Abstract
Purpose.
Bacterial keratitis is a sight-threatening infection of the cornea that is one of the leading causes of blindness globally. In this report, we analyze the role of moxifloxacin susceptibility in the relationship between causative organisms and clinical outcome in bacteria keratitis.
Methods.
A mediation analysis is used to assess the role of moxifloxacin susceptibility in the relationship between causative organisms and clinical outcome in bacterial keratitis using data collected in a randomized, controlled trial.
Results.
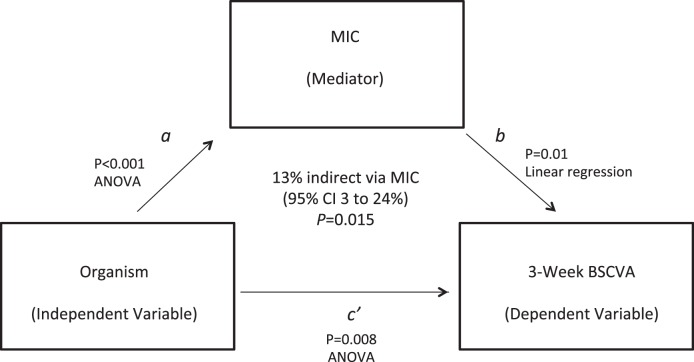
In the Steroids for Corneal Ulcers Trial (SCUT), 500 corneal infections were treated with topical moxifloxacin. The outcome of 3-week best spectacle-corrected visual acuity was significantly associated with an organism (Streptococcus pneumoniae, Pseudomonas aeruginosa, etc., P = 0.008). An indirect effects mediation model suggests that MIC accounted for approximately 13% (95% confidence interval, 3%–24%, P = 0.015) of the effect of the organism on 3-week visual acuity.
Conclusions.
Moxifloxacin mediates the relationship between causative organisms and clinical outcome in bacterial keratitis, and is likely on the causal pathway between the organism and outcome. (ClinicalTrials.gov number, NCT00324168.)
Different etiologic organisms are thought to result in different clinical outcomes. Here, we found that moxifloxacin susceptibility mediates this relationship, suggesting it is on the causal pathway.
Introduction
Corneal opacity, including scarring from infectious keratitis, is a leading cause of blindness globally.1 Bacteria are a leading cause of infectious keratitis. In bacterial keratitis, while eradication of the infection is generally successful with appropriate antibiotic treatment, patients are frequently left with scars and vision loss can remain severe. Susceptibility of the causative organism to the antibiotic plays a role in predicting clinical outcomes, including visual acuity, in bacterial and fungal keratitis.2–7 Susceptibility has also been shown to correlate with the causative organism in these infections.4,6 It is commonly thought that clinical outcomes differ by causative organisms in systemic and ocular infections. For example, in bacterial keratitis, infection with Pseudomonas aeruginosa has been suggested to be associated with poor outcome.8 If both causative organisms and susceptibility are predictive of clinical outcome, and susceptibility correlates with the organism, a mediation analysis can be performed to assess how much of the effect organism has on outcome is actually due to susceptibility. In a recent large clinical trial, the Steroids for Corneal Ulcers Trial (SCUT), clinical outcomes were associated with antibiotic susceptibility.6 Here, we assess the relationship between organisms and clinical outcome and the portion of the effect that the organism has on outcome that can be attributed to differences in susceptibility to moxifloxacin as measured by the minimum inhibitory concentration (MIC) in SCUT.
Materials and Methods
Trial Methods
SCUT was a National Eye Institute–funded, randomized, placebo-controlled, double-masked, multicenter clinical trial comparing outcomes in patients randomized to receive topical prednisolone phosphate 1% (Bausch and Lomb Pharmaceuticals, Inc., Tampa, FL) or placebo (0.9% NaCl and preservative, prepared by Leiter's Pharmacy, San Jose, CA) as adjunctive therapy for the treatment of culture-positive bacterial keratitis. All patients received topical moxifloxacin 0.5% (Vigamox; Alcon, Fort Worth, TX) for 3 weeks from enrollment. The moxifloxacin regimen consisted of one drop applied every hour while awake for the first 48 hours, then one drop applied every 2 hours until re-epithelialization, and then four times a day until 3 weeks from enrollment.
Patients were examined every 3 days (+/– 1 day) until reepithelialization, and at 3 weeks and 3 months from enrollment. The primary outcome for the trial was best spectacle-corrected visual acuity (BSCVA) at 3 months from enrollment. Topical moxifloxacin was administered according to an identical protocol for all patients for 3 weeks. Complete trial methods have been previously described.9
Institutional review board approval was granted by the University of California, San Francisco Committee on Human Research, the Dartmouth Medical School Committee for the Protection of Human Subjects, and the Aravind Eye Care System Institutional Review Board. This study conformed to the tenets of the Declaration of Helsinki, and written informed consent was obtained from all subjects.
Microbiological Methods
Microbiological methods have been described previously.6,9 In brief, corneal scrapings for smear and culture were obtained at the presentation visit after slit lamp examination. Two scrapings were smeared on Gram stain and potassium hydroxide (KOH) wet mount. Three further scrapings were inoculated onto sheep's blood agar, chocolate agar, and potato dextrose or Sabouraud's agar for bacterial and fungal cultures. Any evidence of fungus on culture or smear resulted in exclusion from the trial. The criterion for a positive bacterial culture was growth of the organism on one solid medium at the site of inoculation, except for Staphylococcus epidermis and diphtheroids, which were considered positive only if moderate growth was seen on at least two solid media or on one solid medium plus a Gram-stained corneal smear.10 Antibiotic susceptibility testing for moxifloxacin was performed using the antimicrobial resistance testing method (E-test; AB BIODISK, Solna, Sweden).2 Further antibiotic susceptibility testing for a variety of antibiotics other than the treating antibiotic was performed using Kirby-Bauer disc diffusion.
Statistical Methods
A log2-transformed MIC was used in all statistical analyses. Visual acuity was analyzed as log10 of the minimum angle of resolution (logMAR). ANOVA was used to assess differences in continuous outcome variables across groups of the organism. Three-week visual acuity was used as the outcome variable for mediation analysis since antibiotics were given for 3 weeks per study protocol. Mediation analysis was performed using an indirect effects mediation model consisting of two linear regression models.11 One model consisted of the independent variable (organism) predicting the mediator variable (MIC), including enrollment acuity as a covariate, and the second model consisted of the independent and mediator variables predicting the outcome variable (visual acuity at 3 weeks), adjusting for enrollment acuity. This model generated a coefficient for each organism's effect on MIC and visual acuity at 3 weeks. The indirect effect of MIC on 3-week visual acuity was calculated by nonlinear combination of these estimates. The estimate of the direct effect of the organism on 3-week visual acuity was achieved by linear combination of the coefficients. The proportion of the total effect that was attributable indirectly to moxifloxacin susceptibility was then calculated as: indirect/(indirect + direct). Standard errors were estimated using bootstrap resampling and were used to calculate P values and 95% confidence limits. Sensitivity analyses were performed, restricting the analysis only to patients with >1.7 logMAR visual acuity at baseline and including a covariate for treatment arm (steroid or placebo). All statistical analyses were performed using analytical software (Stata 10.0; StataCorp, College Station, TX).
Results
Between September 2006 and February 2010, 500 patients were enrolled in SCUT. Of these patients, eight had no growth for MIC testing, six had a mixed infection, and six were culture-positive; but the organism could not be identified. Thus, 480 isolates were available for this analysis. Of these, 467 (97%) had complete follow-up information at 3 weeks from enrollment. The most commonly isolated organisms were Streptococcus pneumoniae (N = 247, 51%); Pseudomonas aeruginosa (N = 109, 23%); and Nocardia species (N = 55, 11%, Table). In SCUT cases, 89.3% (292/327, 95% confidence interval [CI], 85.4%–92.4%) of ulcers with an MIC <2 ug/mL had an improvement in acuity from presentation to 3 weeks, whereas only 59.3% (83/140, 50.7%–67.5%) of those with an MIC ≥2 ug/mL had an improvement in acuity at 3 weeks. The Table shows MIC data for moxifloxacin as well as Kirby-Bauer disc diffusion susceptibilities for several antibiotics. By Kirby-Bauer disc diffusion, moxifloxacin had the highest percentage of susceptible organisms (85% susceptible).
Table. .
Susceptibility for Bacterial Keratitis Isolates in the Steroids for Corneal Ulcers Trial
|
|
N |
Moxifloxacin (MIC50, ug/mL) |
Susceptible by Disc Diffusion N (%) |
||||
|
Moxifloxacin |
Vancomycin |
Tobramycin |
Cefazolin |
Amikacin |
|||
| Bacillus spp | 1 | 19 | 1 (100%) | 1 (100%) | N/A* | 0 (0%) | N/A* |
| Corynebacterium spp | 4 | 0.75 | 3 (75%) | 4 (100%) | 2 (50%) | 3 (75%) | 4 (100%) |
| Mycobacteria spp | 1 | 1 | 0 (0%) | 0 (0%) | N/A* | N/A* | 1 (100%) |
| Nocardia spp | 55 | 2 | 38 (69%) | 32 (60%)† | 21 (53%)† | 1 (2%)† | 53 (96%) |
| S. aureus | 11 | 1 | 6 (60%)† | 11 (100%) | 5 (56%)† | 10 (91%) | 4 (67%)† |
| Staphylococcus, coagulase-negative | 21 | 0.25 | 14 (67%) | 21 (100%) | 14 (70%)† | 15 (71%) | 14 (88%)† |
| S. pneumoniae | 247 | 0.25 | 246 (99%) | 244 (100%)† | 16 (14%)† | 247 (100%) | 4 (4%)† |
| Streptococcus, viridans group | 10 | 0.22 | 8 (100%)† | 5 (63%)† | 1 (14%)† | 6 (75%)† | 2 (33%)† |
| Enterobacter spp | 2 | 0.25 | 2 (100%) | 0 (0%)† | 2 (100%) | 0 (0%) | 2 (100%) |
| Klebsiella spp | 3 | 0.125 | 2 (67%) | 0 (0%)† | 3 (100%) | 2 (67%) | 2 (67%) |
| Moraxella spp | 13 | 0.09 | 10 (91%)† | 2 (40%)† | 10 (100%)† | 7 (88%)† | 9 (90%)† |
| Pseudomonas spp, non-aeruginosa | 3 | 2 | 3 (100%) | 0 (0%)† | 3 (100%) | 0 (0%)† | 3 (100%) |
| Pseudomonas aeruginosa | 109 | 3 | 71 (66%)† | 2 (7%)† | 98 (92%)† | 1 (2%)† | 98 (92%)† |
| Total | 480 | 0.38 | 404 (85%) | 322 (85%) | 193 (60%) | 292 (74%) | 196 (61%) |
Disc diffusion not performed.
Disc diffusion not performed for all isolates.
Visual acuity at 3 weeks was significantly different in different organisms (P = 0.008, ANOVA, Fig.). Previously, we showed that MIC was predictive of 3-week visual acuity and correlated with causative organisms, and thus we were able to do a mediation analysis.6 Mediation analysis estimated that approximately 13% (95% CI, 3%–24%, P = 0.015) of the effect of organisms on 3-week visual acuity was indirect via MIC. Controlling for randomized treatment arm, we found that this effect was 12.8% (P = 0.018). Excluding the subgroup of patients with baseline visual acuity of counting fingers (logMAR 1.7) or worse, this effect was 14.9% (P = 0.019). Therefore, MIC partially mediated the relationship between effect of organisms and visual acuity.
Figure.
Mediation diagram depicting relationship between causative organism, MIC, and BSCVA at 3 weeks from enrollment. Causative organism is the independent variable (IV); BSCVA at 3 weeks is the dependent variable (DV); and MIC is the mediator (M). P values and corresponding statistical tests associated with each arrow show each individual relationship, and the overall relationship of the mediator on the relationship between the independent and dependent variables is shown in bold in the middle of the diagram.
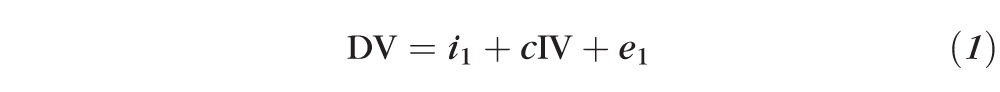 |
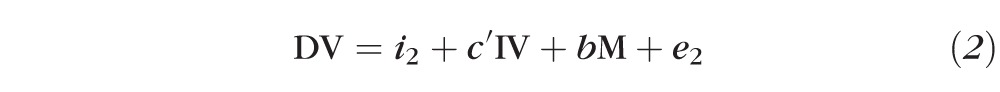 |
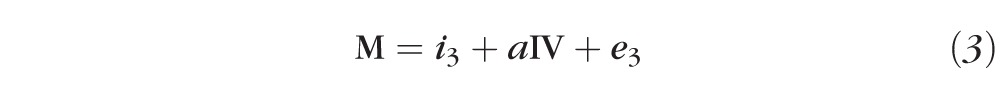 |
Discussion
The relationship between the causative organism, moxifloxacin susceptibility (as measured by MIC), and clinical outcome in bacterial infections has not been clearly defined. Previously, SCUT showed that a higher MIC was predictive of a worse 3-week visual acuity, although the effect size was relatively small.6 In addition, MICs varied significantly in different organisms, with the highest MIC50 of moxifloxacin found with P. aeruginosa.6 In this analysis, we attempted to further elucidate the relationship of the organism, MIC, and clinical outcome. Susceptibility varies significantly across organisms. Susceptibility is predictive of outcome visual acuity, and outcome acuity differs across causative organisms. It is reasonable that moxifloxacin susceptibility lies on the causal pathway between organisms and outcome visual acuity; specific organisms may have poor outcomes because they are more difficult to treat. We found that MIC is a mediator of the relationship between organisms and outcome, although the effect of MIC is not large; approximately 13% of the effect of an organism on outcome can be explained by antibiotic susceptibility.
Antibiotic susceptibility is only one of many organism-related factors associated with outcome in bacterial keratitis. Ulcer location, degree of inflammation, and toxins are all associated with both the organism and outcome, and may share partial mediation effects.12 SCUT demonstrated that while overall there was no effect of topical corticosteroid treatment on outcome visual acuity, there was a beneficial effect in the subgroup with the most severe keratitis at presentation. Various organisms may differ in their inflammatory processes, and corticosteroids may thus affect outcome differentially due to how they regulate the inflammatory response. Sensitivity analyses controlling for a randomized study arm (corticosteroid or placebo) and restricting the analysis only to patients who had an enrollment visual acuity better than counting fingers (i.e., excluding the most severe cases at presentation) did not significantly change the results, indicating that randomization to receive corticosteroids had little influence on this analysis.
The “90-60 rule” suggests that susceptible organisms respond 90% of the time and resistant organisms respond 60% of the time.13 In SCUT, 89% of susceptible isolates (defined as an MIC <2 μg/mL) had an improvement in visual acuity over the first 3 weeks of the trial, compared with 59% of resistant isolates. Thus, we expect that antibiotic susceptibility should explain only some of the variance in outcome; it is not difficult to show that the 90-60 rule implies that a maximum of 12.6% of the variance in outcome can be explained by knowing whether an organism is susceptible or resistant to the antibiotic used. Our results suggesting that 13% of the effect of an organism on outcome is due to moxifloxacin resistance are consistent with the estimate derived from the 90-60 rule that 12.6% of the variance in outcome can be explained by resistance of the organism. Both our results and the 90-60 rule demonstrate that susceptibility testing can yield useful information for appropriate antibiotic choice, but there are many other factors at play that affect the outcome of the infection.
The epidemiology of etiologic bacterial organisms in this study differs somewhat from what has been previously reported. Etiologic organisms have been shown to differ in their epidemiology geographically and temporally.14,15 In SCUT, the most commonly isolated organism was S. pneumoniae, consistent with other studies in South India.16–18 S. pneumoniae was overrepresented in this study compared with studies outside of South India. Of particular note, Nocardia species was the third most commonly isolated organism, an organism that is rarely reported outside of South Asia. Other reports have listed P. aeruginosa and Staphylococcus species as the commonest bacterial organisms.14,15,19–22 Staphylococcus species were relatively rare in this study, and no methicillin-resistant S. aureus (MRSA) or methicillin-resistant S. epidermidis (MRSE) strains were identified. It is likely that the difference in distribution of organisms between South India and other geographic locations has to do with geography and climactic factors. South India has a humid tropical climate, which may facilitate the growth of certain organisms. We cannot assume that other locations, where pneumococcus is not the most common isolate, will have similar results.
This analysis may suffer from several limitations. Here, we analyzed genus—and in some cases species of organism—but we were unable to analyze specific strains of organisms. P. aeruginosa, for example, consists of multiple different strains of organisms, with different virulence factors. There may be differences in susceptibility and outcome within these strains that is not identified in this analysis. The most severe cases at presentation were excluded from the trial (those with impending corneal perforation), which would likely have worse visual acuity at 3 weeks from enrollment. However, we adjusted for enrollment visual acuity in our models and thus were able to analyze how MIC and organisms affected acuity over the course of treatment in the trial. It is impossible to know how the inclusion of these cases would affect these results. Another limitation to our analysis could be potential measurement error in the organism, acuity, and MIC. Acuity was shown to be reproducible in the trial, and previous studies have found the antimicrobial resistance testing method (AB BIODISK) to be very reproducible in this setting. Finally, given that the standardized antibiotic therapy in this trial was moxifloxacin, we were unable to assess the effect of different antibiotics, including those with a better or a worse MIC than moxifloxacin. Therefore, we cannot comment on the effect of other antibiotics with different susceptibility patterns to moxifloxacin as mediators in this relationship. Other antibiotics' susceptibilities ranged widely by the organism in the trial, and future research could focus on specific organisms, antibiotics other than moxifloxacin, or combinations of antibiotics and this relationship. Moxifloxacin was chosen as the standardized antibiotic in the trial to isolate the effect of corticosteroids, and because it is a broad-spectrum antibiotic commonly used in practice.23,24 Overall among isolates in the trial, susceptibility to moxifloxacin was high, especially among S. pneumoniae isolates. Given the large number of S. pneumoniae isolates and the lack of MRSA, susceptibility in this study may have been higher than would be expected in settings with less S. pneumoniae or more MRSA, which could affect the generalizability of these results.
In conclusion, susceptibility is a mediator between an organism and 3-week visual acuity in bacterial keratitis, which is consistent with harder-to-treat organisms having a worse clinical result. While certain organisms have worse outcomes in general, the susceptibility of organisms to moxifloxacin explains a portion of the variance in outcome visual acuity. However, the amount of the outcome explained by susceptibility is small, indicating that there are other mechanisms that ultimately determine the majority of outcome. These results support the determination of the causative organism and in vitro susceptibility as prognostic indicators, and suggest the importance of appropriate antibiotic choice. Further work should be done characterizing additional explanations of poor outcomes.
Acknowledgments
We would like to thank the research staff at each of the trial sites. We are also grateful for the invaluable guidance and advice of the SCUT data and safety monitoring board: Marian Fisher (chair), Anthony Aldave, Donald Everett, Jacqueline Glover, K. Ananda Kannan, Steven Kymes, Gudlavalleti V. S. Murthy, and Ivan Schwab.
Footnotes
Supported by the National Eye Institute, U10 EY015114; a National Institute of Allergy and Infectious Disease Grant, T32AI007535 (CEO); a National Eye Institute K23EY017897 Grant and a Research to Prevent Blindness Award (NRA). The Department of Ophthalmology at UCSF is supported by a core grant from the National Eye Institute, EY02162; an unrestricted grant from Research to Prevent Blindness, New York, New York; and That Man May See, Inc., San Francisco, California. The authors alone are responsible for the content and writing of the paper.
Presented in part at the Infectious Disease Society of America, Boston, Massachusetts, October 22, 2011.
Disclosure: C.E. Oldenburg, None; P. Lalitha, None; M. Srinivasan, None; P. Manikandan, None; M.J. Bharathi, None; R. Rajaraman, None; M. Ravindran, None; J. Mascarenhas, None; N. Nardone, None; K.J. Ray, None; D.V. Glidden, None; N.R. Acharya, Alcon (F); T.M. Lietman, Alcon (F)
References
- 1. Resnikoff S, Pascolini D, Etya'ale D, et al. Global data on visual impairment in the year 2002. Bull World Health Organ. 2004; 82: 844–851 [PMC free article] [PubMed] [Google Scholar]
- 2. Chen A, Prajna L, Srinivasan M, et al. Does In Vitro Susceptibility Predict Clinical Outcome in Bacterial Keratitis? Am J Ophthalmol. 2008; 145: 409–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kaye S, Tuft S, Neal T, Tole D, Leeming J. Bacterial susceptibility to topical antimicrobials and clinical outcome in bacterial keratitis. Invest Ophthalmol Vis Sci. 2010; 51: 362–368 [DOI] [PubMed] [Google Scholar]
- 4. Lalitha P, Prajna NV, Oldenburg CE, et al. Organism, minimum inhibitory concentration, and outcome in a fungal corneal ulcer clinical trial. Cornea. 2012; 31: 662–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shapiro BL, Lalitha P, Loh AR. Susceptibility testing and clinical outcome in fungal keratitis. Br J Ophthalmol. 2010; 94: 384–385 [DOI] [PubMed] [Google Scholar]
- 6. Lalitha P, Srinivasan M, Manikandan P, et al. Relationship of in vitro susceptibility to moxifloxacin and in vivo clinical outcome in bacterial keratitis. Clin Infect Dis. 2012; 54: 1381–1387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wilhelmus KR, Abshire RL. Influence of fluoroquinolone susceptibility on the therapeutic response of fluoroquinolone-treated bacterial keratitis. Arch Ophthalmol. 2003; 121: 1229–1233 [DOI] [PubMed] [Google Scholar]
- 8. Green M, Apel A, Stapleton F. Risk Factors and Causative Organisms in Microbial Keratitis. Cornea. 2008; 27: 22–27 [DOI] [PubMed] [Google Scholar]
- 9. Srinivasan M, Mascarenhas J, Rajaraman R, et al. The Steroids for Corneal Ulcers Trial: study design and baseline characteristics. Arch Ophthalmol. 2012; 130: 151–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wilhelmus KR, Liesegang TJ, Osato MS. Laboratory diagnosis of ocular infections. In: Specter SC. ed Cumitech. Washington, DC: American Society for Microbiology; 1994. [Google Scholar]
- 11. Preacher KJ, Hayes AF. SPSS. and SAS procedures for estimating indirect effects in simple mediation models. Behav Res Methods Instrum Comput. 2004; 36: 717–731 [DOI] [PubMed] [Google Scholar]
- 12. Fleiszig SM, Evans DJ. The pathogenesis of bacterial keratitis: studies with Pseudomonas aeruginosa. Clin Exp Optom. 2002; 85: 271–278 [DOI] [PubMed] [Google Scholar]
- 13. Rex JH, Pfaller MA. Has antifungal susceptibility testing come of age? Clin Infect Dis. 2002; 35: 982–989 [DOI] [PubMed] [Google Scholar]
- 14. Shah A, Sachdev A, Coggon D, Hossain P. Geographic variations in microbial keratitis: an analysis of the peer-reviewed literature. Br J Ophthalmol. 2011; 95: 762–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lichtinger A, Yeung SN, Kim P, et al. Shifting trends in bacterial keratitis in Toronto: an 11-year review. Ophthalmology. 2012; 119: 1785–1790 [DOI] [PubMed] [Google Scholar]
- 16. Bharathi MJ, Ramakrishnan R, Vasu S, Meenakshi R, Palaniappan R. Aetiological diagnosis of microbial keratitis in South India - A study of 1618 cases. Indian J Med Microbiol. 2002; 20: 19–24 [PubMed] [Google Scholar]
- 17. Bharathi MJ, Ramakrishnan R, Meenakshi R, Padmavathy S, Shivakumar C, Srinivasan M. Microbial keratitis in South India: influence of risk factors, climate, and geographical variation. Ophthalmic Epidemiol. 2007; 14: 61–69 [DOI] [PubMed] [Google Scholar]
- 18. Leck AK, Thomas PA, Hagan M, et al. Aetiology of suppurative corneal ulcers in Ghana and south India, and epidemiology of fungal keratitis. Br J Ophthalmol. 2002; 86: 1211–1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dhakwa K, Sharma MK, Bajimaya S, Dwivedi AK, Rai SK. Causative organisms in microbial keratitis, their sensitivity pattern and treatment outcome in western Nepal. Nep J Oph. 2012; 4: 119–127 [DOI] [PubMed] [Google Scholar]
- 20. de Rojas V, Llovet F, Martínez M, et al. Infectious keratitis in 18, 651 laser surface ablation procedures. J Cataract Refract Surg. 2011; 37: 1822–1831 [DOI] [PubMed] [Google Scholar]
- 21. Darugar A, Gaujoux T, Goldschmidt P. Clinical, microbiological, and therapeutic features of severe bacterial keratitis. J Fr Ophthalmol. 2011; 34: 362–368 [DOI] [PubMed] [Google Scholar]
- 22. Prokosch V, Gatzioufas Z, Thanos S, Stupp T. Microbiological findings and predisposing risk factors in corneal ulcers. Graefes Arch Clin Exp Ophthalmol. 2011; 250: 369–374 [DOI] [PubMed] [Google Scholar]
- 23. Srinivasan M, Mascarenhas J, Rajaraman R, et al. Corticosteroids for bacterial keratitis: the Steroids for Corneal Ulcers Trial (SCUT). Arch Ophthalmol. 2012; 130: 143–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hsu HY, Nacke R, Song JC, Yoo SH. Community opinions in the management of corneal ulcers and ophthalmic antibiotics: a survey of 4 states. Eye Contact Lens. 2010; 36: 195–200 [DOI] [PubMed] [Google Scholar]