Abstract
Purpose
The present study evaluated the effects of inherent envelope modulation and the availability of cues across frequency on behavioral gap detection with noise-band stimuli in school-age children.
Methods
Listeners were normal-hearing adults and 5.2- to 15.6-year-olds. Stimuli were continuous bands of noise centered on 2000 Hz, either 1000 or 25 Hz wide. In addition to Gaussian noise at these bandwidths, there were conditions using 25-Hz-wide noise bands modified to either accentuate or minimize inherent envelope modulation (staccato and low-fluctuation noise, respectively).
Results
Within the 25-Hz-wide conditions, adults’ gap detection thresholds were highest in the staccato, lower in the Gaussian, and lowest in the low-fluctuation noise. Similar trends were evident in children’s thresholds, although inherent envelope modulation had a smaller effect on children than adults. Whereas adults’ thresholds were comparable for the 1000-Hz-wide Gaussian and 25-Hz-wide low-fluctuation stimulus, children’s performance converged on adults’ at a younger age for the 1000-Hz-wide Gaussian stimulus.
Conclusions
Results are consistent with the idea that children are less susceptible to the disruptive effects of inherent envelope modulation than adults when detecting a gap in a narrowband noise. Further, the ability to use spectrally distributed gap detection cues appears to mature relatively early in childhood.
I. INTRODUCTION
Deficits in temporal processing have been implicated in the failure of some children to develop normal language skills (Basu, Krishnan, & Weber-Fox, 2010; Tallal, Stark, & Mellits, 1985). Poor temporal resolution could delay linguistic development by limiting access to important acoustic features of speech, such as the temporal cues underlying the discrimination of some consonants (Phillips, 1999; Tyler, Summerfield, Wood, & Fernandes, 1982). Understanding the maturation of temporal processing in typically developing children is critical to evaluating these abilities in special populations. Despite its importance, there is relatively little consensus on the question of when in development temporal processing begins to resemble that of adults. While there are a number of paradigms used to characterize temporal resolution (reviewed by Buss, He, Grose, & Hall, 2013), one of the most widely adopted methods is gap detection. Using the gap detection paradigm, some studies have found adult-like performance by 5 to 6 years of age (Trehub, Schneider, & Henderson, 1995; Wightman, Allen, Dolan, Kistler, & Jamieson, 1989), whereas others indicate that maturation extends into later childhood (Davis & McCroskey, 1980; Irwin, Ball, Kay, Stillman, & Rosser, 1985) or early adolescence (Fischer & Hartnegg, 2004).
Electrophysiological studies of gap detection indicate mature encoding of temporal information relatively early in infancy, despite poor behavioral thresholds (Trainor, Samuel, Desjardins, & Sonnadara, 2001; Werner, Folsom, Mancl, & Syapin, 2001). For example, Werner et al. (2001) measured gap detection thresholds in a broadband noise based on either auditory brainstem response (ABR) or behavioral responses. In that study, behavioral thresholds of 3-month-olds were an order of magnitude higher than those of adults, but the ABR thresholds for detecting the gap did not differ between age groups. Similarly, Trainor et al. (2001) used mismatch negativity to characterize gap detection for Gaussian-modulated 2000-Hz tone-pips. Data from 6- to 7-month-olds indicated sensitivity to gaps as short as 4 ms, similar to adult data. These findings of mature electrophysiological responses in infancy implicate high-level aspects of auditory processing in the relatively prolonged maturation of behavioral gap detection in children.
If the temporal features of gapped stimuli are well represented in the auditory system in infancy, the question then becomes why young children are poor at using this information psychophysically. One possibility, considered by Wightman et al. (1989), is that relatively high rates of inattention could be responsible for poor gap detection in children. That study measured behavioral gap detection in 3- to 7-year-olds using half-octave bands of noise centered on 400 or 2000 Hz, and demonstrated that children’s thresholds converged on adults’ at around 6 years of age. The poor performance of younger children in that study was modeled in terms of transient lapses in attention, assuming access to an adult-like temporal cue on non-lapse trials and random guessing on lapse trails. Using this ‘all or none’ model of inattention, data of 3-year-olds were consistent with lapses on approximately 50% of trials. Wightman et al. (1989) argued that this lapse rate was likely to be unrealistically high, and while they did not rule out an effect of inattention in the maturation of gap detection, they concluded that inattention alone was unlikely to account for the age effects observed. The present study addressed the possibility that stimulus factors could be important in the maturation of behavioral gap detection, with a focus on stimulus fluctuation and the availability of cues in multiple frequency channels.
In adults, sensitivity to the introduction of a gap in a band of noise improves as the bandwidth of the noise increases (Eddins, Hall, & Grose, 1992; Fitzgibbons, 1983; Shailer & Moore, 1983). There are two factors thought to contribute to this bandwidth effect. First, increasing the stimulus bandwidth increases the equivalent rate of inherent envelope modulation, reducing the perceptual similarity between the inherent modulation of the envelope and a temporal gap (Glasberg & Moore, 1992; Grose, Buss, & Hall, 2008). Higher rates of inherent envelope modulation could also be associated with reductions in the effective modulation depth of the stimulus as it is represented in the auditory system, due to limitations in temporal resolution (Viemeister, 1979), or to modulation masking (Bacon & Grantham, 1989; Dau, Verhey, & Kohlrausch, 1999; Ewert & Dau, 2004). Beneficial effects of reduced modulation depth have been demonstrated with low-fluctuation noise bands, characterized by relatively flat envelopes. In adults, low-fluctuation noise supports better gap detection than narrow bands of Gaussian noise (Grose, et al., 2008). Second, increasing the noise bandwidth beyond the critical band introduces an opportunity to combine cues to the presence of the gap across auditory channels (Eddins, et al., 1992; Grose, 1991; Grose & Hall, 1988). In one demonstration of this effect, Grose (1991) showed that gap detection thresholds are better for a set of five widely dispersed 20-Hz wide bands of noise than for the same set of bands presented contiguously in frequency. While gap detection effects related to inherent envelope modulation and the availability of cues in multiple auditory channels have been demonstrated in adults, very little is known about these effects in young children.
The detection of a temporal gap requires the listener to monitor stimulus intensity over time and identify a reduction in that intensity. It has been suggested that the limiting factor in children’s gap detection performance may be related to the processing of intensity (Smith, Trainor, & Shore, 2006), which is known to be immature in school-age children (e.g., Buss, Hall, & Grose, 2013). One way that children and adults differ is in terms of the disruptive effects of stimulus level jitter on intensity discrimination. Buss et al. (2006) showed that jittering stimulus intensity in an intensity discrimination task has a smaller effect on thresholds of young children than adults, a result which may be interpreted in terms of internal noise: if performance is limited by the combination of internal (listener-based) and external (stimulus-based) noise, then listeners with high levels of internal noise should be less susceptible to the disruptive effects of external noise. In the data of Buss et al. (2006), this would imply that intensity jitter had a smaller effect on intensity discrimination in young children because those listeners had higher levels of internal noise. Applying this logic to gap detection, if children’s internal representation of the stimulus envelope is less precise than adults’ (a form of internal noise), this should result in a more modest detrimental effect of inherent envelope modulation (a form of external noise) when compared to adults.
Whereas arguments based on greater internal noise in children support the prediction that inherent envelope modulation should impact children’s gap detection less than that of adults, other data support the prediction of greater effects of inherent envelope modulation in children. It is thought that inherent envelope modulation disrupts adults’ gap detection by introducing envelope features that resemble a gap, an effect that could be considered a form of informational masking (Grose, et al., 2008). Children have been shown to be more susceptible than adults to informational masking in many paradigms (Leibold & Neff, 2007; Oh, Wightman, & Lutfi, 2001; Wightman, Kistler, & O'Bryan, 2010), so it is reasonable to expect inherent envelope modulation to have a particularly detrimental effect on gap detection in young listeners for the same reason. Several studies have cited increased susceptibility to confusion between inherent envelope modulation and temporal gaps as a rationale for avoiding the use of narrowband noise stimuli in assessing gap detection in infants and children (Trehub, et al., 1995; Werner, Marean, Halpin, Spetner, & Gillenwater, 1992). Thus, whereas previous informational masking data suggest that gap detection in children might be particularly subject to deleterious effects related to inherent envelope modulation, previous developmental effects on level rove in intensity discrimination suggest the opposite. The present study was designed to resolve these differences in expectation.
Like the effects of stimulus fluctuation, data pertinent to children’s ability to use gap detection cues that are distributed across auditory channels are also mixed. Grose et al. (1993) measured children’s ability to detect a 400-ms pure tone in a steady or 10-Hz square-wave amplitude-modulated noise masker, with masker bandwidths of either 76 or 240 Hz. Young children obtained a smaller benefit from masker modulation than adults, particularly for the 76-Hz bandwidth. Grose et al. (1993) hypothesized that the narrow masker bandwidth could be associated with higher processing demands than the wide masker bandwidth due to the absence of across-channel masker envelope cues, which can help differentiate the signal from the masker (Hall, Haggard, & Fernandes, 1984; Moore & Glasberg, 1982; Puleo & Pastore, 1980). This result suggests that young children are relatively adept at making use of temporal cues distributed across auditory channels. This is not entirely consistent, however, with recent data obtained for a brief tonal signal presented at different temporal positions relative to the square-wave masker modulation (Buss, He, et al., 2013). For brief tone detection, 5- to 10-year-olds appeared to be less able to benefit from an increased masking bandwidth than adults. It is unclear how the ability to utilize cues distributed across auditory channels in tone detection relates to that ability in gap detection. One important difference between paradigms is that detection of an added signal may rely on segregation of the signal and masker, whereas detection of a gap could be based on information that has been integrated across frequency.
Existing data on gap detection in children are qualitatively consistent with the hypothesis that stimulus bandwidth affects the age at which gap detection thresholds reach adult-like durations. Whereas Wightman et al. (1989) demonstrated adult-like thresholds by 6 years of age for a half-octave band of noise at 400 or 2000 Hz, two early studies of gap detection in children reported adult-like performance by around 10 years of age, one using broadband noise (Irwin, et al., 1985) and the other using pure tones (Davis & McCroskey, 1980). One caveat is that Davis and McCroskey (1980) used rapid (1-ms) onset and offset ramps, introducing splatter; the absence of a frequency effect between 250 and 4000 Hz in these data, however, undermines the possibility that splatter cues played a large role in their results. A more prolonged time-course of development for tonal stimuli than bandpass noise is consistent with the idea that decreasing internal noise as a function of age is apparent when assessed with minimal external noise (e.g., tones), but that this age effect is masked when using stimuli with more pronounced external noise (e.g., bandpass noise). Similarly, a more prolonged time-course of development for wide than narrow stimuli is consistent with greater ability of adults to benefit from cues distributed across auditory channels. One limitation to comparisons of data across these studies is that they used different stimulus parameters and methods. Further, while there are within-subject data for stimuli using different bandwidths, this variable tends to co-vary with center frequency in the studies performed to date (Irwin, et al., 1985; Wightman, et al., 1989). Frequency effects have been observed in the development of a handful of psychoacoustical tasks (Grose, et al., 1993; Trehub, Schneider, Morrongiello, & Thorpe, 1988), including temporal tasks (He, Buss, & Hall, 2010), so it is important to differentiate these factors in understanding potential effects of bandwidth.
The goal of the present study was to better understand bandwidth effects, particularly the possible contributions of inherent envelope modulation depth and the availability of cues in multiple auditory channels in the maturation of gap detection in school-age children. To that end, thresholds were measured for two bandwidths of Gaussian noise: 25 and 1000 Hz. In addition, the depth of inherent envelope modulation of a 25-Hz-wide band of noise was manipulated using an iterative technique to either reduce or accentuate fluctuation. The resulting stimuli are referred to as low-fluctuation and staccato noise, respectively. The procedures used to generate the 25-Hz-wide bands closely resemble those described in Grose et al. (2008); the primary exception was the use of a 2000-Hz center frequency in the present experiment.
II. METHODS
A. Listeners
Potential listeners were screened for normal hearing, defined as pure-tone thresholds at or below 20 dB HL at octave frequencies between 250 and 8000 Hz (ANSI, 2010). Exclusionary criteria included daily use of aspirin, a history of chronic ear disease or a history of speech, language, or learning disorders, by self or parental report. There were 12 adult listeners, 18.5 to 28.8 years of age, with a mean age of 23.0 years. There were 34 child listeners, 5.2 to 15.6 years of age, with an approximately uniform distribution on the logarithm of age. This range and distribution of child ages was intended to span the range previously associated with the maturation of gap detection, with reduced representation at the higher ages due to the expectation of decelerating maturation. Four children began but did not complete the study, one due to scheduling constraints and three due to an inability to master the task (5.7, 6.0 and 8.8 yrs).
B. Stimuli
The stimuli were bands of noise centered on 2000 Hz that played continuously at 70 dB SPL, except for introduction of the temporal gap. In two conditions these bands were Gaussian noise, either 25 or 1000 Hz wide. Two additional 25-Hz-bandwidth conditions differed with respect to the temporal envelope of the noise band, which was described as either low-fluctuation or staccato noise. Stimuli in each of four conditions were generated prior to the experiment and saved to disk. Each stimulus was based on a broadband noise sample, transformed to the frequency domain, restricted in frequency by setting the magnitude of components outside the passband to zero, and transformed back into the time domain. Methods for generating the low-fluctuation and staccato noise were the same as those used by Grose et al. (2008). In the low-fluctuation noise condition, a 25-Hz wide band of noise was iteratively divided by its Hilbert envelope and restricted to the original bandwidth (Kohlrausch et al., 1997), a process that was repeated eight times. The staccato noise band was generated using a similar iterative technique, but in this case the envelope was multiplied with a target waveform prior to bandpass filtering. This target waveform was an array of zeros and ones, generated based on the temporal envelope of the 25-Hz wide band of Gaussian noise in the first iteration: zeros corresponded to envelope amplitudes at or below 80% of the envelope mean, and ones corresponded to higher amplitudes. Staccato noise samples were generated with 20 iterations. Each stimulus sample consisted of 217 points, which repeated seamlessly once every 10.7 sec when played out at 12207 Hz.
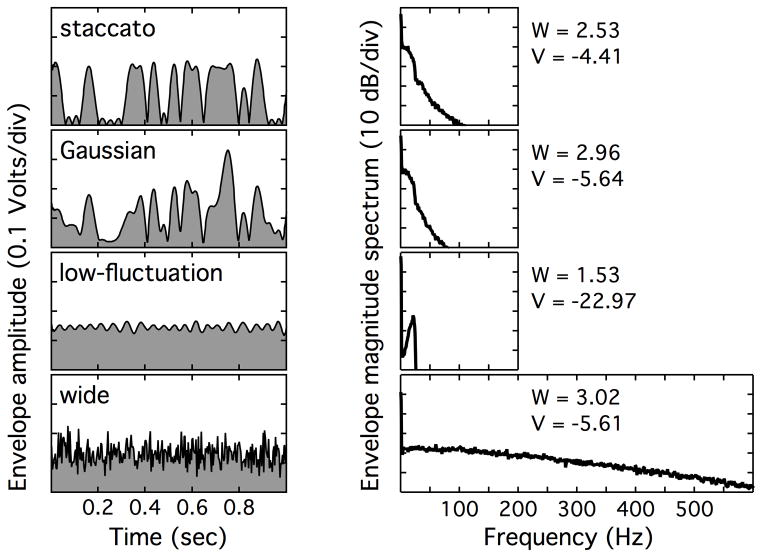
Figure 1 illustrates features of the stimulus envelopes, as they might be represented in the auditory system. To generate this figure, stimuli were passed through a roex filter centered on 2000 Hz (Moore & Glasberg, 1987), and the Hilbert envelope was extracted. The roex filter had no appreciable effect on the envelopes of the 25-Hz-wide stimuli, but it reduced the high-frequency envelope fluctuations of the wide masker. The left column of Figure 1 shows the time-domain envelope of a 1-sec sample for each stimulus, and the right column shows the magnitude spectrum of the envelope of the entire 10.7-sec sample for each stimulus. Following Kohlrausch, et al. (1997), modulation depth of these stimuli was quantified in two ways: the stimulus fourth moment (W), and the ratio of the envelope standard deviation to its mean, in dB (V). In both cases, lower values reflect a flatter envelope. Values of W and V associated with each stimulus appear at the far right of Figure 1.
Figure 1.
Illustrations of the inherent envelope modulation characteristics of the four stimulus conditions at the output of a roex filter centered on 2000 Hz. The left column of panels shows the envelopes of 1-sec samples, plotted as a function of time. The right column of panels shows the envelope magnitude spectra of the full 10.7-sec samples. Values of W (fourth moment) and V (ratio of std and mean, in dB) appear to the far right of the right column of panels. Stimuli expected to produce the lowest thresholds (wide and low-fluctuation noise) appear in the bottom rows, whereas those expected to produce higher thresholds (Gaussian and staccato noise) appear in the top rows.
This figure illustrates two important points. First, in the wide stimulus condition the envelope includes more high-frequency energy than the envelope in the 25-Hz-bandwidth conditions. While early work on modulation detection hypothesized that envelope modulation frequencies above 64 Hz might be attenuated (Viemeister, 1979), more recent work suggests that reduced sensitivity to high-frequency envelope modulation is due to a combination of modulation masking and peripheral filtering (Dau, et al., 1999). Second, the two statistics used to characterize modulation depth do not predict the same pattern of gap detection thresholds as observed in adults. Values of both W and V are consistent with lower thresholds in the low-fluctuation noise than either the Gaussian or the staccato noise. However, these statistics make contradictory predictions with respect to gap detection for the Gaussian and staccato stimuli. One reason for the discrepancy is that the fourth moment tends to be dominated by high-amplitude epochs, and the staccato noise envelope tends to have relatively uniform peaks compared to Gaussian noise. This difference in envelope statistics can be seen in the left column of Figure 1, around 0.75 sec, where the staccato sample has a flat plateau and the Gaussian envelope has a sharp peak. Given that gap thresholds tend to be higher in adults for staccato noise than Gaussian noise (Grose, et al., 2008), these statistics (V and W) do not seem to capture all features of the stimuli relevant to the task.
The temporal gap, when present, was introduced using 40-ms raised-cosine ramps for the 25-Hz-bandwidth conditions and 4-ms raised-cosine ramps for the 1000-Hz-bandwidth condition. The longer duration ramp for the narrowband stimulus was used to prevent reliance on spectral as opposed to temporal cues. For both bandwidths, the ramps resemble a half-period of inherent modulation at the output of an auditory filter. Gap duration was defined as the interval between the beginning of the offset and the beginning of the subsequent onset, such that a 0-ms gap is equivalent to a continuous (ungapped) interval. In conditions for which the gap duration was less than the ramp duration, the offset and onset overlapped, and the stimulus amplitude in the center of the gap did not fall to zero.
C. Procedures
Thresholds were measured in a two-down one-up track estimating the duration associated with 71% correct. Gap duration was varied by a factor 1.41 prior to the first two track reversals and by a factor of 1.19 thereafter. The maximum allowable gap duration was 500 ms; whenever the stepping rule called for a value greater than this maximum, a value of 500 ms was substituted, and a ceiling bump was recorded. A track continued for a total of eight reversals, and threshold was the geometric mean of the duration at the last six reversals. Each listener completed the four stimulus conditions in random order, providing three or four estimates in each, dependent on the testing time available. All estimates obtained were included in the geometric means reported below. All listeners were paid an hourly rate for participation.
The task was a three-alternative forced-choice gap detection. Listening intervals were 500 ms, separated by 500-ms inter-stimulus intervals. Each interval was indicated visually on a computer screen, and listeners entered their responses using a mouse or a touchscreen. A cartoon picture was revealed over the course of a threshold estimation track, in the style of a jigsaw puzzle, with one piece revealed following each correct response. The puzzle display remained unchanged following an incorrect response. A progress bar at the top of the screen showed the proportion of desired track reversals obtained. At the end of a track the remaining pieces of the puzzle were revealed, and the resulting image performed a 2-sec animation. Data were collected in a double-walled sound-attenuating booth. The experiment was controlled using a custom script written in MATLAB (MathWorks), which loaded stimuli from disk into a real-time processor (RP2, TDT) and controlled stimulus gating using ActiveX (RPvds, TDT). Stimuli were routed from the real-time processor to a headphone buffer (HB7, TDT) and presented monaurally to the listener’s left ear (Sennheiser, HD 265).
D. Data analyses
All statistical analyses were performed on the logarithm of gap duration. This is a standard approach in studies of gap detection, based on the observation that the perceptual effect of the gap changes proportional to duration in these units. Similarly, the logarithm of age (in years) was used to evaluate maturational effects based on developmental data indicating that maturation appears to proceed more rapidly in the youngest listeners and slows down with progressing age (Mayer & Dobson, 1982; Moller & Rollins, 2002). Imposing a maximum duration of 500 ms in the adaptive track could introduce a bias to underestimate thresholds. Because thresholds were computed based on the gap duration at the last six track reversals, this bias would occur when the 500-ms ceiling was imposed after the second track reversal. To avoid this bias, data from tracks with one or more ceiling bumps after the second reversal were fitted with a logistic function, using the methods described by Wichmann and Hill (2001). The duration associated with 71% correct on that function was then adopted as threshold.
III. RESULTS AND DISCUSSION
Ceiling bumps after the second track reversal occurred in only 1.5% of tracks collected with child listeners. This included tracks for three children in the Gaussian noise condition (ages 6.5, 6.6, and 7.6 yrs) and two different children in the staccato noise condition (5.5 and 5.9 yrs). On average, threshold estimates based on logistic fits were 20% higher than those estimated by the duration-limited stepping rule. No ceiling bumps were observed in adult data.
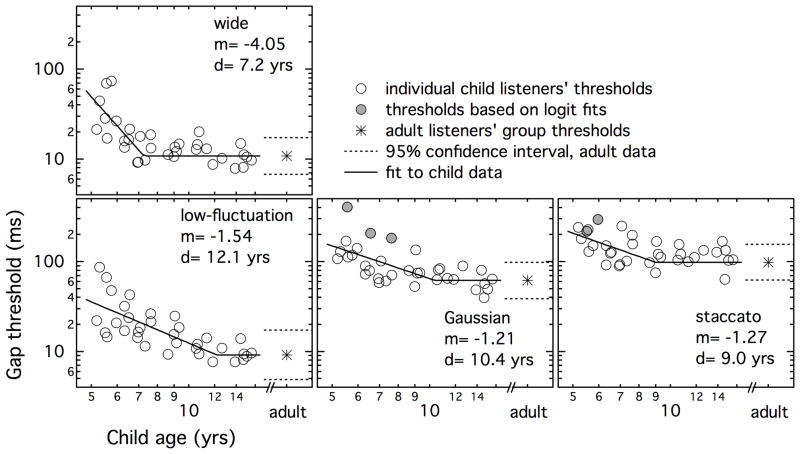
Thresholds for individual children are plotted as circles in Figure 2 as a function of age, with data for each stimulus condition plotted in a separate panel. Points shaded with gray indicate data in which one or more thresholds were based on psychometric function fits, due to ceiling bumps in the original track. The means of adult data are plotted at the far right in each panel, with dotted horizontal lines indicating the 95% confidence interval around each mean. Recall that narrowband stimuli were gated with 40-ms ramps, and the wideband stimulus was gated with 4-ms ramps. Listeners’ thresholds were comparable to or higher than the ramp duration in the Gaussian, staccato and wide stimulus conditions. This was not the case for the low-fluctuation condition, however, where most listeners’ thresholds were lower than 40 ms. In these cases, listeners detected a reduction in stimulus level.
Figure 2.
Gap detection thresholds for individual child listeners, plotted as a function of listener age. Circles show individual child listeners’ thresholds as a function of age, with shading indicating data where one or more threshold estimates were based on a psychometric function fits. Stars indicate the geometric mean of adult thresholds, and dotted lines indicate the 95% confidence interval around those means. Solid lines and the text in each panel indicate fits to child data.
In general, the youngest listeners had the poorest thresholds, whereas thresholds for the oldest children tested resembled those of adults. As expected based on previous data in adults (Grose, et al., 2008), overall performance in the narrowband noise conditions was best for the low-fluctuation noise, intermediate for the Gaussian noise, and worst for the staccato noise; this trend was evident for all age groups. In the youngest and oldest listeners, performance for the wideband noise was similar to that for the low-fluctuation noise. Of particular interest here, there is some indication that these stimulus factors affected listeners’ performance in an age-dependent fashion. For example, 8 year-olds appear to perform at the same level as adults for the wide stimulus but not for the low-fluctuation stimulus. The effects of envelope modulation and stimulus bandwidth as a function of listener age were evaluated in two ways: comparing group data, including adults and four age groups of children, and treating child age as a continuous variable.
A. Group data
Table 1 shows the geometric means for gap detection thresholds in four age groups of child listeners, approximately equally spaced on the logarithm of age in years, and in adults. The first analysis evaluated the effect of stimulus on gap detection thresholds in adults. Thresholds were subjected to a repeated-measures analysis of variance (ANOVA), with four levels of stimulus condition. There was a main effect of condition (F3,33=291.99, p<0.001). Repeated contrasts indicated that thresholds in the wide and low-fluctuation noise conditions were not significantly different (p=0.262). However, for the 25-Hz wide noise stimuli, thresholds were lower for the low-fluctuation than the Gaussian noise (p<0.001), and lower for the Gaussian than the staccato noise (p<0.001). There are two possible explanations for the comparably good thresholds in the wide and low-fluctuation noise conditions. One is that the relatively flat envelope of the low-fluctuation noise and the 1000-Hz bandwidth of the wide noise had comparably beneficial effects with respect to the internal representation of the envelope in the auditory system, removing envelope features that could be mistaken for a gap. Given the comparable frequency resolution of children and adults (Hall & Grose, 1991), these effects would be expected to be similar for children and adults. Another interpretation is that reduced envelope modulation depth was responsible for good performance for the low-fluctuation noise, and the presence of cues in multiple auditory channels was responsible for good performance in the wide noise. If the ability to use these different cues matures at different rates, then the wide and low-fluctuation stimuli should be associated with different thresholds in children.
Table 1.
Gap detection thresholds by age group. Bold text indicates child thresholds that are significantly higher than the associated adult thresholds.
| age group | Group geometric mean thresholds (ms)
|
||||
|---|---|---|---|---|---|
| (yrs) | n | wide | low- fluctuation | Gaussian | staccato |
| <6.5 | 9 | 28.8 ** | 29.4 ** | 131.4 ** | 177.3 ** |
| 6.5 – 8.5 | 8 | 13.8 | 20.3 ** | 91.7 * | 133.9 * |
| 8.5 – 11.0 | 8 | 13.5 | 13.4 * | 78.0 | 116.1 |
| >11.0 | 9 | 10.3 | 9.8 | 60.2 | 112.6 |
|
| |||||
| adult | 12 | 10.9 | 9.2 | 61.8 | 98.5 |
higher than adult thresholds at p = 0.001 (one tailed)
higher than adult thresholds at p = 0.05 (one tailed)
In all conditions, thresholds for the youngest children (5.2- to 6.5-year-olds) were higher than adults’, and those for the oldest children (11.0- to 15.6-year-olds) were very similar to adults’. Results for the intermediate age groups appeared to differ between stimulus conditions, however. A repeated-measures ANOVA was performed to evaluate the significance of these observations, with four levels of stimulus and five levels of age group. As in the data of adults, there was a main effect of stimulus condition (F3,123=841.15, p<0.001). There was also a main effect of listener age group (F4,41=15.51, p<0.001) and an interaction between condition and group (F12,123=2.72, p=0.003). Simple contrasts using low-fluctuation noise as the reference indicated a condition-by-group interaction with the wide noise (F4,41=2.65, p=0.047) and the staccato noise (F4,41=3.69, p=0.012) conditions; there was a non-significant trend for a condition- by-group interaction with the low-fluctuation compared to the Gaussian stimulus conditions (F4,41=2.13, p=0.094). Greater insight into the interaction between age and condition was gained with simple effects testing, using Fisher's Least Significant Difference (LSD) test and one-tailed significance criteria, following the prediction of lower thresholds in adults than children. Group thresholds that differed significantly from adults’ are indicated with bold formatting in Table 1. These results are consistent with the conclusion that the time-course of maturation is more protracted for the low-fluctuation noise stimulus than for the other three stimuli.
If just one stimulus had been selected for assessing temporal resolution, the conclusion regarding when children attain adult-like performance would depend heavily on which stimulus was used. Results with the wide masker indicate adult-like performance by approximately 6.5 years of age, whereas the low-fluctuation stimulus indicates continued improvement out to approximately 11 years of age. Results for the Gaussian and staccato stimuli are intermediate, consistent with adult-like performance at approximately 8.5 years of age. The similar developmental trajectory in Gaussian and staccato noise likely reflects the relatively modest difference between gap detection in these conditions, as observed in adult thresholds. The different results for wide and low-fluctuation stimuli are consistent with a difference in the time-course of maturation in this task. However, interpretation of age effects across other stimulus pairs is somewhat problematic due to differences in the range of thresholds across age. For example, the interaction between age and stimulus condition for the low-fluctuation/staccato noise pair could be due either to more prolonged maturation for the low-fluctuation stimulus, or to a greater magnitude of change in threshold (on log units) as a function of age in the low-fluctuation than the staccato stimulus. Fits to individual data were undertaken to differentiate between range effects and differences in the time-course of maturation.
B. Child age as a continuous variable
While the group analyses reported above indicate different age effects in the different stimulus conditions, it is not clear how these results are impacted by differences in the range of performance across groups in each condition. For example, comparing 5.0- to 6.5-year-olds to adults, thresholds differ by a factor 3.2 for the low-fluctuation noise and by a factor of 1.8 for the staccato noise. While this is consistent with the idea that children are less affected by prominent inherent envelope modulation, it is still of interest to determine the point at which children’s performance is adult-like. Age effects were therefore evaluated in greater detail by fitting the child data in each condition with a ‘broken stick’ model, treating age as a continuous variable. This approach assumes linear improvement in thresholds as a function of age (in log units), up to the point where thresholds converge on those of adults. The model was defined as:
where y is child threshold, a is the mean of adult thresholds, x is child age, d is the age at which child thresholds converge on the adult mean (a) , and m is the slope of the line characterizing maturation. Both age parameters (x and d) are represented in years, whereas gap duration (y and a) parameters are represented in log units. This model was fitted to data in each condition separately using SPSS. The resulting parameters are shown in Table 2, and the fits are shown in Figure 2 with solid lines.
Table 2.
Fits to child data, by stimulus condition. The parameter m is the slope defining improvement in threshold (log of duration in ms) as a function of age (log of age in yrs) in young listeners. The parameter d is the age (yrs) at which that line reaches the adult mean. The standard error of the mean is indicated below each parameter estimate.
| stimulus | m | d | R2 |
|---|---|---|---|
| wide | −4.05 (0.98) | 7.2 (0.36) | 0.55 |
| low-fluctuation | −1.53 (0.32) | 12.1 (1.37) | 0.55 |
| Gaussian | −1.21 (0.44) | 10.4 (1.7) | 0.38 |
| staccato | −1.28 (0.55) | 9.0 (1.39) | 0.18 |
The parameter d can be interpreted as the age at which children become adult-like in their detection of a temporal gap. Estimates of d indicate relatively later maturation for the low-fluctuation noise (12.1 yrs) than for the Gaussian (10.4 yrs) and staccato noise (9.0 yrs). This is consistent with the expectation that stimulus fluctuation impacts younger listeners less than adults. Despite the relatively small standard error associated with estimates of d, however, this trend for a difference in the age of mature performance was not significant. This was determined by performing two fits to the 25-Hz bandwidth data – one with a different value of d for each condition, and one with a single value. The slope associated with each condition (m) was always a free parameter, and the asymptotic adult threshold (a) was always condition-specific. A partial F-test (Kleinbaum, Kupper, & Muller, 1988) was used to evaluate the improvement in the data fit with the inclusion of condition-specific values of d, which was not significant (p ≈ 1.0). One reason for this result is that fixing d resulted in compensatory changes in m. The trading relation between values of d and m was corroborated by the observation that these values were highly correlated across iterations in the single-condition fits. Another factor in the failure to find a significant difference in estimates of d across stimulus conditions could be the relatively poor fit obtained for the Gaussian and staccato noise conditions, with 38% and 18% of the variance accounted for, respectively.
As in the analyses of group data, fits to child data for the low-fluctuation and wide stimulus conditions suggest a different time-course of maturation in these two conditions. Whereas children’s thresholds resembled those of adults by age 7.2 yrs for the wide stimulus, they were not adult-like until age 12.1 yrs in the low-fluctuation stimulus. This difference was evaluated by performing two fits to the data in these conditions: one with condition-specific d parameters, and one with a single value for d fitted to data from both stimulus conditions. The model with condition-specific values of d was significantly better than that with the single value (F1,64=13.67, p<0.001). This result indicates that the age at which gap detection matures is significantly lower for the wide than the low-fluctuation masker.
Interestingly, the time-course of maturation was not significantly different for the 25- and 1000-Hz bands of Gaussian noise. Estimates of d differed for the wide and Gaussian stimulus conditions in the condition-specific fits, with values of 7.2 and 10.4 years, respectively. However, this trend for condition-specific values of d to outperform a single fixed value just missed significance (F1,64=3.55, p=0.064). As previously suggested, a failure to demonstrate significant differences despite large differences in mean values in d could be due, at least in part, to the relatively poor quality of the fit for data with the 25-Hz Gaussian stimulus. The quality of these fits could be related to the relatively greater contribution of external noise to thresholds in the Gaussian stimulus, which would tend to reduce the size of the age effect by elevating adults’ thresholds and increasing the proportion of variance due to measurement error.
IV. GENERAL DISCUSSION
The present study was designed to evaluate the role of inherent envelope modulation depth and availability of cues across auditory channels in children’s detection of a temporal gap. To that end, gap detection thresholds were measured in 5.2 to 15.6 year olds, as well as adults, for bands of noise centered on 2000 Hz. The prominence of inherent envelope modulation was manipulated in three 25-Hz-bandwidth stimulus conditions, and performance was also assessed for a 1000-Hz-bandwidth stimulus. The pattern of improvement with listener age depended on the stimulus. For narrow stimuli, younger children were less detrimentally affected by inherent envelope modulation than older children and adults. This result is consistent with the idea that internal variability in the processing of temporal gaps, a form of internal noise, plays a larger role in determining thresholds of younger children than older children and adults. Whereas adults were equally sensitive to gaps in low-fluctuation and wide noise, the time-course of development was different for these two stimuli. The wide stimulus supported adult-like performance at an earlier age than low-fluctuation noise, a result that could reflect earlier maturation of the ability to benefit from cues distributed across frequency channels than the ability to benefit from reduced inherent envelope modulation depth. The finding of different patterns of maturation within a single group of listeners bolsters the idea that inattention cannot fully account for maturation of gap detection in school-age children (Wightman, et al., 1989). Whereas Irwin et al. (1985) demonstrated different patterns of maturation for stimuli with different center frequencies and bandwidths, the present results indicate that stimulus features related to bandwidth (e.g. inherent envelope modulation) also impact results for stimuli at a fixed center frequency. From a practical perspective, these findings highlight the importance of selecting stimuli that resemble the spectral and temporal features of speech (Phillips, 1999) when using gap detection to evaluate the maturation of temporal resolution as it pertains to speech perception.
A. Effects related to inherent envelope modulation
The effect of inherent envelope modulation for 25-Hz-bandwidth stimuli was similar to that observed by Grose et al. (2008) in adults. That study estimated the gap duration associated with 79% correct for 25-Hz-wide bands centered on 1000 Hz; in a group of adults, thresholds were 24 ms (low-fluctuation), 67 ms (Gaussian) and 111 ms (staccato). Thresholds for adults in the present study were slightly below those in the previous study, which could be due to the fact that the present study tracked 71% correct. The pattern of thresholds was the same in both datasets, however, consistent with the idea that the more pronounced inherent envelope modulation is associated with higher gap detection thresholds. A similar pattern was evident in the thresholds of children, with the caveat that stimulus type had a smaller effect on thresholds of younger children than older children and adults. Whereas thresholds of 5.2- to 6.5-year olds were a factor of 3.2 higher than those of adults for low-fluctuation noise, that age effect was only a factor of 2.1 for Gaussian noise and 1.8 for staccato noise. This is consistent with the idea that internal noise in the representation of stimulus envelope has a dominant effect on young children’s ability to detect a gap, such that increasing levels of external noise (in the form of inherent envelope modulation) has less of a detrimental effect on gap detection. Such a finding is consistent with relatively modest effects of intensity jitter on intensity discrimination in young children (Buss, Hall, & Grose, 2009).
If this interpretation in terms of internal noise is correct, then children’s performance should become adult-like at a younger age for stimuli characterized by prominent inherent envelope modulation. If performance is dominated by the larger of two noise sources – internal or external – then performance for a stimulus with minimal envelope modulation should predominantly reflect internal noise, whereas a stimulus with pronounced envelope modulation should tend to equalize performance for all listeners. Fits to individual child data in each stimulus condition were consistent with earlier maturation of gap detection for stimuli with more prominent inherent envelope modulation, providing some support for this prediction. One important caveat is that the age associated with adult-like performance, based on broken-stick fits, was not significantly different for the three 25-Hz-bandwidth conditions, perhaps due to the relatively large individual differences and poor fits to the data collected with Gaussian and staccato noise stimuli.
While previous studies of gap detection in children have not used noise stimuli as narrow as those in the present experiment, it is of interest to compare our results with low-fluctuation noise with those obtained previously for tonal stimuli. Davis and McCroskey (1980) played pairs of tones, each 17 ms including 1-ms ramps, and asked 3- to 12-year olds whether they heard one or two tones. Each tone in a tone pair was the same frequency (250, 500, 1000250, 500, 2000 or 4000 Hz) and level (20, 40 or 60 dB SL), but neither frequency nor level affected the pattern of results with respect to listener age. Mean thresholds improved between 3 and approximately 9–10 years of age, with no difference in mean performance of 10 and 11 year olds. Of interest here, thresholds of 5-year-olds were on the order of 15 ms, whereas those of the oldest children were approximately 5 ms. Thresholds in the low-fluctuation noise of the present study were approximately a factor of two larger than those reported by Davis and McCroskey (1980), a difference that is qualitatively consistent with the shorter onset/offset ramps used in that study (1-ms vs 40-ms). Despite the methodological and stimulus differences, however, the general conclusions regarding the maturation of gap detection for stimuli with little or no inherent envelope modulation are similar: that maturation continues through 9-10 years of age. These results contrast with those of Trehub et al. (1995), however. In that study infants, children and adults detected a gap between two Gaussian-enveloped 500-Hz tones, each with a half-rise duration of 4.7 ms. Thresholds, defined by a performance criterion of d’=0.5, were similar for 5-year-olds and adults (5.6 vs 5.2 ms), and those of infants were only modestly higher (11 ms). It is unclear how to think about the very modest developmental effects in this paradigm.
B. Effects related to the availability of cues across frequency
The finding in adults of comparable thresholds in the low-fluctuation and the wide stimulus conditions could be interpreted in two ways. First, both stimuli are relatively free of temporal events that resemble the signal, and gap detection is based on the same within-channel cue in both cases. Second, the wide stimulus contains more temporal events that resemble the target gap than the low-fluctuation stimulus, but the presence of cues across frequency in the wide stimulus can be used to disambiguate those confusions, such that slightly different cues are used in the two conditions. If the cue were the same in both conditions (option 1), then the prediction would be for maturation to follow the same trajectory in the two conditions. Earlier maturation of gap detection in the wide than the low-fluctuation noise conditions, however, suggests that performance in these two conditions may be determined by different cues (option 2). Whereas modest envelope modulation may be the dominant factor supporting good performance for the low-fluctuation noise, the presence of cues in multiple auditory channels separated in frequency may contribute to performance for the wide stimulus. Adult-like gap detection for the wide stimulus at an early age could therefore reflect the relatively early emergence of an ability to benefit from across-channel cues. This is consistent with data showing that even young school-age children benefit from across-channel masker coherence in the detection of a long-duration tone in a square-wave amplitude modulated noise masker (Grose, et al., 1993), although it is less consistent with the finding that even 6.5- to 10-year-olds appeared to be less able to benefit from across-channel cues than adults when the signal is a brief tone (Buss, He, et al., 2013).
Children’s gap detection thresholds in the wide stimulus can be compared to those reported by Irwin, et al. (1985). In the second experiment of that report, thresholds were measured for octave-wide bands of noise, gated on and off with 1-ms ramps and presented in a background masker to prevent spectral cues from dominating performance of the task. Whereas thresholds improved between 6- and 12-years of age for the band centered on 500 Hz, no significant effects of age were observed at the 2000-Hz frequency; thresholds hovered around 6 to 8 ms for 6- to 12-year-olds. In the present study, thresholds for the wide stimulus centered on 2000 Hz ranged from 10.3 to 28.8 ms across child age groups. One factor to consider in comparing these studies is that about half of the 6-year-olds tested by Irwin et al. (1985) were unable to complete the experiment. In contrast, for the 5.2- to 6.5-year olds tested here, only two of eleven were excused due to an inability to master the task. It is therefore possible that the youngest group of listeners tested here was more representative of the range in performance in this population.
Another consideration is the stimulus differences between studies. The present study used a 0.74-octave bandwidth with abrupt spectral edges, whereas Irwin et al. (1985) used a 1-octave bandwidth that was shaped with filters that fell off at 45 dB/oct outside the passband. If gap detection matures earlier at higher frequencies, as temporal processing appears to in other paradigms (Grose, et al., 1993; He, et al., 2010; Trehub, et al., 1988), these spectral differences could account for the discrepancy between the results of the present study and those of Irwin et al. (1985). Another factor to consider in comparing gap detection thresholds in the wide and low-fluctuation conditions is the use of different ramp durations (4 and 40 ms, respectively), chosen to restrict the availability of splatter cues and to resemble the inherent envelope modulations at the output of the auditory filter centered on 2000 Hz. In light of these differences in ramp duration, it is possible that gap detection matures earlier when stimuli are gated using briefer ramps. This interpretation is broadly consistent with the fact that Irwin et al. (1985) used 1-ms ramps and found earlier maturation of gap detection than found here. It is inconsistent, however, with the fact that Davis and McCroskey (1980) used 1-ms ramps and found a more extended time-course of maturation. Both comparisons to the present data should be treated cautiously, however, given the other procedural differences among studies. While effects of ramp duration cannot be ruled out based on the available data, an interpretation based on across-frequency cues seems more parsimonious at this juncture. Studies evaluating the possible effects of ramp duration are presently under way.
V. CONCLUSIONS
The present data support the following conclusions:
Even for a fixed center frequency, the characteristics of the noise stimulus used to evaluate the maturation of behavioral gap detection had a marked effect on the age at which performance became adult-like.
Inherent envelope modulation of narrowband noise stimuli had a less detrimental effect on gap detection of younger children. As such, the maturation of gap detection may appear more prolonged when evaluated with low-fluctuation bands than stimuli with more pronounced envelope fluctuation.
The time-course of maturation differed significantly for a narrow (25-Hz-wide) band of low-fluctuation noise and a wide (1000-Hz-wide) band of Gaussian noise, despite the fact that performance was similar in these two conditions for adults. Children attained adult-like performance earlier for the wider stimulus. This could be due to relatively early development of the ability to combine gap detection cues across frequency, but differences in gap ramp duration could also have played a role.
Acknowledgments
This work was supported by NIH NIDCD Grant No. R01 DC000397 (JWH).
References
- ANSI. ANSI S3.6-2010, American National Standard Specification for Audiometers. New York: American National Standards Institute; 2010. [Google Scholar]
- Bacon SP, Grantham DW. Modulation masking: effects of modulation frequency, depth, and phase. Journal of the Acoustical Society of America. 1989;85(6):2575–2580. doi: 10.1121/1.397751. [DOI] [PubMed] [Google Scholar]
- Basu M, Krishnan A, Weber-Fox C. Brainstem correlates of temporal auditory processing in children with specific language impairment. Developmental Science. 2010;13(1):77–91. doi: 10.1111/j.1467-7687.2009.00849.x. [DOI] [PubMed] [Google Scholar]
- Buss E, Hall JW, 3rd, Grose JH. Factors affecting the processing of intensity in school-aged children. Journal of Speech, Language, and Hearing Research. 2013;56(1):71–80. doi: 10.1044/1092-4388(2012/12-0008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss E, Hall JW, Grose JH. Development and the role of internal noise in detection and discrimination thresholds with narrow band stimuli. Journal of the Acoustical Society of America. 2006;120(5 Pt 1):2777–2788. doi: 10.1121/1.2354024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss E, Hall JW, Grose JH. Psychometric functions for pure tone intensity discrimination: Slope differences in school-aged children and adults. Journal of the Acoustical Society of America. 2009;125(2):1050–1058. doi: 10.1121/1.3050273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss E, He S, Grose JH, Hall JW. The monaural temporal window based on masking period pattern data in school-aged children and adults. Journal of the Acoustical Society of America. 2013;133(3):1586–1597. doi: 10.1121/1.4788983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dau T, Verhey J, Kohlrausch A. Intrinsic envelope fluctuations and modulation-detection thresholds for narrow-band noise carriers. Journal of the Acoustical Society of America. 1999;106(5):2752–2760. doi: 10.1121/1.428103. [DOI] [PubMed] [Google Scholar]
- Davis SM, McCroskey RL. Auditory Fusion in Children. Child Development. 1980;51(1):75–80. [PubMed] [Google Scholar]
- Eddins DA, Hall JW, 3rd, Grose JH. The detection of temporal gaps as a function of frequency region and absolute noise bandwidth. Journal of the Acoustical Society of America. 1992;91(2):1069–1077. doi: 10.1121/1.402633. [DOI] [PubMed] [Google Scholar]
- Ewert SD, Dau T. External and internal limitations in amplitude-modulation processing. Journal of the Acoustical Society of America. 2004;116(1):478–490. doi: 10.1121/1.1737399. [DOI] [PubMed] [Google Scholar]
- Fischer B, Hartnegg K. On the development of low-level auditory discrimination and deficits in dyslexia. Dyslexia. 2004;10(2):105–118. doi: 10.1002/dys.268. [DOI] [PubMed] [Google Scholar]
- Fitzgibbons PJ. Temporal gap detection in noise as a function of frequency, bandwidth, and level. Journal of the Acoustical Society of America. 1983;74(1):67–72. doi: 10.1121/1.389619. [DOI] [PubMed] [Google Scholar]
- Glasberg BR, Moore BC. Effects of envelope fluctuations on gap detection. Hearing Research. 1992;64(1):81–92. doi: 10.1016/0378-5955(92)90170-r. [DOI] [PubMed] [Google Scholar]
- Grose JH. Gap detection in multiple narrow bands of noise as a function of spectral configuration. Journal of the Acoustical Society of America. 1991;90(6):3061–3068. doi: 10.1121/1.401780. [DOI] [PubMed] [Google Scholar]
- Grose JH, Buss E, Hall JW. Gap detection in modulated noise: Across-frequency facilitation and interference. Journal of the Acoustical Society of America. 2008;123(2):998–1007. doi: 10.1121/1.2828058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grose JH, Hall JW. Basic Issues in Hearing. New York: Academic; 1988. Across-frequency processing in temporal gap detection; pp. 308–316. [Google Scholar]
- Grose JH, Hall JW, Gibbs C. Temporal analysis in children. Journal of Speech and Hearing Research. 1993;36(2):351–356. doi: 10.1044/jshr.3602.351. [DOI] [PubMed] [Google Scholar]
- Hall JW, Grose JH. Notched-noise measures of frequency selectivity in adults and children using fixed-masker-level and fixed-signal-level presentation. Journal of Speech and Hearing Research. 1991;34(3):651–660. doi: 10.1044/jshr.3403.651. [DOI] [PubMed] [Google Scholar]
- Hall JW, Haggard MP, Fernandes MA. Detection in noise by spectro-temporal pattern analysis. Journal of the Acoustical Society of America. 1984;76(1):50–56. doi: 10.1121/1.391005. [DOI] [PubMed] [Google Scholar]
- He S, Buss E, Hall JW. Monaural temporal integration and temporally selective listening in children and adults. Journal of the Acoustical Society of America. 2010;127(6):3643–3653. doi: 10.1121/1.3397464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin RJ, Ball AK, Kay N, Stillman JA, Rosser J. The development of auditory temporal acuity in children. Child Development. 1985;56(3):614–620. [PubMed] [Google Scholar]
- Kleinbaum DG, Kupper LL, Muller KE. Applied regression analysis and other multivariable methods. 2. Boston, Mass: PWS-Kent Pub. Co; 1988. [Google Scholar]
- Kohlrausch A, Fassel R, van der Heijden M, Kortekaas R, van de Par S, Oxenham AJ. Detection of tones in low-noise noise: Further evidence for the role of envelope fluctuations. Acustica. 1997;83:659–669. [Google Scholar]
- Leibold LJ, Neff DL. Effects of masker-spectral variability and masker fringes in children and adults. Journal of the Acoustical Society of America. 2007;121(6):3666–3676. doi: 10.1121/1.2723664. [DOI] [PubMed] [Google Scholar]
- Mayer DL, Dobson V. Visual acuity development in infants and young children, as assessed by operant preferential looking. Vision Research. 1982;22(9):1141–1151. doi: 10.1016/0042-6989(82)90079-7. [DOI] [PubMed] [Google Scholar]
- Moller AR, Rollins PR. The non-classical auditory pathways are involved in hearing in children but not in adults. Neuroscience Letters. 2002;319(1):41–44. doi: 10.1016/s0304-3940(01)02516-2. [DOI] [PubMed] [Google Scholar]
- Moore BC, Glasberg BR. Formulae describing frequency selectivity as a function of frequency and level, and their use in calculating excitation patterns. Hearing Research. 1987;28(2–3):209–225. doi: 10.1016/0378-5955(87)90050-5. [DOI] [PubMed] [Google Scholar]
- Moore BCJ, Glasberg BR. Contralateral and ipsilateral cueing in forward masking. Journal of the Acoustical Society of America. 1982;71(4):942–945. doi: 10.1121/1.387574. [DOI] [PubMed] [Google Scholar]
- Oh EL, Wightman F, Lutfi RA. Children's detection of pure-tone signals with random multitone maskers. Journal of the Acoustical Society of America. 2001;109(6):2888–2895. doi: 10.1121/1.1371764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips DP. Auditory gap detection, perceptual channels, and temporal resolution in speech perception. Journal of the American Academy of Audiology. 1999;10(6):343–354. [PubMed] [Google Scholar]
- Puleo JS, Pastore RE. Contralateral cueing effects in backward masking. Journal of the Acoustical Society of America. 1980;67(3):947–951. doi: 10.1121/1.383973. [DOI] [PubMed] [Google Scholar]
- Shailer MJ, Moore BCJ. Gap detection as a function of frequency, bandwidth, and level. Journal of the Acoustical Society of America. 1983;74(2):467–473. doi: 10.1121/1.389812. [DOI] [PubMed] [Google Scholar]
- Smith NA, Trainor LJ, Shore DI. The development of temporal resolution: Between-channel gap detection in infants and adults. Journal of Speech, Language, and Hearing Research. 2006;49(5):1104–1113. doi: 10.1044/1092-4388(2006/079). [DOI] [PubMed] [Google Scholar]
- Tallal P, Stark RE, Mellits D. The relationship between auditory temporal analysis and receptive language development: Evidence from studies of developmental language disorder. Neuropsychologia. 1985;23(4):527–534. doi: 10.1016/0028-3932(85)90006-5. [DOI] [PubMed] [Google Scholar]
- Trainor LJ, Samuel SS, Desjardins RN, Sonnadara RR. Measuring temporal resolution in infants using mismatch negativity. Neuroreport. 2001;12(11):2443–2448. doi: 10.1097/00001756-200108080-00031. [DOI] [PubMed] [Google Scholar]
- Trehub SE, Schneider BA, Henderson JL. Gap detection in infants, children, and adults. Journal of the Acoustical Society of America. 1995;98(5 Pt 1):2532–2541. doi: 10.1121/1.414396. [DOI] [PubMed] [Google Scholar]
- Trehub SE, Schneider BA, Morrongiello BA, Thorpe LA. Auditory sensitivity in school-age children. Journal of Experimental Child Psychology. 1988;46(2):273–285. doi: 10.1016/0022-0965(88)90060-4. [DOI] [PubMed] [Google Scholar]
- Tyler RS, Summerfield Q, Wood EJ, Fernandes MA. Psychoacoustic and phonetic temporal processing in normal and hearing-impaired listeners. Journal of the Acoustical Society of America. 1982;72(3):740–752. doi: 10.1121/1.388254. [DOI] [PubMed] [Google Scholar]
- Viemeister NF. Temporal modulation transfer functions based upon modulation thresholds. Journal of the Acoustical Society of America. 1979;66(5):1364–1380. doi: 10.1121/1.383531. [DOI] [PubMed] [Google Scholar]
- Werner LA, Folsom RC, Mancl LR, Syapin CL. Human auditory brainstem response to temporal gaps in noise. Journal of Speech, Language, and Hearing Research. 2001;44(4):737–750. doi: 10.1044/1092-4388(2001/058). [DOI] [PubMed] [Google Scholar]
- Werner LA, Marean GC, Halpin CF, Spetner NB, Gillenwater JM. Infant auditory temporal acuity: Gap detection. Child Development. 1992;63(2):260–272. [PubMed] [Google Scholar]
- Wichmann FA, Hill NJ. The psychometric function: I. Fitting, sampling, and goodness of fit. Perception and Psychophysics. 2001;63(8):1293–1313. doi: 10.3758/bf03194544. [DOI] [PubMed] [Google Scholar]
- Wightman F, Allen P, Dolan T, Kistler D, Jamieson D. Temporal resolution in children. Child Development. 1989;60(3):611–624. [PubMed] [Google Scholar]
- Wightman FL, Kistler DJ, O'Bryan A. Individual differences and age effects in a dichotic informational masking paradigm. Journal of the Acoustical Society of America. 2010;128(1):270–279. doi: 10.1121/1.3436536. [DOI] [PMC free article] [PubMed] [Google Scholar]