ABSTRACT
Superinfection exclusion is a widespread phenomenon that prevents secondary infections by closely related viruses. The vaccinia virus A56 and K2 proteins in the cell membrane can prevent superinfection by interacting with the entry-fusion complex of subsequent viruses. Here, we described another form of exclusion that is established earlier in infection and does not require the A56 or K2 protein. Cells infected with one or more infectious virions excluded hundreds of superinfecting vaccinia virus particles. A related orthopoxvirus, but neither a flavivirus nor a rhabdovirus, was also excluded, indicating selectivity. Although superinfecting vaccinia virus bound to cells, infection was inhibited at the membrane fusion step, thereby preventing core entry into the cytoplasm and early gene expression. In contrast, A56/K2 protein-mediated exclusion occurred subsequent to membrane fusion. Induction of resistance to superinfection depended on viral RNA and protein synthesis by the primary virus but did not require DNA replication. Although superinfection resistance correlated with virus-induced changes in the cytoskeleton, studies with mutant vaccinia viruses indicated that the cytoskeletal changes were not necessary for resistance to superinfection. Interferon-inducible transmembrane proteins, which can inhibit membrane fusion in other viral systems, did not prevent vaccinia virus membrane fusion, suggesting that these interferon-inducible proteins are not involved in superinfection exclusion. While the mechanism remains to be determined, the early establishment of superinfection exclusion may provide a “winner-take-all” reward to the first poxvirus particles that successfully initiate infection and prevent the entry and genome reproduction of defective or less fit particles.
IMPORTANCE The replication of a virus usually follows a defined sequence of events: attachment, entry into the cytoplasm or nucleus, gene expression, genome replication, assembly of infectious particles, and spread to other cells. Although multiple virus particles may enter a cell at the same time, mechanisms exist to prevent infection by subsequent viruses. The latter phenomenon, known as superinfection exclusion, can occur by a variety of mechanisms that are not well understood. We showed that superinfection by vaccinia virus was prevented at the membrane fusion step, which closely followed virion attachment. Thus, neither gene expression nor genome replication of the superinfecting virus occurred. Expression of early proteins by the primary virus was necessary and sufficient to induce the superinfection-resistant state. Superinfection exclusion may be beneficial to vaccinia virus by selecting particles that can infect cells rapidly, excluding defective particles and synchronizing the replication cycle.
INTRODUCTION
The ability of an established virus infection to interfere with a secondary infection by a homologous virus was first described in bacteriophages and subsequently in animal and plant viruses with RNA and DNA genomes (1). The wide occurrence of superinfection exclusion (SIE) suggests that it has important consequences for virus replication, pathogenesis, and evolution. The mechanisms of SIE are varied and in many cases incompletely understood. Poxvirus SIE was observed in several early studies (2, 3) and characterized for vaccinia virus (VACV) by Christen et al. (4). They concluded, mainly based on UV inactivation of virus particles, that early gene expression by the primary virus was responsible for resistance to superinfection and that early gene expression by the secondary virus was prevented. Subsequent studies provided evidence that SIE can be mediated by a heterodimer formed by the A56 and K2 proteins on the cell membrane (5, 6), which interact with a protein complex on the virus surface that is required for fusion and entry (7, 8). Whether this mechanism, which was demonstrated at a late phase of virus replication, is related to the early SIE was not assessed. The exclusion mechanism(s) described above prevent infection by the mature virion (MV), which is composed of a nucleoprotein core surrounded by a single membrane that contains the fusion proteins (9). A second infectious form, called the extracellular enveloped virion (EV), contains an additional nonfusogenic membrane surrounding the mature virion (10). Doceul and collaborators (11) described another form of SIE in which the EV is repulsed from infected cells that have expressed the A33 and A36 proteins. Thus, poxviruses appear to have multiple ways of preventing superinfection.
Since the initial studies of SIE, much has been learned about the biology of poxviruses, making it worthwhile to reassess MV exclusion mechanisms (12). Four proteins are known to mediate attachment of MVs (13), and 11 or more proteins participate in the membrane fusion step (9). VACV entry can occur at the plasma membrane at neutral pH or through endocytic vesicles at low pH, resulting in the entry of the virus core into the cytoplasm (14, 15). The initial step of VACV entry consists of lipid mixing of the outer leaflets of viral and cellular membranes, a process known as hemifusion (16, 17). However, there are still significant gaps in our knowledge of the fusion mechanism and the roles of cellular signaling and receptor proteins in entry (18–20). The VACV core contains the ∼200,000-bp double-stranded DNA genome and a set of enzymes that enable the synthesis and modification of more than 100 early mRNAs. The early mRNAs encode proteins involved in host cell interactions, DNA replication, and intermediate-stage transcription; the intermediate and late mRNAs encode proteins for maturation and packaging of DNA and virion assembly (21, 22). Progeny virus particles are formed following genome replication and intermediate and late gene expression (23).
In the present study, we demonstrated that a primary VACV infection prevented entry of superinfecting virions at the membrane lipid-mixing (hemifusion) step. Induction of superinfection resistance required viral early RNA and protein synthesis by the primary virus and was differentiated from A56/K2-mediated exclusion. Thus, it is important to distinguish SIE of the secondary virus and induction of superinfection resistance by the primary virus, which occur at different steps. The ability of a single infectious virion to prevent the subsequent entry and replication of numerous virus particles suggests that early SIE provides a powerful selection for rapid virus entry and gene expression with important consequences for maintaining virus fitness.
MATERIALS AND METHODS
Cells and viruses.
Human HeLa and African green monkey kidney BS-C-1 and Vero cells were maintained in minimum essential medium with Earle's salts (EMEM) supplemented with 2.5% fetal bovine serum (FBS), 2 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin (Quality Biologicals). HEK293 cells (ATCC CCL-1573) and HEK293 cells expressing A56/K2 (6) were maintained in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% FBS and 2 mM l-glutamine. HEK293 cells expressing A56/K2 were cultured in the presence of 100 μg/ml of zeocin (Life Technologies). We used the following strains of VACV: Western Reserve (WR; ATCC VR-1354; GenBank accession number NC_006998), International Health Department-J (IHD-J; from S. Dales), MVA (ATCC VR-1508); and cowpox virus (CPXV) strain Brighton Red (ATCC VR-302). Recombinant strains of VACV WR included deletion mutants WRΔA56R, WRΔK2L, and WRΔA56RΔK2L (7); inducible mutants vA21Li (24), vG3Li (17), vH2Ri (25), vL1Ri (26), and vL3Li (27); yellow fluorescent protein (YFP) fusion protein mutant WR YFP-A4 (28); and WRvFire expressing luciferase (LUC) under a VACV synthetic early/late promoter (14). VACV strain IHD-J and CPXV constructs included IHD-J GFP-A4 (29), IHD-JvFire (29), and CPXVvFire (30). The VACV WR recombinant possessing two premature stop codons in the F11L open reading frame (ORF) was a kind gift of Nissin Moussatche (University of Florida). Vesicular stomatitis virus (VSV) strain Indiana was from ATCC (VR-1238); West Nile virus (WNV) reporter virus particles (RVPs) expressing renilla LUC (31) were a generous gift of Theodore Pierson (NIAID, NIH).
Recombinant VACV construction.
Recombinant VACV (parental strain WR) expressing green fluorescent protein (GFP) under a synthetic early/late VACV promoter (32) was generated by inserting the GFP ORF between the VACV F12L and F13L gene loci. Recombinant VACV (parental strain IHD-J) expressing the far-red fluorescent protein HcRed1 under a synthetic early/late VACV promoter was generated by inserting the HcRed1 ORF between the VACV F12L and F13L gene loci. The recombinant IHD-J ΔF11L was generated by replacing the F11L ORF with that of GFP (under a synthetic early/late VACV promoter). In each case, plaques containing recombinant viruses expressing the appropriate fluorescent protein were identified, and clonal isolates were obtained after five rounds of plaque purification. The correct site of recombination was verified by PCR and sequence analysis.
Virus purification and titration.
BS-C-1 cells were infected with VACV (in the presence or absence of inducer) for 48 h. Intracellular virus particles were purified by sedimentation twice through a sucrose cushion and banding on a sucrose gradient as described previously and stored at −80°C, and the infectious titer was determined by plaque assay on BS-C-1 cells (33, 34). The number of virus particles in purified virus was estimated from the optical density at 260 nm (33).
LUC entry assay.
HeLa cells seeded in 48-well plates were uninfected or infected with 10 PFU/cell of primary virus. Purified LUC-encoding MVs (3 PFU/cell) were added, and the cells were incubated at 37°C for 150 min unless indicated otherwise. Cells were washed with phosphate-buffered saline (PBS) and incubated with cell culture lysis reagent (Promega) for 30 min at room temperature with gentle agitation. LUC activity in cellular extracts was measured according to the manufacturer's protocol (luciferase assay system; Promega) and quantified on a Berthold Sirius luminometer (Berthold Detection Systems).
Flow cytometry of dually infected cells.
HeLa cells were uninfected or infected with IHD HcRed-encoding virus at several multiplicities for 180 min at 37°C. Secondary VACV WR GFP-encoding virus at 4 PFU/cell then was added and cultures incubated for approximately 16 h at 37°C. In a similar manner, cells were coinfected with IHD HcRed-encoding virus at several multiplicities and WR GFP-encoding virus (4 PFU/cell). Cells were fixed in PBS containing 4% paraformaldehyde and analyzed using a FACSCalibur flow cytometer. Data were processed with FloJo software for the percentage of HcRed+ cells and the levels of GFP expression (mean fluorescence intensity [MFI]) among HcRed+ cells.
Virus-cell binding assay.
HeLa cells seeded in 48-well plates (8.0 × 104 cells per well) were uninfected or infected with primary virus at a multiplicity of 10 PFU per cell, chilled to 4°C, and incubated with 5 PFU per cell of secondary WR YFP-A4 virions for 60 min at 4°C. Cells were washed twice with cold PBS (Quality Biological) to remove unbound virus and fixed in 4% paraformaldehyde–PBS for 30 min at 4°C. YFP-positive cells were quantified using a FACSCalibur flow cytometer (BD Biosciences), and data were processed with FloJo software (Tree Star, Inc.).
Fluorescent labeling of virus particles and virus-cell membrane fusion assay.
The loading of purified MVs with DiD (Life Technologies) for assessment of virus-cell membrane fusion has been described (17). HeLa cells were left uninfected or were infected with 10 PFU/cell of primary virus for various times and superinfected with DiD-loaded secondary virus. After 90 min, cells were washed, trypsinized, sedimented, and fixed in PBS containing 4% paraformaldehyde. DiD-positive cells were quantified using a FACSCalibur flow cytometer, and data were processed with FloJo software. The loading of purified MVs with R18 (Life Technologies) for assessment of the kinetics of virus-cell membrane fusion has been described (17). R18 fluorescence was monitored with a Fluoro-Max3 spectrofluorometer outfitted with a Peltier sample cooler (Horiba Jobin Yvon) and a temperature control unit to maintain the samples at 37°C.
Stimulation of virus entry by low-pH treatment.
Low-pH stimulation of virus entry was performed as described previously (14). Infected cells were incubated for 3 min in prewarmed 37°C PBS with Ca2+ and Mg2+ at pH 7.4 or PBS with Ca2+ and Mg2+ supplemented with 1 mM 2-morpholinoethanesulfonic acid adjusted to pH 5.0 with HCl. After removal of PBS, the pH was neutralized by one wash with EMEM containing 2.5% FBS. Cells were incubated in prewarmed EMEM with 2.5% FBS for 120 min at 37°C, and LUC was quantified as described above.
Antibody neutralization of VACV.
Equivalent amounts of purified primary WR virus were incubated with preimmune rabbit sera (1:450 dilution; Covance), anti-VACV polyclonal rabbit sera (1:450 dilution) (35), or 2.2 μg/ml anti-L1 monoclonal antibody (36) in EMEM without serum for 30 min at room temperature. Infectious virus titers were determined by serial dilution and plaque assay as described above.
Inhibitor treatments.
HeLa cells were left untreated or were pretreated with the indicated concentrations of the inhibitors (Sigma-Aldrich) amanitin (10 μg/ml), cordycepin (40 μg/ml), actinomycin D (4 μg/ml), emetine (2 μM), cycloheximide (CHX; 66 μM), and anisomycin (1 μM) for 30 min at 37°C. Inhibitor concentrations were maintained throughout the course of the experiments.
UV irradiation of primary virus.
Equivalent numbers of purified MVs (2.8 × 108 PFU) of VACV IHD-J and recombinant IHD-J GFP-A4 and IHD-JvFire were diluted into approximately one ml of PBS plus 0.2% bovine serum albumin (BSA). Virus aliquots were placed in a 6-well tissue culture dish on ice and exposed to UV for the indicated length of time using a SuperBright II 3000 series box equipped with an SW lamp (UV peak, 254 nm) placed approximately 35 mm above the virus samples. Virus samples then were assessed for cell attachment, virus-cell membrane fusion, early and late reporter gene expression, and infectivity utilizing the assays as described above. Virus recovery following UV exposure was assessed by analyzing viral 4b core protein (encoded by the A3L gene) by Western blotting using rabbit polyclonal antibody raised against A3 protein (R. Doms and B. Moss, unpublished data).
Actin staining of cells.
HeLa cells plated on glass coverslips were left untreated or were pretreated with actinomycin D (4 μg/ml) or anisomycin (1 μM) for 30 min at 37°C. Inhibitor concentrations were maintained throughout the course of the experiments. Cells were cooled and incubated with 10 PFU/cell of purified virions (strain IHD-J unless otherwise indicated) for 60 min at 4°C. Cells then were washed twice with cold PBS to remove unbound virus, and prewarmed EMEM plus 2.5% FBS was added. Cells were incubated at 37°C for the indicated length of time, at which point cells were washed and fixed in PBS with 4% paraformaldehyde for 10 min at room temperature. Cells then were permeabilized with PBS plus 0.1% Triton X-100 for 10 min at room temperature, washed, blocked in PBS plus 1% BSA for 30 min at room temperature, and then incubated with Alexa Fluor 488 phalloidin (Life Technologies) for 20 min at room temperature. Coverslips were mounted using ProLong Gold (Life Technologies) and imaged on a Leica SP5 inverted four-channel microscope. Time zero indicates that samples were fixed immediately after the 4°C binding stage.
Electron microscopy.
Infected HeLa cells in 60-mm-diameter wells were prepared for cryosectioning and immunogold labeling as described previously (37). Cryosections were picked up on grids, thawed, washed free of sucrose, and stained with rabbit antibody to GFP (Abcam, Eugene, OR) and protein A conjugated to 10-nm gold spheres (University Medical Center, Utrecht, Netherlands). Specimens were viewed with a FEI Tecnai Spirit transmission electron microscope (FEI, Hillsboro, OR).
Heterologous virus infections and superinfections.
For VSV superinfection, HeLa or Vero cells were left untreated or were pretreated with dynasore (80 μM; Sigma-Aldrich) for 30 min at 37°C; where indicated, dynasore concentrations were maintained throughout the experiment. Cells then were left uninfected or were infected with primary IHD-J VACV (10 PFU/cell) for 240 min at 37°C. Secondary VSV (3 PFU/cell) was added and cultures incubated for 21 h at 37°C. Whole-cell lysates were resolved by SDS-PAGE on 4 to 12% Novex NuPAGE acrylamide gels (Life Technologies), and proteins were transferred to nitrocellulose membranes. Membranes were blocked with 5% nonfat milk in PBS containing 0.05% Tween 20, incubated with primary antibody overnight at 4°C, washed, incubated with species-appropriate secondary antibody conjugated with IRDye 680 (LI-COR Biosciences), and analyzed using a LI-COR Odyssey infrared imager (LI-COR Biosciences). The intensity of protein bands was quantitated with ImageJ software (NIH). Primary antibodies against VSV G protein (Sigma-Aldrich) and cellular glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Covance) were used.
For WNV superinfection, HeLa cells were left uninfected or were infected with primary VACV WR (10 PFU/cell) as indicated for 180 min at 37°C. Secondary WNV reporter particles (3 particles/cell) were added and cultures incubated for 20 h at 37°C. Cells then were incubated with renilla luciferase assay buffer (Promega) for 30 min at room temperature with gentle agitation. Renilla LUC activity in cellular extracts was measured according to the manufacturer's protocol (renilla luciferase assay system; Promega) and quantified on a Berthold Sirius luminometer.
RESULTS
Resistance to superinfection.
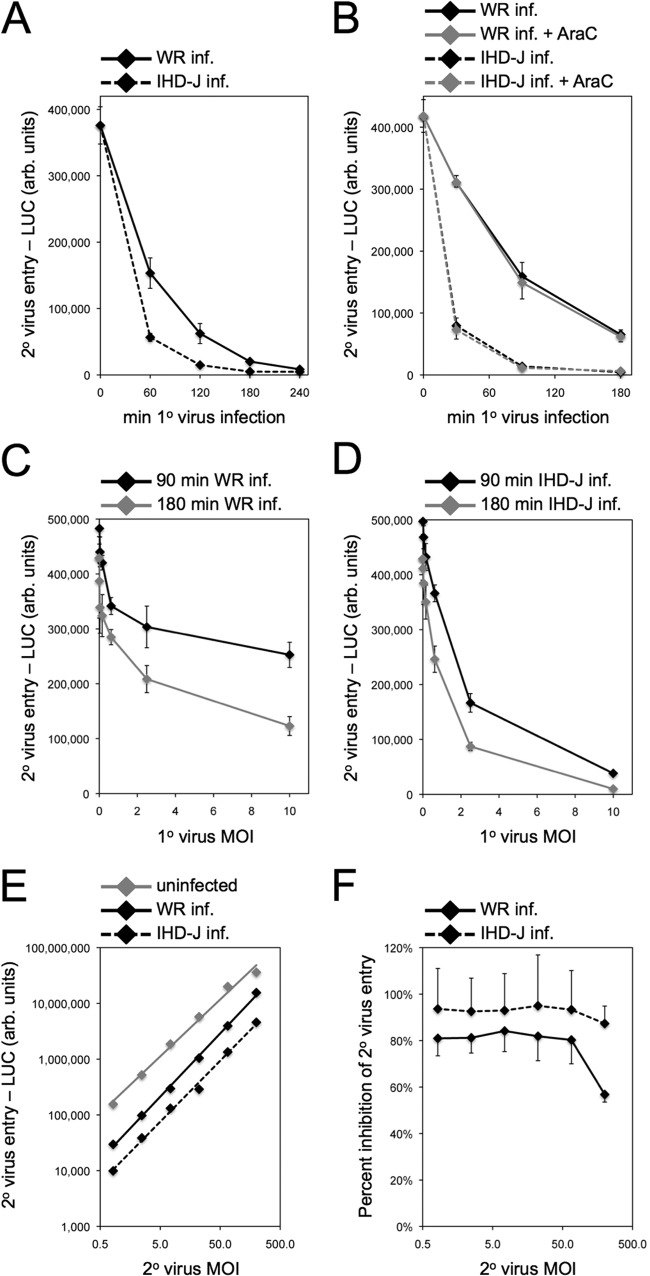
We employed a sensitive and quantitative assay using VACV WRvFire (14), which encodes firefly LUC regulated by an early/late promoter, to detect entry and gene expression by a superinfecting virus. Two commonly used laboratory strains of VACV, WR and IHD-J, were tested for their ability to induce superinfection resistance. HeLa cells, which are permissive for both VACV strains, were infected with 10 PFU/cell of WR or IHD-J and, at intervals, were secondarily infected with WRvFire. At 150 min after each WRvFire infection, LUC activity was measured. Resistance to superinfection was established between 1 and 4 h after primary virus infection, as judged by a profound reduction in LUC activity following secondary infection with WRvFire (Fig. 1A). The accelerated induction of resistance by IHD-J correlated with its more rapid entry and early gene expression compared to that of WR (data not shown). The kinetics of establishing resistance was unaltered by the presence of cytosine arabinoside (AraC), an inhibitor of DNA replication (Fig. 1B), consistent with the early time frame of the process. The establishment of resistance was dependent on a primary virus multiplicity sufficient to infect all cells (Fig. 1C and D). Remarkably, the extent of SIE was constant from 50 to 200 PFU of superinfecting virus (Fig. 1E and F). SIE could be established by primary infection of a variety of cells, including BS-C-1, RK13, Vero, BHK-21, CHO, and 293T, in addition to HeLa (data not shown).
FIG 1.
Kinetics of acquisition of resistance to superinfection and effects of primary (1°) and secondary (2°) virus multiplicities. (A and B) Kinetics. HeLa cells were infected (inf.) with 10 PFU/cell of VACV strain WR or IHD-J for 0 to 240 min in the absence or presence of AraC. At intervals, cells were superinfected with 3 PFU/cell of recombinant WRvFire for 150 min. LUC activity was determined and plotted as arbitrary (arb.) units versus length of time of primary virus infection prior to superinfection. (C and D) Effect of primary virus multiplicity. Cells were infected with 0 to 10 PFU/cell of VACV WR or IHD-J for either 90 or 180 min and superinfected as described for panels A and B. LUC levels were assayed and plotted as a function of primary virus multiplicity of infection (MOI). (E and F) Effect of superinfecting virus multiplicity. Cells were uninfected or infected with 10 PFU/cell of VACV WR or IHD-J for 180 min and then superinfected with 0.5 to 200 PFU/cell of VACV WR encoding LUC for 150 min. LUC was assayed and plotted as a function of secondary virus MOI in panel E and is plotted in panel F as percent inhibition of secondary virus entry as determined from LUC activities.
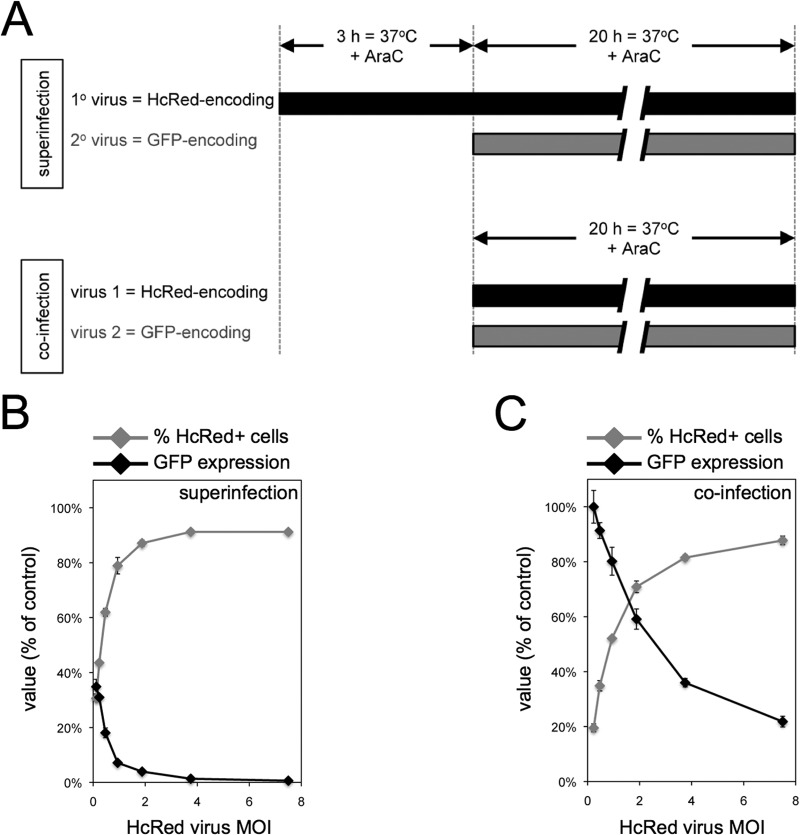
The results described above suggested that no more than a few infectious virus particles per cell were required to establish potent resistance to superinfection. Flow cytometry was used to more quantitatively evaluate this parameter (Fig. 2A). Cells were infected with a primary virus encoding red fluorescent protein (HcRed) under an early viral promoter at a multiplicity of 0 to 7.5 PFU per cell. The cells were coinfected or superinfected after 3 h with a second virus encoding GFP under an early viral promoter at a multiplicity of 4 PFU per cell. A DNA synthesis inhibitor was added to prevent progeny virus formation and spread. The cells were analyzed in a flow cytometer, and the percentage of HcRed+ cells at each multiplicity and their GFP fluorescence intensities (relative to that of an infection without HcRed virus) were determined (Fig. 2B). When the multiplicity of the primary virus infection was 7.5 PFU/cell, approximately 91% of the cells were HcRed+, and GFP expression in those cells was less than 1% of that of control cells not infected with HcRed-encoding virus. As the multiplicity of the primary virus infection decreased toward 1 PFU/cell, the population of HcRed+ cells was reduced; however, the level of GFP expression remained low among those cells scored as HcRed+ (Fig. 2B). For example, a primary virus multiplicity of 0.47 PFU/cell resulted in 62% HcRed+ cells in which GFP expression was reduced by an average of 82% compared to control cells not infected with HcRed-encoding virus. Under the latter conditions, the median inhibition of GFP expression in HcRed+ cells compared to that of HcRed− cells was approximately 90%. These results suggest that a single primary infectious particle can trigger resistance to superinfection within 3 h. In contrast, high GFP expression occurred under coinfection conditions: a virus multiplicity of 0.47 PFU/cell of HcRed-expressing virus together with 4 PFU/cell of GFP-expressing virus resulted in approximately 35% HcRed+ cells in which there was only a 9% reduction in GFP expression compared to that of control cells not infected with HcRed-encoding virus (Fig. 2C).
FIG 2.
Single infectious primary virus can trigger resistance to superinfection. (A) Schematic of superinfection and coinfection protocols. Under superinfection conditions, HeLa cells were infected with 0 to 7.5 PFU/cell of primary HcRed-encoding virus for 3 h in the presence of AraC. Cells then were superinfected with 4 PFU/cell of secondary GFP-encoding virus for 20 h in the presence of AraC. Under coinfection conditions, cells were infected with a mixture of HcRed-encoding virus and 4 PFU/cell of GFP-encoding virus for 20 h in the presence of AraC. Cells were harvested and analyzed by flow cytometry for HcRed and GFP fluorescence. For superinfection (B) and coinfection (C) conditions, the percentages of HcRed+ cells were quantified and plotted as a function of HcRed-encoding virus MOI. The GFP mean fluorescence in HcRed+ cells was quantified and plotted as the percentage of fluorescence in control cells infected with GFP-encoding virus.
Determination of the step at which superinfection was halted.
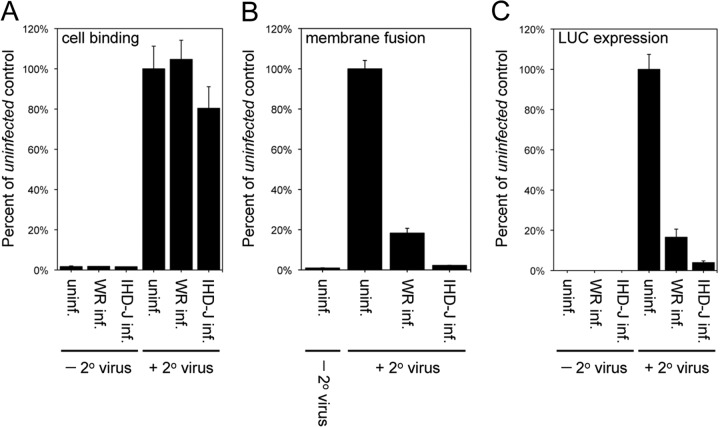
We investigated the ability of the superinfecting virus to undergo cell attachment, virus-cell membrane fusion, and gene expression following virus core entry. For virus attachment, uninfected cells or cells that had been infected with VACV strain WR or IHD-J for 120 min were cooled to 4°C and incubated for 60 min with the recombinant virus WR YFP-A4, in which a core protein is fused to YFP. Cells then were washed and processed by flow cytometry to determine the association of the secondary virus with cells. Uninfected cells and cells infected with either WR or IHD-J primary virus permitted attachment of the YFP-A4 virus to similar levels (Fig. 3A), indicating the SIE occurs at a subsequent step.
FIG 3.
SIE occurs at the virus-cell membrane fusion step. (A) Cell binding step. Cells were left uninfected or infected with 10 PFU/cell of VACV WR or IHD-J for 120 min and incubated with 5 PFU/cell of YFP-tagged secondary VACV WR at 4°C for 60 min. Cells were analyzed by flow cytometry to determine the number and mean fluorescence of YFP+ cells. Data are presented as the percentage of YFP-tagged virus bound to primary virus-infected cells compared to uninfected control cells. (B) Hemifusion step. Cells were uninfected or infected with 10 PFU/cell of VACV WR or IHD-J for 120 min. The cells were superinfected with 3 PFU/cell of DiD-loaded secondary VACV WR at 37°C for 90 min and analyzed by flow cytometry to determine the number of DiD+ cells and the DiD mean fluorescence intensity. The latter values were normalized to the values obtained for the uninfected control cells infected with DiD-loaded virus. (C) LUC expression. Cells were uninfected or infected with 10 PFU/cell of VACV WR or IHD-J for 120 min and superinfected with 3 PFU/cell of WRvFire for 150 min. Cells then were lysed and LUC activity quantified; the data were normalized to values obtained for the uninfected control cells infected with WRvFire.
The next stage in VACV entry is fusion of viral and cell membrane lipid bilayers, a process known as hemifusion (16, 17). Lipid mixing can be measured by loading a self-quenching dye in the viral membrane; when fusion with the cell membrane occurs, the dye is diluted, resulting in increased fluorescence. We incubated virus labeled with the fluorescent membrane probe DiD with uninfected cells or cells that had been infected with WR or IHD-J. The fluorescence was reduced by 80% and 95% in cells that had been previously infected with WR and IHD-J, respectively, compared to uninfected cells (Fig. 3B). The extent of inhibition at the virus-cell membrane fusion stage was similar to that measured by LUC expression (Fig. 3C). Thus, SIE occurs at a step immediately after attachment.
Analysis of SIE by electron microscopy.
Transmission electron microscopy was used to directly visualize the fate of the superinfecting virus. HeLa cells were infected with 10 PFU/cell of IHD-J and subsequently infected at neutral or low pH with 100 PFU/cell of recombinant WR containing YFP fused to the A4 core protein. Control cells were infected with only the WR YFP-A4 virus. The cells were fixed at 10 min after low-pH infection, which synchronizes fusion at the plasma membrane, and 30 min after neutral-pH infection to allow greater endosomal uptake. Immunogold labeling with antibody that recognized YFP was performed to distinguish the superinfecting virus from the primary virus, which had no YFP. Since immunostaining was performed following cryosectioning, the intact MVs located at the surface and in endocytic vesicles as well as cytoplasmic cores were labeled with antibody. We distinguished cores from MVs in endosomes by resolving their membranes, and representatives are shown in Fig. 4A to D. Examination of 50 control cells revealed 140 and 192 immunogold-labeled cores under neutral- and low-pH conditions, respectively, whereas 0 and 25 immunogold-labeled cores were detected after superinfection at neutral and low pH. Although labeled MVs were found in cytoplasmic vesicles of superinfected and control cells, the numbers were too low for accurate quantification. These data indicated that core entry was inhibited regardless of pathway.
FIG 4.
Analysis of SIE by transmission electron microscopy. HeLa cells were mock infected (A and B) or infected (C and D) with IHD-J (10 PFU/cell). After 4 h, the cells were inoculated with 100 PFU/cell of VACV WR containing YFP fused to the A4 core protein. After adsorption, cells were briefly incubated with buffer at pH 7.3 (A and C) or pH 5.0 (B and D) at 37°C, and the incubation continued in regular medium. The cells were fixed at 30 min after pH 7.3 treatment or 10 min after pH 5.0 treatment. After cryosectioning, the cells were stained with antibody to YFP followed by 10-nm gold spheres attached to protein A. Arrows point to cores; arrowheads point to MVs in endosomes. Magnifications are indicated.
Primary virus attachment and entry required for establishing resistance to superinfection.
Thus far, we mainly have been describing the step at which superinfection is blocked. We now turn to the requirements for establishing resistance to superinfection by the primary virus. The rapid establishment of resistance, even in the presence of AraC, indicated that the block was triggered at an early step in infection. The steps preceding viral DNA replication include virus attachment, membrane fusion, core entry, early gene transcription, and translation. To differentiate these steps, we incubated the primary virus with polyclonal antibody made in rabbits infected with VACV, which prevents virus attachment (J. Laliberte, unpublished data), or with a monoclonal antibody to the viral L1 protein, which allows attachment and hemifusion but prevents core entry (17). At the concentrations used, the polyclonal and monoclonal antibodies reduced infectivity by 88% and 97%, respectively (data not shown). Treatment with either antibody prevented the primary virus from inducing resistance to superinfection (Fig. 5A), suggesting that the critical event performed by the primary virus occurred after the initial membrane fusion step.
FIG 5.
Cell attachment and entry of the primary virus are necessary to induce resistance to superinfection. (A) Effect of antibodies on establishment of superinfection resistance. VACV WR that had been incubated with PBS, control (cntrl) serum, polyclonal VACV antibody, or monoclonal L1 antibody was adsorbed to cells at 37°C for 150 min. Cells then were washed extensively and incubated with 3 PFU/cell of WRvFire at 37°C for 120 min, and LUC activity was determined. Data are presented as percentages of secondary virus entry into uninfected control cells (set to 100%). (B) Relative abilities of EFC-positive and EFC-negative viruses to establish resistance to superinfection. Cells were uninfected or infected with equivalent numbers of IHD-J or EFC-positive or EFC-negative virus particles at 37°C for 180 min and then superinfected with 3 PFU/cell of WRvFire for 120 min. LUC activity was determined and is presented as a percentage of secondary virus entry into uninfected control cells.
The poxvirus entry-fusion complex (EFC) consists of 11 or more viral proteins, embedded in the MV membrane, that are required for membrane fusion and core entry (9, 17). The availability of conditional lethal inducible WR mutants allowed us to prepare MVs containing or lacking the specific EFC proteins A21, G3, H2, and L1. The MVs formed in the absence of inducer and which lacked one of the above-mentioned EFC proteins were impaired in infectivity by 95 to 99% compared to the controls formed in the presence of inducer (data not shown). Cells were infected with an equivalent number of EFC− and EFC+ virus particles for 3 h and then superinfected with WRvFire. The EFC− virus particles, unlike the EFC+ virus particles, were unable to prevent LUC expression by the superinfecting virus (Fig. 5B), indicating the requirement for core entry by the primary virus to induce superinfection resistance.
Primary virus gene expression required for establishing resistance to superinfection.
We next determined whether gene expression by the primary virus was needed to induce resistance to superinfection. However, the LUC assay is unsuitable for this purpose, as potent inhibitors of RNA and protein synthesis are not readily reversible. Therefore, we used membrane fusion as the read-out, since lipid mixing occurs independently of RNA and protein synthesis. Cordycepin and actinomycin D are general inhibitors of RNA synthesis, affecting both viral and cellular transcription, whereas α-amanitin is a specific inhibitor of cellular RNA polymerase II. Preliminary experiments showed that cordycepin and actinomycin D, in contrast to α-amanitin, inhibited VACV-mediated LUC expression by more than 99% (data not shown). To measure hemifusion, uninfected cells or cells infected with primary virus in the absence or presence of transcription inhibitors were superinfected with DiD-labeled virus particles. Cells were analyzed by flow cytometry for DiD fluorescence. In the absence of a primary virus infection, transcription inhibitors had no effect on fusion of DiD-loaded particles with cells (Fig. 6A). As expected, cells that were infected with WR or IHD-J in the absence of inhibitors became resistant to superinfection, as demonstrated by inhibition of lipid mixing following infection with DiD-loaded virus (Fig. 6A). In contrast, cells infected with primary virus in the presence of cordycepin or actinomycin D did not become resistant to superinfection, as shown by the similarity of virus-cell membrane fusion relative to controls (Fig. 6A). The inability of α-amanitin to prevent the induction of resistance to superinfection further suggested that viral RNA synthesis, rather than cellular mRNA synthesis, was required.
FIG 6.
Transcriptional and translational requirements for establishment of superinfection resistance. (A) Effects of transcription inhibitors. Cells were left untreated (UT) or were treated with α-amanitin (10 μg/ml), cordycepin (40 μg/ml), or actinomycin D (act. D; 4 μg/ml) for the duration of the experiment. Following 30 min of inhibitor pretreatment, cells were uninfected (uninf.) or infected with 10 PFU/cell of VACV WR or IHD-J for 150 min at 37°C. Cells then were superinfected with 3 PFU of DiD-loaded secondary VACV WR at 37°C for 90 min. Cells were analyzed by flow cytometry to determine DiD mean fluorescence (DiD fluor.) intensities (MFI), which were normalized to the values obtained for the untreated and uninfected control (cntrl) cells infected with DiD-loaded virus. (B) Effects of translation inhibitors. Protocols were the same as those in panel A, except that emetine (2 μM), cycloheximide (CHX; 66 μM), and anisomycin (1 μM) were used. (C) Inability of UV-irradiated VACV to establish superinfection resistance. Equivalent PFU of IHD-J were irradiated with UV light for the indicated number of seconds (sec UV irr.). Cells were uninfected or infected with irradiated virus particles (preirradiation equivalent of 10 PFU/cell) at 37°C for 180 min. Cells then were superinfected with 3 PFU/cell of LUC-expressing secondary VACV for 150 min. Cells then were lysed and LUC levels measured. (D) Inability of transcription-deficient VACV to establish resistance to superinfection determined by LUC expression. Cells were left uninfected or were infected with 10 PFU/cell of primary VACV containing (+) or lacking (−) L3 protein for various lengths of time at 37°C. Cells then were superinfected with 3 PFU/cell of WRvFire for 150 min at 37°C. LUC activity was determined and plotted as a percentage of the uninfected cell control value. (E) Inability of transcription-deficient VACV to establish resistance to superinfection determined by hemifusion assay. Cells were untreated (UT) or were treated with the indicated inhibitors for the duration of the experiment as described for panels A and B. Following 30 min of inhibitor pretreatment, cells were left uninfected or were infected with 10 PFU/cell of vL3+ or vL3− VACV for 150 min at 37°C. Cells then were superinfected with 3 PFU/cell of DiD-loaded VACV WR for 90 min at 37°C and analyzed by flow cytometry to determine the MFI. Data for DiD MFI were normalized to the values obtained for the untreated and uninfected control cells infected with DiD-loaded virus.
The inhibition of viral mRNA synthesis by cordycepin and actinomycin D also results in the inhibition of viral protein synthesis. However, we could readily distinguish between these two steps in gene expression, since VACV early transcription is not dependent on de novo protein synthesis. Indeed, viral early RNA synthesis is enhanced by translation inhibitors (38). Three potent translation inhibitors, emetine, cycloheximide, and anisomycin, were employed. Preliminary experiments indicated that these inhibitors reduced LUC expression by more than 99% (data not shown). To measure their effect on the induction of resistance to superinfection, uninfected cells or cells infected with primary virus in the absence or presence of the protein synthesis inhibitors were incubated with DiD-labeled secondary virus particles and analyzed by flow cytometry. In the absence of a primary virus infection, translation inhibitors had no effect on virus-cell membrane fusion (Fig. 6B). Cells that were infected with VACV WR or IHD-J in the absence of inhibitors became resistant to superinfection, as demonstrated by inhibition of lipid mixing following infection with DiD-loaded virus (Fig. 6B). In contrast, cells infected with primary virus in the presence of translation inhibitors did not acquire resistance, as shown by the similarity of virus-cell membrane fusion of the superinfecting virus relative to controls (Fig. 6B). Accordingly, virus transcription by the primary virus was not sufficient to induce resistance to superinfection.
Except for α-amanitin, the inhibitors used above prevented both viral and cellular RNA and protein synthesis. We used two additional approaches to distinguish between viral and cellular functions needed to establish resistance to superinfection. The first method was to UV inactivate the primary virus. The recovery of virus after irradiation was assessed by a Western blot probed with antibody to the 4b core protein. Cell binding appeared moderately reduced, but virus-cell membrane fusion actually increased with increasing irradiation (data not shown). However, UV irradiation reduced infectivity and gene expression to nearly background levels (data not shown). Significantly, irradiation of the primary virus reduced its ability to induce resistance to superinfection (Fig. 6C), consistent with a need for virus gene expression.
For another approach to the question of whether viral or cellular gene expression was needed for resistance to superinfection, we used the recombinant VACV vL3Li, which is an inducible mutant that produces virus particles lacking L3 protein under nonpermissive conditions that are defective in early gene expression (27). Cells were infected with equivalent numbers of L3+ and L3− virions for various lengths of time and then superinfected with WRvFire and assayed for LUC expression as an indicator of successful secondary virus entry. Compared to the superinfection resistance triggered by the L3+ primary virus, L3− particles allowed superinfection of secondary virus measured by LUC expression (Fig. 6D). Similar results were obtained using DiD-labeled secondary virus and measuring hemifusion fluorescence by flow cytometry: L3+ virus induced resistance to superinfection, whereas L3− virus permitted membrane fusion, as did L3+ virus in the presence of actinomycin D or anisomycin (Fig. 6E).
SIE correlated with but was not dependent on extensive actin cytoskeletal changes.
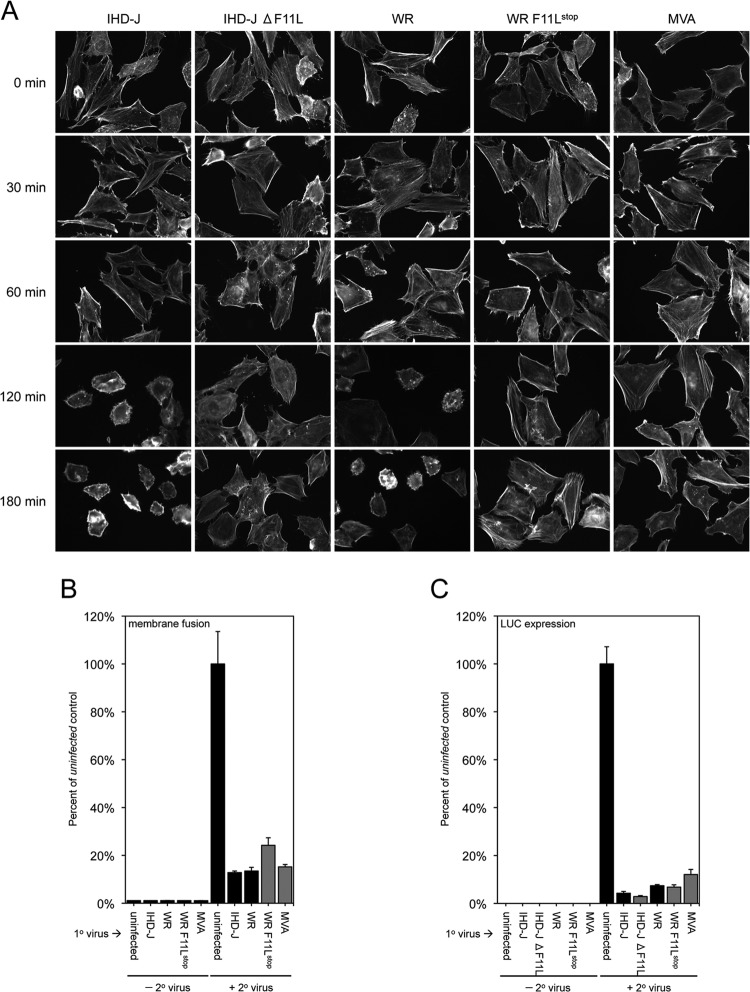
We considered that changes in the actin cytoskeleton were involved in SIE for the following reasons. Like SIE, VACV-induced cytoskeletal changes are dependent on viral early protein synthesis (39, 40). In addition, inhibitors of actin dynamics prevent VACV entry at the hemifusion stage (17). Following primary infection, cell rounding, loss of actin stress fibers, and intense actin staining of the periphery was seen as previously described (41). These changes were evident at 30 min after infection and still were present at 180 min, the time during which resistance to superinfection was established. Although cytoskeletal changes correlated with acquisition of resistance to superinfection, it did not mean that these changes were the cause of the resistance. However, the observation that the cytoskeletal changes were prevented by actinomycin D and anisomycin (data not shown), conditions in which resistance to superinfection was not established, was consistent with a causative role.
We further considered that if the acquisition of resistance to superinfection was dependent on cytoskeletal changes, then a mutant VACV defective in this process should be unable to establish resistance. The F11 protein was previously shown to be involved in remodeling the actin network, cell motility, and release of virus particles (42–46). To test our hypothesis, we acquired an F11L mutant with stop codons near the N terminus in the VACV WR background (47), constructed a new F11L deletion mutant in the VACV IHD-J background, and also employed the attenuated MVA strain of VACV, which has an interruption in the F11L ORF (48). We confirmed that each of these mutants was defective in causing the extensive cytoskeletal changes that occur following infection with wild-type VACV (Fig. 7A). Nevertheless, each of the mutants induced potent resistance to superinfection, as measured by either hemifusion or LUC expression (Fig. 7B and C). Thus, the F11 protein was not the mediator of superinfection resistance, and the establishment of such resistance was not dependent on extensive cytoskeletal changes.
FIG 7.
Ability of F11L mutants to establish resistance to superinfection. (A) Inability of F11L mutants to induce cytoskeletal changes. HeLa cells were mock infected or infected with 10 PFU/cell of the indicated VACV. At various times, the cells were stained with Alexa Fluor 488 phalloidin and examined by confocal microscopy. The same magnification was used for all samples. (B) Hemifusion step. HeLa cells were uninfected or infected with 10 PFU/cell of the indicated VACV for 180 min. The cells then were superinfected with 3 PFU/cell of DiD-loaded secondary VACV WR at 37°C for 90 min and analyzed by flow cytometry to determine the number of DiD+ cells and the DiD mean fluorescence intensity. The latter values were normalized to the values obtained for the uninfected control cells infected with DiD-loaded virus. (C) LUC expression. HeLa cells were uninfected or infected with 10 PFU/cell of the indicated VACV for 180 min and then superinfected with 3 PFU/cell of WRvFire for 150 min. Cells then were lysed and LUC activity quantified; the data were normalized to values obtained for the uninfected control cells infected with WRvFire.
A56 and K2 proteins not required for early acquisition of resistance to superinfection.
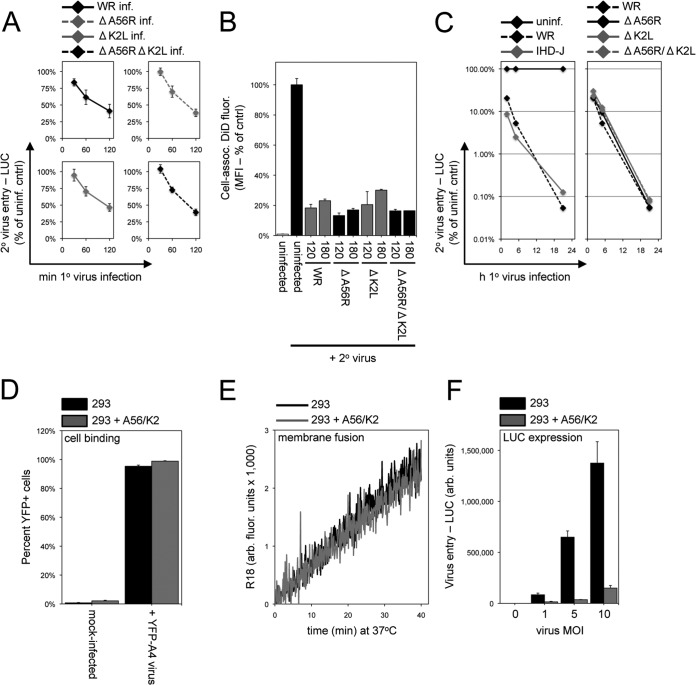
Previous studies established a role for the poxvirus A56R and K2L gene products in preventing superinfection of MVs at late times after infection (5) and in stably transfected uninfected cells (6). Although A56 is expressed early in infection, K2 is a late protein, making it unlikely that the early establishment of resistance to superinfection described here could be due to the combined action of the two proteins. Nevertheless, we decided to test this possibility directly using the replication-competent single-deletion A56R (WRΔA56R) and K2L (WRΔK2L) mutant viruses as well as the double-deletion (WRΔA56RΔK2L) mutant virus (7). Cells were infected with the wild-type WR and deletion mutants and then superinfected with WRvFire. The kinetics and extent of SIE, measured by reduction of LUC expression, were similar in each case (Fig. 8A). We also tested the acquisition of superinfection resistance by analyzing virus-cell membrane fusion through flow cytometry. An 80 to 90% inhibition of virus-cell membrane fusion between secondary DiD-labeled virions with cells infected first with WR, A56R, and K2L deletion viruses was observed at early times after primary virus infection (Fig. 8B). Thus, neither A56 nor K2 was required for the establishment of superinfection resistance, assayed by either membrane fusion or early gene expression. In view of the previous study (5), we considered the possibility that the early acquisition of resistance was time limited in duration and that A56 and K2 might be needed to maintain resistance at late times. Nonetheless, we found that the ability to inhibit superinfection continued to increase with time in cells infected with WR or the A56 and K2 deletion mutants (Fig. 8C).
FIG 8.
A56/K2 was not required for early SIE. (A) Establishment of superinfection resistance by A56/K2 deletion mutants analyzed by LUC expression. Cells were uninfected or infected with 10 PFU/cell of VACV WR or the WR deletion mutant ΔA56R, ΔK2L, or ΔA56RΔK2L at 37°C for various lengths of time and then superinfected with 3 PFU/cell of WRvFire for 150 min at 37°C. LUC activity was determined and is plotted as a percentage of the uninfected cell control activity. (B) Establishment of superinfection resistance by A56/K2 deletion mutants analyzed by hemifusion assay. Cells were uninfected or infected with 10 PFU/cell of wild-type VACV WR or equivalent numbers of particles of deletion mutants for 120 or 180 min and then superinfected with 3 PFU/cell of DiD-loaded VACV WR at 37°C for 90 min. The DiD MFI of the cells was determined by flow cytometry and normalized to the values obtained for the uninfected control cells infected with DiD-loaded virus. (C) Duration of superinfection resistance established by A56/K2 deletion mutants. Cells were uninfected or infected with 10 PFU/cell of wild-type WR or IHD-J or deletion for up to 21 h at 37°C and then superinfected with 3 PFU/cell of WRvFire for 150 min at 37°C. LUC activity was determined and is plotted as a percentage of the uninfected cell control activity. (D) Virus binding to A56/K2 cell line. Parental and A56/K2 293 cells were incubated with 20 PFU/cell of YFP-tagged VACV at 4°C for 60 min. Cells then were analyzed by flow cytometry to determine the number of YFP+ cells. (E) Hemifusion of A56/K2 cells. R18-loaded VACV particles (3 PFU/cell) were incubated with parental and A56/K2 293 cells for 60 min at 4°C. The temperature then was raised to 37°C, and R18 fluorescence was monitored over the next 40 min and quantified as arbitrary fluorescence units. (F) LUC expression. Parental and A56/K2 293 cells were infected with the indicated multiplicities of WRvFire for 120 min at 37°C. Cells then were lysed and LUC activity measured.
The ability of A56 and K2 to prevent superinfection independently of other viral proteins was demonstrated by making a cell line that expresses the two proteins on the plasma membrane (6). However, the precise stage at which exclusion occurs had not been delineated. Using the previously constructed cell line, we confirmed the block in core entry and early gene expression (Fig. 8F). However, neither cell attachment nor virus-cell membrane hemifusion was inhibited in the A56/K2 cell line (Fig. 8D and E). Thus, A56/K2 inhibits virus entry at a posthemifusion step, unlike the resistance to superinfection established early during infection.
SIE was poxvirus specific.
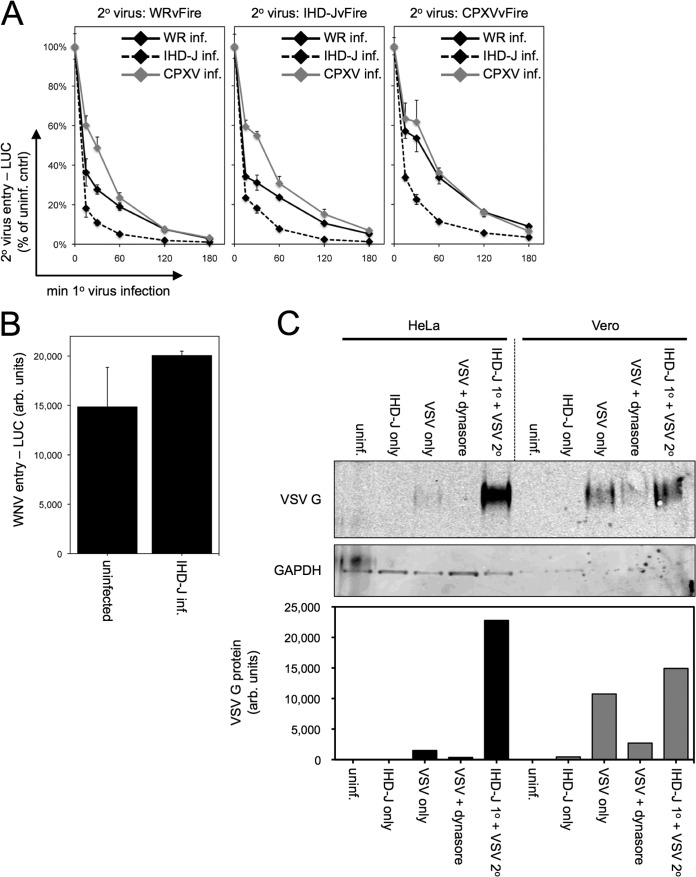
Cowpox virus (CPXV), which is closely related to VACV, could induce superinfection resistance that inhibited entry and LUC expression of WRvFire, IHD-JvFire, and CPXVvFire itself (Fig. 9A). In addition, entry and LUC expression of CPXVvFire was inhibited by primary infection with WR and IHD-J. The kinetics of acquisition of resistance was IHD-J > WR > CPXV, which parallels the kinetics of gene expression by these viruses.
FIG 9.
Selectivity of SIE. (A) CPXV. Cells were uninfected or infected with 10 PFU/cell of VACV strain WR or IHD-J or CPXV strain Brighton at 37°C for various lengths of time and then superinfected with 3 PFU/cell of WRvFire, IHD-JvFire, or CPXVvFire at 37°C for 150 min. LUC activity then was measured and plotted as a percentage of the uninfected cell control. (B) WNV. Cells were uninfected or infected with VACV at 10 PFU/cell of IHD-J at 37°C for 180 min. WNV reporter virus particles (3 particles per cell) were added, and cells were incubated at 37°C for 20 h. Cells were lysed and renilla LUC activity in cellular extracts measured. (C) VSV. Equivalent numbers of HeLa and Vero cells were uninfected or infected with 10 PFU/cell VACV IHD-J for 240 min at 37°C. Cells then were superinfected with 3 PFU/cell of VSV for 21 h at 37°C. Cells were lysed, and VSV G and cellular GAPDH proteins were analyzed by Western blotting. As a specificity control, cells were treated with dynasore to prevent VSV entry and G protein expression. Image J was used to quantify VSV G protein in each sample as arbitrary units.
The establishment of resistance to superinfection at the membrane fusion step and the accompanying cytoskeletal changes raised the possibility that it also prevents cell entry of unrelated enveloped viruses. Therefore, we tested whether VACV could prevent flavivirus entry. Uninfected HeLa cells or HeLa cells infected with VACV WR for 3 h were superinfected with WNV reporter particles encoding renilla LUC (31). LUC was expressed to similar levels under both conditions, indicating that VACV-induced resistance to superinfection did not prevent WNV infection (Fig. 9B).
We also investigated whether VACV-induced superinfection resistance would prevent infection by vesicular stomatitis virus (VSV), a small negative-stranded RNA virus that belongs to the rhabdovirus family. VSV is extremely sensitive to interferon (IFN), and previous studies had shown that coinfection as well as preinfection of certain cells with VACV enhanced replication of VSV due to the ability of VACV to counter interferon action (49, 50). We tested whether VACV could exclude VSV in HeLa cells, which produce interferon, and Vero cells, which do not (51). After 3 h, mock- or IHD-J-infected HeLa and Vero cells were superinfected with VSV. Entry and gene expression were monitored by measuring VSV G protein synthesis at 21 h. VACV primary infection greatly enhanced VSV replication, as measured by Western blotting of VSV G protein in HeLa cells, and slightly augmented VSV replication in Vero cells (Fig. 9C). As a specificity control, treatment of HeLa or Vero cells with dynasore, an inhibitor of dynamin GTPase whose activity is necessary for efficient VSV entry (52, 53), reduced VSV replication (Fig. 9C). These results suggest that superinfection resistance established by VACV was poxvirus specific.
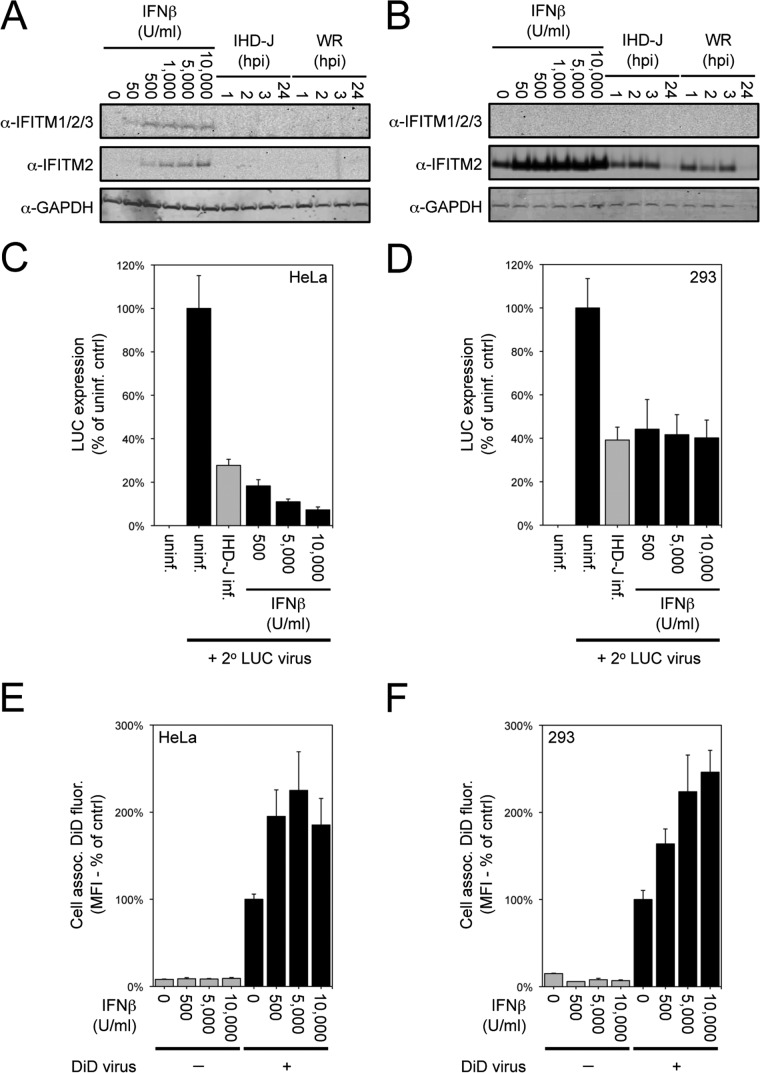
Recent studies indicated that cellular IFITMs inhibit entry of several viruses and restrict viral membrane hemifusion (54, 55). The similarity in the stage of inhibition led us to investigate a possible role of IFITMs in poxvirus SIE. First, we checked whether VACV infection increased the level of IFITMs as measured by Western blotting. Control experiments indicated that IFITMs detected by antibody to IFITM1/2/3 or to IFITM2 increased in HeLa cells (Fig. 10A) and 293 cells (Fig. 10B) treated with IFN-β, although only the IFITM2 antibody gave positive results with 293 cells. Neither VACV WR nor VACV IHD-J increased IFITM levels over uninfected cell levels (Fig. 10A and B), indicating that increased synthesis of IFITMs was not correlated with the development of resistance to superinfection.
FIG 10.
IFN-β induction of cellular IFITM protein and effects on VACV reporter gene expression and virus-cell membrane fusion. (A and B) Induction of IFITM by IFN-β. HeLa cells (A) or 293 cells (B) were left untreated, were treated for 24 h with the indicated concentration of IFN-β (Antigenix America Inc.), or were untreated and then infected with 10 PFU/cell of VACV IHD-J or WR strain for 1, 2, 3, or 24 h. Cells were lysed and analyzed by Western blotting with antibodies to IFITM1/2/3 (Santa Cruz Biotechnology), IFITM2 (Proteintech), and GAPDH (Covance). IFITM was not detected with the Santa Cruz antibody in 293 cell extracts. (C and D) Inhibition of VACV replication by IFN-β. HeLa cells (C) and 293 cells (D) were uninfected, infected with 10 PFU/cell of VACV IHD-J for 120 min, or treated for 24 h with the indicated concentration of IFN-β. Except for an uninfected control, cells were infected with 3 PFU/cell of WRvFire for 120 min. Cells were lysed and LUC activity quantified. Data were normalized to values obtained for the uninfected cells that were then infected with WRvFire. (E and F) Hemifusion of IFN-β-treated cells. HeLa cells (E) or 293 cells (F) were left untreated or were treated for 24 h with the indicated concentration of IFN-β. Cells then were infected with 3 PFU/cell of DiD-loaded VACV particles for 90 min and analyzed by flow cytometry to determine the mean fluorescence intensities (MFI) of cell-associated DiD fluorescence. Data were normalized to the values obtained for the untreated control cells infected with DiD-loaded virus.
However, since levels of IFITMs increased upon treatment with IFN-β, we asked whether this treatment could prevent hemifusion mediated by VACV. Although overnight treatment with IFN-β profoundly inhibited VACV WRvFire infection in HeLa cells and, to a lesser extent, in 293 cells as measured by LUC expression (Fig. 10C and D), hemifusion was actually increased (Fig. 10E and F). Thus, induction of IFITMs does not reproduce the block at which VACV SIE occurs.
DISCUSSION
The present study addressed four main questions regarding VACV SIE. (i) At what step in superinfection is secondary virus excluded? (ii) What functions are needed by the primary virus to establish resistance to superinfection? (iii) Is the presently described resistance to superinfection dependent on the A56 and K2 proteins, which were previously shown to mediate SIE at late times of infection? (iv) Is SIE specific for the homologous virus? Studies with other animal viruses have indicated that SIE can act by preventing the homologous secondary virus from entering the cell (56, 57), inhibiting translation of viral RNA (58), or preventing genome replication (59, 60). The early steps in VACV infection include cell attachment, virus-cell membrane fusion, core entry, and early gene expression. Attachment, which we measured by the cell association of virus particles containing a fluorescent core fusion protein, occurred following superinfection. In contrast, hemifusion, which entails lipid mixing of the outer leaflets of the viral and cellular membranes, failed to occur. This step was measured by loading virus particles with a self-quenching fluorescent dye, allowing virus attachment and then measuring fluorescence either with a fluorometer or by flow cytometry. The arrest occurred at the same step for both the WR and IHD-J strains of VACV, which differ in their requirement for endosomal acidification (29). The fate of superinfecting virus was analyzed by electron microscopy. Virus particles were found on the plasma membrane and in endosomal vesicles, but there were very few cores in the cytoplasm even when the medium was acidified. Thus, superinfection was arrested even before the virus core could enter the cytoplasm, and subsequent events, such as early gene expression, failed to occur.
With regard to the second question, we found that resistance to superinfection was established within the first few hours and did not require viral DNA replication by the primary virus. We took advantage of the lipid mixing fusion assay, which does not depend on RNA or protein synthesis by the secondary virus, to show that transcription and translation inhibitors prevented the induction of superinfection resistance by the primary virus. Further experiments indicated that viral early protein synthesis was required to establish superinfection resistance. UV-irradiated virus particles and a conditional lethal mutant unable to synthesize viral early mRNA were unable to induce resistance. The induction of resistance to superinfection in the presence of α-amanitin, however, suggested that cellular mRNA synthesis was not required. Thus, SIE occurs at the membrane fusion step prior to early gene expression, whereas the induction of resistance requires early gene expression by the primary virus.
The third question was whether the SIE characterized in this study involves the A56 and K2 proteins. The A56 protein, also known as hemagglutinin, is a transmembrane protein synthesized before and after genome replication, whereas K2 is a secreted protein made only after DNA replication. Nevertheless, the two proteins interact to form a heterodimer on the plasma membrane of infected cells (61). Deletion or mutation of either of these proteins results in the formation of syncytia at neutral pH (62–66). The finding that the A56/K2 heterodimer interacts specifically with two interacting proteins of the EFC provided a mechanism for preventing syncytium formation (7, 8). Turner and Moyer (5) reported that cells infected with A56 or K2 mutants have increased susceptibility to superinfection at late times. Wagenaar and Moss (6) found that cells transiently or stably transfected with both A56 and K2 are resistant to VACV infection. We confirmed the latter results in the present study and demonstrated that infection was blocked at a posthemifusion stage, distinguishing A56/K2-mediated SIE from early SIE described here. Furthermore, VACV mutants with deletions of A56, K2, or both were able to establish rapid and long-lasting resistance to superinfection as well as the wild-type virus can. The primary role of A56/K2 may be to prevent fusion of infected cells rather than to prevent superinfection by free virus, which is blocked earlier in the infection cycle.
The fourth question was whether other viruses that depend on membrane fusion for entry also would be excluded by a primary VACV infection. CPXV was able to exclude VACV and was itself excluded by VACV, which was expected because they are closely related members of the same orthopoxvirus genus. We also tested two heterologous virus systems: a WNV pseudovirion and VSV as representatives of flavivirus and rhabdovirus families, respectively. Both viruses enter by clathrin-dependent endocytosis and require acidification (53, 67, 68). Neither virus was excluded by primary infection with VACV, indicating that SIE shows specificity for orthopoxviruses, although testing of members of additional virus families would be needed to generalize this conclusion.
An important remaining question is how a few or even one primary infectious virus particle can modify the cell membrane to prevent hemifusion of hundreds of superinfecting particles. We considered that the involvement of IFITM proteins, which are widely expressed in cells, can be activated by virus infection and inhibit entry of several viruses (54, 55). Moreover, recent data indicate that IFITM proteins restrict virus membrane hemifusion (55). However, IFITM proteins are unlikely to play an important role in VACV SIE for the following reasons. First, primary VACV infection did not induce IFITM proteins as determined by Western blotting. Second, the induction of IFITM proteins with IFN-β did not prevent VACV hemifusion. Third, a primary VACV infection did not prevent entry of an IFITM-sensitive flavivirus or rhabdovirus.
A recent report indicates that baculovirus SIE is correlated with actin reorganization (69). Extensive actin remodeling occurs following VACV infection (15, 18, 43, 70), and we found a striking correlation between the cytoskeletal alterations induced by VACV and SIE. However, this appears to be a red herring, since VACV F11L mutants were able to induce superinfection resistance without causing extensive cytoskeletal changes. Which of the more than 100 other early genes are needed to establish SIE remains to be determined.
Lastly, we consider the question of the advantage of SIE to poxviruses. A number of possibilities have been suggested for other viruses, including prevention of detrimental recombination events and maintenance of cellular resources for the primary virus. In the case of VACV, we found that the superinfecting virus is still infectious (J.L., unpublished); therefore, it could infect a neighboring uninfected cell. It is attractive to view SIE as a powerful selection for viruses that rapidly infect cells and initiate expression. SIE could prevent defective and less fit viruses from contributing to the gene pool, analogous to fertilization of an egg by the swiftest sperm.
ACKNOWLEDGMENTS
We thank Liliana Maruri-Avidal and Andrea Weisberg for carrying out the electron microscopy analysis, Catherine Cotter for cells, Theodore Pierson for WNV reporter virus particles, and other members of the Laboratory of Viral Diseases for helpful discussions. Lily Koo in the Biological Imaging Section, NIAID, provided expert assistance with microscopy. Nissin Moussatche of the University of Florida kindly provided the VACV WR F11L stop codon mutant.
Footnotes
Published ahead of print 11 June 2014
REFERENCES
- 1.Folimonova SY. 2012. Superinfection exclusion is an active virus-controlled function that requires a specific viral protein. J. Virol. 86:5554–5561. 10.1128/JVI.00310-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Joklik WK. 1964. The intracellular uncoating of poxvirus DNA. I. The fate of radioactively-labeled rabbitpox virus. J. Mol. Biol. 8:263–276 [DOI] [PubMed] [Google Scholar]
- 3.Moss B, Rosenblum EN, Grimley PM. 1971. Assembly of virus particles during mixed infection with wild-type vaccinia and a rifampicin-resistant mutant. Virology 45:135–148. 10.1016/0042-6822(71)90120-6 [DOI] [PubMed] [Google Scholar]
- 4.Christen L, Seto J, Niles EG. 1990. Superinfection exclusion of vaccinia virus in virus-infected cell cultures. Virology 174:35–42. 10.1016/0042-6822(90)90051-R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Turner PC, Moyer RW. 2008. The vaccinia virus fusion inhibitor proteins SPI-3 (K2) and HA (A56) expressed by infected cells reduce the entry of superinfecting virus. Virology 380:226–233. 10.1016/j.virol.2008.07.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wagenaar TR, Moss B. 2009. Expression of the A56 and K2 proteins is sufficient to inhibit vaccinia virus entry and cell fusion. J. Virol. 83:1546–1554. 10.1128/JVI.01684-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wagenaar TR, Moss B. 2007. Association of vaccinia virus fusion regulatory proteins with the multicomponent entry/fusion complex. J. Virol. 81:6286–6293. 10.1128/JVI.00274-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wagenaar TR, Ojeda S, Moss B. 2008. Vaccinia virus A56/K2 fusion regulatory protein interacts with the A16 and G9 subunits of the entry fusion complex. J. Virol. 82:5153–5160. 10.1128/JVI.00162-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moss B. 2012. Poxvirus cell entry: how many proteins does it take? Viruses 4:688–707. 10.3390/v4050688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Law M, Carter GC, Roberts KL, Hollinshead M, Smith GL. 2006. Ligand-induced and non-fusogenic dissolution of a viral membrane. Proc. Natl. Acad. Sci. U. S. A. 103:5989–5994. 10.1073/pnas.0601025103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doceul V, Hollinshead M, van der Linden L, Smith GL. 2010. Repulsion of superinfecting virions: a mechanism for rapid virus spread. Science 327:873–876. 10.1126/science.1183173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moss B. 2013. Poxviridae, p 2129–2159 In Knipe DM, Howley PM. (ed), Fields virology, 6th ed, vol 2 Wolters Kluwer/Lippincott Williams & Wilkins, Philadelphia, PA [Google Scholar]
- 13.Lin CL, Chung CS, Heine HG, Chang W. 2000. Vaccinia virus envelope H3L protein binds to cell surface heparan sulfate and is important for intracellular mature virion morphogenesis and virus infection in vitro and in vivo. J. Virol. 74:3353–3365. 10.1128/JVI.74.7.3353-3365.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Townsley AC, Weisberg AS, Wagenaar TR, Moss B. 2006. Vaccinia virus entry into cells via a low pH-dependent-endosomal pathway. J. Virol. 80:8899–8908. 10.1128/JVI.01053-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mercer J, Helenius A. 2008. Vaccinia virus uses macropinocytosis and apoptotic mimicry to enter host cells. Science 320:531–535. 10.1126/science.1155164 [DOI] [PubMed] [Google Scholar]
- 16.Doms RW, Blumenthal R, Moss B. 1990. Fusion of intra- and extracellular forms of vaccinia virus with the cell membrane. J. Virol. 64:4884–4892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laliberte JP, Weisberg AS, Moss B. 2011. The membrane fusion step of vaccinia virus entry is cooperatively mediated by multiple viral proteins and host cell components. PLoS Pathog. 7:e1002446. 10.1371/journal.ppat.1002446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang CY, Lu TY, Bair CH, Chang YS, Jwo JK, Chang W. 2008. A novel cellular protein, VPEF, facilitates vaccinia virus penetration into HeLa cells through fluid phase endocytosis. J. Virol. 82:7988–7999. 10.1128/JVI.00894-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Izmailyan R, Hsao JC, Chung CS, Chen CH, Hsu PWC, Liao CL, Chang W. 2012. Integrin beta 1 mediates vaccinia virus entry through activation of PI3K/Akt signaling. J. Virol. 86:6677–6687. 10.1128/JVI.06860-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schroeder N, Chung CS, Chen CH, Liao CL, Chang W. 2012. The lipid raft-associated protein CD98 is required for vaccinia virus endocytosis. J. Virol. 86:4868–4882. 10.1128/JVI.06610-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang Z, Bruno DP, Martens CA, Porcella SF, Moss B. 2011. Genome-wide analysis of the 5′ and 3′ ends of vaccinia virus early mRNAs delineates regulatory sequences of annotated and anomalous transcripts. J. Virol. 85:5897–5909. 10.1128/JVI.00428-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang Z, Reynolds SE, Martens CA, Bruno DP, Porcella SF, Moss B. 2011. Expression profiling of the intermediate and late stages of poxvirus replication. J. Virol. 85:9899–9908. 10.1128/JVI.05446-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Condit RC, Moussatche N, Traktman P. 2006. In a nutshell: structure and assembly of the vaccinia virion. Adv. Virus Res. 66:31–124. 10.1016/S0065-3527(06)66002-8 [DOI] [PubMed] [Google Scholar]
- 24.Townsley A, Senkevich TG, Moss B. 2005. Vaccinia virus A21 virion membrane protein is required for cell entry and fusion. J. Virol. 79:9458–9469. 10.1128/JVI.79.15.9458-9469.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Senkevich TG, Moss B. 2005. Vaccinia virus H2 protein is an essential component of a complex involved in virus entry and cell-cell fusion. J. Virol. 79:4744–4754. 10.1128/JVI.79.8.4744-4754.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bisht H, Weisberg AS, Moss B. 2008. Vaccinia virus L1 protein is required for cell entry and membrane fusion. J. Virol. 82:8687–8694. 10.1128/JVI.00852-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Resch W, Moss B. 2005. The conserved poxvirus L3 virion protein is required for transcription of vaccinia virus early genes. J. Virol. 79:14719–14729. 10.1128/JVI.79.23.14719-14729.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Katsafanas GC, Moss B. 2007. Colocalization of transcription and translation within cytoplasmic poxvirus factories coordinates viral expression and subjugates host functions. Cell Host Microbe 2:221–228. 10.1016/j.chom.2007.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bengali Z, Townsley AC, Moss B. 2009. Vaccinia virus strain differences in cell attachment and entry. Virology 389:132–140. 10.1016/j.virol.2009.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bengali Z, Satheshkumar PS, Moss B. 2012. Orthopoxvirus species and strain differences in cell entry. Virology 433:506–512. 10.1016/j.virol.2012.08.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pierson TC, Sanchez MD, Puffer BA, Ahmed AA, Geiss BJ, Valentine LE, Altamura LA, Diamond MS, Doms RW. 2006. A rapid and quantitative assay for measuring antibody-mediated neutralization of West Nile virus infection. Virology 346:53–65. 10.1016/j.virol.2005.10.030 [DOI] [PubMed] [Google Scholar]
- 32.Chakrabarti S, Sisler JR, Moss B. 1997. Compact, synthetic, vaccinia virus early/late promoter for protein expression. Biotechniques 23:1094–1097 [DOI] [PubMed] [Google Scholar]
- 33.Earl PL, Moss B, Wyatt LS, Carroll MW. 2001. Generation of recombinant vaccinia viruses. Curr. Protoc. Mol. Biol. Chapter 16:Unit 16.17. 10.1002/0471142727.mb1617s43 [DOI] [PubMed] [Google Scholar]
- 34.Earl PL, Cooper N, Wyatt LS, Moss B, Carroll MW. 2001. Preparation of cell cultures and vaccinia virus stocks. Curr. Protoc. Mol. Biol. Chapter 16:Unit 16.16. 10.1002/0471142727.mb1616s43 [DOI] [PubMed] [Google Scholar]
- 35.Earl PL, Americo JL, Moss B. 2003. Development and use of a vaccinia virus neutralization assay based on flow cytometric detection of green fluorescent protein. J. Virol. 77:10684–10688. 10.1128/JVI.77.19.10684-10688.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wolffe EJ, Vijaya S, Moss B. 1995. A myristylated membrane protein encoded by the vaccinia virus L1R open reading frame is the target of potent neutralizing monoclonal antibodies. Virology 211:53–63. 10.1006/viro.1995.1378 [DOI] [PubMed] [Google Scholar]
- 37.Senkevich TG, Wyatt LS, Weisberg AS, Koonin EV, Moss B. 2008. A conserved poxvirus NlpC/P60 superfamily protein contributes to vaccinia virus virulence in mice but not to replication in cell culture. Virology 374:506–514. 10.1016/j.virol.2008.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Woodson B. 1967. Vaccinia mRNA synthesis under conditions which prevent uncoating. Biochem. Biophys. Res. Commun. 27:169–175. 10.1016/S0006-291X(67)80057-3 [DOI] [PubMed] [Google Scholar]
- 39.Bablanian R. 1968. The prevention of early vaccinia-virus-induced cytopathic effects by inhibition of protein synthesis. J. Gen. Virol. 3:51–61. 10.1099/0022-1317-3-1-51 [DOI] [PubMed] [Google Scholar]
- 40.Meyer RK, Burger MM, Tschannen R, Schafer R. 1981. Actin filament bundles in vaccinia virus infected fibroblasts. Arch. Virol. 67:11–18. 10.1007/BF01314597 [DOI] [PubMed] [Google Scholar]
- 41.Schepis A, Schramm B, de Haan CA, Locker JK. 2006. Vaccinia virus-induced microtubule-dependent cellular rearrangements. Traffic 7:308–323. 10.1111/j.1600-0854.2005.00381.x [DOI] [PubMed] [Google Scholar]
- 42.Arakawa Y, Cordeiro JV, Way M. 2007. F11L-mediated inhibition of RhoA-mDia signaling stimulates microtubule dynamics during vaccinia virus infection. Cell Host Microbe 1:213–226. 10.1016/j.chom.2007.04.007 [DOI] [PubMed] [Google Scholar]
- 43.Arakawa Y, Cordeiro JV, Schleich S, Newsome TP, Way M. 2007. The release of vaccinia virus from infected cells requires RhoA-mDia modulation of cortical actin. Cell Host Microbe 1:227–240. 10.1016/j.chom.2007.04.006 [DOI] [PubMed] [Google Scholar]
- 44.Valderrama F, Cordeiro JV, Schleich S, Frischknecht F, Way M. 2006. Vaccinia virus-induced cell motility requires F11L-mediated inhibition of RhoA signaling. Science 311:377–381. 10.1126/science.1122411 [DOI] [PubMed] [Google Scholar]
- 45.Morales I, Carbajal MA, Bohn S, Holzer D, Kato SEM, Greco FAB, Moussatche N, Locker JK. 2008. The vaccinia virus F11L gene product facilitates cell detachment and promotes migration. Traffic 9:1283–1298. 10.1111/j.1600-0854.2008.00762.x [DOI] [PubMed] [Google Scholar]
- 46.Cordeiro JV, Guerra S, Arakawa Y, Dodding MP, Esteban M, Way M. 2009. F11-mediated inhibition of RhoA signalling enhances the spread of vaccinia virus in vitro and in vivo in an intranasal mouse model of infection. PLoS One 4:e8506. 10.1371/journal.pone.0008506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kato SEM, Greco FAB, Damaso CRA, Condit RC, Moussatche N. 2004. An alternative genetic method to test essential vaccinia virus early genes. J. Virol. Methods 115:31–40. 10.1016/j.jviromet.2003.09.011 [DOI] [PubMed] [Google Scholar]
- 48.Antoine G, Scheiflinger F, Dorner F, Falkner FG. 1998. The complete genomic sequence of the modified vaccinia Ankara strain: comparison with other orthopoxviruses. Virology 244:365–396. 10.1006/viro.1998.9123 [DOI] [PubMed] [Google Scholar]
- 49.Whitaker-Dowling P, Youngner JS. 1983. Vaccinia rescue of VSV from interferon-induced resistance: reversal of translation block and inhibition of protein kinase activity. Virology 131:128–136. 10.1016/0042-6822(83)90539-1 [DOI] [PubMed] [Google Scholar]
- 50.Chang HW, Watson JC, Jacobs BL. 1992. The E3L gene of vaccinia virus encodes an inhibitor of the interferon-induced, double-stranded RNA-dependent protein kinase. Proc. Natl. Acad. Sci. U. S. A. 89:4825–4829. 10.1073/pnas.89.11.4825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Desmyter J, Melnick JL, Rawls WE. 1968. Defectiveness of interferon production and of rubella virus interference in a line of African green monkey kidney cells (Vero). J. Virol. 2:955–961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Macia E, Ehrlich M, Massol R, Boucrot E, Brunner C, Kirchhausen T. 2006. Dynasore, a cell-permeable inhibitor of dynamin. Dev. Cell 10:839–850. 10.1016/j.devcel.2006.04.002 [DOI] [PubMed] [Google Scholar]
- 53.Cureton DK, Massol RH, Saffarian S, Kirchhausen TL, Whelan SP. 2009. Vesicular stomatitis virus enters cells through vesicles incompletely coated with clathrin that depend upon actin for internalization. PLoS Pathog. 5:e1000394. 10.1371/journal.ppat.1000394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Diamond MS, Farzan M. 2013. The broad-spectrum antiviral functions of IFIT and IFITM proteins. Nat. Rev. Immunol. 13:46–57. 10.1038/nri3344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li K, Markosyan RM, Zheng YM, Golfetto O, Bungart B, Li M, Ding S, He Y, Liang C, Lee JC, Gratton E, Cohen FS, Liu SL. 2013. IFITM proteins restrict viral membrane hemifusion. PLoS Pathog. 9:e1003124. 10.1371/journal.ppat.1003124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Steck FT, Rubin H. 1966. The mechanism of interference between an avian leukosis virus and Rous sarcoma virus. II. Early steps of infection by RSV of cells under conditions of interference. Virology 29:642–653 [DOI] [PubMed] [Google Scholar]
- 57.Lindwasser OW, Chaudhuri R, Bonifacino JS. 2007. Mechanisms of CD4 downregulation by the Nef and Vpu proteins of primate immunodeficiency viruses. Curr. Mol. Med. 7:171–184. 10.2174/156652407780059177 [DOI] [PubMed] [Google Scholar]
- 58.Schaller T, Appel N, Koutsoudakis G, Kallis S, Lohmann V, Pietschmann T, Bartenschlager R. 2007. Analysis of hepatitis C virus superinfection exclusion by using novel fluorochrome gene-tagged viral genomes. J. Virol. 81:4591–4603. 10.1128/JVI.02144-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Adams RH, Brown DT. 1985. BHK cells expressing Sindbis virus-induced homologous interference allow the translation of nonstructural genes of superinfecting virus. J. Virol. 54:351–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee YM, Tscherne DM, Yun SI, Frolov I, Rice CM. 2005. Dual mechanisms of pestiviral superinfection exclusion at entry and RNA replication. J. Virol. 79:3231–3242. 10.1128/JVI.79.6.3231-3242.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Turner PC, Moyer RW. 2006. The cowpox virus fusion regulator proteins SPI-3 and hemagglutinin interact in infected and uninfected cells. Virology 347:88–99. 10.1016/j.virol.2005.11.012 [DOI] [PubMed] [Google Scholar]
- 62.Ichihashi Y, Dales S. 1971. Biogenesis of poxviruses: interrelationship between hemagglutinin production and polykaryocytosis. Virology 46:533–543. 10.1016/0042-6822(71)90057-2 [DOI] [PubMed] [Google Scholar]
- 63.Seki M, Oie M, Ichihashi Y, Shida H. 1990. Hemadsorption and fusion inhibition activities of hemagglutinin analyzed by vaccinia virus mutants. Virology 175:372–384. 10.1016/0042-6822(90)90422-N [DOI] [PubMed] [Google Scholar]
- 64.Law KM, Smith GL. 1992. A vaccinia serine protease inhibitor which prevents virus-induced cell fusion. J. Gen. Virol. 73:549–557. 10.1099/0022-1317-73-3-549 [DOI] [PubMed] [Google Scholar]
- 65.Turner PC, Moyer RW. 1992. An orthopoxvirus serpin-like gene controls the ability of infected cells to fuse. J. Virol. 66:2076–2085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhou J, Sun XY, Fernando GJP, Frazer IH. 1992. The vaccinia virus K2L gene encodes a serine protease inhibitor which inhibits cell-cell fusion. Virology 189:678–686. 10.1016/0042-6822(92)90591-C [DOI] [PubMed] [Google Scholar]
- 67.Cureton DK, Massol RH, Whelan SP, Kirchhausen T. 2010. The length of vesicular stomatitis virus particles dictates a need for actin assembly during clathrin-dependent endocytosis. PLoS Pathog. 6:e1001127. 10.1371/journal.ppat.1001127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pierson TC, Kielian M. 2013. Flaviviruses: braking the entering. Curr. Opin. Virol. 3:3–12. 10.1016/j.coviro.2012.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Beperet I, Irons SL, Simon O, King LA, Williams T, Possee RD, Lopez-Ferber M, Caballero P. 2014. Superinfection exclusion in alphabaculovirus infections is concomitant with actin reorganization. J. Virol. 88:3548–3556. 10.1128/JVI.02974-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Locker JK, Kuehn A, Schleich S, Rutter G, Hohenberg H, Wepf R, Griffiths G. 2000. Entry of the two infectious forms of vaccinia virus at the plasma membrane is signaling-dependent for the IMV but not the EEV. Mol. Biol. Cell 11:2497–2511. 10.1091/mbc.11.7.2497 [DOI] [PMC free article] [PubMed] [Google Scholar]