Abstract
IMPORTANCE
Binocular summation (BiS) is defined as the superiority of visual function for binocular over monocular viewing. Binocular summation decreases with age and large interocular differences in visual acuity. To our knowledge, BiS has not heretofore been well studied as a functional measure of binocularity in strabismus.
OBJECTIVE
To evaluate the effect of strabismus on BiS using a battery of psychophysical tasks that are clinically relevant and easy to use and to determine whether strabismus is associated with binocular inhibition in extreme cases.
DESIGN
Case-control study.
SETTING
University-based eye institute.
PARTICIPANTS
Strabismic patients recruited during 2010 to 2012 from a preoperative clinic and control participants with no history of eye disease other than refractive error.
INTERVENTION
A battery of psychophysical and electrophysiological tests including Early Treatment Diabetic Retinopathy Study visual acuity, Sloan low-contrast acuity (LCA) (2.5% and 1.25%), Pelli-Robson contrast sensitivity, and sweep visual evoked potential contrast sensitivity.
MAIN OUTCOME AND MEASURE
Binocular summation was calculated as the ratio between binocular and better-eye individual scores.
RESULTS
Sixty strabismic and 80 control participants were prospectively examined (age range, 8–60 years). Mean BiS was significantly lower in the strabismic patients than controls for LCA (2.5% and 1.25%, P = .005 and <.001, respectively). For 1.25% LCA, strabismic patients had a mean BiS score less than 1, indicating binocular inhibition (ie, the binocular score was less than that of the better eye’s monocular score). There was no significant difference in BiS for contrast thresholds on Early Treatment Diabetic Retinopathy Study visual acuity, Pelli-Robson contrast sensitivity, or sweep visual evoked potential contrast sensitivity. Regression analysis revealed a significant worsening of BiS with strabismus for 2.5% (P = .009) and 1.25% (P = .002) LCA, after accounting for age.
CONCLUSIONS AND RELEVANCE
Strabismic patients demonstrate subnormal BiS and even binocular inhibition for LCA, suggesting that strabismus impairs visual function more than previously appreciated. This may explain why strabismic patients who are not diplopic close 1 eye in visually demanding situations. This finding clarifies the visual deficits impacting quality of life in strabismic patients and may represent a novel measure by which to evaluate and monitor function in strabismus.
Strabismus is a common ocular disease, occurring in 2% to 5% of the population.1 Most clinicians who treat strabismic patients are aware of multiple inexplicable visual complaints in this population, including preference to close 1 eye to perform complex visual tasks, or in visually confusing settings, even when diplopia is absent. Although effective treatments for strabismus exist, our understanding of the functional binocular visual deficits in strabismus lags behind our knowledge of treatment. Currently, binocular function is assessed in clinical settings by evaluating fusion and stereoacuity.2 However, some tests of stereoacuity and fusion have questionable validity because of monocular cues or dissociative testing methods. All tests of stereoacuity and fusion require a minimum level of visual acuity in each eye to assess binocular status. In addition, patients with early-onset and/or long-standing strabismus typically perform poorly on such tests and show little improvement with treatment, so stereoacuity and fusion are not useful as clinical trial outcomes or in patient management in these cohorts.
Binocular summation (BiS), defined as the superiority of binocular over monocular performance on visual threshold tasks,3 is a measure of binocular function that is not well characterized in strabismic patients. Unlike stereoacuity, BiS is not affected by monocular cues and can be reasonably assessed in patients with poor vision in 1 eye or who have had childhood strabismus interfering with the development of fusion potential. For 50 years, BiS has been well studied in normal participants, and several important details have been elucidated. First, BiS improves performance on psychophysical tests at low contrast in normal participants by approximately 40%4,5 or greater.6–9 In addition, it is known that advanced age10 and interocular differences in visual acuity (VA) both impair BiS.3,10–12 When interocular differences in VA are very large, a destructive neural interaction can occur, known as binocular inhibition, diminishing the participants’ binocular score compared with that of the better eye. In these cases, participants see better monocularly than binocularly. Binocular inhibition, or a lack of BiS, may explain some of the previously inexplicable symptoms described by patients with strabismus.
It is tempting to think that strabismus would impair BiS. Based on previous studies showing that interocular differences in VA play a key role in determining a subject’s capacity for BiS, we hypothesized that when a manifest strabismus causes an image to fall extrafoveally in the deviated eye, this may lead to an induced interocular difference in resolution of a target for a particular visual task, and therefore decrease BiS, or even produce binocular inhibition. The purpose of this study was to evaluate the effect of strabismus on BiS using a battery of psychophysical tasks that are clinically relevant and easy to use and to determine whether strabismus is associated with binocular inhibition in extreme cases.
Methods
This study was approved by the University of California, Los Angeles institutional review board and conformed to the requirements of the US Health Insurance Portability and Accountability Act, and informed consent was received from the participants. Strabismic patients were recruited during 2010 to 2012 from the preoperative clinic of 4 of us (S.L.P., J.L.D., F.G.V., and S.J.I.) during preoperative visits. Exclusion criteria included any history of amblyopia, age younger than 3 years or older than 80 years, dissociated vertical or horizontal deviation as the sole form of strabismus, pathologic nystagmus, neurologic disease, or any structural lesion causing an interocular difference exceeding 0.3 logMAR. Nonstrabismic control participants were recruited among staff at the Jules Stein Eye Institute, as well as family members of patients who were seen between 2010 and 2011. Control participants were included only if they had no history of eye disease other than refractive error.
All participants underwent a screening examination in which their VA was tested using the Early Treatment Diabetic Retinopathy Study (ETDRS) protocol with their habitual refractive correction.13 If visual acuity was worse than 0.20 log-MAR in either eye, a manifest refraction was performed and the study tests were performed with this refraction. Next, binocular alignment was measured at distance (5 m) and near (30 cm) using cover/uncover and alternate prism cover testing. Right eye, left eye, and binocular testing were performed in an order randomly assigned prior to testing that was consistently maintained for each subject for the various psychophysical and electrophysiological tests. All testing was performed by trained technicians experienced in the examination of patients for research studies, with adherence to detailed standard protocols, including written scripts and instructions for testing. The following tests were performed (in order of presentation to the participants).
High-Contrast Visual Acuity
Visual acuity was tested using the ETDRS protocol13 at 3 m. The score VA was the number of letters identified correctly, with a maximum score of 70 (Snellen equivalent 20/12.5).
Low-Contrast Visual Acuity
Sloan acuity was tested (Precision Vision) at low-contrast levels of 2.5%, followed by 1.25%, using the ETDRS protocol at 3 min a dimly lit room. Sloan charts have a similar format to the ETDRS charts (5 letters per line), with each Sloan chart corresponding to a different contrast level. The low-contrast acuity (LCA) score is the number of letters identified correctly, with a maximum score of 70 (14 lines). For participants who were unable to see any letters (letter score of zero), a score of 1 was substituted for the individual eye scores such that the BiS fraction could still be created (without having to divide by zero). A second analysis excluding these patients was also performed to evaluate for bias induced by this method of score replacement. Pelli-Robson charts (Metropia Ltd) were also used to test contrast sensitivity at 1 m for each eye individually and binocularly.
Sweep Visual Evoked Potential
Sweep visual evoked potential (VEP) was tested using the PowerDiva (digital infant vision assessment) sweep VEP system in a dark room.14 The stimuli were phase-reversal sinewave gratings presented on a 17.5 × 29 × 38-cm high-resolution video monitor 1 m away at 50.3 cd/m2 mean luminance. The active electrode was positioned on the midline of the scalp 1 cm above the inion, referenced to an ear clip on the right ear and a ground on the left ear. Stimuli consisted of a horizontal square-wave grating (fixed spatial frequency of 1 cycle/degree)with a contrast range swept from 2%to 90% at 7.2-Hz fixed temporal frequency. Contrast thresholds were measured by sweeping 10 contrast levels over a 10-second trial with log steps. Log steps were used because contrast response functions are monotonically increasing functions associated linearly with increasing log contrast over a range of near-threshold contrasts. At least 5 sweeps were obtained, and sweeps with a signal to noise ratio more than 3:1 were averaged to calculate contrast threshold.15
Statistical Analysis
The demographic features of control and strabismic participants were compared using the t test for continuous variables and χ2 test for categorical variables. For letter charts (ETDRS and Sloan 2.5% and 1.25%), BiS was calculated by dividing the better-eye score into the binocular score (binocular/better-eye score). For contrast threshold tasks (Pelli-Robson and sweep VEP), the contrast threshold was converted to contrast sensitivity (1/contrast threshold), and then BiS was calculated by dividing the better-eye contrast sensitivity score into the binocular score. As a conservative correction for test variability, a BiS score exceeding 1.1 was required to demonstrate BiS. A value of 1.1 would indicate a 5-letter binocular improvement over that of the better eye. Similarly, binocular inhibition was considered to exist when the BiS score was 0.9 or less, to indicate a 5-letter or more decrement in vision with binocular vision compared with the better eye alone. The mean BiS score for strabismic participants was compared with that of control participants using a t test. The percentages of patients in each group demonstrating BiS and binocular inhibition were compared using the Fisher exact test. Linear regression analysis was then performed to evaluate the effect of age, a known covariate for BiS, and the presence or absence of strabismus. Finally, additional covariates such as age of strabismus onset (based on clinical history), age at surgery, and angle of deviation were evaluated using linear regression.
Results
Demographic Features
Sixty strabismic and 80 control participants were enrolled. The mean (SD) age of the control participants was 34 (15) years (range, 2.5–66 years; median, 32 years) and 39 (26) years (range, 2.2–80 years; median, 34 years) for the strabismic participants (P = .14). Demographicand VA information are summarized in Table 1. Thirty-nine percent of control participants and 55% of strabismic participants were male (P = .08). Subtypes of strabismus included early-onset esotropia with onset before age 1.5 years (n = 8), childhood esotropia with onset between ages 1.5 and 8 years (n = 5), esotropia acquired after 8 years of age (n = 11), intermittent exotropia (n = 11), consecutive exotropia after surgery for infantile esotropia (n = 7), acquired exotropia with onset after age 1.5 years (n = 2), presumed congenital superior oblique palsy (n = 6), acquired hypertropia after age 1.5 years (n = 7), and mixed acquired horizontal and vertical strabismus with horizontal and vertical components each larger than 10 prism diopters in central distance gaze (n = 3).
Table 1.
Demographic and VA Information for Participants
| Mean (SD); Median [Range] | |||||
|---|---|---|---|---|---|
| Age, y | VA OU, Letters |
IOD VA, Letters |
Angle of Strabismus, Δ |
% With Diplopia |
|
| Control participants | 34 (15); 32 [2.5–66] | 60 (6) | 3.8 (4) | 0 (0) | 0 |
| Strabismic participants | 39 (26); 34 [2.2–80] | 56 (7) | 4.8 (10) | 22 (15) | 31 |
| P valuea | .14 | .01 | .05 | … | … |
Abbreviations: ellipses, not applicable; IOD, interocular difference in visual acuity; OU, each eye; VA, visual acuity; Δ, prism diopter.
Paired t test.
Binocular Summation
Mean BiS is summarized in Table 2 for all groups. There was a significant decrement in BiS in strabismic participants for the 2.5% (P = .005) and 1.25% (P < .001) low-contrast Sloan charts. There was no significant difference between groups for high-contrast ETDRS VA and contrast threshold tasks (Pelli-Robson chart and sweep VEP contrast sensitivity). For the lowest contrast level (1.25%), strabismic patients overall demonstrated binocular inhibition with a mean BiS of 0.9. Therefore, overall, this group showed a detrimental effect of binocularity compared with monocular viewing with thebetter eye. In contrast, the control participants on average had a 50% improvement(ratio = 1.5)during binocularviewing compared with better-eye monocular viewing. This analysis was repeated with censoring of participants in whom zero letters were seen and who had a substitution of a letter score of “1” for their individual eye scores on the 2.5% or 1.25% LCA charts (n = 5). These results were similar (eTable in the Supplement) to those with substituted values.
Table 2.
Mean Binocular Summation Scoresa
| Mean (SD) | |||||
|---|---|---|---|---|---|
| ETDRS VA | 2.5% Sloan LCA |
1.25% Sloan LCA |
Pelli-Robson CS |
Sweep VEP CS |
|
| Strabismic participants (n = 60) |
1.01 (0.07) | 1.0 (0.3) | 0.9 (0.4) | 0.99 (0.1) | 0.99 (0.45) |
| Control participants (n = 80) |
1.02 (0.05) | 1.3 (0.4) | 1.5 (1.0) | 0.97 (0.06) | 1.03 (0.49) |
| P valueb | .90 | .005 | <.001 | .40 | .70 |
Abbreviations: CS, contrast sensitivity; ETDRS, Early Treatment Diabetic Retinopathy Study; LCA, low-contrast acuity; VA, visual acuity; VEP, visual evoked potential.
Binocular summation score calculated as a ratio between the binocular letter score and the better-eye letter score (binocular score/better-eye score).
Two-tailed t test.
For comparison, the percentage of participants with BiS (BiS score >1.1) and binocular inhibition (BIS score <0.9) were compared (Table 3). The percentage of patients demonstrating BiS for the 2.5% LCA and 1.25% LCA charts was significantly higher in control than in strabismic participants (P < .001 and P < .001, respectively), while the percentage of patients demonstrating binocular inhibition for the 2.5% and 1.25% LCA contrast thresholds was significantly higher in strabismic participants (P < .001 for both). For the 2.5% and 1.25% LCA tests, the percentage of participants not demonstrating any summation or inhibition (BiS ratio between 0.9–1.1) was 25% and 25% for the control participants and 59% and 65% for the strabismic participants for the 2.5% and 1.25% LCA charts, respectively. Of these participants, the majority of the control participants (58% and 53%) had BiS ratios greater than 1 while the majority of the strabismic participants (52% and 80%) had BiS ratios less than 1 for the 2.5% and 1.25% LCA charts, respectively.
Table 3.
| % of Participants | |||
|---|---|---|---|
| With Binocular Summation (BiS >1.1) |
With Binocular Inhibition (BiS <0.9) |
||
| ETDRS letters | |||
| Strabismic participants | 7 | 5 | |
| Control participants | 10 | 0 | |
| P value | .80 | .10 | |
| 2.5% Sloan letters | |||
| Strabismic participants | 25 | 16 | |
| Control participants | 75 | 0 | |
| P value | <.001 | <.001 | |
| 1.25% Sloan letters | |||
| Strabismic participants | 21 | 44 | |
| Control participants | 64 | 11 | |
| P value | <.001 | <.001 | |
| Pelli-Robson CS | |||
| Strabismic participants | 9 | 3 | |
| Control participants | 0 | 12 | |
| P value | .10 | .10 | |
| Sweep VEP CS | |||
| Strabismic participants | 32 | 49 | |
| Control participants | 36 | 46 | |
| P value | .70 | .90 | |
Abbreviations: BiS, binocular summation score; CS, contrast sensitivity; ETDRS, Early Treatment Diabetic Retinopathy Study; VEP, visual evoked potential.
Binocular summation score calculated as a ratio between the binocular letter score and the better-eye letter score for the ETDRS and 2.5% and 1.25% Sloan letter charts. For the Pelli-Robson CS and sweep VEP CS tests, the BiS was calculated as a ratio between the better-eye CS score and the binocular CS score.
P values calculated by 2-tailed Fisher exact test.
Multiple Linear Regression Model
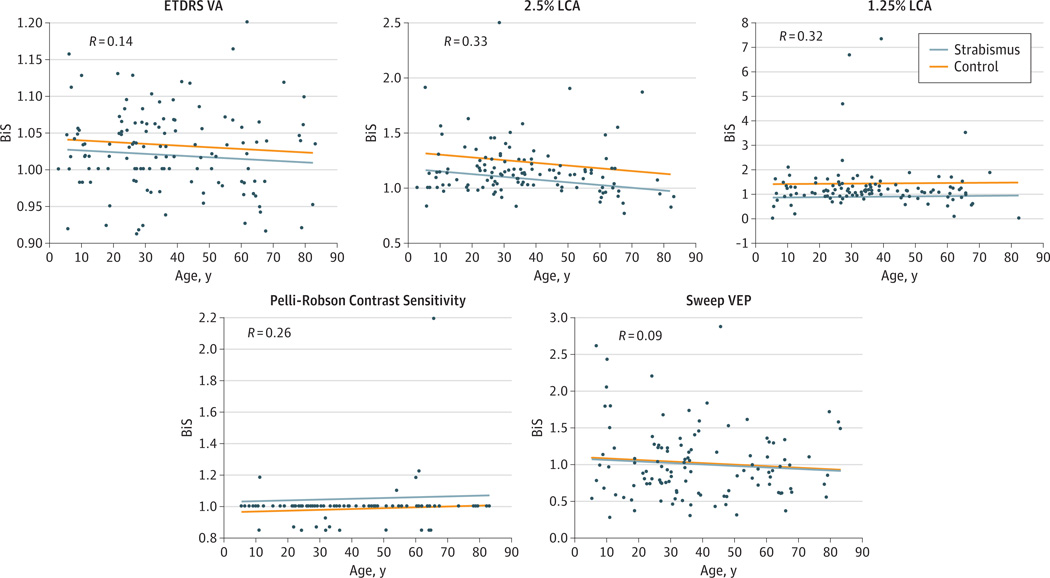
Given the known decrement in BiS with increasing age, a multiple linear regression model of BiS scores was developed incorporating as covariates age and strabismus vs control status. Scatterplots of BiS ratio vs age are presented in the Figure for both the strabismic and control groups. P values for the regression coefficient for the strabismus vs control variable were statistically significant for the 2.5% LCA chart (P = .007), 1.25% LCA chart (P < .001), and Pelli-Robson contrast sensitivity (P = .02) but not for ETDRS VA or sweep VEP contrast sensitivities; this suggests that strabismus is highly associated with decreased BiS even after correcting for age for Sloan LCA letter charts but not for high-contrast ETDRS charts and sweep VEP contrast thresholds. In addition, other covariates were examined by linear regression to evaluate potential associations with diminished BiS, including age at onset, age at surgery, presence of diplopia, and angle of deviation. No additional significant associations were found; however, the study was not sufficiently powered to rule out these possible associations.
Figure. Scatterplot Representations for Linear Regression Models of Binocular Summation (BiS) Accounting for Age and Strabismic vs Control Status.
R values are depicted for each visual outcome. Strabismic vs control status was the only statistically significant variable for 2.5% Sloan low-contrast acuity (LCA) (P = .007), 1.25% Sloan LCA (P < .001), and Pelli-Robson contrast sensitivity (P = 0.02). Neither age nor strabismus vs control status were significantly associated with increased BiS for high-contrast Early Treatment Diabetic Retinopathy Study visual acuity chart (ETDRS VA) or sweep visual evoked potential (VEP) contrast sensitivity.
Discussion
Strabismic patients often have visual complaints that are difficult to characterize, but frequently, these patients prefer to close 1 eye to achieve their best vision. Although the presence of diplopia can explain diminished binocular vision in many cases, it is not always perceived and is seldom the sole explanation for visual deficits. Our data suggest that strabismic patients often have decreased BiS and even binocular inhibition for very low-contrast tasks.
Currently, clinicians use tests of stereoacuity and fusion to diagnose and monitor binocular visual deficits in strabismus. The present results demonstrate that other functional binocular deficits existing in this patient group. Furthermore, some standard tests of stereopsis and fusion are less useful in this population because they are subject to monocular cues or use dissociative measures that might affect their validity. In addition, tests of stereoacuity and fusion require a minimum level of vision in both eyes and often require a baseline level of ocular alignment during the formative period of childhood development during which stereopsis is acquired.16 Therefore, in long-standing cases of strabismus, such as adult-onset consecutive deviations, other functional measures of binocularity may be necessary to provide insight into the functionality of binocular vision and for use in following up functional binocular vision over time, as well as for assessing the impact of ocular realignment surgery on binocular function. Many cases of adult strabismus without diplopia are thought only to impact the psychosocial aspects of the subject’s life; however, in this study, we found that there are uncharacterized changes in binocularity that can be demonstrated by BiS measurements in participants with strabismus.
Binocular summation can be attributed either to (1) “probability summation,” which assumes complete independence of the 2 eyes and predicts enhancement of binocular over monocular vision due to the statistical consideration that a binocular observer has 2 opportunities to detect weak signals, or (2) “neural summation,” the result of binocularly enhanced performance that exceeds what would be expected from probability summation alone. Neural BiS for various electrophysiological and psychophysical tasks has been shown to arise from interactions most likely in cortical layer VI and is most easily demonstrated at low contrast.17 Previous studies have shown that in normal patients, BiS often approximates √2 or greater, corresponding to a 40% improvement.17 Our data confirm these studies in that the mean (SD) BiS ratios for control participants were 1.3 (0.4) and 1.5 (1.0) for the 2.5% and 1.25% LCA charts, respectively. Interestingly, control participants did not exhibit BiS for the contrast threshold tasks (Pelli-Robson chart and the sweep VEP contrast sensitivity), with mean (SD) BiS values of 0.96 (0.07) and 1.03 (0.49). These tests may be less sensitive clinical measures of BiS than the 2.5% and 1.25% LCA charts. It is not surprising that control participants did not exhibit mean BiS more than 1.0 for the ETDRS charts since it is well known that BiS is more enhanced for lower-contrast tests. Interestingly, there were a few control participants (n = 9) who demonstrated binocular inhibition for the 1.25% LCA test. We believe this may be in part due to test-retest variability or patient fatigue. In addition, age or undiagnosed interocular difference for LCA may have contributed to the finding of binocular inhibition in these participants. Despite this, we still found a strongly significant difference between the control and strabismic participants. Similarly, we might have expected more than 64% of the control participants to exhibit BiS for the 1.25% LCA test. One potential explanation for this finding is our stringent definition of summation to be a BiS ratio greater than 1.1 instead of 1.0. If we used the definition of 1.0, then we wouldhavefoundthat 71 patients (89%) would havehad summation. Therefore, this finding can largely be explained by a small subgroupof patients who displayed summation but did not meet our criteria. There is also the potential that some of our control participants were not visually “normal.” Our screening criteria consisted of VA, refraction, and ocular motility examinations. Therefore, the possibility of participants with subtle abnormalities in contrast sensitivity may have been inadvertently included.
There is strong published evidence that advanced age10 and large interocular VA differences both have detrimental effects on BiS. In addition, studies of the role of retinal correspondence have shown that stimulation of noncorresponding points outside of the fusional range decreases neural summation.18–21 In cases that exceed the tolerated range of interocular VA differences, binocular inhibition occurs (BiS<1.0). The mechanism of binocular inhibition is not well defined but is likely related to interocular suppressive mechanisms in layer VI22,23 and most commonly occurs in participants with large interocular differences in VA. Based on the foregoing studies, we hypothesized that if a manifest strabismus exists, the degraded image that falls outside of the fovea of the deviated eye may lead to an induced difference in resolution of a particular target and therefore cause a decrease in BiS, or even binocular inhibition on clinical testing. Our data support this notion in that the mean BiS for the 1.25% LCA chart was 0.9 for strabismic participants, and only 21% of the strabismic participants demonstrated BiS for this measure, compared with 64% of control participants (P < .001).
Binocular summation has been studied in amblyopic participants in several conflicting reports. Early studies argued that amblyopic participants showed decreased BiS, or even binocular inhibition, when compared with normal controls.24,25 The degree of BiS loss (and binocular inhibition) appears to be directly related to interocular difference in VA.26 However, more recent studies have demonstrated that although BiS for contrast sensitivity is decreased in amblyopic participants, it can be improved by normalizing the interocular difference with neutral density filters,27 revealing that individuals with amblyopia likely retain the neural mechanisms for BiS but are at a disadvantage secondary to interocular differences in VA. We chose to exclude amblyopic participants from our study for this reason.
Binocular summation has been less well studied in non-amblyopic strabismic populations than in purely amblyopic populations; the few existing reports evaluated mainly participants with infantile-onset strabismus. Most published studies used fewer than 20 participants28–36 and reported BiS using flash or pattern visual evoked response, often in participants younger than 5 years. Results of these previous studies are conflicting, probably secondary to small sample sizes, differing experimental conditions, and the variability in strabismus subtypes being compared. However, the majority found that BiS may be decreased in some forms of strabismus, including largeangle esotropia,29,30,32,35 decompensated exotropia,30 and simulated vertical strabismus in normal participants.28,36 Most of these studies used only visual evoked responses as their primary outcome and did not evaluate clinically accessible outcomes, such as letter chart LCA.
In the current study, the decrement in BiS due to strabismus was most significant on the Sloan 2.5% and 1.25% letter charts, which are readily available for clinical use. The finding of decreased BiS (and a mean binocular inhibition for the 1.25% letter chart) continued to be highly significant even after accounting for the known covariate of age, as well as other predicted covariates such as the angle or type of strabismus.
In addition to describing a novel method by which to assess and potentially track binocular function in strabismic patients, we have also shown that BiS is most readily demonstrated using Sloan LCA letter charts. Both the 2.5% and 1.25% contrast levels were highly useful in differentiating strabismic from control participants. This finding has been similarly described in a large cohort of patients with multiple sclerosis.12 In addition, when BiS was calculated as a ratio between the binocular score and the better-eye score, the control subject mean was close to the estimated 1.414 (or V2) that has been commonly reported for BiS in other laboratory psychophysical measures.
The results of our study should be understood within the context of the study’s limitations. First, sweep VEP measurements were performed at only 1 spatial frequency and at 1 temporal frequency. Since BiS for VEP contrast sensitivity is dependent on spatial and temporal frequency,37 it is possible that different results might have been possible at different spatial or temporal frequencies. This study included a wide range of ages and strabismus subtypes. Multiple types of patients were included to evaluate the overarching hypothesis that BiS is diminished by strabismus. However, we were unable to look at specific strabismus subgroups. We are currently recruiting larger subgroups of specific strabismus types so that we may address this question. In addition, we are following up these patients longitudinally to evaluate the impact that strabismus surgery has on BiS. Although the study was designed to include similar patient groups based on visual acuity and age, there was a clinically small (<0.1 logMAR) but statistically significant difference in high-contrast visual acuity and interocular difference between the strabismic and control groups. Finally, there were a few patients (n = 5) who were unable to provide a measurable response for the lowest-contrast 1.25% LCA task; these patients were assigned to a default value of 1 so that the BiS ratio score could be computed. However, our secondary analysis excluding these patients revealed strikingly similar results, thereby diminishing concern related to induced bias from these 5 participants.
Despite its limitations, to our knowledge, this study represents the largest cohort of strabismic patients and control participants tested with clinically available tests to evaluate BiS. Binocular summation is an easily measured parameter representing a difference in binocular visual function between strabismic patients and normal participants that may represent a parameter that can be followed up over time to monitor for changes in binocular function and postoperative changes after strabismus surgery. Our data from strabismic participants showing subnormal BiS and possible binocular inhibition suggest that strabismus impairs binocular vision more than previously appreciated.
Supplementary Material
Acknowledgments
Funding/Support: This work was supported by National Institutes of Health National Eye Institute grant K23EY021762, the Knights Templar Eye Foundation, and the Oppenheimer Family Foundation.
Role of the Sponsor: The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Supplemental content at jamaophthalmology.com
Author Contributions: Dr Pineles had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Pineles, Velez, Birch, Nusinowitz, Demer.
Acquisition of data: Pineles, Velez, Isenberg, Fenoglio.
Analysis and interpretation of data: Pineles, Birch, Nusinowitz, Demer.
Drafting of the manuscript: Pineles, Nusinowitz.
Critical revision of the manuscript for important intellectual content: Pineles, Velez, Isenberg, Birch, Demer.
Statistical analysis: Pineles.
Obtained funding: Pineles.
Administrative, technical, or material support: Pineles, Velez, Isenberg, Fenoglio, Nusinowitz.
Study supervision: Pineles, Velez, Birch, Demer.
Conflict of Interest Disclosures: None reported.
Previous Presentation: This paper was presented at the annual meeting of the American Association for Pediatric Ophthalmology and Strabismus; April 6, 2013; Boston, Massachusetts.
REFERENCES
- 1.Friedman DS, Repka MX, Katz J, et al. Prevalence of amblyopia and strabismus in white and African American children aged 6 through 71 months the Baltimore Pediatric Eye Disease Study. Ophthalmology. 2009;116(11):2128–2134. e1–e2. doi: 10.1016/j.ophtha.2009.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Diagnostic techniques for strabismus and amblyopia. American Academy of Ophthalmology. 2013–2014 Basic and Clinical Science Course, Section 6: Pediatric Ophthalmology and Strabismus. Vol. 87 San Francisco, CA: American Academy of Ophthalmology; 2011. [Google Scholar]
- 3.Blake R, Sloane M, Fox R. Further developments in binocular summation. Percept Psychophys. 1981;30(3):266–276. doi: 10.3758/bf03214282. [DOI] [PubMed] [Google Scholar]
- 4.Campbell FW, Green DG. Monocular versus binocular visual acuity. Nature. 1965;208(5006):191–192. doi: 10.1038/208191a0. [DOI] [PubMed] [Google Scholar]
- 5.Legge GE. Binocular contrast summation, I: detection and discrimination. Vision Res. 1984;24(4):373–383. doi: 10.1016/0042-6989(84)90063-4. [DOI] [PubMed] [Google Scholar]
- 6.Meese TS, Georgeson MA, Baker DH. Binocular contrast vision at and above threshold. J Vis. 2006;6(11):1224–1243. doi: 10.1167/6.11.7. [DOI] [PubMed] [Google Scholar]
- 7.Meese TS, Hess RF. Low spatial frequencies are suppressively masked across spatial scale, orientation, field position, and eye of origin. J Vis. 2004;4(10):843–859. doi: 10.1167/4.10.2. [DOI] [PubMed] [Google Scholar]
- 8.Meese TS, Hess RF. Interocular suppression is gated by interocular feature matching. Vision Res. 2005;45(1):9–15. doi: 10.1016/j.visres.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 9.Rose D, Pardhan S. Selective attention, ideal observer theory and ‘early’ visual channels. Spat Vis. 2000;14(1):77–80. doi: 10.1163/156856801741378. [DOI] [PubMed] [Google Scholar]
- 10.Gagnon RW, Kline DW. Senescent effects on binocular summation for contrast sensitivity and spatial interval acuity. Curr Eye Res. 2003;27(5):315–321. doi: 10.1076/ceyr.27.5.315.17225. [DOI] [PubMed] [Google Scholar]
- 11.Jiménez JR, Ponce A, Anera RG. Induced aniseikonia diminishes binocular contrast sensitivity and binocular summation. Optom Vis Sci. 2004;81(7):559–562. doi: 10.1097/00006324-200407000-00019. [DOI] [PubMed] [Google Scholar]
- 12.Pineles SL, Birch EE, Talman LS, et al. One eye or two:a comparison of binocular and monocular low-contrast acuity testing in multiple sclerosis. Am J Ophthalmol. 2011;152(1):133–140. doi: 10.1016/j.ajo.2011.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Early Treatment Diabetic Retinopathy Study design and baseline patient characteristics: ETDRS report number 7. Ophthalmology. 1991;98(5 suppl):741–756. doi: 10.1016/s0161-6420(13)38009-9. [DOI] [PubMed] [Google Scholar]
- 14.Norcia AM. PowerDiva Manual. San Francisco, CA: Smith Kettlewell Eye Research Institute; 1999. [Google Scholar]
- 15.Norcia AM, Tyler CW, Hamer RD, Wesemann W. Measurement of spatial contrast sensitivity with the swept contrast VEP. Vision Res. 1989;29(5):627–637. doi: 10.1016/0042-6989(89)90048-5. [DOI] [PubMed] [Google Scholar]
- 16.Tycheson L. Infantile esotropia: current neurophysiologic concepts. In: Rosenbaum AL, Santiago AP, editors. Clinical Strabismus Management. Philadelphia, PA: Saunders; 1999. [Google Scholar]
- 17.Blake R, Wilson H. Binocular vision. Vision Res. 2011;51(7):754–770. doi: 10.1016/j.visres.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blake R, Martens W, Di Gianfilippo A. Reaction time as a measure of binocular interaction in human vision. Invest Ophthalmol Vis Sci. 1980;19(8):930–941. [PubMed] [Google Scholar]
- 19.Harwerth RS, Smith EL, Levi DM. Suprathreshold binocular interactions for grating patterns. Percept Psychophys. 1980;27(1):43–50. [Google Scholar]
- 20.Thorn F, Boynton RM. Human binocular summation at absolute threshold. Vision Res. 1974;14(7):445–458. doi: 10.1016/0042-6989(74)90033-9. [DOI] [PubMed] [Google Scholar]
- 21.Westendorf DH, Fox R. Binocular detection of disparate light flashes. Vision Res. 1977;17(6):697–702. doi: 10.1016/s0042-6989(77)80005-9. [DOI] [PubMed] [Google Scholar]
- 22.Baker DH, Meese TS, Summers RJ. Psychophysical evidence for two routes to suppression before binocular summation of signals in human vision. Neuroscience. 2007;146(1):435–448. doi: 10.1016/j.neuroscience.2007.01.030. [DOI] [PubMed] [Google Scholar]
- 23.Moradi F, Heeger DJ. Inter-ocular contrast normalization in human visual cortex. J Vis. 2009;9(3):1–22. doi: 10.1167/9.3.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vedamurthy I, Suttle CM, Alexander J, Asper LJ. A psychophysical study of human binocular interactions in normal and amblyopic visual systems. Vision Res. 2008;48(14):1522–1531. doi: 10.1016/j.visres.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 25.Wanger P, Persson HE. Visual evoked responses to pattern-reversal stimulation in childhood amblyopia. Acta Ophthalmol (Copenh) 1980;58(5):697–706. doi: 10.1111/j.1755-3768.1980.tb06682.x. [DOI] [PubMed] [Google Scholar]
- 26.Pardhan S, Gilchrist J. Binocular contrast summation and inhibition in amblyopia: the influence of the interocular difference on binocular contrast sensitivity. Doc Ophthalmol. 1992;82(3):239–248. doi: 10.1007/BF00160771. [DOI] [PubMed] [Google Scholar]
- 27.Baker DH, Meese TS, Mansouri B, Hess RF. Binocular summation of contrast remains intact in strabismic amblyopia. Invest Ophthalmol Vis Sci. 2007;48(11):5332–5338. doi: 10.1167/iovs.07-0194. [DOI] [PubMed] [Google Scholar]
- 28.Campos EC, Chiesi C. Binocularity in comitant strabismus, II: objective evaluation with visual evoked responses. Doc Ophthalmol. 1983;55(4):277–293. doi: 10.1007/BF00161285. [DOI] [PubMed] [Google Scholar]
- 29.Chiesi C, Sargentini AD, Bolzani R. Binocular visual perception in strabismics studied by means of visual evoked responses. Doc Ophthalmol. 1984;58(1):51–56. doi: 10.1007/BF00140898. [DOI] [PubMed] [Google Scholar]
- 30.Giuseppe N, Andrea F. Binocular interaction in visual-evoked responses: summation, facilitation and inhibition in a clinical study of binocular vision. Ophthalmic Res. 1983;15(5):261–264. doi: 10.1159/000265268. [DOI] [PubMed] [Google Scholar]
- 31.Shea SL, Aslin RN, McCulloch D. Binocular VEP summation in infants and adults with abnormal binocular histories. Invest Ophthalmol Vis Sci. 1987;28(2):356–365. [PubMed] [Google Scholar]
- 32.Leguire LE, Rogers GL, Bremer DL. Visual-evoked response binocular summation in normal and strabismic infants: defining the critical period. Invest Ophthalmol Vis Sci. 1991;32(1):126–133. [PubMed] [Google Scholar]
- 33.Rogers GL, Bremer DL, Leguire LE, Fellows RR. Clinical assessment of visual function in the young child: a prospective study of binocular vision. J Pediatr Ophthalmol Strabismus. 1986;23(5):233–235. doi: 10.3928/0191-3913-19860901-08. [DOI] [PubMed] [Google Scholar]
- 34.Kozma P, Deák A, Janáky M, Benedek G. Effect of late surgery for acquired esotropia on visual evoked potential. J Pediatr Ophthalmol Strabismus. 2001;38(2):83–88. doi: 10.3928/0191-3913-20010301-09. [DOI] [PubMed] [Google Scholar]
- 35.Leguire LE, Rogers GL, Bremer DL. Flash visual evoked response binocular summation in normal subjects and in patients with early-onset esotropia before and after surgery. Doc Ophthalmol. 1995;89(3):277–286. doi: 10.1007/BF01203381. [DOI] [PubMed] [Google Scholar]
- 36.Srebro R. The visually evoked response: binocular facilitation and failure when binocular vision is disturbed. Arch Ophthalmol. 1978;96(5):839–844. doi: 10.1001/archopht.1978.03910050445009. [DOI] [PubMed] [Google Scholar]
- 37.Apkarian PA, Nakayama K, Tyler CW. Binocularity in the human visual evoked potential: facilitation, summation and suppression. Electroencephalogr Clin Neurophysiol. 1981;51(1):32–48. doi: 10.1016/0013-4694(81)91507-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.