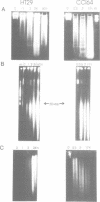
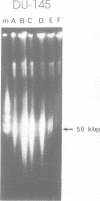
Abstract
To date, apoptosis has been characterized biochemically by the production of 180-200 bp internucleosomal DNA fragments resulting from the activation of an endonuclease(s). The principal morphological feature of apoptosis is the condensation of chromatin and it has been assumed that this may reflect the oligonucleosomal fragmentation pattern. We have re-examined this dogma by comparing the biochemical and morphological features of cell death in several epithelial cell types (HT-29-I1 colon adenocarcinoma, CC164 mink lung, DU-145 human prostatic carcinoma and MCF-7 human breast adenocarcinoma) and one mesenchymal cell line (H11ras-R3 ras-transformed rat fibroblasts). Cell death was induced either by serum deprivation, TGF-beta 1 or etoposide, or by leaving cells to reach confluence. Cell death was assessed with respect to detachment from monolayers, morphological changes and DNA integrity. The DNA-binding fluorophore Hoechst 33258 revealed chromatin condensation patterns consistent with apoptotic cell death in all cell types except MCF-7 cells. Using field inversion gel electrophoresis in conjunction with conventional 2% agarose gel electrophoresis, cleavage of DNA to 50 kbp fragments was observed in all cases except MCF-7 cells. This preceded the appearance of oligonucleosomal fragments in HT-29-I1, CC164 and H11ras-R3 cells. Although the DNA of DU-145 cells fragmented into 50 kbp units, and although the cells exhibited classical apoptotic morphology, no subsequent internucleosomal cleavage was observed. These results suggest that changes in the integrity of DNA indicative of the release of chromatin loop domains occur before cleavage at internucleosomal sites is initiated and that the latter is not an essential step in the apoptotic process.
Full text
PDF

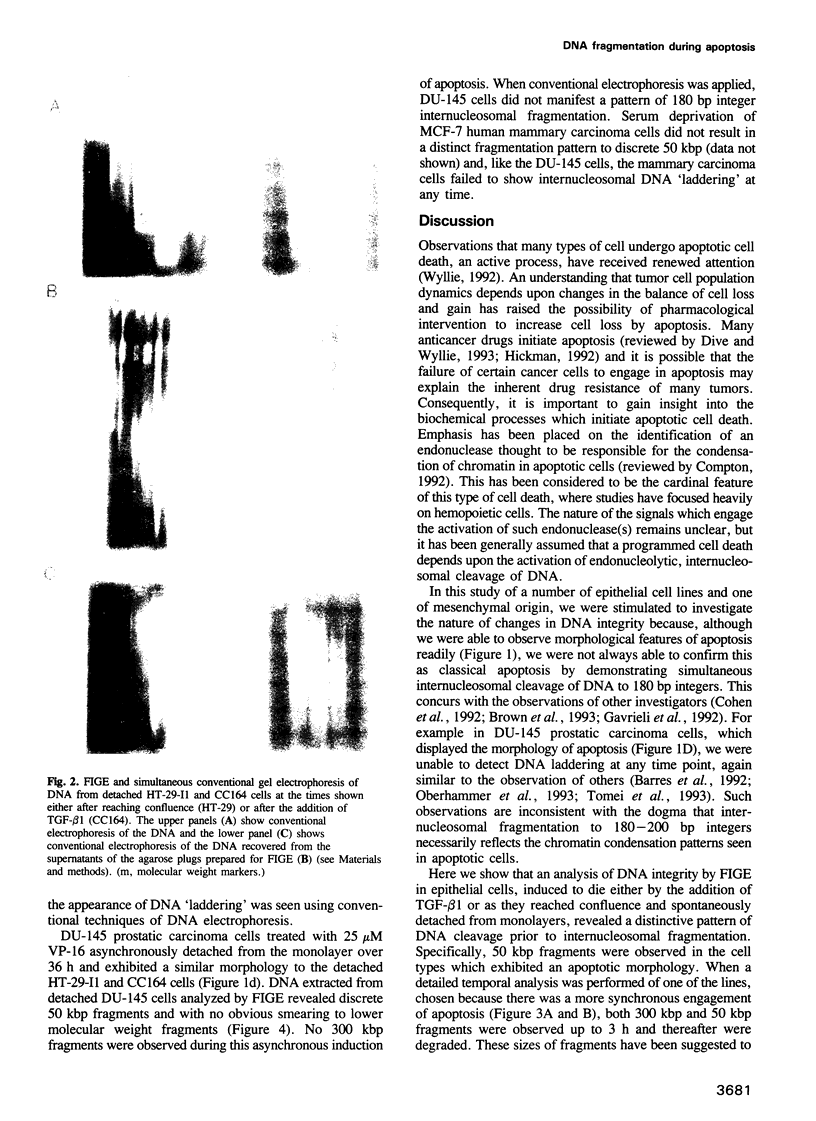
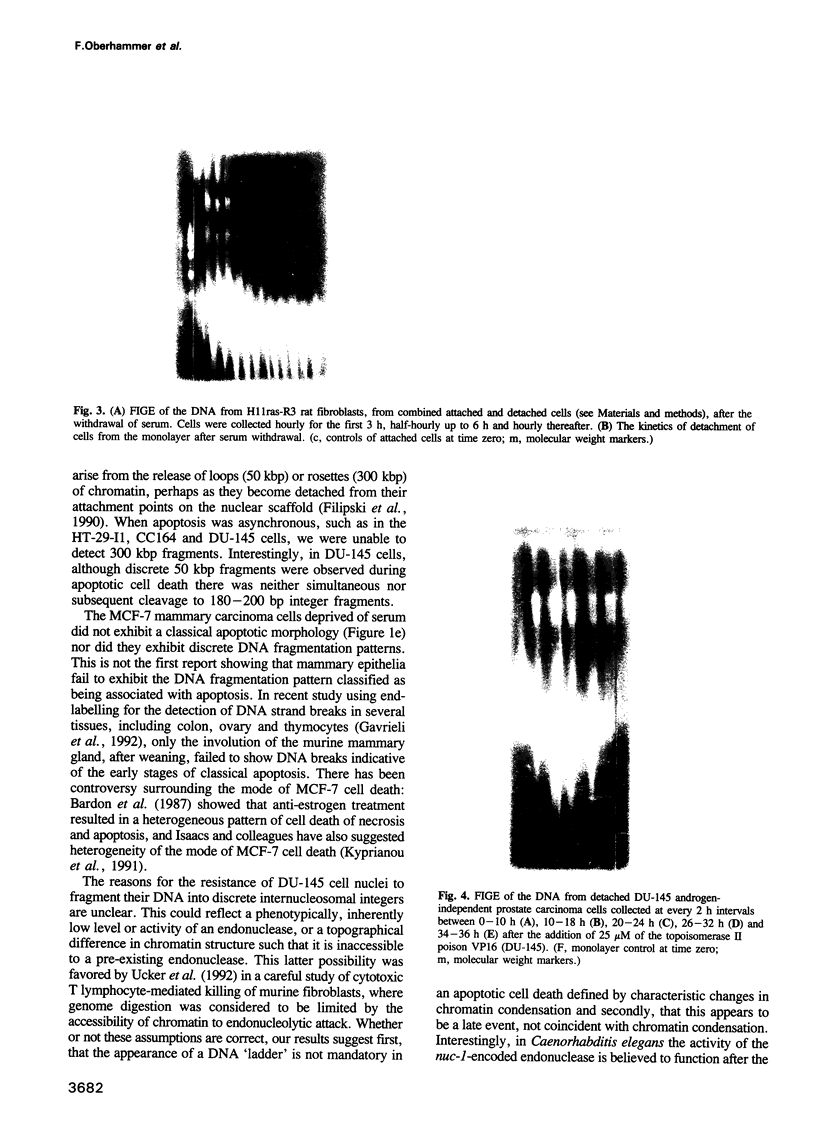


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi Y., Luke M., Laemmli U. K. Chromosome assembly in vitro: topoisomerase II is required for condensation. Cell. 1991 Jan 11;64(1):137–148. doi: 10.1016/0092-8674(91)90215-k. [DOI] [PubMed] [Google Scholar]
- Arends M. J., Morris R. G., Wyllie A. H. Apoptosis. The role of the endonuclease. Am J Pathol. 1990 Mar;136(3):593–608. [PMC free article] [PubMed] [Google Scholar]
- Bardon S., Vignon F., Montcourrier P., Rochefort H. Steroid receptor-mediated cytotoxicity of an antiestrogen and an antiprogestin in breast cancer cells. Cancer Res. 1987 Mar 1;47(5):1441–1448. [PubMed] [Google Scholar]
- Barres B. A., Hart I. K., Coles H. S., Burne J. F., Voyvodic J. T., Richardson W. D., Raff M. C. Cell death and control of cell survival in the oligodendrocyte lineage. Cell. 1992 Jul 10;70(1):31–46. doi: 10.1016/0092-8674(92)90531-g. [DOI] [PubMed] [Google Scholar]
- Barry M. A., Eastman A. Identification of deoxyribonuclease II as an endonuclease involved in apoptosis. Arch Biochem Biophys. 1993 Jan;300(1):440–450. doi: 10.1006/abbi.1993.1060. [DOI] [PubMed] [Google Scholar]
- Brown D. G., Sun X. M., Cohen G. M. Dexamethasone-induced apoptosis involves cleavage of DNA to large fragments prior to internucleosomal fragmentation. J Biol Chem. 1993 Feb 15;268(5):3037–3039. [PubMed] [Google Scholar]
- Carle G. F., Frank M., Olson M. V. Electrophoretic separations of large DNA molecules by periodic inversion of the electric field. Science. 1986 Apr 4;232(4746):65–68. doi: 10.1126/science.3952500. [DOI] [PubMed] [Google Scholar]
- Caron-Leslie L. M., Schwartzman R. A., Gaido M. L., Compton M. M., Cidlowski J. A. Identification and characterization of glucocorticoid-regulated nuclease(s) in lymphoid cells undergoing apoptosis. J Steroid Biochem Mol Biol. 1991;40(4-6):661–671. doi: 10.1016/0960-0760(91)90288-g. [DOI] [PubMed] [Google Scholar]
- Cohen G. M., Sun X. M., Snowden R. T., Dinsdale D., Skilleter D. N. Key morphological features of apoptosis may occur in the absence of internucleosomal DNA fragmentation. Biochem J. 1992 Sep 1;286(Pt 2):331–334. doi: 10.1042/bj2860331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton M. M. A biochemical hallmark of apoptosis: internucleosomal degradation of the genome. Cancer Metastasis Rev. 1992 Sep;11(2):105–119. doi: 10.1007/BF00048058. [DOI] [PubMed] [Google Scholar]
- Ellis R. E., Horvitz H. R. Two C. elegans genes control the programmed deaths of specific cells in the pharynx. Development. 1991 Jun;112(2):591–603. doi: 10.1242/dev.112.2.591. [DOI] [PubMed] [Google Scholar]
- Filipski J., Leblanc J., Youdale T., Sikorska M., Walker P. R. Periodicity of DNA folding in higher order chromatin structures. EMBO J. 1990 Apr;9(4):1319–1327. doi: 10.1002/j.1460-2075.1990.tb08241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavrieli Y., Sherman Y., Ben-Sasson S. A. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992 Nov;119(3):493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickman J. A. Apoptosis induced by anticancer drugs. Cancer Metastasis Rev. 1992 Sep;11(2):121–139. doi: 10.1007/BF00048059. [DOI] [PubMed] [Google Scholar]
- Jacobson M. D., Burne J. F., King M. P., Miyashita T., Reed J. C., Raff M. C. Bcl-2 blocks apoptosis in cells lacking mitochondrial DNA. Nature. 1993 Jan 28;361(6410):365–369. doi: 10.1038/361365a0. [DOI] [PubMed] [Google Scholar]
- Kaufmann S. H. Induction of endonucleolytic DNA cleavage in human acute myelogenous leukemia cells by etoposide, camptothecin, and other cytotoxic anticancer drugs: a cautionary note. Cancer Res. 1989 Nov 1;49(21):5870–5878. [PubMed] [Google Scholar]
- Kokileva L. Endogenous degradation of rat liver chromatin studied by agar gel electrophoresis of nuclei. Mol Biol Rep. 1988;13(3):139–143. doi: 10.1007/BF00444309. [DOI] [PubMed] [Google Scholar]
- Kyprianou N., English H. F., Davidson N. E., Isaacs J. T. Programmed cell death during regression of the MCF-7 human breast cancer following estrogen ablation. Cancer Res. 1991 Jan 1;51(1):162–166. [PubMed] [Google Scholar]
- Mirkovitch J., Mirault M. E., Laemmli U. K. Organization of the higher-order chromatin loop: specific DNA attachment sites on nuclear scaffold. Cell. 1984 Nov;39(1):223–232. doi: 10.1016/0092-8674(84)90208-3. [DOI] [PubMed] [Google Scholar]
- Oberhammer F., Fritsch G., Schmied M., Pavelka M., Printz D., Purchio T., Lassmann H., Schulte-Hermann R. Condensation of the chromatin at the membrane of an apoptotic nucleus is not associated with activation of an endonuclease. J Cell Sci. 1993 Feb;104(Pt 2):317–326. doi: 10.1242/jcs.104.2.317. [DOI] [PubMed] [Google Scholar]
- Peitsch M. C., Polzar B., Stephan H., Crompton T., MacDonald H. R., Mannherz H. G., Tschopp J. Characterization of the endogenous deoxyribonuclease involved in nuclear DNA degradation during apoptosis (programmed cell death). EMBO J. 1993 Jan;12(1):371–377. doi: 10.1002/j.1460-2075.1993.tb05666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberge M., Gasser S. M. DNA loops: structural and functional properties of scaffold-attached regions. Mol Microbiol. 1992 Feb;6(4):419–423. doi: 10.1111/j.1365-2958.1992.tb01485.x. [DOI] [PubMed] [Google Scholar]
- Roy C., Brown D. L., Little J. E., Valentine B. K., Walker P. R., Sikorska M., Leblanc J., Chaly N. The topoisomerase II inhibitor teniposide (VM-26) induces apoptosis in unstimulated mature murine lymphocytes. Exp Cell Res. 1992 Jun;200(2):416–424. doi: 10.1016/0014-4827(92)90190-j. [DOI] [PubMed] [Google Scholar]
- Tomei L. D., Shapiro J. P., Cope F. O. Apoptosis in C3H/10T1/2 mouse embryonic cells: evidence for internucleosomal DNA modification in the absence of double-strand cleavage. Proc Natl Acad Sci U S A. 1993 Feb 1;90(3):853–857. doi: 10.1073/pnas.90.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ucker D. S., Obermiller P. S., Eckhart W., Apgar J. R., Berger N. A., Meyers J. Genome digestion is a dispensable consequence of physiological cell death mediated by cytotoxic T lymphocytes. Mol Cell Biol. 1992 Jul;12(7):3060–3069. doi: 10.1128/mcb.12.7.3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker P. R., Smith C., Youdale T., Leblanc J., Whitfield J. F., Sikorska M. Topoisomerase II-reactive chemotherapeutic drugs induce apoptosis in thymocytes. Cancer Res. 1991 Feb 15;51(4):1078–1085. [PubMed] [Google Scholar]
- Wyllie A. H. Apoptosis and the regulation of cell numbers in normal and neoplastic tissues: an overview. Cancer Metastasis Rev. 1992 Sep;11(2):95–103. doi: 10.1007/BF00048057. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H. Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature. 1980 Apr 10;284(5756):555–556. doi: 10.1038/284555a0. [DOI] [PubMed] [Google Scholar]