Abstract
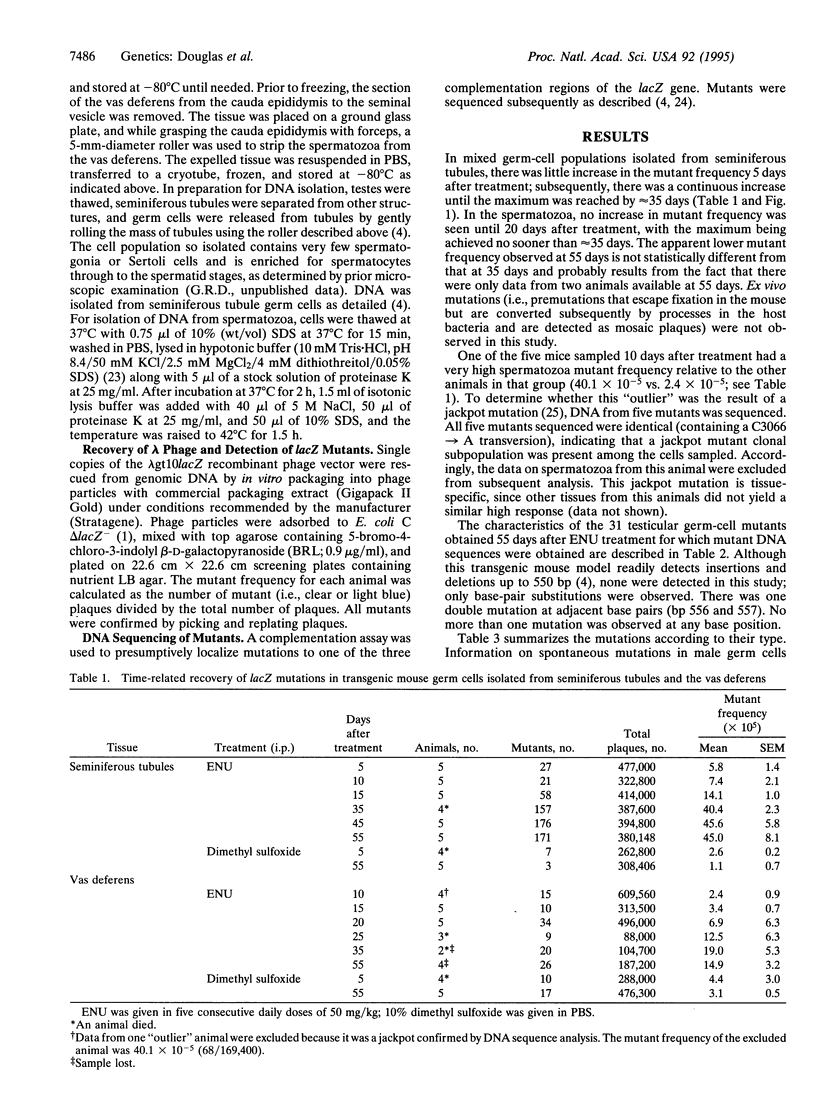
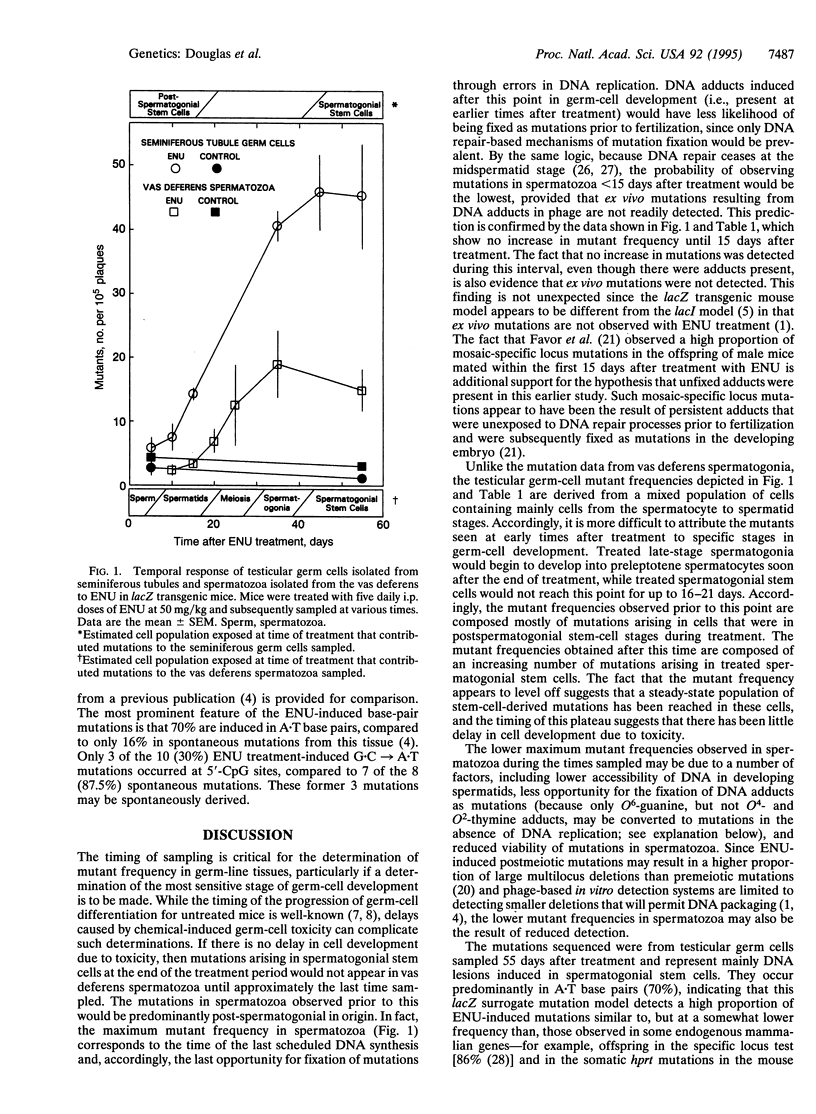
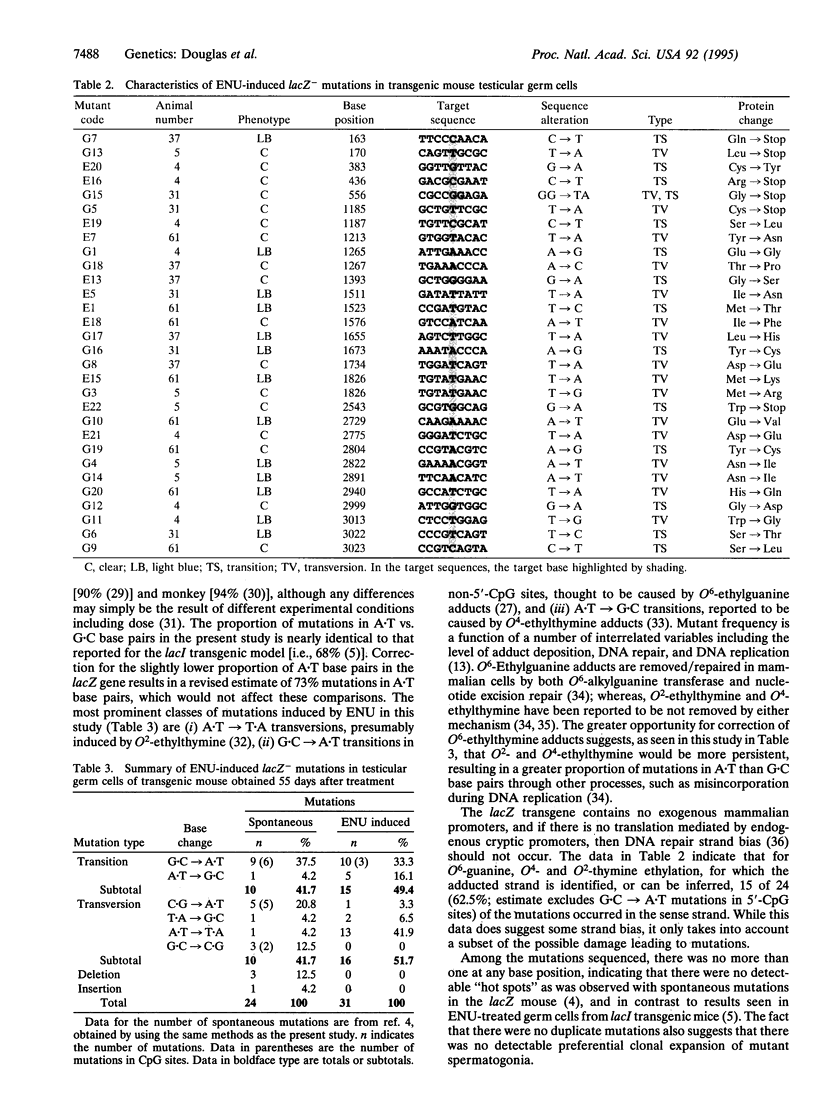
The lacZ transgenic mouse (Muta mouse) model was used to examine the timing of ethylnitrosourea (ENU)-induced mutations in germ cells. The spectrum of mutations was also determined. Animals received five daily treatments with ENU at 50 mg/kg and were sampled at times up to 55 days after treatment. In mixed germ-cell populations isolated from seminiferous tubules, there was little increase in the mutant frequency 5 days after treatment; subsequently, there was a continuous increase until the maximum (17.5-fold above background) was reached by approximately 35 days. In the spermatozoa, an increase in mutant frequency was not seen until 20 days after treatment, with the maximum (4.3-fold above background) being achieved no sooner than approximately 35 days. Based on the timing of sampling, these data demonstrate the detection of both spermatogonial and postspermatogonial, mutations. The most prominent feature of the ENU-induced base-pair mutations in testicular germ cells sampled 55 days after treatment is that 70% are induced in A.T base pairs, compared to only 16% in spontaneous mutations. These findings are consistent with comparable data from ENU studies using assays for inherited germ-cell mutations in mice. This study has demonstrated the utility and potential of the transgenic mouse lacZ model (Muta mouse) for the detection and study of germ-cell mutations and provides guidance in the selection of simplified treatment and sampling protocols.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhanot O. S., Grevatt P. C., Donahue J. M., Gabrielides C. N., Solomon J. J. In vitro DNA replication implicates O2-ethyldeoxythymidine in transversion mutagenesis by ethylating agents. Nucleic Acids Res. 1992 Feb 11;20(3):587–594. doi: 10.1093/nar/20.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakey D. H., Douglas G. R., Huang K. C., Winter H. J. Cytogenetic mapping of lambda gt10 lacZ sequences in the transgenic mouse strain 40.6 (Muta Mouse). Mutagenesis. 1995 Mar;10(2):145–148. doi: 10.1093/mutage/10.2.145. [DOI] [PubMed] [Google Scholar]
- Bronstein S. M., Cochrane J. E., Craft T. R., Swenberg J. A., Skopek T. R. Toxicity, mutagenicity, and mutational spectra of N-ethyl-N-nitrosourea in human cell lines with different DNA repair phenotypes. Cancer Res. 1991 Oct 1;51(19):5188–5197. [PubMed] [Google Scholar]
- Bronstein S. M., Skopek T. R., Swenberg J. A. Efficient repair of O6-ethylguanine, but not O4-ethylthymine or O2-ethylthymine, is dependent upon O6-alkylguanine-DNA alkyltransferase and nucleotide excision repair activities in human cells. Cancer Res. 1992 Apr 1;52(7):2008–2011. [PubMed] [Google Scholar]
- Douglas G. R., Gingerich J. D., Gossen J. A., Bartlett S. A. Sequence spectra of spontaneous lacZ gene mutations in transgenic mouse somatic and germline tissues. Mutagenesis. 1994 Sep;9(5):451–458. doi: 10.1093/mutage/9.5.451. [DOI] [PubMed] [Google Scholar]
- Douglas G. R., Gingerich J. D., Soper L. M. Evidence for in vivo non-mutagenicity of the carcinogen hydrazine sulfate in target tissues of lacZ transgenic mice. Carcinogenesis. 1995 Apr;16(4):801–804. doi: 10.1093/carcin/16.4.801. [DOI] [PubMed] [Google Scholar]
- Favor J. A comparison of the dominant cataract and recessive specific-locus mutation rates induced by treatment of male mice with ethylnitrosourea. Mutat Res. 1983 Aug;110(2):367–382. doi: 10.1016/0027-5107(83)90153-7. [DOI] [PubMed] [Google Scholar]
- Favor J., Neuhäuser-Klaus A., Ehling U. H. The frequency of dominant cataract and recessive specific-locus mutations and mutation mosaics in F1 mice derived from post-spermatogonial treatment with ethylnitrosourea. Mutat Res. 1990 Apr;229(2):105–114. doi: 10.1016/0027-5107(90)90084-h. [DOI] [PubMed] [Google Scholar]
- Gossen J. A., de Leeuw W. J., Bakker A. Q., Vijg J. DNA sequence analysis of spontaneous mutations at a LacZ transgene integrated on the mouse X chromosome. Mutagenesis. 1993 May;8(3):243–247. doi: 10.1093/mutage/8.3.243. [DOI] [PubMed] [Google Scholar]
- Gossen J. A., de Leeuw W. J., Tan C. H., Zwarthoff E. C., Berends F., Lohman P. H., Knook D. L., Vijg J. Efficient rescue of integrated shuttle vectors from transgenic mice: a model for studying mutations in vivo. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7971–7975. doi: 10.1073/pnas.86.20.7971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanawalt P. C. Heterogeneity of DNA repair at the gene level. Mutat Res. 1991 Apr;247(2):203–211. doi: 10.1016/0027-5107(91)90016-h. [DOI] [PubMed] [Google Scholar]
- Harbach P. R., Filipunas A. L., Wang Y., Aaron C. S. DNA sequence analysis of spontaneous and N-ethyl-N-nitrosourea-induced hprt mutations arising in vivo in cynomolgus monkey T-lymphocytes. Environ Mol Mutagen. 1992;20(2):96–105. doi: 10.1002/em.2850200205. [DOI] [PubMed] [Google Scholar]
- Heddle J. A., Gingerich J. D., Urlando C., Pagura M., Shepson P., Khan M. A. Detection of somatic mutations in vivo in lung fibroblasts, I. Spontaneous frequencies in Chinese hamsters and F344 rats. Mutat Res. 1992 Dec;272(3):195–203. doi: 10.1016/0165-1161(92)91532-v. [DOI] [PubMed] [Google Scholar]
- Hess R. A., Chen P. Computer tracking of germ cells in the cycle of the seminiferous epithelium and prediction of changes in cycle duration in animals commonly used in reproductive biology and toxicology. J Androl. 1992 May-Jun;13(3):185–190. [PubMed] [Google Scholar]
- Klein J. C., Bleeker M. J., Lutgerink J. T., van Dijk W. J., Brugghe H. F., van den Elst H., van der Marel G. A., van Boom J. H., Westra J. G., Berns A. J. Use of shuttle vectors to study the molecular processing of defined carcinogen-induced DNA damage: mutagenicity of single O4-ethylthymine adducts in HeLa cells. Nucleic Acids Res. 1990 Jul 25;18(14):4131–4137. doi: 10.1093/nar/18.14.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler S. W., Provost G. S., Kretz P. L., Dycaico M. J., Sorge J. A., Short J. M. Development of a short-term, in vivo mutagenesis assay: the effects of methylation on the recovery of a lambda phage shuttle vector from transgenic mice. Nucleic Acids Res. 1990 May 25;18(10):3007–3013. doi: 10.1093/nar/18.10.3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis S. E., Johnson F. M., Skow L. C., Popp D., Barnett L. B., Popp R. A. A mutation in the beta-globin gene detected in the progeny of a female mouse treated with ethylnitrosourea. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5829–5831. doi: 10.1073/pnas.82.17.5829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirsalis J. C., Monforte J. A., Winegar R. A. Transgenic animal models for measuring mutations in vivo. Crit Rev Toxicol. 1994;24(3):255–280. doi: 10.3109/10408449409021608. [DOI] [PubMed] [Google Scholar]
- Mirsalis J. C., Provost G. S., Matthews C. D., Hamner R. T., Schindler J. E., O'Loughlin K. G., MacGregor J. T., Short J. M. Induction of hepatic mutations in lacI transgenic mice. Mutagenesis. 1993 May;8(3):265–271. doi: 10.1093/mutage/8.3.265. [DOI] [PubMed] [Google Scholar]
- OAKBERG E. F. Duration of spermatogenesis in the mouse and timing of stages of the cycle of the seminiferous epithelium. Am J Anat. 1956 Nov;99(3):507–516. doi: 10.1002/aja.1000990307. [DOI] [PubMed] [Google Scholar]
- Peters J., Andrews S. J., Loutit J. F., Clegg J. B. A mouse beta-globin mutant that is an exact model of hemoglobin Rainier in man. Genetics. 1985 Aug;110(4):709–721. doi: 10.1093/genetics/110.4.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popp R. A., Bailiff E. G., Skow L. C., Johnson F. M., Lewis S. E. Analysis of a mouse alpha-globin gene mutation induced by ethylnitrosourea. Genetics. 1983 Sep;105(1):157–167. doi: 10.1093/genetics/105.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provost G. S., Short J. M. Characterization of mutations induced by ethylnitrosourea in seminiferous tubule germ cells of transgenic B6C3F1 mice. Proc Natl Acad Sci U S A. 1994 Jul 5;91(14):6564–6568. doi: 10.1073/pnas.91.14.6564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rank K., McManus T. P., Ginsberg L. C., Ficsor G. Preparation of mouse-sperm DNA for PCR. Mutat Res. 1991 Oct;264(2):67–69. doi: 10.1016/0165-7992(91)90047-8. [DOI] [PubMed] [Google Scholar]
- Russell W. L., Hunsicker P. R., Carpenter D. A., Cornett C. V., Guinn G. M. Effect of dose fractionation on the ethylnitrosourea induction of specific-locus mutations in mouse spermatogonia. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3592–3593. doi: 10.1073/pnas.79.11.3592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sega G. A., Rohrer C. R., Harvey H. R., Jetton A. E. Chemical dosimetry of ethyl nitrosourea in the mouse testis. Mutat Res. 1986 Jan-Feb;159(1-2):65–74. doi: 10.1016/0027-5107(86)90113-2. [DOI] [PubMed] [Google Scholar]
- Shibuya T., Morimoto K. A review of the genotoxicity of 1-ethyl-1-nitrosourea. Mutat Res. 1993 Jul;297(1):3–38. doi: 10.1016/0165-1110(93)90005-8. [DOI] [PubMed] [Google Scholar]
- Skopek T. R., Walker V. E., Cochrane J. E., Craft T. R., Cariello N. F. Mutational spectrum at the Hprt locus in splenic T cells of B6C3F1 mice exposed to N-ethyl-N-nitrosourea. Proc Natl Acad Sci U S A. 1992 Sep 1;89(17):7866–7870. doi: 10.1073/pnas.89.17.7866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su L. K., Kinzler K. W., Vogelstein B., Preisinger A. C., Moser A. R., Luongo C., Gould K. A., Dove W. F. Multiple intestinal neoplasia caused by a mutation in the murine homolog of the APC gene. Science. 1992 May 1;256(5057):668–670. doi: 10.1126/science.1350108. [DOI] [PubMed] [Google Scholar]
- Tao K. S., Urlando C., Heddle J. A. Comparison of somatic mutation in a transgenic versus host locus. Proc Natl Acad Sci U S A. 1993 Nov 15;90(22):10681–10685. doi: 10.1073/pnas.90.22.10681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei S. J., Chang R. L., Bhachech N., Cui X. X., Merkler K. A., Wong C. Q., Hennig E., Yagi H., Jerina D. M., Conney A. H. Dose-dependent differences in the profile of mutations induced by (+)-7R,8S-dihydroxy-9S,10R-epoxy-7,8,9,10-tetrahydrobenzo(a)pyrene in the coding region of the hypoxanthine (guanine) phosphoribosyltransferase gene in Chinese hamster V-79 cells. Cancer Res. 1993 Jul 15;53(14):3294–3301. [PubMed] [Google Scholar]



