Abstract
Degeneration alters the biochemical composition of the disc, affecting the mechanical integrity leading to spinal instability. Quantitative T2* MRI probes water mobility within the macromolecular network, a potentially more sensitive assessment of disc health. We determined the relationship between T2* relaxation time and proteoglycan content, collagen content, and compressive mechanics throughout the degenerative spectrum. Eighteen human cadaveric lumbar (L4–L5) discs were imaged using T2* MRI. The T2* relaxation time at five locations (nucleous pulposus or NP, anterior annulus fibrosis or AF, posterior AF, inner AF, and outer AF) was correlated with sulfated-glycosaminoglycan (s-GAG) content, hydroxyproline content, and residual stress and strain at each location. T2* relaxation times were significantly correlated with s-GAG contents in all test locations and were particularly strong in the NP (r = 0.944; p < 0.001) and inner AF (r = 0.782; p < 0.001). T2* relaxation times were also significantly correlated with both residual stresses and excised strains in the NP (r = 0.857; p < 0.001: r = 0.816; p < 0.001), inner AF (r = 0.535; p = 0.022: r = 0.516; p = 0.028), and outer AF (r = 0.668; p = 0.002: r = 0.458; p = 0.041). These strong correlations highlight T2* MRI’s ability to predict the biochemical and mechanical health of the disc. T2* MRI assessment of disc health is a clinically viable tool showing promise as a biomarker for distinguishing degenerative changes.
Keywords: disc degeneration, quantitative magnetic resonance imaging, T2* (T2 star), proteoglycan, biomechanics
The intervertebral disc (IVD) affords the spine its extensive multidirectional motion due to the complex interaction between two morphologically, biomechanically, and biochemically distinct regions: the annulus fibrosus (AF) and the nucleus pulposus (NP). The AF consists of highly organized, concentric rings of fibro-cartilaginous material surrounding the NP, which is composed of a hydrated, disorganized matrix of collagen and proteoglycans.1 When a load is applied to the spine, the hydrated NP acts like a pressure vessel, placing the AF in tension.2 This unique system allows the disc to absorb and dissipate the large compressive loads applied to the spine.
Degeneration of the IVD is a progressive phenomenon that occurs in response to age, injury, pathology, or more likely a combination of these factors.2,3 Increased or abnormal mechanical loading leads to alterations in biochemical composition.2 These changes lead to diminished mechanical competency resulting in the disc’s inability to maintain its structure and function, potentially causing discogenic pain or pain due to cord occlusion, neural compressive lesions, or nerve root pinching, from altered spinal stability.4 Detecting these changes early in the degeneration process would allow timely use new therapeutics including biologics and other treatments.
Direct measurement of disc biochemical content is impossible in vivo; therefore, magnetic resonance imaging (MRI) is used to evaluate disc health. Unfortunately, current clinical imaging techniques do not adequately assess degeneration, especially in the early stages.5 New quantitative MRI techniques are being developed to overcome the subjectivity in interpreting imaging findings. These techniques include T1ρ, magnetization transfer, T2 mapping, and T2* (T2 star) mapping. Although some of these are promising, quantitative T2* is a multi-echo gradient-echo technique, rather than the multi-echo spin-echo technique of traditional T2 mapping. The relaxation time is a combination of the inherent “true” T2 relaxation and additional relaxation due to magnetic inhomogeneities (1/T2* = 1/T2 + 1/T2′, where T2′ is the relaxation due to magnetic field inhomogeneities).6 Therefore, T2* can measure ultrashort relaxation times, providing the added benefit of a short acquisition time, high signal-to-noise ratio, along with 3D capabilities.7–9 T2* is a clinically available scan approved by the FDA. Traditional T2 mapping correlates with hydration, but to a lesser degree with proteoglycan and collagen concentration and organization.10–12 T2* relaxation times provide information regarding the biochemical properties of the tissue, specifically interrogating water mobility within the macromolecular network, and is beneficial in cartilage imaging.7–9,13–19 Recent studies expanded T2* imaging to the intervertebral disc.7,20 Welsch et al.7 reported a significant correlation between T2* relaxation time and the degree of degeneration based on Pfirrmann grades in subjects with low back pain. Hoppe et al.20 utilized axial T2* maps and also found a significant correlation between T2* relaxation time and Pfirrmann grade.
IVD degeneration typically begins in the NP with the breakdown of hydrophilic proteoglycans.1,21 This leads to a loss of hydration resulting in a decrease in the hydrostatic pressure and compressive residual stress and strain of the disc.22–25 The loss of hydration affects the interactions between the NP and AF, resulting in a diminished ability to absorb loads. Later stages of degeneration include modification in collagen synthesis and degradation and eventually disc collapse. Altogether the degradation in biochemical content affects the mechanical response and structural integrity of the disc. Noninvasive evaluation of the biochemical composition of the IVD could profoundly impact assessment of degeneration.
Although T2* is correlated with disc degeneration, the relationship between T2* relaxation time and IVD constituents has yet to be established. We determined the relationship between regional quantitative T2* measures and corresponding sulfated-glycosaminoglycan, total collagen content, and residual mechanical properties. We hypothesized that T2* relaxation time can predict site-specific parameters: (i) sulfated-glycosaminoglycan content, (ii) hydroxyproline content, (iii) water content, (iv) residual stress, and (v) and excised strain.
METHODS
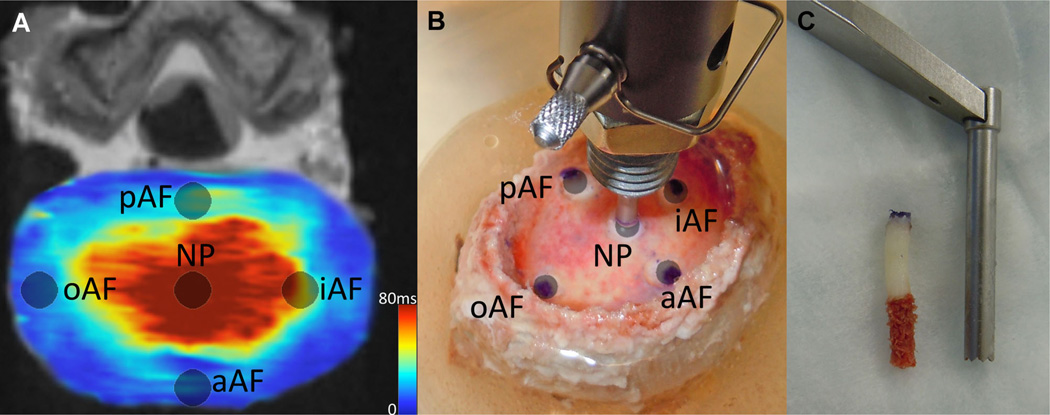
Eighteen human cadaveric lumbar (L4–L5) IVDs (53.2 ± 15.5 years; range: 21–71 years) were imaged using a Siemens 3T MRI scanner (Magnetom Trio; Siemens Healthcare, Erlangen, Germany, Fig. 1). Each specimen was placed in the prone position inside a brain coil where traditional T2 weighted and quantitative T2* MR images were acquired in the sagittal plane. The scans were performed consecutively. To provide context for relative disc health, Pfirrmann grade was assessed independently by seven neurosurgical/orthopedics spine surgeons and 3 Ph.D. level experienced spine researchers based on the T2 weighted MRI.26 These scores were averaged and rounded to the nearest integer. Quantitative T2* relaxation maps (MapIt, Siemens Healthcare, Erlangen, Germany) were obtained using the following imaging parameters [TR(ms): 500; TE(ms): 4.18, 11.32, 18.46, 25.60, 32.74, 39.88; voxel size (mm): 0.5 × 0.5 × 3.0, slices: 33]. Five test sites were isolated across the transverse plane of the IVD, and mean T2* relaxation time was recorded using Osirix Imaging Software at each region of interest (ROI; Fig. 2A). The sites included one in the center NP and four in the AF, including three outer AF areas: anterior (aAF), posterior (pAF), and lateral (oAF), and one inner lateral region (iAF). The lateral test sites were randomized left or right based on a block design based on Pfirrmann grade. A correlational study design was used to examine the relationship between quantitative T2* relaxation time and IVD constituents and residual mechanics properties.
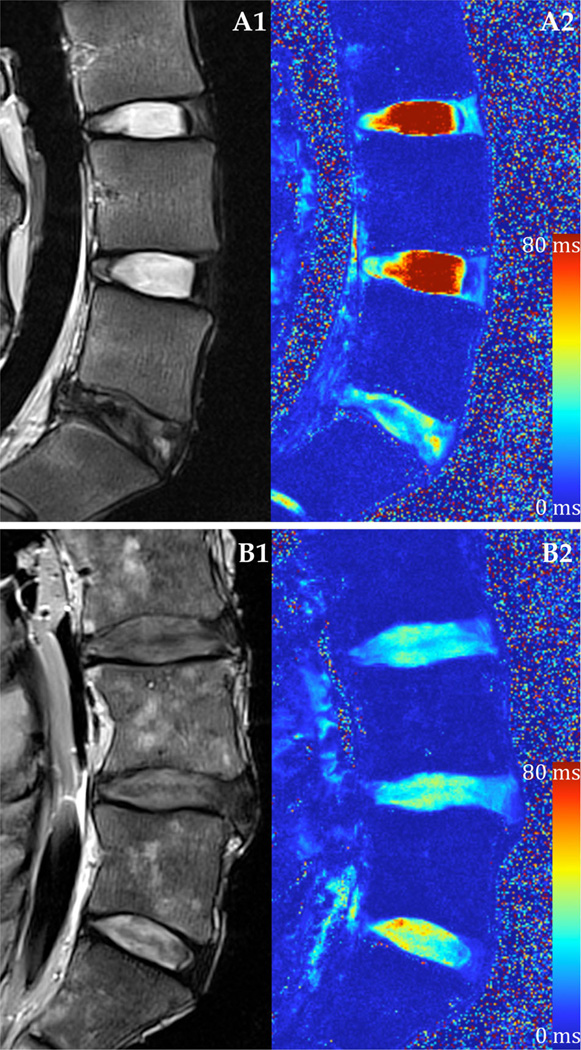
Figure 1.
Representative T2 weighted MRI and T2* relaxation time mapping. (A) Healthy lumbar IVDs and (B) degenerated IVDs using a (1) conventional T2 weighted imaging sequence and (2) quantitative T2* mapping techniques.
Figure 2.
IVD test sites. (A) Site locations on a transverse slice of a quantitative T2* map. The average T2* relaxation time was measured at each site. (B) Same test locations on a specimen undergoing stress relaxation tests using hybrid confined/in situ indentation methodology to compute the residual stress and excised strain of the tissue. (C) A 3 mm punch was used to remove a plug of tissue at the same locations where indentation tests were performed and where MRI relaxation times were recorded. The endplate and inferior trabecular bone were removed prior to biochemical quantification. In a randomized fashion, the superior or inferior section was used to quantify either s-GAG and water content or hydroxyproline content.
Following imaging, the residual stress and strain was detremined at each location using a hybrid confined/in situ indentation methodology (Fig. 2B).24 This technique utilizes the cartilaginous endplate as a porous indenter by releasing a 3mm diameter portion of the endplate, while leaving the superior endplate contiguous with the IVD inferiorly. Following excision of the endplate, the released section increased in height, indicating the presence of an internal residual stress. The height increase was used to calculate the excised strain (εR) by dividing the change in height increase by the original site-specific disc height, calculated from the T2 weighted MR images using the linear measurement tool in Imaging Software (Osirix Imaging Software, Pixmeo, Geneva, Switzerland) to measure the distance between endplates at each ROI.
Stress relaxation tests were performed using an Instron microtester (5548 Instron, Norwood, MA) equipped with a 3mm diameter indenter tip. The endplate was re-positioned back to its original height with the indenter and allowed to equilibrate for 900 s; the equilibrium residual stress was determined by dividing the applied equilibrium force over the 3 mm indenter tip area.
Subsequent biochemical analysis was performed at each test site investigating sulfated-glycosaminoglycan (s-GAG) and hydroxyproline content. A sharp 3 mm punch was used to remove the endplate-disc-endplate construct at each test site (Fig. 2C). Both endplates were then removed, and the cylindrical disc sample was divided in half along the transverse axis. In a randomized fashion, the superior or inferior section was used to quantify either s-GAG or hydroxyproline content. To determine s-GAG content, disc samples were dried at 60°C to constant dry weight, and a commercially available 1,9 dimethylmethylene blue assay was used (Astart Biologics Rheumera, Proteoglycan Detection Kit, Redmond, WA); s-GAG content was normalized by dry weight (µg/µg).27 Total protein content was determined as described previously.28 Hydroxyproline content was determined as described previously, omitting lyophilization.29 Hydroxyproline content was normalized by total protein content (µg/µg). Dry weights were unavailable for the hydroxyproline sections. Water content (%H2O) was also calculated as (wet weight − dry weight)/wet weight at each location.
While Pfirrmann grading provided a categorical value for each sample’s independent variable, it was used to contextualize the findings, and all results were assessed as continuous data without grouping. Pearson’s correlation tests were performed between regional T2* relaxation time (ms) and corresponding biochemical content and residual stress and strain to define the relationship between imaging parameters and the disc’s constituents. Each hypothesis was tested; acceptance was established based on an alpha of 0.05.
RESULTS
Pfirrmann grade was significantly correlated with average T2* relaxation time (ranged from 9.1 to 91.6 ms) in the NP (r = −0.891; p < 0.001) and iAF (r = −0.749; p < 0.001), but not pAF (r = −0.460; p = 0.055), oAF (r = −0.387; p = 0.113), or aAF (r = −0.297; p = 0.232). Correlations were found between the imaging parameters and the discs’ constituents. The mean T2* relaxation time was positively correlated with s-GAG normalized by dry weight in all five regions of interest (Table 1). This relationship was particularly strong in the NP (r = 0.944; p < 0.001) and inner AF (r = 0.782; p < 0.001). Figure 3 displays the correlations between T2* relaxation time and s-GAG (3A), excised strain (3B), and residual stress (3C) for NP (black) and inner AF (gray). T2* relaxation times of the NP, inner AF, and outer AF were significantly correlated with both excised strain and residual stress. The posterior AF T2* time was also correlated with residual stress. Hydroxyproline normalized to total protein was only significantly correlated with the outer AF (r = −0.568; p = 0.014). Water content (%H2O) was significantly correlated with T2* value in the posterior AF region only. All metrics were significantly correlated with the T2* relaxation time when all locations were grouped.
Table 1.
Correlations Between T2* Relaxation Time (ms) and s-GAG, Hydroxyproline, Water Content, and Residual Mechanics
| T2* Relaxation Time (ms) | ||||||
|---|---|---|---|---|---|---|
| NP | iAF | oAF | aAF | pAF | All | |
| s-GAG/dry weight | ||||||
| r | 0.944 | 0.782 | 0.647 | 0.569 | 0.474 | 0.586 |
| p | <0.001* | <0.001* | 0.004* | 0.014* | 0.047* | <0.001* |
| Hydroxyproline/total protein | ||||||
| r | 0.071 | 0.322 | −0.568 | −0.153 | 0.226 | −0.217 |
| p | 0.778 | 0.193 | 0.014* | 0.543 | 0.368 | 0.040* |
| Excised strain | ||||||
| r | 0.816 | 0.516 | 0.485 | −0.040 | 0.405 | 0.412 |
| p | <0.001* | 0.028* | .041* | 0.874 | 0.095 | <0.001* |
| Residual stress | ||||||
| r | 0.857 | 0.535 | 0.668 | 0.242 | 0.527 | 0.347 |
| p | <0.001* | 0.022* | 0.002* | 0.332 | 0.025* | 0.001* |
| Water content | ||||||
| r | 0.465 | 0.330 | 0.341 | 0.032 | 0.572 | 0.427 |
| p | 0.052 | 0.181 | 0.166 | 0.900 | 0.013* | <0.001* |
r, Pearson’s correlation coefficient; p-value
represents significance at p = 0.05.
Figure 3.
T2* Relaxation time correlational plots of the nucleus pulposus and inner annulus fibrosus regions. NP data are shown in black and inner AF data in gray. Pearson’s correlational coefficient, r, and p-value are displayed on corresponding plots along with the linear regression equation. (A) T2* versus s-GAG content (%dry weight). (B) T2* versus excised strain (%strain). (C) T2* versus residual stress (MPa).
DISCUSSION
Quantitative T2* MRI is a multi-echo gradient-echo technique where the relaxation time is a combination of the inherent “true” T2 relaxation and additional relaxation due to magnetic inhomogeneities. T2* mapping is beneficial in cartilage imaging, providing information regarding the spatial macromolecular architecture and interaction with water mobility.8,9 T2* relaxation times are related to histological grades of degeneration in the hip joint, where a decrease in T2* relaxation time was significantly correlated with a higher degree of cartilage degeneration. Also, a significant correlation was determined between T2* relaxation time and disc health assessed via Pfirrmann grade.7,20 Our study expands upon these principals and utilizes the T2* technique to investigate the relationship between T2* relaxation times and the corresponding biochemical composition and mechanical competency of the IVD.
The loss of proteoglycans is a marker of the early stage of disc degeneration; therefore, it is of critical interest to estimate the proteoglycan content of the IVD noninvasively. As hypothesized, we found T2* relaxation time at all 5 locations (NP, iAF, oAF, aAF, pAF) were significantly and positively correlated with the s-GAG content. An especially strong linear relationship was discovered in the NP (r = 0.944; p < 0.001) and inner AF (r = 0.782; p < 0.001). Interestingly, there was not a strong correlation between T2* relaxation time and the water content, as hypothesized at individual test sites, other than the oAF, though there was weak correlation observed on the whole disc and a trend in the NP that approached significance. This suggests T2* may be able to detect the proteoglycans of the disc based on their interactions with water, not just the hydration level alone.
Our results are contrary to previous work investigating the relationship between proteoglycan content of the disc and imaging parameters from various MRI techniques, such as T2 mapping, T1, and T1ρ. Weidenbaum et al.10 reported a lack of significant correlation between 1/T2 and proteoglycan content; however, the scans were performed in a low strength (0.5 tesla) magnet. Marinelli et al.12 reported a moderate correlation between T2 relaxation time and proteoglycan content in the NP (r2 = 0.73; p = 0.06), but not in the AF (r2 = 0.45; p = 0.21). Each of these studies reported a significant correlation between T2 and water content.10,12 Marinelli et al. were limited to a low sample size of human disc material (n = 5). Benneker et al.30 conducted a similar study with a larger sample from the entire degenerative spectrum and found a significant relationship between T2 values and both water and proteoglycan content. The inconsistent data surrounding T2 weighted MRI led to the development of other imaging protocols, such as T1ρ.
Johannessen et al.31 reported a correlation coefficient of r = 0.67 (p < 0.01) between T1ρ value and s-GAG per dry weight in non-degenerative discs with a Pfirrmann grade of ≤3.5. They also found a significant correlation between T1ρ and water content. Other studies also established the relationship between T1ρ and proteoglycans in articular cartilage and disc.32–34 Nguyen et al.35 aimed to evaluate the functionality of T1ρ in more degenerative discs and determine the relationship between T1ρ values and the mechanics of the NP. The previous relationships with proteoglycans (r = 0.69; p < 0.05) and water content (r = 0.53; p < 0.05) were confirmed, but were not significant when corrected for the intercorrelations within the same spine. They did find a significant correlation with the swelling pressure (r = 0.59; p < 0.05) of the NP.
As postulated, we found T2* values to be significantly correlated with the residual stress in all test locations except the anterior AF. The excised strain was also significantly correlated with T2* relaxation time in the NP, inner AF, and outer AF. In particular, the NP displayed a strong correlation with residual stress (r = 0.857; p < 0.001) and excised strain (r = 0.816; p < 0.001), indicating T2* mapping’s ability to detect changes in the mechanical behavior of the disc. The disc’s excised strain ranged from a 30% increase in height following release of the endplate to no change, while the residual stress ranged from 0.20 to 0 MPa as discs progressed from healthy to severely degenerated. These values are similar to previously published work using this technique and those reported for pressures in vitro and in vivo.24,35–37 Representative T2* MR images of a healthy, moderately degenerated, and severely degenerated disc are displayed in Figure 4 with their respective T2* relaxation time, s-GAG content, and residual stress overlaid at each test location. These plots provide a glimpse into the progression of disc degeneration as it affects the biochemistry and biomechanics in a site-specific fashion.
Figure 4.
Representative transverse T2* MR images of a healthy, moderate, and severely degenerated IVD with corresponding T2* relaxation times, s-GAG content, and residual stress. Bars displayed atop each MR image denote T2* relaxation time, s-GAG content, and residual stress at representative test locations: NP, anterior AF, posterior AF, lateral outer AF, and lateral inner AF. The color of the bar is dictated by the T2* relaxation time according to the scale. Healthy: Pfirrmann Grade 1. Moderate: Pfirrmann Grade 3. Severe: Pfirrmann Grade 5.
The limitations of our study include the use of cadaveric tissue, where imaging was conducted without any external soft tissue on a motionless specimen at room temperature. Although care was taken to minimize the effect from the adjacent test sites on the current test site during the indentation protocol, this interaction was not characterized. Dry weights were unavailable for the samples used to quantify hydroxyproline content, therefore they were normalized by total protein, which is less reported and more difficult to contextualize and compare against other studies. Multiple freeze-thaw processes necessary for other aspects of the entire study could have affected the hydration levels of the disc, although attempts were made to standardize hydration protocols.
The inclusion of discs from the entire degenerative spectrum allows for the evaluation of the T2* technique. This sequence seems to be sensitive in detecting the early changes in the NP, which is reflected by the large differences in relaxation times at the “healthier” end of the spectrum. The absolute changes become less for the more degenerative tissues, while still maintaining the observed linear relationship. In other words, the T2* relaxation time drastically decreases early in the degeneration process, similar to the proteoglycan content, then slows down during later stages. The majority of the discs included in our study were healthy to moderately degenerated (Pfirrmann Grades 1–III), and this technique was able to stratify these discs. Since there was no a priori knowledge of these relationships, a linear fit was attempted. Future studies should explore the possibility of more complex interactions. Furthermore, since these correlations do not reveal causality, future research should examine the molecular mechanisms whereby T2* relaxation is affected by proteoglycan content. However, a potential explanation of this relationship is the T2* signal is not only influenced by the true T2 relaxation, but also the inhomogeneities of the local magnetic field. The disorganized matrix of the hydrophilic proteoglycans in the disc cause disturbances in the local magnetic field and thus impacts the T2* signal.
The strong correlations between T2* relaxation time and s-GAG, residual stress, and excised strain highlight this technique’s ability to predict the health of the disc on a biochemical and mechanical level. T2* relaxation time may serve as a biomarker of proteoglycan content and mechanical function in the detection of disc degeneration using a currently available technology. T2* mapping is fast, reliable, and available on all clinical 3T MRI systems and may serve as a biomarker for disc health, thus improving patient stratification allowing for more specifically targeted strategies with the development of new therapeutics.
ACKNOWLEDGMENTS
Funding was provided through NIH/NIAMS grants T32 AR050938 and T32 AR056950. The authors thank Peter Kollasch from the Center of Magnetic Resonance Research at the University of Minnesota for help acquiring MR images. The authors acknowledge Jonathan Sembrano M.D., Edward Santos M.D., Charles Ledonio M.D., Matthew Hunt M.D., Ann Parr M.D., Ph.D., Robert Morgan M.D., and Hitesh Mehta M.D., Ph.D. Also, thanks to Sandy Johnson for her aid with the biochemical analysis.
Footnotes
Conflict of interest: None.
REFERENCES
- 1.Roughley PJ. Biology of intervertebral disc aging and degeneration: involvement of the extracellular matrix. Spine (Phila Pa 1976) 2004;29:2691–2699. doi: 10.1097/01.brs.0000146101.53784.b1. [DOI] [PubMed] [Google Scholar]
- 2.Adams MA, Roughley PJ. What is intervertebral disc degeneration, and what causes it? Spine (Phila Pa 1976) 2006;31:2151–2161. doi: 10.1097/01.brs.0000231761.73859.2c. [DOI] [PubMed] [Google Scholar]
- 3.Urban JP, Roberts S. Degeneration of the intervertebral disc. Arthritis Res Ther. 2003;5:120–130. doi: 10.1186/ar629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adams MA. Biomechanics of back pain. Acupunct Med. 2004;22:178–188. doi: 10.1136/aim.22.4.178. [DOI] [PubMed] [Google Scholar]
- 5.Luoma K, Vehmas T, Riihimaki H, et al. Disc height and signal intensity of the nucleus pulposus on magnetic resonance imaging as indicators of lumbar disc degeneration. Spine (Phila Pa 1976) 2001;26:680–686. doi: 10.1097/00007632-200103150-00026. [DOI] [PubMed] [Google Scholar]
- 6.Chavhan GB, Babyn PS, Thomas B, et al. Principles, techniques, and applications of T2*-based MR imaging and its special applications. Radiographics. 2009;29:1433–1449. doi: 10.1148/rg.295095034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Welsch GH, Trattnig S, Paternostro-Sluga T, et al. Parametric T2 and T2* mapping techniques to visualize intervertebral disc degeneration in patients with low back pain: initial results on the clinical use of 3.0 Tesla MRI. Skeletal Radiol. 2011;40:543–551. doi: 10.1007/s00256-010-1036-8. [DOI] [PubMed] [Google Scholar]
- 8.Krause FG, Klammer G, Benneker LM, et al. Biochemical T2* MR quantification of ankle arthrosis in pes cavovarus. J Orthop Res. 2010;28:1562–1568. doi: 10.1002/jor.21192. [DOI] [PubMed] [Google Scholar]
- 9.Mamisch TC, Hughes T, Mosher TJ, et al. T2 star relaxation times for assessment of articular cartilage at 3 T: a feasibility study. Skeletal Radiol. 2012;41:287–292. doi: 10.1007/s00256-011-1171-x. [DOI] [PubMed] [Google Scholar]
- 10.Weidenbaum M, Foster RJ, Best BA, et al. Correlating magnetic resonance imaging with the biochemical content of the normal human intervertebral disc. J Orthop Res. 1992;10:552–561. doi: 10.1002/jor.1100100410. [DOI] [PubMed] [Google Scholar]
- 11.Tertti M, Paajanen H, Laato M, et al. Disc degeneration in magnetic resonance imaging. A comparative biochemical, histologic, and radiologic study in cadaver spines. Spine (Phila Pa 1976) 1991;16:629–634. doi: 10.1097/00007632-199106000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Marinelli NL, Haughton VM, Munoz A, et al. T2 relaxation times of intervertebral disc tissue correlated with water content and proteoglycan content. Spine (Phila Pa 1976) 2009;34:520–524. doi: 10.1097/BRS.0b013e318195dd44. [DOI] [PubMed] [Google Scholar]
- 13.Welsch GH, Trattnig S, Hughes T, et al. T2 and T2* mapping in patients after matrix-associated autologous chondrocyte transplantation: initial results on clinical use with 3.0-Tesla MRI. Eur Radiol. 2010;20:1515–1523. doi: 10.1007/s00330-009-1669-y. [DOI] [PubMed] [Google Scholar]
- 14.Williams A, Qian Y, Bear D, et al. Assessing degeneration of human articular cartilage with ultra-short echo time (UTE) T2* mapping. Osteoarthr Cartilage. 2010;18:539–546. doi: 10.1016/j.joca.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bittersohl B, Miese FR, Hosalkar HS, et al. T2* mapping of hip joint cartilage in various histological grades of degeneration. Osteoarthritis Cartilage. 2012;20:653–660. doi: 10.1016/j.joca.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 16.Qian Y, Williams AA, Chu CR, et al. Multicomponent T2* mapping of knee cartilage: technical feasibility ex vivo. Magn Reson Med. 2010;64:1426–1431. doi: 10.1002/mrm.22450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williams A, Qian Y, Chu CR. UTE-T2 * mapping of human articular cartilage in vivo: a repeatability assessment. Osteoarthr Cartilage. 2011;19:84–88. doi: 10.1016/j.joca.2010.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marik W, Apprich S, Welsch GH, et al. Biochemical evaluation of articular cartilage in patients with osteochondrosis dissecans by means of quantitative T2- and T2-mapping at 3T MRI: a feasibility study. Eur J Radiol. 2012;81:923–927. doi: 10.1016/j.ejrad.2011.01.124. [DOI] [PubMed] [Google Scholar]
- 19.Miese FR, Zilkens C, Holstein A, et al. Assessment of early cartilage degeneration after slipped capital femoral epiphysis using T2 and T2* mapping. Acta Radiol. 2011;52:106–110. doi: 10.3109/02841851.2010.516015. [DOI] [PubMed] [Google Scholar]
- 20.Hoppe S, Quirbach S, Mamisch TC, et al. Axial T2 mapping in intervertebral discs: a new technique for assessment of intervertebral disc degeneration. Eur Radiol. 2012;22:2013–2019. doi: 10.1007/s00330-012-2448-8. [DOI] [PubMed] [Google Scholar]
- 21.Antoniou J, Steffen T, Nelson F, et al. The human lumbar intervertebral disc: evidence for changes in the biosynthesis and denaturation of the extracellular matrix with growth, maturation, ageing, and degeneration. J Clin Invest. 1996;98:996–1003. doi: 10.1172/JCI118884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pearce RH, Grimmer BJ, Adams ME. Degeneration and the chemical composition of the human lumbar intervertebral disc. J Orthop Res. 1987;5:198–205. doi: 10.1002/jor.1100050206. [DOI] [PubMed] [Google Scholar]
- 23.Buckwalter JA. Aging and degeneration of the human intervertebral disc. Spine (Phila Pa 1976) 1995;20:1307–1314. doi: 10.1097/00007632-199506000-00022. [DOI] [PubMed] [Google Scholar]
- 24.Ellingson AM, Nuckley DJ. Intervertebral disc viscoelastic parameters and residual mechanics spatially quantified using a hybrid confined/in situ indentation method. J Biomech. 2012;45:491–496. doi: 10.1016/j.jbiomech.2011.11.050. [DOI] [PubMed] [Google Scholar]
- 25.Johannessen W, Elliott DM. Effects of degeneration on the biphasic material properties of human nucleus pulposus in confined compression. Spine. 2005;30:E724–E729. doi: 10.1097/01.brs.0000192236.92867.15. [DOI] [PubMed] [Google Scholar]
- 26.Pfirrmann CW, Metzdorf A, Zanetti M, et al. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine (Phila Pa 1976) 2001;26:1873–1878. doi: 10.1097/00007632-200109010-00011. [DOI] [PubMed] [Google Scholar]
- 27.Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986;883:173–177. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- 28.Starcher B. A ninhydrin-based assay to quantitate the total protein content of tissue samples. Anal Biochem. 2001;292:125–129. doi: 10.1006/abio.2001.5050. [DOI] [PubMed] [Google Scholar]
- 29.Stegemann H, Stalder K. Determination of hydroxyproline. Clin Chim Acta. 1967;18:267–273. doi: 10.1016/0009-8981(67)90167-2. [DOI] [PubMed] [Google Scholar]
- 30.Benneker LM, Heini PF, Anderson SE, et al. Correlation of radiographic and MRI parameters to morphological and biochemical assessment of intervertebral disc degeneration. Eur Spine J. 2005;14:27–35. doi: 10.1007/s00586-004-0759-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johannessen W, Auerbach JD, Wheaton AJ, et al. Assessment of human disc degeneration and proteoglycan content using T1rho-weighted magnetic resonance imaging. Spine (Phila Pa 1976) 2006;31:1253–1257. doi: 10.1097/01.brs.0000217708.54880.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Auerbach JD, Johannessen W, Borthakur A, et al. In vivo quantification of human lumbar disc degeneration using T(1rho)-weighted magnetic resonance imaging. Eur Spine J. 2006;15:S338–S344. doi: 10.1007/s00586-006-0083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blumenkrantz G, Majumdar S. Quantitative magnetic resonance imaging of articular cartilage in osteoarthritis. Eur Cell Mater. 2007;13:76–86. doi: 10.22203/ecm.v013a08. [DOI] [PubMed] [Google Scholar]
- 34.Wheaton AJ, Dodge GR, Borthakur A, et al. Detection of changes in articular cartilage proteoglycan by T(1rho) magnetic resonance imaging. J Orthop Res. 2005;23:102–108. doi: 10.1016/j.orthres.2004.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nguyen AM, Johannessen W, Yoder JH, et al. Noninvasive quantification of human nucleus pulposus pressure with use of T1-weighted magnetic resonance imaging. J Bone Joint Surg. 2008;90:796–802. doi: 10.2106/JBJS.G.00667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Andersson GB, Ortengren R, Nachemson A. Intradiskal pressure, intra-abdominal pressure and myoelectric back muscle activity related to posture and loading. Clin Orthop Relat Res. 1977:156–164. doi: 10.1097/00003086-197711000-00018. [DOI] [PubMed] [Google Scholar]
- 37.Wilke H, Neef P, Hinz B, et al. Intradiscal pressure together with anthropometric data–a data set for the validation of models. Clin Biomech (Bristol, Avon) 2001;16:S111–S126. doi: 10.1016/s0268-0033(00)00103-0. [DOI] [PubMed] [Google Scholar]