Abstract
Depression is one of the most common, disabling, and costly conditions encountered in older primary care patients. Using the case of a 69-year-old woman who struggled with prolonged depression and comorbid medical illnesses, this article summarizes effective strategies for detection and treatment of late-life depression. Clinicians should screen older patients for depression using a standard rating scale, initiate effective treatment such as antidepressant medications or evidence-based psychotherapies, and carefully follow for improvement of depression symptoms. Patients who are not improving should be considered for psychiatric consultation and additional treatments such as electroconvulsive therapy (ECT). Several changes in treatment are often needed before patients achieve remission, and maintenance treatment and relapse prevention planning can reduce the risk of relapse. Evidence-based collaborative programs, in which primary care providers work closely with special mental health providers, following a measurement-based treatment-to-target approach have been shown to be significantly more effective than usual primary care.
The Patient's Story
In early 2001, Mrs B, then a 69-year-old retired cafeteria worker, developed a severe depressive episode. At the time, she was married, and was the primary caretaker for a son suffering from leukemia. A few months earlier, she had developed asthma-like symptoms (later diagnosed as gastroesophageal reflux disease, causing wheezing), which were treated with corticosteroids. She developed a corticosteroid-induced psychosis with visual hallucinations, reported that she had “never felt better in her life” and called people in the middle of the night. Her psychotic and manic symptoms resolved after discontinuing corticosteroids and by March 2001, she felt “depressed 24 hours a day.” She had “lost all interest in things.” Her concentration was poor, making it difficult for her to read a newspaper or follow television programs. She became increasingly disabled; unable to make even simple decisions and ultimately unable to get out of bed. Although she had worked in food services for many years, her daughter recalled, “She couldn't even fry an egg.”
She became increasingly anxious with related physical symptoms, including trouble swallowing, choking sensations, nausea, and vomiting. She reported being “in constant fear,” worrying that she was never going to get well. She had thoughts of death and suicide but no specific plan to end her life.
She slept poorly, and experienced severe appetite loss, with a 50-pound weight loss over 18 months. During this period, treatments with several antidepressant and anxiolytic medications proved unsuccessful. Her condition deteriorated and she had the first of what became several psychiatric hospitalizations. Her then psychiatrist recommended a course of electroconvulsive therapy (ECT) but her husband opposed this recommendation. Mrs B was discharged, additional medications were substituted and added, but she did not improve. Approximately 6 months after the onset of her depression, Mrs B's husband died unexpectedly. She was readmitted for a second time in December 2001, for a 50-day inpatient stay, again treated with a variety of medications without much improvement. In March 2002, after this second hospitalization, Mrs B's family arranged for her to live in an assisted living facility because she was too disabled to live at home or with family. Mrs B was readmitted for a third time for a 90-day inpatient stay, during which she had 8 ECTs, ending in the summer of 2002. She was judged to have a partial response with better appetite and interest. Still on several antidepressant medications, she was discharged to weekly outpatient psychotherapy.
Nonetheless, she remained depressed, stating “My days are hell.” She was independently able to carry out some ADLs, but still needed help in virtually all IADLs. In September 2002, her daughter took her to a university-based department of psychiatry, where she was seen by Dr F. At the time of her initial evaluation by Dr F, she was on bupropion SR 100 mg orally twice daily, mirtazapine 15 mg orally at bedtime, quetiapine 100 mg orally at bedtime, alprazolam 0.5 mg orally 3 times daily, and temazepam 30 mg orally at bedtime as needed. Dr F saw her every 2 to 4 weeks for several months. She increased the bupropion to 3 times daily, but saw little response.
In November 2002, she was admitted for a 35-day stay on an inpatient psychiatric unit. Dr F recommended electroconvulsive therapy. Her family strongly supported this recommendation and Mrs B consented. Upon admission, Mrs B's medical history included a hiatal hernia with gastroesophageal reflux disease, osteoporosis, and non-insulin dependent diabetes mellitus. Mrs B reported one prior episode of depression and anxiety. Her family history included a grandfather who had been in a mental hospital, a brother with depression and anxiety, and a sister with anxiety. She had recently lost her husband and her son.
Physical examination was essentially normal. She scored 26/30 points on the Mini Mental Status Examination, missing 4 points on “serial sevens.“ She exhibited limited eye contact, depressed mood, and restricted and tearful affect. She had no psychotic symptoms and no thoughts of harming herself or others. Routine laboratory testing including electrolytes, blood counts, thyroid function tests, B12, and folate were normal.
Mrs B was seen by geriatric, cardiology, and anesthesia consultants and deemed an acceptable candidate for ECT. She began the ECT in December 2002, receiving 12 treatments at 2- to 3-day intervals. She reported some short-term memory loss between treatments but cognitive testing revealed no significant long-term changes in memory. Over the next 4 weeks, her mood, appetite, and activity level gradually improved and she was discharged to live with her sister-inlaw. With outpatient maintenance ECT and medication management, her depression continued to improve over the ensuing 2 months and she was able to live independently at home again.
Mrs B's post-hospital ECT course was complicated by chest pain, which required emergent hospitalization and percutaneous transluminal coronary angioplasty and stent placement. While recuperating, maintenance ECT was discontinued for 2 months and the patient's depression again worsened. ECT was restarted, with the patient on aspirin, clopidrogel, and atenolol. One month after restarting ECT, Mrs B's mood improved. Over the next 9 months, her depression completely resolved and she was able to resume complete self-care. ECT was discontinued and Mrs B continued on maintenance antidepressant medications. She also pursued psychotherapy to deal with long-term difficulties related to her childhood and marriage and with bereavement related to losing her son and her husband.
Perspectives
Mrs B, her daughter, and Mrs B's psychiatrist, were interviewed by a Care of the Aging Patient editor in April and May 2011.
Mrs B: It was couple years when I didn't know anything about what was happening... I didn't eat, I got real sad. I don't remember any of this. They say that I was very, very depressed.
Mrs B's daughter: What she doesn't remember ... it's a blessing. Think about depression, who'd want to remember it?
Dr F: She was severely depressed...she was not functioning....being dependent in all IADLs... at age 69, who, before the depression was completely independent in everything.
Methods
We searched MEDLINE for English language articles on the epidemiology and treatment of late-life depression published between January 1995 and June 2012. This paper focuses on evidence from meta-analyses and randomized controlled trials (see online for a full description).
Results of Evidence Review: Evidence and Clinical Strategies
Epidemiology of late-life depression
Depression is one of the most common mental disorders in late-life1. Two to 5% of community dwelling adults aged 65 years and older meet diagnostic criteria for major depression2, 3. Up to 10% of older adults seen in primary care4 and 30-50% of those in institutional and long-term care facilities clinically significant depression5, 6. When it is not successfully treated, depression becomes a persistent problem in as many as 40% of older adults7, 8. Rates of chronic depression are particularly high in individuals with chronic medical illnesses9. Yet, depression can be successfully treated, restoring patients to a high quality of life10.
Mrs B had several risk factors for depression11, 12 (e.g. being female, personal or family history of depression, loss and grief, care-taking responsibilities, comorbid medical conditions) and several risk factors associated with poor response to depression treatment (e.g. high initial symptom severity and chronicity, comorbid anxiety and somatic symptoms, a family history of mood disorders, and a history of childhood abuse and neglect).
Screening
The U.S. Preventive Services Task Force (USPSTF) found at least fair evidence that screening adults for depression improves health outcomes and that benefits outweigh harms (B rating), and recommends screening for depression if practices have systems in place to assure accurate diagnosis, effective treatment, and follow-up13. Based on these recommendations, the Centers for Medicare & Medicaid Services (CMS) recently determined to cover annual screening for depression for Medicare beneficiaries in primary care settings with staff-assisted depression care supports (see resources).
Brief depression screening tools (see resources) are available and can be easily administered by office staff or physicians during a primary care visit. A simple question ‘Do you often feel sad or depressed?’ can be a good starting point. The Patient Health Questionnaire-2 (PHQ-2)14 asks patients about depressed mood and anhedonia. To address possible ‘false negatives,’ clinicians can ask additional questions to patients who appear depressed, who have difficulty engaging in care, or whose functional impairment seems inconsistent with objective medical illness. A positive screen for depression should trigger further investigation such as an interview supported by the 9-item Patient Health Questionnaire (PhQ-9)15, which systematically explores the 9 DSM-IV16 symptoms of major depression. A score of 10 or greater on the PHQ-9 indicates an elevated level of depressive symptomatology and increased risk of clinically significant depression. Both PHQ-2 and PHQ-9 have good sensitivity (83% and 88% respectively) and specificity (92% and 88% respectively) for detecting depressive disorders14,15.
Major Depression in Older Adults
Mrs B experienced all of the DSM-IV symptoms of major depression (Box 1): depressed mood, difficulty concentrating and making decisions, feelings of guilt, lack of energy, psychomotor retardation, poor sleep, poor appetite and significant weight loss, and thoughts of death or suicide. Unlike Mrs B, not all older adults fit this typical picture of depression 17. Depressed mood may be less evident and some older adults do not report feeling sad at all 18. In such patients, anhedonia and the absence of positive affect may be better indicators of depression. Patients with anhedonia and at least four of the remaining DSM-IV symptoms still meet criteria for major depression 19. In addition to these ‘core symptoms’ of major depression, patients may present with high levels of anxiety and somatic symptoms20, 21, concerns about cognitive impairment and physical disability22. Depressed older adults may find it hard to participate in physical and social activities and in medical treatments such as physical therapy. Expressions such as “I just can't do this” or “nothing will help me” can be signs of decreased motivation, self-efficacy or hopelessness. Statements such as “I am not needed” or “I am in everyone's way” may indicate feelings of low self-worth or loneliness.
Evaluation
All depressed older adults should be asked about thoughts of harming themselves. If positive, clinicians should inquire about specific plans, develop a treatment plan that ensures the patient's safety and consider consultation with a mental health specialist. There is no evidence that inquiries about the self-harm increase the risk of suicide. Depressed older adults should also be asked about a history of mania, a time when they may have experienced rapid thoughts or speech, excess energy or activity, a reduced need for sleep, or behaviors that others thought were excessive or risky. Patients with bipolar disorder commonly present in a clinician's office in the depressive phase of the disorder. Taking a careful history of manic symptoms is important because treatment with antidepressants can exacerbate bipolar disorder and treatment with mood stabilizing medications such as Lithium is indicated instead. Clinicians should also explore the patient's day-to-day functioning because it would be easy to miss the profound level of functional impairment that can occur in older adults with severe depression.
A thorough history and physical examination should focus on medical conditions such as neurological illnesses that may contribute to or worsen depression. Cognitive screening is important to identify impairments that may be related to depression, delirium or dementia. Box 3 lists factors suggesting that cognitive deficits may be related to depression rather than dementia. In patients without a prior history of cognitive impairment, effective treatment of depression may improve cognitive functioning, although cognitive impairment first presenting in the context of depression may also be a sign of early dementia. Persistent cognitive deficits after treatment of depression should be evaluated to rule out dementia. Patients should be asked about new medications such as interferon or corticosteroids, about the use of alcohol, prescription sedatives, hypnotics, opioids, or illicit drugs which can exacerbate depressive symptoms.
Grief and bereavement are important factors to consider in late-life depression. Mrs B experienced grief and bereavement related to the losses of her son and husband. Grieving individuals may experience sorrow, emptiness, numbness, guilt, anger, and sadness. With time, these feelings gradually ease and individuals accept the loss. Major depression on the other hand is pervasive and persistent, and it can impair an older person's ability to overcome feelings of grief and move forward. Mrs B was suffering from a major depressive episode in addition to grief. She noted later that she was not able to grieve properly and to cope with her losses until she had received effective treatment for depression.
Management
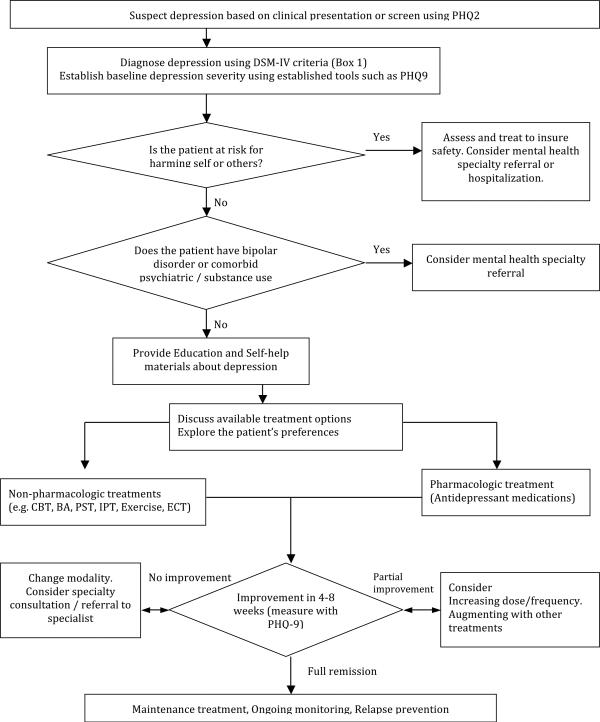
Several treatment options are available for late-life depression: antidepressant medications, psychotherapy, or a combination. Other options include exercise programs and electroconvulsive therapy (ECT). Figure 1 summarizes key steps in the management of late-life depression.
Figure 1.
Flowchart of late-life depression treatment.
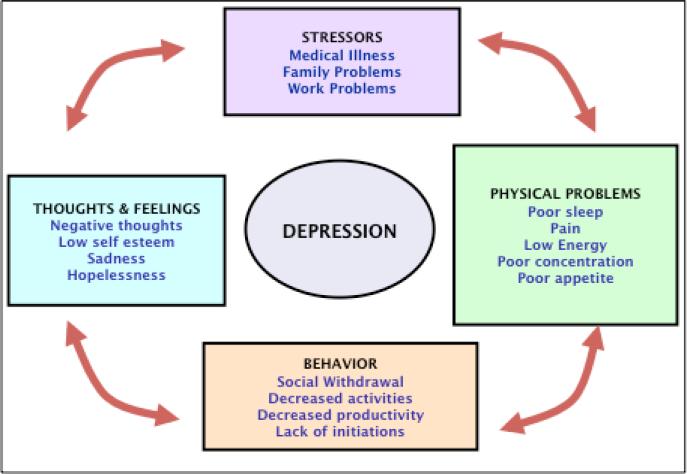
In selecting specific treatments, clinicians should discuss available treatment options and explore prior experiences with treatments, preferences and concerns with patients and family members. Individual preferences about depression treatment are strongly associated with treatment adherence and outcomes23. A conceptual model called the ‘cycle of depression (Figure 2)’ can help clinicians explain how symptoms of depression may be related to one another and how specific treatments can address these symptoms. Mrs B, for example, reported multiple life stressors, physical symptoms and poor sleep, which led to her feeling tired and unable to concentrate the next day. She became increasingly inactive, and began to feel progressively depressed and hopeless, a “vicious cycle” that she could not escape from. Treatments may be aimed at the various ‘components’ of depression. For example, medications may target somatic symptoms, sleep, and energy. Brief counseling strategies such as behavioral activation can help with the inactivity often associated with depression, and psychotherapy can help address negative thoughts and help the patient learn strategies to solve current and future life problems.
Figure 2.
The ‘Cycle of Depression.’ This conceptual model can help clinician explain how symptoms of depression may be related to one another and discuss treatment options aimed at the various ‘components’ of depression with patients.
Patients whose depression has remitted should have a relapse prevention plan that summarizes early warning signs for depression, maintenance treatments such as medications, and other strategies to reduce the risk of relapse (e.g., regular physical activity or pleasant activities). They should also be reassessed for recurrence of depressive symptoms with a screening tool such as the PHQ-9 on future visits.
Pharmacological management
In primary care, treatment commonly starts with an antidepressant medication, but clinicians should also consider non-pharmacologic treatment options as alternatives or in addition to medication management. More than 20 antidepressant medications have been approved by the FDA for treatment of depression in older adults, Table 1 lists commonly used antidepressants. Older adults may require lower doses of medications than younger patients because of changes in pharmacokinetics (e.g., decreased renal or hepatic clearance) or drug-drug interactions. On the other hand, medication doses should be titrated upwards to full adult doses in patients who experience partial responses without substantial side effects. Older adults may be more sensitive to certain medication side effects (e.g., the anticholinergic effects of tricyclic antidepressants). Recent meta-analyses24, 25 conclude that newer generations of antidepressants such as SSRIs and SNRIs are often preferred to older medications such as tricyclic antidepressants because of their more tolerable side effect profile.
Table 1.
Commonly used antidepressant for late-life depression
| Medication | Starting dose | Common Therapeutic Doses | Half-life (hour) | Common side effects | Comments |
|---|---|---|---|---|---|
| SSRI (Selective serotonin-reuptake inhibitors): | Nausea, dyspepsia, anorexia, tremors, anxiety, insomnia, sexual dysfunction, jitteriness, hyponatremia | Risk of serotonin syndrome if combined with certain drugs. | |||
| Fluoxetine | 5 mg | 10 – 40 mg once daily | 70–80 | Very long acting. | |
| Sertraline | 12.5 mg | 50 – 200 mg once daily | 25–30 | Loose stools, diarrhea | |
| Citalopram | 10 mg | 20 – 40 mg once daily | 40–50 | ||
| Escitalopram | 2.5 mg | 10– 30 mg once daily | 40–50 | ||
| Paroxetine | 10 mg | 20 – 50 mg once daily | 10–20 | Dry mouth, drowsiness, fatigue, weight gain | More anticholinergic side effects. High risk of discontinuation syndrome if drug stopped abruptly. |
| SNRIs (Serotonin-norepinephrine reuptake inhibitors) | Nausea, drowsiness, fatigue, weight gain, hyponatremia, diastolic hypertension at higher doses | Risk of serotonin syndrome if combined with certain drugs. High risk of discontinuation syndrome if medication stopped abruptly. | |||
| Venlafaxine XR | 37.5 mg | 75-225g once daily | 5 –9 | ||
| Duloxetine | 20 mg | 20-60mg once daily | 8 –17 | ||
| Other newer antidepressants | |||||
| Mirtazapine | 15mg | 15-45mg at bedtime | 20 –40 | Sedation, increased appetite / weight gain | No sexual side effects. |
| Bupropion SR | 100mg | 100-150 mg twice daily | 15 | No sexual side effects. Contraindicated in patients with seizures. Not recommended for patients with comorbid anxiety. | |
| TCAs (Tricyclic antidepressants) | Sedation, weight gain, dry mouth, urinary retention, constipation, blurry vision, orthostatic hypotension, impairment of cardiac conduction | High risk in overdose: 10 days of typical daily dose may result in a fatal cardiac arrhythmia. Get baseline ECG and follow- up ECG at steady state or if new cardiac symptoms occur. | |||
| Nortriptyline | 10mg | 50-125mg every night | 18 –56 | Fatigue | Therapeutic blood levels range from 50 to 150 ng/mL |
| Desipramine | 25mg | 100-200mg once daily | 12 –28 | Insomnia, agitation | |
A recent AHRQ report comparing second generation antidepressants26 found no evidence that specific medications are more effective than others, leaving the choice of individual medications up to physicians and patients. When choosing antidepressant medication, patient's and family members’ treatment history, patient's treatment preferences, side effect profile, safety in overdose, drug-drug interactions, availability and cost of medication should be discussed with the patient. In Mrs B's case, for example, bupropion is not likely to help with her anxiety and might even exacerbate this problem and SNRIs or SSRIs may exacerbate her problems with nausea and vomiting. A drug like mirtazapine may help with her depressive symptoms, insomnia, and loss of appetite and weight loss. For most older adults, maintenance treatment with antidepressants (usually for at least 2 years) can substantially reduce the risk of depression relapse compared to placebo27.
Nonpharmacological options for depression treatment include psychotherapy and exercise programs (Table 2). These approaches can be used alone or in combination with antidepressants. A meta-analysis study of 89 controlled studies (pooled observation=5,328 older adults) concluded that psychotherapy and medication did not show strong differences in effect sizes (effect sizes ranged from 0.62 for pharmacotherapy to 0.83 for psychotherapy studies) and recommended that the choice of treatment should be based on other criteria, such as contraindications, treatment access or patients preferences28, 29.
Table 2.
Meta-analyses of non-pharmacologic treatment for late-life depression
| Treatment type Name of the studies | Efficacy* | |
|---|---|---|
| Cognitive Behavioral Therapy (CBT): Challenge and change negative or dysfunctional thoughts, behaviors, and associated feelings. | ||
| Wilson et al 20 0831 | 5 RCTs (pooled N=153 older adults) | CBT more efficacious than waitlist control [WMD=9.85 (95% CI: −11.97, −7.73)] |
| Behavioral activation (BA): Increase pleasant activities and experiences of success to improve sense of self-efficacy, mastery, and mood. | ||
| Cuijpers et al 200734 | 16 RCTs (pooled N=780) (5 RCTs with older adults) | BA equally effective as cognitive therapy [PES=0.02 (95% CI:–0.21, 0.25)] Large effect size [MSE= 0.87 (95% CI: 0.60~1.15)] |
| Mazzuccheli et al 200935 | 34 RCTs (pooled N=2,055) (5 RCTs with older adults) | BA more efficacious than control groups (waitlist or treatment as usual) [PES=0.74 (95% CI: 0.57, 0.97)] |
| Ekers et al 20 0736 | 17 RCTs (pooled N=1,109) (3 studies with older adults) | BA more efficacious than control groups [SDM= –0.70 (95% CI: –1.00, –0.39)], brief psychotherapy [SDM=–0.66 (95% CI: –1.00, –0.12)], supportive therapy [SDM=-0.75 (95% CI: –1.37, –0.14)], and equal to CBT [SDM=0.08 (95% CI: –0.14, –0.30)] |
| Problem solving therapy (PST): Increase understanding the link between depression and life problems. Teach a technique to resolve problems in a structured way. Provide positive experiences related to problem solving. | ||
| Bell et al 20 0933 | 21 RCTs (pooled N=1,264) (2 studies with older adults) | PST significantly more efficacious in reducing depression symptom than supportive therapy and attention control group [SDM = .45 (p<.001)], likely be more effective than waitlist control group [SDM=2.38 (p=.09)] |
| Interpersonal psychotherapy (IPT): Develop interpersonal skills to help adapt to current interpersonal roles and situations. | ||
| de Mello et al 200532 | 13 RCTs (pooled N=2,199) (2 studies with older adults) | IPT was superior in efficacy to placebo [WMD=–3.57 (95% CI: –5.9, –1.16)] and significantly better than CBT [WMD=–2.16 (95% CI: –4.16, –0.15)]. |
| Exercise: Improve physicial activity, fitness, resilience, sense of mastery. | ||
| Sjosten et al 200640 | 13 RCTs (pooled N=1,264 older adults) | The majority of studies reported statistically significant improvement in exercise group |
| Blake et al 200941 | 11 RCTs (pooled N=641 older adults) | compared to control group. No pooled effect sizes were provided |
Studies have reported different outcomes rneasures for efficacy: WMD= weighted mean differences. MES= mean effect size. PES= pooled effect size. SMD= standardized mean difference
Psychotherapy
Data from IMPACT study showed that older adults find various types of psychological services acceptable and more patients prefer psychotherapy (57%) over medications (43%)30. Recent meta-analyses show that several psychotherapeutic treatments are effective for late-life depression31, 32: cognitive behavioral therapy (CBT)28, 33, interpersonal therapy (IPT)34, and problem solving treatment (PST)35. Briefer strategies such as Activity Scheduling and Behavioral Activation (BA) may also be useful 36-38. Maintenance treatment with a combination of psychotherapy and antidepressants has been associated with fewer relapses than either treatment alone 39. Although such treatments can be effectively provided to older adults in primary care40, 41, they are not widely available in most primary care settings today and we encourage primary care providers(PCPs) to develop relationships with mental health specialists who can offer such treatments or to train a staff member in the clinic to provide evidence-based psychotherapies.
Exercise
Several RCTs and a recent meta-analysis have found that exercise can be effective treatment or treatment adjunct for late-life depression 42, 43. It can be challenging, however, to engage severely depressed patients in exercise programs. Everyday physical activities (e.g. walking, taking stairs) and light housework such as gardening may be a good start. For fragile elders, low intensity exercises using a chair or wheelchair can build strength and improve a sense of self-efficacy.
Electroconvulsive Treatment (ECT)
ECT is an important and viable treatment option for severely depressed older adults. In Mrs B's case, two courses of ECT were required to improve her depression. ECT should be strongly considered for patients who have severe, persistent depression that does not respond to several trials of pharmacological or psychotherapy and/or puts patient at high risk of harm (e.g. severe weight loss, malnutrition, refusal of food or suicidal ideation). Poor tolerance or limited response to medications and a history of successful treatment with ECT are also indicatinons for ECT. Data from the 1993 Healthcare Cost and Utilization Project suggested that the likelihood of receiving ECT was greater for individulas 65 years of age and older (21%)44.
Physical side effects of ECT are usually short lived and can be treated with medications. They include nausea, headache, jaw pain, or muscle aches. Other adverse effects related to ECT include an increased risk of falls. Serious morbidity and mortality from ECT are low with an estimated complication rate of less than 1%. Even medically frail patients usually tolerate ECT well45, 46. However, increased blood pressure and cardiopulmonary events can occur during the seizure or in the immediate peri-ECT period, making adequate preoperative evaluation essential. Patients with coronary heart disease may experience ischemic events during seizures, a time of increased cardiac activity and oxygen demand47. Unstable cardiopulmonary disease, recent intracranial surgery, an intracranial mass with raised intracranial pressure, unstable vascular aneurysm, recent cerebral hemorrhage or stroke, or pheochromocytoma are contraindications for ECT. Mrs B's ECT had to be interrupted until her coronary heart disease was stabilized.
Despite the paucity of randomized evidence for ECT in elderly populations48, literature suggests that ECT is safe and effective in acute treatment of late-life depression49, 50. Patients who improve with ECT are at high risk for relapse 50, 51 and maintenance treatment with ECT or appropriate pharmacotherapy is highly recommended to reduce this risk 52, 53 Maintenance ECT (ranging from weekly to monthly) has been found to be safe and effective in depressed older adults46. Mrs B's ECT was started on an inpatient basis and followed-up with outpatient maintenance ECT for almost one year. ECT is available in most academic medical centers and in many hospitals with inpatient psychiatric units. Clinicians should consult a psychiatrist who specializes in this procedure when considering ECT for their patients.
Patients immediately after ECT may awaken with varying degrees of confusion that resolves within hours of treatment. Anterograde amnesia, which can occur after ECT, will usually resolve within 4-8 weeks of stopping ECT. Memory for events during the time shortly before or during ECT may not be fully recovered.. Older adults with cognitive impairment from dementia may be at increased risk for cognitive side effects. On the other hand, depression can contribute to cognitive impairment and cognition may improve after successful ECT for depression. A recent review of 27 studies concluded that studies have mixed results, but except for a deterioration in information processing speed during a course of ECT, there is neglibigle deterioration in the cognitition of older adults who receive ECT 54.
Other Treatment Considerations
Minimize adverse effects: first, do no harm
Mrs B's daughter: My mom was on so much medication it was unbelievable. I think I counted from May until October that she had been on between maybe 50 and 60 different kinds of medications.
All depression treatments have potential adverse effects, especially in medically ill older adults who are on multiple medications. A careful review of all medications is recommended before starting new medications. Discontinuing medications that do not appear helpful or simplifying complex medication regimens can reduce side effects and improve medication adherence and thus effectiveness.
Measure outcomes and treat to target
Systematic tracking of depression severity with rating scales such as the PHQ-9 can help address clinical inertia which is a frequent barrier to improvement and suggest when it is time for treatment adjustments or a specialty consultation55. As many as50-70% of depressed patients will not improve with initial treatment and need adjustments such as medication changes and/or addition of psychotherapy56. With systematic treatment adjustments, the majority of patients who fail an initial antidepressant trial eventually improve57.
Patients with persistent depression despite multiple courses of antidepressant medications and psychotherapy like Mrs B should be reevaluated for potential contributors to persistent depression (Box 4) and considered for referral to a geriatric psychiatrist.
Overcome barriers to care
A clear and consistent message of hope is an important step to keeping patients engaged even if initial treatments are not immediately effective. Stigma can be a formidable barrier to depression care and clinicians should emphasize that depression is not a sign of personal weakness or simply a part of ‘normal aging58, 59. Common fears about losing autonomy60 and concerns about treatments (e.g. becoming addicted to antidepressant medications) should be addressed as well as cultural and individual variations in the presentation of depression and in treatment preferences.
Clinicians should pay particular attention to populations at high risk for not receiving depression care. These include older adults who are poor, less educated, from ethnic minority groups, or with low social support61-64. Older men are also less likely to receive depression care65.
Include family caregivers
Engaged and active family members and caregivers can play critical roles , especially when an older adult's ability to advocate for oneself is decreased due to depression. Family members can facilitate adherence to treatment 66, 67 and improve depression outcomes 68-70. Dr. F reported, for example, that “the buy-in from the family” for ECT was instrumental for successful treatment of Mrs. B.
Translate evidence into clinical practice
Mrs B's long journey to recovery from depression is not unique. System barriers to effective depression care include a fragmented health care system where PCPs and mental health providers cannot collaborate effectively, limited availability of mental health specialists, especially for evidence-based psychotherapies71, and a lack of systematic approaches for detecting and managing depression in primary care72.
Models of care in which PCPs and mental health specialists collaborate effectively, have gained significant momentum nationally and internationally and are supported by a robust efficacy and effectiveness literature 73-75. In such programs, a depression care manager (typically a nurse, social worker, or psychologist) works closely with the patient's PCP to provide education, support treatment adherence, and track treatment effectiveness. Care managers may coach patients in pleasant events scheduling and behavioral activation, support antidepressant medications prescribed by a PCP, or offer evidence-based psychotherapies such as PST40 or IPT76. A designated psychiatrist regularly reviews patients who are not improving and provides treatment recommendations to the PCP. The IMPACT model (Improving Mood: Promoting Access to Collaborative Treatment for Late-life Depression) was demonstrated in a large RCT to more than double the effectiveness of usual care for depression, improve patient functioning, and to reduce long-term health care costs77.
Conclusions
Within a few months of completing her second course of ECT, Mrs B was living back in her home. She was going to church regularly, played cards with friends, made lunches for people, and attended her grandson's sports events. She entertained 32 people in her home for her daughter's 50th birthday party and her daughter reported that this was the happiest she had ever seen her mother. At age 78, nine years after developing severe depression, Mrs B continues on one antidepressant medication (venlafaxine) to prevent a relapse. She lives independently, drives, is socially active and enjoys her life.
Mrs B: Every Thursday night, I go out with my friends to eat. On Friday nights, I play cards. We have a card club, there's 10 of us: 5 guys and 5 women. My girlfriend and I go to all the local basketball and football games and volleyball games. I go to church. I like to go grocery shopping, though I don't like to put the groceries away...
Even severe depression in late-life can be treated in most cases. Patients, family members, and clinicians should be prepared to systematically pursue treatment until an effective approach is found.
Box 1: Diagnostic criteria for major depression (adapted from DSM-IV 16).
Depressed mood
Marked diminished interest or pleasure (anhedonia)
Sleep disturbance (insomnia or hypersomnia)
Appetite or weight disturbance (markedly increased or decreased appetite and / or weight) when not dieting
Persistent fatigue or loss of energy
Diminished ability to concentrate or indecisiveness
Feelings of worthlessness or excessive or inappropriate guilt
Psychomotor retardation or agitation (oba change in mental and physical speed perceived by other people)
Recurrent thoughts of death or suicide (not just fear of dying)
Note: To meet criteria for a major depressive episode, a patient must have 1 of the 2 core symptoms (depressed mood or anhedonia) and at least 5 of these 9 symptoms nearly every day for at least 2 weeks. They must also experience functional impairment related to these depressive symptoms and the symptoms should not be due to bipolar disorder or a mixed episode (with depressive and hypomanic symptoms), medications, a drug of abuse, or a medical condition (e.g., hypothyroidism).
Box 2: ”Red flags” for consideration of depression.
Irritability
Agitation/anxiety/excessive worrying
Excessive or unexplained somatic complaints
Concerns about cognitive impairment
Decreased problem-solving capacity
Excessive guilt
Social withdrawal
Alcohol or substance abuse
Statements such as: “I just can't do this” “Nothing will help me” “I am not needed” “I am in everyone's way”
Box 3: Factors that suggest cognitive impairment may be related to depression.
Depressed affect/mood
Neurovegetative signs such as poor energy, appetite, or sleep
Long response latency
Frequent responses of “I don't know”
Little effort; quick to give up
Slow speech and movements
Errors of omission rather than errors of commission
Patient is concerned about cognitive difficulties. “I think I am getting Alzheimer's. I cannot remember things.”
Movements, writing, and speaking may be slow but no clear signs of aphasia, apraxia, or agnosia.
Greater similarity to subcortical dementias, such as Parkinson Disease
Box 4: Factors to consider in the evaluation of patients with persistent depression.
Wrong Diagnosis (eg, does the patient have bipolar disorder?)
Comorbid psychiatric disorders (eg, dementia, posttraumatic stress disorder)
Untreated or ineffectively treated chronic pain
Insomnia
Alcohol or substance abuse
Medical problems or medications that may cause or worsen depression
Severe psychological or social stressors
Problems with treatment adherence
Insufficient dose / duration of treatment
Side effects
Initial treatments may have been appropriate but are simply not effective
Acknowledgment
The Authors thank Wayne Katon, MD at the University of Washington and Randall Espinoza, MD, MPH at the University of California, Los Angeles for their review and feedback on an earlier version of this manuscript. Both scholars were not compensated for their work. We also thank Mrs B, her daughter, and Dr F for sharing and giving permission to publish their story. Jürgen Unützer, MD, MA, MPH has received grant and contract funding from the National Institute of Health (NIMH), the John A. Hartford Foundation, the Alaska Mental Health Trust Authority, the George Foundation, the Hogg Foundation for Mental Health, and the Henry M. Jackson Foundation. He provides technical assistance and / or consultation to Community Health Plan of Washington, Public Health of Seattle & King County, AARP Services Incorporated, the National Council of Community Behavioral Health Care (NCCBH), and he serves as an advisor to the Carter Center Mental Health Program, the Institute for Clinical Systems Improvement (ICSI), and the World Health Organization. Mijung Park PhD, RN is funded by Geriatric Mental Health Services Research Fellowship by NIH (NIMH 2 T32 MH 73553-6). She has no conflict of interest to declare.
Contributor Information
Jürgen Unützer, Psychiatry and Behavioral Sciences Chief of Psychiatry, University of Washington Medical Center Director, UW AIMS Center (http://uwaims.org) Director, IMPACT Implementation Program (http://impact-uw.org) 1959 NE Pacific Street Box 356560 Seattle, Washington 98195-6560.
Mijung Park, Postdoctoral fellow of Geriatric Mental Health Services Research Department of Psychiatry and Behavioral Sciences University of Washington.
References
- 1.Centers for Disease Control and Prevention and National Association of Chronic Disease Directors . The State of Mental Health and Aging in America Issue Brief 2: Addressing Depression in Older Adults: Selected Evidence-Based Programs. National Association of Chronic Disease Directors; Atlanta, GA: 2009. [Google Scholar]
- 2.Mojtabai R, Olfson M. Major depression in community-dwelling middle-aged and older adults: prevalence and 2- and 4-year follow-up symptoms. Psychological Medicine. 2004;34(04):623–634. doi: 10.1017/S0033291703001764. [DOI] [PubMed] [Google Scholar]
- 3.Byers AL, Yaffe K, Covinsky KE, Friedman MB, Bruce ML. High Occurrence of Mood and Anxiety Disorders Among Older Adults: The National Comorbidity Survey Replication. Arch Gen Psychiatry. 2010;67(5):489–496. doi: 10.1001/archgenpsychiatry.2010.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lyness JM, Caine ED, King DA, Cox C, Yoediono Z. Psychiatric Disorders in Older Primary Care Patients. Journal of General Internal Medicine. 1999;14(4):249–254. doi: 10.1046/j.1525-1497.1999.00326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Teresi J, Abrams R, Holmes D, Ramirez M, Eimicke J. Prevalence of depression and depression recognition in nursing homes. Social Psychiatry and Psychiatric Epidemiology. 2001;36(12):613–620. doi: 10.1007/s127-001-8202-7. [DOI] [PubMed] [Google Scholar]
- 6.Hoover DR, Siegel M, Lucas J, et al. Depression in the first year of stay for elderly long-term nursing home residents in the U.S.A. International Psychogeriatrics. 2010:1–11. doi: 10.1017/S1041610210000578. First View. [DOI] [PubMed] [Google Scholar]
- 7.Licht-Strunk E, van der Windt DlA, van Marwijk HW, de Haan M, Beekman AT. The prognosis of depression in older patients in general practice and the community. A systematic review. Family Practice. 2007;24(2):168–180. doi: 10.1093/fampra/cml071. [DOI] [PubMed] [Google Scholar]
- 8.Alexopoulos G, Chester JG. Outcomes on geriatric depression. Vol. 8. Elsevier; New York, NY: 1992. [PubMed] [Google Scholar]
- 9.Iosifescu DV, Nierenberg AA, Alpert JE, et al. Comorbid Medical Illness and Relapse of Major Depressive Disorder in the Continuation Phase of Treatment. Psychosomatics. 2004;45(5):419–425. doi: 10.1176/appi.psy.45.5.419. [DOI] [PubMed] [Google Scholar]
- 10.Unutzer J. Clinical practice. Late-life depression. N Engl J Med. 2007 Nov 29;357(22):2269–2276. doi: 10.1056/NEJMcp073754. [DOI] [PubMed] [Google Scholar]
- 11.Vink D, Aartsen MJ, Schoevers RA. Risk factors for anxiety and depression in the elderly: A review. Journal of Affective Disorders. 2008;106(1-2):29–44. doi: 10.1016/j.jad.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 12.Cole M, Dendukuri N. Risk factors for depression among elderly community subjects: a systematic review and meta-analysis. Am. J. Psychiatry. 2003;160:1147. doi: 10.1176/appi.ajp.160.6.1147. [DOI] [PubMed] [Google Scholar]
- 13.U.S. Preventive Services Task Force Screening for Depression: Recommendations and Rationale. 2002 http://www.uspreventiveservicestaskforce.org/3rduspstf/depression/depressrr.htm. Accessed October 30, 2011.
- 14.Kroenke K, Spitzer RL, Williams JBW. The Patient Health Questionnaire-2: Validity of a Two-Item Depression Screener. Medical care. 2003;41(11):1284–1292. doi: 10.1097/01.MLR.0000093487.78664.3C. [DOI] [PubMed] [Google Scholar]
- 15.Kroenke K, Spitzer RL, Williams JBW, L√∂we B. The Pa ent Health Ques onnaire Soma c, Anxiety, and Depressive Symptom Scales: a systematic review. General hospital psychiatry. 2010;32(4):345–359. doi: 10.1016/j.genhosppsych.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 16.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders : DSMIV-TR. 4th ed. Washington, DC: 2000. [Google Scholar]
- 17.Mitchell AJ, Rao S, Vaze A. Do Primary Care Physicians Have Particular Difficulty Identifying Late-Life Depression? A Meta-Analysis Stratified by Age. Psychotherapy and Psychosomatics. 2010;79(5):285–294. doi: 10.1159/000318295. [DOI] [PubMed] [Google Scholar]
- 18.Gallo J, Rabins P, Lyketsos C, Tien A, Anthony J. Depression without sadness: functional outcomes of nondysphoric depression in later life. J. Am. Geriatr. Soc. 1997;45:570. doi: 10.1111/j.1532-5415.1997.tb03089.x. [DOI] [PubMed] [Google Scholar]
- 19.Evans DL, Charney DS, Lewis L, et al. Mood Disorders in the Medically Ill: Scientific Review and Recommendations. Biological Psychiatry. 2005;58(3):175–189. doi: 10.1016/j.biopsych.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 20.Yu DSF, Lee DTF. Do medically unexplained somatic symptoms predict depression in older Chinese? International Journal of Geriatric Psychiatry. 2012;27(2):119–126. doi: 10.1002/gps.2692. [DOI] [PubMed] [Google Scholar]
- 21.Sheehan B, Banerjee S. Review: somatization in the elderly. International Journal of Geriatric Psychiatry. 1999;14(12):1044–1049. doi: 10.1002/(sici)1099-1166(199912)14:12<1044::aid-gps55>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 22.Beekman A, de Beurs E, van Balkom A, Deeg D, van Dyck R, van Tilburg W. Anxiety and depression in later life: co-occurrence and communality of risk factors. Am. J. Psychiatry. 2000;157:89. doi: 10.1176/ajp.157.1.89. [DOI] [PubMed] [Google Scholar]
- 23.Fawzi W, Abdel Mohsen MY, Hashem AH, Moussa S, Coker E, Wilson KCM. Beliefs about medications predict adherence to antidepressants in older adults. International Psychogeriatrics. 2012;24(01):159–169. doi: 10.1017/S1041610211001049. [DOI] [PubMed] [Google Scholar]
- 24.Kok RM, Heeren TJ, Nolen WA. Continuing Treatment of Depression in the Elderly: A Systematic Review and Meta- Analysis of Double-Blinded Randomized Controlled Trials With Antidepressants. American Journal of Geriatric Psych. 2011;19(3):249–255. doi: 10.1097/jgp.0b013e3181ec8085. 210.1097/JGP.1090b1013e3181ec8085. [DOI] [PubMed] [Google Scholar]
- 25.Nelson JC, Delucchi K, Schneider L. Efficacy of Second Generation Antidepressants in Late-Life Depression: A Meta-Analysis of the Evidence. American Journal of Geriatric Psychiatry. 2008;16(7):558–567. doi: 10.1097/JGP.0b013e3181693288. [DOI] [PubMed] [Google Scholar]
- 26.Gartlehner G, Gaynes BN, Hansen RA, et al. Comparative Benefits and Harms of Second-Generation Antidepressants: Background Paper for the American College of Physicians. Annals of Internal Medicine. 2008 Nov 18;149(10):734–750. doi: 10.7326/0003-4819-149-10-200811180-00008. 2008. [DOI] [PubMed] [Google Scholar]
- 27.Reynolds CF, Dew MA, Pollock BG, et al. Maintenance Treatment of Major Depression in Old Age. New England Journal of Medicine. 2006;354(11):1130–1138. doi: 10.1056/NEJMoa052619. [DOI] [PubMed] [Google Scholar]
- 28.Pinquart M, Duberstein PR, Lyness JM. Treatments for Later-Life Depressive Conditions: A Meta-Analytic Comparison of Pharmacotherapy and Psychotherapy. Am J Psychiatry. 2006 Sep 1;163(9):1493–1501. doi: 10.1176/ajp.2006.163.9.1493. 2006. [DOI] [PubMed] [Google Scholar]
- 29.Lauren R, Annabel P, Alison E, Koravangattu V, J HI, Matthew H. Antidepressants for depression in physically ill people. Cochrane Database of Systematic Reviews. 2010:3. doi: 10.1002/14651858.CD007503.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gum AM, Arean PA, Hunkeler E, et al. Depression treatment preferences in older primary care patients. Gerontologist. 2006 Feb;46(1):14–22. doi: 10.1093/geront/46.1.14. [DOI] [PubMed] [Google Scholar]
- 31.Cuijpers P, van Straten A, Smit F. Psychological treatment of late-life depression: a meta-analysis of randomized controlled trials. International Journal of Geriatric Psychiatry. 2006;21(12):1139–1149. doi: 10.1002/gps.1620. [DOI] [PubMed] [Google Scholar]
- 32.Pinquart M, Duberstein PR, Lyness JM. Effects of psychotherapy and other behavioral interventions on clinically depressed older adults: A meta-analysis. Aging and Mental Health. 2007;11(6):645–657. doi: 10.1080/13607860701529635. [DOI] [PubMed] [Google Scholar]
- 33.Wilson K, Mottram PG, Vassilas C. Psychotherapeutic treatments for older depressed people. Cochrane Database of Systematic Reviews. 2008:1. doi: 10.1002/14651858.CD004853.pub2. [DOI] [PubMed] [Google Scholar]
- 34.de Mello MF, de Jesus Mari J, Bacaltchuk J, Verdeli H, Neugebauer R. A systematic review of research findings on the efficacy of interpersonal therapy for depressive disorders. European Archives of Psychiatry and Clinical Neuroscience. 2005;255(2):75–82. doi: 10.1007/s00406-004-0542-x. [DOI] [PubMed] [Google Scholar]
- 35.Bell AC, D'Zurilla TJ. Problem-solving therapy for depression: A meta-analysis. Clinical Psychology Review. 2009;29(4):348–353. doi: 10.1016/j.cpr.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 36.Cuijpers P, van Straten A, Warmerdam L. Behavioral activation treatments of depression: A meta-analysis. Clinical Psychology Review. 2007;27(3):318–326. doi: 10.1016/j.cpr.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 37.Mazzucchelli T, Kane R, Rees C. Behavioral Activation Treatments for Depression in Adults: A Meta-analysis and Review. Clinical Psychology: Science and Practice. 2009;16(4):383–411. [Google Scholar]
- 38.Ekers D, Richards D, Gilbody S. A meta-analysis of randomized trials of behavioural treatment of depression. Psychological Medicine. 2008;38:611 – 623. doi: 10.1017/S0033291707001614. [DOI] [PubMed] [Google Scholar]
- 39.Reynolds CF, Frank E, Perel JM, et al. Nortriptyline and Interpersonal Psychotherapy as Maintenance Therapies for Recurrent Major Depression. JAMA: The Journal of the American Medical Association. 1999;281(1):39–45. doi: 10.1001/jama.281.1.39. [DOI] [PubMed] [Google Scholar]
- 40.Arean P, Hegel M, Vannoy S, Fan M-Y, Unuzter J. Effectiveness of Problem-Solving Therapy for Older, Primary Care Patients With Depression: Results From the IMPACT Project. The Gerontologist. 2008;48(3):311–323. doi: 10.1093/geront/48.3.311. [DOI] [PubMed] [Google Scholar]
- 41.Schulberg HC, Post EP, Raue PJ, Have TT, Miller M, Bruce ML. Treating late-life depression with interpersonal psychotherapy in the primary care sector. International Journal of Geriatric Psychiatry. 2007;22(2):106–114. doi: 10.1002/gps.1700. [DOI] [PubMed] [Google Scholar]
- 42.Sjösten N, Kivelä S. The effects of physical exercise on depressive symptoms among the aged: a systematic review. Int. J. Geriatr. Psychiatry. 2006;21:410. doi: 10.1002/gps.1494. [DOI] [PubMed] [Google Scholar]
- 43.Blake H, Mo P, Malik S, Thomas S. How effective are physical activity interventions for alleviating depressive symptoms in older people? A systematic review. Clinical Rehabilitation. 2009;23(10):873–887. doi: 10.1177/0269215509337449. [DOI] [PubMed] [Google Scholar]
- 44.Olfson M, Marcus S, Sackeim HA, Thompson J, Pincus HA. Use of ECT for the Inpatient Treatment of Recurrent Major Depression. Am J Psychiatry. 1998;155:22–29. doi: 10.1176/ajp.155.1.22. [DOI] [PubMed] [Google Scholar]
- 45.Damm J, Eser D, Schüle C, et al. Influence of Age on Effectiveness and Tolerability of Electroconvulsive Therapy. The Journal of ECT. 2010;26(4):282–288. doi: 10.1097/YCT.0b013e3181cadbf5. 210.1097/YCT.1090b1013e3181cadbf1095. [DOI] [PubMed] [Google Scholar]
- 46.van Schaik AM, Comijs HC, Sonnenberg CM, Beekman AT, Sienaert P, Stek ML. Efficacy and Safety of Continuation and Maintenance Electroconvulsive Therapy in Depressed Elderly Patients: A Systematic Review. American Journal of Geriatric Psych. 2012;20(1):5–17. doi: 10.1097/JGP.0b013e31820dcbf9. 10.1097/JGP.1090b1013e31820dcbf31829. [DOI] [PubMed] [Google Scholar]
- 47.Tess AV, Smetana GW. Medical Evaluation of Patients Undergoing Electroconvulsive Therapy. New England Journal of Medicine. 2009;360(14):1437–1444. doi: 10.1056/NEJMra0707755. [DOI] [PubMed] [Google Scholar]
- 48.Stek M, Wurff van der FF, Hoogendijk W, Beekman A. Electroconvulsive therapy for the depressed elderly. Cochrane Database of Systematic Reviews. 2003:2. doi: 10.1002/14651858.CD003593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dombrovski AY, Mulsant BH. ECT: the preferred treatment for severe depression in late life. International Psychogeriatrics. 2007;19(01):10–14. doi: 10.1017/S1041610206224384. [DOI] [PubMed] [Google Scholar]
- 50.van der Wurff FB, Stek ML, Hoogendijk WJG, Beekman ATF. The efficacy and safety of ECT in depressed older adults: a literature review. International Journal of Geriatric Psychiatry. 2003;18(10):894–904. doi: 10.1002/gps.944. [DOI] [PubMed] [Google Scholar]
- 51.Kellner CH, Knapp RG, Petrides G, et al. Arch Gen Psychiatry. 2006;Continuation Electroconvulsive Therapy vs Pharmacotherapy for Relapse Prevention in Major Depression: A Multisite Study From the Consortium for Research in Electroconvulsive Therapy (CORE).63(12):1337–1344. doi: 10.1001/archpsyc.63.12.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gagné GG, Furman MJ, Carpenter LL, Price LH. Efficacy of Continuation ECT and Antidepressant Drugs Compared to Long-Term Antidepressants Alone in Depressed Patients. Am J Psychiatry. 2000;157:1960–1965. doi: 10.1176/appi.ajp.157.12.1960. [DOI] [PubMed] [Google Scholar]
- 53.Sackeim HA, Haskett RF, Mulsant BH, et al. Continuation Pharmacotherapy in the Prevention of Relapse Following Electroconvulsive Therapy. JAMA: The Journal of the American Medical Association. 2001;285(10):1299–1307. doi: 10.1001/jama.285.10.1299. [DOI] [PubMed] [Google Scholar]
- 54.Gardner BK, O'Connor DW. A Review of the Cognitive Effects of Electroconvulsive Therapy in Older Adults. The Journal of ECT. 2008;24(1):68–80. doi: 10.1097/YCT.0b013e318165c7b0. 10.1097/YCT.1090b1013e318165c318167b318160. [DOI] [PubMed] [Google Scholar]
- 55.Henke RM, Zaslavsky AM, McGuire TG, Ayanian JZ, Rubenstein LV. Clinical Inertia in Depression Treatment. Medical care. 2009;47(9):959–967. doi: 10.1097/MLR.0b013e31819a5da0. 910.1097/MLR.1090b1013e31819a31815da31810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Simon G, Perlis R. Personalized Medicine for Depression: Can We Match Patients With Treatments? Am J Psychiatry. 2010;167(12):1445–1455. doi: 10.1176/appi.ajp.2010.09111680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and Longer-Term Outcomes in Depressed Outpatients Requiring One or Several Treatment Steps: A STAR*D Report. Am J Psychiatry. 2006 Nov 1;163(11):1905–1917. doi: 10.1176/ajp.2006.163.11.1905. 2006. [DOI] [PubMed] [Google Scholar]
- 58.Drayer RA, Mulsant BH, Lenze EJ, et al. Somatic symptoms of depression in elderly patients with medical comorbidities. International Journal of Geriatric Psychiatry. 2005;20(10):973–982. doi: 10.1002/gps.1389. [DOI] [PubMed] [Google Scholar]
- 59.Sarkisian CA, Lee-Henderson MH, Mangione CM. Do depressed older adults who attribute depression to “old age” believe it is important to seek care? Journal of General Internal Medicine. 2003;18(12):1001–1005. doi: 10.1111/j.1525-1497.2003.30215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Snowden M, Steinman L, Frederick J. Treating depression in older adults: challenges to implementing the recommendations of an expert panel. Prev Chronic Dis. 2008;5(1) [PMC free article] [PubMed] [Google Scholar]
- 61.Strothers HS, Rust G, Minor P, Fresh E, Druss B, Satcher D. Disparities in Antidepressant Treatment in Medicaid Elderly Diagnosed with Depression. Journal of the American Geriatrics Society. 2005;53(3):456–461. doi: 10.1111/j.1532-5415.2005.53164.x. [DOI] [PubMed] [Google Scholar]
- 62.Crystal S, Sambamoorthi U, Walkup JT, Akıncıgil A. Diagnosis and Treatment of Depression in the Elderly Medicare Population: Predictors, Disparities, and Trends. Journal of the American Geriatrics Society. 2003;51(12):1718–1728. doi: 10.1046/j.1532-5415.2003.51555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Alegria M, Chatterji P, Wells K, et al. Disparity in Depression Treatment Among Racial and Ethnic Minority Populations in the United States. Psychiatr Serv. 2008;59(11):1264–1272. doi: 10.1176/appi.ps.59.11.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Akincigil A, Olfson M, Siegel M, Zurlo KA, Walkup JT, Crystal S. Racial and Ethnic Disparities in Depression Care in Community-Dwelling Elderly in the United States. American Journal of Public Health. 2011;102(2):319–328. doi: 10.2105/AJPH.2011.300349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hinton L, Zweifach M, Oishi S, Tang L, Unutzer J. Gender disparities in the treatment of late-life depression: qualitative and quantitative findings from the IMPACT trial. Am J Geriatr Psychiatry. 2006;14(10):884–892. doi: 10.1097/01.JGP.0000219282.32915.a4. [DOI] [PubMed] [Google Scholar]
- 66.Greenberger H, Litwin H. Caregiver Resources and Facilitation of Elderly Care Recipient Adherence to Health Regimens. Canadian Journal on Aging. 2003;22(4):395–405. [Google Scholar]
- 67.Voils CI, Steffens DC, Flint EP, Bosworth HB. Social Support and Locus of Control as Predictors of Adherence to Antidepressant Medication in an Elderly Population. American Journal of Geriatric Psych. 2005;13(2):157–165. doi: 10.1176/appi.ajgp.13.2.157. [DOI] [PubMed] [Google Scholar]
- 68.Martire LM, Lustig AP, Schulz R, Miller GE, Helgeson VS. Is It Beneficial to Involve a Family Member? A Meta-Analysis of Psychosocial Interventions for Chronic Illness. Health Psychology. 2004;23(6):599–611. doi: 10.1037/0278-6133.23.6.599. [DOI] [PubMed] [Google Scholar]
- 69.Sörensen S, Pinquart M, Duberstein P. How Effective Are Interventions With Caregivers? An Updated Meta-Analysis. The Gerontologist. 2002;42(3):356–372. doi: 10.1093/geront/42.3.356. [DOI] [PubMed] [Google Scholar]
- 70.Chesla CA. Do Family Interventions Improve Health? Journal of Family Nursing. 2010;16(4):355–377. doi: 10.1177/1074840710383145. [DOI] [PubMed] [Google Scholar]
- 71.Akincigil A, Olfson M, Walkup JT, et al. Diagnosis and Treatment of Depression in Older Community-Dwelling Adults: 1992–2005. Journal of the American Geriatrics Society. 2011;59(6):1042–1051. doi: 10.1111/j.1532-5415.2011.03447.x. [DOI] [PubMed] [Google Scholar]
- 72.McCall L, Clarke D, Rowle G. A questionnaire to measure general practitioners’ attitudes to their role in the management of patients with depression and anxiety. Australian family physician. 2002;31:299–303. [PubMed] [Google Scholar]
- 73.Unutzer J, Katon WJ, Fan MY, et al. Long-term cost effects of collaborative care for late-life depression. Am J Manag Care. 2008 Feb;14(2):95–100. [PMC free article] [PubMed] [Google Scholar]
- 74.Gilbody S, Bower P, Fletcher J, Richards D, Sutton AJ. Collaborative Care for Depression: A Cumulative Meta-analysis and Review of Longer-term Outcomes. Arch Intern Med. 2006;166(21):2314–2321. doi: 10.1001/archinte.166.21.2314. [DOI] [PubMed] [Google Scholar]
- 75.Gilbody S, Bower P, Whitty P. Costs and consequences of enhanced primary care for depression: Systematic review of randomised economic evaluations. British Journal of Psychiatry. 2006;189:297 – 308. doi: 10.1192/bjp.bp.105.016006. [DOI] [PubMed] [Google Scholar]
- 76.Bruce M, Ten Have T, Reynolds C, Katz I, Schulberg H. Reducing suicidal ideation and depressive symptoms in depressed older primary care patients: a randomized controlled trial. JAMA. 2004;291:1081. doi: 10.1001/jama.291.9.1081. [DOI] [PubMed] [Google Scholar]
- 77.Unutzer J, Katon W, Callahan CM, et al. Collaborative Care Management of Late-Life Depression in the Primary Care Setting: A Randomized Controlled Trial. Jama. 2002 Dec 11;288(22):2836–2845. doi: 10.1001/jama.288.22.2836. 2002. [DOI] [PubMed] [Google Scholar]