Abstract
In 2012, an estimated 8.6 million people developed tuberculosis (TB) and 1.3 million died from the disease. With its recent resurgence with the human immunodeficiency virus (HIV); TB prevention and management has become further challenging. We systematically evaluated the effectiveness of community based interventions (CBI) for the prevention and treatment of TB and a total of 41 studies were identified for inclusion. Findings suggest that CBI for TB prevention and case detection showed significant increase in TB detection rates (RR: 3.1, 95% CI: 2.92, 3.28) with non-significant impact on TB incidence. CBI for treating patients with active TB showed an overall improvement in treatment success rates (RR: 1.09, 95% CI: 1.07, 1.11) and evidence from a single study suggests significant reduction in relapse rate (RR: 0.26, 95% CI: 0.18, 0.39). The results were consistent for various study design and delivery mechanism. Qualitative synthesis suggests that community based TB treatment delivery through community health workers (CHW) not only improved access and service utilization but also contributed to capacity building and improving the routine TB recording and reporting systems. CBI coupled with the DOTS strategy seem to be an effective approach, however there is a need to evaluate various community-based integrated delivery models for relative effectiveness.
Keywords: Community-based interventions, Tuberculosis, DOTS, integrated delivery, CHWs
Multilingual abstracts
Please see Additional file 1 for translations of the abstract into the six official working languages of the United Nations.
Introduction
Tuberculosis (TB) remains a major global health problem. In 2012, an estimated 8.6 million people developed TB and 1.3 million died from the disease [1]. The number of TB deaths is unacceptably large given that most of these deaths are preventable. With the recent resurgence related to the human immunodeficiency virus (HIV), TB prevention and management has become further challenging [2-4]. TB is preventable as well as curable and its transmission could be prevented by prompt identification and treatment of the infected person. However; ensuring treatment completion is crucial for the prevention of relapse and secondary drug resistance. The World Health Organization (WHO) recommends Stop TB Strategy based on the Directly Observed Therapy, Short-course (DOTS) to control TB. The strategy aims to ensure that patients take a standard short-course of chemotherapy under guided supervision to cure the disease as well as to prevent transmission. Patients are assisted through their treatment regimen and encouraged to treatment completion in order to prevent resistance to the available anti-TB drugs. DOTS has been delivered by health workers, community volunteers, lay health workers and even family members [5]. For further details on TB burden, epidemiology and intervention coverage, refer to previous paper in this series [6].
Considering the recent shift in epidemiological presence of TB, there is a legitimate call for integration of therapeutic services especially with HIV [7]. Since both diseases require long term treatment regimens, community based support may play a defining role towards prevention and control of these syndemic diseases of poverty. Moreover, integration of services in low-income countries may prove beneficial in terms of cost-effectiveness and decrease demand on health service infrastructure. However, there is a need to gauge whether these strategies lead to effective treatment outcomes. This paper aims to evaluate the effectiveness of community based interventions (CBI) for the prevention and treatment of TB.
Methods
We systematically reviewed literature published by September 2013 to identify studies evaluating CBI for TB as outlined in our conceptual framework [8]. Our priority was to select existing randomized controlled trials (RCT), quasi-experimental and before/after studies in which the intervention was delivered within community settings and the reported outcomes were relevant. A comprehensive search strategy was developed using appropriate key words, Medical Subject Headings (MeSH) and free text terms. The search was conducted in PubMed, Cochrane libraries, EMBASE and WHO Regional Databases; additional studies were identified by hand searching references from included studies. Studies were excluded if the intervention was purely facility-based or had a facility-based component. Studies that met the inclusion criteria were selected and double data abstracted on a standardized abstraction sheet. Quality assessment of the included RCT was done using the Cochrane risk of bias assessment tool [9]. We conducted meta-analysis for individual studies using the software Review Manager 5.1. Pooled statistics were reported as the relative risk (RR) for categorical variables and standard mean difference (SMD) for continuous variables between the experimental and control groups with 95% confidence intervals (CI). Subgroup analysis was conducted for therapeutic and preventive (screening) CBI, integrated and non-integrated CBI and by type of studies. The detailed methodology is described in previous paper [8].
Review
A total of 7,772 titles were identified from all databases and 107 full texts were screened. After screening, forty one [10-50] studies met the inclusion criteria; 34 RCT and 7 before/after studies (Figure 1). From the included RCT, 18 were adequately randomized while five studies reported adequate sequence generation (Table 1). Due to the nature of the intervention, blinding of the participants and assessors was not possible. Studies provided insufficient information on selective reporting which limited us from making any judgment. Ten of the included studies focused on TB prevention and case detection while 31 studies were on treatment of patients with active TB. Interventions involved community based delivery of DOTS; community mobilization and support; education and training; and monetary incentives for treatment adherence. Most of the CBI utilized community health workers (CHW) or family members as part of the delivery strategy. Table 2 describes the characteristics of included studies.
Figure 1.
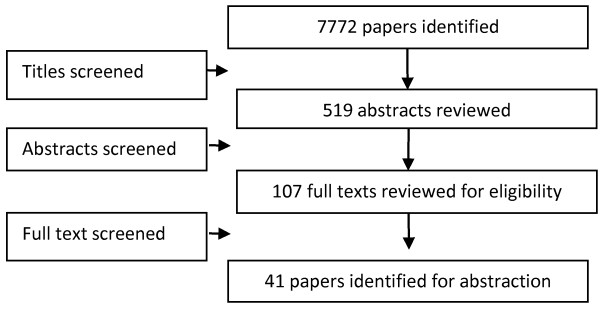
Search flow diagram.
Table 1.
Quality assessment of the included RCTs
| Article | Randomization | Sequence generation | Allocation concealment | Blinding of participants | Blinding of assessors | Selective reporting |
|---|---|---|---|---|---|---|
| Atkins 2011 [11] |
No |
No |
No |
Not clear |
Not clear |
Not clear |
| Barker 2002 [12] |
No |
No |
Not clear |
Not clear |
Not clear |
No |
| Clarke 2005 [15] |
Yes |
No |
Yes |
No |
No |
No |
| Colvin 2003 [16] |
No |
No |
No |
Not clear |
Not clear |
Yes |
| Corbett 2010 [17] |
Yes |
Not clear |
Yes |
No |
Yes |
No |
| Dudley 2003 [19] |
No |
No |
No |
Not clear |
Not clear |
No |
| Fairall 2005 [20] |
Yes |
Yes |
Not clear |
No |
Yes |
No |
| Ferreira 2011 [21] |
No |
No |
No |
Not clear |
Not clear |
No |
| Filho 2009 [14] |
No |
No |
No |
Not clear |
Not clear |
Yes |
| Kironde 2002 [23] |
No |
No |
No |
Not clear |
Not clear |
No |
| Vieira 2011 [31] |
No |
No |
No |
Not clear |
Not clear |
Not clear |
| White 2002 [33] |
Yes |
Yes |
Yes |
Not clear |
Not clear |
No |
| Zwarenstein 2000 [35] |
Yes |
Yes |
Yes |
No |
No |
No |
| Vassal 2002 [30] |
Not clear |
Not clear |
Not clear |
Not clear |
Not clear |
Not clear |
| Prado 2011 [27] |
No |
No |
No |
No |
No |
No |
| Mafigiri 2012 [24] |
No |
No |
No |
No |
Not clear |
No |
| Niazi 2003 [26] |
No |
No |
No |
No |
Not clear |
No |
| Uwimana 2012 [28] |
Yes |
Not clear |
Not clear |
Not clear |
Not clear |
No |
| Yassin 2013 [34] |
No |
No |
No |
Not clear |
Not clear |
No |
| CDI group |
Yes |
Not clear |
Not clear |
Not clear |
Not clear |
Not clear |
| Miti 2003 [25] |
No |
No |
No |
Not clear |
Not clear |
No |
| Zwarenstein 1998 [50] |
Yes |
Not clear |
Not clear |
No |
No |
No |
| Chaisson 2001 [37] |
Yes |
Yes |
Yes |
Not clear |
Not clear |
No |
| Heal 1998 [39] |
No |
No |
No |
No |
No |
No |
| Kamolratanakul 1999 [40] |
Yes |
Yes |
Yes |
Yes |
No |
No |
| Khan 2002 [41] |
Yes |
Not clear |
Not clear |
Not clear |
Not clear |
No |
| Lwilla 2003 [42] |
Yes |
Not clear |
Not clear |
No |
No |
Yes |
| MacIntyre 2003 [43] |
No |
No |
No |
No |
Yes |
Yes |
| Malotte 2001 [44] |
Yes |
No |
Yes |
Not clear |
Not clear |
No |
| Newell 2006 [45] |
Yes |
No |
Yes |
No |
Yes |
No |
| Ollé-Goig 2001 [46] |
No |
No |
No |
No |
No |
No |
| Walley 2001 [47] |
Yes |
Yes |
Yes |
No |
Yes |
No |
| Wandwalo 2004 [48] |
Yes |
No |
No |
No |
No |
No |
| Wright 2004 [49] | Yes | No | Yes | Not clear | Not clear | No |
Table 2.
Characteristics of the included studies
| Study | Study design | Country | Intervention | Target Population | Integrated/Non-Integrated |
|---|---|---|---|---|---|
| Gandhi 2008 [38] |
Before/after |
South Africa |
DOTS therapy integrated with anti-retroviral therapy on a community level |
TB and HIV co-infected adults |
Integrated |
| Chaisson 2001 [37] |
RCT |
USA |
Preventive Isoniazid therapy for injection drug users |
Injection drug users |
Non-integrated |
| Zwarenstein 1998 [50] |
RCT |
South Africa |
Self-supervised treatment compared against therapy observed in clinic |
Adult pulmonary TB patients |
Non-integrated |
| Heal 1998 [39] |
Quasi-trial |
Canada |
Self-administered preventive therapy compared against preventive therapy observed in a clinic |
All aboriginals in British Columbia undergoing preventive therapy for TB |
Non-integrated |
| Kamolratanakul 1999 [40] |
RCT |
Thailand |
DOTS therapy compared against self-supervised therapy |
All smear positive pulmonary TB patients |
Non-integrated |
| Khan 2002 [41] |
RCT |
Pakistan |
DOTS therapy compared against self-supervised therapy |
Adults with TB |
Non-integrated |
| Lwilla 2003 [42] |
RCT |
Tanzania |
Community-based DOTS compared against institution-based DOTS |
All patients diagnosed with TB at selected health centres |
Non-integrated |
| MacIntyre 2003 [43] |
Quasi-trial |
Australia |
Family member supervised DOTS compared against non-observed therapy |
All patients diagnosed with TB at selected health centres |
Non-integrated |
| Malotte 2001 [44] |
RCT |
USA |
INH therapy of latent TB infections given either by outreach workers or at a facility |
People with active or recent history of drug use |
Non-integrated |
| Newell 2006 [45] |
RCT |
Nepal |
Comparison between community-members DOTS and family member DOTS |
All new smear positive cases of pulmonary TB |
Non-integrated |
| Ollé-Goig 2001 [46] |
Quasi-trial |
Haiti |
DOTS compared with non-observed therapy |
Adult TB patients |
Non-integrated |
| Walley 2001 [47] |
RCT |
Pakistan |
DOTS by family member compared with DOTS by healthcare worker and non-observed therapy |
Adult TB patients |
Non-integrated |
| Wandwalo 2004 [48] |
RCT |
Tanzania |
Community-based DOTS compared against healthcare worker DOTS |
TB patients of all ages |
Non-integrated |
| Wright 2004 [49] |
RCT |
Swaziland |
Community health worker DOTS compared with family member DOTS |
TB patients of all ages |
Non-integrated |
| Atkins 2011 [11] |
Quasi-trial |
South Africa |
Enhanced tuberculosis treatment adherence |
Adult TB patients |
Integrated |
| Barker 2002 [12] |
Quasi-trial |
South Africa |
Community-based DOTS compared against healthcare worker DOTS |
TB patients of all ages |
Non-integrated |
| Clarke 2005 [15] |
RCT |
South Africa |
Comparison between conventional TB treatment and lay health worker DOTS |
Adult TB patients |
Non-integrated |
| Colvin 2003 [16] |
Quasi-trial |
South Africa |
Traditional healers mobilized as DOTS supervisors |
TB patients of all ages |
Non-integrated |
| Corbett 2010 [17] |
RCT |
Zimbabwe |
Door-to-door and mobile van announcements compared as strategies to increase TB detection |
All people in a specific community |
Integrated |
| Diez 1996 [18] |
Before/after |
Spain |
Social support for deserving TB patients |
Adult TB patients |
Non-integrated |
| Dudley 2003 [19] |
Quasi-trial |
South Africa |
DOTS compared with non-observed therapy |
Adult TB patients |
Non-integrated |
| Fairall 2005 [20] |
RCT |
South Africa |
Educational outreach to nurses to increase TB case detection |
Patients attending specific clinics |
Integrated |
| Ferreira 2011 [21] |
Quasi-trial |
Brazil |
DOTS compared with non-observed therapy |
TB patients of all ages |
Non-integrated |
| Filho 2009 [14] |
Quasi-trial |
Brazil |
Food baskets offered to patient to assess effect on treatment outcomes |
Adult TB patients |
Integrated |
| Kamineni 2011 [22] |
Before/after |
India |
Increasing case detection and treatment adherence, decreasing stigma and discrimination, empowering affected people, and mobilising political commitment and resources |
TB patients of all ages |
Non-integrated |
| Kirondea 2002 [23] |
Quasi-trial |
South Africa |
Assessing the feasibility of using lay volunteers as DOTS supervisors |
Adult TB patients |
Non-integrated |
| Vieira 2011 [31] |
Quasi-trial |
Brazil |
DOTS compared with non-observed therapy |
Adult TB patients |
Non-integrated |
| Weis 1994 [32] |
Before/after |
USA |
DOTS compared with non-observed therapy |
TB patients of all ages |
Non-integrated |
| White 2002 [33] |
RCT |
USA |
Incentivized treatment compared with no incentive |
Susceptible population in a county jail |
Non-integrated |
| Zwarenstein 2000 [35] |
RCT |
South Africa |
DOTS compared with non-observed therapy |
TB patients of all ages |
Non-integrated |
| Prado 2011 [27] |
Quasi-trial |
Brazil |
Community health worker DOTS compared with family member DOTS |
Adult TB patients |
Non-integrated |
| Vassall 2002 [30] |
Quasi-trial |
Syria and Egypt |
Community-based DOTS compared against institution-based DOTS |
TB patients of all ages |
Integrated |
| CDI study group |
RCT |
Nigeria, Uganda and Cameroon |
Integration of community interventions to counter multiple diseases through a single framework |
TB patients of all ages |
Integrated |
| Miti 2003 [25] |
Quasi-trial |
Zambia |
Integration of HIV and TB services |
Adult TB patients |
Integrated |
| Amo-Adjei 2013 [10] |
Before/after |
Ghana |
Improvements in diagnosis, community TB care and stigma reduction among community and health workers towards TB patients |
Adult TB patients |
Integrated |
| Brust 2012 [13] |
Before/after |
South Africa |
Integration of HIV and TB services |
Adult TB patients |
Integrated |
| Mafigiri 2012 [24] |
Quasi-trial |
Uganda |
Community-based DOTS compared against institution-based DOTS |
TB patients of all ages |
Non-integrated |
| Niazi 2003 [26] |
Quasi-trial |
Iraq |
Community-based DOTS compared against institution-based DOTS |
Adult TB patients |
Non-integrated |
| Uwimana 2012 [28] |
RCT |
South Africa |
Training community care workers (CCWs) to provide integrated care |
All members of localities where the CCWs were based |
Integrated |
| Uwimana 2013 [29] |
Before/after |
South Africa |
Training community care workers (CCWs) to provide integrated care |
All members of localities where the CCWs were based |
Integrated |
| Yassin 2013 [34] | Quasi-trial | Ethiopia | Training, engaging stakeholders and communities and active case-finding by female Health Extension Workers (HEWs) at village level | All members of localities where the HEWs were based | Non-integrated |
Quantitative synthesis
Overall, CBI for TB prevention and case detection showed significant increase in TB detection rates (RR: 3.1, 95% CI: 2.92, 3.28) (Figure 2) while there was a non-significant impact on TB incidence, although this evidence is from a single study. Subgroup analysis showed consistent results for various study designs and whether the interventions were delivered in an integrated or a non-integrated manner. CBI for treating patients with active TB showed an overall improvement in treatment success rates (RR: 1.09, 95% CI: 1.07, 1.11) (Figure 3) and evidence from a single study suggests significant reduction in relapse rate (RR 0.26, 95% CI: 0.18, 0.39). The results were consistent for various study design and delivery mechanism. The results are summarized in Table 3.
Figure 2.
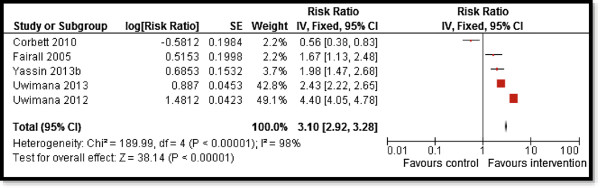
Forest plot for the impact of CBI on TB case detection.
Figure 3.
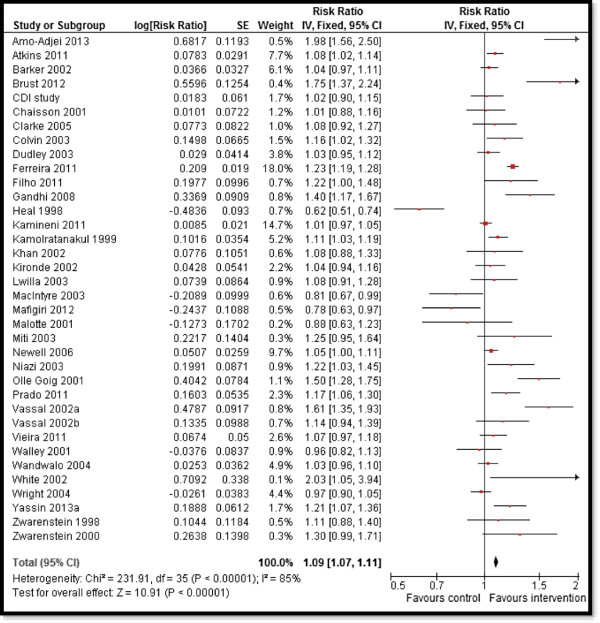
Forest plot for the impact of CBI on treatment success rate.
Table 3.
Results for overall and sub-group analysis according to type of study, intervention and treatment
| Outcomes | Estimates (95% CI) | |||||
|---|---|---|---|---|---|---|
| |
Prevention/Screening |
Therapeutic management |
RCT/Quasi |
Pre-post studies |
Integrated |
Non-integrated |
|
CBI for TB case detection | ||||||
|
TB detection |
3.10 [2.92, 3.28] |
- |
3.71 [3.44, 4.01] |
2.43 [2.22, 2.65] |
3.15 [2.97, 3.34] |
1.98 [1.47, 2.68] |
| 5 datasets, 5 studies |
|
4 datasets, 4 studies |
1 dataset, 1 study |
4 datasets, 4 studies |
1 dataset, 1 study |
|
|
TB incidence |
0.63 [0.36, 1.09] |
- |
- |
0.63 [0.36, 1.09] |
- |
0.63 [0.36, 1.09] |
| 1 dataset, 1 study |
|
|
1 dataset, 1 study |
|
1 dataset, 1 study |
|
|
CBI for patients with Active TB | ||||||
|
Treatment success |
- |
1.09 [1.07, 1.11] |
1.10 [1.08, 1.12] |
1.06 [1.02, 1.10] |
1.29 [1.21, 1.38] |
1.08 [1.06, 1.10] |
| 36 datasets 35 studies |
32 datasets, 31 studies |
4 datasets, 4 studies |
8 datasets, 7 studies |
28 datasets, 28 studies |
||
|
Relapse |
- |
0.26 [0.18, 0.39] |
- |
0.26 [0.18, 0.39] |
- |
0.26 [0.18, 0.39] |
| 1 dataset, 1 study | 1 dataset, 1 study | 1 dataset, 1 study | ||||
Italics denote statistically significant estimates.
Qualitative synthesis
Included studies suggest that CBI for TB have the potential to improve access to diagnostic and treatment services for poor rural communities and vulnerable population including women and children. Community based TB treatment delivery through CHW not only improved access and service utilization but also contributed to capacity building and improving routine TB recording and reporting systems through regular supportive supervision [34]. Better outcomes were reported when DOTS was provided together with CHW program as it enabled treatment continuation; thus achieving higher treatment success rates [21]. Studies also support the feasibility of integrating cadres of CHW through establishing supportive structures and supervision [28,29]. Besides treatment, community based untargeted periodic active case finding for symptomatic smear-positive TB also made a substantial contribution to diagnosis and control of infectious TB [17]. This is a significant finding as the slow rate at which patients with tuberculosis report to health facilities is a major limitation in global efforts to control TB. However, especial emphasis needs to be given for training, close supervision and support for CHW to achieve job satisfaction and sustainability [34].
Despite considerable advocacy for increased collaboration and integration of TB and HIV care, few models of integration have been implemented, evaluated and reported [11,17,20,28,29]. However, existing evidence favors integrating TB and HIV care for improving active case finding and early diagnosis of TB, which in turn, reduces the risk of TB transmission [28]. TB/HIV co-infected patients receiving concurrent antiretroviral and TB therapy achieved high levels of adherence and excellent TB and HIV outcomes [28]. Integrated provision of TB, HIV and prevention of mother to child transmission (PMTCT) services at community level through CHW is feasible, acceptable and successful [28,29]. Training CHW to provide a comprehensive package of TB ⁄HIV⁄PMTCT prevention, case finding and treatment support services can bridge the current gaps in service delivery through vertical TB, HIV and PMTCT programs. Evidence also suggests that DOTS strategy can be successfully implemented at primary health care clinics [31]. However such integrations should follow careful planning and caution with greater investments in developing and implementing infection control and laboratory infrastructure [28].
Key components reported for a successful community delivery strategy to prevent and treat TB included a preexisting TB DOTS infrastructure, patient treatment literacy training, and adherence support from trained CHW and family members [21]. Involvement of non-governmental organization (NGO) has also been reported as an essential component of TB programs [22]. Formation of community groups also have reported to contribute towards improved awareness and knowledge about TB and treatment adherence. Community groups help bridge gaps between health system and community through support and coordination. Multi-sectoral community mobilization events that engage community leaders is also one of the enabling tools for successful community based programs in TB⁄HIV⁄PMTCT care [28,29]. Engagement of successfully treated patients can also assist in reducing community stigma and discrimination [22]. Other strategies for organizing, coordinating and managing health care include continuous education and direct supervision of health providers; establishment of goals and regular monitoring of process and result indicators; and incentives for effective use of recommended guidelines [21].
One of the major reported barriers in the success of TB programs is non-adherence mainly due to the lack of support. The intensity of support for patients is reported to diminish in the continuation phase of treatment [11]. Lack of incentives, difficult treatment access, poor communication between health providers and patients, poor application of DOTS, lack of active search for missing patients, and limitations of supervision in treatment units are recognized barriers to treatment success [21]. In addition, the presence of multiple cadres of CHW providing TB and HIV services in silos has hindered the enhancement of collaborative TB⁄HIV activities in community, as well as their supervision [28,29]. Inconsistency in the supply of commodities such as test kits need to be resolved to increase uptake of HIV testing and counseling [28,29].
Discussion
Our review findings suggest that CBI are effective in TB detection and treatment but its role in preventing TB cases has not been comprehensively evaluated. Community based delivery of DOTS may be more feasible and effective for TB case detection and treatment as community workers are familiar with the layout of community and have community member’s trust which healthcare officials would have to develop. Moreover, a community-based approach helps empower each community to deal with its own problems and also provides patient with a greater degree of autonomy and satisfaction with the treatment regime [51]. This involvement of respected, responsible and resourceful community and family members increases the trust that is required to initiate treatment and provides close supervision thereby maximizing adherence which is crucial in such a lengthy treatment regime. Limited coverage of public health services has continued to impede accelerated access to TB control services due to inadequate health service infrastructure, insufficient decentralization of services and inadequate human, material and financial resources. Hence, community delivery platforms offer improved access and equitable distribution of care.
High incidence of TB and its significant financial burden makes it imperative to find a plausible strategy to cope with this disease. The fact that it effects lower socio-economic groups further compounds the problem. Gender inequality, social stigma, and poverty are also recognized as important barriers for successful TB prevention and control programs [52-55]. In light of the above situation, DOTS provides a successful and cost-effective strategy to deal with the burden of TB [27,30,41]. CBI coupled with DOTS seems to be an effective approach as they have the potential to maximize the outreach and minimize the cost. Community based TB control also offers many lessons for the control of HIV epidemic. With the emergence of HIV and consequent TB resurgence, a comprehensive and equitable strategy is needed to stem the worsening double burden of these two infections in poor countries [56].
The WHO currently advocates home-based care and integrated management of dually infected TB/AIDS patients [57]. It recommends a 12 point package of collaborative TB/HIV activities based on creating a mechanism of collaboration between TB and HIV programs, reducing the burden of TB among people living with HIV and reducing the burden of HIV among TB patients. CHW delivering DOTS can be further trained to carry out this additional task and studies are needed to evaluate the feasibility, relative effectiveness and cost effectiveness of this approach [23,58]. However, such integration would involve CHW training and time; improved collaboration between community and facility; and strengthening referral services [59,60].
Conclusion
Well-designed operational research is needed to pragmatically evaluate various models of community based delivery. There is a need to evaluate and address context specific barriers to community based implementation, especially for collaborative TB⁄HIV activities in the community to avoid duplication of labor and resources. Future studies should focus on evaluating novel community delivery models for their success in larger and more diverse populations and impact TB prevention and active case detection.
Abbreviations
CBI: Community based interventions; CHW: Community health workers; CI: Confidence interval; DOTS: Direct observed therapy; HIV: Human immunodeficiency virus; MDR-TB: Multi drug resistant tuberculosis; NGO: Non-governmental organization; PMTCT: Prevention of mother-to-child transmission; RCT: Randomized controlled trial; RR: Relative risk; SMD: Standard mean difference; TB: Tuberculosis; WHO: World Health organization.
Competing interest
The authors declare that they have no financial or non-financial competing interests.
Authors’ contribution
ZAB was responsible for designing and coordinating the review. AA and IN were responsible for: data collection, screening the search results, screening retrieved papers against inclusion criteria, appraising quality of papers and abstracting data. RAS, JKD and ZSL were responsible for data interpretation and writing the review. ZAB critically reviewed and modified the manuscript. All authors read and approved the final manuscript.
Supplementary Material
Multilingual abstracts in the six official working languages of the United Nations.
Contributor Information
Ahmed Arshad, Email: ahmedarshd@gmail.com.
Rehana A Salam, Email: rehana.salam@aku.edu.
Zohra S Lassi, Email: zohra.lassi@aku.edu.
Jai K Das, Email: jai.das@aku.edu.
Imama Naqvi, Email: imama.naqvi@gmail.com.
Zulfiqar A Bhutta, Email: zulfiqar.bhutta@aku.edu.
Acknowledgements
The collection of scoping reviews in this special issue of Infectious Diseases of Poverty was commissioned by the UNICEF/UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases (TDR) in the context of a Contribution Agreement with the European Union for “Promoting research for improved community access to health interventions in Africa”.
References
- World Health Organization. Global Tuberculosis Report. 2013. Availabel at http://apps.who.int/iris/bitstream/10665/91355/1/9789241564656_eng.pdf?ua=1.
- WHO. Global Tuberculosis Control: Epidemiology, Strategy, Financing: WHO, 2009. Geneva, Switzerland: WHO; 2009. [Google Scholar]
- Rose AM, Watson JM, Graham C, Nunn AJ, Drobniewski F, Ormerod LP, Darbyshire JH, Leese J. Tuberculosis at the end of the 20th century in England and Wales: results of a national survey in 1998. Thorax. 2001;56(3):173–179. doi: 10.1136/thorax.56.3.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluger NW, Burzynski J. Tuberculosis and HIV infection: epidemiology, immunology, and treatment. HIV Clin Trials. 2001;2(4):356–365. doi: 10.1310/TUNH-UAKU-N0E4-1PXF. [DOI] [PubMed] [Google Scholar]
- WHO. WHO. Geneva: World Health Organization; 2003. Community contribution to TB care: practice and policy. [Google Scholar]
- Bhutta ZA, Sommerfeld J, Lassi ZS, Salam RA, Das JK. Global burden, distribution and interventions for the infectious diseases of poverty. Infect Dis Pov. 2014;3:21. doi: 10.1186/2049-9957-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood R. The case for integrating tuberculosis and HIV treatment services in South Africa. J Infect Dis. 2007;196(Suppl 3):S497–S499. doi: 10.1086/521118. [DOI] [PubMed] [Google Scholar]
- Lassi ZS, Salam RA, Das JK, Bhutta ZA. Conceptual framework and assessment methodology for the systematic review on community based interventions for the prevention and control of IDoP. Infect Dis Pov. 2014;3:22. doi: 10.1186/2049-9957-3-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. [updated March 2011]. The Cochrane Collaboration, 2011. Available from [ http://www.cochrane-handbook.org]
- Amo-Adjei J, Awusabo-Asare K. Reflections on tuberculosis diagnosis and treatment outcomes in Ghana. Arch Public Health. 2013;71(1):22. doi: 10.1186/2049-3258-71-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins S, Lewin S, Jordaan E, Thorson A. Lay health worker-supported tuberculosis treatment adherence in South Africa: an interrupted time-series study. Int J Tuberc Lung Dis. 2011;15(1):84–89. i. [PubMed] [Google Scholar]
- Barker RD, Millard FJ, Nthangeni ME. Unpaid community volunteers–effective providers of directly observed therapy (DOT) in rural South Africa. S Afr Med J. 2002;92(4):291–294. [PubMed] [Google Scholar]
- Brust JC, Shah NS, Scott M, Chaiyachati K, Lygizos M, van der Merwe TL, Bamber S, Radebe Z, Loveday M, Moll AP, Margot B, Lalloo UG, Friedland GH, Gandhi NR. Integrated, home-based treatment for MDR-TB and HIV in rural South Africa: an alternate model of care. Int J Tuberc Lung Dis. 2012;16(8):998–1004. doi: 10.5588/ijtld.11.0713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantalice Filho JP. Food baskets given to tuberculosis patients at a primary health care clinic in the city of Duque de Caxias, Brazil: effect on treatment outcomes. J Bras Pneumol. 2009;35(10):992–997. doi: 10.1590/s1806-37132009001000008. [DOI] [PubMed] [Google Scholar]
- Clarke M, Dick J, Zwarenstein M, Lombard CJ, Diwan VK. Lay health worker intervention with choice of DOT superior to standard TB care for farm dwellers in South Africa: a cluster randomised control trial. Int J Tuberc Lung Dis. 2005;9(6):673–679. [PubMed] [Google Scholar]
- Colvin M, Gumede L, Grimwade K, Maher D, Wilkinson D. Contribution of traditional healers to a rural tuberculosis control programme in Hlabisa, South Africa. Int J Tuberc Lung Dis. 2003;7(9 Suppl 1):S86–S91. [PubMed] [Google Scholar]
- Corbett EL, Bandason T, Duong T, Dauya E, Makamure B, Churchyard GJ, Williams BG, Munyati SS, Butterworth AE, Mason PR, Mungofa S, Hayes RJ. Comparison of two active case-finding strategies for community-based diagnosis of symptomatic smear-positive tuberculosis and control of infectious tuberculosis in Harare, Zimbabwe (DETECTB): a cluster-randomised trial. Lancet. 2010;376(9748):1244–1253. doi: 10.1016/S0140-6736(10)61425-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diez E, Claveria J, Serra T, Cayla JA, Jansa JM, Pedro R, Villalbi JR. Evaluation of a social health intervention among homeless tuberculosis patients. Tuber Lung Dis. 1996;77(5):420–424. doi: 10.1016/s0962-8479(96)90114-8. [DOI] [PubMed] [Google Scholar]
- Dudley L, Azevedo V, Grant R, Schoeman JH, Dikweni L, Maher D. Evaluation of community contribution to tuberculosis control in Cape Town, South Africa. Int J Tuberc Lung Dis. 2003;7(9 Suppl 1):S48–S55. [PubMed] [Google Scholar]
- Fairall LR, Zwarenstein M, Bateman ED, Bachmann M, Lombard C, Majara BP, Joubert G, English RG, Bheekie A, van Rensburg D, Mayers P, Peters AC, Chapman RD. Effect of educational outreach to nurses on tuberculosis case detection and primary care of respiratory illness: pragmatic cluster randomised controlled trial. BMJ. 2005;331(7519):750–754. doi: 10.1136/bmj.331.7519.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira V, Brito C, Portela M, Escosteguy C, Lima S. DOTS in primary care units in the city of Rio de Janeiro. Southeastern Brazil Rev Saude Publica. 2011;45(1):40–48. doi: 10.1590/s0034-89102010005000055. [DOI] [PubMed] [Google Scholar]
- Kamineni VV, Turk T, Wilson N, Satyanarayana S, Chauhan LS. A rapid assessment and response approach to review and enhance advocacy, communication and social mobilisation for tuberculosis control in Odisha state. India BMC Public Health. 2011;11:463. doi: 10.1186/1471-2458-11-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kironde S, Kahirimbanyi M. Community participation in primary health care (PHC) programmes: lessons from tuberculosis treatment delivery in South Africa. Afr Health Sci. 2002;2(1):16–23. [PMC free article] [PubMed] [Google Scholar]
- Mafigiri DK, McGrath JW, Whalen CC. Task shifting for tuberculosis control: a qualitative study of community-based directly observed therapy in urban Uganda. Glob Public Health. 2012;7(3):270–284. doi: 10.1080/17441692.2011.552067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miti S, Mfungwe V, Reijer P, Maher D. Integration of tuberculosis treatment in a community-based home care programme for persons living with HIV/AIDS in Ndola. Zambia Int J Tuberc Lung Dis. 2003;7(9 Suppl 1):S92–S98. [PubMed] [Google Scholar]
- Niazi AD, Al-Delaimi AM. Impact of community participation on treatment outcomes and compliance of DOTS patients in Iraq. East Mediterr Health J. 2003;9(4):709–717. [PubMed] [Google Scholar]
- Prado TN, Wada N, Guidoni LM, Golub JE, Dietze R, Maciel EL. Cost-effectiveness of community health worker versus home-based guardians for directly observed treatment of tuberculosis in Vitoria, Espirito Santo State. Brazil Cad Saude Publica. 2011;27(5):944–952. doi: 10.1590/s0102-311x2011000500012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uwimana J, Zarowsky C, Hausler H, Jackson D. Training community care workers to provide comprehensive TB/HIV/PMTCT integrated care in KwaZulu-Natal: lessons learnt. Trop Med Int Health. 2012;17(4):488–496. doi: 10.1111/j.1365-3156.2011.02951.x. [DOI] [PubMed] [Google Scholar]
- Uwimana J, Zarowsky C, Hausler H, Swanevelder S, Tabana H, Jackson D. Community-based intervention to enhance provision of integrated TB-HIV and PMTCT services in South Africa. Int J Tuberc Lung Dis. 2013;17(10 Suppl 1):48–55. doi: 10.5588/ijtld.13.0173. [DOI] [PubMed] [Google Scholar]
- Vassall A, Bagdadi S, Bashour H, Zaher H, Maaren PV. Cost-effectiveness of different treatment strategies for tuberculosis in Egypt and Syria. Int J Tuberc Lung Dis. 2002;6(12):1083–1090. [PubMed] [Google Scholar]
- Vieira AA, Ribeiro SA. Compliance with tuberculosis treatment after the implementation of the directly observed treatment, short-course strategy in the city of Carapicuiba. Brazil J Bras Pneumol. 2011;37(2):223–231. doi: 10.1590/s1806-37132011000200013. [DOI] [PubMed] [Google Scholar]
- Weis SE, Slocum PC, Blais FX, King B, Nunn M, Matney GB, Gomez E, Foresman BH. The effect of directly observed therapy on the rates of drug resistance and relapse in tuberculosis. N Engl J Med. 1994;330(17):1179–1184. doi: 10.1056/NEJM199404283301702. [DOI] [PubMed] [Google Scholar]
- White MC, Tulsky JP, Goldenson J, Portillo CJ, Kawamura M, Menendez E. Randomized controlled trial of interventions to improve follow-up for latent tuberculosis infection after release from jail. Arch Intern Med. 2002;162(9):1044–1050. doi: 10.1001/archinte.162.9.1044. [DOI] [PubMed] [Google Scholar]
- Yassin MA, Datiko DG, Tulloch O, Markos P, Aschalew M, Shargie EB, Dangisso MH, Komatsu R, Sahu S, Blok L, Cuevas LE, Theobald S. Innovative community-based approaches doubled tuberculosis case notification and improve treatment outcome in Southern Ethiopia. PLoS One. 2013;8(5):e63174. doi: 10.1371/journal.pone.0063174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwarenstein M, Schoeman JH, Vundule C, Lombard CJ, Tatley M. A randomised controlled trial of lay health workers as direct observers for treatment of tuberculosis. Int J Tuberc Lung Dis. 2000;4(6):550–554. [PubMed] [Google Scholar]
- Group C. Community-directed interventions for priority health problems in Africa: results of a multicountry study. Bull World Health Organ. 2010;88(7):509–518. doi: 10.2471/BLT.09.069203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaisson RE, Barnes GL, Hackman J, Watkinson L, Kimbrough L, Metha S, Cavalcante S, Moore RD. A randomized, controlled trial of interventions to improve adherence to isoniazid therapy to prevent tuberculosis in injection drug users. Am J Med. 2001;110(8):610–615. doi: 10.1016/s0002-9343(01)00695-7. [DOI] [PubMed] [Google Scholar]
- Gandhi NR, Moll AP, Lalloo U, Pawinski R, Zeller K, Moodley P, Meyer E, Friedland G. Successful integration of tuberculosis and HIV treatment in rural South Africa: the Sizonq'oba study. J Acquir Immune Defic Syndr. 2009;50(1):37–43. doi: 10.1097/QAI.0b013e31818ce6c4. [DOI] [PubMed] [Google Scholar]
- Heal G, Elwood RK, FitzGerald JM. Acceptance and safety of directly observed versus self-administered isoniazid preventive therapy in aboriginal peoples in British Columbia. Int J Tuberc Lung Dis. 1998;2(12):979–983. [PubMed] [Google Scholar]
- Kamolratanakul P, Sawert H, Lertmaharit S, Kasetjaroen Y, Akksilp S, Tulaporn C, Punnachest K, Na-Songkhla S, Payanandana V. Randomized controlled trial of directly observed treatment (DOT) for patients with pulmonary tuberculosis in Thailand. Trans R Soc Trop Med Hyg. 1999;93(5):552–557. doi: 10.1016/s0035-9203(99)90379-6. [DOI] [PubMed] [Google Scholar]
- Khan MA, Walley JD, Witter SN, Imran A, Safdar N. Costs and cost-effectiveness of different DOT strategies for the treatment of tuberculosis in Pakistan. Directly Observed Treatment. Health Policy Plan. 2002;17(2):178–186. doi: 10.1093/heapol/17.2.178. [DOI] [PubMed] [Google Scholar]
- Lwilla F, Schellenberg D, Masanja H, Acosta C, Galindo C, Aponte J, Egwaga S, Njako B, Ascaso C, Tanner M, Alonso P. Evaluation of efficacy of community-based vs. institutional-based direct observed short-course treatment for the control of tuberculosis in Kilombero district, Tanzania. Trop Med Int Health. 2003;8(3):204–210. doi: 10.1046/j.1365-3156.2003.00999.x. [DOI] [PubMed] [Google Scholar]
- MacIntyre CR, Goebel K, Brown GV, Skull S, Starr M, Fullinfaw RO. A randomised controlled clinical trial of the efficacy of family-based direct observation of anti-tuberculosis treatment in an urban, developed-country setting. Int J Tuberc Lung Dis. 2003;7(9):848–854. [PubMed] [Google Scholar]
- Malotte CK, Hollingshead JR, Larro M. Incentives vs outreach workers for latent tuberculosis treatment in drug users. Am J Prev Med. 2001;20(2):103–107. doi: 10.1016/s0749-3797(00)00283-x. [DOI] [PubMed] [Google Scholar]
- Newell JN, Baral SC, Pande SB, Bam DS, Malla P. Family-member DOTS and community DOTS for tuberculosis control in Nepal: cluster-randomised controlled trial. Lancet. 2006;367(9514):903–909. doi: 10.1016/S0140-6736(06)68380-3. [DOI] [PubMed] [Google Scholar]
- Olle-Goig JE, Alvarez J. Treatment of tuberculosis in a rural area of Haiti: directly observed and non-observed regimens. The experience of H pital Albert Schweitzer. Int J Tuberc Lung Dis. 2001;5(2):137–141. [PubMed] [Google Scholar]
- Walley JD, Khan MA, Newell JN, Khan MH. Effectiveness of the direct observation component of DOTS for tuberculosis: a randomised controlled trial in Pakistan. Lancet. 2001;357(9257):664–669. doi: 10.1016/S0140-6736(00)04129-5. [DOI] [PubMed] [Google Scholar]
- Wandwalo E, Kapalata N, Egwaga S, Morkve O. Effectiveness of community-based directly observed treatment for tuberculosis in an urban setting in Tanzania: a randomised controlled trial. Int J Tuberc Lung Dis. 2004;8(10):1248–1254. [PubMed] [Google Scholar]
- Wright J, Walley J, Philip A, Pushpananthan S, Dlamini E, Newell J, Dlamini S. Direct observation of treatment for tuberculosis: a randomized controlled trial of community health workers versus family members. Trop Med Int Health. 2004;9(5):559–565. doi: 10.1111/j.1365-3156.2004.01230.x. [DOI] [PubMed] [Google Scholar]
- Zwarenstein M, Schoeman JH, Vundule C, Lombard CJ, Tatley M. Randomised controlled trial of self-supervised and directly observed treatment of tuberculosis. Lancet. 1998;352(9137):1340–1343. doi: 10.1016/S0140-6736(98)04022-7. [DOI] [PubMed] [Google Scholar]
- WHO. Community Involvement in Tuberculosis Care and Prevention. Towards Partnerships for Health. 2008. Available at http://whqlibdoc.who.int/publications/2008/9789241596404_eng.pdf. [PubMed]
- Atre SR, Kudale AM, Morankar SN, Rangan SG, Weiss MG. Cultural concepts of tuberculosis and gender among the general population without tuberculosis in rural Maharashtra. India Trop Med Int Health. 2004;9(11):1228–1238. doi: 10.1111/j.1365-3156.2004.01321.x. [DOI] [PubMed] [Google Scholar]
- Long NH, Johansson E, Diwan VK, Winkvist A. Fear and social isolation as consequences of tuberculosis in VietNam: a gender analysis. Health Policy. 2001;58(1):69–81. doi: 10.1016/s0168-8510(01)00143-9. [DOI] [PubMed] [Google Scholar]
- Weiss MG, Somma D, Karim F, Abouihia A, Auer C, Kemp J, Jawahar MS. Cultural epidemiology of TB with reference to gender in Bangladesh, India and Malawi. Int J Tuberc Lung Dis. 2008;12(7):837–847. [PubMed] [Google Scholar]
- Aryal S, Badhu A, Pandey S, Bhandari A, Khatiwoda P, Khatiwada P, Giri A. Stigma related to tuberculosis among patients attending DOTS clinics of Dharan municipality. Kathmandu Univ Med J (KUMJ) 2012;10(37):48–52. doi: 10.3126/kumj.v10i1.6914. [DOI] [PubMed] [Google Scholar]
- Farmer P, Laandre F, Mukherjee J, Gupta R, Tarter L, Kim JY. Community-based treatment of advanced HIV disease: introducing DOT-HAART (directly observed therapy with highly active antiretroviral therapy) Bull World Health Organ. 2001;79(12):1145–1151. [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. WHO Policy on Collaborative TB/HIV Activities: Guidelines for National Programmes and other Stakeholders. Geneva: World Health Organization; 2012. [PubMed] [Google Scholar]
- Kironde S. In: South African Health Review. Ntuli A, Crisp N, Clarke E, Baron P, editor. Durban: Health Systems Trust; 2000. Tuberculosis; pp. 335–349. [Google Scholar]
- Legido-Quigley H, Montgomery CM, Khan P, Atun R, Fakoya A, Getahun H, Grant AD. Integrating tuberculosis and HIV services in low and middle-income countries: a systematic review. Trop Med Int Health. 2013;18(2):199–211. doi: 10.1111/tmi.12029. [DOI] [PubMed] [Google Scholar]
- Howard AA, El-Sadr WM. Integration of tuberculosis and HIV services in sub-Saharan Africa: lessons learned. Clin Infect Dis. 2010;50(Supplement 3):S238–S244. doi: 10.1086/651497. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Multilingual abstracts in the six official working languages of the United Nations.


