cell responses. This is particularly advantageous for an HIV vaccine given the enormous diversity of the virus. CD40L could enhance the breadth of SIV-specific T cell response by providing costimulation to DCs for subdominant epitopes. The costimulatory effect of CD40L could also have enhanced cytokine production, altering the polyfunctionality of CD4 T cells elicited by the DNA/MVA SIV vaccine.
Studies have recently used SIVE660 for mucosal challenges to test the efficacy of various vaccines. It is difficult to compare the level of protection between these studies and ours, since different challenge stocks, challenge doses, and numbers of challenges were used (39, 40). In addition to these differences, the rate of SIV acquisition with our challenge stock was not significantly influenced by the TRIM5α genotype of the animal, whereas acquisition was dependent on TRIM5α genotype in studies using two other challenge stocks (39, 41). Nevertheless, we used a challenge dose that infects >30% of the unvaccinated controls at the first challenge and impressively protected nearly 70% of the vaccinated animals after 11 challenges with our CD40L-adjuvanted vaccine, highlighting the potential use of CD40L as an adjuvant for an HIV vaccine.
The protection we achieved with the CD40L adjuvant is similar to what we achieved using a granulocyte-macrophage colony-stimulating factor (GM-CSF)-adjuvanted DNA/MVA vaccine (43). Both of these studies were performed in parallel, and we used the same challenge stock and dose for weekly intrarectal challenges. Interestingly, in both of these studies, higher avidity of vaccine-elicited antibody against the E660 Env correlated with enhanced protection. However, there were some differences for immune responses elicited by these two adjuvants. The CD40L adjuvant but not GM-CSF enhanced the breadth of SIV-specific CD4 and CD8 T cell responses and influenced the cytokine coexpression profile of SIV-specific CD4 T cells. Whereas the GM-CSF adjuvant but not CD40L enhanced the neutralization against tier 1 E660 isolate. More in-depth qualitative analysis of T and B cell responses will need to be performed to further understand how similar or dissimilar these two adjuvants are in their effects on anti-SIV immunity.
The novelty in our approach is in achieving targeted adjuvant activity by presenting CD40L on the surface of the SIV VLP. In addition, our study is the first demonstration of the biological effects of CD40L adjuvant on vaccine responses in nonhuman primates. Because we did not compare the immunogenicity and efficacy of VLP-expressed CD40L with other forms of CD40L, such as a secreted multimeric form, it is difficult to conclude that adjuvant activity requires the expression of multimeric CD40L on the surface of VLP. However, we consider cis expression important because SIV VLPs containing CD40L both target antigen and stimulate DCs and B cells. In conclusion, these results reveal the potential of CD40L expressed with viral antigens in VLPs to serve as an adjuvant for enhancing the maturation of humoral immunity and the breadth of cellular immunity and suggest that CD40L can serve as an important adjuvant for HIV vaccines.
ACKNOWLEDGMENTS
We thank Vanessa Hirsch for SIVE660 challenge stock, Welkin Johnson for typing the TRIM5α genotype, and Helen Drake-Perrow for administrative support. We thank the Yerkes Division of Research Resources for animal care, the Emory CFAR Virology Core for viral load assays, the NIH AIDS Research and Reference Reagent Program for the provision of peptides, the Resource for Nonhuman Primate Immune Reagents at Yerkes for the macaque CD40L gene, and the Robert P. Apkarian Integrated Electron Microscopy Core of Emory University for help with electron microscopy.
This study was supported by NIH/National Institute of Allergy and Infectious Disease (NIAID) grants R01 AI057029, R01 AI071852, R01 AI074417, and P01 AI088575 to R.R.A., NCRR (currently supported by the Office of Research Infrastructure Programs) grant P51OD011132 to Yerkes National Primate Research Center, NIH grant P30 AI050409 to Emory CFAR, an HHSN27201100016C grant to D.C.M., and Division of Intramural Research, NIAID/NIH, support to B.M.
R.R.A. and H.L.R. are coinventors of the DNA/MVA vaccine technology, and Emory University licensed this technology to Geovax, Inc. H.L.R. is a founder and CSO of Geovax, Inc.
Footnotes
Published ahead of print 11 June 2014
REFERENCES
- 1.Grewal IS, Flavell RA. 1998. CD40 and CD154 in cell-mediated immunity. Annu. Rev. Immunol. 16:111–135. 10.1146/annurev.immunol.16.1.111 [DOI] [PubMed] [Google Scholar]
- 2.Bennett SR, Carbone FR, Karamalis F, Flavell RA, Miller JF, Heath WR. 1998. Help for cytotoxic-T-cell responses is mediated by CD40 signaling. Nature 393:478–480. 10.1038/30996 [DOI] [PubMed] [Google Scholar]
- 3.Schoenberger SP, Toes RE, van der Voort EI, Offringa R, Melief CJ. 1998. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature 393:480–483. 10.1038/31002 [DOI] [PubMed] [Google Scholar]
- 4.Ridge JP, Di Rosa F, Matzinger P. 1998. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature 393:474–478. 10.1038/30989 [DOI] [PubMed] [Google Scholar]
- 5.Elgueta R, Benson MJ, de Vries VC, Wasiuk A, Guo Y, Noelle RJ. 2009. Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol. Rev. 229:152–172. 10.1111/j.1600-065X.2009.00782.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burger JA, Mendoza RB, Kipps TJ. 2001. Plasmids encoding granulocyte-macrophage colony-stimulating factor and CD154 enhance the immune response to genetic vaccines. Vaccine 19:2181–2189. 10.1016/S0264-410X(00)00382-0 [DOI] [PubMed] [Google Scholar]
- 7.Harcourt JL, Brown MP, Anderson LJ, Tripp RA. 2003. CD40 ligand (CD154) improves the durability of respiratory syncytial virus DNA vaccination in BALB/c mice. Vaccine 21:2964–2979. 10.1016/S0264-410X(03)00119-1 [DOI] [PubMed] [Google Scholar]
- 8.Ihata A, Watabe S, Sasaki S, Shirai A, Fukushima J, Hamajima K, Inoue J, Okuda K. 1999. Immunomodulatory effect of a plasmid expressing CD40 ligand on DNA vaccination against human immunodeficiency virus type-1. Immunology 98:436–442. 10.1046/j.1365-2567.1999.00879.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maue AC, Waters WR, Palmer MV, Whipple DL, Minion FC, Brown WC, Estes DM. 2004. CD80 and CD86, but not CD154, augment DNA vaccine-induced protection in experimental bovine tuberculosis. Vaccine 23:769–779. 10.1016/j.vaccine.2004.07.019 [DOI] [PubMed] [Google Scholar]
- 10.Mendoza RB, Cantwell MJ, Kipps TJ. 1997. Immunostimulatory effects of a plasmid expressing CD40 ligand (CD154) on gene immunization. J. Immunol. 159:5777–5781 [PubMed] [Google Scholar]
- 11.Sin JI, Kim JJ, Zhang D, Weiner DB. 2001. Modulation of cellular responses by plasmid CD40L: CD40L plasmid vectors enhance antigen-specific helper T cell type 1 CD4+ T cell-mediated protective immunity against herpes simplex virus type 2 in vivo. Hum. Gene Ther. 12:1091–1102. 10.1089/104303401750214302 [DOI] [PubMed] [Google Scholar]
- 12.Wienhold D, Armengol E, Marquardt A, Marquardt C, Voigt H, Buttner M, Saalmuller A, Pfaff E. 2005. Immunomodulatory effect of plasmids coexpressing cytokines in classical swine fever virus subunit gp55/E2-DNA vaccination. Vet. Res. 36:571–587. 10.1051/vetres:2005019 [DOI] [PubMed] [Google Scholar]
- 13.French RR, Chan HT, Tutt AL, Glennie MJ. 1999. CD40 antibody evokes a cytotoxic T-cell response that eradicates lymphoma and bypasses T-cell help. Nat. Med. 5:548–553. 10.1038/8426 [DOI] [PubMed] [Google Scholar]
- 14.Sotomayor EM, Borrello I, Tubb E, Rattis FM, Bien H, Lu Z, Fein S, Schoenberger S, Levitsky HI. 1999. Conversion of tumor-specific CD4+ T-cell tolerance to T-cell priming through in vivo ligation of CD40. Nat. Med. 5:780–787. 10.1038/10503 [DOI] [PubMed] [Google Scholar]
- 15.van Mierlo GJ, den Boer AT, Medema JP, van der Voort EI, Fransen MF, Offringa R, Melief CJ, Toes RE. 2002. CD40 stimulation leads to effective therapy of CD40− tumors through induction of strong systemic cytotoxic T lymphocyte immunity. Proc. Natl. Acad. Sci. U. S. A. 99:5561–5566. 10.1073/pnas.082107699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barr TA, McCormick AL, Carlring J, Heath AW. 2003. A potent adjuvant effect of CD40 antibody attached to antigen. Immunology 109:87–92. 10.1046/j.1365-2567.2003.01634.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahonen CL, Doxsee CL, McGurran SM, Riter TR, Wade WF, Barth RJ, Vasilakos JP, Noelle RJ, Kedl RM. 2004. Combined TLR and CD40 triggering induces potent CD8+ T cell expansion with variable dependence on type I IFN. J. Exp. Med. 199:775–784. 10.1084/jem.20031591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schneider P, Holler N, Bodmer JL, Hahne M, Frei K, Fontana A, Tschopp J. 1998. Conversion of membrane-bound Fas(CD95) ligand to its soluble form is associated with downregulation of its proapoptotic activity and loss of liver toxicity. J. Exp. Med. 187:1205–1213. 10.1084/jem.187.8.1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suda T, Hashimoto H, Tanaka M, Ochi T, Nagata S. 1997. Membrane Fas ligand kills human peripheral blood T lymphocytes, and soluble Fas ligand blocks the killing. J. Exp. Med. 186:2045–2050. 10.1084/jem.186.12.2045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kehry MR, Castle BE, Hodgkin PD. 1992. B-cell activation mediated by interactions with membranes from helper T cells. Adv. Exp. Med. Biol. 323:139–148. 10.1007/978-1-4615-3396-2_18 [DOI] [PubMed] [Google Scholar]
- 21.Kishore U, Reid KB. 1999. Modular organization of proteins containing C1q-like globular domain. Immunopharmacology 42:15–21. 10.1016/S0162-3109(99)00011-9 [DOI] [PubMed] [Google Scholar]
- 22.Kishore U, Gaboriaud C, Waters P, Shrive AK, Greenhough TJ, Reid KB, Sim RB, Arlaud GJ. 2004. C1q and tumor necrosis factor superfamily: modularity and versatility. Trends Immunol. 25:551–561. 10.1016/j.it.2004.08.006 [DOI] [PubMed] [Google Scholar]
- 23.Crouch E, Persson A, Chang D, Heuser J. 1994. Molecular structure of pulmonary surfactant protein D (SP-D). J. Biol. Chem. 269:17311–17319 [PubMed] [Google Scholar]
- 24.Holmskov U, Thiel S, Jensenius JC. 2003. Collections and ficolins: humoral lectins of the innate immune defense. Annu. Rev. Immunol. 21:547–578. 10.1146/annurev.immunol.21.120601.140954 [DOI] [PubMed] [Google Scholar]
- 25.Hoppe HJ, Reid KB. 1994. Collectins–soluble proteins containing collagenous regions and lectin domains—and their roles in innate immunity. Protein Sci. 3:1143–1158. 10.1002/pro.5560030801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu J, Teh C, Kishore U, Reid KB. 2002. Collectins and ficolins: sugar pattern recognition molecules of the mammalian innate immune system. Biochim. Biophys. Acta 1572:387–400. 10.1016/S0304-4165(02)00320-3 [DOI] [PubMed] [Google Scholar]
- 27.van de Wetering JK, van Golde LM, Batenburg JJ. 2004. Collectins: players of the innate immune system. Eur. J. Biochem. 271:1229–1249. 10.1111/j.1432-1033.2004.04040.x [DOI] [PubMed] [Google Scholar]
- 28.Stone GW, Barzee S, Snarsky V, Kee K, Spina CA, Yu XF, Kornbluth RS. 2006. Multimeric soluble CD40 ligand and GITR ligand as adjuvants for human immunodeficiency virus DNA vaccines. J. Virol. 80:1762–1772. 10.1128/JVI.80.4.1762-1772.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miyahira Y, Akiba H, Katae M, Kubota K, Kobayashi S, Takeuchi T, Garcia-Sastre A, Fukuchi Y, Okumura K, Yagita H, Aoki T. 2003. Cutting edge: a potent adjuvant effect of ligand to receptor activator of NF-κB gene for inducing antigen-specific CD8+ T cell response by DNA and viral vector vaccination. J. Immunol. 171:6344–6348. 10.4049/jimmunol.171.12.6344 [DOI] [PubMed] [Google Scholar]
- 30.Sumida SM, McKay PF, Truitt DM, Kishko MG, Arthur JC, Seaman MS, Jackson SS, Gorgone DA, Lifton MA, Letvin NL, Barouch DH. 2004. Recruitment and expansion of dendritic cells in vivo potentiate the immunogenicity of plasmid DNA vaccines. J. Clin. Invest. 114:1334–1342. 10.1172/JCI22608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hammarlund E, Lewis MW, Hansen SG, Strelow LI, Nelson JA, Sexton GJ, Hanifin JM, Slifka MK. 2003. Duration of antiviral immunity after smallpox vaccination. Nat. Med. 9:1131–1137. 10.1038/nm917 [DOI] [PubMed] [Google Scholar]
- 32.Lai L, Kwa SF, Kozlowski PA, Montefiori DC, Nolen TL, Hudgens MG, Johnson WE, Ferrari G, Hirsch VM, Felber BK, Pavlakis GN, Earl PL, Moss B, Amara RR, Robinson HL. 2012. SIVmac239 MVA vaccine with and without a DNA prime, similar prevention of infection by a repeated dose SIVsmE660 challenge despite different immune responses. Vaccine 30:1737–1745. 10.1016/j.vaccine.2011.12.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kannanganant S, Gangadhara S, Lai L, Lawson B, Kozlowski PA, Robinson HL, Amara RR. 2014. Local control of repeat dose rectal challenges in DNA/MVA vaccinated macaques protected against a first series of SIV challenges. J. Virol. 88:5864–5869. 10.1128/JVI.00145-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patel V, Jalah R, Kulkarni V, Valentin A, Rosati M, Alicea C, von Gegerfelt A, Huang W, Guan Y, Keele BF, Bess JW, Piatak M, Lifson JD, Williams WT, Shen X, Tomaras GD, Amara RR, Robinson HL, Johnson W, Broderick KE, Sardesai NY, Venzon DJ, Hirsch VM, Felber BK, Pavlakis GN. 2013. DNA and virus particle vaccination protects against acquisition and confers control of viremia upon heterologous simian immunodeficiency virus challenge. Proc. Natl. Acad. Sci. U. S. A. 110:2975–2980. 10.1073/pnas.1215393110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Giorgi JV, Hultin LE, McKeating JA, Johnson TD, Owens B, Jacobson LP, Shih R, Lewis J, Wiley DJ, Phair JP, Wolinsky SM, Detels R. 1999. Shorter survival in advanced human immunodeficiency virus type 1 infection is more closely associated with T lymphocyte activation than with plasma virus burden or virus chemokine coreceptor usage. J. Infect. Dis. 179:859–870. 10.1086/314660 [DOI] [PubMed] [Google Scholar]
- 36.Xiao P, Zhao J, Patterson LJ, Brocca-Cofano E, Venzon D, Kozlowski PA, Hidajat R, Demberg T, Robert-Guroff M. 2010. Multiple vaccine-elicited nonneutralizing antienvelope antibody activities contribute to protective efficacy by reducing both acute and chronic viremia following simian/human immunodeficiency virus SHIV89.6P challenge in rhesus macaques. J. Virol. 84:7161–7173. 10.1128/JVI.00410-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pegu P, Vaccari M, Gordon S, Keele BF, Doster M, Guan Y, Ferrari G, Pal R, Ferrari MG, Whitney S, Hudacik L, Billings E, Rao M, Montefiori D, Tomaras G, Alam SM, Fenizia C, Lifson JD, Stablein D, Tartaglia J, Michael N, Kim J, Venzon D, Franchini G. 2013. Antibodies with high avidity to the gp120 envelope protein in protection from simian immunodeficiency virus SIVmac251 acquisition in an immunization regimen that mimics the RV-144 Thai trial. J. Virol. 87:1708–1719. 10.1128/JVI.02544-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Amara RR, Villinger F, Altman JD, Lydy SL, O'Neil SP, Staprans SI, Montefiori DC, Xu Y, Herndon JG, Wyatt LS, Candido MA, Kozyr NL, Earl PL, Smith JM, Ma HL, Grimm BD, Hulsey ML, Miller J, McClure HM, McNicholl JM, Moss B, Robinson HL. 2001. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science 292:69–74. 10.1126/science.1058915 [DOI] [PubMed] [Google Scholar]
- 39.Letvin NL, Rao SS, Montefiori DC, Seaman MS, Sun Y, Lim SY, Yeh WW, Asmal M, Gelman RS, Shen L, Whitney JB, Seoighe C, Lacerda M, Keating S, Norris PJ, Hudgens MG, Gilbert PB, Buzby AP, Mach LV, Zhang J, Balachandran H, Shaw GM, Schmidt SD, Todd JP, Dodson A, Mascola JR, Nabel GJ. 2011. Immune and genetic correlates of vaccine protection against mucosal infection by SIV in monkeys. Sci. Transl. Med. 3:81ra36. 10.1126/scitranslmed.3002351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reynolds MR, Weiler AM, Piaskowski SM, Kolar HL, Hessell AJ, Weiker M, Weisgrau KL, Leon EJ, Rogers WE, Makowsky R, McDermott AB, Boyle R, Wilson NA, Allison DB, Burton DR, Koff WC, Watkins DI. 2010. Macaques vaccinated with simian immunodeficiency virus SIVmac239Delta nef delay acquisition and control replication after repeated low-dose heterologous SIV challenge. J. Virol. 84:9190–9199. 10.1128/JVI.00041-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reynolds MR, Sacha JB, Weiler AM, Borchardt GJ, Glidden CE, Sheppard NC, Norante FA, Castrovinci PA, Harris JJ, Robertson HT, Friedrich TC, McDermott AB, Wilson NA, Allison DB, Koff WC, Johnson WE, Watkins DI. 2011. The TRIM5α genotype of rhesus macaques affects acquisition of simian immunodeficiency virus SIVsmE660 infection after repeated limiting-dose intrarectal challenge. J. Virol. 85:9637–9640. 10.1128/JVI.05074-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kannanganat S, Nigam P, Velu V, Earl PL, Lai L, Chennareddi L, Lawson B, Wilson RL, Montefiori DC, Kozlowski PA, Moss B, Robinson HL, Amara RR. 2010. Preexisting vaccinia virus immunity decreases SIV-specific cellular immunity but does not diminish humoral immunity and efficacy of a DNA/MVA vaccine. J. Immunol. 185:7262–7273. 10.4049/jimmunol.1000751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lai L, Kwa S, Kozlowski PA, Montefiori DC, Ferrari G, Johnson WE, Hirsch V, Villinger F, Chennareddi L, Earl PL, Moss B, Amara RR, Robinson HL. 2011. Prevention of infection by a granulocyte-macrophage colony-stimulating factor coexpressing DNA/modified vaccinia Ankara simian immunodeficiency virus vaccine. J. Infect. Dis. 204:164–173. 10.1093/infdis/jir199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Amara RR, Villinger F, Staprans SI, Altman JD, Montefiori DC, Kozyr NL, Xu Y, Wyatt LS, Earl PL, Herndon JG, McClure HM, Moss B, Robinson HL. 2002. Different patterns of immune responses but similar control of a simian-human immunodeficiency virus 89.6P mucosal challenge by modified vaccinia virus Ankara (MVA) and DNA/MVA vaccines. J. Virol. 76:7625–7631. 10.1128/JVI.76.15.7625-7631.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nigam P, Earl PL, Americo JL, Sharma S, Wyatt LS, Edghill-Spano Y, Chennareddi LS, Silvera P, Moss B, Robinson HL, Amara RR. 2007. DNA/MVA HIV-1/AIDS vaccine elicits long-lived vaccinia virus-specific immunity and confers protection against a lethal monkeypox challenge. Virology 366:73–83. 10.1016/j.virol.2007.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]