Abstract
Distraction and reappraisal are two commonly used forms of cognitive emotion regulation. Functional neuroimaging studies have shown that each one depends upon interactions between prefrontal cortex, interpreted as implementing cognitive control, and limbic regions, interpreted as mediating emotional responses. However, no study has directly compared distraction with reappraisal, and it thus remains unclear whether they draw upon different neural mechanisms and have different emotional consequences. The present fMRI study compared distraction and reappraisal, and found both similarities and differences between the two forms of emotion regulation. Both resulted in decreased negative affect, decreased activation in the amygdala, and increased activation in prefrontal and cingulate regions. Relative to distraction, reappraisal led to greater decreases in negative affect, and greater increases in a network of regions associated with processing affective meaning (medial prefrontal and anterior temporal cortices). Relative to reappraisal, distraction led to greater decreases in amygdala activation, and greater increases in activation in prefrontal and parietal regions. Taken together, these data suggest that distraction and reappraisal differentially engage neural systems involved in attentional deployment and cognitive reframing, and have different emotional consequences.
The ability to influence how we experience and express emotions – known as emotion regulation – is a crucial contributor to mental health (Amstadter, 2008; Gross, 2007; Taylor & Liberzon, 2007). Among the most powerful and flexible forms of emotion regulation are cognitive strategies that either alter the way we attend to a stimulus (distraction) or the way we interpret the meaning of a stimulus (reappraisal). Indeed, distraction and reappraisal are among the best-studied forms of emotion regulation (Craske, Street, & Barlow, 1989; Li & Lambert, 2007; Ochsner & Gross, 2005, 2008; Sheppes & Meiran, 2007; Totterdell & Parkinson, 1999).
Distraction involves the use of selective attention to limit the extent to which the emotionally evocative aspects of an event are attended and appraised. Distraction has been shown to be effective for reducing various kinds of negative affective responses, including dysphoric mood (Rusting, 1998), negative cognitions (Fennell & Teasdale, 1984), anger (Gerin, Davidson, Christenfeld, Goyal, & Schwartz, 2006; Rusting, 1998), and stress (Bennett, Phelps, Brain, Hood, & Gray, 2007). Neuroimaging studies have shown that performing a variety of demanding tasks diminishes the aversiveness of pain, as measured by self-reported experience, as well as activation in pain-related regions such as the insula and medial prefrontal cortex (Bantick et al., 2002; Frankenstein, Richter, McIntyre, & Remy, 2001; Seminowicz & Davis, 2007; Wiech et al., 2005). Typically, reductions in indices of pain response are accompanied by greater activation in regions linked to cognitive control, such as lateral prefrontal cortex (lPFC) and dorsal anterior cingulate cortex (dACC; (Bantick et al., 2002; Frankenstein, Richter, McIntyre, & Remy, 2001; Kalisch, Wiech, Herrmann, & Dolan, 2006; Seminowicz & Davis, 2007).
Reappraisal involves cognitively changing one’s appraisal of the affective meaning of a stimulus. Reappraisal is recognized as a key component of one of the most successful interventions for the treatment of mood and anxiety disorders, cognitive behavioral therapy or CBT (Beck, Rush, Shaw, & Emery, 1979). In laboratory studies, reappraisal has been shown to be successful in decreasing negative emotional responding, as measured by self-reports of emotional experience (Gross, 1998a), peripheral physiological measures of arousal and negative affect (Jackson, Malmstadt, Larson, & Davidson, 2000; Ohira et al., 2006), and activation in brain regions involved in the processing of negative emotion such as the amygdala and insula (Eippert et al., 2007; Kim & Hamann, 2007; Ochsner, Bunge, Gross, & Gabrieli, 2002; Ochsner, Ray et al., 2004; Phan et al., 2005; Urry et al., 2006). Neuroimaging studies have shown that successful reappraisal is supported by activation in dorsal and ventral lateral PFC, medial PFC and dorsal ACC – all regions associated with various aspects of cognitive control (Miller & Cohen, 2001).
Although growing evidence supports the efficacy of both distraction and reappraisal as emotion regulation strategies, it has been difficult to directly compare distraction and reappraisal for at least three reasons. First, distraction manipulations, which include verbal fluency, Stroop, multi-source interference, and self-generation tasks, typically involve multiple kinds of processing, at least some of which are controlled and deliberative. It is not clear whether control-related activations reflect processes supporting cognitive task performance, regulation of emotion to reduce interference with the cognitive task, or both. Second, some studies have failed to show reductions in self-reported negativity or stress as a result of distraction (Chua, Krams, Toni, Passingham, & Dolan, 1999; Kalisch, Wiech, Herrmann, & Dolan, 2006), making it difficult to determine whether prefrontal and cingulate activity reflects successful emotion regulation or simply the effort to successfully perform the cognitive task in the face of an affective distracter. The clinical utility of distraction indicates that it can influence self-reported affect, but it is unclear why the experimental designs in these prior studies failed to show that influence. Third, and most importantly, although distraction and reappraisal both have been shown to alter emotional responding, studies have differed in the kinds of stimuli presented and the specificity of the instructions employed, and no study has directly compared them. This makes it impossible to determine whether these two emotion regulation strategies engage similar or dissimilar brain mechanisms and whether or not they have comparable affective consequences.
The goal of this study was to directly compare distraction and reappraisal. Based on previous research, we predicted that both distraction and reappraisal would decrease self-reported negative emotion and amygdala activation. However, given that distraction has had relatively inconsistent effects on self-reported emotion, we expected that reappraisal would lead to greater decreases in this measure of emotional responding. According to our process conception of emotion regulation (Gross, 1998b), our general expectation for regulation-related regions was that both strategies would recruit lateral prefrontal regions (implicated in cognitive control), anterior cingulate regions (implicated in monitoring the success of regulation) and parietal regions (implicated in shifting attention; Ochsner & Gross, 2005; 2008). At the same time, we also expected to see different regions recruited by each of the strategies. Employing a cognitive process perspective, we hypothesized that distraction and reappraisal differentially depend upon processes supporting selective attention as opposed to those involved in the generation or manipulation of an emotional narrative, respectively. Therefore, we predicted that reappraisal would recruit additional dlPFC regions that are known to be involved in generating higher-level cognitive strategies, which in this case would involve the specific re-interpretations of images that are part and parcel of reappraisal (Ochsner & Gross, 2005). In addition, we predicted that relative to distraction, reappraisal would differentially recruit dmPFC regions that are known to support higher-level appraisals of emotional stimuli and monitoring of one’s own emotional state (Lane & McRae, 2004; Ochsner, Ray et al., 2004; Teasdale et al., 1999). Finally, we predicted that relative to reappraisal, distraction would differentially recruit areas of prefrontal and parietal cortex that are known to be involved in directing one’s attention towards an external stimulus (Wager, Jonides, & Reading, 2004).
Methods
Participants
Eighteen female subjects (mean age = 24.4 years, S.D. = 3.5) participated. Potential participants were recruited via paper and electronic flyers from the campus community in Stanford, California. Interested participants were screened via email and invited to participate provided they met the following criteria: 1) right-hand dominance. 2) English as a native language, 3) fMRI compatibility (no embedded metal in body, not pregnant, not claustrophobic), 4) no current psychiatric diagnosis, and 5) no current use of psychoactive medications.
Task Training
Participants were trained on the experimental procedure in a separate session 3–5 days before scanning in which an experimenter guided the participants through the different instructions presented during the task. When they saw the word “ATTEND,” participants were instructed to pay attention and respond naturally to the subsequent stimulus, allowing to unfold whatever reaction the picture would naturally evoke in them. When they saw the word “DECREASE,” they were asked to re-interpret the situation depicted in the picture in a way that made them feel less negative about it. When they saw a 6-letter string (the distraction instruction), they were instructed to try to keep all six letters in mind during the picture presentation, and were told they would be probed for memory directly after the presentation of each picture. Training began with several practice trials of each type (using images not repeated during the experiment). For reappraisal practice images, the experimenter required that all subjects verbalize their reappraisals to ensure 1) that when reappraising participants used the instructed strategy of reinterpreting the affects/dispositions, outcomes and contexts depicted in images (this is known as ‘situation focused’ reappraisal (Ochsner et al, 2004) or “reinterpretation” more generally (Ochsner & Gross, 2008)), and 2) that participants were not actually using distraction (looking away from the picture or only attending to the non-emotional aspects of the picture) or any other regulation strategy (such as expressive suppression). Participants were reminded of the previous task training and re-read the description of the different instructions immediately before the scanning session.
To check whether participants were actually engaging in the desired form of emotion regulation, after scanning, participants were asked to write down the strategies they used to decrease their negative affect during the reappraisal trials, and what percentage of the time they used each strategy. Only two participants reported any non-reappraisal strategies on any proportion of the trials, one that used distraction on 10% of the reappraisal trials, and one that reported being unable to reappraise on less than 25% of the reappraisal trials. To address the possibility that participants’ self-ratings of negative affect might be influenced by demand characteristics, participants were asked to complete the Marlowe-Crown Social Desirability Scale (MCSDS). No correlation between self-reports of negative affect and MCSDS scores were obtained (r = 0.062, p = 0.862). To confirm that participants were actually performing the distraction task, we examined performance accuracy on the forced choice recognition probe of the 6-letter string. Mean accuracy was 94.03% correct (S.E.M. = 0.081%).
Task
Participants viewed pictures drawn from the International Affective Picture System (IAPS; (Lang, Bradley, & Cuthbert, 2001) as well as pictures from an in-house set that were rated in a separate sample for comparable valence and arousal to those from the IAPS. Pictures normatively rated as negative (valence: M = 2.38, S.D. = 0.57; arousal: M = 6.06, S.D. = 1.18) and neutral (valence: M = 4.97, S.D. = 0.42; arousal: M = 3.40, S.D. = 1.08) were selected.
In order to compare distraction and reappraisal to unregulated responding, negative pictures were seen with three preceding 2-second displays: the word “decrease” (the reappraisal condition), a 6-letter string (the distraction condition) and the word “attend” (the look, or non-regulation condition). In order to provide a neutral baseline condition, neutral pictures were also presented in the look condition. Three other instruction conditions (the letter string paired with a neutral picture, and both the letter string and look instructions presented before a fixation cross) were interspersed with these conditions. Data from these trials are not of interest to the present report and will be reported elsewhere.
To address the possibility that differences in brain activation or the behavioral consequences of reappraisal and distraction could be attributable to differences in task difficulty, we conducted a separate pilot study designed to equate the distraction and reappraisal trials for effort. For this study, 23 female participants (a separate sample drawn from the same community as the present sample) completed the same regulatory task as did the scanner participants, with the exception that at the end of the study they completed a post-test rating (on a 7 point scale, 1 = not at all effortful and 7 = very effortful) of how much effort they exerted to hold in mind the letter string or to reappraise on each trial. Average ratings indicated that reappraisal (M= 4.12, S.D. = 0.68) and distraction (M= 3.93, S.D. = 0.61) were rated as requiring significantly more effort than the non-regulation negative picture condition (M= 2.96, S.D. = 0.21; (t(22) = 3.62 p < 0.002 for distraction condition, and t(22) = 4.04, p < 0.001 for the reappraisal condition. Crucially, the distraction and reappraisal conditions were not rated as significantly different in effort (t(22) = 1.18, p = 0.25).
Following the 8-second presentation of each picture, participants were presented with one letter for 4 seconds and asked to respond with a keypress to indicate if the letter was part of the 6-letter set they saw before the picture (for the distraction trials) or to press any key (for the look and reappraisal trials; this kept motor responses constant across trial types). Next, participants were asked to indicate how negative they felt. To decrease demand effects, the experimenter emphasized that this rating should correspond to their honest assessment of negative affect, and explicitly mentioned the possibility that reappraisals could fail to decrease negative affect. This response was made using a scale that consisted of a horizontal rectangular bar labeled ‘strength of negative affect’ with anchors of 0 and 7 labeled ‘weak’ and ‘strong’ respectively. At the beginning of the 4-s rating period, the bar grew from left to right and participants pressed a key when the bar had grown to a size that corresponded to the strength of their current negative feeling. This bar provided a continuous index of participants’ subjective experience of negative emotion. Lastly, a screen that read “relax” was presented for 2.5 seconds at the end of each trial.
One-hundred sixty trials were presented in an event-related fashion in four different stimulus presentation orders, which ensured the counterbalanced pairing of individual negative and neutral stimuli across the different instruction conditions.
Imaging Parameters
Twenty-four axial slices (4.4 mm thick) were collected on a 1.5T (GE Signa LX Horizon Echospeed) scanner with a T2* sensitive gradient echo spiral-in-out pulse sequence (TR= 2.00, TE= 40ms, 80° flip angle, 24-cm field of view, 64 × 64 data acquisition matrix). Two hundred twenty whole-brain images were taken in each of eight 7-minute, 20-second runs. T2-weighted flow-compensated spin echo scans were acquired for anatomical localization using identical slice prescription as the functional scans. We evaluated signal drop-out in the amygdala, and in accordance with previously reported findings, observed 0% drop-out in the amygdala (Preston, Thomason, Ochsner, Cooper & Glover, 2004).
fMRI Pre-processing
For the fMRI data, each participant’s sequential functional volumes were realigned to the first scan and co-registered to his or her anatomical MRI using an automated rigid-body transformation algorithm using statistical parametric mapping software (SPM2; Wellcome Department of Imaging Neuroscience, University College London, UK). Default SPM2 settings were used to warp volumetric MRIs to fit a standardized template (16 non-linear iterations), and normalization parameters were applied to subjects’ co-registered functional images. Normalized images were re-sampled into 2 × 2 × 2 mm voxels. Finally, images were smoothed with a 6 mm full width at half maximum kernel.
fMRI Analyses
Basic Contrasts
Preprocessed images were entered into a GLM in SPM that modeled the canonical hemodynamic response function convolved with a 12-second boxcar representing the instruction and picture-viewing period. Because encoding-related activity in working memory tasks often extends into the delay period (Postle, Zarahn, & D'Esposito, 2000) it was necessary to model the instruction period and the picture-viewing period together. Consequently, any preparatory activity during the reappraisal instruction that reflects re-interpretations or strategies generated before the onset of the stimulus was included as well. These models were used to create contrasts between conditions of interest (look negative > look neutral, reappraise > look negative, distract > look negative, and reappraise > distract) for each subject. These individual contrasts were then entered into a one-sample t-test to perform a random-effects group analysis. Because SPM does not correct jointly at the voxel and extent levels, as in prior work (e.g. (Ochsner, Knierim et al., 2004) we employed AlphaSim, a Monte Carlo simulation bootstrapping program in the AFNI library, to correct for multiple comparisons. AlphaSim takes into account the voxel-wise and cluster-volume thresholds to establish a cluster-wise p-value that protects against false positive detection of activation clusters (Forman, Fitzgerald, Eddy, Mintun, & Noll, 1995). For whole-brain analyses, the cluster extent threshold was 42 with a voxel threshold of p < 0.001 to protect against false positives at a rate of p < 0.05 overall.
In order to identify regions that were significantly more active during both distraction and reappraisal, we used the Fisher method for combining probabilities as used for conjunction analyses in previous work (Kampe, Frith, & Frith, 2003; Ochsner, Ray et al., 2004). The voxels identified in the reappraise > look contrast at a threshold of p < 0.01 as a mask to display the distraction > look contrast at the same display threshold, for a Fisher combined probability of p < 0.001 that a given region was active in both contrasts. When directly comparing distraction and reappraisal, a different masking approach was used. To identify reappraisal-related regions that were not recruited during distraction, the reappraise > distract contrast was masked by the reappraise > look contrast. This ensured that only regions more active during reappraising than during the baseline look condition could be identified as reappraisal-specific by the reappraise > distract contrast. Similarly, distraction-related regions were identified by masking the distract > reappraise contrast with the distract > look contrast.
Region of Interest (ROI) Analyses
We performed ROI analyses that identified functionally activated voxels falling within an anatomically defined amygdala ROI (defined at the group level, using the AAL atlas; Brett, Anton, Valabregue & Poline, 2002) as an a priori region of interest. This analysis tested for the effects of reactivity (look negative > look neutral contrast) and both types of regulation (look negative > reappraise, look negative > distract). The comparison of each regulation condition with neutral was used to mask direct comparisons between reappraisal and distraction, providing the inverse of the masked contrasts described above. As a region of a priori interest, the threshold for all amygdala ROI analyses was p < 0.05 with an extent threshold of 5. To display the time course of the response in these regions, we used in-house percent signal change code, which extracted and averaged the time series for all voxels that were above threshold in the group-level contrasts. For these voxels, each run’s time course was individually filtered, averaged across time, and then each time point was divided by the average and multiplied by 100. In addition, the values immediately preceding each event were averaged and subtracted from the event’s time course. These values for each subject were fitted with robust regression to compute the mean effect at each time point, and the standard errors displayed were computed from this robust regression.
Correlation Comparison Analyses
To determine whether activations in different regions of the brain could be predicted by each individual’s drop in self-reported negative affect, we conducted whole-brain robust regression analyses (that are especially resistant to outliers (Wager, Keller, Lacey, & Jonides, 2005)). For both reappraisal and distraction, we correlated decreases in negative affect relative to the look negative condition with the whole-brain activity in the reappraise > look negative and distract > look negative contrasts.
Results
Self-Reported Negative Affect
Common effects of distraction and reappraisal
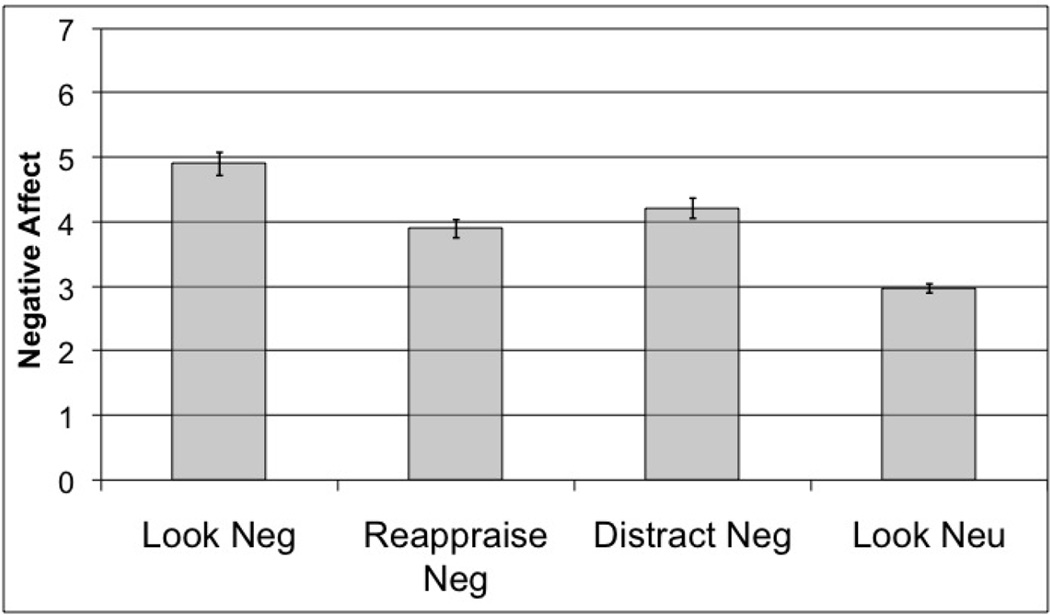
Self reports of negative affect were entered into a repeated measures GLM in SPSS. Instruction condition was entered as a within-subject factor with four levels (reappraisal negative, distraction negative, look negative, and look neutral; means in Figure 1). The main effect of condition was significant (F (3,15) = 60.75, p <0.001), and planned t-tests were performed to examine differences across conditions. First, the negative stimuli elicited greater negative affect than the neutral stimuli (t(17) = 13.32, p <0.001). Second, both forms of emotion regulation were successful in reducing negative affect relative to the look-negative condition (reappraisal, t(17) = 6.33, p < 0.001; distraction, t(17) = 5.18, p < 0.001).
Figure 1.
Self-reported negative affect in response to pictures presented in four conditions. Means in all conditions significantly differ from one another (p < 0.05) Error bars represent standard error of the mean (SEM).
Differential effects of distraction and reappraisal
Because ratings of self-reported negative affect differed by condition, we used planned t-tests to investigate the direct comparison of ratings during distraction and reappraisal. This analysis showed that reappraisal led to a greater reduction in negative affect than distraction (t(17)= 2.19, p <0.043).
Functional Imaging
Common effects of distraction and reappraisal
We had two goals with respect to identifying regions commonly involved in distraction and reappraisal. First, we sought to determine whether activity was decreased in similar regions during the employment of the two strategies. Relative to the look negative condition, we found significant reductions for both strategies in the ROI for our a priori region of interest, the amygdala (right amygdala peak at [24 2 −24], T = 2.62, p <0.005, uncorrected, left amygdala peak at [−24 0 −28], T = 2.24, p <0.02, uncorrected), and in whole brain analyses we observed common reductions in the left insula, right inferior parietal lobe and middle temporal gyrus as well (see Table 1a).
Table1. Common effects of distraction and reappraisal, or conjunction analyses.
Voxel threshold was p <0.01 with an extent threshold of 5 for each contrast, resulting in an overall threshold of p <0.001.
| 1a: Regions in which activity decreased during both Reappraisal and Distraction; Look Negative > Reappraise masked with Look Negative > Distract. | |||||||
|---|---|---|---|---|---|---|---|
| Region | Brodmann area | Extent | T | MNI X | MNI Y | MNI Z | Hemisphere |
| Temporal Lobe | |||||||
| Middle Temporal Gyrus | 21 | 69 | 4.1 | 42 | 0 | −24 | Right |
| Amygdala* | 8 | 4.83 | 36 | 2 | −24 | Right | |
| Amygdala* | 17 | 2.96 | 28 | 2 | −24 | Right | |
| Amygdala* | 9 | 2.29 | −26 | 0 | −28 | Left | |
| Parietal Lobe | |||||||
| Inferior Parietal Lobule | 40 | 582 | 5.44 | 58 | −24 | 30 | Right |
| Subcortical | |||||||
| Insula | 13 | 77 | 3.58 | −42 | −12 | 0 | Left |
| 1b: Regions in which activity increased during both Reappraisal and Distraction; Reappraise > Look Negative, masked with Distract > Look Negative. | |||||||
|---|---|---|---|---|---|---|---|
| Region | Brodmann Area | Extent | T | MNI X |
MNI Y |
MNI Z |
Hemisphere |
| Frontal Lobe | |||||||
| Superior Frontal Gyrus | 6 | 701 | 5.84 | −6 | 10 | 62 | Left |
| Middle Frontal Gyrus | 10 | 367 | 5.06 | −36 | 62 | 12 | Left |
| Middle Frontal Gyrus | 9 | 1013 | 4.51 | −42 | 22 | 30 | Left |
| Inferior Frontal Gyrus | 47 | 112 | 4.36 | 36 | 20 | −4 | Right |
| Middle Frontal Gyrus | 10 | 88 | 3.9 | 38 | 64 | 14 | Right |
| Middle Frontal Gyrus | 9 | 58 | 3.59 | 42 | 30 | 34 | Right |
| Parietal Lobe | |||||||
| Inferior Parietal Lobule | 40 | 100 | 4.99 | −42 | −60 | 46 | Left |
| Subcortical | |||||||
| Lentiform Nucleus | 350 | 4.51 | −16 | 10 | 8 | Left | |
The amygdala was explored as an a priori region of interest with a voxel threshold of p <0.05, extent threshold of 5.
Second, we sought to identify regions in which activity increased during both distraction and reappraisal relative to the look negative condition. Regions commonly active for distraction and reappraisal included left-sided middle and inferior lateral prefrontal cortices, and dorsomedial prefrontal cortex, extending into the dorsal anterior cingulate. These regions are listed in Table 1b.
Differential effects of distraction and reappraisal
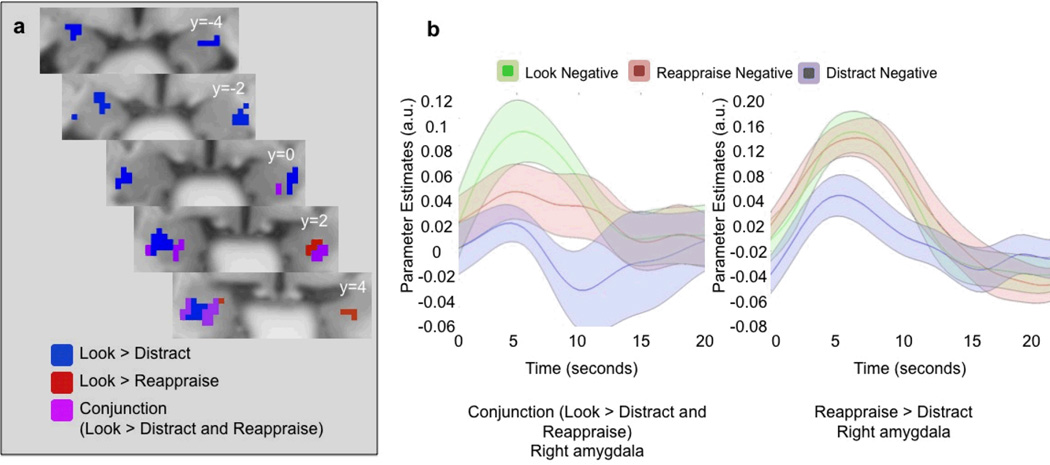
We also had two goals when directly comparing the neural effects of these two strategies. First, we sought to determine whether they had different modulatory effects on the amygdala using an amygdala ROI. As shown in Figure 2, significant clusters were observed in the amygdala that showed greater reduction in activity during distraction than reappraisal (reappraise > distract; right amygdala peak at [24 −8 −12], T=4.13, p <0.001 uncorrected; left amygdala peak at [−28 −6 −18], T = 3.04, p <0.005, uncorrected). No clusters in the amygdala showed greater activity for distraction than reappraisal.
Figure 2.
a. Voxels in the amygdala down-regulated by reappraisal (Look Negative > Reappraise; red), distraction (Look Negative > Distract; blue) and both reappraisal and distraction (conjunction; purple). The display threshold was p < 0.05, with an extent threshold of 5 voxels. b. Time-courses from the right amygdala overlap voxels (top) and the right amygdala voxels from the Reappraise > Distract contrast (bottom). Means (solid center line) for each time course were estimated robustly, and the SEM (transparent surround) was computed from the standard mean. The time course means and standard errors were then smoothed and interpolated using cubic spline to a 0.5-second resolution.
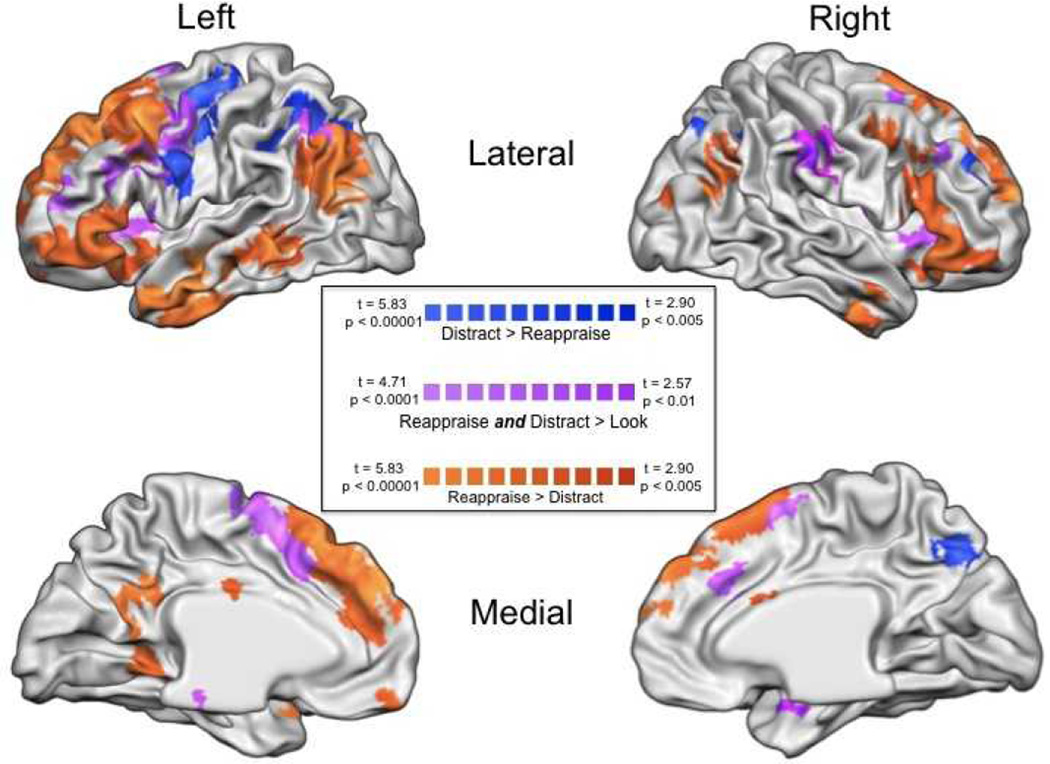
Second, to identify regions differentially associated with implementing each type of strategy, we directly contrasted whole-brain activation during distraction and reappraisal. Several regions were identified in the reappraise negative > distract negative contrast, including clusters in dorsomedial prefrontal cortex, bilateral dorsal and ventral lateral prefrontal cortex, and several left-sided clusters in temporal cortex. These regions are listed in Table 2a. Only a few regions were identified in the distract > reappraise contrast. These regions included left inferolateral prefrontal cortex, right lateral prefrontal cortex, and bilateral clusters in superior parietal cortex. These regions are listed in Table 2b.
Table 2.
Unique effects of distraction and reappraisal.
| 2a: Regions in which activity was greater for Reappraisal than Distraction; Reappraise Negative > Distract Negative (masked with Reappraise Negative> Look Negative) | |||||||
|---|---|---|---|---|---|---|---|
| Region | Brodmann Area | Extent | T | MNI X |
MNI Y |
MNI Z | Hemisphere |
| Frontal Lobe | |||||||
| Middle Frontal Gyrus | 8 | 6844 | 8.91 | −40 | 14 | 46 | Left |
| Middle Frontal Gyrus | 6 | 70 | 4.87 | 48 | 4 | 46 | Right |
| Middle Frontal Gyrus | 47 | 4.65 | 48 | 50 | −10 | Right | |
| Limbic Lobe | |||||||
| Amygdala* | 72 | 3.04 | −28 | −6 | −18 | Left | |
| Amygdala* | 106 | 4.13 | 24 | −8 | −12 | Right | |
| Parahippocampal Gyrus | 36 | 399 | 5.9 | −22 | −44 | −10 | Left |
| Temporal Lobe | |||||||
| Middle Temporal Gyrus | 21 | 3723 | 9.16 | −54 | −12 | −18 | Left |
| Middle Temporal Gyrus | 39 | 2924 | 8.94 | −56 | −74 | 18 | Left |
| Superior Temporal Gyrus | 38 | 1572 | 6.83 | 48 | 18 | −26 | Right |
| Middle Temporal Gyrus | 39 | 1004 | 5.76 | 58 | −72 | 22 | Right |
| Parietal Lobe | |||||||
| Precuneus | 7 | 773 | 5.75 | −6 | −60 | 32 | Left |
| 2b: Regions in which activity was greater for Distraction than Reappraisal; Distract Negative > Reappraise Negative (masked with Distract Negative> Look Negative). | |||||||
|---|---|---|---|---|---|---|---|
| Region | Brodmann Area | Extent | T | MNI X | MNI Y | MNI Z | Hemisphere |
| Frontal Lobe | |||||||
| Precentral Gyrus | 6 | 293 | 7.76 | −56 | −4 | 48 | Left |
| Middle Frontal Gyrus | 64 | 4.9 | 48 | 42 | 32 | Right | |
| Parietal Lobe | |||||||
| Superior Parietal Lobule | 7 | 324 | 5.61 | −26 | −66 | 42 | Left |
The amygdala was explored as an a priori region of interest with a voxel threshold of p <0.05, extent threshold of 5.
Correlations with self-reported negative affect
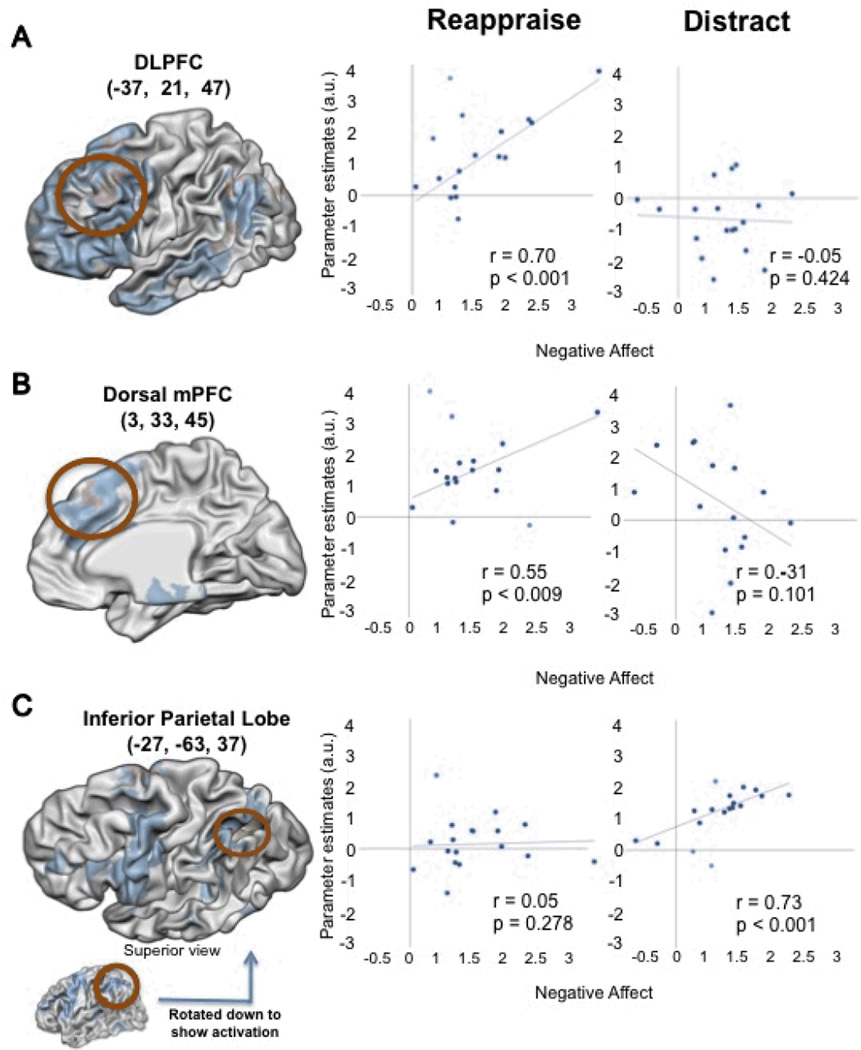
Separate regression analyses for each strategy were used to identify the regions that correlated with the difference in negative affect between the non-regulation condition (look negative) and each regulation condition (distraction, reappraisal). Several regions were correlated with decreases in self-report during to reappraisal, including left lateral prefrontal cortex, dorsomedial prefrontal cortex, and an activation in caudate that extended into the ventral striatum. By contrast, only a few regions, including inferior parietal cortex, were correlated with decreases in self-report during distraction. These results are shown in Figure 4 and Table 3.
Figure 4.
Whole-brain correlations with decreases due to negative affect during reappraisal (A and B) and distraction (C). Regions in blue represent the main effect (regulation > attend). Regions in orange show a significant correlation with decreases in self-reported negative affect during reappraisal (A and B) and during distraction (C).
Table 3.
Regions correlated with decreases in self-reported negative affect during reappraisal or distraction. The regions listed for each contrast were identified in the regression with that contrast, but r and p values for the other contrast are listed for comparison purposes.
| Region | Brodmann Area | Extent |
Pearson r Reappraise |
p |
Pearson r Distract |
p | x | y | z | Hemisphere |
|---|---|---|---|---|---|---|---|---|---|---|
| Reappraise > Look | ||||||||||
| Frontal Lobe | ||||||||||
| Inferior Frontal Gyrus | 47 | 35 | 0.74 | < 0.001 | −0.07 | 0.400 | −45 | 29 | −11 | Left |
| Superior Frontal Gyrus | 6 | 54 | 0.68 | 0.002 | 0.01 | 0.487 | −17 | 19 | 67 | Left |
| Superior Frontal Gyrus | 10 | 55 | 0.75 | < 0.001 | −0.25 | 0.178 | −27 | 57 | 29 | Left |
| Inferior Frontal Gyrus | 47 | 77 | 0.65 | 0.003 | −0.19 | 0.241 | −57 | 25 | −9 | Left |
| Middle Frontal Gyrus | 8 | 192 | 0.72 | 0.001 | −0.05 | 0.429 | −37 | 21 | 47 | Left |
| Middle Frontal Gyrus | 6 | 37 | 0.61 | 0.005 | −0.12 | 0.323 | 59 | 3 | 49 | Right |
| Middle Frontal Gyrus | 46 | 51 | 0.65 | 0.003 | −0.24 | 0.183 | 55 | 27 | 29 | Right |
| Medial Frontal Gyrus | 8 | 61 | 0.59 | 0.007 | −0.32 | 0.111 | 3 | 33 | 45 | Right |
| Middle Frontal Gyrus | 10 | 66 | 0.68 | 0.002 | 0.22 | 0.206 | 33 | 61 | 9 | Right |
| Middle Frontal Gyrus | 11 | 78 | 0.77 | < 0.001 | −0.12 | 0.325 | 45 | 51 | −13 | Right |
| Parietal Lobe | ||||||||||
| Superior Parietal Lobule | 7 | 244 | 0.63 | 0.004 | 0.29 | 0.138 | −31 | −71 | 51 | Left |
| Occipital Lobe | ||||||||||
| Superior Occipital Gyrus | 19 | 37 | 0.64 | 0.003 | 0.13 | 0.311 | 35 | −95 | 19 | Right |
| Temporal Lobe | ||||||||||
| Superior Temporal Gyrus | 22 | 51 | 0.6 | 0.006 | −0.11 | 0.337 | −61 | −55 | 19 | Left |
| Subcortical | ||||||||||
| Lentiform Nucleus | Putamen | 91 | 0.64 | 0.004 | 0.36 | 0.081 | 21 | 17 | 3 | Right |
| Distract > Look | ||||||||||
| Frontal Lobe | ||||||||||
| Superior Frontal Gyrus | 6 | 51 | 0.33 | 0.107 | 0.72 | 0.001 | −7 | −1 | 71 | Left |
| Parietal Lobe | ||||||||||
| Angular Gyrus | 39 | 121 | 0.05 | 0.425 | 0.77 | < 0.001 | −27 | −63 | 37 | Left |
| Superior Parietal Lobule | 7 | 98 | 0.25 | 0.174 | 0.68 | 0.002 | 31 | −71 | 49 | Right |
| Occipital Lobe | ||||||||||
| Cuneus | 30 | 34 | 0.39 | 0.065 | 0.66 | 0.002 | −21 | −75 | 7 | Left |
| Subcortical | ||||||||||
| Lentiform Nucleus | Putamen | 63 | −0.22 | 0.202 | 0.71 | 0.001 | −21 | 11 | 15 | Left |
Discussion
This study provided the first direct comparison of the behavioral and neural correlates of attentional distraction and cognitive reappraisal. We found both similarities and differences between them. On one hand, both strategies decreased negative affect, decreased activation in the amygdala, and increased activation in prefrontal and cingulate regions that have been implicated in the control of cognition and emotion. On the other hand, there were differential effects of each strategy that provide insight into the processes that define them. Reappraisal resulted in greater decreases in negative affect and increases in activation medial prefrontal and anterior temporal regions associated with processing affective meaning. Distraction resulted in greater decreases in activation in the amygdala and increases in activation of prefrontal and parietal regions associated with selective attention. As discussed below, these data inform both our specific understanding of distraction and reappraisal and our understanding of the neural architecture supporting emotion regulation more generally.
Common Effects of Distraction and Reappraisal
One of the striking results of this study was that distraction and reappraisal utilized overlapping prefrontal networks to decrease both amygdala activity and self-reported negative emotion. These findings fit with prior work generally implicating prefrontal-amygdala dynamics in the cognitive control of emotion (Kim & Hamann, 2007; Lieberman et al., 2007; Ochsner & Gross, 2008; Stein et al., 2007; Urry et al., 2006), but go beyond them by implicating specific neural systems as involved in this regulatory process regardless of the strategy employed.
The network active during both distraction and reappraisal could reflect their mutual reliance on a set of control operations that play important roles in both strategies. Common activations included a large medial prefrontal region that included the dorsal anterior cingulate, which has been implicated in signaling the need for cognitive control (Lungu, Binenstock, Pline, Yeaton, & Carey, 2007; Milham, Banich, Claus, & Cohen, 2003; Procyk, Tanaka, & Joseph, 2000) and controlling attention to emotional stimuli (Hutcherson et al., 2005; McRae, Reiman, Fort, Chen, & Lane, in press), as well as left inferior parietal cortex, which may also reflect recruitment of attentional control processes in both strategies (Mayer et al., 2007). Also commonly active were regions of left lateral prefrontal cortex associated with verbal or working memory (Paulesu, Frith, & Frackowiak, 1993; Wager & Smith, 2003) and regions of right inferior prefrontal cortex associated with inhibition of motor responses (Aron, Robbins, & Poldrack, 2004) and other verbal strategies that can be used to down-regulate negative affective responses (Lieberman et al., 2007; Ochsner, Ray et al., 2004). These prefrontal activations may reflect the need to keep in mind the goals and contents of each strategy – letters in the case of distraction and an interpretation of the image in the case of reappraisal – as well as the need to withhold prepotent affective appraisals while doing so.
Differential Effects of Distraction and Reappraisal
Perhaps more salient than the common effects of each strategy were their differential effects on emotional responding, which in turn depended on the differential recruitment of specific control systems. These differences provide insight into the distinguishing characteristics of each strategy.
Differential Modulation of Emotional Responses
Distraction and reappraisal led to intriguing differences in self-reported negative emotion and amygdala activity. Considering the self report effects first, prior work is mixed with respect to the question of which strategy more effectively down-regulates negative emotion. On one hand, there is some laboratory evidence that both strategies are effective at down-regulating emotions (Sheppes & Merian, 2007). On the other hand, a study of everyday strategy use found that distraction was rated as less effective than reappraisal (Totterdell & Parkinson, 1999), a finding that fits with previous behavioral work showing that distraction may be less effective than cognitive reappraisal for down-regulating depressive affect (Kross & Ayduk, in press), and imaging work showing that distraction may not effectively diminish pain affect (Chua, Krams, Toni, Passingham, & Dolan, 1999; Kalisch, Wiech, Herrmann, & Dolan, 2006). In part, the inconsistent effects of distraction on self reports of emotional responding may be due to the variation in the types of distracting tasks used. Overall, our findings dovetail with prior work suggesting that reappraisal is one of the most effective ways to down-regulate self-reported emotion.
Distraction, however, down-regulated amygdala activity to a greater extent than reappraisal. Amygdala activity is thought to signal the degree to which a stimulus that is detected in the environment requires some sort of further processing – whether it be to bring the stimulus into focal attention, to prepare motor responses, or to enhance encoding into memory (LeDoux, 1996; Phelps, 2006; Whalen et al., 2004). On this view, distraction should decrease amygdala activity because individuals are not attending to or encoding all the emotional aspects of a stimulus. By contrast, reappraisal involves focusing one’s attention on a stimulus and reinterpreting its meaning. In this case, amygdala activity might decrease to the extent that a cognitive reinterpretation of an aversive stimulus renders it neutral and unarousing (e.g. “That man is tired, not sick”). In other cases, however, reappraising a negative stimulus as positive (e.g. “The crying women are joyful, not sad”) may maintain some level of arousal, albeit with a different valence (Cunningham, Van Bavel, & Johnsen, 2008; Hamann, Ely, Hoffman, & Kilts, 2002; McRae, Ochsner, Mauss, Gabrieli, & Gross, 2008). Thus, because reappraisal involves attending to the emotional stimulus, and possibly reframing its meaning as positive but still arousing, we not might expect amygdala activity to be entirely diminished during reappraisal.
The possibility that reappraisal results in relatively sustained amygdala activation compared to distraction is supported by two additional results reported here. The first is that reappraisal and distraction appear to down-regulate amygdala voxels that are not entirely overlapping (Figure 2). Although it is possible that these two strategies could have their maximal effects in different parts of the amygdala, our imaging parameters do not permit precise localization of activations with in amygdala sub-nuclei, and so it is impossible for the present data to address this. The second result is the relationship between self-reported decreases in negative affect due to reappraisal and reappraisal-related activation. The ventral striatum, which has a strong role in processing reward and positive affect, was more active in those that had greater reappraisal-related decreases in negative affect (McRae, Ochsner, Mauss, Gabrieli, & Gross, 2008; Wager, Davidson, Hughes, Lindquist, & Ochsner, 2008). This was not true of the decreases in negative affect due to distraction. Future work should investigate the difference of reappraising to a positive versus a neutral target.
Differential Reliance on Control Systems
As outlined above, a network of regions generally implicated in cognitive control was recruited by both distraction and reappraisal. The regions that distinguish between the two strategies, however, provide important clues as to the key processes that uniquely define them. Whereas distraction depended more on right prefrontal and pariental regions implicated in the control of attention (Mayer et al., 2007), reappraisal depended more on regions implicated in appraising the an affective stimulus in the context of one’s current individual goals and context (Teasdale et al., 1999; Van Overwalle, 2008). This network included mPFC, which has been associated with emotional awareness and mental state attribution (Gilbert et al., 2006; Olsson & Ochsner, 2008; Teasdale et al., 1999), vlPFC regions that may reflect a stimulus’s current affective value (Bender, Hellwig, Resch, & Weisbrod, 2007; Boettiger & D'Esposito, 2005; Hornak et al., 2004), and inferior temporal regions important for recognizing social cues (Britton et al., 2006; Fletcher et al., 1995; Tsukiura et al., 2002).
Here it is noteworthy that a subset of the regions that positively covaried with decreases in self-reported negative affect during reappraisal negatively covaried with decreases in self-reported negative affect during distraction (see Figure 4, panel b). These findings are consistent with the argument advanced earlier that in order to reappraise an emotional stimulus, the initial appraisal (or affective meaning) of the stimulus must be attended and then altered so that the emotional meaning is changed (Scherer, 2001). By contrast, when effective, distraction can prevent the affective meaning of a stimulus from being processed. Thus, one of the primary differences between distraction and reappraisal is the degree to which the affective meaning of the stimulus is attended and appraised.
It also is worth noting that, as can be seen in Figure 3, clusters showing greater activation for reappraisal, for distraction, or that were activated by them both, are in some cases spatially close to one another. This raises the question as to whether such spatially similar, but statistically distinct, activation peaks reflect differential dependence on distinct cognitive processes or quantitative differences in the recruitment of similar processes. This question is, of course, not unique to this study, and within cognitive neuroscience there currently are no clear criteria for determining the answer. That being said, we favor the idea that in regions showing distinct but spatially similar peaks for reappraisal and distraction, the two strategies recruit similar processes, but to different degrees. Future replication and extension of the present study may serve to better address this issue, including determining whether small differences in the specific foci of activation may be affected by the constellation of other regions that are simultaneously engaged during performance of each strategy.
Figure 3.
Whole-brain results from three contrasts. Orange: (Reappraise > Distract masked with Reappraise > Look Negative) Blue: (Distract > Reappraise masked with Distract > Look Negative) Purple: (Reappraise > Look Negative masked by Distract > Look Negative).
Implications for the Use of Distraction and Reappraisal
Our interpretation of the behavioral and imaging results has several implications for understanding the consequences of using distraction and reappraisal both in everyday life and in the course of clinical interventions. Some of these implications follow from the finding that a set of prefrontal and cingulate regions associated with cognitive control supported the performance of both strategies. This suggests that success in implementing distraction or reappraisal depends in part on the integrity of domain-general control processes. This could have important implications for understanding how individual differences in general control abilities may influence the efficacy of emotion regulation. For example, developing adolescents who show impairments in “cold” cognitive control abilities such as working memory and inhibition of prepotent responses might be expected to be less effective emotion regulators. In like fashion, structural or functional deficits in the commonly recruited prefrontal and cingulate regions might also be expected to impact both distraction and reappraisal. For instance, older adults or individuals with schizophrenia show deficits in structure and function in cingulate and prefrontal cortices and might be expected to show relatively diminished capacities to regulate using either strategy, just as they would show impaired abilities to perform other types of higher-level cognitive control (Andreasen et al., 1994; Bell-McGinty et al., 2005; Carter & Barch, 2007; Davidson & Heinrichs, 2003; Grieve, Williams, Paul, Clark, & Gordon, 2007).
Many other implications of our data and interpretations follow from the finding that reappraisal involves greater processing of the affective meaning of the stimulus, whereas distraction involves lesser attention to and poorer encoding of affective meaning than during unregulated responding. This suggests superior memory for items viewed while reappraising, which has been demonstrated in several studies (Dillon, Ritchey, Johnson, & LaBar, 2007; Richards & Gross, 2000; Sheppes & Meiran, 2007) and impaired memory for items viewed during distraction, which also has been observed (Sheppes & Meiran, 2007).
These findings further suggest divergent effects of reappraisal and distraction upon unregulated re-exposure to emotional stimuli. In particular, one might predict that when re-exposed to stimuli initially viewed under instructions to reappraise, to the extent that prior reappraisal altered the meaning of the stimulus and that this new meaning is re-accessed, then several aspects of the emotional response would be diminished. However, if one is re-exposed to stimuli previously viewed during distraction, the stimulus may be processed as if it was being seen for the first time. This idea has been borne out by laboratory studies showing that cognitive reinterpretation has long-lasting effects, whereas distraction only has immediate effects (Kross & Ayduk, in press). This suggests that distraction may be best used in situations when ignoring the affective meaning of the eliciting stimulus is permissible – such as situations that do not require memory for the stimulus, or when re-exposure is unlikely. Reappraisal, while requiring more extensive processing of affective meaning, might be more appropriate when this meaning must be addressed, manipulated, remembered, and re-exposure is likely. These predictions are consistent with the intuition that it is often permissible to use distraction when watching a gruesome movie or listening to a sad story about a stranger, because there are very few consequences of poor encoding of the affective meaning of the story. However, when one is faced with a more personally relevant situation, such as handling the sickness or death of a loved one, often times it is important to be aware of and to reappraise the meaning of the emotionally charged aspects of the situation so that future reminders of the event do not retain the power to continually re-evoke the sadness or trauma of the past event.
Limitations and Future Directions
One important direction for future research concerns stimulus generalizability. One strength of the present study is that our use of picture stimuli permits contact with a large number of previous studies using an emotional picture paradigm to study cognitive reappraisal (e.g. Ochsner et al., 2002; Urry et al., 2006). In this context, the present results replicate and extend prior work. By contrast, much of the work in distraction has used painful stimulation rather than image-based paradigms (e.g. Bantick et al. 2002, Kalisch et al 2006). Therefore, a direct comparison of distraction and reappraisal in the context of pain anticipation or delivery – or in the context of other types of emotionally charged stimuli– might further illuminate the similarities and differences between the two strategies.
Another important direction for future research concerns subtypes of distraction and reappraisal. Based on previous comparisons of different types of reappraisal strategies (Ochsner et al., 2004), we might expect different types of reappraisal to compare with distraction in different ways. Similarly, the specific way in which one is distracted may also be important. We selected a well-characterized verbal working memory task that was intended to depend on verbal rehearsal processes like those thought to be involved in reappraisal and that was matched to reappraisal in terms of subjective effort. Previous studies of distraction have used other types of demanding cognitive tasks that involve various kinds and combinations of control processes whose relative level of effort is not clear. Therefore, comparisons of several methods of distraction and several types of reappraisals may be important in future research.
The distraction and reappraisal conditions also differed in an important psychological respect. The reappraisal instruction necessarily called the participant’s attention to the fact that successful reduction of negative affect could be taking place. This might have created a situation of greater experimental demand during the reappraisal condition compared to distraction. Although we took several precautions against these demand effects, we cannot rule out the possibility that experimenter demand may be reflected in some of the results reported here.
A final direction for future research concerns the role that individual and group differences play in determining whether reappraisal or distraction is more effective. In the present study, we chose to include only women so as to avoid gender-related factors that might influence emotional responding (Bradley, Codispoti, Sabatinelli, & Lang, 2001; Wrase et al., 2003) or emotion regulation (McRae, Ochsner, Mauss, Gabrieli, & Gross, 2008; Rusting, 1998). It is possible that the relationship between distraction and reappraisal is different in men, and these gender differences should be investigated in the future. The presence and nature of a clinical disorder may be another important factor determining whether distraction or reappraisal is more effective. For instance, distraction has been shown to be a successful emotion regulation strategy for those who suffer from past or current major depression (Fennell & Teasdale, 1984; Joorman, Siemer, & Gotlib, 2007). However, paradoxical effects of distraction have been reported when it is used during exposure therapy for specific phobias (Craske, Street, Jayaraman, & Barlow, 1991; Foa & Kozak, 1986; Telch et al., 2004). Future work could examine whether and how clinical contexts dictate when distraction can facilitate the down-regulation of negative emotion.
Concluding Comment
The present study compared the emotional effects and neural bases of two commonly used emotion regulation strategies: attentional distraction and cognitive reappraisal. We found that both strategies successfully reduced emotional experience and amygdala activity while engaging prefrontal regions important for working memory, selective attention and cognitive control more generally. In addition, reappraisal preferentially activated a network associated with processing affective meaning, and resulted in more successful down-regulation of emotional experience than distraction. Distraction, by contrast, preferentially activated regions associated with the allocation of attention, and resulted in down-regulation of amygdala activity to a greater extent than reappraisal. We interpret these results as indicating that reappraisal requires attending to and processing affective meaning of the stimulus to be regulated whereas distraction results in decreased processing of affective meaning. Future work should identify the situational and clinical contexts in which enhancing or ignoring affective meaning results in maximally effective emotion regulation.
Acknowledgements
The authors would like to thank Jeffrey Cooper, Rebecca D. Ray, PhD, Matthew Davidson, Jochen Weber, Elaine Robertson and Sean Pereira for their assistance with data analysis and display. Completion of this research was supported by NIH Grants MH076137 (K.N.O.) and MH58147 and MH66957 (J.J.G).
Footnotes
Ten female participants between the ages of 18 and 22 years were asked to view negative IAPS pictures under the instructions to reappraise. They were asked to indicate when they were finished with a keypress. The average time to finish was 6.93 seconds (s.d. = 3.52 seconds). The time in seconds it took people to indicate they were finished was moderately related to their degree of down-regulation of negative affect (r = 0.60, p <0.07).
References
- Amstadter A. Emotion regulation and anxiety disorders. Journal of Anxiety Disorders. 2008;22(2):211–221. doi: 10.1016/j.janxdis.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen NC, Flashman L, Flaum M, Arndt S, Swayze V, O'Leary DS, et al. Regional brain abnormalities in schizophrenia measured with magnetic resonance imaging. Journal of the American Medical Association. 1994;272(22):1763–1769. [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends in Cognitive Sciences. 2004;8(4):170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Bantick SJ, Wise RG, Ploghaus A, Clare S, Smith SM, Tracey I. Imaging how attention modulates pain in humans using functional MRI. Brain. 2002;125(2):310–319. doi: 10.1093/brain/awf022. [DOI] [PubMed] [Google Scholar]
- Beck AT, Rush S, Shaw P, Emery N. Cognitive therapy of depression. New York: Guilford Press; 1979. [Google Scholar]
- Bell-McGinty S, Lopez OL, Meltzer CC, Scanlon JM, Whyte EM, DeKosky ST, et al. Differential Cortical Atrophy in Subgroups of Mild Cognitive Impairment. Arch Neurol. 2005;62(9):1393–1397. doi: 10.1001/archneur.62.9.1393. [DOI] [PubMed] [Google Scholar]
- Bender S, Hellwig S, Resch F, Weisbrod M. Am I safe? The ventrolateral prefrontal cortex `detects' when an unpleasant event does not occur. NeuroImage. 2007;38(2):367–385. doi: 10.1016/j.neuroimage.2007.07.044. [DOI] [PubMed] [Google Scholar]
- Bennett P, Phelps C, Brain K, Hood K, Gray J. A randomized controlled trial of a brief self-help coping intervention designed to reduce distress when awaiting genetic risk information. Journal of Psychosomatic Research. 2007;63(1):59–64. doi: 10.1016/j.jpsychores.2007.01.016. [DOI] [PubMed] [Google Scholar]
- Boettiger CA, D'Esposito M. Frontal Networks for Learning and Executing Arbitrary Stimulus-Response Associations. J. Neurosci. 2005;25(10):2723–2732. doi: 10.1523/JNEUROSCI.3697-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley MM, Codispoti M, Sabatinelli D, Lang PJ. Emotion and motivation II: Sex differences in picture processing. Emotion. 2001;1(3):300–319. [PubMed] [Google Scholar]
- Brett M, Anton JL, Valabregue R, Poline J-B. Region of interest analysis using an SPM toolbox. Neuroimage. 2002;16 abstract 497. [Google Scholar]
- Britton JC, Phan KL, Taylor SF, Welsh RC, Berridge KC, Liberzon I. Neural correlates of social and nonsocial emotions: An fMRI study. NeuroImage. 2006;31(1):397–409. doi: 10.1016/j.neuroimage.2005.11.027. [DOI] [PubMed] [Google Scholar]
- Carter CS, Barch DM. Cognitive neuroscience-based approaches to measuring and improving treatment effects on cognition in schizophrenia: The CNTRICS initiative. Schizophr Bull. 2007;33(5):1131–1137. doi: 10.1093/schbul/sbm081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua P, Krams M, Toni I, Passingham R, Dolan R. A functional anatomy of anticipatory anxiety. Neuroimage. 1999;9(6 Pt 1):563–571. doi: 10.1006/nimg.1999.0407. [DOI] [PubMed] [Google Scholar]
- Craske MG, Street L, Barlow DH. Instructions to focus upon or distract from internal cues during exposure treatment of agoraphobic avoidance. Behaviour Research and Therapy. 1989;27(6):663–672. doi: 10.1016/0005-7967(89)90150-2. [DOI] [PubMed] [Google Scholar]
- Craske MG, Street LL, Jayaraman J, Barlow DH. Attention versus distraction during in vivo exposure: Snake and spider phobias. Journal of Anxiety Disorders. 1991;5(3):199–211. [Google Scholar]
- Cunningham WA, Van Bavel JJ, Johnsen IR. Affective Flexibility: Evaluative Processing Goals Shape Amygdala Activity. Psychological Science. 2008;19(2):152–160. doi: 10.1111/j.1467-9280.2008.02061.x. [DOI] [PubMed] [Google Scholar]
- Davidson LL, Heinrichs RW. Quantification of frontal and temporal lobe brain-imaging findings in schizophrenia: a meta-analysis. Psychiatry Research: Neuroimaging. 2003;122(2):69–87. doi: 10.1016/s0925-4927(02)00118-x. [DOI] [PubMed] [Google Scholar]
- Dillon DG, Ritchey M, Johnson BD, LaBar KS. Dissociable effects of conscious emotion regulation strategies on explicit and implicit memory. Emotion. 2007;7(2):354–365. doi: 10.1037/1528-3542.7.2.354. [DOI] [PubMed] [Google Scholar]
- Eippert F, Veit R, Weiskopf N, Erb M, Birbaumer N, Anders S. Regulation of emotional responses elicited by threat-related stimuli. Human Brain Mapping. 2007;28(5):409–423. doi: 10.1002/hbm.20291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fennell MJ, Teasdale JD. Effects of distraction on thinking and affect in depressed patients. British Journal of Clinical Psychology. 1984;23:65–66. doi: 10.1111/j.2044-8260.1984.tb00628.x. [DOI] [PubMed] [Google Scholar]
- Fletcher PC, Happe F, Frith U, Baker SC, Dolan RJ, Frackowiak RSJ, et al. Other minds in the brain: a functional imaging study of "theory of mind" in story comprehension. Cognition. 1995;57(2):109–128. doi: 10.1016/0010-0277(95)00692-r. [DOI] [PubMed] [Google Scholar]
- Foa EB, Kozak MJ. Emotional processing of fear: Exposure to corrective information. Psychological Bulletin. 1986;99(1):20–35. [PubMed] [Google Scholar]
- Forman SCJD, Fitzgerald M, Eddy W, Mintun M, Noll D. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magnetic Resonance in Medicine. 1995;(33):636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Frankenstein UN, Richter W, McIntyre MC, Remy F. Distraction Modulates Anterior Cingulate Gyrus Activations during the Cold Pressor Test. NeuroImage. 2001;14(4):827–836. doi: 10.1006/nimg.2001.0883. [DOI] [PubMed] [Google Scholar]
- Gerin W, Davidson KW, Christenfeld NJS, Goyal T, Schwartz JE. The Role of Angry Rumination and Distraction in Blood Pressure Recovery From Emotional Arousal. Psychosom Med. 2006;68(1):64–72. doi: 10.1097/01.psy.0000195747.12404.aa. [DOI] [PubMed] [Google Scholar]
- Gilbert SJ, Spengler S, Simons JS, Steele JD, Lawrie SM, Frith CD, et al. Functional Specialization within Rostral Prefrontal Cortex (Area 10): A Meta-analysis. Journal of Cognitive Neuroscience. 2006;18(6):932–948. doi: 10.1162/jocn.2006.18.6.932. [DOI] [PubMed] [Google Scholar]
- Grieve SM, Williams LM, Paul RH, Clark CR, Gordon E. Cognitive Aging, Executive Function, and Fractional Anisotropy: A Diffusion Tensor MR Imaging Study. Am J Neuroradiol. 2007;28(2):226–235. [PMC free article] [PubMed] [Google Scholar]
- Gross JJ. Antecedent- and response-focused emotion regulation: divergent consequences for experience, expression, and physiology. Journal of Personality and Social Psychology. 1998a;74:224–237. doi: 10.1037//0022-3514.74.1.224. [DOI] [PubMed] [Google Scholar]
- Gross JJ. The emerging field of emotion regulation: An integrative review. Review of General Psychological. 1998b;2:271–299. [Google Scholar]
- Gross JJ, editor. Handbook of emotion regulation. New York, NY: Guilford Press; 2007. [Google Scholar]
- Hamann S, Ely TD, Hoffman JM, Kilts CD. Ecstasy and agony: activation of the human amygdala in positive and negative emotion. Psychological Science. 2002;13:135–141. doi: 10.1111/1467-9280.00425. [DOI] [PubMed] [Google Scholar]
- Hornak J, O'Doherty J, Bramham J, Rolls ET, Morris RG, Bullock PR, et al. Reward-related Reversal Learning after Surgical Excisions in Orbito-frontal or Dorsolateral Prefrontal Cortex in Humans. Journal of Cognitive Neuroscience. 2004;16(3):463–478. doi: 10.1162/089892904322926791. [DOI] [PubMed] [Google Scholar]
- Hutcherson CA, Goldin PR, Ochsner KN, Gabrieli JDE, Feldman Barrett L, Gross JJ. Attention and Emotion: Does Rating Emotion Alter Neural Responses to Amusing and Sad Films? NeuroImage. 2005;27(3):656–668. doi: 10.1016/j.neuroimage.2005.04.028. [DOI] [PubMed] [Google Scholar]
- Jackson DC, Malmstadt JR, Larson CL, Davidson RJ. Suppression and enhancement of emotional responses to unpleasant pictures. Psychophysiology. 2000;37(4):515–522. [PubMed] [Google Scholar]
- Joorman J, Siemer M, Gotlib IH. Mood regulation in depression: Differential effects of distraction and recall of happy memories on sad mood. Journal of Abnormal Psychology. 2007;116(3):484–490. doi: 10.1037/0021-843X.116.3.484. [DOI] [PubMed] [Google Scholar]
- Kalisch R, Wiech K, Herrmann K, Dolan RJ. Neural correlates of self-distraction from anxiety and a process model of cognitive emotion regulation. J Cogn Neurosci. 2006;18(8):1266–1276. doi: 10.1162/jocn.2006.18.8.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampe KKW, Frith CD, Frith U. "Hey John": Signals Conveying Communicative Intention toward the Self Activate Brain Regions Associated with "Mentalizing," Regardless of Modality. J. Neurosci. 2003;23(12):5258–5263. doi: 10.1523/JNEUROSCI.23-12-05258.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Hamann S. Neural Correlates of Positive and Negative Emotion Regulation. Journal of Cognitive Neuroscience. 2007;19(5):776–798. doi: 10.1162/jocn.2007.19.5.776. [DOI] [PubMed] [Google Scholar]
- Kross E, Ayduk O. Facilitating Adaptive Emotional Analysis: Distinguishing Distanced-Analysis of Depressive Experiences From Immersed-Analysis and Distraction. Personality and Social Psychology Bulletin. doi: 10.1177/0146167208315938. (in press) [DOI] [PubMed] [Google Scholar]
- Lane RD, McRae K. Neural substrates of conscious emotional experience: A cognitive-neuroscientific perspective. In: Beauregard M, editor. Consciousness, Emotional Self-Regulation and the Brain. Amsterdam: John Benjamins; 2004. [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. The University of Florida: The Center for Research in Psychophysiology; 2001. International Affective Picture System (IAPS). Instruction Manual and Affective Ratings. Technical Report. [Google Scholar]
- LeDoux JE. The emotional brain: The mysterious underpinnings of emotional life. New York, NY: Simon & Schuster; 1996. [Google Scholar]
- Li J, Lambert VA. Coping strategies and predictors of general well-being in women with breast cancer in the People's Republic of China. Nursing & Health Sciences. 2007;9(3):199–204. doi: 10.1111/j.1442-2018.2007.00325.x. [DOI] [PubMed] [Google Scholar]
- Lieberman MD, Eisenberger NI, Crockett MJ, Tom SM, Pfeifer JH, Way BM. Putting Feelings Into Words: Affect Labeling Disrupts Amygdala Activity in Response to Affective Stimuli. Psychological Science. 2007;18(5):421–428. doi: 10.1111/j.1467-9280.2007.01916.x. [DOI] [PubMed] [Google Scholar]
- Lungu OV, Binenstock MM, Pline MA, Yeaton JR, Carey JR. Neural Changes in Control Implementation of a Continuous Task. J. Neurosci. 2007;27(11):3010–3016. doi: 10.1523/JNEUROSCI.5051-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer JS, Bittner RA, Nikolic D, Bledowski C, Goebel R, Linden DEJ. Common neural substrates for visual working memory and attention. NeuroImage. 2007;36(2):441–453. doi: 10.1016/j.neuroimage.2007.03.007. [DOI] [PubMed] [Google Scholar]
- McRae K, Ochsner KN, Mauss IB, Gabrieli JD, Gross JJ. Gender differences in emotion regulation: An fMRI study of cognitive reappraisal. Group Processes and Intergroup Relations. 2008;11(2):143–162. doi: 10.1177/1368430207088035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae K, Reiman EM, Fort CL, Chen K, Lane RD. Association between trait emotional awareness and dorsal anterior cingulate activity during emotion is arousal-dependent. NeuroImage. doi: 10.1016/j.neuroimage.2008.02.030. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milham MP, Banich MT, Claus ED, Cohen NJ. Practice-related effects demonstrate complementary roles of anterior cingulate and prefrontal cortices in attentional control. NeuroImage. 2003;18(2):483–493. doi: 10.1016/s1053-8119(02)00050-2. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual Review of Neuroscience. 2001;24(1):167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD. Rethinking feelings: An FMRI study of the cognitive regulation of emotion. Journal of Cognitive Neuroscience. 2002;14:1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends in Cognitive Sciences. 2005;9(5):242. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. Cognitive emotion regulation: Insights from social cognitive and affective neuroscience. Current Directions in Psychological Science. 2008;17(2):153–158. doi: 10.1111/j.1467-8721.2008.00566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Knierim K, Ludlow D, Hanelin J, Ramachandran T, Mackey S. Reflecting upon feelings: An fMRI study of neural systems supporting the attribution of emotion to self and other. Journal of Cognitive Neuroscience. 2004;16(10):1746–1772. doi: 10.1162/0898929042947829. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JD, et al. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage. 2004;23:483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Ohira H, Nomura M, Ichikawa N, Isowa T, Iidaka T, Sato A, et al. Association of neural and physiological responses during voluntary emotion suppression. NeuroImage. 2006;29(3):721–733. doi: 10.1016/j.neuroimage.2005.08.047. [DOI] [PubMed] [Google Scholar]
- Olsson A, Ochsner KN. The role of social cognition in emotion. Trends in Cognitive Sciences. 2008;12(2):65–71. doi: 10.1016/j.tics.2007.11.010. [DOI] [PubMed] [Google Scholar]
- Paulesu E, Frith CD, Frackowiak RSJ. The neural correlates of the verbal component of working memory. Nature. 1993;362:342–345. doi: 10.1038/362342a0. [DOI] [PubMed] [Google Scholar]
- Phan KL, Fitzgerald DA, Nathan PJ, Moore GJ, Uhde TW, Tancer ME. Neural substrates for voluntary suppression of negative affect: A functional magnetic resonance imaging study. Biol Psychiatry. 2005;57(3):210–219. doi: 10.1016/j.biopsych.2004.10.030. [DOI] [PubMed] [Google Scholar]
- Phelps EA. Emotion and Cognition: Insights from Studies of the Human Amygdala. Annual Review of Psychology. 2006;57(1):27–53. doi: 10.1146/annurev.psych.56.091103.070234. [DOI] [PubMed] [Google Scholar]
- Postle BR, Zarahn E, D'Esposito M. Using event-related fMRI to assess delay-period activity during performance of spatial and nonspatial working memory tasks. Brain Research Protocols. 2000;5(1):57–66. doi: 10.1016/s1385-299x(99)00053-7. [DOI] [PubMed] [Google Scholar]
- Preston AR, Thomason ME, Ochsner KN, Cooper JC, Glover GH. Comparison of spiral-in/out and spiral-out BOLD fMRI at 1.5 and 3 T. NeuroImage. 2004;21:291–301. doi: 10.1016/j.neuroimage.2003.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Procyk E, Tanaka YL, Joseph JP. Anterior cingulate activity during routine and non-routine sequential behaviors in macaques. Nature Neuroscience. 2000;3:502–508. doi: 10.1038/74880. [DOI] [PubMed] [Google Scholar]
- Richards JM, Gross JJ. Emotion regulation and memory: The cognitive costs of keeping one's cool. Journal of Personality and Social Psychology. 2000;79:410–424. doi: 10.1037//0022-3514.79.3.410. [DOI] [PubMed] [Google Scholar]
- Rusting CL. Personality, mood, and cognitive processing of emotional information: three conceptual frameworks. Psychological Bulletin. 1998;124:165–196. doi: 10.1037/0033-2909.124.2.165. [DOI] [PubMed] [Google Scholar]
- Scherer KR, Schorr A, Johnstone T. Appraisal processes in emotion: Theory, methods, research. New York: Oxford University Press; 2001. [Google Scholar]
- Seminowicz DA, Davis KD. Interactions of Pain Intensity and Cognitive Load: The Brain Stays on Task. Cereb. Cortex. 2007;17(6):1412–1422. doi: 10.1093/cercor/bhl052. [DOI] [PubMed] [Google Scholar]
- Sheppes G, Meiran N. Better Late Than Never? On the Dynamics of Online Regulation of Sadness Using Distraction and Cognitive Reappraisal. Pers Soc Psychol Bull. 2007;33(11):1518–1532. doi: 10.1177/0146167207305537. [DOI] [PubMed] [Google Scholar]
- Stein JL, Wiedholz LM, Bassett DS, Weinberger DR, Zink CF, Mattay VS, et al. A validated network of effective amygdala connectivity. NeuroImage. 2007;36(3):736–745. doi: 10.1016/j.neuroimage.2007.03.022. [DOI] [PubMed] [Google Scholar]
- Taylor SF, Liberzon I. Neural correlates of emotion regulation in psychopathology. Trends in Cognitive Sciences. 2007;11(10):413–418. doi: 10.1016/j.tics.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Teasdale JD, Howard RJ, Cox SG, Ha Y, Brammer MJ, Williams SCR, et al. Functional MRI Study of the Cognitive Generation of Affect. Am J Psychiatry. 1999;156(2):209–215. doi: 10.1176/ajp.156.2.209. [DOI] [PubMed] [Google Scholar]
- Telch MJ, Valentiner DP, Ilai D, Young PR, Powers MB, Smits JAJ. Fear activation and distraction during the emotional processing of claustrophobic fear. Journal of Behavior Therapy and Experimental Psychiatry. 2004;35(3):219–232. doi: 10.1016/j.jbtep.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Totterdell P, Parkinson B. Use and effectiveness of self-regulation strategies for improving mood in a group of trainee teachers. Journal of Occupational Health Psychology. 1999;4(3):219. doi: 10.1037//1076-8998.4.3.219. [DOI] [PubMed] [Google Scholar]
- Tsukiura T, Fujii T, Fukatsu R, Otsuki T, Okuda J, Umetsu A, et al. Neural Basis of the Retrieval of People's Names: Evidence from Brain-Damaged Patients and fMRI. Journal of Cognitive Neuroscience. 2002;14(6):922–937. doi: 10.1162/089892902760191144. [DOI] [PubMed] [Google Scholar]
- Urry HL, van Reekum CM, Johnstone T, Kalin NH, Thurow ME, Schaefer HS, et al. Amygdala and Ventromedial Prefrontal Cortex Are Inversely Coupled during Regulation of Negative Affect and Predict the Diurnal Pattern of Cortisol Secretion among Older Adults. J. Neurosci. 2006;26(16):4415–4425. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Overwalle F. Human Brain Mapping. 9999. Vol. 9999. NA: 2008. Social cognition and the brain: A meta-analysis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN. Prefrontal-Subcortical Pathways Mediating Successful Emotion Regulation. Neuron. 2008;59(6):1037–1050. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Jonides J, Reading S. Neuroimaging studies of shifting attention: a meta-analysis. NeuroImage. 2004;22(4):1679–1693. doi: 10.1016/j.neuroimage.2004.03.052. [DOI] [PubMed] [Google Scholar]
- Wager TD, Keller MC, Lacey SC, Jonides J. Increased sensitivity in neuroimaging analyses using robust regression. NeuroImage. 2005;26:99–113. doi: 10.1016/j.neuroimage.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Wager TD, Smith EE. Neuroimaging studies of working memory: A meta-analysis. Cognitive, Affective & Behavioral Neuroscience. 2003;3(4):255–274. doi: 10.3758/cabn.3.4.255. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Kagan J, Cook RG, Davis FC, Kim H, Polis S, et al. Human amygdala responsivity to masked fearful eye whites. Science. 2004;306(5704):2061. doi: 10.1126/science.1103617. [DOI] [PubMed] [Google Scholar]
- Wiech K, Seymour B, Kalisch R, Enno Stephan K, Koltzenburg M, Driver J, et al. Modulation of pain processing in hyperalgesia by cognitive demand. NeuroImage. 2005;27(1):59–69. doi: 10.1016/j.neuroimage.2005.03.044. [DOI] [PubMed] [Google Scholar]
- Wrase J, Klein S, Gruesser SM, Hermann D, Flor H, Mann K, et al. Gender differences in the processing of standardized emotional visual stimuli in humans: a functional magnetic resonance imaging study. Neuroscience Letters. 2003;348(1):41–45. doi: 10.1016/s0304-3940(03)00565-2. [DOI] [PubMed] [Google Scholar]