Abstract
4′-Ethynyl-2-fluoro-2′-deoxyadenosine (EFdA) is a novel nucleoside analog of great interest because of its superior activity against wild-type and multidrug-resistant HIV-1 strains, and favorable safety profiles in vitro and in vivo. The aim of this work was to provide preformulation information of EFdA important for delivery system development. A simple, accurate and specific reverse-phase high performance liquid chromatographic (RP-HPLC) method with UV detection was developed for quantification of EFdA. In addition, physicochemical characterizations including pH solubility profile, octanol/water partition coefficient (Log Po/w), DSC analysis, field emission scanning electron microscopy, and stability studies under various conditions were conducted. EFdA existed in planar or flake shape, with a melting point of ~130 °C, and had a pH dependent solubility. The log Po/w value of EFdA was −1.19. The compound was stable upon exposure to pH levels from 3 to 9 and showed good stability at elevated temperature (65 °C). In vitro cytotoxicity assessments were performed in two different epithelial cell lines. In cell-based studies, the EFdA selectivity index (50% cytotoxic concentration [CC50] values/50% effective concentration [EC50]) was found to be greater than 1 × 103. Permeability studies using cell- and tissue-based models showed that EFdA had an apparent permeability coefficient (Papp) <1 × 10−6cm/s and that the paracelluar pathway was the dominant transport route for EFdA. Overall, EFdA possesses favorable characteristics for further formulation development.
Keywords: Caco-2 cells, EFdA, permeability, preformulation, solubility, transport
Introduction
As of 2010, there were an estimated 34 million people were living with HIV worldwide, with nearly 70% of these individuals in sub- Saharan Africa1. To date, HIV reverse transcriptase inhibitors, including non-nucleoside reverse transcriptase inhibitors (NNRTIs), nucleoside reverse transcriptase inhibitors (NRTIs) and nucleotide reverse transcriptase inhibitors (NtRTIs) are major components of highly active antiretroviral therapy (HAART) used as first-line therapies for the treatment of HIV infection. Although HAART has dramatically improved the quality of life and prognosis of patients infected by HIV-1, a number of side effects are associated with this therapy such as lipid and gastrointestinal abnormalities. Furthermore, this therapy can be associated with the development of HIV resistance. For these reasons new drugs are needed to maintain therapeutic options for patients failing on currently available therapies.
Furthermore, preventive measures such as microbicides (oral and topical pre-exposure prophylaxis) and vaccines are urgently needed to curb the continued spread of HIV infection. Advanced drug delivery systems and proper administration routes for anti-HIV compounds should be taken into consideration in order to prevent HIV transmission effectively, given that sexual transmission through the lower genital and rectal mucosa is a major pathway for HIV infection2. Currently, vaginal drug delivery has been widely used for different types of therapeutic agents such as antibacterials, antifugals, spermicides and steroids. At present, several promising vaginal microbicides are being explored for prevention of HIV-1 sexual transmission at the research and development or early clinical trial stage. These candidates consist of viral entry inhibitors (including broadly neutralizing monoclonal antibodies), reverse transcriptase inhibitors and integrase inhibitors. The most advanced of the microbicide candidates is the nucleotide reverse transcriptase inhibitor (NtRTI) tenofovir. A tenofovir gel product is currently being evaluated in a Phase III clinical trial (FACTS 001) as a topical microbicide to prevent HIV-1 infection. Previously it was evaluated in two Phase IIb trials (CAPRISA 004 and VOICE). The 1% tenofovir vaginal gel was found to reduce HIV-1 incidence by 39% in the CAPRISA 004 trail. However, the tenofovir vaginal gel arm in the VOICE clinical trial was discontinued for futility. In addition to gel products, intravaginal rings containing antiretrovirals such as zidovudine (nucleoside reverse transcriptase inhibitor, NRTI), dapivirine (non-nucleoside reverse transcriptase inhibitor, NNRTI), tenofovir and MIV-150 (NNRTI) and vaginal films containing tenofovir, dapivirine, RC-101 (retrocyclin analog) and IQP-0528 are under development by several research groups as they may potentially provide improved user compliance and adherence3–11. As an ideal anti-HIV microbicide, it should possess the properties11: (1) potent activity against most HIV strains and other sexually transmitted pathogens; (2) effective against both cell-free and cell-associated HIV; (3) no effect on the integrity of vagina and cervical mucosal epithelium; (4) no effect on vaginal commensal flora, especially lactobacilli; (5) resistant to acidic pH and stable at higher, tropical temperatures; (6) odorless, colorless and tasteless and (7) easy to use and low cost.
Recently, a novel NRTI, 4′-ethynyl-2-fluoro-2′-deoxyadenosine (EFdA), was reported to exert highly potent antiretroviral activity both in vitro12–14 and in vivo15,16. EFdA retains potent activity against a variety of drug-resistant and multi-drug resistant strains of HIV, and has very favorable toxicity profiles in vitro12 and in vivo16. Thus, EFdA may be a very promising drug candidate for use in both HIV therapeutic and preventive modalities. In order to direct the development of EFdA in a vaginal pharmaceutical dosage form, it is important to assess the fundamental properties of the drug substance that are important factors for the development of a stable, safe, effective and marketable formulated product such as tablet, gel, film or ring. The aim of the present studies was to provide preclinical preformulation data for EFdA to facilitate the development of EFdA related drug delivery systems. To this end, we carried out a series of preformulation work including analytical method development and validation, physicochemical properties such as pH-solubility profile, Log Po/w, field emission scanning electron microscopy, DSC analysis, pH and thermal stability, in vitro cytotoxicity and bioactivity, and drug permeability across cell-and human tissue-based models.
Materials and methods
Materials
EFdA was a generous gift from Yamasa Corp. (Chiba, Japan). BD Falcon™ cell culture inserts, methanol (HPLC grade), 1-octanol, DMSO, HEPES and fetal bovine serum (FBS) potassium biphthalate, potassium phosphate monobasic, potassium chloride, sodium chloride, certified 0.2M sodium hydroxide and hydrochloric acid solutions were obtained from Fisher Scientific (Pittsburgh, PA). HBSS was purchased from Lonza (Walkersville, MD). MTT was purchased from Sigma-Aldrich (St. Louis, MO). Methanesulfonic acid was obtained from Acros (Morris Plains, NJ). Phosphate buffered saline (PBS, pH7.4), RPMI1640, Dulbecco’s modified Eagle medium (DMEM) and penicillin-streptomycin were purchased from Mediatech Inc (Manassas, VA). All other chemicals were analytical grade. Ultrapure water was obtained in-house from a MilliQ water purification unit.
HPLC analysis
An HPLC system (Waters Corporation, Milford, MA) equipped with an auto injector (model 717), a quaternary pump (model 600), and a Photodiode Array Detector (PDA, model 2996) was used for analytical method development. Empower Pro 2 software was used to control the HPLC system. Separation of the compound of interest was achieved by using a Zorbax Eclipse XDB C18 column (3.5μm, 100 × 4.6 mm). The mobile phase consisted of (A) 0.4% phosphoric acid in MilliQ water and (B) methanol using a gradient elution of 10–40% B at 0–5 min, 40–60% B at 5–10 min 60% B at 10–11 min, 60–10% B at 11–13 min, and 10% B at 13–20 min at a flow rate of 0.8 ml/min. Sample injection volume was 10 μl and EFdA was determined by UV detection at 260 nm. All experiments were performed at room temperature and the total area of peak was used to quantify EFdA.
Physicochemical characterization
pH-solubility profile
Buffer systems in the range of 3~9 were used for pH-solubility profile studies. Standard buffer solutions were 0.2M and included acid phthalate buffer (pH 3.0 or 4.0), neutralized phthalate buffer (pH 5.0), PBS (pH 5.0, 6.0 or 7.0), and alkaline borate buffer (pH 9.0) prepared according to USP. In order to investigate the effect of ionic strength on the solubility of EFdA, the ionic strength of different buffer solutions at pH 4, 7 and 9 was adjusted to 0.5 and 1.0 by adding sodium chloride. An excess amount of EFdA was added to microcentrifuge tubes containing 1.0 ml of the different buffer solutions. The samples were mixed using a Multi-Purpose Rotator with moderate rotation speed at ambient temperature for 120 h. The samples were then filtered through a 0.2 μm membrane filter and the filtrate was analyzed by HPLC as described above. All measurements were conducted in triplicate.
Octanol/water partition coefficient (Log Po/w) determination
Octanol-aqueous solutions comprising octanol and MilliQ water, acetate buffer (pH 4.1) or PBS (pH 7.4) in the ratio of 1:2 (v/v) were mixed and allowed to pre-saturate for 24 h at room temperature. Known amounts of EFdA were added to the different pre-saturated mixtures, and the samples were rotated end-over-end at room temperature for 120 h. Aliquots of the aqueous phase were withdrawn and assessed for EFdA content by HPLC. The concentration of EFdA in the octanol phase was calculated based on distribution of the known amount of EFdA into the aqueous phase. Log Po/w was calculated as the logarithm of the ratio of EFdA concentration in the octanol to that in the aqueous phase. All determinations were done in duplicate. Calculated Log Po/w (cLog Po/w) was determined using Marvin sketch software (version 5.5).
Field emission scanning electron microscopy
Surface morphology of EFdA was imaged by field emission scanning electron microscopy system (Philips XL30 FEG) at an accelerating voltage of 10 kV. Samples for field emission scanning electron microscopy (FESEM) were mounted on aluminum holders by carbon conductive glue and coated with a platinum layer by platinum sputter coater before scanning.
Differential scanning calorimetry
Differential Scanning Calorimetry (DSC) was performed using Perkin-Elmer DSC 7, TAC 7/DX Thermal Analysis Controller (Boston, MA), and Pyris software. DSC thermograms were obtained by heating from 30 to 300 °C at a heating rate of 20 °C/min under a constant nitrogen purge of 20 ml/min.
X-ray diffraction
X-ray diffraction (XRD) analysis of EFdA powders was conducted by an X-ray diffractometer (Philips PW1830/00, the Netherlands) equipped with a Cu Kα radiation source (40 kV, 30 mA, λ = 0.15406 nm). EFdA powders were pressed onto the sample holder to form a thin EFdA layer. The samples were measured from 2.5° to 45° at a rate of 0.04°/s.
Polarized light microscopy
Microscopic observations were performed using the Zeiss Axioskop 40 inverted phase-contrast microscope with polarized light filter. Images were acquired using AxioCam MRc5 color video camera and analyzed using AxioVision Rel 4.7 software. EFdA powders were mounted on glass slides, smeared with cytoseal™ 60, covered with cover slips and observed under polarized light.
Dynamic vapor sorption
Dynamic vapor sorption (DVS) analysis was conducted at Micromeritics Pharmaceutical Services (Norcross, GA) using DVS-Intrinsic (Surface measurement systems Ltd, UK) to investigate hygroscopicity behavior of EFdA. A drying step at 0% relative humidity (RH) was started and held for at least 2 h at 40 °C. For typical hygroscopicity assessment, approximately 7 mg of sample was used and the experiment was performed under isothermal conditions at 25 °C. The relative humidity (%RH) is stepped from a low initial level (0 %RH) to a high level (90 %RH), then back down to 0 %RH. Data was collected using DVS-Intrinsic control software and exported to an Excel spreadsheet for graphing.
Stability study
Known concentrations of EFdA were prepared in various buffer solutions ranging from pH 3–9 and incubated at 25 °C in the dark to avoid complications from potential photo-decomposition. Aliquots were removed at various times over a period of 21 days and the amount of EFdA was quantified by HPLC. Thermal stability of EFdA was assessed by incubation of aqueous solutions of the drug at 25, 40 or 65 °C for up to 21 days. Oxidative stability was evaluated by incubating a solution of EFdA in 0.02% hydrogen peroxide. Photolytic stability was assessed by exposing an aqueous solution of EFdA to light (Philips Daylight, 20 W). In all stability studies, the EFdA levels were quantified at different incubation times and compared to those of the starting solution. All determinations were in triplicate.
In vitro cytotoxicity
Two human epithelial carcinoma cell lines, CaSki (cervical origin) and A 431 (epidermal origin), were obtained from ATCC and the cells were cultured at 37 °C with 5% CO2 under fully humidified conditions. The A 431 cells were cultured in DMEM medium, supplemented with 10% fetal bovine serum (FBS), 100 IU/ml penicillin and 100 μg/ml streptomycin sulfate, and the CaSki cells were cultured in RPMI 1640 medium, supplemented with 10% FBS, 100 IU/ml penicillin and 100 μg/ml streptomycin sulfate.
CaSki and A 431 cells were seeded in 96-well plates at a density of 1 × 104 cells per well, respectively. After 24 h of incubation at 37 °C, the growth medium was replaced with 200 μl medium containing the drug samples with concentrations ranging from 1 ng/ml to 50 μg/ml. After 24, 48 or 72 h incubation, cell survival was measured using MTT assay. Briefly, drug-containing medium was removed and 180 μl of fresh growth medium and 20 μl of MTT (5 mg/ml) solution were added to each well. The plate was incubated for an additional 4 h at 37 °C and the media was removed, and then 200 μl of DMSO was added to each well to dissolve any purple formazan crystals formed. The plates were vigorously shaken before measuring the relative color intensity. The absorbance at 595 nm of each well was measured by a microplate reader (Beckman Coulter® DTX 880, US).
Transport of EFdA across Caco-2 cell monolayers
Caco-2 cells were cultured in DMEM medium, supplemented with 10% FBS, 1% non-essential amino acid solution, 1% L-glutamine, 1% penicillin–streptomycin at 37 °C with 5% CO2 under fully humidified conditions. The cells were grown to 90% confluence and harvested by trypsinization using a 0.25% trypsin and 0.02% EDTA solution. For transport studies, Caco-2 cells were seeded at a density of 2.5 × 105 onto polycarbonate inserts (4.2 cm2) in 6-well tissue culture plates. The medium was changed every second day for the first week and then replaced daily. Caco- 2 cell monolayers were used between days 21 and 24 post-seeding. Cell passages between 27 and 32 were used in the experiment. The quality of the monolayers grown on the permeable membrane was assessed before and after the transport studies by the transepithelial electrical resistance (TEER) of the monolayers at 37 °C using a Millicell-ERS apparatus (Millipore, Bedford, MA). Only monolayers displaying TEER values >600 Ωcm2 were used in transport studies17,18.
Bidirectional transport of EFdA, apical-to-basolateral (a–b, absorptive) and basolateral-to-apical (b-a, secretory) was measured using Caco-2 cell monolayers prepared as described above. The incubation medium was Hank’s balanced salt solution (HBSS) buffered either with 10mM methanesulfonic acid (pH 5.5 and 6.5), or with 25mM HEPES (pH 7.4)19. Before each experiment, the cell monolayers were washed twice with HBSS and then the monolayers were preincubated at 37 °C for 20 min with HBSS at the appropriate pH, followed by the measurement of TEER values. To investigate the effect of apical pH on the transport of EFdA from a–b, 1.5 ml of HBSS (pH 5.5, 6.5 or 7.4) containing EFdA (25 μg/ml) was added onto the apical side and 2.0 ml of HBSS (pH 7.4) without the test compound was added on the basolateral side. Additionally, in order to investigate the bidirectional transport of EFdA across Caco-2 cell monolayers, an expanded concentration range of EFdA (2.5~250 μg/ml) was applied. Briefly, 1.5 ml of HBSS (pH 7.4) containing EFdA was added onto the apical side and 2.0 ml of HBSS (pH 7.4) without EFdA was added on the basolateral side. Conversely, for the determination of b-a transport, 2.0 ml of HBSS (pH 7.4) containing EFdA was added onto the basolateral side and 1.5 ml of HBSS (pH 7.4) without the drug was added on the apical side. The Caco-2 cell monolayers were incubated at 37 °C for 2 h with moderate shaking. 200 μl medium was taken from each donor or receptor compartment at 0, 15, 30, 45, 60, 90 and 120 min, and then the same volume of fresh HBSS (pH 7.4) was supplemented. After the final time point, TEER values were measured again. EFdA in all samples was analyzed using HPLC as described above.
The apparent permeability coefficient (Papp; cm/s) of EFdA was determined from the amount of compound transported over time. Papp was calculated according to the following equation:
| (1) |
where dQ/dt is the steady-state flux (μg/s), A is the surface area of exposure (cm2) and C0 is the initial concentration in the donor chamber (μg/ml). The ratio of the transport in the b-a direction to that in the a–b direction was calculated in order to highlight any asymmetry in the transport of the compounds. Efflux ratio was calculated using the following equation:
| (2) |
Human tissue permeability study
Freshly excised human ectocervical tissue was obtained from the Tissue Procurement Facility at Magee-Womens Hospital under IRB approved protocol. Tissue samples were from 3 women with median age of 40 years undergoing hysterectomy for benign conditions. For each tissue, three replicates were performed for permeability studies. All tissue specimens were obtained within 2 h of surgical excision. Tissues were held at 4 °C in Dulbecco’s modified Eagle medium (DMEM) during transfer from surgery to the laboratory. Excessive stromal tissue was removed and the epithelial layer was isolated using a Thomas-Stadie Riggs tissue slicer (Thomas Scientific, Swedesboro, NJ). The thickness of each tissue was measured by placing the tissue between two slides and the thickness was measured using a micrometer.
Tissue permeability studies were conducted using a Franz cell system (PermeGear, Nazareth, PA). The Franz cell system was a two-compartment system consisting of an upper chamber (donor compartment) and a lower chamber (receptor compartment). The system was water-jacketed and temperature was maintained at 37 °C throughout the experiment via a circulating water bath. The isolated epithelial sections of each tissue were placed between the donor and receptor compartments with the epithelial side of the tissue oriented towards the donor compartment which provided a diffusion area of 0.385 cm2. PBS (pH 7.4) solution was used in the receptor chamber. The latter chamber (5.0 ml volume) was continuously stirred by a magnetic stir bar. The tissue was equilibrated with PBS in the donor compartment for 5 min prior to the permeability study. After the equilibration period, PBS was removed from the donor chamber and replaced with 450 μL of EFdA (500 μg/ml in PBS, pH 7.4). 50 μL was removed from the donor compartment for mass balance. At various time intervals over a 6 h period, 200 μL aliquots were removed from the receptor compartment. Receptor compartment medium was replenished with fresh medium after removal of each aliquot. EFdA in the receptor compartment aliquots was quantified by HPLC.
Bioactivity analysis
Two types of bioactivity tests were carried out, (i) standard antiviral assessments in which cells were simultaneously exposed to varying concentrations of drug and HIV, with drug being present throughout the infection process and (ii) protective or memory effect assessments in which cells were pretreated with varying concentrations of drug for 16 h, then exogenous drug was removed by extensive washing and the cells exposed to HIV in the absence of exogenous drug. HIV replication was evaluated in single replication cycle HIV assays, using P4R5 HIV infection indicator cells (from Dr John Mellors, University of Pittsburgh). Cells were maintained in DMEM/10% FBS supplemented with puromycin (0.5 g/ml). P4R5 cells express CD4, CXCR4 and CCR5 as well as a β-galactosidase reporter gene under the control of an HIV LTR promoter. Viral infectivity was assessed in 96-well microplate assays seeded with P4R5 cells at a density of 5 × 103 cells/well. Cells were inoculated with 25 ng HIV-1 p24/well and the extent of infection was evaluated 48 h post-infection using a fluorescence-based β-galactosidase detection assay. Briefly, infected cells were washed, then incubated with 100μL lysis buffer (60mM Na2HPO4, 40mM NaH2PO4 (pH 7.2), 1mM MgSO4, 100mM -mercaptoethanol, 2% [v/v] Triton X-100) for 1 h at 37 °C. β-Galactosidase activity was assessed by addition of 50μL 4-MUG to a final concentration of 0.5 mM, incubation for 1 h at 37 °C, and then quenched with 150 μL 0.2M Na2CO3 (pH 11.2). Fluorescence intensity was assessed with a SPECTRA max GEMINI XS dual-scanning microplate spectrofluorometer (Molecular Devices, Sunnyvale, CA) using an excitation wavelength of 355 nm and an emission wavelength of 480 nm, with cutoff filter set to 475 nm.
Statistical analysis
Statistical analysis was performed by Student’s t-test for two groups and one-way ANOVA for multiple groups. All results were expressed as the mean ± standard deviation (SD). A p value <0.05 is considered statistically significant.
Results and discussion
Analytical method for EFdA
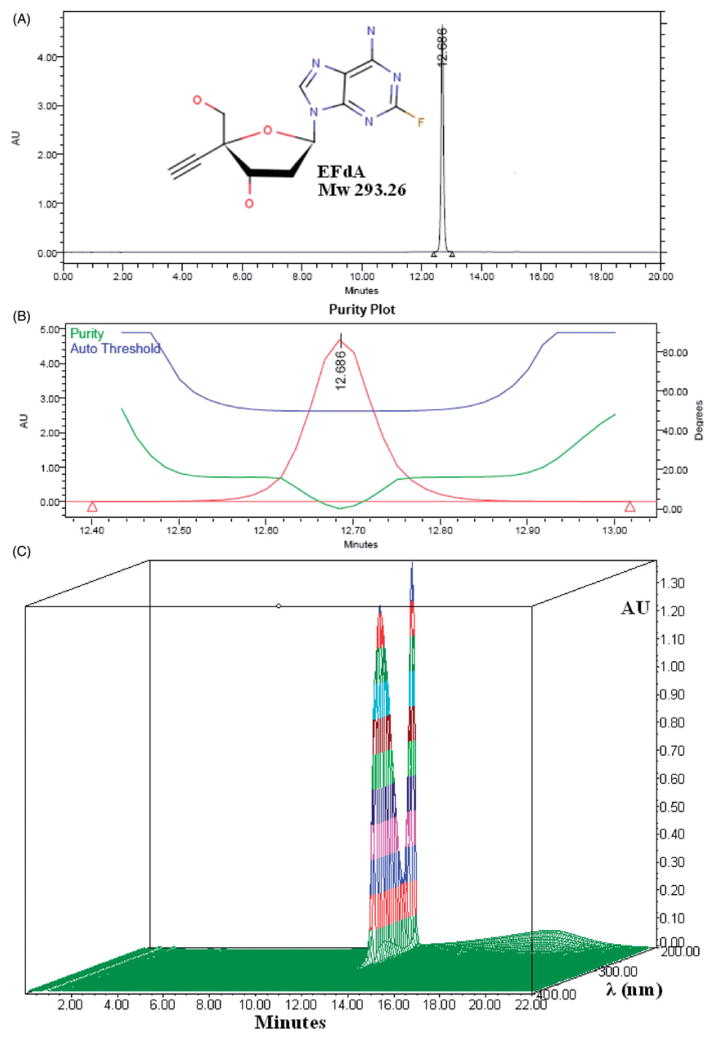
Figure 1(A) shows a typical chromatogram of EFdA, with a retention time of approximately 13 min. The purity angle of EFdA was less than the purity threshold, indicating that there is no evidence of spectral heterogeneity (Figure 1B). Linearity of the standard curve for quantification of EFdA was determined by linear least squares regression analysis of a plot of peak area versus EFdA concentration, using seven standard solutions of EFdA over a range of 0.2~200 μg/ml. Standard variation was <1.5%. The Limit of Detection (LOD) and Limit of Quantification (LOQ) for EFdA were estimated at a signal-to-noise ratio (S/N) of 3:1 and 10:1, respectively. The LOD was 0.05 μg/ml and LOQ was determined to be 0.15 μg/ml. As presented in Figure 1(C), the maximum absorbance for EFdA was at 260 nm.
Figure 1.
(A) Typical HPLC chromatogram of EFdA; (B) EFdA peak purity plot; (C) HPLC-DAD analysis of EFdA.
Physicochemical characterization
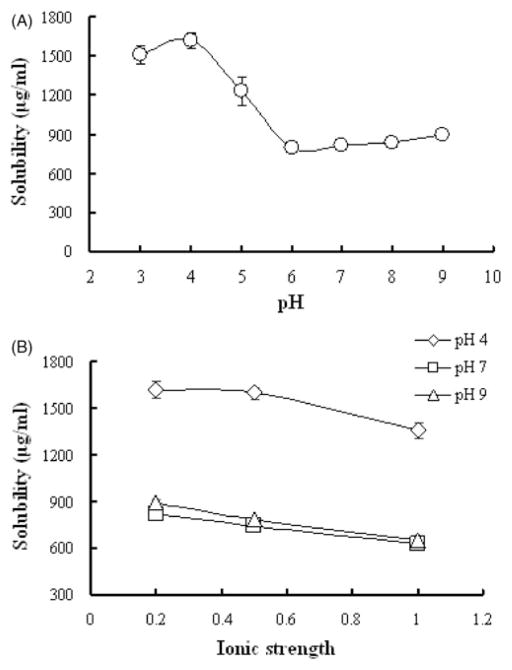
The solubility of EFdA was found to be pH dependent (Figure 2A). An increase in pH from 3 to 6 resulted in a significant decrease in EFdA solubility (from 1508.7±68.4 to 799.2±8.7 μg/ml, p<0.05), indicating that EFdA may be partially protonated under acidic conditions. From pH 6–9, no obvious change in solubility was observed for EFdA. Figure 2(B) shows the solubility of EFdA as a function of ionic strength under three different pH conditions. At pH 4.0, increase in ionic strength from 0.2 to 1.0, resulted in a slight decrease in solubility of EFdA (from 1620.8±55.9 to 1358.3±48.3 μg/ml, p>0.05). As for pH 7.0 and 9.0, a statistically significant decrease in the solubility of EFdA was observed with increase in ionic strength (p<0.05), suggesting that the high concentration of the salts in the solution may have negative impact on the solubility of EFdA. Additionally, it was found that the solubility of EFdA in Vaginal Fluid Simulant (VFS, pH = 4.2) was 887.4 μg/ml, less than that of acid phthalate buffer (pH 4.0), possibly due to the increased ionic strength in VFS. EFdA was also found to be soluble in DMSO (>20 mg/ml), ethanol (>10 mg/ml) and acetonitrile (>1 mg/ml), respectively.
Figure 2.
(A) pH-solubility profile of EFdA in different buffer solutions at various pH; (B) The effect of ionic strength on solubility of EFdA. Each point represents mean ± SD (n = 3).
Drug octanol/water partition coefficient (Log Po/w) is commonly used to estimate the potential for drug absorption. Log Po/w describes the ability of a drug molecule to partition into a lipophilic phase, octanol, which is assumed to have comparable lipophilicity to that of biological membranes20. The experimental partition coefficients determined for water, PBS (pH 7.4) and acetate buffer (pH 4.1) were −1.19, −0.85 and −0.87, respectively, suggesting that EFdA was hydrophilic. In addition, the cLog P predicted by Marvin sketch software was −0.82, which correlates well with the experimental Log Po/w (−1.19).
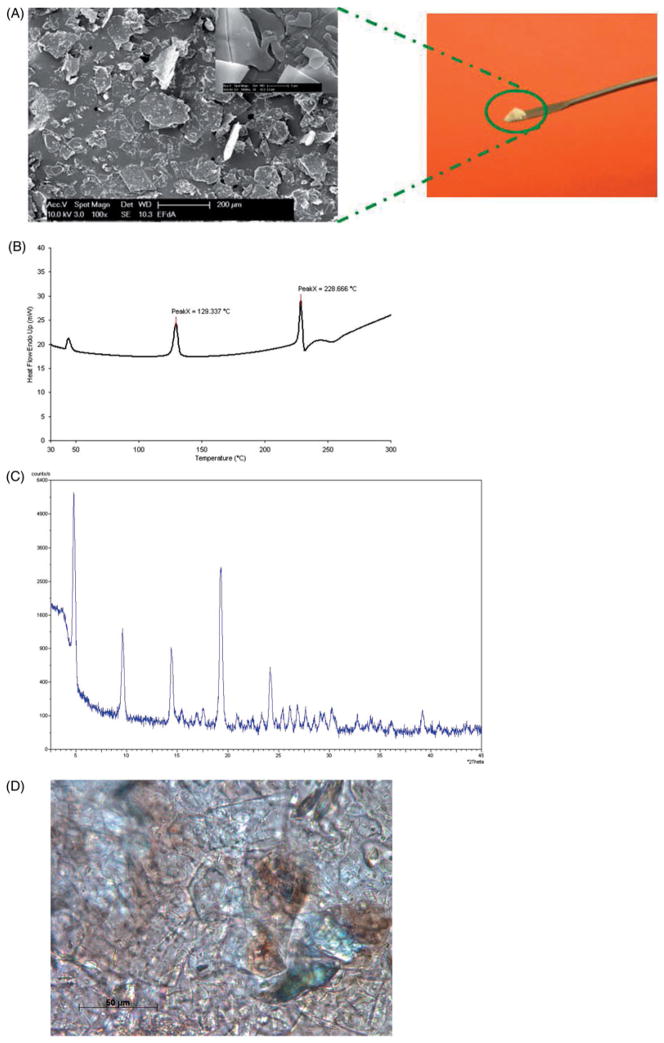
FESEM was used to better understand the crystal structure for EFdA. The FESEM images (Figure 3A) showed that EFdA crystals existed in planar or flaky shape of a non-uniform particle size. Aggregates of crystals were also observed. As presented in Figure 3(B), two endothermic events were obtained in the EFdA DSC thermogram, one at 129.3 °C and the other at 228.7 °C. The first endothermic peak observed in DSC may be attributed to the melting of crystalline drug powder, while the second endothermic event at 228.7 °C followed by a small exothermic peak in DSC might be due to the thermal decomposition of EFdA at higher temperature. XRD analysis was also performed to elucidate the crystalline state of EFdA. It was observed that EFdA powder presented high crystallinity by its sharp and intense diffractive peaks at 4.8°, 9.5°, 14.4°, 19.3° and 24.2° (Figure 3C). Crystallinity of EFdA was further confirmed by the presence of birefringence as observed using polarized light microscopy (Figure 3D). Results of dynamic vapor sorption study (Figure 4) indicated that EFdA was hygroscopic in nature and absorbed approximately 10% w/w moisture at 25°C/80% RH. Additionally, no sharp weight decrease was found in the desorption profile, suggesting that no hydrate was formed.
Figure 3.
(A) Observation of drug powder by field emission scanning electron microscopy (FESEM). Insert shows FESEM image of EFdA with the scale bar = 2 μm. (B) DSC thermogram for EFdA. DSC analysis was performed with Perkin-Elmer DSC 7, TAC 7/DX Thermal Analysis Controller (Boston, MA), and Pyris software. (C) X-ray diffractograms of EFdA powders. X-ray diffraction was conducted by an X-ray diffractometer (Philips PW1830/00, Netherlands) equipped with a Cu Kα radiation source (40 kV, 30 mA, λ = 0.15406 nm). (D) Polarized light microscopic image of EFdA with the scale bar = 50 μm.
Figure 4.
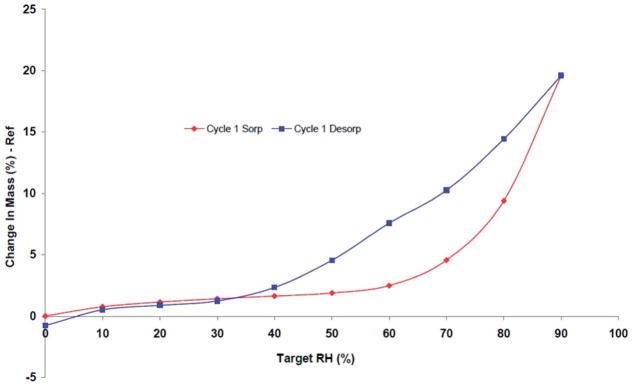
DVS isotherm plot of EFdA.
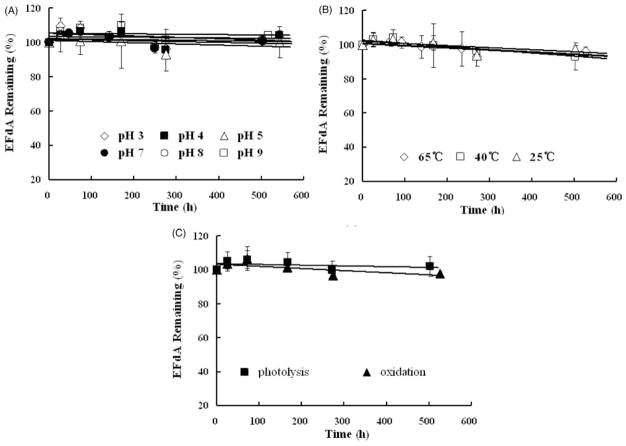
To elucidate the primary potential degradation pathways of EFdA, forced degradation studies (acid hydrolysis, base hydrolysis, photolysis, oxidative and thermal stability) were performed (Figure 5A–C). EFdA was very stable under all test conditions studied, with >95% of the drug remaining following exposure to accelerated condition. These data showed that EFdA was stable to light, 0.02% hydrogen peroxide and from pH 3.0–9.0. This data indicates that EFdA is stable at the physiological pH of the vagina (approximately 4.2). Additionally, its observed stability at relatively high temperature (~65 °C) is important for formulation development options such as solid dispersion preparation, film casting or nanoparticle fabrication.
Figure 5.
Kinetic stability of EFdA (200 μg/ml) in buffer solutions against time under different pH (A), temperature (B), light and oxidation (C) conditions. Each point represents mean ± SD (n = 3).
In vitro cytotoxicity
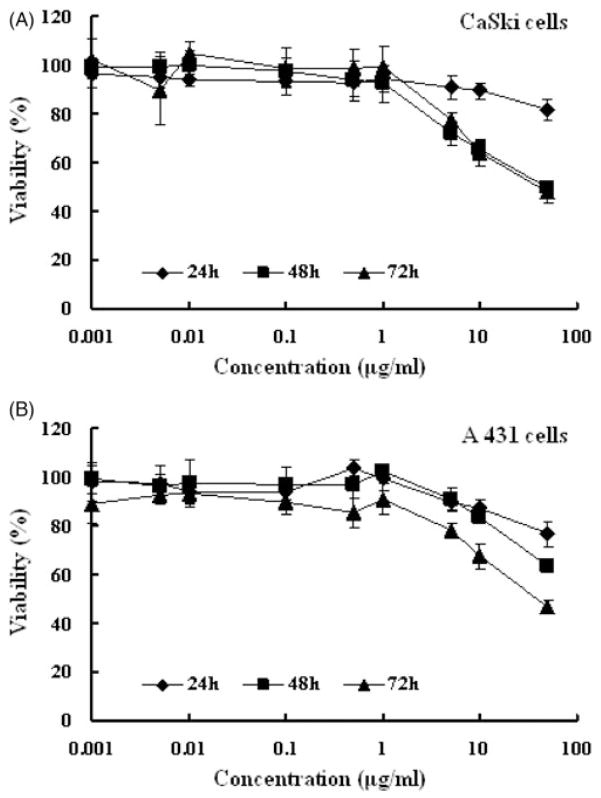
In order to reduce the failure rate of a microbicide formulation in animal and clinical testing, it was important to assess potential safety issues, since local inflammation and disruption of epithelial barriers could lead to an increased risk of HIV acquisition or infection21. Therefore, cell viability assays were performed to determine the effect of EFdA exposure on CaSki and A 431 cell lines21–23. As illustrated in Figure 6(A), the EFdA provided a time-dependent slight increase in cytotoxicity in CaSki cells, with CC50 values greater than 50 μg/ml following 24 h treatment, 47.81±12.40 μg/ml at 48 h and 41.11±8.78 μg/ml at 72 h. Similar results were noted for the A 431 cell line (Figure 6B). The low cytotoxicity found in the present study correlates well with previous assessments of EFdA toxicity in vitro12–14 and in vivo15,16.
Figure 6.
In vitro cytotoxicy of EFdA at various concentrations against CaSki (A) and A 431 (B) cell lines at 24, 48 and 72 h. Each point represents mean ± SD (n = 8).
Transport study across Caco-2 cell monolayers
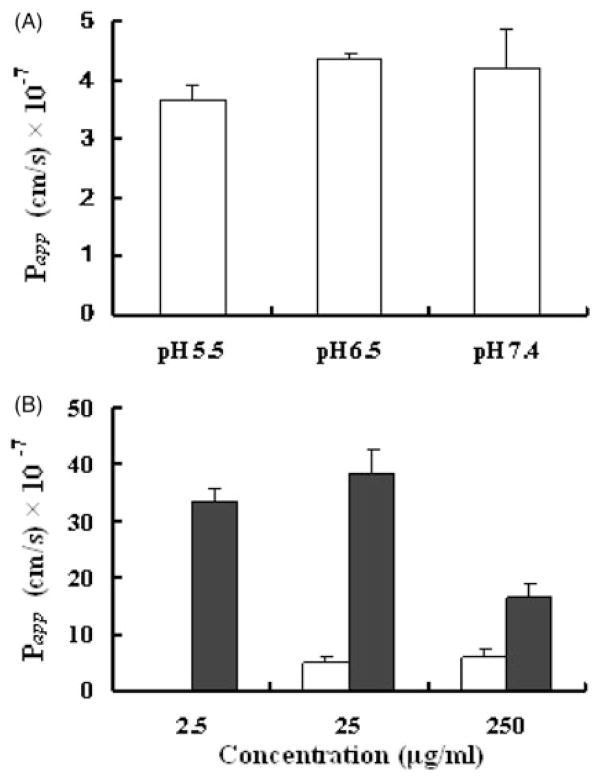
Caco-2 cell monolayers have become the standard for in vitro prediction of intestinal drug permeability24,25. We used this system to evaluate EFdA transport. In our study, the pH of the apical medium was varied (5.5, 6.5 or 7.4) while maintaining the basolateral pH at 7.4 since it is known that oral absorption of drugs can be pH-dependent due to altered solubilities of weakly acidic or basic drugs26,27. As shown in Figure 7(A), the Papp values from apical side to basolateral side under different pH conditions were similar (3.67±0.27) × 10−7 cm/s for pH 5.5, (4.36±0.09) × 10−7 cm/s for pH 6.5 and (4.20±0.66) × 10−7 cm/s for pH 7.4, indicating that the transport of EFdA across Caco-2 cell monolayers was independent of pH within the range studied. This is consistent with the constant solubility of EFdA over this pH range, as described above.
Figure 7.
(A) Effect of pH on the apparent permeability coefficient (Papp) of EFdA across Caco-2 monolayers. Caco-2 monolayers were incubated with 25 μg/ml EFdA on the apical side at 37 °C. The pH of apical side was 5.5, 6.5, 7.4, and the pH of the basolateral side was maintained at pH 7.4. (B) Bidirectional transport of EFdA across Caco-2 cell monolayers at concentrations of 2.5, 25 or 250 μg/ml (Open: a–b; Filled: b-a). The buffer pH was the same on both sides of the monolayer (pH 7.4). Each value indicates mean ± SD (n = 3).
The influence of EFdA concentration on bidirectional transport of EFdA across Caco-2 cell monolayers was also determined. As presented in Figure 7(B), from apical to basolateral, the Papp (a–b) values of EFdA were (4.80±1.24) × 10−7 and (5.95±1.44) × 10−7 cm/s for 25 μg/ml and 250 μg/ml treatments, respectively. The differences between these values was not significant (p>0.05), suggesting that passive diffusion played a primary role in EFdA transport. As for the absorptive transport (a–b) of EFdA at the concentration of 2.5 μg/ml, no EFdA was detected in the receptor compartment, probably due to the LOD of HPLC method (0.05 μg/ml). For the transport of EFdA from basolateral to apical side, no significant difference in Papp (b-a) value was observed between low and medium concentration groups (p>0.05). However, the Papp (b-a) value obtained for the high concentration group was about 2-fold lower than those of low and medium concentration treatments, suggesting the possibility of a saturable transport process. These data suggest that there was no indication of saturation up to 25 μg/ml which was several orders of magnitude higher than the EC50 of EFdA (~0.07 nM)13. The efflux ratios for the medium and high concentration groups were 8.01 and 2.74, respectively, both greater than 1, suggesting that the efflux transporters such as P-glycoprotein (P-gp), multidrug resistance-associated proteins (MRPs) and breast cancer resistance protein (BRCP) or certain influx transporters expressed on the basolateral side might be involved in the transport of EFdA across Caco-2 cell monolayers.
Human tissue permeability study
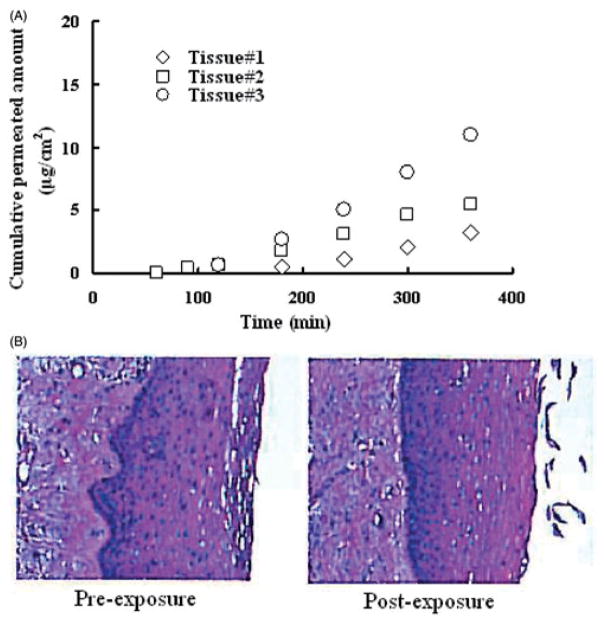
The potent anti-HIV activity of EFdA suggests that it may have significant potential as a microbicide candidate for use in HIV prevention. For this application, EFdA could be formulated into dosage forms such as films, gels or rings for vaginal use by women. To understand the potential for systemic uptake of EFdA when delivered topically, a permeability study for EFdA using excised human ectocervical tissues was performed. EFdA transport through human ectocervical tissues was determined by quantitating the amount of EFdA found in the receptor compartment at predetermined time intervals using developed HPLC methods. The amount of drug which permeated the tissue was used to calculate the apparent permeability coefficient (Papp) as described previously28. The average Papp value obtained from 3 separate excised human cervical tissues was (8.34±4.50) × 10−7 cm/s (Figure 8A), which correlated well with previous results of transport studies conducted in Caco-2 cell monolayers. Additionally, both inter- and intra-patient variability were observed among EFdA diffusion profiles obtained from different human ectocervical explants. It is well known that lipophilic compounds are usually transported through the transcellular route while hydrophilic drugs are primarily transported through the paracellular pathway which consists of small watery channels and pores within the incontinuous or interrupted lipid membrane28. The average molecular size cutoff for the paracellular route of passive diffusion is approximately 500 Da29. Based on the negative Log Po/w value of EFdA and permeability study results (Caco-2 cell monolayers and excised human cervical tissues), we propose that the paracellular route is the dominant pathway for EFdA to penetrate the vaginal epithelia. Furthermore, the lack of any significant morphological changes in human ectocervical tissues exposed to EFdA suggests the low tissue toxicity of EFdA (Figure 8B).
Figure 8.
(A) The cumulative amount of EFdA transported through the tissue versus time obtained from permeability experiments with 3 different fresh human ectocervical tissues. (B) Comparison of morphology of human ectocervical tissues pre- and post-treatment.
Bioactivity analysis
UC781 was the first potent NNRTI to be considered for use in microbicidal applications30. We compared the anti-HIV activity of EFdA to that of UC781 using a cell-based assay that effectively limits HIV replication to a single cycle. Both EFdA and UC781 were found to be highly potent inhibitors under conditions in which the drugs remain present at constant levels throughout the HIV infection process (Figure 9A). However, EFdA was found to about two-fold more potent than UC781 under these conditions. The protective or “memory” effect imparted by EFdA was significantly superior to that of UC781 (Figure 9B). In these experiments, designed to assess the pre-exposure prophylactic activity of microbicide candidates, uninfected cells are exposed to drug, the exogenous drug is then removed and the pretreated cells are exposed to HIV. As seen in Figure 9(B), cells pretreated with EFdA retained anti-HIV protection similar to that seen when EFdA was present throughout the infection (Figure 9A). In contrast, cells pretreated with identical concentrations of UC781 showed substantial diminution of anti-HIV protection as compared to conditions where UC781 was present throughout infection.
Figure 9.
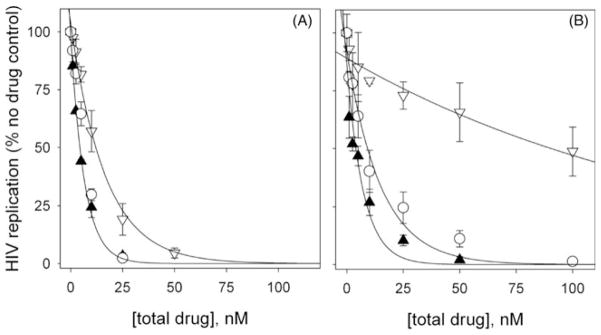
Bioactivity analysis of EFdA (▲), the NNRTI UC781 (▽) and an equimolar combination of EFdA+UC781 (○). Details of the experimental protocol are in Materials and Methods. (A) Antiviral activity. P4R5 cells were simultaneously exposed to HIV-1 and the indicated concentrations of the drug. Drug was maintained in the culture throughout the 48 h infection period. (B) Protective activity or “memory” effect. Uninfected P4R5 cells were preincubated with the indicated concentration of drug for 16 h. The cells were washed free of exogenous drug and then exposed to infectious HIV. No exogenous drug was present throughout the 48 h infection period.
Conclusions
Preformulation studies revealed that EFdA is relatively soluble in water, exists in planar or flaky structure, and has good stability upon exposure to different pH conditions and increased temperature over a period of 21 days. In vitro cytotoxicity of EFdA was performed in different human epithelial cell lines, and the cytotoxic profiles for EFdA were found to be dependent on incubation time and cell line origin. Transport studies conducted in Caco-2 cell monolayers suggest that both passive and active transport mechanisms may be involved in bidirectional transport of EFdA. In addition, the apparent permeability coefficient (Papp) of EFdA was found to be 8.34 × 10−7 cm/s in an excised human ectocervical tissue permeability study. Taken together, the low cytotoxicity, potent anti-HIV activity, and good stability profile for EFdA provide rationale for the development of this drug substance into an anti-HIV pharmaceutical product.
Acknowledgments
We gratefully acknowledge the staff associated with the Materials Micro- Characterization Laboratory, Department of Mechanical Engineering and Materials Science, University of Pittsburgh, for assistance with the scanning electron microscopy conducted in this study. We would like to thank Marilyn R. Cost for her technical support in the human tissue processing.
Research reported in this publication was supported in part by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number AI 076119 and AI 079801. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Declaration of interest
Wei Zhang, Michael A. Parniak, Stefan G. Sarafianos, Phillip W. Graebing and Lisa C. Rohan report no declaration of interest. Hiroaki Mitsuya is a named inventor on patent US 7339053 which describes EFdA and its activity against HIV-1.
References
- 1.UNAIDS. Global Report: UNAIDS world AIDS day report. 2011 [Google Scholar]
- 2.Hladik F, McElrath MJ. Setting the stage: host invasion by HIV. Nat Rev Immunol. 2008;8:447–57. doi: 10.1038/nri2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rohan LC, Moncla BJ, Ayudhya RP, et al. In vitro and ex vivo testing of tenofovir shows it is effective as an HIV-1 microbicide. PLoS One. 2010;5:e9310. doi: 10.1371/journal.pone.0009310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akil A, Parniak MA, Dezzutti CS, et al. Development and characterization of a vaginal film containing dapivirine, a non-nucleoside reverse transcriptase inhibitor (NNRTI), for prevention of HIV-1 sexual transmission. Drug Deliv Transl Res. 2011;1:511–17. doi: 10.1007/s13346-011-0022-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sassi AB, Cost MR, Cole AL, et al. Formulation development of retrocyclin 1 ananlog RC-101 as an anti-HIV vaginal microbicide product. Antimicrob Agents Chemother. 2011;55:2282–9. doi: 10.1128/AAC.01190-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ham AS, Rohan LC, Boczar A, et al. Vaginal film drug delivery of the pyrimidinedione IQP-0528 for the prevention of HIV infection. Pharm Res. 2012;29:1897–907. doi: 10.1007/s11095-012-0715-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geonnotti AR, Katz DF. Compartmental transport model of microbicide delivery by an intravaginal ring. J Pharm Sci. 2010;99:3514–21. doi: 10.1002/jps.22120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ndesendo VM, Pillay V, Choonara YE, et al. In vivo evaluation of the release of zidovudine and polystyrene sulfonate from a dual intravaginal bioadhesive polymeric device in the pig model. J Pharm Sci. 2011;100:1416–35. doi: 10.1002/jps.22365. [DOI] [PubMed] [Google Scholar]
- 9.Moss JA, Malone AM, Smith TJ, et al. Safety and pharmacokinetics of intravaginal rings delivering tenofovir in pig-tailed macaques. Antimicrob Agents Chemother. 2012;56:5952–60. doi: 10.1128/AAC.01198-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singer R, Mawson P, Derby N, et al. An intravaginal ring that releases the NNRTI MIV-150 reduces SHIV transmission in macaques. Sci Transl Med. 2012;4:150ra123. doi: 10.1126/scitranslmed.3003936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Balzarini J, Van Damme L. Microbicide drug candidates to prevent HIV infection. Lancet. 2007;369:787–97. doi: 10.1016/S0140-6736(07)60202-5. [DOI] [PubMed] [Google Scholar]
- 12.Nakata H, Amano M, Koh Y, et al. Activity against human immunodeficiency virus type 1, intracellular metabolism, and effects on human DNA polymerases of 4′-ethynyl-2-fluoro-2′-deoxyadenosine. Antimicrob Agents Chemother. 2007;51:2701–8. doi: 10.1128/AAC.00277-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kawamoto A, Kodama E, Sarafianos SG, et al. 2′-Deoxy-4′-C-ethynyl- 2-halo-adenosines active against drug-resistant human immunodeficiency virus type 1 variants. Int J Biochem Cell Biol. 2008;40:2410–20. doi: 10.1016/j.biocel.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 14.Michailidis E, Marchand B, Kodama EN, et al. Mechanism of inhibition of HIV-1 reverse transcriptase by 4′-ethynyl-2-fluoro-2′-deoxyadenosine triphosphate, a translocation defective reverse transcriptase inhibitor. J Biol Chem. 2009;284:35681–91. doi: 10.1074/jbc.M109.036616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hattori S, Ide K, Nakata H, et al. Potent activity of a nucleoside reverse transcriptase inhibitor, 4′-ethynyl-2-fluoro-2′-deoxyadenosine, against human immunodeficiency virus type 1 infection in a model using human peripheral blood mononuclear cell-transplanted Nod/SCID janus kinase 3 knockout mice. Antimicrob Agents Chemother. 2009;53:3887–93. doi: 10.1128/AAC.00270-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murphey-Corb M, Rajakumar P, Michael H, et al. Response of simian immunodeficiency virus to the novel nucleoside reverse transcriptase inhibitor 4′-ethynyl-2-fluoro-2′-deoxyadenosine in vitro and in vivo. Antimicrob Agents Chemother. 2009;53:3887–93. doi: 10.1128/AAC.00723-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sha XY, Fang XL. Transport characteristics of 9-nitrocamptothecin in the human intestinal cell line Caco-2 and everted gut sacs. Int J Pharm. 2004;272:161–71. doi: 10.1016/j.ijpharm.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 18.Leonard F, Collnot EM, Lehr CM. A three-dimensional coculture of enterocytes, monocytes and dendritic cells to model inflamed intestinal mucosa in vitro. Mol Pharm. 2010;7:2103–19. doi: 10.1021/mp1000795. [DOI] [PubMed] [Google Scholar]
- 19.Hubatsch I, Ragnarsson EG, Artursson P. Determination of drug permeability and prediction of drug absorption in Caco-2 monolayers. Nat Protoc. 2007;2:2111–19. doi: 10.1038/nprot.2007.303. [DOI] [PubMed] [Google Scholar]
- 20.Artursson P, Palm K, Luthman K. Caco-2 monolayers in experimental and theoretical predictions of drug transport. Adr Drug Deliv Rev. 2001;46:27–43. doi: 10.1016/s0169-409x(00)00128-9. [DOI] [PubMed] [Google Scholar]
- 21.Gali Y, Delezay O, Brouwers J, et al. In vitro evaluation of viability, integrity, and inflammation in genital epithelia upon exposure to pharmaceutical excipients and candidate microbicides. Antimicrob Agents Chemother. 2010;54:5105–14. doi: 10.1128/AAC.00456-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krebs FC, Miller SR, Catalone BJ, et al. Comparative in vitro sensitivities of human immune cell lines, vaginal and cervical epithelial cell lines, and primary cells to candidate microbicides nonoxynol 9, C31G, and sodium dodecyl sulfate. Antimicrob Agents Chemother. 2002;46:2292–8. doi: 10.1128/AAC.46.7.2292-2298.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Christensen ND, Reed CA, Culp TD, et al. Papillomavirus microbicidal activities of high-molecular-weight cellulose sulfate, dextran sulfate, and polystyrene sulfonate. Antimicrob Agents Chemother. 2001;45:3427–32. doi: 10.1128/AAC.45.12.3427-3432.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Artursson P. Epithelial transport of drugs in cell culture. I: A model for studying the passive diffusion of drugs over intestinal absorptive (Caco-2) cells. J Pharm Sci. 1990;79:476–82. doi: 10.1002/jps.2600790604. [DOI] [PubMed] [Google Scholar]
- 25.Artursson P, Karlsson J. Correlation between oral drug absorption in humans and apparent drug permeability coefficients in human intestinal epithelial (Caco-2) cells. Biochem Biophys Res Comm. 1991;175:880–5. doi: 10.1016/0006-291x(91)91647-u. [DOI] [PubMed] [Google Scholar]
- 26.Neuhoff S, Ungell AL, Zamora I, Artursson P. pH-dependent bidirectional transport of weakly basic drugs across Caco-2 monolayers: implications for drug–drug interactions. Pharm Res. 2003;20:1141–8. doi: 10.1023/a:1025032511040. [DOI] [PubMed] [Google Scholar]
- 27.Neuhoff S, Ungell AL, Zamora I, Artursson P. pH-dependent passive and active transport of acidic drugs across Caco-2 cell monolayers. Eur J Pharm Sci. 2005;25:211–20. doi: 10.1016/j.ejps.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 28.Sassi AB, Mccullough KD, Cost MR, et al. Permeability of tritiated water through human cervical and vaginal tissue. J Pharm Sci. 2004;93:2009–16. doi: 10.1002/jps.20107. [DOI] [PubMed] [Google Scholar]
- 29.He YL, Murby S, Warhurst G, et al. Species differences in size discrimination in the paracellular pathway reflected by oral bioavailability of poly (ethylene glycol) and D-peptides. J Pharm Sci. 1998;87:626–33. doi: 10.1021/js970120d. [DOI] [PubMed] [Google Scholar]
- 30.Barnard J, Borkow G, Parniak MA. The thiocarboxanilide nonnucleoside UC781 is a tight-binding inhibitor of HIV-1 reverse transcriptase. Biochemistry. 1997;36:7786–92. doi: 10.1021/bi970140u. [DOI] [PubMed] [Google Scholar]