Abstract
Interleukin-21 (IL-21) can be produced by CD8 T cells from HIV-1-infected individuals and those with autoimmune disease, but the mechanism remains poorly understood. Here we demonstrate that IL-21-producing CD8 T cells are not associated with CD4 depletion and are absent in patients with idiopathic CD4 lymphocytopenia. Instead, IL-21 production by CD8 T cells was associated with high levels of activation, suggesting that these cells emerge as a consequence of excessive chronic immune activation rather than CD4 lymphopenia.
TEXT
As a product of CD4 T cells, interleukin-21 (IL-21) has emerged as a key factor for control of chronic viral infections (1–8). Accumulating evidence suggests that CD8 T cells secrete IL-21, and populations of these cells have been described in HIV-1 infection and autoimmunity (9–13). We have demonstrated that HIV-1-specific IL-21-producing CD8 T cells are enriched in HIV-1 elite controllers, whereas polyclonally stimulated IL-21-producing CD8 T cells are increased in patients who lack viral control (12).
The basis for the apparent division of IL-21 competency between CD4 and CD8 T cell subsets is unclear, but one possibility is that CD4-negative T cells may acquire CD4 T-helper function (14–20). Given that CD4 T cell depletion is a hallmark of HIV-1 infection, such a compensatory mechanism could allow for CD8 T cells to acquire IL-21 competency and helper function. On the other hand, CD8 T cell production of IL-21 could also be seen with immune perturbations such as seen in HIV-1 infection and autoimmunity, diseases associated with this unusual cellular phenotype (9–13).
We sought to define IL-21-competent CD8 T cells and determine their relation to conventional CD4 T cells. Cryopreserved peripheral blood mononuclear cells (PBMCs) from 30 chronically HIV-1-infected individuals off antiretroviral therapy (ART) (median plasma viral load [pVL], 18,282 copies/ml; median CD4 count, 581 cells/mm3) and 19 HIV-1-seronegative subjects were thawed, activated with phorbol 12,13-dibutyrate and ionomycin (PDBu and ionomycin), and stained as previously described (12). The institutional review boards (IRBs) of the University of Alabama at Birmingham (UAB) and National Institute of Allergy and Infectious Diseases (NIAID) approved this study, and written informed consent was obtained from study participants.
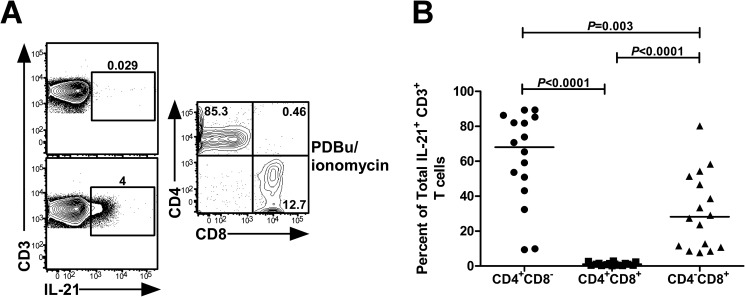
Given that IL-21 is traditionally regarded as a CD4 T cell-derived cytokine, we evaluated the extent to which IL-21-producing CD8 T cells expressed CD4 to exclude the possibility that these cells may represent CD4 T cells. We found that the majority of IL-21-competent CD8 T cells lacked surface expression of CD4 (Fig. 1A and B), validating the existence of mutually exclusive IL-21+ CD4 and CD8 T cell subsets.
FIG 1.
IL-21-producing CD8 T cells do not coexpress CD4. PBMCs from 16 HIV-1-infected individuals were polyclonally stimulated for 5 h by PDBu/ionomycin. Cells were gated on CD3+ IL-21+ T lymphocytes and subsequently plotted for CD4 versus CD8 expression. (A) Representative flow cytometric analysis of CD4 and CD8 expression on IL-21-producing T cells. The left column shows IL-21 production from CD3+ T cells in unstimulated cells (top) and PDBu/ionomycin-treated cells (bottom). (B) Cumulative data for the percentages of CD4+ CD8−, CD4+ CD8+, and CD4− CD8+ IL-21-producing T cells in HIV-1-infected subjects.
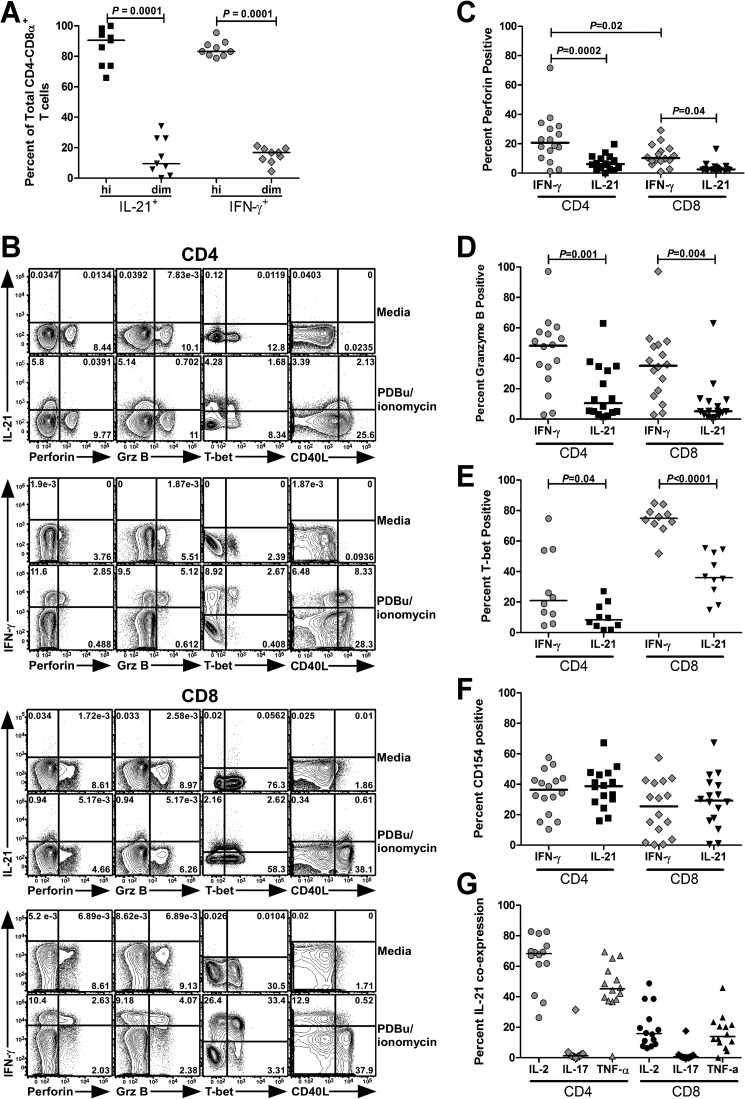
Studies in African green monkeys (AGM) have shown that the decrease in CD4+ T cells negatively correlates with the increased expansion of a population of CD4− CD8αdim T cells eliciting functional characteristics of CD4 T cells (17). To address whether IL-21-producing CD8 T cells had a similar CD4− CD8αdim phenotype, we compared the degree to which IL-21+ and gamma interferon-positive (IFN-γ+) CD4− T cells from HIV-1-infected individuals expressed the CD8α chain. Both IL-21 and IFN-γ-responsive CD4− T cells resided predominantly within the CD8αhi compartment, suggesting that IL-21-producing CD8 T cells do not develop from CD4 T cells that have lost CD4 and upregulated CD8α (Fig. 2A).
FIG 2.
IL-21-producing CD4− CD8+ T cells exhibit a few characteristics of CD4 T cells. Phenotypic and functional analysis of IL-21-producing T cell populations in HIV-1-infected individuals as determined by flow cytometry. PBMCs were cultured in the presence of anti-CD28/CD49d, GolgiStop, and GolgiPlug, polyclonally activated with PDBu/ionomycin for 5 h, and subsequently stained with fluorochrome-conjugated monoclonal antibodies (MAbs) against IL-21 (clone 3A3.N21) and IFN-γ (clone B27). Cells were then gated on live CD3+ CD4− lymphocytes and analyzed for high (“hi”) or low (“dim”) expression of the CD8α chain (clone RPA-T8). Data are expressed as the percentage of total IL-21+ or IFN-γ+ CD4− CD8α+ T cells (A). Panels B to F contain representative flow cytometric plots (B) and compilation graphs showing the percentages of total IL-21+ or IFN-γ+ CD8− CD4+ and CD4− CD8+ T cells that expressed perforin (clone BD48) (C), granzyme B (GrzB) (clone GB11) (D), T-bet (clone 4B10) (E), or upregulated CD40L (clone TRAP-1) (F). For assessment of CD40L expression, cells were cocultured with CD154 MAb in the presence of GolgiStop. The percentages of total IL-21-producing CD4 and CD8 T cells that coexpressed IL-2 (clone MQ1-17H12), IL-17 (clone N49-653), or TNF-α (clone MAb11) are also shown (G). Statistical significance was determined using the two-tailed Mann-Whitney U test; horizontal bars represent median.
We next asked whether this subset of CD8 T cells would be devoid of effector functions typically associated with cytotoxic CD8 T cells. To test this hypothesis, CD8 T cells producing IL-21 and IFN-γ were assessed for coexpression of cytolytic effector molecules perforin, granzyme B, and the transcription factor T-bet, a critical regulator of CD8 T cell differentiation and lytic gene expression and function (21, 22). A comparative analysis revealed that IL-21+ CD8 T cells exhibited a limited propensity to coexpress perforin, granzyme B, and T-bet than did their IFN-γ+ counterparts (Fig. 2B to E). To probe for the existence of IL-21-producing CD8 T cells with CD4-like characteristics, we evaluated their ability to upregulate CD40 ligand (CD40L) upon stimulation. Indeed, no differences were noted between IL-21-producing CD8 and CD4 T cells expressing CD40L or the frequency of CD40L-expressing IFN-γ+ CD8 T cells (Fig. 2B and F). Approximately 20% of IL-21-producing CD8 T cells expressed tumor necrosis factor alpha (TNF-α) and IL-2, with a nearly undetectable induction of IL-21/IL-17 coproducers (Fig. 2G). Importantly, we did not observe a correlation between production of IL-2 by CD4 T cells and IL-21 by CD8 T cells (data not shown), unlike what was noted in mouse models, whereby limited IL-2 production was associated with increased IL-21 production by CD4 T cells (2, 23). To ascertain whether IL-21-producing CD8 T cells manifested traits that resemble T follicular helper (Tfh) cells (24, 25), we determined the frequency of IL-21-producing CD8 T cells that coexpressed Tfh-associated markers CXCR5 and inducible costimulator (ICOS). We found a negligible fraction of IL-21-producing cells expressed these molecules (data not shown). These results demonstrate that IL-21-competent CD8 T cells share a few characteristics of classical CD4 T cells yet are phenotypically distinct from Tfh CD4 T cells. While we have not performed functional studies of CD8+ IL-21+ T cells, previous work has shown that CD40L+ CD8 T cells can indeed provide B-cell help (26). Interestingly, these CD40L+ CD8 T cells expressed low levels of IL-21 mRNA, providing evidence that IL-21+ CD8 T cells may have the capacity to execute immunologic helper functions (26).
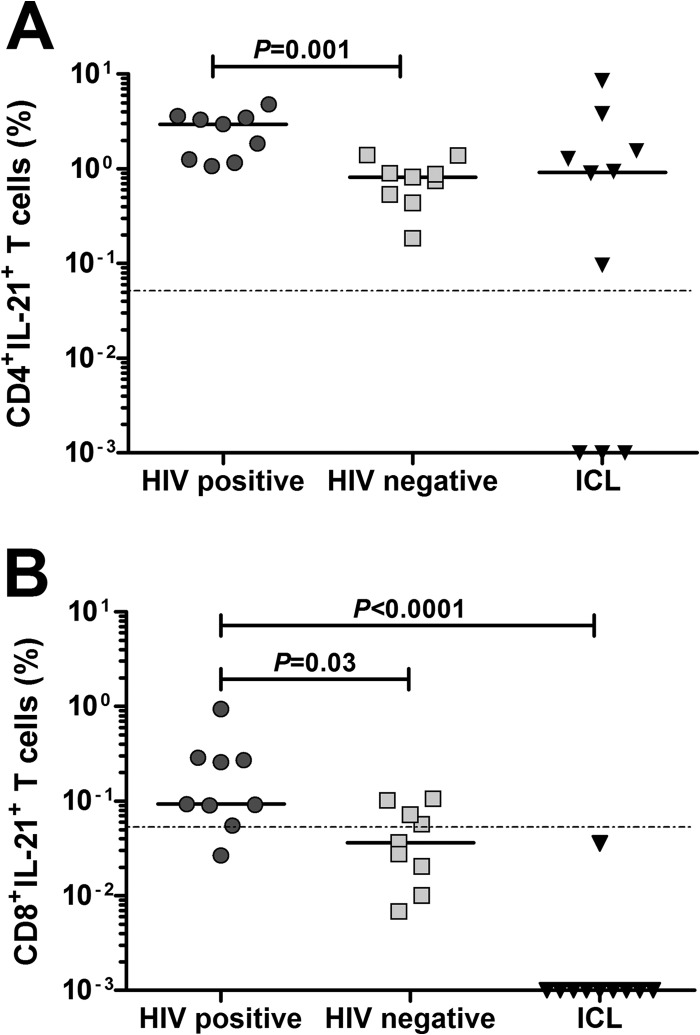
It remained unclear whether the emergence of IL-21-producing CD8 T cells was a direct consequence of CD4 T cell deficiency or HIV-1 disease. To address this issue, we evaluated the presence of IL-21-producing CD8 T cells in 10 patients with idiopathic CD4 lymphocytopenia (ICL), a syndrome of unknown etiology characterized by low CD4 T cell counts. Importantly, many clinical manifestations associated with ICL are akin to those in HIV-1-infection (27, 28). Notably, IL-21-producing CD4 T cells were comparable in patients with ICL and HIV-1-infected subjects (Fig. 3A), concomitant with the absence of IL-21+ CD8 T cells in ICL patients (Fig. 3B). Furthermore, there was no association between the frequency of CD8+ IL-21+ T cells and CD4 T cell count or viral load (data not shown). These findings further solidify that expansion of IL-21-producing CD8 T cells in chronic, progressive HIV-1 infection is independent of CD4 T cell loss.
FIG 3.
Absence of IL-21-producing CD8 T cells in patients with idiopathic CD4 lymphocytopenia. PBMCs from HIV-1-infected patients (n = 10), HIV-1-seronegative subjects (n = 9), and patients with idiopathic CD4 lymphocytopenia (ICL) (n = 10) were stimulated with PDBu/ionomycin for 5 h and stained for IL-21. The magnitude of CD4 (A) or CD8 (B) T cells producing IL-21 is shown, and horizontal bars indicate the median value for each cohort. Statistical significance was determined using the two-tailed nonparametric Mann-Whitney U test. The dotted line discriminates between a positive response and negative response.
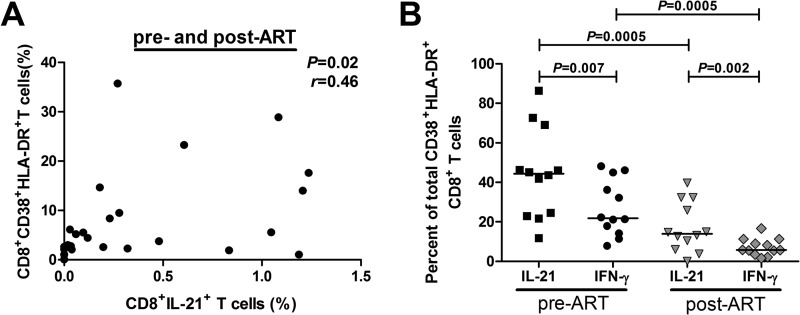
Elevated frequencies of IL-21-competent CD8 T cells have been described in the context of autoimmunity (9, 11, 13). Uncontrolled T cell responses feature prominently in both autoimmunity and HIV-1 infection, suggesting a common mechanism by which IL-21-producing CD8 T cells arise (29, 30). We reasoned that persistent immune activation drives the acquisition of CD8 T cell IL-21 production during HIV-1 infection. To explore this possibility, we studied longitudinal samples from 12 patients prior to ART initiation (median pVL, 30,086 copies/ml; median CD4 count, 375 cells/mm3) and after achieving fully suppressed VL (median time, 6 months; median pVL, 49 copies/ml; median CD4 count, 573 cells/mm3). We found that the frequency of CD8 T cells producing IL-21 modestly correlated with the percentages of activated CD8 T cells, as measured by coexpression of CD38 and HLA-DR (Fig. 4A). This association was primarily seen in samples taken from pre-ART time points (r = 0.57, P = 0.04) (data not shown). We then compared the extent to which IL-21-producing CD8 T cells displayed an activated phenotype relative to IFN-γ-producing cells. Overall, IL-21-producing CD8 T cells showed higher levels of activation than IFN-γ-responsive cells irrespective of ART-induced viral suppression (Fig. 4B). These results provide further evidence that immune activation, rather than perturbations in CD4 T cell numbers, drives CD8 T cell IL-21 production.
FIG 4.
CD8 T cell IL-21 production is associated with CD8 T cell activation levels. (A) Spearman rank correlation between the frequency of IL-21+ CD8 T cells and levels of activated CD38+ HLA-DR+ CD8 T cells obtained from HIV-1-infected individuals before and 6 months after achieving full viral suppression with ART. (B) Percentage of total CD38+ HLA-DR+ CD8 T cells that produced IL-21 or IFN-γ in a cohort of HIV-1-infected individuals (n = 12) with longitudinal samples collected prior to ART initiation and 6 to 12 months after achieving fully suppressed viremia.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grants R01 AI082966 and AI084772 and Bill & Melinda Gates Foundation grant 37874 to P.A.G. and NIH grants T32 AI007051, F31 AI085970, and T32 AI007392 to L.D.W. I.S. was supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases. Flow cytometry was performed, in part, at the UAB Center for AIDS Research Flow Cytometry Core (funded by NIH grant P30 AI027767) and the Duke University Center for AIDS Research Flow Cytometry Facility (funded by NIH grant P30 AI64510).
We are grateful for technical assistance from Eleanor M. P. Wilson, William L. Thompson, and Amrit Singh in performing flow cytometry experiments at the NIH. We thank the patients and staff of the UAB 1917 and Alabama Vaccine Research Clinics and NIAID HIV-Clinic for their participation in this study.
All authors declare they have no financial conflict of interest for this study.
L.D.W., A.B., P.A.G., S.S., S.L.H, and I.S. contributed to the conception and design of the experiments. L.D.W. and N.A. performed the experiments. L.D.W, N.A., A.B, and P.A.G. analyzed the data. S.L.H., P.A.G, and I.S. provided clinical care for the patients and helped identify patients for the study. L.D.W., A.B, and P.A.G. wrote the manuscript. P.A.G. supervised the entire project.
Footnotes
Published ahead of print 18 June 2014
REFERENCES
- 1.Chevalier MF, Julg B, Pyo A, Flanders M, Ranasinghe S, Soghoian DZ, Kwon DS, Rychert J, Lian J, Muller MI, Cutler S, McAndrew E, Jessen H, Pereyra F, Rosenberg ES, Altfeld M, Walker BD, Streeck H. 2011. HIV-1-specific interleukin-21+ CD4+ T cell responses contribute to durable viral control through the modulation of HIV-specific CD8+ T cell function. J. Virol. 85:733–741. 10.1128/JVI.02030-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Elsaesser H, Sauer K, Brooks DG. 2009. IL-21 is required to control chronic viral infection. Science 324:1569–1572. 10.1126/science.1174182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frohlich A, Kisielow J, Schmitz I, Freigang S, Shamshiev AT, Weber J, Marsland BJ, Oxenius A, Kopf M. 2009. IL-21R on T cells is critical for sustained functionality and control of chronic viral infection. Science 324:1576–1580. 10.1126/science.1172815 [DOI] [PubMed] [Google Scholar]
- 4.Iannello A, Boulassel MR, Samarani S, Debbeche O, Tremblay C, Toma E, Routy JP, Ahmad A. 2010. Dynamics and consequences of IL-21 production in HIV-infected individuals: a longitudinal and cross-sectional study. J. Immunol. 184:114–126. 10.4049/jimmunol.0901967 [DOI] [PubMed] [Google Scholar]
- 5.Yi JS, Cox MA, Zajac AJ. 2010. Interleukin-21: a multifunctional regulator of immunity to infections. Microbes Infect. 12:1111–1119. 10.1016/j.micinf.2010.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yi JS, Du M, Zajac AJ. 2009. A vital role for interleukin-21 in the control of a chronic viral infection. Science 324:1572–1576. 10.1126/science.1175194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yi JS, Ingram JT, Zajac AJ. 2010. IL-21 deficiency influences CD8 T cell quality and recall responses following an acute viral infection. J. Immunol. 185:4835–4845. 10.4049/jimmunol.1001032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yue FY, Lo C, Sakhdari A, Lee EY, Kovacs CM, Benko E, Liu J, Song H, Jones RB, Sheth P, Chege D, Kaul R, Ostrowski MA. 2010. HIV-specific IL-21 producing CD4+ T cells are induced in acute and chronic progressive HIV infection and are associated with relative viral control. J. Immunol. 185:498–506. 10.4049/jimmunol.0903915 [DOI] [PubMed] [Google Scholar]
- 9.Dolff S, Abdulahad WH, Westra J, Doornbos-van der Meer B, Limburg PC, Kallenberg CG, Bijl M. 2011. Increase in IL-21 producing T-cells in patients with systemic lupus erythematosus. Arthritis Res. Ther. 13:R157. 10.1186/ar3474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mittal A, Murugaiyan G, Beynon V, Hu D, Weiner HL. 2012. IL-27 induction of IL-21 from human CD8+ T cells induces granzyme B in an autocrine manner. Immunol. Cell Biol. 90:831–835. 10.1038/icb.2012.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ortega C, Fernandez AS, Carrillo JM, Romero P, Molina IJ, Moreno JC, Santamaria M. 2009. IL-17-producing CD8+ T lymphocytes from psoriasis skin plaques are cytotoxic effector cells that secrete Th17-related cytokines. J. Leukoc. Biol. 86:435–443. 10.1189/JLB.0109046 [DOI] [PubMed] [Google Scholar]
- 12.Williams LD, Bansal A, Sabbaj S, Heath SL, Song W, Tang J, Zajac AJ, Goepfert PA. 2011. Interleukin-21-producing HIV-1-specific CD8 T cells are preferentially seen in elite controllers. J. Virol. 85:2316–2324. 10.1128/JVI.01476-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu X, Ma D, Zhang J, Peng J, Qu X, Ji C, Hou M. 2010. Elevated interleukin-21 correlated to Th17 and Th1 cells in patients with immune thrombocytopenia. J. Clin. Immunol. 30:253–259. 10.1007/s10875-009-9353-1 [DOI] [PubMed] [Google Scholar]
- 14.Locksley RM, Reiner SL, Hatam F, Littman DR, Killeen N. 1993. Helper T cells without CD4: control of leishmaniasis in CD4-deficient mice. Science 261:1448–1451. 10.1126/science.8367726 [DOI] [PubMed] [Google Scholar]
- 15.Pearce EL, Shedlock DJ, Shen H. 2004. Functional characterization of MHC class II-restricted CD8+ CD4− and CD8− CD4− T cell responses to infection in CD4−/− mice. J. Immunol. 173:2494–2499. 10.4049/jimmunol.173.4.2494 [DOI] [PubMed] [Google Scholar]
- 16.Rahemtulla A, Kundig TM, Narendran A, Bachmann MF, Julius M, Paige CJ, Ohashi PS, Zinkernagel RM, Mak TW. 1994. Class II major histocompatibility complex-restricted T cell function in CD4-deficient mice. Eur. J. Immunol. 24:2213–2218. 10.1002/eji.1830240942 [DOI] [PubMed] [Google Scholar]
- 17.Beaumier CM, Harris LD, Goldstein S, Klatt NR, Whitted S, McGinty J, Apetrei C, Pandrea I, Hirsch VM, Brenchley JM. 2009. CD4 downregulation by memory CD4+ T cells in vivo renders African green monkeys resistant to progressive SIVagm infection. Nat. Med. 15:879–885. 10.1038/nm.1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murayama Y, Amano A, Mukai R, Shibata H, Matsunaga S, Takahashi H, Yoshikawa Y, Hayami M, Noguchi A. 1997. CD4 and CD8 expressions in African green monkey helper T lymphocytes: implication for resistance to SIV infection. Int. Immunol. 9:843–851. 10.1093/intimm/9.6.843 [DOI] [PubMed] [Google Scholar]
- 19.Murayama Y, Mukai R, Inoue-Murayama M, Yoshikawa Y. 1999. An African green monkey lacking peripheral CD4 lymphocytes that retains helper T cell activity and coexists with SIVagm. Clin. Exp. Immunol. 117:504–512. 10.1046/j.1365-2249.1999.00999.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vinton C, Klatt NR, Harris LD, Briant JA, Sanders-Beer BE, Herbert R, Woodward R, Silvestri G, Pandrea I, Apetrei C, Hirsch VM, Brenchley JM. 2011. CD4-like immunological function by CD4− T cells in multiple natural hosts of simian immunodeficiency virus. J. Virol. 85:8702–8708. 10.1128/JVI.00332-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Intlekofer AM, Banerjee A, Takemoto N, Gordon SM, Dejong CS, Shin H, Hunter CA, Wherry EJ, Lindsten T, Reiner SL. 2008. Anomalous type 17 response to viral infection by CD8+ T cells lacking T-bet and eomesodermin. Science 321:408–411. 10.1126/science.1159806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pearce EL, Mullen AC, Martins GA, Krawczyk CM, Hutchins AS, Zediak VP, Banica M, DiCioccio CB, Gross DA, Mao CA, Shen H, Cereb N, Yang SY, Lindsten T, Rossant J, Hunter CA, Reiner SL. 2003. Control of effector CD8+ T cell function by the transcription factor eomesodermin. Science 302:1041–1043. 10.1126/science.1090148 [DOI] [PubMed] [Google Scholar]
- 23.Khattar M, Miyahara Y, Schroder PM, Xie A, Chen W, Stepkowski SM. 2014. Interleukin-21 is a critical regulator of CD4 and CD8 T cell survival during priming under interleukin-2 deprivation conditions. PLoS One 9:e85882. 10.1371/journal.pone.0085882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crotty S. 2011. Follicular helper CD4 T cells (TFH). Annu. Rev. Immunol. 29:621–663. 10.1146/annurev-immunol-031210-101400 [DOI] [PubMed] [Google Scholar]
- 25.Vinuesa CG, Linterman MA, Goodnow CC, Randall KL. 2010. T cells and follicular dendritic cells in germinal center B-cell formation and selection. Immunol. Rev. 237:72–89. 10.1111/j.1600-065X.2010.00937.x [DOI] [PubMed] [Google Scholar]
- 26.Frentsch M, Stark R, Matzmohr N, Meier S, Durlanik S, Schulz AR, Stervbo U, Jürchott K, Gebhardt F, Heine G, Reuter MA, Betts MR, Busch D, Thiel A. 2013. CD40L expression permits CD8+ T cells to execute immunologic helper functions. Blood 122:405–412. 10.1182/blood-2013-02-483586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee PI, Ciccone EJ, Read SW, Asher A, Pitts R, Douek DC, Brenchley JM, Sereti I. 2009. Evidence for translocation of microbial products in patients with idiopathic CD4 lymphocytopenia. J. Infect. Dis. 199:1664–1670. 10.1086/598953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zonios DI, Falloon J, Bennett JE, Shaw PA, Chaitt D, Baseler MW, Adelsberger JW, Metcalf JA, Polis MA, Kovacs SJ, Kovacs JA, Davey RT, Lane HC, Masur H, Sereti I. 2008. Idiopathic CD4+ lymphocytopenia: natural history and prognostic factors. Blood 112:287–294. 10.1182/blood-2007-12-127878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spolski R, Leonard WJ. 2008. The Yin and Yang of interleukin-21 in allergy, autoimmunity and cancer. Curr. Opin. Immunol. 20:295–301. 10.1016/j.coi.2008.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spolski R, Leonard WJ. 2008. Interleukin-21: basic biology and implications for cancer and autoimmunity. Annu. Rev. Immunol. 26:57–79. 10.1146/annurev.immunol.26.021607.090316 [DOI] [PubMed] [Google Scholar]