ABSTRACT
Initiation of antiretroviral therapy during the earliest stages of HIV-1 infection may limit the seeding of a long-lasting viral reservoir, but long-term effects of early antiretroviral treatment initiation remain unknown. Here, we analyzed immunological and virological characteristics of nine patients who started antiretroviral therapy at primary HIV-1 infection and remained on suppressive treatment for >10 years; patients with similar treatment duration but initiation of suppressive therapy during chronic HIV-1 infection served as controls. We observed that independently of the timing of treatment initiation, HIV-1 DNA in CD4 T cells decayed primarily during the initial 3 to 4 years of treatment. However, in patients who started antiretroviral therapy in early infection, this decay occurred faster and was more pronounced, leading to substantially lower levels of cell-associated HIV-1 DNA after long-term treatment. Despite this smaller size, the viral CD4 T cell reservoir in persons with early treatment initiation consisted more dominantly of the long-lasting central-memory and T memory stem cells. HIV-1-specific T cell responses remained continuously detectable during antiretroviral therapy, independently of the timing of treatment initiation. Together, these data suggest that early HIV-1 treatment initiation, even when continued for >10 years, is unlikely to lead to viral eradication, but the presence of low viral reservoirs and durable HIV-1 T cell responses may make such patients good candidates for future interventional studies aiming at HIV-1 eradication and cure.
IMPORTANCE Antiretroviral therapy can effectively suppress HIV-1 replication to undetectable levels; however, HIV-1 can persist despite treatment, and viral replication rapidly rebounds when treatment is discontinued. This is mainly due to the presence of latently infected CD4 T cells, which are not susceptible to antiretroviral drugs. Starting treatment in the earliest stages of HIV-1 infection can limit the number of these latently infected cells, raising the possibility that these viral reservoirs are naturally eliminated if suppressive antiretroviral treatment is continued for extremely long periods of time. Here, we analyzed nine patients who started on antiretroviral therapy within the earliest weeks of the disease and continued treatment for more than 10 years. Our data show that early treatment accelerated the decay of infected CD4 T cells and led to very low residual levels of detectable HIV-1 after long-term therapy, levels that were otherwise detectable in patients who are able to maintain a spontaneous, drug-free control of HIV-1 replication. Thus, long-term antiretroviral treatment started during early infection cannot eliminate HIV-1, but the reduced reservoirs of HIV-1 infected cells in such patients may increase their chances to respond to clinical interventions aiming at inducing a drug-free remission of HIV-1 infection.
INTRODUCTION
Acute HIV-1 infection is characterized by extremely high levels of viral replication that subsequently decline to the viral set point (1, 2). Based on a number of different considerations, this disease stage has been regarded as a window of opportunity for initiation of antiretroviral therapy to improve clinical outcomes of HIV-1 infection (3, 4) and to reduce viral transmission from highly viremic individuals (5). A series of prior studies have indeed shown that short-term antiretroviral treatment in acute or early HIV-1 infection can preserve B-cell-mediated (6) and T-cell-mediated (7) immune function, supports the development of HIV-1-specific CD4 T cell responses (8), limits the diversity of circulating viral strains (9), and in some cases facilitates spontaneous control of low-level HIV-1 viremia after treatment discontinuation (10, 11). Recently, data from three prospective, randomized-controlled clinical trials indicated that a 1- to 2-year antiretroviral treatment course started during acute or early HIV-1 infection can lead to reduced HIV-1 set point viremia after treatment discontinuation (12–14), providing compelling evidence for beneficial effects of antiretroviral therapy initiation during the earliest stages of HIV-1 infection. Nevertheless, such effects were modest and not sustained long term, indicating that short-term therapy in primary infection may not significantly affect the eventual HIV-1 disease outcome.
The virological and immunological effects of long-term antiretroviral therapy started in early HIV-1 infection have been less clearly investigated. Previous studies have shown that antiretroviral therapy initiated during the earliest stage of HIV-1 infection and continued for several years may reduce the reservoir of latently infected CD4 T cells, which harbor a transcriptionally silent form of HIV-1 and likely serve as the major source for virological rebound after treatment discontinuation (15–18). Such low reservoirs of HIV-1-infected cells are also observed in “elite controllers,” a small group of HIV-1 patients who maintain undetectable levels of HIV-1 replication in the absence of antiretroviral therapy (19–21), and in “posttreatment controllers,” who develop a controller phenotype after completing several years of suppressive antiretroviral therapy initiated during primary HIV-1 infection (10, 22). Together, these data suggest that low viral reservoirs may, at least in selected patients, contribute to the ability to achieve a long-term drug-free remission of HIV-1 infection.
In the present study, we systematically investigated virological and immunological characteristics of a unique cohort of HIV-1 patients who started antiretroviral therapy during primary HIV-1 infection and continuously remained on suppressive treatment for the subsequent 10 years, giving us an opportunity to analyze long-term effects of early antiretroviral treatment initiation. Our studies indicate that long-term antiretroviral therapy started during early infection can accelerate the decay of the HIV-1 reservoir while maintaining strong HIV-1-specific T cell responses. Such characteristics may make these patients attractive candidates for future interventional strategies to reduce HIV-1 persistence despite HAART.
MATERIALS AND METHODS
Patients.
Study participants were recruited from the Massachusetts General Hospital (Boston, MA) and the University Hospital Germans Trias i Pujol (Badalona, Spain). Peripheral blood mononuclear cell (PBMC) samples were used according to protocols approved by the institutional review boards of both centers. Study subjects gave written informed consent to participate in accordance with the Declaration of Helsinki. Early antiretroviral therapy (ART) was defined as initiation of ART within the first 6 months after HIV-1 infection. Starting ART during the chronic phase of infection was defined as initiation of suppressive ART at least 2 years after HIV-1 diagnosis.
CD4 isolation and sorting of CD4 T cell subsets.
We used a Dynabeads untouched human CD4 T cells kit (Invitrogen) for CD4 isolation. The purity of extraction was greater than 95% (data not shown). For CD4 subset isolation, PBMC were stained with monoclonal antibodies directed against CD4, CD3, CD45RA, CCR7, CD62L, CD122, and CD95. After 20 min, CCR7+ CD45RA+ naive CD4 T cells, CCR7+ CD45RA− central memory CD4 T cells, CCR7− CD45RA− effector memory CD4 T cells, CCR7− CD45RA+ terminally differentiated CD4 T cells, and CCR7+ CD45RA+ CD62L+ CD95+ CD122+ T memory stem CD4 T cells were live sorted in a specifically designated biosafety cabinet (Baker Hood), using a FACS Aria cell sorter (BD Biosciences) at 70 lb/in2.
Assessment of cell-associated HIV-1 DNA.
Isolated CD4 T cells were digested as previously described (23) to extract cell lysates. We amplified total HIV-1 DNA with primers and probes previously described (24). Integrated HIV-1 DNA was detected using nested PCR with Alu-1/Alu-2 primers and the HIV-1 long terminal repeat (LTR) primer L-M667 for first-round PCR and LTR primer AA55M, lambda T primers, and probe MH603 for second-round quantitative PCR, as described previously (25). Serial dilutions of HIV-1 DNA from cell lysates of the HIV-1-infected cell line 293T (provided by F. Bushman, University of Pennsylvania, Philadelphia, PA, USA) were used for reference purposes. 2-LTR HIV-1 DNA was quantified as previously described (26).
Analysis of cell-associated HIV-1 RNA and plasma viremia.
RNA was extracted from CD4+ T cells using the mirVana miRNA isolation kit (Ambion). RNA was reverse transcribed according to standard protocols. HIV-1 cDNA was amplified using TaqMan-based quantitative PCR as previously described (27). Data were normalized to a standard HIV-1 RNA sample run in serial dilutions, and final results were calculated as the number of HIV-1 RNA copies per microgram of total RNA. Residual HIV-1 plasma viremia was measured by single-copy assay (SCA) as previously described (27).
Viral outgrowth assay.
Viral outgrowth assays were performed as previously described with some modifications (38). CD4+ T cells isolated from 50 to 100 million PBMCs were seeded at 50,000 cells/well in round-bottom 96-well plates. Subsequently, cells were stimulated with phytohemagglutinin (PHA) (2 μg/ml), recombinant human interleukin 2 (rhIL-2) (100 units/ml), and irradiated allogeneic PBMCs from HIV-negative healthy donors. CD8-depleted, PHA-stimulated PBMCs from HIV-negative donors were added to each well on day 3 and again on days 7 and 14 of culture. The cultures were subjected to removal of 33% of the cell suspension every 7 days and replenished with fresh medium containing rhIL-2 (100 U/ml). After 14 to 21 days, cell supernatant from each well was harvested, and the number of wells containing infectious HIV-1 was assessed by incubation of the supernatant with TZM-bl cells, a permissive HeLa cell clone that contains HIV-1 Tat-responsive reporter genes for firefly luciferase under the control of the HIV-1 LTR, permitting sensitive and accurate measurements of infection (29). Luciferase activity was quantified by luminescence and is directly proportional to the number of infectious virus particles present in the initial inoculum. Estimated frequencies of cells with replication-competent HIV-1 were calculated using limiting dilution analysis as described in reference 30.
Intracellular cytokine staining.
Peripheral blood mononuclear cells (0.5 to 1 million) were stimulated with a pool of overlapping HIV-1 Gag peptides (clade B consensus sequence; final concentration, 4 μg/ml) and incubated with anti-CD28 and anti-CD49d antibodies (1 μg/ml each; BD Biosciences) for 6 h at 37°C and 5% CO2. Brefeldin A (10 μg/ml; Sigma) and monensin (6 μg/ml; BD Biosciences) were added after the initial hour of incubation. Afterwards, cells were washed and stained with surface antibodies directed against CD3, CD8, CD45RA, and CCR7 (all antibodies from BD Biosciences). Cells were then washed again, fixed and permeabilized using a fixation/permeabilization kit (Biolegend), and stained for intracellular expression of gamma interferon (IFN-γ). Cells were acquired on an LSRII flow cytometer (BD Biosciences), using fluorescence-activated-cell-sorter DiVa software. Electronic compensation was performed with antibody capture beads (BD Biosciences) stained separately with individual antibodies used in the test samples. Flow data were analyzed with the FlowJo software package (Treestar, Ashland, OR).
Western blotting for HIV-1 detection.
HIV-1 Western blots were performed with a commercial kit according to the manufacturer's protocol (Maxim Biomedical Inc., Rockville, MD, USA).
Statistical analysis.
Data are summarized as individual data plots. Spearman's correlation coefficient was calculated to analyze correlations. Differences between nominal data were tested for statistical significance by a 2-tailed Student's t test, Mann-Whitney U-test, analysis of variance (ANOVA), or paired-Wilcoxon test as appropriate. A P value of <0.05 was considered significant. For calculation of HIV-1 DNA decay in CD4 T cells, Tobit regression for censored data (31) was applied using a standard Markov chain-Monte Carlo method as described in reference 32, applying noninformative normally distributed priors. The uncertainty in the measured data was computed as the fraction of two density-dependent lognormally distributed random variables (i.e., the measured copy numbers of HIV DNA and CCR5, respectively), with density-dependent lognormal variance computed according to the equations described in reference 33. The censoring limit chosen was 7 HIV-1 DNA copies in the sample, consistent with the censoring limits described in reference 27.
RESULTS
Lower reservoirs of HIV-1 infected CD4 T cells after long-term antiretroviral therapy initiated in primary HIV-1 infection.
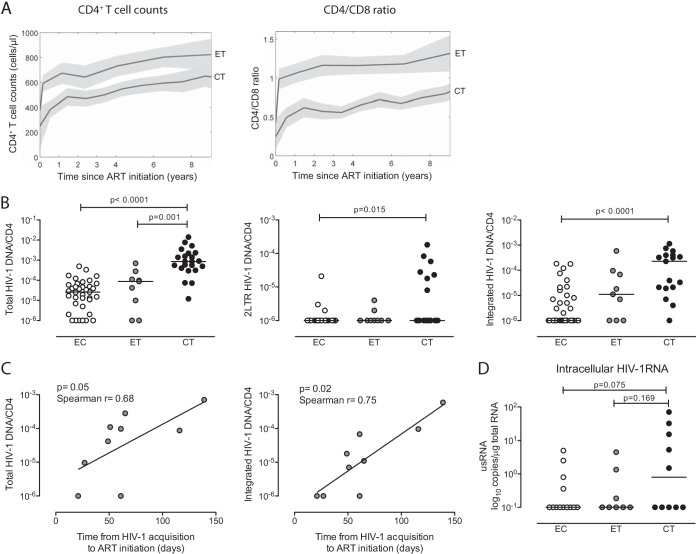
To investigate the effects of uninterrupted long-term antiretroviral therapy on the reservoir of infected CD4 T cells, we focused on a group of nine HIV-1 patients who began treatment during primary HIV-1 infection and a reference cohort of 26 persons who initiated suppressive treatment during chronic HIV-1 infection (2 years after HIV-1 diagnosis). Both patient groups remained on suppressive therapy for at least 10 years. Clinical and demographic characteristics of the study patients are summarized in Table 1. Notably, study cohorts were not statistically different in terms of gender, baseline plasma viremia, total baseline CD4 T cell counts, and time until undetectable plasma HIV-1 viral loads were achieved (Table 1), but increases in both CD4 T cell counts and the ratio of CD4/CD8 T cells after treatment initiation were more pronounced in persons with early treatment initiation (Fig. 1A). A cohort of elite controllers who maintained undetectable HIV-1 replication for at least 8 years in the absence of antiretroviral treatment was recruited as a reference population.
TABLE 1.
Clinical and demographical characteristics of the study cohorts
| Characteristic | Value for groupa |
P (ET vs. CT) | ||
|---|---|---|---|---|
| Early treated (ET) n = 9 | Elite controllers (EC) n = 37 | Chronic treated (CT) n = 26 | ||
| Age (yrs)b | 45.7 ± 10.2 | 48.8 ± 9.0 | 51.7 ± 7.3 | 0.04 |
| No. of females (%)c | 4 (44.4) | 9 (24.3) | 5 (19.2) | 0.27 |
| Time (days) between HIV-1 infectione and ART initiation | 65.5 ± 58.4 | NA | 1,587 ± 1,317 | <0.001 |
| Time (yrs) of undetectable viral load after ART initiationb | 10.2 ± 1.0 | NA | 10.7 ± 2.2 | 0.26 |
| Plasma viremia (log RNA copies/ml) | ||||
| Baselineb | 5.2 [4.4–5.5] | NA | 5.1 [4.7–5.5] | 0.95 |
| Yr 10 (ET and CT) or yr 8 (EC)a | 1.6 [1.6–1.6] | 1.6 [1.6–1.6] | 1.6 [1.6–1.6] | NA |
| P within group (baseline vs. yr 10)d | <0.001 | <0.001 | ||
| CD4+ T cells (absolute no. of cells/μl) | ||||
| Baselineb | 412 [263–575] | NA | 308 [123–402] | 0.10 |
| Yr 10 (ET and CT) or yr 8b | 860 [497–1018] | 815 [774–1048] | 836 [466–983] | 0.74 |
| P within group (baseline vs. year 10)d | <0.001 | <0.001 | ||
Values are in the form mean ± SD or median [IQR] unless otherwise specified.
Mann-Whitney U test.
Pearson's chi square test.
Wilcoxon signed rank test.
Estimated according to previously-described algorithm (4).
FIG 1.
Long-term antiretroviral therapy initiated in primary HIV-1 infection reduces the viral reservoir to levels seen in elite controllers. (A) Trajectories of CD4 T cell counts and CD4/CD8 ratio with 95% pointwise confidence intervals bands in patients with treatment initiation in early (n = 9) versus chronic (n = 16) infection. (B) Copies per cell of total, 2-LTR, and integrated HIV-1 DNA in CD4 T cells isolated from individuals started on suppressive antiretroviral therapy in early HIV-1 infection (ET) and chronic HIV-1 infection (CT) and from elite controllers (EC). Study patients had undetectable viremia for >8 years (EC) or >10 years (ET and CT). (C) Correlation between time from HIV-1 acquisition to antiretroviral treatment initiation and viral reservoir size after 10 years of continuous antiretroviral therapy in HIV-1 patients with treatment initiation in primary HIV-1 infection. (D) Levels of CD4 T cell-associated HIV-1 RNA in the indicated study groups.
In an initial cross-sectional analysis, we compared levels of total, 2-LTR, and integrated HIV-1 DNA in isolated CD4 T cells from the different study cohorts after long-term suppression of HIV-1 replication. In line with previous findings (19–21), we observed that all HIV-1 DNA forms were significantly lower in elite controllers than in patients who had been on long-term suppressive antiretroviral therapy initiated during chronic HIV-1 infection (Fig. 1B). Interestingly, levels of HIV-1 DNA in patients who had been on long-term antiretroviral therapy started in early HIV-1 infection closely resembled those of elite controllers while being substantially lower than those in patients started on treatment during chronic infection. Moreover, within the cohort of patients treated in early infection, we observed a statistical correlation between the time from HIV-1 acquisition (estimated according to a previously described algorithm [4]) to initiation of therapy and total or integrated HIV-1 DNA levels after >10 years of continuous treatment (Fig. 1C). Interestingly, we also noted a trend toward lower levels of cell-associated HIV-1 RNA in CD4 T cells from elite controllers and patients started on treatment in early HIV-1 infection than in those from patients started on treatment in chronic HIV-1 infection (Fig. 1D). Residual HIV-1 plasma viremia, determined using assays with single-copy resolution, was undetectable in most study subjects for whom sufficient material was available and did not differ between the two study cohorts on antiretroviral therapy (data not shown).
We subsequently analyzed levels of replication-competent HIV-1 in our highly active ART (HAART)-treated study cohorts after long-term antiretroviral treatment, using a functional ex vivo viral outgrowth assay. These studies demonstrated that replication-competent virus was retrievable from 5 of 8 individuals with early treatment initiation, in 3 of 8 elite controllers, and in 9 of 11 persons started on treatment in chronic infection. The estimated frequency of CD4 T cells harboring replication-competent virus was significantly higher in persons with treatment initiation during chronic HIV-1 infection than in elite controllers, and a trend toward higher frequency (P = 0.052) was also observed in comparison to persons with early start of therapy (Fig. 2A). Of note, there was a statistically significant correlation between the frequency of cells with replication-competent HIV-1, and the total amount of HIV-1 DNA determined by real-time PCR (Spearman r = 0.73, P = 0.0009) (Fig. 2B). However, within the individual study cohorts, such an association was observed only for patients who initiated treatment in chronic infection (Spearman r = 0.68, P = 0.05) (data not shown), not for persons starting treatment in early infection (r = 0.22, P = 0.58), for reasons that are currently unclear. Interestingly, levels of activated CD4 and CD8 T cells (measured as the proportion of HLA-DR+ T cells) were not significantly associated with the levels of CD4 T cell-associated HIV-1 DNA or the amount of replication-competent viruses and were not different between patients starting therapy in early versus chronic HIV-1 infection, although both study cohorts showed elevated levels of immune activation compared to healthy individuals (data not shown). Together, these data indicate that long-term antiretroviral therapy initiated during primary HIV-1 infection reduces the viral reservoir to levels that are otherwise seen in person with spontaneous control of HIV-1.
FIG 2.
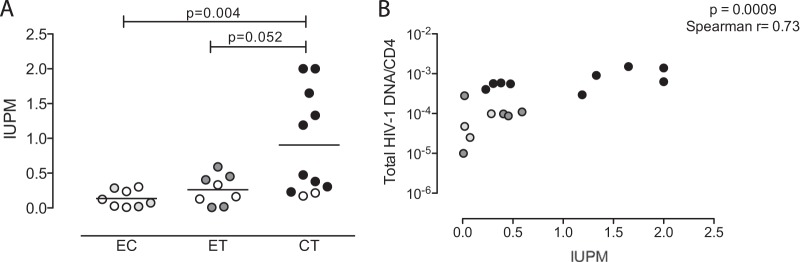
Reduced frequencies of CD4 T cells with replication-competent HIV-1 after long-term antiretroviral therapy initiated in primary HIV-1 infection. (A) Infectious units per million CD4 T cells (IUPM) isolated from the study subjects after 10 years of antiretroviral therapy initiated during early (ET) or chronic (CT) HIV-1 infection and from elite controllers (EC). (B) Spearman correlation between the frequencies and corresponding levels of HIV-1 DNA per CD4 T cell. All data pairs with detectable IUPM and total HIV-1 DNA data were included. Open symbols reflect test results below detection threshold.
Early treatment initiation accelerates the decay of HIV-1 infected CD4 T cells.
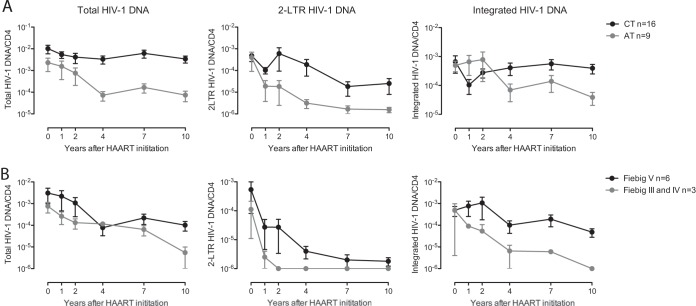
We subsequently investigated the longitudinal evolution of per-cell levels of HIV-1 DNA in isolated CD4 T cells from the two HAART-treated study cohorts. Notably, persons with treatment initiation in early versus chronic infection did not differ significantly in pretreatment levels of HIV-1 DNA, although there was a slight trend toward lower levels in persons who started treatment early. Using a mixed-effects model with log-order reduction in HIV-1 DNA following the initiation of antiviral therapy, we observed statistically significant longitudinal decreases in total, integrated, and 2-LTR HIV-1 DNA in persons started on treatment in early infection (Table 2; Fig. 3A). This decay was particularly pronounced in patients starting therapy prior to HIV-1 seroconversion (Fiebig stage III-IV) (Fig. 3B). In individuals who initiated treatment in chronic infection, decay rates of total and 2-LTR HIV-1 DNA were lower than those in persons who began treatment in early HIV-1 infection, and the decay of integrated HIV-1 DNA was not statistically significant in comparison to baseline levels (Table 2; Fig. 3). Overall, these data indicate faster decay kinetics of multiple HIV-1 DNA forms in patients who started treatment during the earliest stages of HIV-1 infection, suggesting that more rapid initiation of treatment can accelerate the decline of the reservoir of HIV-1-infected CD4 T cells.
TABLE 2.
Decay rate of HIV-1 DNA in isolated CD4 T cells from the study cohortsa
| Measurementb | Value for group |
P value between groups | |
|---|---|---|---|
| Early treated (n = 9) | Chronic treated (n = 16) | ||
| Total HIV-1 DNA decay | 0.13 ± 0.01 | 0.01 ± 0.007 | <1e-5 |
| P value within group | <1e-5 | 0.02 | |
| 2-LTR HIV-1 DNA decay | 0.32 ± 0.03 | 0.11 ± 0.02 | 0.0008 |
| P value within group | <1e-5 | 0.02 | |
| Integrated HIV-1 DNA decay | 0.07 ± 0.01 | −0.01 ± 0.01 | <1e-5 |
| P value within group | <1e-5 | 0.10 | |
Tobit regression for censored data was applied using a standard Markov chain-Monte Carlo method (31).
Decay values are log10 decay/year (mean ± SD); P values are for 0 versus 10 years.
FIG 3.
Antiretroviral treatment initiation in primary HIV-1 infection accelerates HIV-1 DNA decay. (A) Longitudinal evolution of copies per cell of total, 2-LTR, and integrated HIV-1 DNA in CD4 T cells from patients with antiretroviral treatment initiation in early (ET) (n = 9) or chronic (CT) (n = 16) HIV-1 infection. (B) Longitudinal decline of HIV-1 DNA after antiretroviral treatment initiation in primary HIV-1 infection, stratified according to treatment commencement before HIV-1 seroconversion (Fiebig stage III-IV) (n = 3) or after HIV-1 seroconversion (Fiebig stage V) (n = 6). Data are pointwise maximum-likelihood estimates of the means and 95% confidence interval estimates of the mean.
Initiation of treatment during primary HIV-1 infection results in an altered composition of the viral reservoir.
The memory CD4 T cell pool consists of a heterogenous mixture of cell subsets with distinct developmental programs, which most likely evolve in a sequential hierarchical order from immature, long-lasting CD4 T memory stem cells and central-memory cells to more short-lived effector memory and terminally differentiated CD4 T cells (34–38). To determine if treatment initiated at different phases of the infection modifies the composition of the viral reservoir in different CD4+ T cells after long-term therapy, we determined levels of HIV-1 DNA in sorted CD4 T cell subsets from our study cohorts from whom sufficient PBMCs were available after >10 years of continuous antiretroviral therapy. In patients started on treatment during the early phase of the infection, per-cell levels of HIV-1 DNA were lower in most CD4 T cell subsets; however, these differences were significant only for effector memory and terminally differentiated CD4 T cells and were less obvious for central-memory and T memory stem cells, in which HIV-1 levels typically remains very stable over time (Fig. 4A). HIV-1 2-LTR DNA was below the threshold of detection in naive, T memory stem cells and central-memory and terminally differentiated CD4 T cells, irrespective of the study cohort; however, 2-LTR DNA was observed in effector memory CD4 T cells in 5 of 10 subjects treated during chronic infection but not in any of the subjects with early treatment initiation (P = 0.05, chi-square test) (data not shown). To further investigate the composition of the viral CD4 T cell reservoir in our study patients, we analyzed the contribution of CD4 T subsets to the total HIV-1 reservoir in CD4 T cells after long-term treatment. Consistent with higher per-cell levels of HIV-1 DNA in effector-memory and terminally differentiated CD4 T cell subsets in persons started on treatment in chronic infection, we observed that these cell populations made a larger contribution to the total CD4 T cell reservoir in persons with late treatment initiation (Fig. 4B). In contrast, in patients who initiated treatment during early HIV-1 infection, the more immature, long-lived T cell subsets accounted for a significantly larger proportion of the total viral reservoir in CD4 T cells than in patients who started treatment in chronic HIV-1 infection, despite the fact that the contribution of these T cell subsets to the total CD4 T cell pool did not differ between the two study cohorts. These data show that long-term antiretroviral treatment started in early infection leads to a viral CD4 T cell reservoir that is significantly smaller than that in patients who delayed treatment until chronic infection but includes a larger fraction of HIV-1 DNA in the more immature, long-lasting central-memory and T memory stem cells.
FIG 4.
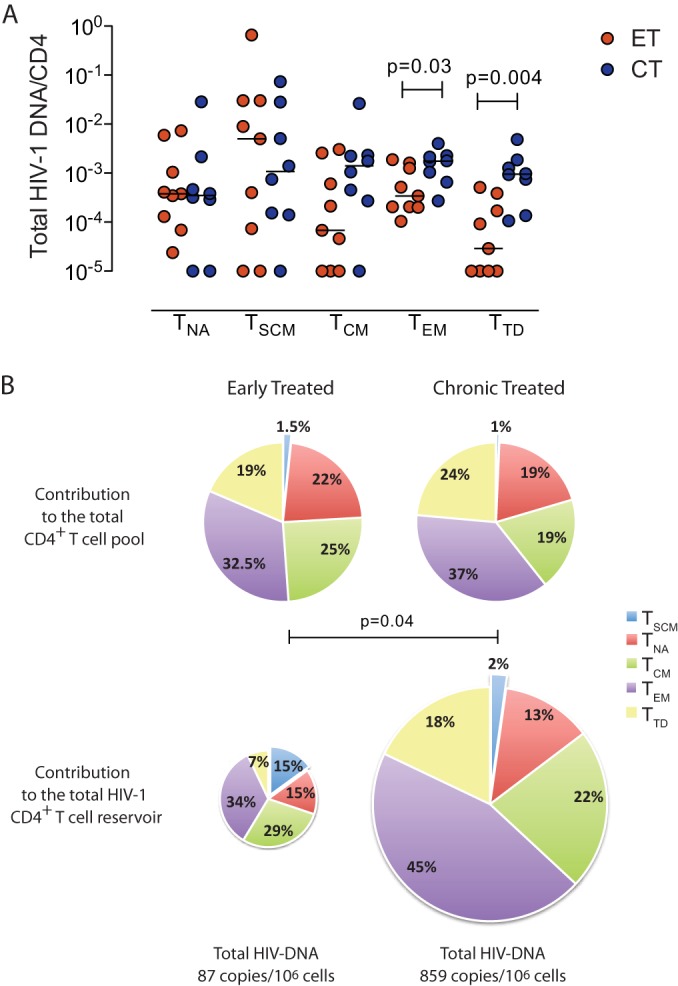
Differential composition of the HIV-1 CD4 T cell reservoir in patients started on antiretroviral treatment in early versus chronic HIV-1 infection. (A) Per-cell levels of total HIV-1 DNA in isolated naive (TNA), memory stem cell (TSCM), central-memory (TCM), effector memory (TEM), and terminally differentiated (TTD) CD4 T cells in persons with treatment initiation in early versus chronic HIV-1 infection. (B) Contribution of individual CD4 T cell subsets to the total CD4 T cell pool (top) and to the total HIV-1 CD4 T cell reservoir (bottom) in patients with treatment initiation in early (n = 8) versus chronic (n = 9) HIV-1 infection. The sizes of the pie charts in the lower panel correspond to the smaller size of the total HIV-1 reservoir in CD4 T cells from individuals with early HIV-1 treatment initiation. All measurements were performed with samples after a median of 10 years after treatment initiation.
HIV-1-specific T and B cell immunity after treatment initiation in primary infection.
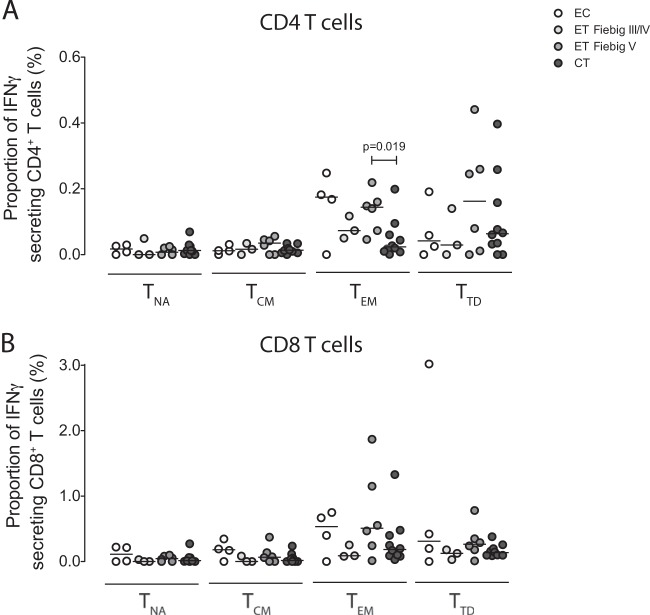
To compare the effects of long-term antiretroviral therapy on HIV-1-specific antibody responses, HIV-1 Western blots were performed on samples from our study cohorts after long-term treatment. The number of detectable antibody responses to HIV-1 proteins ranged from 1 to 9 in elite controllers, 7 to 9 in patients started on treatment in chronic infection, and 2 to 9 in individuals who initiated treatment in early HIV-1 infection (data not shown). Only 55% of patients treated early after infection showed responses to all proteins tested; in contrast, 79% of elite controllers and 71% of chronic treated individuals were positive for all Western blot bands. Importantly, in 4 study subjects started on treatment in early HIV-1 infection, we observed a reduction in the number of Western blot bands after 10 years, compared to baseline assessments performed during early HIV-1 infection; this is consistent with a partial seroreversion. To further investigate immune activity against HIV-1 in our study patients after 10 years for continuous treatment, we analyzed Gag-specific CD4 and CD8 T cell responses by antigen-specific IFN-γ secretion. Persons who started antiretroviral treatment after seroconversion (Fiebig stage V) had high frequencies of Gag-specific effector T cells that were not significantly different from those in elite controllers but were slightly higher than those in patients who started treatment in chronic HIV-1 infection. Patients who started antiretroviral treatment prior to HIV-1 seroconversion (Fiebig stage III-IV) had the lowest levels of Gag-specific effector T cell responses, but these responses were still detectable in most patients (Fig. 5). Together, these findings indicate that HIV-1-specific T cell responses, and to a lesser extent HIV-1-specific B cell responses, can persist long term, even when viral antigen levels are pharmacologically suppressed from the earliest stages of viral infection.
FIG 5.
Long-term persistence of HIV-1-specific T cell responses despite suppressive antiretroviral therapy. The proportion of IFN-γ-secreting HIV-1-specific CD4 (A) and CD8 (B) T cells with the naive, central-memory, effector memory, or terminally differentiated phenotype from elite controllers and individuals with antiretroviral treatment initiation in primary or chronic HIV-1 infection is shown.
DISCUSSION
Reducing the reservoir of CD4 T cells with latent HIV-1 infection may help HIV-1 patients to reach at least a transient drug-free remission of their disease. Whether long-term antiretroviral treatment initiated during very early stages of the infection can facilitate such a status and lead to a more effective reduction of such residual reservoirs of latently infected CD4 T cells remains unknown. Here, we focused on a small number of patients who initiated antiretroviral therapy in early HIV-1 infection and remained on fully suppressive treatment for more than 10 consecutive years. Interestingly, our data indicated that a measurable decline of HIV-1 DNA in CD4 T cells occurred primarily in the initial 3 to 4 years of treatment, independent of whether treatment was initiated in early or chronic infection. However, in persons started on treatment early, this decline occurred faster and was more pronounced, leading to substantially larger differences in the size of the viral reservoir between the two cohorts after 10 years of treatment than at baseline. Nevertheless, despite 10 years of effective treatment, HIV-1 remained detectable in almost all patients, indicating that antiretroviral treatment, even when initiated early and continuously maintained for >10 years, is insufficient to reduce viral reservoirs to a point that approaches viral eradication.
The initial decline of HIV-1 DNA in CD4 T cells during the first three to 4 years, followed by near-constant levels of HIV-1 DNA during the subsequent time of evaluation, is consistent with recent studies (16, 39, 40, 46) and mathematical models (15) showing that the viral reservoir is heterogeneously structured and includes more short-lived elements as well as extremely long-lasting components that are largely unaffected by antiretroviral treatment and seem to persist indefinitely. Early treatment initiation seemed to accelerate viral decay and cause a more profound relative and absolute reduction in the frequency of infected cells, for reasons that are not entirely clear. It is possible that during primary HIV-1 infection, a larger fraction of HIV-1 DNA in CD4 T cells consists of extrachromosomal forms with reduced stability and faster decay than chromosomally integrated HIV-1 DNA; however, levels of integrated HIV-1 DNA at the time of treatment initiation were not significantly different between individuals who started treatment early and those who started late. Alternatively, short-lived effector memory CD4 T cells with accelerated intrinsic decay kinetics are typically most susceptible to HIV-1 and may be preferentially infected when antiretroviral therapy is initiated rapidly during early HIV-1 infection. Whether the HIV-1 reservoir in primary infection was indeed biased toward less durable CD4 T cell subsets in our patients is uncertain; unfortunately, samples from our patients were insufficient to analyze the composition of the viral CD4 T cell reservoir at the beginning of treatment initiation. Nevertheless, we observed that after more than 10 years of therapy, HIV-1 DNA was readily detectable in all T cell subsets and that central-memory and T memory stem cells made a larger contribution to the viral CD4 T cell reservoir when treatment was begun early. Together, these findings suggest that seeding of the viral reservoir in CD4 T memory stem cells and central-memory CD4 T cells can occur despite early treatment initiation and that central-memory/T memory stem cells may represent the population of extremely long-lasting and treatment-refractory components of the viral reservoir that were previously hypothesized based on theoretical and mathematical considerations (15). Notably, differences in per-cell levels of HIV-1 DNA between individuals with treatment initiation in early versus chronic infection were most obvious in effector memory and terminally differentiated T cells, consistent with the observation that after treatment initiation in early infection, HIV-1 DNA preferentially declines in these short-lived CD4 T cell subsets while remaining relatively stable in central-memory and T memory stem cells (38).
In addition to an altered T cell subset distribution of the viral reservoir, accelerated HIV-1 decay kinetics after early treatment initiation may also reflect a more complete suppression of viral replication. In the vast majority of our patients who had been on suppressive therapy for more than 10 years, we were unable to detect residual HIV-1 replication using ultrasensitive assays. However, levels of CD4 T cell-associated 2-LTR HIV-1 DNA, which may reflect ongoing HIV-1 replication more reliably than single-copy viremia (26, 41), tended to be higher in persons with treatment initiation during later disease stages. This difference was particularly obvious in effector memory CD4 T cells, in which detectable levels of 2-LTR HIV-1 DNA were noticed in several patients with treatment initiation in chronic infection, even after >10 years of continuous suppressive therapy; this suggests that low levels of viral replication may be ongoing in these cells even after prolonged antiretroviral treatment. Residual ongoing replication in this CD4 T cell subset would be consistent with a larger viral reservoir observed in effector memory CD4 T cells after chronic treatment initiation than in patients who started on treatment early.
An important finding in this work is that after 10 years of continuous antiretroviral therapy, substantial numbers of HIV-1-specific effector CD8 T cells remained detectable, even in patients who started treatment in early HIV-1 infection, when HIV-1-specific T cell responses are typically weak and narrowly focused on a selected number of epitopes (42, 43). This indicates that long-term treatment started during the earliest stages of HIV-1 infection does not prevent the emergence or maintenance of a visible T cell response against HIV-1 and attests to the ability of the immune system to maintain antigen-specific T cell responses for prolonged periods of time in the absence of detectable active generation of viral antigen. Interestingly, antibody responses against HIV-1 appeared to show weaker persistence than cellular immune responses to HIV-1, and a partial seroreversion was observed in some of our patients who started treatment early, as previously described (44).
Eradication of HIV-1 and induction of a long-term drug-free remission of HIV-1 infection may require a simultaneous combination of immunologic and virologic interventions (45). The low residual levels of the viral reservoir, paired with persistence of HIV-1-specific CD8 T cells, may make patients with early treatment initiation more likely to respond to such interventions in a clinically significant way. Nevertheless, our data indicate that even when antiretroviral treatment is initiated early, an extremely long-lasting reservoir of HIV-1 infected CD4 T cells is effectively established, likely through infection of very long-lived CD4 T cell subsets. Our data suggest that the small number of HIV-1 patients with long-term treatment started in primary infection may represent good candidates for future pilot clinical trials aiming at reducing HIV-1 persistence.
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health (grants AI098487 and AI106468 to M.L.; AI089339, AI078799, and AI098484 to X.G.Y.; AI100699 to J.Z.L.) and by the American Foundation for AIDS Research (grant 108302-51-RGRL). M.L. is a recipient of the Clinical Scientist Development Award from the Doris Duke Charitable Foundation (grant 2009034). M.J.B. is supported by the Tosteson postdoctoral fellowship award from Massachusetts General Hospital. M.J.B. and E.G. are recipients of the Scholar Award from HU CFAR NIH/NIAID fund 5P30AI060354-10. J.M-P. is supported by the Spanish Ministry of Science and Innovation (SAF2013-49042-R) and the HIVACAT program. Patient blood sample collection was supported by the National Institutes of Health (grant AI074415), by the Mark and Lisa Schwartz Foundation, and by the Bill & Melinda Gates Foundation.
Footnotes
Published ahead of print 25 June 2014
REFERENCES
- 1.Kahn JO, Walker BD. 1998. Acute human immunodeficiency virus type 1 infection. N. Engl. J. Med. 339:33–39. 10.1056/NEJM199807023390107 [DOI] [PubMed] [Google Scholar]
- 2.Schacker T, Collier AC, Hughes J, Shea T, Corey L. 1996. Clinical and epidemiologic features of primary HIV infection. Ann. Intern. Med. 125:257–264. 10.7326/0003-4819-125-4-199608150-00001 [DOI] [PubMed] [Google Scholar]
- 3.Hecht FM, Wang L, Collier A, Little S, Markowitz M, Margolick J, Kilby JM, Daar E, Conway B, Holte S. 2006. A multicenter observational study of the potential benefits of initiating combination antiretroviral therapy during acute HIV infection. J. Infect. Dis. 194:725–733. 10.1086/506616 [DOI] [PubMed] [Google Scholar]
- 4.Le T, Wright EJ, Smith DM, He W, Catano G, Okulicz JF, Young JA, Clark RA, Richman DD, Little SJ, Ahuja SK. 2013. Enhanced CD4+ T-cell recovery with earlier HIV-1 antiretroviral therapy. N. Engl. J. Med. 368:218–230. 10.1056/NEJMoa1110187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McNairy ML, El-Sadr WM. 2014. Antiretroviral therapy for the prevention of HIV transmission: what will it take? Clin. Infect. Dis. 58:1003–1011. 10.1093/cid/ciu018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moir S, Buckner CM, Ho J, Wang W, Chen J, Waldner AJ, Posada JG, Kardava L, O'Shea MA, Kottilil S, Chun TW, Proschan MA, Fauci AS. 2010. B cells in early and chronic HIV infection: evidence for preservation of immune function associated with early initiation of antiretroviral therapy. Blood 116:5571–5579. 10.1182/blood-2010-05-285528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fidler S, Fox J, Touloumi G, Pantazis N, Porter K, Babiker A, Weber J. 2007. Slower CD4 cell decline following cessation of a 3 month course of HAART in primary HIV infection: findings from an observational cohort. AIDS 21:1283–1291. 10.1097/QAD.0b013e3280b07b5b [DOI] [PubMed] [Google Scholar]
- 8.Rosenberg ES, Billingsley JM, Caliendo AM, Boswell SL, Sax PE, Kalams SA, Walker BD. 1997. Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science 278:1447–1450. 10.1126/science.278.5342.1447 [DOI] [PubMed] [Google Scholar]
- 9.Delwart E, Magierowska M, Royz M, Foley B, Peddada L, Smith R, Heldebrant C, Conrad A, Busch M. 2002. Homogeneous quasispecies in 16 out of 17 individuals during very early HIV-1 primary infection. AIDS 16:189–195. 10.1097/00002030-200201250-00007 [DOI] [PubMed] [Google Scholar]
- 10.Saez-Cirion A, Bacchus C, Hocqueloux L, Avettand-Fenoel V, Girault I, Lecuroux C, Potard V, Versmisse P, Melard A, Prazuck T, Descours B, Guergnon J, Viard JP, Boufassa F, Lambotte O, Goujard C, Meyer L, Costagliola D, Venet A, Pancino G, Autran B, Rouzioux C. 2013. Post-treatment HIV-1 controllers with a long-term virological remission after the interruption of early initiated antiretroviral therapy ANRS VISCONTI Study. PLoS Pathog. 9:e1003211. 10.1371/journal.ppat.1003211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosenberg ES, Altfeld M, Poon SH, Phillips MN, Wilkes BM, Eldridge RL, Robbins GK, D'Aquila RT, Goulder PJ, Walker BD. 2000. Immune control of HIV-1 after early treatment of acute infection. Nature 407:523–526. 10.1038/35035103 [DOI] [PubMed] [Google Scholar]
- 12.Fidler S, Porter K, Ewings F, Frater J, Ramjee G, Cooper D, Rees H, Fisher M, Schechter M, Kaleebu P, Tambussi G, Kinloch S, Miro JM, Kelleher A, McClure M, Kaye S, Gabriel M, Phillips R, Weber J, Babiker A. 2013. Short-course antiretroviral therapy in primary HIV infection. N. Engl. J. Med. 368:207–217. 10.1056/NEJMoa1110039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grijsen ML, Steingrover R, Wit FW, Jurriaans S, Verbon A, Brinkman K, van der Ende ME, Soetekouw R, de Wolf F, Lange JM, Schuitemaker H, Prins JM. 2012. No treatment versus 24 or 60 weeks of antiretroviral treatment during primary HIV infection: the randomized Primo-SHM trial. PLoS Med. 9:e1001196. 10.1371/journal.pmed.1001196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hogan CM, Degruttola V, Sun X, Fiscus SA, Del Rio C, Hare CB, Markowitz M, Connick E, Macatangay B, Tashima KT, Kallungal B, Camp R, Morton T, Daar ES, Little S. 2012. The setpoint study (ACTG A5217): effect of immediate versus deferred antiretroviral therapy on virologic set point in recently HIV-1-infected individuals. J. Infect. Dis. 205:87–96. 10.1093/infdis/jir699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Archin NM, Vaidya NK, Kuruc JD, Liberty AL, Wiegand A, Kearney MF, Cohen MS, Coffin JM, Bosch RJ, Gay CL, Eron JJ, Margolis DM, Perelson AS. 2012. Immediate antiviral therapy appears to restrict resting CD4+ cell HIV-1 infection without accelerating the decay of latent infection. Proc. Natl. Acad. Sci. U. S. A. 109:9523–9528. 10.1073/pnas.1120248109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Strain MC, Little SJ, Daar ES, Havlir DV, Gunthard HF, Lam RY, Daly OA, Nguyen J, Ignacio CC, Spina CA, Richman DD, Wong JK. 2005. Effect of treatment, during primary infection, on establishment and clearance of cellular reservoirs of HIV-1. J. Infect. Dis. 191:1410–1418. 10.1086/428777 [DOI] [PubMed] [Google Scholar]
- 17.Ananworanich J, Schuetz A, Vandergeeten C, Sereti I, de Souza M, Rerknimitr R, Dewar R, Marovich M, van Griensven F, Sekaly R, Pinyakorn S, Phanuphak N, Trichavaroj R, Rutvisuttinunt W, Chomchey N, Paris R, Peel S, Valcour V, Maldarelli F, Chomont N, Michael N, Phanuphak P, Kim JH. 2012. Impact of multitargeted antiretroviral treatment on gut T cell depletion and HIV reservoir seeding during acute HIV infection. PLoS One 7:e33948. 10.1371/journal.pone.0033948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ngo-Giang-Huong N, Deveau C, Da Silva I, Pellegrin I, Venet A, Harzic M, Sinet M, Delfraissy JF, Meyer L, Goujard C, Rouzioux C. 2001. Proviral HIV-1 DNA in subjects followed since primary HIV-1 infection who suppress plasma viral load after one year of highly active antiretroviral therapy. AIDS 15:665–673. 10.1097/00002030-200104130-00001 [DOI] [PubMed] [Google Scholar]
- 19.Dalmasso C, Carpentier W, Meyer L, Rouzioux C, Goujard C, Chaix ML, Lambotte O, Avettand-Fenoel V, Le Clerc S, de Senneville LD, Deveau C, Boufassa F, Debre P, Delfraissy JF, Broet P, Theodorou I. 2008. Distinct genetic loci control plasma HIV-RNA and cellular HIV-DNA levels in HIV-1 infection: the ANRS Genome Wide Association 01 study. PLoS One 3:e3907. 10.1371/journal.pone.0003907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Descours B, Avettand-Fenoel V, Blanc C, Samri A, Melard A, Supervie V, Theodorou I, Carcelain G, Rouzioux C, Autran B. 2012. Immune responses driven by protective human leukocyte antigen alleles from long-term nonprogressors are associated with low HIV reservoir in central memory CD4 T cells. Clin. Infect. Dis. 54:1495–1503. 10.1093/cid/cis188 [DOI] [PubMed] [Google Scholar]
- 21.Lambotte O, Boufassa F, Madec Y, Nguyen A, Goujard C, Meyer L, Rouzioux C, Venet A, Delfraissy JF. 2005. HIV controllers: a homogeneous group of HIV-1-infected patients with spontaneous control of viral replication. Clin. Infect. Dis. 41:1053–1056. 10.1086/433188 [DOI] [PubMed] [Google Scholar]
- 22.Hocqueloux L, Saez-Cirion A, Rouzioux C. 2013. Immunovirologic control 24 months after interruption of antiretroviral therapy initiated close to HIV seroconversion. JAMA Intern. Med. 173:475–476. 10.1001/jamainternmed.2013.2176 [DOI] [PubMed] [Google Scholar]
- 23.Chomont N, El-Far M, Ancuta P, Trautmann L, Procopio FA, Yassine-Diab B, Boucher G, Boulassel MR, Ghattas G, Brenchley JM, Schacker TW, Hill BJ, Douek DC, Routy JP, Haddad EK, Sekaly RP. 2009. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat. Med. 15:893–900. 10.1038/nm.1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liszewski MK, Yu JJ, O'Doherty U. 2009. Detecting HIV-1 integration by repetitive-sampling Alu-gag PCR. Methods 47:254–260. 10.1016/j.ymeth.2009.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brussel A, Delelis O, Sonigo P. 2005. Alu-LTR real-time nested PCR assay for quantifying integrated HIV-1 DNA. Methods Mol. Biol. 304:139–154. 10.1385/1-59259-907-9:139 [DOI] [PubMed] [Google Scholar]
- 26.Buzon MJ, Massanella M, Llibre JM, Esteve A, Dahl V, Puertas MC, Gatell JM, Domingo P, Paredes R, Sharkey M, Palmer S, Stevenson M, Clotet B, Blanco J, Martinez-Picado J. 2010. HIV-1 replication and immune dynamics are affected by raltegravir intensification of HAART-suppressed subjects. Nat. Med. 16:460–465. 10.1038/nm.2111 [DOI] [PubMed] [Google Scholar]
- 27.Palmer S, Wiegand AP, Maldarelli F, Bazmi H, Mican JM, Polis M, Dewar RL, Planta A, Liu S, Metcalf JA, Mellors JW, Coffin JM. 2003. New real-time reverse transcriptase-initiated PCR assay with single-copy sensitivity for human immunodeficiency virus type 1 RNA in plasma. J. Clin. Microbiol. 41:4531–4536. 10.1128/JCM.41.10.4531-4536.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Reference deleted.
- 29.Wei X, Decker JM, Liu H, Zhang Z, Arani RB, Kilby JM, Saag MS, Wu X, Shaw GM, Kappes JC. 2002. Emergence of resistant human immunodeficiency virus type 1 in patients receiving fusion inhibitor (T-20) monotherapy. Antimicrob. Agents Chemother. 46:1896–1905. 10.1128/AAC.46.6.1896-1905.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu Y, Smyth GK. 2009. ELDA: extreme limiting dilution analysis for comparing depleted and enriched populations in stem cell and other assays. J. Immunol. Methods 347:70–78. 10.1016/j.jim.2009.06.008 [DOI] [PubMed] [Google Scholar]
- 31.Tobin J. 1958. Estimation of relationships for limited dependent variables. Econometrica 1:24–36 [Google Scholar]
- 32.Huang Y, Wu H, Acosta EP. 2010. Hierarchical Bayesian inference for HIV dynamic differential equation models incorporating multiple treatment factors. Biom J. 52:470–486. 10.1002/bimj.200900173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luo R, Piovoso MJ, Zurakowski R. 2012. Modeling uncertainty in single-copy assays for HIV. J. Clin. Microbiol. 50:3381–3382. 10.1128/JCM.01254-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gattinoni L, Klebanoff CA, Restifo NP. 2012. Paths to stemness: building the ultimate antitumour T cell. Nat. Rev. Cancer. 12:671–684. 10.1038/nrc3322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lanzavecchia A, Sallusto F. 2002. Progressive differentiation and selection of the fittest in the immune response. Nat. Rev. Immunol. 2:982–987. 10.1038/nri959 [DOI] [PubMed] [Google Scholar]
- 36.Sallusto F, Geginat J, Lanzavecchia A. 2004. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu. Rev. Immunol. 22:745–763. 10.1146/annurev.immunol.22.012703.104702 [DOI] [PubMed] [Google Scholar]
- 37.Gattinoni L, Lugli E, Ji Y, Pos Z, Paulos CM, Quigley MF, Almeida JR, Gostick E, Yu Z, Carpenito C, Wang E, Douek DC, Price DA, June CH, Marincola FM, Roederer M, Restifo NP. 2011. A human memory T cell subset with stem cell-like properties. Nat. Med. 17:1290–1297. 10.1038/nm.2446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Buzon MJ, Sun H, Li C, Shaw A, Seiss K, Ouyang Z, Martin-Gayo E, Leng J, Henrich TJ, Li JZ, Pereyra F, Zurakowski R, Walker BD, Rosenberg ES, Yu XG, Lichterfeld M. 2014. HIV-1 persistence in CD4 T cells with stem cell-like properties. Nat. Med. 20:139–142. 10.1038/nm.3445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chun TW, Justement JS, Moir S, Hallahan CW, Maenza J, Mullins JI, Collier AC, Corey L, Fauci AS. 2007. Decay of the HIV reservoir in patients receiving antiretroviral therapy for extended periods: implications for eradication of virus. J. Infect. Dis. 195:1762–1764. 10.1086/518250 [DOI] [PubMed] [Google Scholar]
- 40.Hocqueloux L, Avettand-Fenoel V, Jacquot S, Prazuck T, Legac E, Melard A, Niang M, Mille C, Le Moal G, Viard JP, Rouzioux C. 2013. Long-term antiretroviral therapy initiated during primary HIV-1 infection is key to achieving both low HIV reservoirs and normal T cell counts. J. Antimicrob. Chemother. 68:1169–1178. 10.1093/jac/dks533 [DOI] [PubMed] [Google Scholar]
- 41.Sharkey M, Babic DZ, Greenough T, Gulick R, Kuritzkes DR, Stevenson M. 2011. Episomal viral cDNAs identify a reservoir that fuels viral rebound after treatment interruption and that contributes to treatment failure. PLoS Pathog. 7:e1001303. 10.1371/journal.ppat.1001303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lichterfeld M, Yu XG, Cohen D, Addo MM, Malenfant J, Perkins B, Pae E, Johnston MN, Strick D, Allen TM, Rosenberg ES, Korber B, Walker BD, Altfeld M. 2004. HIV-1 Nef is preferentially recognized by CD8 T cells in primary HIV-1 infection despite a relatively high degree of genetic diversity. AIDS 18:1383–1392. 10.1097/01.aids.0000131329.51633.a3 [DOI] [PubMed] [Google Scholar]
- 43.Altfeld M, Rosenberg ES, Shankarappa R, Mukherjee JS, Hecht FM, Eldridge RL, Addo MM, Poon SH, Phillips MN, Robbins GK, Sax PE, Boswell S, Kahn JO, Brander C, Goulder PJ, Levy JA, Mullins JI, Walker BD. 2001. Cellular immune responses and viral diversity in individuals treated during acute and early HIV-1 infection. J. Exp. Med. 193:169–180. 10.1084/jem.193.2.169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kassutto S, Johnston MN, Rosenberg ES. 2005. Incomplete HIV type 1 antibody evolution and seroreversion in acutely infected individuals treated with early antiretroviral therapy. Clin. Infect. Dis. 40:868–873. 10.1086/428127 [DOI] [PubMed] [Google Scholar]
- 45.Katlama C, Deeks SG, Autran B, Martinez-Picado J, van Lunzen J, Rouzioux C, Miller M, Vella S, Schmitz JE, Ahlers J, Richman DD, Sekaly RP. 2013. Barriers to a cure for HIV: new ways to target and eradicate HIV-1 reservoirs. Lancet 381:2109–2117. 10.1016/S0140-6736(13)60104-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ananworanich J, Vandergeeten C, Chomchey N, Chomont N. 2013. Early ART intervention restricts the seeding of the HIV reservoir in long-lived central memory CD4 T cells, abstr. 57 In Program and abstracts of the 20th Conference on Retroviruses and Opportunistic Infections, Atlanta, GA [Google Scholar]