Abstract
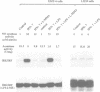
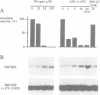
Biosynthesis of nitric oxide (NO) from L-arginine modulates activity of iron-dependent enzymes, including mitochondrial acontiase, an [Fe-S] protein. We examined the effect of NO on the activity of iron regulatory factor (IRF), a cytoplasmic protein which modulates both ferritin mRNA translation and transferrin receptor mRNA stability by binding to specific mRNA sequences called iron responsive elements (IREs). Murine macrophages were activated with interferon-gamma and lipopolysaccharide to induce NO synthase activity and cultured in the presence or absence of NG-substituted analogues of L-arginine which served as selective inhibitors of NO synthesis. Measurement of the nitrite concentration in the culture medium was taken as an index of NO production. Mitochondria-free cytosols were then prepared and aconitase activity as well as IRE binding activity and induction of IRE binding activity were correlated and depended on NO synthesis after IFN-gamma and/or LPS stimulation. Authentic NO gas as well as the NO-generating compound 3-morpholinosydnonimine (SIN-1) also conversely modulated aconitase and IRE binding activities of purified recombinant IRF. These results provide evidence that endogenously produced NO may modulate the post-transcriptional regulation of genes involved in iron homeostasis and support the hypothesis that the [Fe-S] cluster of IRF mediates iron-dependent regulation.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. O., Hamilton T. A. The cell biology of macrophage activation. Annu Rev Immunol. 1984;2:283–318. doi: 10.1146/annurev.iy.02.040184.001435. [DOI] [PubMed] [Google Scholar]
- Amber I. J., Hibbs J. B., Jr, Taintor R. R., Vavrin Z. Cytokines induce an L-arginine-dependent effector system in nonmacrophage cells. J Leukoc Biol. 1988 Jul;44(1):58–65. doi: 10.1002/jlb.44.1.58. [DOI] [PubMed] [Google Scholar]
- Aziz N., Munro H. N. Iron regulates ferritin mRNA translation through a segment of its 5' untranslated region. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8478–8482. doi: 10.1073/pnas.84.23.8478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babij P. Tissue-specific and developmentally regulated alternative splicing of a visceral isoform of smooth muscle myosin heavy chain. Nucleic Acids Res. 1993 Mar 25;21(6):1467–1471. doi: 10.1093/nar/21.6.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beinert H., Kennedy M. C. 19th Sir Hans Krebs lecture. Engineering of protein bound iron-sulfur clusters. A tool for the study of protein and cluster chemistry and mechanism of iron-sulfur enzymes. Eur J Biochem. 1989 Dec 8;186(1-2):5–15. doi: 10.1111/j.1432-1033.1989.tb15170.x. [DOI] [PubMed] [Google Scholar]
- Beinert H. Recent developments in the field of iron-sulfur proteins. FASEB J. 1990 May;4(8):2483–2491. doi: 10.1096/fasebj.4.8.2185975. [DOI] [PubMed] [Google Scholar]
- Bourgeade M. F., Silbermann F., Kühn L., Testa U., Peschle C., Mémet S., Thang M. N., Besançon F. Post-transcriptional regulation of transferrin receptor mRNA by IFN gamma. Nucleic Acids Res. 1992 Jun 25;20(12):2997–3003. doi: 10.1093/nar/20.12.2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd T. F., Horwitz M. A. Interferon gamma-activated human monocytes downregulate transferrin receptors and inhibit the intracellular multiplication of Legionella pneumophila by limiting the availability of iron. J Clin Invest. 1989 May;83(5):1457–1465. doi: 10.1172/JCI114038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey J. L., Hentze M. W., Koeller D. M., Caughman S. W., Rouault T. A., Klausner R. D., Harford J. B. Iron-responsive elements: regulatory RNA sequences that control mRNA levels and translation. Science. 1988 May 13;240(4854):924–928. doi: 10.1126/science.2452485. [DOI] [PubMed] [Google Scholar]
- Constable A., Quick S., Gray N. K., Hentze M. W. Modulation of the RNA-binding activity of a regulatory protein by iron in vitro: switching between enzymatic and genetic function? Proc Natl Acad Sci U S A. 1992 May 15;89(10):4554–4558. doi: 10.1073/pnas.89.10.4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding A. H., Nathan C. F., Stuehr D. J. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J Immunol. 1988 Oct 1;141(7):2407–2412. [PubMed] [Google Scholar]
- Drapier J. C., Hibbs J. B., Jr Differentiation of murine macrophages to express nonspecific cytotoxicity for tumor cells results in L-arginine-dependent inhibition of mitochondrial iron-sulfur enzymes in the macrophage effector cells. J Immunol. 1988 Apr 15;140(8):2829–2838. [PubMed] [Google Scholar]
- Drapier J. C., Hibbs J. B., Jr Murine cytotoxic activated macrophages inhibit aconitase in tumor cells. Inhibition involves the iron-sulfur prosthetic group and is reversible. J Clin Invest. 1986 Sep;78(3):790–797. doi: 10.1172/JCI112642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drapier J. C., Pellat C., Henry Y. Generation of EPR-detectable nitrosyl-iron complexes in tumor target cells cocultured with activated macrophages. J Biol Chem. 1991 Jun 5;266(16):10162–10167. [PubMed] [Google Scholar]
- Drapier J. C., Wietzerbin J., Hibbs J. B., Jr Interferon-gamma and tumor necrosis factor induce the L-arginine-dependent cytotoxic effector mechanism in murine macrophages. Eur J Immunol. 1988 Oct;18(10):1587–1592. doi: 10.1002/eji.1830181018. [DOI] [PubMed] [Google Scholar]
- Feelisch M., Ostrowski J., Noack E. On the mechanism of NO release from sydnonimines. J Cardiovasc Pharmacol. 1989;14 (Suppl 11):S13–S22. [PubMed] [Google Scholar]
- Fiskum G., Craig S. W., Decker G. L., Lehninger A. L. The cytoskeleton of digitonin-treated rat hepatocytes. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3430–3434. doi: 10.1073/pnas.77.6.3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawron O., Waheed A., Glaid A. J., 3rd, Jaklitsch A. Iron and aconitase activity. Biochem J. 1974 Jun;139(3):709–714. doi: 10.1042/bj1390709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger D. L., Taintor R. R., Cook J. L., Hibbs J. B., Jr Injury of neoplastic cells by murine macrophages leads to inhibition of mitochondrial respiration. J Clin Invest. 1980 Feb;65(2):357–370. doi: 10.1172/JCI109679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green S. J., Nacy C. A., Meltzer M. S. Cytokine-induced synthesis of nitrogen oxides in macrophages: a protective host response to Leishmania and other intracellular pathogens. J Leukoc Biol. 1991 Jul;50(1):93–103. doi: 10.1002/jlb.50.1.93. [DOI] [PubMed] [Google Scholar]
- Haile D. J., Rouault T. A., Harford J. B., Kennedy M. C., Blondin G. A., Beinert H., Klausner R. D. Cellular regulation of the iron-responsive element binding protein: disassembly of the cubane iron-sulfur cluster results in high-affinity RNA binding. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):11735–11739. doi: 10.1073/pnas.89.24.11735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haile D. J., Rouault T. A., Tang C. K., Chin J., Harford J. B., Klausner R. D. Reciprocal control of RNA-binding and aconitase activity in the regulation of the iron-responsive element binding protein: role of the iron-sulfur cluster. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7536–7540. doi: 10.1073/pnas.89.16.7536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton T. A., Gray P. W., Adams D. O. Expression of the transferrin receptor on murine peritoneal macrophages is modulated by in vitro treatment with interferon gamma. Cell Immunol. 1984 Dec;89(2):478–488. doi: 10.1016/0008-8749(84)90348-4. [DOI] [PubMed] [Google Scholar]
- Harrell C. M., McKenzie A. R., Patino M. M., Walden W. E., Theil E. C. Ferritin mRNA: interactions of iron regulatory element with translational regulator protein P-90 and the effect on base-paired flanking regions. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4166–4170. doi: 10.1073/pnas.88.10.4166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry Y., Ducrocq C., Drapier J. C., Servent D., Pellat C., Guissani A. Nitric oxide, a biological effector. Electron paramagnetic resonance detection of nitrosyl-iron-protein complexes in whole cells. Eur Biophys J. 1991;20(1):1–15. doi: 10.1007/BF00183275. [DOI] [PubMed] [Google Scholar]
- Hentze M. W., Rouault T. A., Harford J. B., Klausner R. D. Oxidation-reduction and the molecular mechanism of a regulatory RNA-protein interaction. Science. 1989 Apr 21;244(4902):357–359. doi: 10.1126/science.2711187. [DOI] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Taintor R. R., Vavrin Z. Iron depletion: possible cause of tumor cell cytotoxicity induced by activated macrophages. Biochem Biophys Res Commun. 1984 Sep 17;123(2):716–723. doi: 10.1016/0006-291x(84)90288-2. [DOI] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Taintor R. R., Vavrin Z. Macrophage cytotoxicity: role for L-arginine deiminase and imino nitrogen oxidation to nitrite. Science. 1987 Jan 23;235(4787):473–476. doi: 10.1126/science.2432665. [DOI] [PubMed] [Google Scholar]
- Hirling H., Emery-Goodman A., Thompson N., Neupert B., Seiser C., Kühn L. C. Expression of active iron regulatory factor from a full-length human cDNA by in vitro transcription/translation. Nucleic Acids Res. 1992 Jan 11;20(1):33–39. doi: 10.1093/nar/20.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt N. C., Goldin R. D. Nitric oxide production by monocytes in alcoholic liver disease. J Hepatol. 1992 Mar;14(2-3):146–150. doi: 10.1016/0168-8278(92)90150-n. [DOI] [PubMed] [Google Scholar]
- Hyman M. R., Seefeldt L. C., Morgan T. V., Arp D. J., Mortenson L. E. Kinetic and spectroscopic analysis of the inactivating effects of nitric oxide on the individual components of Azotobacter vinelandii nitrogenase. Biochemistry. 1992 Mar 24;31(11):2947–2955. doi: 10.1021/bi00126a015. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J. Haem-dependent activation of guanylate cyclase and cyclic GMP formation by endogenous nitric oxide: a unique transduction mechanism for transcellular signaling. Pharmacol Toxicol. 1990 Jul;67(1):1–7. doi: 10.1111/j.1600-0773.1990.tb00772.x. [DOI] [PubMed] [Google Scholar]
- Kaptain S., Downey W. E., Tang C., Philpott C., Haile D., Orloff D. G., Harford J. B., Rouault T. A., Klausner R. D. A regulated RNA binding protein also possesses aconitase activity. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):10109–10113. doi: 10.1073/pnas.88.22.10109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy M. C., Emptage M. H., Dreyer J. L., Beinert H. The role of iron in the activation-inactivation of aconitase. J Biol Chem. 1983 Sep 25;258(18):11098–11105. [PubMed] [Google Scholar]
- Kennedy M. C., Mende-Mueller L., Blondin G. A., Beinert H. Purification and characterization of cytosolic aconitase from beef liver and its relationship to the iron-responsive element binding protein. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):11730–11734. doi: 10.1073/pnas.89.24.11730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy M. C., Werst M., Telser J., Emptage M. H., Beinert H., Hoffman B. M. Mode of substrate carboxyl binding to the [4Fe-4S]+ cluster of reduced aconitase as studied by 17O and 13C electron-nuclear double resonance spectroscopy. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8854–8858. doi: 10.1073/pnas.84.24.8854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausner R. D., Rouault T. A., Harford J. B. Regulating the fate of mRNA: the control of cellular iron metabolism. Cell. 1993 Jan 15;72(1):19–28. doi: 10.1016/0092-8674(93)90046-s. [DOI] [PubMed] [Google Scholar]
- Kühn L. C., Hentze M. W. Coordination of cellular iron metabolism by post-transcriptional gene regulation. J Inorg Biochem. 1992 Aug 15;47(3-4):183–195. doi: 10.1016/0162-0134(92)84064-t. [DOI] [PubMed] [Google Scholar]
- Lancaster J. R., Jr, Hibbs J. B., Jr EPR demonstration of iron-nitrosyl complex formation by cytotoxic activated macrophages. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1223–1227. doi: 10.1073/pnas.87.3.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lander H. M., Sehajpal P., Levine D. M., Novogrodsky A. Activation of human peripheral blood mononuclear cells by nitric oxide-generating compounds. J Immunol. 1993 Feb 15;150(4):1509–1516. [PubMed] [Google Scholar]
- Leibold E. A., Munro H. N. Cytoplasmic protein binds in vitro to a highly conserved sequence in the 5' untranslated region of ferritin heavy- and light-subunit mRNAs. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2171–2175. doi: 10.1073/pnas.85.7.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepoivre M., Chenais B., Yapo A., Lemaire G., Thelander L., Tenu J. P. Alterations of ribonucleotide reductase activity following induction of the nitrite-generating pathway in adenocarcinoma cells. J Biol Chem. 1990 Aug 25;265(24):14143–14149. [PubMed] [Google Scholar]
- Magrinat G., Mason S. N., Shami P. J., Weinberg J. B. Nitric oxide modulation of human leukemia cell differentiation and gene expression. Blood. 1992 Oct 15;80(8):1880–1884. [PubMed] [Google Scholar]
- McBride A. A., Klausner R. D., Howley P. M. Conserved cysteine residue in the DNA-binding domain of the bovine papillomavirus type 1 E2 protein confers redox regulation of the DNA-binding activity in vitro. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7531–7535. doi: 10.1073/pnas.89.16.7531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer J. Comparison of carbon monoxide, nitric oxide, and nitrite as inhibitors of the nitrogenase from Clostridium pasteurianum. Arch Biochem Biophys. 1981 Aug;210(1):246–256. doi: 10.1016/0003-9861(81)90186-7. [DOI] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Müllner E. W., Kühn L. C. A stem-loop in the 3' untranslated region mediates iron-dependent regulation of transferrin receptor mRNA stability in the cytoplasm. Cell. 1988 Jun 3;53(5):815–825. doi: 10.1016/0092-8674(88)90098-0. [DOI] [PubMed] [Google Scholar]
- Müllner E. W., Neupert B., Kühn L. C. A specific mRNA binding factor regulates the iron-dependent stability of cytoplasmic transferrin receptor mRNA. Cell. 1989 Jul 28;58(2):373–382. doi: 10.1016/0092-8674(89)90851-9. [DOI] [PubMed] [Google Scholar]
- Nathan C. F., Hibbs J. B., Jr Role of nitric oxide synthesis in macrophage antimicrobial activity. Curr Opin Immunol. 1991 Feb;3(1):65–70. doi: 10.1016/0952-7915(91)90079-g. [DOI] [PubMed] [Google Scholar]
- Nathan C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992 Sep;6(12):3051–3064. [PubMed] [Google Scholar]
- Pellat C., Henry Y., Drapier J. C. IFN-gamma-activated macrophages: detection by electron paramagnetic resonance of complexes between L-arginine-derived nitric oxide and non-heme iron proteins. Biochem Biophys Res Commun. 1990 Jan 15;166(1):119–125. doi: 10.1016/0006-291x(90)91919-j. [DOI] [PubMed] [Google Scholar]
- Reif D. W., Simmons R. D. Nitric oxide mediates iron release from ferritin. Arch Biochem Biophys. 1990 Dec;283(2):537–541. doi: 10.1016/0003-9861(90)90680-w. [DOI] [PubMed] [Google Scholar]
- Rouault T. A., Hentze M. W., Caughman S. W., Harford J. B., Klausner R. D. Binding of a cytosolic protein to the iron-responsive element of human ferritin messenger RNA. Science. 1988 Sep 2;241(4870):1207–1210. doi: 10.1126/science.3413484. [DOI] [PubMed] [Google Scholar]
- Rouault T. A., Stout C. D., Kaptain S., Harford J. B., Klausner R. D. Structural relationship between an iron-regulated RNA-binding protein (IRE-BP) and aconitase: functional implications. Cell. 1991 Mar 8;64(5):881–883. doi: 10.1016/0092-8674(91)90312-m. [DOI] [PubMed] [Google Scholar]
- Rousseau D. L., Singh S., Ching Y. C., Sassaroli M. Nitrosyl cytochrome c oxidase. Formation and properties of mixed valence enzyme. J Biol Chem. 1988 Apr 25;263(12):5681–5685. [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Stadler J., Billiar T. R., Curran R. D., Stuehr D. J., Ochoa J. B., Simmons R. L. Effect of exogenous and endogenous nitric oxide on mitochondrial respiration of rat hepatocytes. Am J Physiol. 1991 May;260(5 Pt 1):C910–C916. doi: 10.1152/ajpcell.1991.260.5.C910. [DOI] [PubMed] [Google Scholar]
- Stamler J. S., Simon D. I., Osborne J. A., Mullins M. E., Jaraki O., Michel T., Singel D. J., Loscalzo J. S-nitrosylation of proteins with nitric oxide: synthesis and characterization of biologically active compounds. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):444–448. doi: 10.1073/pnas.89.1.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuehr D. J., Griffith O. W. Mammalian nitric oxide synthases. Adv Enzymol Relat Areas Mol Biol. 1992;65:287–346. doi: 10.1002/9780470123119.ch8. [DOI] [PubMed] [Google Scholar]
- Stuehr D. J., Marletta M. A. Synthesis of nitrite and nitrate in murine macrophage cell lines. Cancer Res. 1987 Nov 1;47(21):5590–5594. [PubMed] [Google Scholar]
- Taetle R., Honeysett J. M. Gamma-interferon modulates human monocyte/macrophage transferrin receptor expression. Blood. 1988 Jun;71(6):1590–1595. [PubMed] [Google Scholar]
- Testa U., Kühn L., Petrini M., Quaranta M. T., Pelosi E., Peschle C. Differential regulation of iron regulatory element-binding protein(s) in cell extracts of activated lymphocytes versus monocytes-macrophages. J Biol Chem. 1991 Jul 25;266(21):13925–13930. [PubMed] [Google Scholar]
- Toledano M. B., Leonard W. J. Modulation of transcription factor NF-kappa B binding activity by oxidation-reduction in vitro. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4328–4332. doi: 10.1073/pnas.88.10.4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traylor T. G., Sharma V. S. Why NO? Biochemistry. 1992 Mar 24;31(11):2847–2849. doi: 10.1021/bi00126a001. [DOI] [PubMed] [Google Scholar]
- WHARTON D. C., GRIFFITHS D. E. Studies on the electron transport system. XXXIX. Assay of cytochrome oxidase. Effect of phospholipids and other factors. Arch Biochem Biophys. 1962 Jan;96:103–114. doi: 10.1016/0003-9861(62)90458-7. [DOI] [PubMed] [Google Scholar]
- Walden W. E., Patino M. M., Gaffield L. Purification of a specific repressor of ferritin mRNA translation from rabbit liver. J Biol Chem. 1989 Aug 15;264(23):13765–13769. [PubMed] [Google Scholar]
- Wharton M., Granger D. L., Durack D. T. Mitochondrial iron loss from leukemia cells injured by macrophages. A possible mechanism for electron transport chain defects. J Immunol. 1988 Aug 15;141(4):1311–1317. [PubMed] [Google Scholar]