Abstract
Heart failure due to nonischemic dilated cardiomyopathy (DCM) contributes significantly to the global burden of cardiovascular disease. Myocarditis is in turn a major cause of acute dilated cardiomyopathy in both men and women. However, recent clinical and experimental evidence suggests that the pathogenesis and prognosis of DCM differ between the sexes. This seminar provides a contemporary perspective on the immune mediators of myocarditis, including interdependent elements of the innate and adaptive immune response. The heart's acute response to injury is influenced by sex hormones that appear to determine the subsequent risk of chronic DCM. Preliminary data suggest additional genetic variations may account for some of the differences in epidemiology, left ventricular recovery and survival between men and women. We highlight the gaps in our knowledge regarding the management of women with acute DCM and discuss emerging therapies, including bromocriptine for the treatment of peripartum cardiomyopathy.
Introduction
Men have an increased incidence and severity of most cardiovascular diseases including atherosclerosis, myocardial infarction, dilated cardiomyopathy (DCM) and heart failure with the exception of hypertension, which is higher in women.1-4 Historically, myocarditis has been considered a male predominant disease,5 but only recently has this sex difference been confirmed in clinical studies.2,6,7 Huber and colleagues were the first to describe that male mice have increased myocarditis compared to females.8 Animal models continue to provide valuable information about the mechanisms that lead to more severe myocarditis, DCM and heart failure in males.
Historical perspective
In 1949, Ludden and Edwards from the Mayo Clinic reported a 2.5:1 ratio of myocarditis in men to women following a poliomyelitis outbreak that occurred in Minnesota in 1946.9 A study in 1968 reported an incidence of myocarditis in men vs. women of approximately 72%.10 Similarly, in 1980, Woodruff conducted a survey of 164 adolescent and adult cases of coxsackievirus B (CVB) myopericarditis reported in reviews from 1957 to 1973 and found that 109, or roughly 67%, of patients were male.5 Although the incidence of myocarditis appeared to be higher in men, there was little information on sex and gender differences in the pathogenesis of myocarditis except for data obtained from animal studies that showed more severe disease in males.11,12
In 2001, the Institute of Medicine released a report examining the importance of sex differences to human health.13 They concluded that increasing evidence of fundamental sex differences in the basic biology of cells, tissues and chronic diseases meant that examining sex differences in clinical and basic research studies was critical. Around this same time, a number of studies emerged indicating important sex and gender differences in cardiac remodeling and heart failure in patients.14,15 However, a decade would pass from the Institute of Medicine report on the importance of sex differences in disease before more specific clinical data would be obtained about sex differences in myocarditis and DCM patients.
Definitions
Sex and gender are not interchangeable terms. “Sex” is a biological term. In its report, “Exploring the Biological Contributions to Human Health: Does Sex Matter?”, the Institute of Medicine defines sex as “the classification of living things, generally as male or female according to their reproductive organs and functions assigned by the chromosomal complement.”13 Examples of sex differences include differing concentrations of sex hormones, different expression of genes on X and Y chromosomes and different internal and external sex organs.
Gender, on the other hand, is “a person's self-representation as male or female, or how that person is responded to by social institutions based on the individual's gender presentation. Gender is rooted in biology and shaped by environment and experience.”13 It is a sociocultural construct that is associated with the properties of women and men that are expressed in the values, beliefs and perceptions that they hold, their psychosocial characteristics, their behaviors and their roles in society. “Women” and “men” are sex categories while “feminine” and “masculine” are gender categories. Aspects of sex do not vary substantially over time, while aspects of gender have varied across time and culture, as different groups have different beliefs about what it means to be a female or male in society.
Myocarditis is defined as inflammation of the myocardium with or without necrosis, and is a rare, potentially life-threatening and underdiagnosed cause of acute heart failure in children and adults.16 Viral infections are the most common cause of myocarditis in developed countries, but rheumatic carditis, Trypanosoma cruzi, and bacterial infections are frequent contributors to this disease in developing countries. Myocarditis leads to a significant minority of DCM cases in the US.17 Idiopathic DCM is defined as DCM of unknown cause that is characterized by left ventricular (LV) or biventricular dilation and impaired myocardial contractility.18,19 Acute or recent onset DCM includes patients with a LV ejection fraction ≤40% and fewer than 6 months of symptoms.2 A combination of T2-weighted cardiac magnetic resonance imaging (MRI) and post-gadolinium early and late T1-weighted MRI is often used to diagnose myocarditis, but a myocardial biopsy is required to confirm the presence of a myocardial inflammatory infiltrate.20-22
Dennis M. McNamara: Classically myocarditis is defined histologically, however most cases of clinically suspected myocarditis never undergo endomyocardial biopsy (EMB). Sampling error and regional variation limits the sensitivity of biopsy for detecting cellular inflammation and EMB remains a poor gold standard for defining myocardial inflammation. Given these limitations MRI may have an increasing diagnostic role.
Peripartum cardiomyopathy (PPCM) is a relatively rare form of acute cardiomyopathy that affects otherwise healthy women in the peripartum period. According to the National Heart Lung, and Blood Institute and the Office of Rare Diseases of the National Institutes of Health Workshop on Peripartum Cardiomyopathy,23 all four of the following criteria must be met to establish the diagnosis of PPCM: 1) development of cardiac failure in the last month of pregnancy or within 5 months of delivery, 2) absence of an identifiable cause for the cardiac failure, 3) absence of recognizable heart disease before the last month of pregnancy, and 4) LV systolic dysfunction demonstrated by classical echocardiographic criteria (i.e. ejection fraction <45% or fractional shortening <30%, or both). More recently, the Heart Failure Association of the European Society of Cardiology Working Group on PPCM slightly modified the criteria, stating that “PPCM is an idiopathic cardiomyopathy presenting with heart failure secondary to LV systolic dysfunction toward the end of pregnancy or in the months following delivery, where no other cause of heart failure is found. It is a diagnosis of exclusion. The left ventricle may not be dilated but the ejection fraction is nearly always reduced below 45%.”24
Dennis M. McNamara: The incidence of inflammation on EMB is similar in PPCM to other forms of recent onset non-ischemic cardiomyopathy, as is the lymphocytic cellular predominance. A significant subset of PPCM is believed to have a similar inflammatory pathogenesis as other forms of myocarditis, with the “trigger” being fetal of placental antigen, rather than viral.
Sex differences in epidemiology
Myocarditis and DCM have a slighter greater prevalence in men than in women. Recent trials and registries of myocarditis report a female to male ratio between 1:1.5 and 1:1.7,25-27 while DCM studies report a female to male ratio between 1:1.3 and 1:1.5.28-30 In 1989, a population-based study in Olmsted County, Minnesota found the age-adjusted female to male ratio for both incidence and prevalence of idiopathic DCM to be 1:3.31 The increased incidence of men developing idiopathic DCM compared to women is not explained by socioeconomic factors, alcohol intake or other similar variables.18,32 Although relatively rare, PPCM is a cause of heart failure unique to women. No systematic epidemiologic studies have been published, and reported incidence seems to vary according to geography and population, with the estimated incidence varying between 1:300 and 1:5500 live births.33
Sex differences in genetics
Dilated cardiomyopathy has been shown to have a familial basis in about 20-35% of cases. Most of the genes implicated are autosomal with primarily dominant transmission. More than 30 genes have been identified, indicating marked locus heterogeneity. The DCM genes identified can be grouped into four main categories: 1) myocyte cytoskeletal proteins, 2) sarcomeric proteins, 3) nuclear envelope constituents, and 4) regulators of calcium homeostasis. Given the inheritance pattern and the roles of the implicated genes, women and men should be affected equally. However, clinical presentation and outcomes can differ between sexes. These differences are likely due to as yet unexplained factors including a multiple mutations model of DCM affecting penetrance, expressivity and even causation, mitochondrial genetics, modifier genes, epigenetics, environmental factors and perhaps even the pathophysiology or pathogenesis of DCM. For example, mutations in troponin T can lead to familial DCM. A study of familial DCM found that the troponin TA171S mutation resulted in differing phenotypic expression of the disease in that adult males demonstrated a more severe phenotype than adult females.34
Sex-related differences in the gene expression within the ventricular myocardium of patients with idiopathic DCM have been reported. In a study of 11 patients (6 women) with idiopathic DCM and 11 age-matched non-failing heart donors, investigators found that 55 genes were differentially expressed in idiopathic DCM female patient samples (37 upregulated and 18 downregulated), while 19 of those genes were found to be significantly upregulated (13 genes) or downregulated (6 genes) in the male patient samples.35 Functional analysis of the gene expression pattern demonstrated that the deregulated genes unique to women were those involved in energy metabolism and regulation of transcription and translation, while the deregulated genes unique to men were related to myocardial contraction. The findings in this study suggest that these genes may serve as novel targets for tailored treatment of DCM in women and men.
Recently, a study analyzed TTN, the gene encoding the sarcomere protein titin, in 312 subjects with DCM and 249 controls.36 TTN mutations were found in approximately 25% of familial DCM cases and in 18% of sporadic cases. Cardiac outcomes were similar in subjects with and those without TTN mutations, but adverse events occurred earlier in male mutation carriers than in female carriers. The mean age at the time of these adverse events in 94 mutation carriers within 19 families was 68±5 years for the 33 women and 56±3 years for the 61 men.
Genetics may play a role in PPCM. Several cases of PPCM patients with either mothers or sisters who have also been diagnosed with PPCM have been reported,37-40 suggesting that these cases may represent familial DCM unmasked by the hemodynamic stress of pregnancy. A study of 90 DCM families in the Netherlands revealed that 5 of the 90 DCM families had family members with PPCM.41 Cardiologic screening of first degree relatives of 3 PPCM patients (not among the 90 DCM families) who had not fully recovered LV function showed undiagnosed DCM in all three families. These findings suggest that in a subset of patients, PPCM may be the initial manifestation of familial DCM. Another study analyzed families in the Familial Dilated Cardiomyopathy Database and found that familial clustering with DCM was present in 23 of 45 unrelated cases.42 Of the 45 PPCM cases, 19 had been resequenced for known DCM genes, five of which carried mutations (3 familial and 2 sporadic), suggesting that genetic mutations associated with DCM may be implicated in the pathogenesis of PPCM. In a genome-wide association study comparing 41 women with PPCM, 49 postmenopausal women without heart failure and 654 women with unknown phenotype, 1 single nucleotide (rs258415 at Chromosome 12p11.22 near the PTHLH gene) met genome-wide significance.43 These results indicate that further investigation of the role of genetic factors in PPCM is warranted.
In a study using mouse and human healthy cardiac tissue samples, researchers found that genes located on sex chromosomes were the most abundant among the sexually dimorphic genes.44 Furthermore, genes on autosomal chromosomes were identified with sex- and/or age-specific expression levels. Genes expressing sex differences in acute heart failure patients include genes for inflammation and cardiac remodeling.45
Sex differences in pathophysiology
Normal cardiovascular physiology
First, it is important to realize that every cell has “a sex” based on the influence of sex chromosomes and hormones from in utero to adulthood, prior to disease.13 It is also clear that sex differences exist in normal heart physiology and function. For example, men have larger hearts than women, as evidenced by increased LV mass, even after adjusting for body surface area.46 Because the number of cardiac myocytes is determined in infancy, increases in LV mass are mostly due to hypertrophy, which is 25-38% greater in men than in women47 (Table 1). Women have increased LV ejection fraction (LVEF) compared to men,48,49 myocardial mass is better preserved in women as they age,50,51 women have smaller coronary vessels than men,52 and premenopausal women have lower blood pressure53-54 but a faster resting heart rate than men.55 In one study of normal hearts from men and women aged 21 to 93 who died of natural causes, apoptosis was found to be 3-fold higher in the hearts of men compared to women.56 Interestingly, sex, rather than age, was found to be the determining factor in that study. According to several microarray and proteomic analyses of individuals that do not have cardiovascular disease, many sex-specific gene differences exist in the hearts of apparently normal men and women.44,57
Table 1. Known effects of testosterone and/or male sex on the hearta.
| Effect | References |
|---|---|
| Hypertrophy | 47, 61, 62 |
| Apoptosis | 56, 65 |
| Testosterone production in the heart | 60, 106 |
| Myocardial inflammation | 8, 11, 12, 25-32, 91, 92 |
| Atherosclerotic plaques/atherosclerosis | 4, 58 |
| Increased AR expression on macrophages | 74 |
| Increased numbers of macrophages and mast cells | 11 |
| Increased TLR4 and IL-1β expression | 11, 91, 92, 100, 101, 102 |
| Increased Th1 immune response | 11, 71, 85, 91,93-97, 105 |
| Increased Serpin A3n (α1 anti-chymotrypsin) | 92, 114 |
| Increased fibrosis/ECM remodeling | 3, 6, 15, 35, 92, 113, 114 |
| DCM | 1, 18, 32, 92, 99, 112 |
| Heart failure | 1,-3, 14, 68, 69, 111, 114, 119 |
Abbreviations: AR, androgen receptor; DCM, dilated cardiomyopathy; ECM, extracellular matrix; IL-1β, interleukin-1β; Th1, T helper cell-type 1; TLR4, Toll-like receptor 4
Sex steroid hormones are able to directly affect cardiac function, endothelial cell function and vascular tone through genomic and non-genomic effects of the androgen and estrogen receptors, which are present on and within vascular endothelial and smooth muscle cells as well as on and within cardiac fibroblasts, myocytes and platelets.3,58 Additionally, sex differences exist in estrogen receptor (ER) and androgen receptor (AR) levels on various cell types; for example, females have higher levels of ERs in their arteries.58 17β-estradiol has been shown to prevent apoptosis in cardiac myocytes, inhibit reactive oxygen species-induced cardiac damage, and oppose mechanisms that lead to cardiac hypertrophy and fibrosis.59
These cardioprotective effects of estradiol are believed to be mediated by signaling through cell membrane ERs rather than via genomic receptors. The direct effects of testosterone have been more difficult to determine, due at least in part to the ability of aromatase to break down testosterone to estradiol. Testosterone levels in cardiac tissues have been reported to be higher in men than women60 and to be 4-fold higher than in skeletal muscle.61 Evidence in animal models suggests that androgens promote hypertrophy under normal conditions, with male gonadectomized rats developing lower heart weight compared to sham operated rats.61,62 Overall, these underlying sex differences in cardiac function and physiology may influence the immune response to infection and injury in the heart. Indeed, men develop atherosclerotic plaques earlier and more extensively than women, while the number of cardiovascular events in women increases strikingly with menopause, suggesting that sex hormones influence heart disease.58,59,63
Sex and gender differences in myocarditis, DCM and heart failure
Male sex is an important risk factor for developing heart failure14 (Table 1). Heart failure can be the consequence of a number of inflammatory cardiovascular conditions including atherosclerosis, myocarditis, DCM and PPCM.63,64 Sheppard et al. found that men with myocarditis or acute DCM had higher cardiac expression of proteins associated with apoptosis, such as Fas and tumor necrosis factor receptor 1, than women, while patients with the highest levels of these apoptosis markers had minimal improvement in LVEF.65
Dennis M. McNamara: Women also had less histologic evidence of fibrosis and hypertrophy on EMB (Teuteberg HFSA, 2006) which supports the gene expression studies and suggests that women were less likely to present with an “irreversible” DCM phenotype than the men in the trial.
Sliwa et al. has shown that women with PPCM had elevated levels of tumor necrosis factor-alpha, interleukin (IL)-6 and Fas/APO-1 compared to healthy controls, and that soluble Fas/APO-1 levels were significantly higher in PPCM patients who died compared with survivors.66
Most cases of heart failure among young and middle-aged persons are caused by reduced myocardial contractile function (i.e. systolic dysfunction). However, heart failure can also occur because of an inability to relax or fill the ventricle during diastole, in part due to myocardial fibrosis in the setting of preserved systolic function. Heart failure with preserved ejection fraction is more common in women than men.67 Heart failure occurs with advancing age in both genders.3 However, women develop heart failure at an older age and have better LV function than men.68,69 The incidence of heart failure increases dramatically in women over 55 years of age, with the strongest predictor of mortality in women being age, whereas in men it is New York Heart Association (NYHA) classification.3 Patients with chronic heart failure that have elevated levels of inflammatory mediators have a worse prognosis.70
The immune system
Sex hormones have a profound influence on the immune system under normal and pathological conditions.71 Sex steroid hormone receptors like ERα, ERβ and the AR are expressed on and within many immune cell populations including mast cells, macrophages, dendritic cells, natural killer cells, T cells and B cells72 (Figure 1). Human and mouse monocytes and macrophages express ERα, ERβ and the AR.72,73 ARs are expressed at higher levels on and within macrophages in men than women.74 In contrast, women have higher ER expression in their arteries than men, which decreases with age and menopause.58 Additionally, ERα/β, AR, and aromatase, the enzyme that converts androgens to estrogens, are expressed on and within vascular endothelial cells, vascular smooth muscle cells, cardiac fibroblasts, and cardiomyocytes in humans and rodents.59 Estrogen via membrane ERβ signaling has been shown to regulate arterial tone and blood pressure, while membrane ERα mediates protection against vascular injury and atherosclerosis.75,76 Additionally, platelets, which are important for induction of thrombosis, express ERβ and the AR and respond to sex steroids.72 Steroid receptors regulate gene expression by classic genomic actions via androgen and estrogen response elements as well as more rapid membrane-associated receptor signaling.75
Figure 1. Sex hormone effects on the immune cell response.
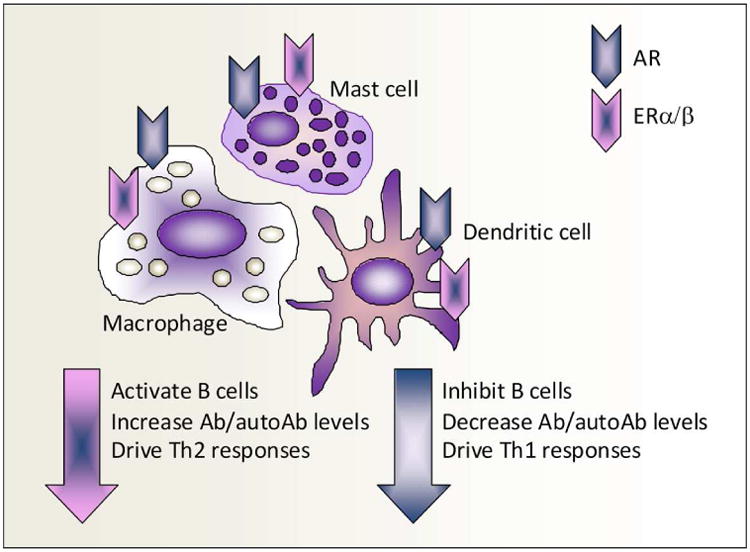
Antigen presenting cells, such as mast cells, dendritic cells and monocyte/macrophages, in mice and humans express androgen receptors (AR) and estrogen receptor-α/β (ERα/β). Men express higher AR levels on macrophages than women. Estrogen via ERα/β has a well known ability to activate B cells, resulting in increased antibody (Ab) and autoAb levels and to drive T helper (Th)2 responses, while testosterone via the AR decreases Ab levels and drives Th1 responses.
Estrogen has a well known ability to activate B cells, resulting in increased antibody responses to infection, vaccines and autoantigens, while androgens have the opposite effect63,71,77,78 (Figure 1). However, depending on the context, estrogen can either drive T helper (Th)1 and/or Th17 responses, increasing inflammation via transcriptional activation of NFκB, or increase the regulatory arm of the adaptive immune response by enhancing tolerogenic dendritic cells, interleukin (IL)-4-driven Th2 responses, transforming growth factor (TGF)-β, regulatory T cell populations, and alternatively activated macrophages.63,79-81 Estrogen's capacity to decrease cell-mediated immune responses by increasing regulatory mechanisms may be due to its ability to inhibit components of the innate immune response including Toll-like receptor (TLR)4-induced IL-1β, IL-6, tumor necrosis factor-α and NFκB activation from macrophages.11,71,82-84 Far less research has been conducted on the role of androgens on immunity, but in general, androgens have been found to increase Th1 responses11,71,78,85 (Figure 1).
One of the difficulties in interpreting studies of the effect of androgens and estrogens on immune cells and immune responses is that most researchers do not take into account whether the cells used in culture are of male or female origin or transformed75,86 and how this could influence the interpretation of the data.87 A similar problem occurs when researchers use animal models of human disease, as either the animal model may not share the same sex differences as the human disease being studied or published research reports do not state the sex of the animal. Another important consideration is whether hormone treatments influence the innate immune response (i.e., initiation of inflammation via antigen presenting cells like mast cells, monocytes and macrophages, which express ERs and the AR) and/or the later adaptive immune response. Other factors include dose, breakdown of testosterone to estrogen, and target organ effects of sex hormones.81 For example, administering a high dose of estrogen may have the opposite effect to administering a low dose.88 These issues lead to a great deal of contradictory findings in the literature and are just a few of the important concerns related to the study of sex differences in disease that urgently need to be addressed.87,89,90
Myocardial inflammation
Currently, our understanding of sex differences in cardiac inflammation during myocarditis comes almost exclusively from animal models of disease. Most clinical cases of myocarditis are not linked to a specific cause, but viral infections like the enterovirus CVB3 are frequently implicated.63 Huber and colleagues were the first to describe that male BALB/c mice have increased CVB3-induced myocarditis compared to females8 (Table 1). Elevated cardiac inflammation in males in response to CVB3 infection is not due to increased viral replication in the heart, which does not differ between the sexes at day 2, 8, 10 or 12 post-infection.91 Infectious virus is cleared from the heart by day 14 after infection and does not reactivate during the development of DCM in either sex.92 Male BALB/c mice with CVB3 myocarditis develop a predominantly Th1-type adaptive immune response with more Th1-induced IgG2a anti-CVB3 antibodies compared to female mice, who develop a Th2 response and more Foxp3+, Tim-3+ and CTLA4+ regulatory T cell populations.11,91,93,94 There is no sex difference in IL-17 levels in the heart during acute CVB3 myocarditis.78 Vγ4+ γδT cells are elevated in the heart of male mice early after CVB3 infection and bias the adaptive immune response to a Th1 phenotype.95 Using culture and animal models, this Th1 vs. Th2 dichotomy, as well as the severity of inflammation, has been shown to be due to testosterone and estradiol, respectively.93,94,96,97
The predominant Th1 response in male mice with CVB3 myocarditis is not due to classical IL-12/STAT4-induced interferon (IFN)-γ production, but TLR4-induced IL-18, a cytokine that strongly induces IFN-γ.11,91 Male mice also have more classically activated M1 macrophages in the heart during acute CVB3 myocarditis, while females have more inhibitory Tim-3+ alternatively activated M2 macrophages.96,98 Surprisingly, TLR4 and IL-1β were not expressed by M1 macrophages in males, but by M2 macrophages,11,96 a macrophage population that increases inflammation and cardiac remodeling in males99,100 (Figure 2). TLR4 mRNA expression has been found to be higher in patients with biopsy-proven myocarditis and DCM than controls and to strongly correlate with enteroviral RNA levels in the heart.101,102 Although sex differences in TLR4 levels were not examined, 66% and 75% of the myocarditis and DCM patients in these studies were men, respectively. Myocarditis patients with active enteroviral replication had higher levels of TLR4 that was associated with lower systolic function and greater LV dilation. Mast cells, which are known to increase myocardial inflammation and fibrosis, are also elevated in number in male mice with CVB3 myocarditis and express more TLR4 compared to female mice11,99,103,104 (Figure 2). Patients with clinically suspected myocarditis or DCM were found to have higher levels of IgG3 autoantibodies against cardiac myosin that strongly correlated with reduced LVEF at baseline and 6 months.105 A higher IFN-γ response was found in patients positive for IgG3, which were predominantly men (p = 0.01). A recent study in mice showed that although sex chromosomes influence CVB3 myocarditis, sex hormones mediate the dominant sex effect.106 A paucity of data exists on sex differences in the inflammatory response in myocarditis and DCM patients, but the findings from animal models summarized here suggest directions for future research.
Figure 2. The effect of testosterone on myocardial inflammation leading to fibrosis and dilated cardiomyopathy (DCM).
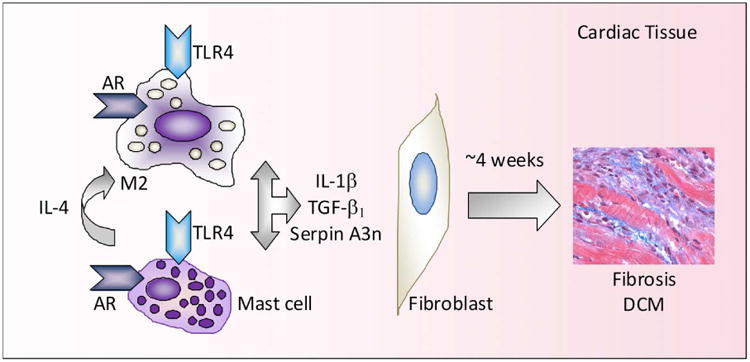
Testosterone increases mast cell and macrophage numbers in the heart and Toll-like receptor (TLR)4 expression on mast cells and macrophages. Mast cell release of interleukin (IL)-4 induces alternative activation of macrophages to induce M2 macrophages that express TLR4 and release IL-1β. Elevated testosterone and IL-1β during acute myocarditis increase serpin A3n levels in the heart, which, along with transforming growth factor (TGF)- β1, causes fibroblasts to produce collagen over the subsequent 4 weeks resulting in fibrosis and the development DCM.
Dennis M. McNamara: Several animal models do suggest important sex differences in myocardial inflammation and the progression to DCM (Kadokami et al., JCI 2000). In a transgenic TNF over expression murine model of myocarditis, female virgin mice are “protected” and do not develop DCM. However, females do develop DCM and heart failure post partum and this TNF transgenic appears to be a interesting model for PPCM.
Cardiac remodeling
Far more data exists regarding sex differences in cardiac remodeling. A critical step in the progression from myocarditis to DCM and heart failure involves extracellular matrix remodeling and fibrosis.107 Cardiac remodeling is defined as structural and functional changes to the myocardium that result in LV dilatation and heart failure. Alterations in the coordinated degradation and synthesis of extracellular matrix components are regulated by enzymes and cytokines released from immune cells, including mast cells and macrophages.92,103,104 Cytokines associated with Th2-type immune responses like IL-4, TGF-β and IL-1β (recall that IL-1β is released from M2 macrophages during acute CVB3 myocarditis), are strongly profibrotic (Figure 2). Interestingly, only males of mouse strains that respond to CVB3 infection with a dominant Th2 response, like BALB/c mice, develop fibrosis and DCM (e.g. elevated LV end systolic dimension by echocardiography) following acute myocarditis92,99,108,109 (Table 1). Female BALB/c mice do not develop DCM following CVB3 infection even though low levels of myocardial inflammation are present (significantly lower compared to male mice) during the chronic stage of disease.92 A similar finding has been observed for severe, clinically symptomatic myocarditis patients, where men were twice as likely to present with visual evidence of myocardial fibrosis then women by cardiac MRI.6 A Th2 response is necessary for male mice to develop DCM following CVB3 myocarditis,99 but disease is far more severe if driven by the Th2 cytokine IL-33 compared to the Th2 cytokine IL-4.108,110 These findings highlight the importance of Th2-driven mast cells and M2 macrophages that express TLR4 and IL-1β in the progression to fibrosis and DCM in males during and following acute myocarditis (Figure 2). This type of Th2-driven immune response appears to be disguised as a classical Th1 response in males due to elevated levels of TLR4-induced IL-18, which increases IFN-γ production and Th1-type immune cells.
Compared to female BALB/c mice, males have significantly elevated TLR4 expression on macrophages and mast cells in the spleen as early as 12 hours after CVB3 infection, and TLR4 expression remains elevated on immune cells in the heart of males with CVB3 myocarditis11 (Figure 2). Recently, microarray analysis of male and female BALB/c mice revealed that males upregulate genes associated with the development of cardiovascular disease and heart failure in the spleen 12 hours after CVB3 infection.111 This gene upregulation is not present in spleens from uninfected mice, further emphasizing the importance of innate inflammation in the progression of disease in males. Genes with well known roles in promoting cardiovascular disease were more highly expressed in the spleen of CVB3 infected males compared to females at 12 hours. These genes included carbonic anhydrase (induces hypertrophy and heart failure), phospholipase A2 (secreted from activated mast cells and macrophages, increases inflammation and atherosclerosis), squalene synthase (increases intracellular cholesterol levels, needed for steroid synthesis), MAP2 kinase 3 (regulates gene activation, proliferation and apoptosis), and the chemokine CXCL12 (regulates neutrophil and macrophage function). Interestingly, phospholipase A2 and MAP2 kinase 3 were found to be more highly expressed in men than women with severe heart failure secondary to idiopathic DCM using microarray of cardiac biopsies.112
Several studies report that men with myocarditis or DCM have a greater induction of extracellular matrix proteins and/or fibrosis in the heart compared to women, including increased collagen and matrix metalloproteinase production3,6,35 (Table 1). Various animal models of heart disease have shown that testosterone is responsible for adverse cardiac remodeling in males.113 and that estrogen signaling via ERb, in particular, prevents cardiac myocyte hypertrophy and fibrosis in females by blocking TGF-β and collagen synthesis.75 Comparison of genes in the cardiac tissue of male and female mice with CVB3 myocarditis or DCM using microarray analysis has revealed that most changes in remodeling genes occur in males during acute myocarditis, but are largely absent at the chronic stage of disease.92 In other words, gene changes that promote remodeling in males occur during acute myocarditis in mice (around day 10 after infection) but only develop into observable fibrosis and dilation several weeks later (by day 35 after infection) (Figure 2). These findings suggest that biomarkers to predict progression to DCM in patients need to be examined in the early stages of disease. This idea is supported by the finding that 33% of genes in a meta-analysis of 11 clinical microarray studies of DCM patients (primarily men) were also elevated in males with acute CVB3 myocarditis, whereas there was only 11% overlap in DCM genes in patients compared to the DCM phase of disease in mice.92,114
Serpin A 3n, also known as α1-anti-chymotrypsin, has been found to be consistently upregulated in 11 separate microarray studies of acute DCM patients and male mice with acute CVB3 myocarditis.92,114 Serpin A 3n is a potent serine protease inhibitor of leukocyte-derived proteases that was found to be increased in the heart of male mice by testosterone and IL-1β and to induce cardiac fibrosis by altering matrix metalloproteinases during acute CVB3 myocarditis92 (Figure 2). Overall, these studies suggest that elevated testosterone levels in men increase cardiac inflammation and fibrosis, leading to DCM and heart failure.
Testosterone
However, a few studies report an association between low serum testosterone levels, cardiovascular disease and mortality in older men.115-117 Testosterone has many beneficial effects, including metabolism of body fat. Conversely, central fat deposits have a high degree of aromatase activity, which metabolizes testosterone to estradiol.115 Testosterone supplementation in older men may reduce cardiovascular disease, particularly in patients with type 2 diabetes.115,117,118 However, one recent trial of testosterone administration to older men via a testosterone gel was discontinued early due to a higher rate of adverse cardiovascular events in the testosterone group compared to the placebo group.119 There are several possible explanations for these contradictory findings. Lower testosterone levels could indicate that the hormone is being consumed.111 A change from a high level of testosterone to a low level with aging could have opposite effects on cardiac tissues or inflammation, as has been shown for estrogens. Another possibility is that elevated tumor necrosis factor-α, IL-1β and IL-6 levels in patients with cardiovascular disease (or other chronic inflammatory conditions) reduce testosterone levels in the sera. These proinflammatory cytokines are known to regulate the hypothalamic-pituitary axis, resulting in reduced testicular production of testosterone.115,120 Additionally, serum testosterone levels may not reflect local tissue or immune cell levels. Cardiac tissues and macrophages, for example, are both able to produce sex steroid hormones. The question that is yet to be resolved, then, is whether testosterone is low in aging men because of the presence of chronic inflammatory disease or if low testosterone is a cardiovascular disease risk factor. It has been suggested that a long-term multi-center randomized trial of testosterone replacement therapy should be conducted with a combined measure of cardiovascular events and death as a primary outcome in order to determine whether low testosterone is beneficial or detrimental.115
Sex and gender differences in clinical manifestation
Men and women with heart failure present with different clinical manifestations. In the Euro Heart Survey, men were found to develop systolic heart failure whereas women more often had preserved ejection fraction and diastolic heart failure3,14 Hypertension, obesity and diabetes are more likely to be risk factors in women, while coronary artery disease and DCM are risk factors in men.1,3,4
The prevalence and severity of symptoms of cardiovascular disease have consistently been reported to be higher in women than men. According to the Studies Of Left Ventricular Dysfunction (SOLVD) database, women with impaired systolic LV function are more likely than men to have dependent edema, murmurs, jugular venous distension, and an S3 gallop.121 A study of 303 patients (65 women) with idiopathic DCM in the Italian Multicenter Cardiomyopathy Study Group showed that women had more severe symptoms, presented more frequently with heart failure, and had higher frequency of left bundle branch block compared to men.122
Like patients with other forms of acute DCM, patients with PPCM frequently present with typical heart failure symptoms including dyspnea, fatigue and peripheral edema. Rarely, patients present with sudden cardiac death. Because many of the signs and symptoms of acute congestive heart failure are similar to those of normal pregnancy and the early postpartum period, the diagnosis of PPCM can easily be missed. Early diagnosis of PPCM is key, as delayed diagnosis may lead to major adverse events including the need for cardiac transplantation, or death.123
Health-related quality of life for patients admitted for heart failure is lower in women compared to men,124 and women with heart failure have greater impairment in activities of daily living.125 A study of middle-aged and elderly Portuguese women and men with symptomatic heart failure found that women had increased depressive symptoms compared to men.126
Sex and gender differences in management
Pharmacologic therapy
Patients with myocarditis, DCM and PPCM are treated with standard heart failure medications. Previous studies involving the general heart failure population have shown that women treated for heart failure are significantly less likely to be prescribed certain evidence-based medications and, when these medications are prescribed for women, dosing tends to be suboptimal.69,127 A study on the impact of physician gender in prescribing appropriate treatment to chronic heart failure patients demonstrated that there was no difference in treatment for male and female patients by female physicians, whereas male physicians used significantly less medication and lower doses of medication for female patients.128 This treatment gap may be changing, however, as a recent study of hospitals participating in the American Heart Association Get With The Guidelines Heart Failure registry, which includes nearly 100,000 patients, found that women were just as likely as men to be prescribed angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs) and β-blockers.129
There is little published on differences in the use of pharmacologic therapies between women and men with either myocarditis or DCM, specifically. The Intervention in Myocarditis and Acute Cardiomyopathy (IMAC) trial, which included 373 patients (38% women) with either myocarditis, acute nonischemic cadiomyopathy or PPCM, showed that there was no significant difference in the use of ACE inhibitors or ARBs and β-blockers between men, women without PPCM, and women with PPCM7 (Table 2). Dosage of these medications was not reported for any group.
Table 2. Post hoc analyses of pharmacologic therapies for cardiovascular disease by sexa.
| Treatment | Survival Benefit | References |
|---|---|---|
| α/β adrenergic receptor blockers | ||
| Bisoprolol | Women = Men | 133, 137 |
| Metoprolol | Women ≤ Men | 134, 137 |
| Carvedilol | Women = Men | 135, 136, 137 |
| Angiotensin-converting enzyme (ACE) inhibitors | Women ≤ Men | 138, 139 |
| Angiotensin receptor blockers | Women = Men | |
| Candesartan | Women = Men | 140 |
| Valsartan | Women = Men | 141 |
| Aldosterone antagonists | ||
| Spironolactone | Women = Men | 142 |
| Eplerenone | Women = Men | 143, 144 |
| Hydralazine + isosorbide dinitrate | Women = Menb | 147, 148 |
| Digoxin | Women < Men | 149, 150, 151 |
Note that no intentional studies of sex differences in the effects of pharmacologic therapies for myocarditis and DCM patients exist
African Americans
Most clinical trials of systolic heart failure did not plan to enroll a certain percentage of women or to prospectively analyze sex differences, thus limiting our ability to draw conclusions about the safety and efficacy of their treatment.130 As a result, current heart failure treatment guidelines are not sex-specific due to under-representation of women and a lack of prospective, randomized clinical trials designed to analyze sex-specific results. As there are discrepancies in clinical presentation, burden of disease, and outcomes between women and men with systolic heart failure, it is essential that the risks and benefits of medical treatment, including “standard medical treatment”, be accurately assessed for both sexes in order to optimize treatment for all patients.
Recently, a sex-specific therapeutic approach has been proposed for PPCM. Preliminary data involving a mouse model for PPCM and a small number of PPCM patients suggest that bromocriptine, which inhibits prolactin secretion, might help prevent death and deterioration of LV function upon subsequent pregnancies by preventing the formation of a 16 kDa prolactin fragment. Increased oxidative stress in the peripartum period triggers the activation of cathepsin D, a ubiquitous lysosomal enzyme that subsequently cleaves serum prolactin into its antiangiogenic and proapoptotic 16 kDa form.131 The 16 kDa fragment induces endothelial inflammation and apoptosis, impairs nitric oxide-mediated vasorelaxation, impairs cardiomyocyte metabolism, and reduces myocardial contraction. A proof-of-concept pilot study of PPCM patients with severely reduced LVEF diagnosed within 1 month of delivery showed that mortality was reduced in patients treated with bromocriptine compared with patients receiving placebo.132 A larger, randomized study in Germany is currently underway in order to confirm the reportedly beneficial effects of bromocriptine during the acute phase of PPCM (http://clinicaltrials.gov/ct2/show/NCT00998556).
Dennis M. McNamara: The proof-of-concept report was limited by an unexpected high mortality in the control group, and the significance of earlier anecdotal reports on the clinical use of bromocriptine is uncertain given the high spontaneous recovery rate in PPCM. The use of bromocriptine for PPCM currently remains quite limited in the US, as most clinician remain hesitant given potential risks. The results of the ongoing European trial will be extremely helpful in determining the future role of bromocriptine in this disorder.
Beta blockers
Investigators have shown that sex hormones play an important role in the regulation of myocardial beta adrenergic receptors, so it seems reasonable that differences in response to medications that target these proteins and receptors would be expected. Most beta blocker trials were not designed to investigate sex differences and recruited, on average, only about 20% female patients. There are no trials that specifically investigate the effects of beta blocker therapy in patients with myocarditis, DCM, or PPCM, so data on the safety and efficacy of beta blocker therapy in patients with these conditions has been extrapolated from trials including patients with LV systolic heart failure due to various causes.
Bisoprolol and metoprolol are both beta1-selective beta adrenergic antagonists. Post hoc analysis of 515 women with NYHA functional class III or IV symptoms and LVEF ≤35% studied in the Cardiac Insufficiency Bisoprolol Study II (CIBIS II) showed that bisoprolol improved survival.133 The survival benefit was similar for women and men (Table 2). Post hoc analysis of 898 women with NYHA functional class II to IV symptoms and LVEF <40% who were enrolled in the Metoprolol CR/XL Randomized Intervention Trial in Heart Failure (MERIT-HF)134 showed that although metoprolol significantly reduced hospitalizations, it provided no survival benefit. In contrast, there was both a significant reduction in heart failure hospitalizations and mortality in the men treated with metoprolol in this study (Table 2).
In contrast to metoprolol and bisoprolol, cardvedilol is a non-selective beta blocker with additional alpha-blocking effects. Post hoc analysis of 256 women with moderate symptoms and LVEF ≤35% participating in the US Carvedilol Heart Failure Study showed that carvedilol improved survival, even though this was not a mortality trial.135 In the Carvedilol Prospective Randomized Cumulative Survival Trial (COPERNICUS), carvedilol reduced the combined end point of death or hospital stay in the 469 women studied with LVEF <25% and severe HF symptoms.136 Most of this benefit, however, was due to a reduction in hospitalizations, as the mortality data had a wide confidence interval that crossed 1.0.
Although the apparently conflicting results of these trials may lead to the conclusion that beta blocker therapy is not as effective in women as in men, when data from three of these trials (i.e. MERIT-HF, COPERNICUS, and CIBIS II) were pooled, beta blockade appears to result in a similar decrease in mortality among women and men137 (Table 2). The beta blocker study results emphasize the need to enroll more women in clinical trials and the importance of performing meta-analyses of data from clinical trials until women are adequately represented in clinical trials in order to determine possible sex differences in treatment effect.
ACE inhibitors
Numerous clinical trials have reported the benefit of ACE inhibitor therapy in patients with reduced LV systolic function and subsequent cardiovascular society guidelines have recommended ACE inhibitors as a key therapy in heart failure patients. Similar to beta blockers, few women participated in the landmark ACE inhibitor studies and sex-specific results were not reported in the initial trials, so it is difficult to estimate the treatment benefit of ACE inhibitor therapy for women with systolic heart failure. There have been no reports analyzing the effect of ACE inhibitor therapy specifically in patients with myocarditis, DCM or PPCM.
A meta-analysis of nearly 7000 patients (23% women) enrolled in 32 randomized trials of ACE inhibitor therapy conducted prior to 1995 demonstrated a 24% decrease in mortality among men, but only a 21% decrease in mortality among women.138 The survival benefit for men, but not for women, was significant, possibly due to the relatively small number of women included in the trials. A subsequent meta-analysis that analyzed the 6 largest ACE inhibitor trials also showed a significant survival benefit for men, but no significant decrease in mortality for women treated with ACE inhibitors139 (Table 2). There was a trend toward benefit of ACE inhibitor therapy in symptomatic, but not asymptomatic women, suggesting that symptomatic women probably benefit from ACE inhibitors but the beneficial effect is likely less than it is in men. It remains unclear whether women with asymptomatic LV systolic dysfunction receive benefit from ACE inhibitor therapy.
Angiotensin receptor blockers
Patients with systolic heart failure who cannot tolerate ACE inhibitor therapy are increasingly being treated with ARBs. Similar to beta blockers and ACE inhibitors, there have been no reports analyzing the effect of ARBs specifically in patients with myocarditis, DCM or PPCM, and there are no prospective sex-specific trials for the efficacy of ARB treatment in patients with heart failure. Current data are limited to post hoc analyses of outcomes in ARB clinical trials. CHARM-Alternative (ARB for patients intolerant of ACEI) and CHARM-Added trials (ARB added to an ACEI) that included 1,188 women with a NYHA functional class II to IV and LVEF ≤40%, show that women who were randomized to candesartan had a 20% reduction in the primary combined end point of cardiovascular death or hospitalization for heart failure.140 The therapeutic effect was similar for men. In the Valsartan Heart Failure Trial (Val-HeFT),141 which included 1,003 women with NYHA functional class II to IV and LVEF ≤40%, women who were treated with valsartan had a 19% reduction in the primary combined endpoint of cardiovascular death and hospitalization for heart failure, but this was nonsignificant, likely due to the relatively low number of women in the trial. Valsartan therapy did not significantly reduce mortality in either men or women. The available data, although limited, suggests that there are no significant sex-specific differences in the therapeutic effect of ARBs in patients with systolic heart failure (Table 2).
Dennis M. McNamara: While more prospective studies would be on interest, new randomized data on safety and efficacy of standard heart failure therapy specifically in women would be extremely difficult to obtain from a human studies perspective unless credible concerns had been raised. As demonstrated by Table 2, for the most part the efficacy of standard therapy appears similar in men and women for beta blockers and ACE inhibitors as well as ARBs and aldosterone receptor antagonists. In contrast to ongoing controversy on racial differences in drug efficacy, the current consensus is that standard heart failure therapies are likely equally efficacious in men and women.
Aldosterone antagonists
In contrast to the subgroup post hoc data on beta blockers, ACE inhibitors and ARBs, data from the Randomized Aldactone Evaluation Study (RALES)142 and Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study (EPHESUS)143 show that both nonspecific and specific aldosterone antagonism seem to decrease mortality in women with symptomatic systolic heart failure.
The RALES trial included 446 (27%) women with either ischemic or nonischemic cardiomyopathy with NYHA functional class III to IV symptoms and LVEF ≤35%.142 Both women and men treated with spironolactone showed an approximately 30% decrease in all-cause mortality. The EPHESUS trial enrolled 1, 918 (29%) women participants with LVEF ≤40% 3-14 days after myocardial infarction.143 Eplerenone was associated with a reduction in all cause mortality in 20% of women and 15% of men, with the difference in survival benefit between sexes nonsignificant. EMPHASIS-HF enrolled 610 (22%) women with NYHA functional class II symptoms and ≤35%.144 Results showed a 24% reduction in all cause mortality in the entire cohort of patients treated with eplerenone, with no significant difference between women and men (Table 2). Given the results of these three trials, it appears that aldosterone antagonism provides a similar survival benefit between women and men with symptomatic systolic heart failure.
Hydralazine and isosorbide dinitrate
Hydralazine plus isosorbide dinitrate has been used in heart failure patients who cannot tolerate an ACE inhibitor or ARB, or are pregnant. The initial clinical trials that supported the use of this combination for improved survival were completed including only men145,146 and to date, there have been no systematic investigations of women using hydralazine plus isosorbide dinitrate as a substitute for an ACE inhibitor or ARB. The African American Heart Failure Trial (A-HeFT)147 studied 420 women and 640 men with NYHA functional class III to IV symptoms already taking beta blockers and an ACE inhibitor or ARB to determine whether adding hydralazine/isosorbide dinitrate would improve outcomes. A significant reduction in both mortality and hospitalizations for heart failure in both women and men prompted early termination of the study.148 Based on this data, the combination of hydralazine and isosorbide dinitrate likely improves outcomes in African American women, but whether this combination has any beneficial effect on women of other races or ethnicities remains uncertain.
Digoxin
The Digitalis Investigation Group (DIG) trial showed that digoxin reduces hospitalization for heart failure but has no effect on mortality.149 A subsequent post hoc subgroup analysis revealed that women not only had a smaller rate of decreased hospitalization compared to men, they also had an increased risk of death that was not observed in men150 (Table 2). The increased mortality was thought to be due to digoxin toxicity, as the mean serum level of digoxin was slightly higher for women than for men at one month, but similar at 12 months. A retrospective analysis of only the men included in the original study showed that higher serum digoxin levels were indeed associated with increased mortality in men with systolic heart failure.151 Based on this retrospective analysis, a digoxin level between 0.5 and 0.9 ng/mL is now generally deemed safe for both men and women, even though the association between drug level and outcomes was not investigated in women.
Pharmacologic considerations for women of childbearing age
Special consideration must be given to treating women with myocarditis, DCM or PPCM during their childbearing years. Although most PPCM patients present postpartum, a number of patients will present while pregnant. Myocarditis most commonly occurs in young adult patients, so women are at risk of developing myocarditis while pregnant. Patients with DCM generally present at an older age, although familial DCM will manifest in some patients while they are pregnant. No pregnant women have been enrolled in any of the randomized cardiovascular pharmacologic therapy trials, so there is no systematic evidence regarding the safety and efficacy for the use of any of the standard heart failure medications during pregnancy.
Treating heart failure patients during pregnancy presents a particular challenge, as potential risks and benefits for both the mother and the fetus must be considered. Although there are no systematic studies of the effects of any of the standard heart failure medications on fetuses, there is animal data and some observational data in humans that suggests that certain standard heart failure pharmacologic therapies can have a detrimental effect. Beta blockers, particularly when administered in the first trimester and in higher doses, have been associated with fetal bradycardia and low birth weight babies. ACE inhibitors and ARBs should be avoided in pregnant women due to potential teratogenic effects including renal malformation and/or dysfunction, intrauterine growth restriction, and fetal or neonatal death. Other afterload reducers, such as hydralazine and nitrates, appear to be safe for both mother and fetus. Aldosterone antagonists should be avoided, as they may cause antiandrogenic effects on the fetus. Digoxin is relatively safe for use during pregnancy, but serum values must be carefully monitored, as plasma digoxin levels tend to fall relative to dose during pregnancy due to increased clearance and then rise again postpartum.
Due to these potential harmful effects on a fetus, patients diagnosed with heart failure during pregnancy have limited pharmacologic options. Even diuretics must be administered cautiously due to the potential for decreased placental blood flow. Women of childbearing age who are diagnosed with heart failure while they are not pregnant must be carefully counseled about the potential harmful effects of each medication prescribed should they become pregnant. Should a woman with heart failure or a history of heart failure wish to become pregnant, preconception counseling is necessary in order to discuss the risks and benefits of all heart failure therapies prior to the onset of pregnancy. This is particularly important with regard to pharmacologic therapy, as the teratogenic effects of some medications may occur quite early in pregnancy.
Devices
Implantable cardiac defibrillator
Women are underrepresented in randomized implantable cardiac defibrillator (ICD) trials and obtain less ICD implantations than men.152,153 The data for safety and efficacy of ICD treatment in women are largely based on post hoc analyses and so we are limited in being able to determine whether women benefit from ICD therapy and, if they do, to what extent. A meta-analysis of five ICD clinical trials (MUSTT, MADIT II, DEFINITE, SCD-HeFT and COMPANION) enrolling 7,229 patients (22% women) with DCM, of whom 1,677 (23%) had nonischemic DCM, showed that there was no significant difference in overall mortality between women and men, but women experienced significantly fewer appropriate ICD interventions. 154 Men, but not women, had a significant survival benefit from prophylactic ICD therapy (Table 3). Another meta-analysis (including data from MUSTT, MADIT II, DINAMIT, DEFINITE and SCD-HeFT) showed that ICD therapy was associated with a significant decrease in total mortality in men, but not in women.155 These findings suggest that ICD therapy could be associated with a decreased survival benefit in women compared to men.
Table 3. Sex differences in the implantation and effectiveness of cardiovascular devices.
| Devicea | Effect | References |
|---|---|---|
| ICD | ||
| F < M ICD implantation | 152-160 | |
| F < M Appropriate ICD shocks | 154, 157 | |
| F < M Reduction in total mortality | 155 | |
| F < M Survival benefit with ICD therapy | 154, 157 | |
| CRT | ||
| F < M CRT implantation | 152, 160-164 | |
| F > M Response to CRT therapy | 161, 162 | |
| F > M Long-term survival | 163, 164 | |
| VADs | ||
| F = M Survivalb | 165 | |
| F < M Need for reoperationb | 165 | |
| F > M Right heart failure following VAD implantationb | 165 | |
| F < M Cardiac transplant following VAD implantation | 167 | |
| F > M Hemorrhagic stroke | 167 | |
| F < M Device lead infection | 167 | |
Abbreviations: CRT, cardiac resynchronization therapy; F, female; ICD, implantable cardiac defibrillator; M, male; VADs, ventricular assist devices.
Patients with acute viral myocarditis
The Defibrillators in Non-Ischemic Cardiomyopathy Treatment Evaluation (DEFINITE) trial enrolled 132 women (29% of study participants) with nonischemic cardiomyopathy, LVEF <36%, and either premature ventricular contractions or nonsustained ventricular tachycardia.156 Trial results showed an overall 35% reduction in all-cause mortality. There was no significant difference in arrhythmic death between women and men after ICD implantation, but there was a relative increase in death among women randomized to ICD therapy compared to women randomized with standard therapy alone.157 This study also showed a trend for women to obtain fewer appropriate ICD shocks.
Women are less likely to undergo ICD implantation than men, particularly for primary prevention. An observational analysis of more than 13,000 patients with and LVEF ≤30% who were hospitalized for heart failure and discharged alive from hospitals in the American Heart Association's Get With the Guidelines Heart Failure quality improvement program between 2005 and 2007 demonstrated that only 35.4% of eligible patients had ICD therapy at discharge.158 ICDs were used in only 29% of eligible women compared to 41% of eligible men. Recently, a follow-up study of patients ≥65 years old with a history of heart failure and a LVEF ≤35% enrolled in the Get With the Guidelines Heart Failure program showed that the use of ICD therapy in 2009 had increased to 32% in eligible women and 50% in eligible men.159 Clearly, gender disparities persist and the gap between women and men is widening. This disparity may be due, in part, on the perception that women do not benefit from ICD therapy based on the results of clinical trials. Given the lower risk of sudden death in women compared to men, however, the number of women enrolled in ICD trials has not been large enough to definitely determine that there is no survival benefit for women treated with ICD therapy.
Patients with myocarditis do not usually require ICD implantation, as tachyarrhythmias often resolve within several weeks after diagnosis. If symptomatic or sustained ventricular tachycardia persists, however, particularly in the setting of persistent LV systolic dysfunction, ICD implantation should be considered. Due to the relatively high rate of LV recovery observed in PPCM patients, ICD implantation is uncommon in this group as well. ICD implantation for primary prevention of sudden death may be considered in PPCM patients whose LVEF remains <30% six months after diagnosis. For patients with either myocarditis or PPCM who present with sudden cardiac death, ICD implantation for secondary prophylaxis is indicated. There are no studies investigating the potential beneficial effects of ICD implantation in patients with either myocarditis or PPCM, so no sex-specific data is available for these patient groups.
Dennis M. McNamara: The use of ICDs and timing of ICD placement was identical in men and non-peripartum women IMAC2, the multicenter investigation of myocardial recovery in recent onset cardiomyopathy The study was too small (n=373) and the incidence of sudden death too rare to address efficacy. The use of ICDs was significantly less in PPCM in IMAC2, likely due to greater myocardial recovery evident in this subset.
Cardiac resynchronization therapy
Cardiac resynchronization therapy (CRT) has increasingly been used to treat patients with advanced heart failure. Similar to ICD trials, CRT trials tend to recruit more men than women, and like ICD utilization studies, women are less likely to receive CRT therapy (Table 3).152,160 This gender disparity is especially notable in light of recent studies which suggest that women are more likely to respond to CRT. A best subset regression analysis of the 1,761 patients enrolled in the Multicenter Automatic Defibrillator Implantation Trial With Cardiac Resynchronization Therapy (MADIT-CRT) identified female sex as one of the seven patient characteristics most predictive of echocardiographic response to CRT therapy.161 A sub-analysis of the Predictors of Response to Cardiac Resynchronization Therapy (PROSPECT) trial also showed that women had significantly better echocardiographic response to CRT than men.162 A study of 233 patients (23% women) found that male gender was associated with worse long-term survival (58 +/−15 months, HR 3.62, CI 1.88-6.99).163 More recently, a study of 578 patients (25% women) who underwent CRT showed that there was a significant difference in long-term survival between women and men, with a 2 year all-cause mortality rate of 15% for men and only 8% for women (p = 0.025).164 Patients with nonischemic DCM had significantly better survival compared to patients with ischemic cardiomyopathy (HR 0.57, CI 0.419-0.776). Women with heart failure due to nonischemic DCM showed the best long-term survival.
Mechanical circulatory support
Ventricular assist devices (VADs) have increasingly been used as a bridge to transplantation or recovery therapy for patients with acute heart failure with hemodynamic compromise and cardiogenic shock, including patients with myocarditis and PPCM. Due to the rarity of myocarditis and PPCM, no large scale clinical trials have investigated the use of these devices specifically in these patient groups. One study of VADs used in 11 patients (6 women) with acute viral myocarditis showed similar survival among women and men.165 However, adverse events differed between the sexes, as more men than women required reoperation, while more women than men developed right heart failure (Table 3). A review of the use of VADs as a bridge to transplant in 6 patients (2 women) with giant cell myocarditis reported that 3 men and 1 woman were still alive after transplant while 1 woman died of an embolic stroke and 1 man died of a hemorrhagic stroke prior to transplant.166 Several case reports have described successful use of ventricular assist device support as either a bridge to transplantation or a bridge to recovery in PPCM patients, but systematic data on the use of VADs in this population remains limited.
Dennis M. McNamara: A recent single center report of the use of LVAD as bridge to recovery (Simon, JCF 2010) reported an incidence of 14% (14/102) of successful LVAD explant without transplantation in non-ischemics. This recovered cohort was predominantly female (10 women including 4 subjects with PPCM) despite being a minority of the overall non-ischemic study group of 34 women and 68 men.
VADs have also been implanted in DCM patients with chronic end stage heart failure as a bridge to transplantation or destination therapy. Few women were enrolled in the early trials investigating pulsatile flow left ventricular flow devices (LVADs) because the devices were often too large for the body size of most women. With the advent of the smaller continuous flow LVADs, more women have been enrolled in clinical trials. Information on sex and gender differences remains limited, however, because sex-specific results are infrequently reported. The single study published to date that systematically compared outcomes in women versus men using LVADs as a bridge to transplantation reported no survival difference between the sexes while on LVAD support, but fewer women than men underwent heart transplantation.167 In the study, more women (72/104, 69%) than men (184/362, 51%) had nonischemic cardiomyopathy. Adverse event rates were similar between women and men with the exception of hemorrhagic stroke, which occurred more frequently in women, and device lead infections, which occurred more frequently in men.
Transplant
Cardiac transplantation is reserved for those patients who are refractory to optimal medical therapy and mechanical circulatory support. Men are more frequently referred for heart transplantation than women. According to the International Society for Heart and Lung Transplantation, women comprised only 23% of patients who underwent heart transplantation between 2005 and 2010.168 The gender imbalance in referral for heart transplantation is even more pronounced for the subset of patients with DCM, as the ratio of referral of women to men has been reported to be 6:1 at the German Heart Center Berlin and 5:1 in the Eurotransplant database.169 The sex differences seem to be due to referral bias, as women in both transplantation cohorts had more severe heart failure but fewer relative contraindications than men at the time of referral. The acceptance rate of patients referred for transplantation in these two cohorts was virtually identical for women and men, and gender did not modify the hazard rate for listing.
The lower rates of transplantation in women may also be at least partially explained by higher levels of panel reactive antibodies in parous women, making finding suitable donors more challenging. Another likely contributing factor is that women are less likely to accept the option for transplantation compared to men.170
In general, survival rates for women versus men after heart transplantation are similar,169 although clinical courses may differ. In patients with idiopathic DCM undergoing heart transplantation, inflammation in explanted hearts occurred more frequently in men than women, and patients with inflammatory DCM had a worse clinical status and a greater dependence on inotropic drugs.32
Recently, a review of the United Network for Organ Sharing Database showed that 42,406 patients (9,419 women, 485 of whom had PPCM as the indication) were transplanted between 1987 and 2010.171 Compared to other women and men, PPCM patients were younger, had higher sensitization, required a higher intensity of cardiovascular support pre-transplant, and had a higher listing status and more post-transplant rejection during the index transplant hospitalization as well as during the first year. In addition, graft survival was inferior and age-adjusted survival was lower for PPCM patients compared to other groups. The authors postulate that “cardio-toxic” autoimmunity may play a role in the higher rate of rejection in PPCM patients, as previous studies found cardiac specific autoantibodies in cardiac biopsy specimens of PPCM patients leading to the hypothesis that dysregulated autoimmunity may cause PPCM.172
Graft survival has recently been reported to be better in women compared to men. Amarellia and colleagues found that male sex was one of the determining factors of early graft failure in the highest-risk group.173 As early graft failure has a poor prognosis, it is not surprising that patients in the highest risk group (93% men) had 100% mortality, while patients in the lowest risk group (27% women) had only 48% mortality. These data support previous reports that women do as well as men after cardiac transplant and suggest that in certain circumstances, women may fare better than men.
Sex Differences in Outcomes
Women with heart failure in general have a better prognosis and longer survival after diagnosis than men with heart failure, possibly due to a lower incidence of ischemic heart disease and a higher incidence of heart failure with preserved ejection fraction. In 1993 Ho et al. reported findings on survival rates after congestive heart failure obtained from 652 participants of the Framingham Heart Study that found that survival was significantly worse for men compared to women.174 This holds true for the subset of heart failure patients with myocarditis and nonischemic DCM as well. Recently, the Intervention in Myocarditis and Acute Cardiomyopathy (IMAC)-2 Study reported that among the 373 patients (38% women) with LVEF <40%, fewer than 6 months of symptom duration, and an evaluation consistent with idiopathic DCM, myocarditis or PPCM, myocardial recovery was significantly better for women than men.2 Baseline LVEF was 24% in women and 23% in men, improving to 43% in women but only 39% in men at 6 months. Transplant-free survival was also significantly better in women, with 1-, 2-, and 4-year transplant-free survival for women of 96%, 96% and 96%,but only 93%, 90% and 84% for men (p = 0.03). This difference was largely driven by a markedly better survival among women compared to men (p = 0.003).
Further analysis of these sex differences among the IMAC-2 study was investigated by subdividing the study cohort into three groups: men (group 1, n = 240), women with nonperipartum cardiomyopathy (group 2, n = 104) and women with PPCM (group 3, n = 39). For groups 1, 2, and 3, the mean LVEF at baseline was 23%, 24% and 27% while the mean LVEF at 6 months was 39%, 42% and 45%, respectively (p = 0.007).7 PPCM patients had a significantly greater likelihood of achieving a LVEF >50% at 6 months than patients in groups 1 or 2 (19%, 34% and 48%, respectively; p = 0.002) (Figure 3) These results show that among patients with acute cardiomyopathy, myocardial recovery is greatest in women with PPCM, poorest in men, and intermediate in women with non-peripartum acute cardiomyopathy.
Figure 3. LVEF at baseline and at 6 months for men, peripartum cardiomyopathy (PPCM) women and non-PPCM women.
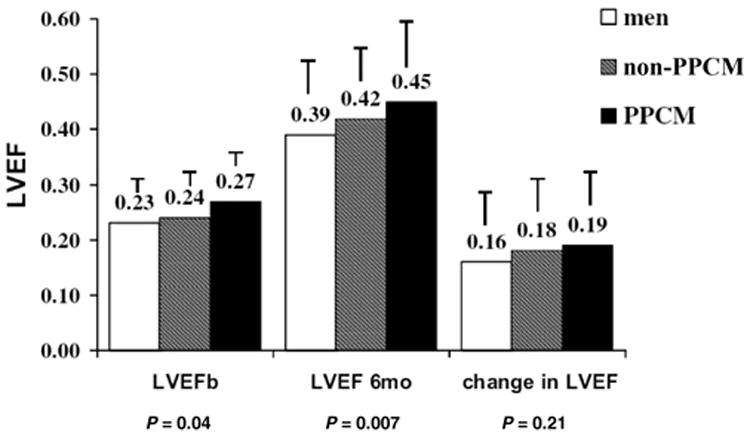
Label on each bar represents the mean left ventricular ejection fraction (LVEF) for the subset ±standard deviation. LVEF was significantly greater in PPCM women at baseline (p = 0.04) and at 6 months (p = 0.007). Men at all points had the lowest LVEF, while non-PPCM women had intermediate LVEF levels. Reproduced with permission from Cooper et al. 2012 (7).
Future directions
The next several years should see an expansion of research efforts in sex-specific pathogenesis and translational science. The rapid progress in the biology of DCM gleaned from murine models will be explored in translational studies of human disease, using blood samples and heart biopsy tissue. Further studies to examine sex differences in myocarditis and other forms of DCM are warranted in order to confirm or establish beneficial effects of current therapies. To facilitate the efforts in translational science, a centralized BioBank of blood samples linked to a well-characterized phenotype should be established. Clinical trials of novel therapeutic strategies for patients with myocarditis and other forms of DCM should be designed to specifically establish efficacy in women. In summary, a heightened understanding of sex differences is critical for improving diagnostic strategies and clinical management that will lead to optimal care for both women and men with these potentially devastating cardiovascular diseases.
Dennis M. McNamara: Differences by sex in the incidence of myocarditis and subsequent clinical outcomes almost certainly underlie the observed differences in the more common cardiovascular disorder, primary non-ischemic dilated cardiomyopathy. The observation of better myocardial recovery in females than males is a consistent finding in murine models of myocarditis and in clinical studies of recent onset cardiomyopathy. The mechanism remains uncertain, but differences in immune function by sex almost certainly play an important role. For myocarditis and inflammatory cardiomyopathy female sex is protective, in sharp contrast to its more adverse impact in more clearly defined autoimmune disorders such as lupus and rheumatoid arthritis. While much can be learned from animal models, the complex genomic influences on the development of DCM makes additional multicenter clinical studies essential to our understanding of th influence of sex on inflammatory pathogenesis and myocardial recovery. Knowledge of the cellular mechanism of this “gender effect” may lead to better targeted therapies for both men and women with myocarditis and dilated cardiomyopathy.
Acknowledgments
Dr. Fairweather would like to thank the National Institutes of Health (R01 HL087033) for funding support.
Abbreviations
- ACE
angiotensin-converting enzyme
- AR
androgen receptor
- ARBs
angiotensin receptor blockers
- CVB3
coxsackievirus B3
- DCM
dilated cardiomyopathy
- ER
estrogen receptor
- ICD
implantable cardiac defibrillator
- IFN
interferon
- IL
interleukin
- LV
left ventricular
- LVEF
left ventricular ejection fraction
- NYHA
New York Heart Association
- PPCM
peripartum cardiomyopathy
- Th
T helper-type immune response
- TGF-β
transforming growth factor-β
- TLR
Toll-like receptor
- VAD
ventricular assist device
Footnotes
Disclosure Statement: There is NOTHING TO DISCLOSE for any of the authors. Dr. Fairweather was supported by funding from the National Institutes of Health (R01 HL087033).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lee LV, Foody JM. Women and heart disease. Cardiol Clin. 2011;29:35–45. doi: 10.1016/j.ccl.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 2.McNamara DM, Starling RC, Cooper LT, Boehmer JP, Mather PJ, Janosko KM, et al. Clinical and demographic predictors of outcomes in recent onset dilated cardiomyopathy: results of the IMAC (Intervention in Myocarditis and Acute Cardiomyopathy)-2 study. J Am Coll Cardiol. 2011;58:1112–18. doi: 10.1016/j.jacc.2011.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Regitz-Zagrosek V, Seeland U. Sex and gender differences in myocardial hypertrophy and heart failure. Wien Med Wochenschr. 2011;161:109–16. doi: 10.1007/s10354-011-0892-8. [DOI] [PubMed] [Google Scholar]
- 4.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, et al. Executive summary: heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation. 2012;125:188–97. doi: 10.1161/CIR.0b013e3182456d46. [DOI] [PubMed] [Google Scholar]
- 5.Woodruff JF. Viral myocarditis. A review. Am J Pathol. 1980;101:425–84. [PMC free article] [PubMed] [Google Scholar]
- 6.Cocker MS, Abdel-Aty H, Strohm O, Friedrich MG. Age and gender effects on the extent of myocardial involvement in acute myocarditis: a cardiovascular magnetic resonance study. Heart. 2009;95:1925–30. doi: 10.1136/hrt.2008.164061. [DOI] [PubMed] [Google Scholar]
- 7.Cooper LT, Mather PJ, Alexis JD, Pauly DF, Torre-Amione G, Wittstein IS, et al. Myocardial recovery in peripartum cardiomyopathy: prospective comparison with recent onset cardiomyopathy in men and nonperipartum women. J Card Fail. 2012;18:28–33. doi: 10.1016/j.cardfail.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lyden DC, Olszewski J, Feran M, Job LP, Huber SA. Coxsackievirus B-3-induced myocarditis Effect of sex steroids on viremia and infectivity of cardiocytes. Am J Pathol. 1987;126:432–8. [PMC free article] [PubMed] [Google Scholar]
- 9.Ludden TE, Edwards JE. Carditis in poliomyelitis; an anatomic study of 35 cases and review of the literature. Am J Pathol. 1949;25:357–81. [PMC free article] [PubMed] [Google Scholar]
- 10.Sainani GS, Krompotic E, Slodki SJ. Adult heart disease due to the Coxsackie virus B infection. Medicine. 1968;47:133–47. doi: 10.1097/00005792-196803000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Frisancho-Kiss S, Davis SE, Nyland JF, Frisancho JA, Cihakova D, Rose NR, et al. Cutting Edge: Cross-regulation by TLR4 and T cell Ig mucin-3 determines sex differences in inflammatory heart disease. J Immunol. 2007;178:6710–14. doi: 10.4049/jimmunol.178.11.6710. [DOI] [PubMed] [Google Scholar]
- 12.Huber SA, Job LP, Auld KR. Influence of sex hormones on Coxsackie B-3 virus infection in Balb/c mice. Cell Immunol. 1982;67:173–9. doi: 10.1016/0008-8749(82)90210-6. [DOI] [PubMed] [Google Scholar]
- 13.Institute of Medicine Report Exploring the Biological Contributions to Human Health: Does Sex Matter? National Academy Press; Washington, DC, USA: 2001. [PubMed] [Google Scholar]
- 14.Cleland JG, Swedberg K, Follath F, Komajda M, Cohen-Solal A, Aguilar JC, et al. The EuroHeart Failure survey programme-a survey on the quality of care among patients with heart failure in Europe Part 1: patient characteristics and diagnosis. Eur Heart J. 2003;24:442–63. doi: 10.1016/s0195-668x(02)00823-0. [DOI] [PubMed] [Google Scholar]
- 15.Luchner A, Brockel U, Muscholl M, Hense HW, Doring A, Riegger GA, et al. Gender-specific differences of cardiac remodeling in subjects with left ventricular dysfunction: a population-based study. Cardiovasc Res. 2002;53:720–7. doi: 10.1016/s0008-6363(01)00510-7. [DOI] [PubMed] [Google Scholar]
- 16.Cooper LT., Jr Myocarditis. N Engl J Med. 2009;360:1526–38. doi: 10.1056/NEJMra0800028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grzybowski J, Bilinska ZT, Ruzyllo W, Kupsc W, Michalak E, Szczesniewska D, et al. Determinants of prognosis of nonischemic dilated cardiomopathy. J Card Fail. 1996;2:77–85. doi: 10.1016/s1071-9164(96)80026-1. [DOI] [PubMed] [Google Scholar]
- 18.Dec GW, Fuster V. Idiopathic dilated cardiomyopathy. N Engl J Med. 1994;331:1564–75. doi: 10.1056/NEJM199412083312307. [DOI] [PubMed] [Google Scholar]
- 19.Elliott P, Andersson B, Arbustini E, Bilinska Z, Cecchi F, Charron P, et al. Classification of the cardiomyopathies: a position statement from the European Society Of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2008;29:270–6. doi: 10.1093/eurheartj/ehm342. [DOI] [PubMed] [Google Scholar]
- 20.Blauwet LA, Cooper LT. Myocarditis. Prog Cardiovasc Dis. 2010;52:274–88. doi: 10.1016/j.pcad.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sagar S, Liu PP, Cooper LT., Jr Myocarditis. Lancet. 2012;379:738–47. doi: 10.1016/S0140-6736(11)60648-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Friedrich MG, Sechtem U, Schulz-Menger J, Holmvang G, Alakija P, Cooper LT, et al. Cardiovascular magnetic resonance in myocarditis: A JACC White Paper. J Am Coll Cardiol. 2009;53:1475–87. doi: 10.1016/j.jacc.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pearson GD, Veille JC, Rahimtoola S, Hsia J, Oakley CM, Hosenpud JD, et al. Peripartum cardiomyopathy: National Heart, Lung, and Blood Institute and Office of Rare Diseases (National Institutes of Health) workshop recommendations and review. JAMA. 2000;283:1183–8. doi: 10.1001/jama.283.9.1183. [DOI] [PubMed] [Google Scholar]
- 24.Sliwa K, Hilfiker-Kleiner D, Petrie MC, Mebazaa A, Pieske B, Buchmann E, et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of peripartum cardiomyopathy: a position statement from the Heart Failure Association of the European Society of Cardiology Working Group on peripartum cardiomyopathy. Eur J Heart Fail. 2010;12:767–78. doi: 10.1093/eurjhf/hfq120. [DOI] [PubMed] [Google Scholar]
- 25.Mason JW, O'Connell JB, Herskowitz A, Rose NR, McManus BM, Billinham ME, et al. A clinical trial of immunosuppressive therapy for myocarditis. The Myocarditis Treatment Trial Investigators. N Engl J Med. 1995;333:269–75. doi: 10.1056/NEJM199508033330501. [DOI] [PubMed] [Google Scholar]
- 26.Caforio AL, Calabrese F, Angelini A, Tona F, Vinci A, Bottaro S, et al. A prospective study of biopsy-proven myocarditis: prognostic relevance of clinical and aetiopathogenetic features at diagnosis. Eur Heart J. 2007;28:1326–33. doi: 10.1093/eurheartj/ehm076. [DOI] [PubMed] [Google Scholar]
- 27.Magnani JW, Danik HJ, Dec GW, Jr, DiSalvo TG. Survival in biopsy-proven myocarditis: a long-term retrospective analysis of the histopathologic, clinical, and hemodynamic predictors. Am Heart J. 2006;151:463–70. doi: 10.1016/j.ahj.2005.03.037. [DOI] [PubMed] [Google Scholar]
- 28.Bagger JP, Baandrup U, Rasmussen K, Moller M, Vesterlund T. Cardiomyopathy in western Denmark. Br Heart J. 1984;52:327–31. doi: 10.1136/hrt.52.3.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gillum RF. Idiopathic cardiomyopathy in the United States, 1970-1982. Am Heart J. 1986;111:752–5. doi: 10.1016/0002-8703(86)90111-0. [DOI] [PubMed] [Google Scholar]
- 30.Coughlin SS, Comstock GW, Baughman KL. Descriptive epidemiology of idiopathic dilated cardiomyopathy in Washington County, Maryland, 1975-1991. J Clin Epidemiol. 1993;46:1003–8. doi: 10.1016/0895-4356(93)90167-y. [DOI] [PubMed] [Google Scholar]
- 31.Codd MB, Sugrue DD, Gersh BJ, Melton LJ., 3rd Epidemiology of idiopathic dilated and hypertrophic cardiomyopathy. A population-based study in Olmsted County, Minnesota, 1975-1984. Circulation. 1989;80:564–72. doi: 10.1161/01.cir.80.3.564. [DOI] [PubMed] [Google Scholar]
- 32.Aguero J, Navarro J, Medina MC, Almenar L, Chirivella M, Martinez-Dolz L, et al. Clinical variables associated with the presence of inflammatory infiltrates in patients with dilated cardiomyopathy undergoing heart transplantation. Transplant Proc. 2008;40:3017–19. doi: 10.1016/j.transproceed.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 33.Blauwet LA, Cooper LT. Diagnosis and management of peripartum cardiomyopathy. Heart. 2011;97:1970–81. doi: 10.1136/heartjnl-2011-300349. [DOI] [PubMed] [Google Scholar]
- 34.Stefanelli CB, Rosenthal A, Borisov AB, Ensing GJ, Russell MW. Novel troponin T mutation in familial dilated cardiomyopathy with gender-dependant severity. Mol Genet Metab. 2004;83:188–96. doi: 10.1016/j.ymgme.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 35.Haddad GE, Saunders LJ, Crosby SD, Carles M, del Monte F, King K, et al. Human cardiac-specific cDNA array for idiopathic dilated cardiomyopathy: sex-related differences. Physiol Genomics. 2008;33:267–77. doi: 10.1152/physiolgenomics.00265.2007. [DOI] [PubMed] [Google Scholar]
- 36.Herman DS, Lam L, Taylor MR, Wang L, Teekakirikul P, Christodoulou D, et al. Truncations of titin causing dilated cardiomyopathy. N Engl J Med. 2012;366:619–28. doi: 10.1056/NEJMoa1110186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Massad LS, Reiss CK, Mutch DG, Haskel EJ. Familial peripartum cardiomyopathy after molar pregnancy. Obstet Gynecol. 1993;81:886–8. [PubMed] [Google Scholar]
- 38.Pearl W. Familial occurrence of peripartum cardiomyopathy. Am Heart J. 1995;129:421–2. doi: 10.1016/0002-8703(95)90032-2. [DOI] [PubMed] [Google Scholar]
- 39.Fett JD, Sundstrom BJ, Etta King M, Ansari AA. Mother-daughter peripartum cardiomyopathy. Int J Cardiol. 2002;86:331–2. doi: 10.1016/s0167-5273(02)00357-1. [DOI] [PubMed] [Google Scholar]
- 40.Pierce JA, Price BO, Joyce JW. Familial occurrence of postpartal heart failure. Arch Intern Med. 1963;111:651–5. doi: 10.1001/archinte.1963.03620290117016. [DOI] [PubMed] [Google Scholar]
- 41.van Spaendonck-Zwarts KY, van Tintelen JP, van Veldhuisen DJ, vand der Werf R, Jongbloed JD, Paulus WJ, et al. Peripartum cardiomyopathy as a part of familial dilated cardiomyopathy. Circulation. 2010;121:2169–75. doi: 10.1161/CIRCULATIONAHA.109.929646. [DOI] [PubMed] [Google Scholar]
- 42.Morales A, Painter T, Li R, Siegfried JD, Li D, Norton N, et al. Rare variant mutations in pregnancy-associated or peripartum cardiomyopathy. Circulation. 2010;121:2176–82. doi: 10.1161/CIRCULATIONAHA.109.931220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Horne BD, Rasmusson KD, Alharethi R, Budge D, Brunisholz KD, Metz T, et al. Genome-wide significance and replication of the chromosome 12p11.22 locus near the PTHLH gene for peripartum cardiomyopathy. Circ Cardiovasc Genet. 2011;4:359–66. doi: 10.1161/CIRCGENETICS.110.959205. [DOI] [PubMed] [Google Scholar]
- 44.Isensee J, Witt H, Pregla R, Hetzer R, Regitz-Zagrosek V, Noppinger PR. Sexually dimorphic gene expression in the heart of mice and men. J Mol Med (Berl) 2008;86:61–74. doi: 10.1007/s00109-007-0240-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Heidecker B, Lamirault G, Kasper EK, Wittstein IS, Chapion HC, Breton E, et al. The gene expression profile of patients with new-onset heart failure reveals important gender-specific differences. Eur Heart J. 2010;31:1188–96. doi: 10.1093/eurheartj/ehp549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Salton CJ, Chuang ML, O'Donnell CJ, Kupka MJ, Larson MG, Kissinger KV, et al. Gender differences and normal left ventricular anatomy in an adult population free of hypertension. A cardiovascular magnetic resonance study of the Framingham Heart Study Offspring cohort. J Am Coll Cardiol. 2002;39:1055–60. doi: 10.1016/s0735-1097(02)01712-6. [DOI] [PubMed] [Google Scholar]
- 47.de Simone G, Devereux RB, Daniels SR, Meyer RA. Gender differences in left ventricular growth. Hypertension. 1995;26:979–83. doi: 10.1161/01.hyp.26.6.979. [DOI] [PubMed] [Google Scholar]
- 48.Chung AK, Das SR, Leonard D, Peshock PM, Kazi F, Abdullah SM, et al. Women have higher left ventricular ejection fractions than men independent of differences in left ventricular volume: the Dallas Heart Study. Circulation. 2006;113:1597–1604. doi: 10.1161/CIRCULATIONAHA.105.574400. [DOI] [PubMed] [Google Scholar]
- 49.Merz CN, Moriel M, Rozanski A, Klein J, Berman DS. Gender-related differences in exercise ventricular function among healthy subjects and patients. Am Heart J. 1996;131:704–9. doi: 10.1016/s0002-8703(96)90274-4. [DOI] [PubMed] [Google Scholar]
- 50.Olivetti G, Giordano G, Corradi D, Melissari M, Lagrasta C, et al. Gender differences and aging: effects on the human heart. J Am Coll Cardiol. 1995;26:1068–79. doi: 10.1016/0735-1097(95)00282-8. [DOI] [PubMed] [Google Scholar]
- 51.Dannenberg AL, Levy D, Garrison RJ. Impact of age on echocardiographic left ventricular mass in a healthy population (the Framingham Study) Am J Cardiol. 1989;64:1066–8. doi: 10.1016/0002-9149(89)90816-3. [DOI] [PubMed] [Google Scholar]
- 52.Kim SG, Apple S, Mintz GS, McMillan T, Canos DA, Maehara A, et al. The importance of gender on coronary artery size: in-vivo assessment by intravascular ultrasound. Clin Cardiol. 2004;27:291–4. doi: 10.1002/clc.4960270511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Burt VL, Whelton P, Roccella EJ, Brown C, Cutler JA, Higgins M, et al. Prevalence of hypertension in the US adult population. Results from the Third National Health and Nutrition Examination Survey, 1988-1991. Hypertension. 1995;25:305–13. doi: 10.1161/01.hyp.25.3.305. [DOI] [PubMed] [Google Scholar]
- 54.Stamler J, Stamler R, Riedlinger WF, Algera G, Roberts RH. Hypertension screening of 1 million Americans Community Hypertension Evaluation Clinic (CHEC) program, 1973 through 1975. JAMA. 1976;235:2299–2306. doi: 10.1001/jama.235.21.2299. [DOI] [PubMed] [Google Scholar]
- 55.Bazett HC. The time relations of the blood-pressure changes after excision of the adrenal glands, with some observations on blood volume changes. J Physiol. 1920;53:320–39. doi: 10.1113/jphysiol.1920.sp001881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mallat Z, Fornes P, Costagliola R, Esposito B, Belmin J, Lecomte D, et al. Age and gender effects on cardiomyocyte apoptosis in the normal human heart. J Gerontol A Biol Sci Med Sci. 2001;56:M719–23. doi: 10.1093/gerona/56.11.m719. [DOI] [PubMed] [Google Scholar]
- 57.Isensee J, Ruiz Noppinger P. Sexually dimorphic gene expression in mammalian somatic tissue. Gend Med. 2007;4(Suppl B):S75–95. doi: 10.1016/s1550-8579(07)80049-0. [DOI] [PubMed] [Google Scholar]
- 58.Vitale C, Mendelsohn ME, Rosano GM. Gender differences in the cardiovascular effect of sex hormones. Nat Rev Cardiol. 2009;6:532–42. doi: 10.1038/nrcardio.2009.105. [DOI] [PubMed] [Google Scholar]
- 59.Regitz-Zagrosek V, Becher E, Mahmoodzadeh S, Schubert C. Sex steroid hormones. In: Bader M, editor. Cardiovascular Hormone Systems: From Molecular Mechanisms to Novel Therapeutics. Weinheim, Germany: Wiley-Blackwell; 2008. pp. 39–64. [Google Scholar]
- 60.Deslypere JP, Vermeulen A. Influence of age on steroid concentrations in skin and striated muscle in women and in cardiac muscle and lung tissue in men. J Clin Endocrinol Metab. 1985;61:648–53. doi: 10.1210/jcem-61-4-648. [DOI] [PubMed] [Google Scholar]
- 61.Melchert RB, Kennedy RH, Acosta D., Jr . Cardiovascular effects of steroidal agents. In: Acosta D, editor. Cardiovascular Toxicology. London: Taylor & Francis; 2001. pp. 425–475. [Google Scholar]
- 62.Scheuer J, Malhotra A, Schaible TF, Capasso J. Effects of gonadectomy and hormonal replacement on rat hearts. Circ Res. 1987;61:12–19. doi: 10.1161/01.res.61.1.12. [DOI] [PubMed] [Google Scholar]
- 63.Fairweather D, Petri MA, Coronado MJ, Cooper LT., Jr Autoimmune heart disease: role of sex hormones and autoantibodies in disease pathogenesis. Expert Rev Clin Immunol. 2012;8:269–84. doi: 10.1586/eci.12.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sliwa K, Forster O, Libhaber E, Fett JD, Sundstron JB, Hilfiker-Kleiner D, et al. Peripartum cardiomyopathy: inflammatory markers as predictors of outcome in 100 prospectively studied patients. Eur Heart J. 2006;27:441–6. doi: 10.1093/eurheartj/ehi481. [DOI] [PubMed] [Google Scholar]
- 65.Sheppard R, Bedi M, Kubota T, Semigran MJ, Dec W, Holubkov R, et al. Myocardial expression of Fas and recovery of left ventricular function in patients with recent-onset cardiomyopathy. J Am Coll Cardiol. 2005;46:1036–42. doi: 10.1016/j.jacc.2005.05.067. [DOI] [PubMed] [Google Scholar]
- 66.Sliwa K, Skudicky D, Bergemann A, Candy G, Puren A, Sareli P. Peripartum cardiomyopathy: analysis of clinical outcome, left ventricular function, plasma levels of cytokines and Fas/APO-1. J Am Coll Cardiol. 2000;35:701–5. doi: 10.1016/s0735-1097(99)00624-5. [DOI] [PubMed] [Google Scholar]
- 67.Bursi F, Weston SA, Redfield MM, Jacobsen SJ, Pakhomov S, Nkomo VT, et al. Systolic and diastolic heart failure in the community. JAMA. 2006;296:2209–16. doi: 10.1001/jama.296.18.2209. [DOI] [PubMed] [Google Scholar]
- 68.Barsheshet A, Brenyo A, Goldenberg I, Moss AJ. Sex-related differences in patients' responses to heart failure therapy. Nat Rev Cardiol. 2012;9(4):234–242. doi: 10.1038/nrcardio.2012.10. [DOI] [PubMed] [Google Scholar]
- 69.Lenzen MJ, Rosengren A, Scholte op Reimer WJ, Follath F, Boersma E, Simoons ML, et al. Management of patients with heart failure in clinical practice: differences between men and women. Heart. 2008;94:e10. doi: 10.1136/hrt.2006.099523. [DOI] [PubMed] [Google Scholar]
- 70.Robinson T, Smith A, Channer KS. Reversible heart failure: the role of inflammatory activation. Postgrad Med J. 2011;87:110–15. doi: 10.1136/pgmj.2010.100123. [DOI] [PubMed] [Google Scholar]
- 71.Lahita RG. Sex hormones and immune function. In: Legato MJ, editor. Principles of Gender-Specific Medicine. 2nd. Burlington (MA): Elsevier; 2010. pp. 615–26. [Google Scholar]
- 72.Gilliver SC. Sex steroids as inflammatory regulators. J Steroid Biochem Mol Biol. 2010;120:105–15. doi: 10.1016/j.jsbmb.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 73.Villablanca AC, Jayachandran M, Banka C. Atherosclerosis and sex hormones: current concepts. Clin Sci. 2010;119:493–513. doi: 10.1042/CS20100248. [DOI] [PubMed] [Google Scholar]
- 74.McCrohon JA, Death AK, Nakhla S, Jessup W, Handelsman DJ, Stanley KK, et al. Androgen receptor expression is greater in macrophages from male than from female donors. A sex difference with implications for atherogenesis. Circulation. 2000;101:224–26. doi: 10.1161/01.cir.101.3.224. [DOI] [PubMed] [Google Scholar]
- 75.Hammes SR, Levin ER. Minireview: recent advances in extranuclear steroid receptor actions. Endocrinol. 2011;152:4489–95. doi: 10.1210/en.2011-1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hodgin JB, Krege JH, Reddick RL, Korach KS, Smithies O, Maeda N. Estrogen receptor alpha is a major mediator of 17beta-estradiol's atheroprotective effects on lesion size in Apoe−/− mice. J Clin Invest. 2001;107:333–40. doi: 10.1172/JCI11320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cook IF. Sexual dimorphism of humoral immunity with vaccines. Vaccine. 2008;26:3551–5. doi: 10.1016/j.vaccine.2008.04.054. [DOI] [PubMed] [Google Scholar]
- 78.Fairweather D, Frisancho-Kiss S, Rose NR. Sex differences in autoimmune disease from a pathologic perspective. Am J Pathol. 2008;173:600–9. doi: 10.2353/ajpath.2008.071008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Papenfuss TL, Powell ND, McClain MA, Bedarf A, Singh A, Gienapp IE, et al. Estriol generates tolergenic dendritic cells in vivo that protect against autoimmunity. J Immunol. 2011;186:3346–55. doi: 10.4049/jimmunol.1001322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rubtsov A, Rubtsova K, Kappler JW, Marrack P. Genetic and hormonal factors in female-biased autoimmunity. Autoimm Rev. 2010;9:494–8. doi: 10.1016/j.autrev.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Straub RH. The complex role of estrogens in inflammation. Endocrine Rev. 2007;28:521–74. doi: 10.1210/er.2007-0001. [DOI] [PubMed] [Google Scholar]
- 82.Deshpande R, Khalili H, Pergolizzi RG, Michael SD, Change MD. Estradiol down-regulates LPS-induced cytokine production and NFκB activation in murine macrophages. Am J Reprod Immunol. 1997;38:46–54. doi: 10.1111/j.1600-0897.1997.tb00275.x. [DOI] [PubMed] [Google Scholar]
- 83.Evans MJ, Eckert A, Lai K, Adelman SJ, Harnish DC. Reciprocal antagonism between estrogen receptor and NF-kappaB activity in vivo. Circ Res. 2001;89:823–30. doi: 10.1161/hh2101.098543. [DOI] [PubMed] [Google Scholar]
- 84.Temple SE, Pham K, Glendenning P, Phillips M, Waterer GW. Endotoxin induced TNF and IL-10 mRNA production is higher in male than female donors: correlation with elevated expression of TLR4. Cell Immunol. 2008;251:69–71. doi: 10.1016/j.cellimm.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 85.Giron-Gonzalez JA, Moral FJ, Elvira J, Garcia-Gil D, Guerrero F, Gavilan I, et al. Consistent production of a higher TH1:TH2 cytokine ratio by stimulated T cells in men compared to women. Eur J Endocrinol. 2000;143:31–6. doi: 10.1530/eje.0.1430031. [DOI] [PubMed] [Google Scholar]
- 86.Saha Roy S, Vadlamudi RK. Role of estrogen receptor signaling in breast cancer metastasis. Int J Breast Cancer. 2012;2012:654698. doi: 10.1155/2012/654698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Miller VM. In pursuit of scientific excellence- sex matters. Am J Physiol Heart Circ Physiol. 2012 Feb 10; doi: 10.1152/ajpheart.00073.2012. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 88.Cutolo M, Brizzolara R, Atzeni F, Capellino S, Straub RH, Puttini PCS. The immunomodulatory effects of estrogens: clinical relevance in immune-mediated rheumatic diseases. Ann NY Acad Sci. 2010;1193:36–42. doi: 10.1111/j.1749-6632.2009.05383.x. [DOI] [PubMed] [Google Scholar]
- 89.Blauwet LA, Hayes SN, McManus D, Redberg RF, Walsh MN. Low rate of sex-specific reporting in cardiovascular trials. Mayo Clin Proc. 2007;82:166–70. doi: 10.4065/82.2.166. [DOI] [PubMed] [Google Scholar]
- 90.The Institute of Medicine of the National Academies. Sex-specific reporting of scientific research: a workshop summary. Washington DC: The National Academies Press; 2012. pp. 1–59. www.national-academies.org. [PubMed] [Google Scholar]
- 91.Frisancho-Kiss S, Nyland JF, Davis SE, Frisancho JA, Barrett MA, Rose NR, et al. Sex differences in coxsackievirus B3-induced myocarditis: IL-12Rβ1 signaling and IFN-γ increase inflammation in males independent from STAT4. Brain Res. 2006;1126:139–47. doi: 10.1016/j.brainres.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 92.Coronado MJ, Brandt JE, Kim E, Bucek A, Bedja D, Abston ED, et al. Testosterone and interleukin-1b increase cardiac remodeling during acute coxsackievirus B3 myocarditis via serpin A 3n. Am J Physiol Heart Circ Physiol. 2012;302:H1726–36. doi: 10.1152/ajpheart.00783.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Huber SA. Coxsackievirus B3-induced myocarditis: infection of females during the estrus phase of the ovarian cycle leads to activation of T regulatory cells. Virology. 2008;378:292–8. doi: 10.1016/j.virol.2008.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Huber SA, Pfaeffle B. Differential Th1 and Th2 cell responses in male and female BALB/c mice infected with coxsackievirus group B type 3. J Virol. 1994;68:5126–32. doi: 10.1128/jvi.68.8.5126-5132.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Huber SA. Increased susceptibility of male BALB/c mice to coxsackievirus B3-induced myocarditis: role of CD1d. Med Microbiol Immunol. 2005;194:121–7. doi: 10.1007/s00430-004-0221-6. [DOI] [PubMed] [Google Scholar]
- 96.Frisancho-Kiss S, Coronado MJ, Frisancho JA, Lau VM, Rose NR, Klein SL, et al. Gonadectomy of male BALB/c mice increases Tim-3+ alternatively activated M2 macrophages, Tim-3+ T cells, Th2 cells and Treg in the heart during acute coxsackievirus-induced myocarditis. Brain Behav Immun. 2009;23:649–57. doi: 10.1016/j.bbi.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Huber SA, Kupperman J, Newell MK. Estradiol prevents and testosterone promotes Fas-dependent apoptosis in CD4+ Th2 cells by altering Bcl2 expression. Lupus. 1999;8:384–7. doi: 10.1177/096120339900800511. [DOI] [PubMed] [Google Scholar]
- 98.Li K, Xu W, Guo Q, Jiang Z, Wang P, Yue Y, et al. Differential macrophage polarization in male and female BALB/c mice infected with coxsackievirus B3 defines susceptibility to viral myocarditis. Circ Res. 2009;105:353–64. doi: 10.1161/CIRCRESAHA.109.195230. [DOI] [PubMed] [Google Scholar]
- 99.Fairweather D, Frisancho-Kiss S, Yusung SA, Barrett MA, Davis SE, Gatewood SJL, et al. IFN-γ protects against chronic viral myocarditis by reducing mast cell degranulation, fibrosis, and the profibrotic cytokines TGF-β1, IL-1β, and IL-4 in the heart. Am J Pathol. 2004;165:1883–94. doi: 10.1016/s0002-9440(10)63241-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fairweather D, Yusung S, Frisancho S, Barrett M, Gatewood S, Steele R, et al. IL-12Rβ1 and TLR4 increase IL-1β and IL-18-associated myocarditis and coxsackievirus replication. J Immunol. 2003;170:4731–7. doi: 10.4049/jimmunol.170.9.4731. [DOI] [PubMed] [Google Scholar]
- 101.Satoh M, Nakamura M, Akatsu T, Iwasaka J, Shimoda Y, Segawa I, et al. Expression of Toll-like receptor 4 is associated with enteroviral replication in human myocarditis. Clin Sci. 2003;104:577–84. doi: 10.1042/CS20020263. [DOI] [PubMed] [Google Scholar]
- 102.Satoh M, Nakamura M, Akatsu T, Iwasaka J, Shimoda Y, Segawa I, et al. Toll-like receptor 4 is expressed with enteroviral replication in myocardium form patients with dilated cardiomyopathy. Lab Invest. 2004;84:173–81. doi: 10.1038/labinvest.3700031. [DOI] [PubMed] [Google Scholar]
- 103.Fairweather D, Frisancho-Kiss S. Mast cells and inflammatory heart disease: potential drug targets. Cardiovasc Hematol Disord Drug Targets. 2008;8:80–90. doi: 10.2174/187152908783884957. [DOI] [PubMed] [Google Scholar]
- 104.Levick SP, Melendez GC, Plante E, McLarty JL, Brower GL, Janicki JS. Cardiac mast cells: the centrepiece in adverse myocardial remodeling. Cardiovasc Res. 2011;89:12–19. doi: 10.1093/cvr/cvq272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Warraich RS, Noutsias M, Kasac I, Seeberg B, Dunn MJ, Schultheiss HP, et al. Immunoglobulin G3 cardiac myosin autoantibodies correlate with left ventricular dysfunction in patients with dilated cardiomyopathy: immunoglobulin G3 and clinical correlates. Am Heart J. 2002;143:1076–84. doi: 10.1067/mhj.2002.124406. [DOI] [PubMed] [Google Scholar]
- 106.Robinson DP, Huber SA, Moussawi M, Roberts B, Teuscher C, Watkins, et al. Sex chromosome complement contributes to sex differences in coxsackievirus B3 but not influenza A virus pathology. Biol Sex Differ. 2011;2:8. doi: 10.1186/2042-6410-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kania G, Blyszczuk P, Eriksson U. Mechanisms of cardiac fibrosis in inflammatory heart disease. Trends Cardiovasc Med. 2009;19:247–52. doi: 10.1016/j.tcm.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 108.Abston ED, Coronado MJ, Bucek A, Bedja D, Shin J, Kim JB, et al. Th2 regulation of viral myocarditis in mice: different roles for TLR3 vs TRIF in progression to chronic disease. Clin Dev Immunol. 2012;2012:129486. doi: 10.1155/2012/129486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fairweather D, Rose NR. Coxsackievirus-induced myocarditis in mice: a model of autoimmune disease for studying immunotoxicity. Methods. 2007;41:118–22. doi: 10.1016/j.ymeth.2006.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Abston ED, Barin JG, Cihakova D, Bucek A, Coronado MJ, Brandt JE, et al. IL-33 independently induces eosinophilic pericarditis and cardiac dilation: ST2 improves cardiac function. Circ Heart Fail. 2012 Mar 27; doi: 10.1161/CIRCHEARTFAILURE.111.963769. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Onyimba JA, Coronado MJ, Garton AE, Kim JB, Bucek A, Bedja D, et al. The innate immune response to coxsackievirus B3 predicts progression to cardiovascular disease and heart failure in male mice. Biol Sex Differ. 2011;2:2. doi: 10.1186/2042-6410-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Boheler KR, Volkova M, Morrell C, Garg R, Zhu Y, Margulies K, et al. Sex- and age-dependent human transcriptome variability: Implications for chronic heart failure. Proc Nat Acad Sci. 2003;100:2754–9. doi: 10.1073/pnas.0436564100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cavasin MA, Tao ZY, Yu AL, Yang XP. Testosterone enhances early cardiac remodeling after myocardial infarction, causing rupture and degrading cardiac function. Am J Physiol Heart Circ Physiol. 2006;290:H2043–50. doi: 10.1152/ajpheart.01121.2005. [DOI] [PubMed] [Google Scholar]
- 114.Asakura M, Kitakaze M. Global gene expression profiling in the failing myocardium. Circ J. 2009;73:1568–76. doi: 10.1253/circj.cj-09-0465. [DOI] [PubMed] [Google Scholar]
- 115.Jones TH. Testosterone deficiency: a risk factor for cardiovascular disease? Trends Endocrinol Metab. 2010;21:496–503. doi: 10.1016/j.tem.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 116.Malkin CJ, Pugh PJ, Morris PD, Asif S, Jones TH, Channer KS. Low serum testosterone and increased mortality in men with coronary heart disease. Heart. 2010;96:1821–5. doi: 10.1136/hrt.2010.195412. [DOI] [PubMed] [Google Scholar]
- 117.Yeap BB. Androgens and cardiovascular disease. Curr Opin Endocrinol Diabetes Obes. 2010;17:269–76. doi: 10.1097/MED.0b013e3283383031. [DOI] [PubMed] [Google Scholar]
- 118.Ohlsson C, Barrett-Conner E, Bhasin S, Orwoll E, Labire F, Karlsson MK, et al. High serum testosterone is associated with reduced risk of cardiovascular events in elderly men. J Am Coll Cardiol. 2011;58:1674–81. doi: 10.1016/j.jacc.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 119.Basaria S, Coviello AD, Travison TG, Storer TW, Farwell WR, Jette AM, et al. Adverse events associated with testosterone administration. N Engl J Med. 2010;363:109–22. doi: 10.1056/NEJMoa1000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hales BB. Interleukin-1 inhibits Leydig cell steriodogenesis primarily by decreasing 17 alpha-hydroxylase/C17-20 lyase cytochrome P450 expression. Endocrinology. 1992;131:2165–72. doi: 10.1210/endo.131.5.1425417. [DOI] [PubMed] [Google Scholar]
- 121.Johnstone D, Limacher M, Rousseau M, Liang CS, Ekelund L, Herman M, et al. Clinical characteristics of patients in studies of left ventricular dysfunction (SOLVD) Am J Cardiol. 1992;70:894–900. doi: 10.1016/0002-9149(92)90734-g. [DOI] [PubMed] [Google Scholar]
- 122.De Maria R, Gavazzi A, Recalcati F, Baroldi G, De Vita C, Camerini F. Comparison of clinical findings in idiopathic dilated cardiomyopathy in women versus men The Italian Multicenter Cardiomyopathy Study Group (SPIC) Am J Cardiol. 1993;72:580–85. doi: 10.1016/0002-9149(93)90355-g. [DOI] [PubMed] [Google Scholar]
- 123.Goland S, Modi K, Bitar F, Janmohamed M, Mirocha JM, Czer LS, et al. Clinical profile and predictors of complications in peripartum cardiomyopathy. J Card Fail. 2009;15:645–50. doi: 10.1016/j.cardfail.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 124.Chin MH, Goldman L. Gender differences in 1-year survival and quality of life among patients admitted with congestive heart failure. Med Care. 1998;36:1033–46. doi: 10.1097/00005650-199807000-00010. [DOI] [PubMed] [Google Scholar]
- 125.Riedinger MS, Dracup KA, Brecht ML, Padilla G, Sarna L, Ganz PA. Quality of life in patients with heart failure: do gender differences exist? Heart Lung. 2001;30:105–16. doi: 10.1067/mhl.2001.114140. [DOI] [PubMed] [Google Scholar]
- 126.Azevedo A, Bettencourt P, Pimenta J, Frioes F, Abreu-Lima C, Hense HW, et al. Clinical syndrome suggestive of heart failure is frequently attributable to non-cardiac disorders--population-based study. Eur J Heart Fail. 2007;9:391–96. doi: 10.1016/j.ejheart.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 127.O'Meara E, Clayton T, McEntegart MB, McMurray JJ, Pina IL, Granger CB, et al. Sex differences in clinical characteristics and prognosis in a broad spectrum of patients with heart failure: results of the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) program. Circulation. 2007;115:3111–20. doi: 10.1161/CIRCULATIONAHA.106.673442. [DOI] [PubMed] [Google Scholar]
- 128.Baumhakel M, Muller U, Bohm M. Influence of gender of physicians and patients on guideline-recommended treatment of chronic heart failure in a cross-sectional study. Eur J Heart Fail. 2009;11:299–303. doi: 10.1093/eurjhf/hfn041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Klein L, Grau-Sepulveda MV, Bonow RO, Hernandez AF, Williams MC, Bhatt DL, et al. Quality of care and outcomes in women hospitalized for heart failure. Circ Heart Fail. 2011;4:589–98. doi: 10.1161/CIRCHEARTFAILURE.110.960484. [DOI] [PubMed] [Google Scholar]
- 130.Hsich EM, Pina IL. Heart failure in women: a need for prospective data. J Am Coll Cardiol. 2009;54:491–8. doi: 10.1016/j.jacc.2009.02.066. [DOI] [PubMed] [Google Scholar]
- 131.Hilfiker-Kleiner D, Kaminski K, Podewski E, Bonda T, Schaefer A, Silwa K, et al. A cathepsin D-cleaved 16 kDa form of prolactin mediates postpartum cardiomyopathy. Cell. 2007;128:589–600. doi: 10.1016/j.cell.2006.12.036. [DOI] [PubMed] [Google Scholar]
- 132.Sliwa K, Blauwet L, Tibazarwa K, Libhaber E, Smedema JP, Becker A, et al. Evaluation of bromocriptine in the treatment of acute severe peripartum cardiomyopathy: a proof-of-concept pilot study. Circulation. 2010;121:1465–73. doi: 10.1161/CIRCULATIONAHA.109.901496. [DOI] [PubMed] [Google Scholar]
- 133.Simon T, Mary-Krause M, Funck-Brentano C, Jaillon P. Sex differences in the prognosis of congestive heart failure: results from the Cardiac Insufficiency Bisoprolol Study (CIBIS II) Circulation. 2001;103:375–80. doi: 10.1161/01.cir.103.3.375. [DOI] [PubMed] [Google Scholar]
- 134.Ghali JK, Pina IL, Gottlieb SS, Deedwania PC, Wikstrand JC. Metoprolol CR/XL in female patients with heart failure: analysis of the experience in Metoprolol Extended-Release Randomized Intervention Trial in Heart Failure (MERIT-HF) Circulation. 2002;105:1585–91. doi: 10.1161/01.cir.0000012546.20194.33. [DOI] [PubMed] [Google Scholar]
- 135.Packer M, Bristow MR, Cohn JN, Colucci WS, Fowler MB, Gilbert EM, et al. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. U.S Carvedilol Heart Failure Study Group. N Engl J Med. 1996;334:1349–55. doi: 10.1056/NEJM199605233342101. [DOI] [PubMed] [Google Scholar]
- 136.Packer M, Coats AJ, Fowler MB, Katus HA, Krum H, Mohacsi P, et al. Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med. 2001;344:1651–58. doi: 10.1056/NEJM200105313442201. [DOI] [PubMed] [Google Scholar]
- 137.Ghali JK, Krause-Steinrauf HJ, Adams KF, Khan SS, Rosenberg YD, Yancy CW, et al. Gender differences in advanced heart failure: insights from the BEST study. J Am Coll Cardiol. 2003;42:2128–34. doi: 10.1016/j.jacc.2003.05.012. [DOI] [PubMed] [Google Scholar]
- 138.Garg R, Yusef S. Overview of randomized trials of angiotensin-converting enzyme inhibitors on mortality and morbidity in patients with heart failure Collaborative Group on ACE Inhibitor Trials. JAMA. 1995;273:1450–6. [PubMed] [Google Scholar]
- 139.Shekelle PG, Rich MW, Morton SC, Atkinson CS, Tu W, Maglione M, et al. Efficacy of angiotensin-converting enzyme inhibitors and beta-blockers in the management of left ventricular systolic dysfunction according to race, gender, and diabetic status: a meta-analysis of major clinical trials. J Am Coll Cardiol. 2003;41:1529–38. doi: 10.1016/s0735-1097(03)00262-6. [DOI] [PubMed] [Google Scholar]
- 140.Young JB, Dunlap ME, Pfeffer MA, Probstfield JL, Cohen-Solal A, Dietz R, et al. Mortality and morbidity reduction with Candesartan in patients with chronic heart failure and left ventricular systolic dysfunction: results of the CHARM low-left ventricular ejection fraction trials. Circulation. 2004;110:2618–26. doi: 10.1161/01.CIR.0000146819.43235.A9. [DOI] [PubMed] [Google Scholar]
- 141.Cohn JN, Tognoni G. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med. 2001;345:1667–75. doi: 10.1056/NEJMoa010713. [DOI] [PubMed] [Google Scholar]
- 142.Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341:709–17. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 143.Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348:1309–21. doi: 10.1056/NEJMoa030207. [DOI] [PubMed] [Google Scholar]
- 144.Zannad F, McMurray JJ, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, et al. Eplerenone patients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364:11–21. doi: 10.1056/NEJMoa1009492. [DOI] [PubMed] [Google Scholar]
- 145.Cohn JN, Archibald DG, Ziesche S, Franciosa JA, Harston WE, Tristani FE, et al. Effect of vasodilator therapy on mortality in chronic congestive heart failure Results of a Veterans Administration Cooperative Study. N Engl J Med. 1986;314:1547–52. doi: 10.1056/NEJM198606123142404. [DOI] [PubMed] [Google Scholar]
- 146.Cohn JN, Johnson G, Ziesche S, Cobb F, Francis G, Tristani F, et al. A comparison of enalapril with hydralazine-isosorbide dinitrate in the treatment of chronic congestive heart failure. N Engl J Med. 1991;325:303–10. doi: 10.1056/NEJM199108013250502. [DOI] [PubMed] [Google Scholar]
- 147.Taylor AL, Ziesche S, Yancy C, Carson P, D'Agostino R, Jr, Ferdinanad K, et al. Combination of isosorbide dinitrate and hydralazine in blacks with heart failure. N Engl J Med. 2004;351:2049–57. doi: 10.1056/NEJMoa042934. [DOI] [PubMed] [Google Scholar]
- 148.Taylor AL, Lindenfeld J, Ziesche S, Walsh MN, Mitchell JE, Adams K, et al. Outcomes by gender in the African-American Heart Failure Trial. J Am Coll Cardiol. 2006;48:2263–7. doi: 10.1016/j.jacc.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 149.The Digitalis Investigation Group. The effect of digoxin on mortality and morbidity in patients with heart failure. N Engl J Med. 1997;336:525–33. doi: 10.1056/NEJM199702203360801. [DOI] [PubMed] [Google Scholar]
- 150.Rathore SS, Wang Y, Krumholz HM. Sex-based differences in the effect of digoxin for the treatment of heart failure. N Engl J Med. 2002;347:1403–11. doi: 10.1056/NEJMoa021266. [DOI] [PubMed] [Google Scholar]
- 151.Rathore SS, Curtis JP, Wang Y, Bristow MR, Krumholz HM. Association of serum digoxin concentration and outcomes in patients with heart failure. JAMA. 2003;289:871–8. doi: 10.1001/jama.289.7.871. [DOI] [PubMed] [Google Scholar]
- 152.Alaeddini J, Wood MA, Amin MS, Ellenbogen KA. Gender disparity in the use of cardiac resynchronization therapy in the United States. Pacing Clin Electrophysiol. 2008;31:468–72. doi: 10.1111/j.1540-8159.2008.01016.x. [DOI] [PubMed] [Google Scholar]
- 153.Curtis LH, Al-Khatib SM, Shea AM, Hammill BG, Hernandez AF, Schulman KA. Sex differences in the use of implantable cardioverter-defibrillators for primary and secondary prevention of sudden cardiac death. JAMA. 2007;298:1517–24. doi: 10.1001/jama.298.13.1517. [DOI] [PubMed] [Google Scholar]
- 154.Santangeli P, Pelargonio G, Dello Russo A, Casella M, Bisceglia C, Bartoletti S, et al. Gender differences in clinical outcome and primary prevention defibrillator benefit in patients with severe left ventricular dysfunction: a systematic review and meta-analysis. Heart Rhythm. 2010;7:876–82. doi: 10.1016/j.hrthm.2010.03.042. [DOI] [PubMed] [Google Scholar]
- 155.Ghanbari H, Dalloul G, Hasan R, Daccarett M, Saba S, David S, et al. Effectiveness of implantable cardioverter-defibrillators for the primary prevention of sudden cardiac death in women with advanced heart failure: a meta-analysis of randomized controlled trials. Arch Intern Med. 2009;169:1500–6. doi: 10.1001/archinternmed.2009.255. [DOI] [PubMed] [Google Scholar]
- 156.Kadish A, Dyer A, Daubert JP, Quigg R, Estes NA, Anderson KP, et al. Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N Engl J Med. 2004;350:2151–8. doi: 10.1056/NEJMoa033088. [DOI] [PubMed] [Google Scholar]
- 157.Albert CM, Quigg R, Saba S, Estes NA, 3rd, Schaechter A, Subacius H et al. Sex differences in outcome after implantable cardioverter defibrillator implantation in nonischemic cardiomyopathy. Am Heart J. 2008;156:367–72. doi: 10.1016/j.ahj.2008.02.026. [DOI] [PubMed] [Google Scholar]
- 158.Hernandez AF, Fonarow GC, Liang L, Al-Khatib SM, Curtis LH, LaBresh KA, et al. Sex and racial differences in the use of implantable cardioverter-defibrillators among patients hospitalized with heart failure. JAMA. 2007;298:1525–32. doi: 10.1001/jama.298.13.1525. [DOI] [PubMed] [Google Scholar]
- 159.Al-Khatib SM, Hellkamp AS, Hernandez AF, Fonarow GC, Thomas KL, Al-Khalidi HR, et al. Trends in use of implantable cardioverter-defibrillator therapy among patients hospitalized for heart failure: have the previously observed sex and racial disparities changed over time? Circulation. 2012;125:1094–1101. doi: 10.1161/CIRCULATIONAHA.111.066605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.McAlister FA, Ezekowitz JA, Wiebe N, Rowe B, Spooner C, Crumley E, Hartling L, Klassen T, Abraham W. Systematic review: cardiac resynchronization in patients with symptomatic heart failure. Ann Intern Med. 2004;141:381–90. doi: 10.7326/0003-4819-141-5-200409070-00101. [DOI] [PubMed] [Google Scholar]
- 161.Goldenberg I, Moss AJ, Hall WJ, Foster E, Goldberger JJ, Santucci P, et al. Predictors of response to cardiac resynchronization therapy in the Multicenter Automatic Defibrillator Implantation Trial with Cardiac Resynchronization Therapy (MADIT-CRT) Circulation. 2011;124:1527–36. doi: 10.1161/CIRCULATIONAHA.110.014324. [DOI] [PubMed] [Google Scholar]
- 162.van Bommel RJ, Bax JJ, Abraham WT, Chung ES, Pires LA, Tavazzi L, et al. Characteristics of heart failure patients associated with good and poor response to cardiac resynchronization therapy: a PROSPECT (Predictors of Response to CRT) sub-analysis. Eur Heart J. 2009;30:2470–7. doi: 10.1093/eurheartj/ehp368. [DOI] [PubMed] [Google Scholar]
- 163.Stabile G, Solimene F, Bertaglia E, La Rocca V, Accoqli M, Scaccia A, et al. Long-term outcomes of CRT-PM versus CRT-D recipients. Pacing Clin Electrophysiol. 2009;32(Suppl 1):S141–5. doi: 10.1111/j.1540-8159.2008.02271.x. [DOI] [PubMed] [Google Scholar]
- 164.Mooyaart EA, Marsan NA, van Bommel RJ, Thijssen J, Borleffs CJ, Delgado V, et al. Comparison of long-term survival of men versus women with heart failure treated with cardiac resynchronization therapy. Am J Cardiol. 2011;108:63–8. doi: 10.1016/j.amjcard.2011.02.345. [DOI] [PubMed] [Google Scholar]
- 165.Topkara VK, Dang NC, Barili F, Martens TP, George I, Cheema FH, et al. Ventricular assist device use for the treatment of acute viral myocarditis. J Thorac Cardiovasc Surg. 2006;131:1190–1. doi: 10.1016/j.jtcvs.2005.08.073. [DOI] [PubMed] [Google Scholar]
- 166.Murray LK, Gonzalez-Costello J, Jonas SN, Sims DB, Morrison KA, Colombo PC, et al. Ventricular assist device support as a bridge to heart transplantation in patients with giant cell myocarditis. Eur J Heart Fail. 2012;14:312–18. doi: 10.1093/eurjhf/hfr174. [DOI] [PubMed] [Google Scholar]
- 167.Bogaev RC, Pamboukian SV, Moore SA, Chen L, John R, Boyle AJ, et al. Comparison of outcomes in women versus men using a continuous-flow left ventricular assist device as a bridge to transplantation. J Heart Lung Transplant. 2011;30:515–22. doi: 10.1016/j.healun.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 168.Stehlik J, Edwards LB, Kucheryavaya AY, Benden C, Christie JD, Dobbels F, et al. The Registry of the International Society for Heart and Lung Transplantation: Twenty-eighth Adult Heart Transplant Report--2011. J Heart Lung Transplant. 2011;30:1078–94. doi: 10.1016/j.healun.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 169.Regitz-Zagrosek V, Petrov G, Lehmkuhl E, Smits JM, Babitsch B, Brunhuber C, et al. Heart transplantation in women with dilated cardiomyopathy. Transplantation. 2010;89:236–44. doi: 10.1097/TP.0b013e3181c35255. [DOI] [PubMed] [Google Scholar]
- 170.Aaronson KD, Schwartz JS, Goin JE, Mancini DM. Sex differences in patient acceptance of cardiac transplant candidacy. Circulation. 1995;91:2753–61. doi: 10.1161/01.cir.91.11.2753. [DOI] [PubMed] [Google Scholar]
- 171.Rasmusson K, Brunisholz K, Budge D, Horne BD, Alharethi R, Folsom J, et al. Peripartum cardiomyopathy: post-transplant outcomes from the United Network for Organ Sharing Database. J Heart Lung Transplant. 2012;31:180–6. doi: 10.1016/j.healun.2011.11.018. [DOI] [PubMed] [Google Scholar]
- 172.Gleicher N, Elkayam U. Perpartum cardiomyopathy, an autoimmune manifestation of allorgraft rejection? Autoimm Rev. 2009;8:384–7. doi: 10.1016/j.autrev.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 173.Amarelli C, De Santo LS, Marra C, Maiello C, Bancone C, Della Corte A. Early graft failure after heart transplant: risk factors and implications for improved donor-recipient matching. Interact Cardiovasc Thorac Surg. 2012 Apr 4; doi: 10.1093/icvts/ivs113. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Ho KK, Anderson KM, Kannel WB, Grossman W, Levy D. Survival after the onset of congestive heart failure in Framingham Heart Study subjects. Circulation. 1993;88:107–15. doi: 10.1161/01.cir.88.1.107. [DOI] [PubMed] [Google Scholar]


