Abstract
Purpose
To evaluate whether progress continues in identifying more effective treatments for children and adolescents with cancer, we examined overall as well as disease-specific childhood cancer mortality rates for the United States, focusing on data for 2000–2010.
Methods
Age-adjusted United States mortality trends for 1975 to 2010 were estimated using joinpoint regression analysis. Analyses of annual percentage change (APC) were performed on the same diagnostic groupings for the time period restricted to 2000–2010 for < 20, < 15 and 15–19 year age groupings.
Results
Following a plateau in mortality rates from 1998–2002 (APC = 0.3), the annual decline in childhood cancer mortality for 2002–2010 (−2.4%) was similar to that observed from 1975 to 1998 (−2.7%). Statistically significant declines in mortality rates for 2000–2010 were noted for acute lymphoblastic leukemia, acute myeloid leukemia, non-Hodgkin lymphoma, Hodgkin lymphoma, neuroblastoma, CNS cancers, and gonadal cancers. For 2000–2010, the rates of decline in mortality for the 15–19 year group were generally equal to or greater than those of the 0–14 year group. Improvements in treatment since 1975 resulted in over 45,000 cancer deaths averted through 2010.
Conclusions
Cancer mortality for both children and adolescents declined from 2000–2010, with significant declines observed for multiple cancer types. However, over 1900 cancer deaths still occur each year among children and adolescents in the United States, and many survivors experience long-term effects that limit their quality of life. Continued research directed towards identifying more effective treatments that produce fewer long-term sequelae is critical to address these remaining challenges.
Introduction
Treatment of childhood cancer is one of the important success stories of 20th century medicine. This success is exemplified by acute lymphoblastic leukemia (ALL), an incurable disease in the 1950s that by the end of the century showed 5-year survival rates approaching 90%. Other childhood cancers also showed marked improvements in outcome in the 20th century, including Wilms tumor, non-Hodgkin lymphoma (NHL), Hodgkin lymphoma, and germ cell tumors.
Despite the successes in identifying effective treatments for some cancer diagnoses, at the end of the twentieth century, more than 20% of children diagnosed with cancer still succumbed to their disease, and many survivors experienced long-term effects which negatively affected their quality of life. Additionally, for some childhood cancers progress was very limited [e.g., diffuse intrinsic brainstem gliomas (DIPG), high-grade gliomas, and metastatic sarcomas]. Of concern, a slowing in the rate of decline in childhood cancer mortality has been described for both European and North American populations, suggesting that a plateau is being reached in the ability of childhood cancer researchers to identify more effective treatments for children with cancer.1, 2
To ascertain whether progress in identifying more effective treatments for children and adolescents with cancer is continuing, we examined overall and disease-specific childhood cancer mortality rates for the United States, focusing on more recent data from 2000–2010.
Methods
Incidence Data
Incident cases that formed the basis for survival estimates included in this report resided in Surveillance, Epidemiology, and End Results (SEER) 9 registries under 20 years of age at the time of diagnosis, between 1975 and 2010. The SEER 9 registries, which cover approximately 10% of the United States population, are Metropolitan Atlanta, Connecticut, Detroit, Hawaii, Iowa, New Mexico, San Francisco-Oakland, Seattle-Puget Sound, and Utah.3 Rates were age-adjusted to the U.S. 2000 standard population.
Mortality Data
Mortality data was based on deaths in the United States that were reported to the Centers for Disease Control and Prevention. Rates were age-adjusted to the U.S. 2000 standard population. For all children under 20, 15–19, and less than 15 years of age mortality rates per 100,000 and the proportion of all United States childhood cancer deaths in 2000–2002, 2003–2006 and 2007–2010 attributable to specific cancer sites was determined for selected sites: leukemia (including acute lymphoblastic leukemia [ALL] and acute myeloid leukemia [AML]), lymphomas (with Hodgkin’s lymphoma and non-Hodgkin’s lymphoma [NHL] separately), brain and other nervous system, neuroblastoma, bone and joint, soft tissue (including heart), kidney and renal pelvis, gonads (ovary and testis), liver and intrahepatic bile duct, all other malignant cancers combined and in situ, benign and unknown behavior tumors.
Mortality Trends
Age-adjusted United States mortality trends were estimated for hematopoietic cancers (leukemia and lymphoma, combined) and for all other cancers combined from 1975–2010 using joinpoint regression analysis (Joinpoint 3.3; Information Management Services, Silver Spring, MD), to fit a series of joined straight lines on a logarithmic scale to annual age-standardized rates.4 A maximum of five joinpoints were allowed. Trends of varying time periods were described by annual percentage change (APC), that is, the slope of the line segment. We also determined the APC for the time period restricted to 2000 to 2010. Statistical significance was defined by rejection of the null hypothesis that the APC is equal to zero with significance level 0.05. We additionally examined absolute declines in mortality rates for the period 2000–2010, specifically comparing mortality in the first three years of the decade (2000–2002) to that in the last four years of the decade (2007–2010) for age < 20 years.
Deaths Averted
The number of childhood malignant cancer deaths averted in the United States from 1975 through 2010 was estimated on the basis of observed deaths per year versus expected deaths, had there been no decrease in rate since 1975. Observed annual age-specific counts of deaths due to malignant cancer were determined by age group: 0, 1 to 4, 5 to 9, 10 to 14, and 15 to 19 years of age. Age-specific expected deaths were estimated by multiplying 1975 age-specific rates by annual age-specific populations from 1975 through 2010. Estimated deaths averted were differences between expected and observed deaths. Total childhood cancer deaths averted were the sum across age strata.
Survival Data
Using the International Childhood Cancer Classification (ICCC), 5-year survival rates for selected childhood age groups were examined during successive 4-year periods from 1975–1978 through 2003–2007. The year 2007 was the last year included in survival analyses, allowing follow-up from time of diagnosis through 2010. Additional information regarding the analysis of survival data are provided in the supplemental materials.
Results
Childhood cancer mortality 2000–2010
We first examined mortality for the period 2000–2010 for children and adolescents < 20 years, < 15 years, and 15–19 years of age. These age groupings were utilized because of differences between histologies presenting in younger children and older adolescents and because of concerns that improvements in adolescent outcomes may lag behind those for younger children. The annual percentage change (APC) was determined for aggregated diagnoses and for specific diagnoses. Statistically significant declines in mortality rates were noted for all age groups when considering all malignant cancers and all leukemias, and were also noted across all age groups for ALL, AML, and NHL considered separately (Table I). Significant declines were additionally observed for Hodgkin lymphoma and germ cell tumors for the < 20 year and 15–19 year age groups, and for neuroblastoma and brain cancers for the < 20 year and <15 year age groups.
Table I.
US childhood cancer mortality rates, 2000–2010
| 2000–2002 | 2003–2006 | 2007–2010 | Percent Change 2000–2002 to 2007–2010 | Annual Percent Change 2000–2010 | |||||
|---|---|---|---|---|---|---|---|---|---|
| Age Group | Site | Rate | 95% CI | Rate | 95% CI | Rate | 95% CI | ||
| <20 years | All malignant cancer | 2.79 | (2.72, 2.86) | 2.64 | (2.59, 2.7) | 2.4 | (2.34, 2.45) | −14.1 | −2.1* |
| Leukemia | 0.88 | (0.84, 0.92) | 0.79 | (0.76, 0.83) | 0.71 | (0.68, 0.73) | −19.9 | −3.1* | |
| Acute lymphocytic leukemia | 0.4 | (0.37, 0.42) | 0.35 | (0.33, 0.37) | 0.31 | (0.29, 0.33) | −23.1 | −3.8* | |
| Acute myeloid leukemia | 0.27 | (0.25, 0.29) | 0.25 | (0.23, 0.27) | 0.23 | (0.21, 0.24) | −16.7 | −2.3* | |
| Brain and ONS | 0.68 | (0.64, 0.71) | 0.68 | (0.65, 0.71) | 0.63 | (0.6, 0.66) | −6.8 | −1.1* | |
| Ganglioneuroblastoma | 0.23 | (0.21, 0.25) | 0.23 | (0.21, 0.25) | 0.2 | (0.18, 0.21) | −13.3 | −1.9* | |
| Bone and joint | 0.22 | (0.21, 0.24) | 0.23 | (0.21, 0.24) | 0.21 | (0.19, 0.22) | −8.5 | −1.3 | |
| Soft tissue including heart | 0.18 | (0.17, 0.2) | 0.19 | (0.17, 0.2) | 0.18 | (0.17, 0.2) | −0.5 | −0.1 | |
| non-Hodgkin lymphoma | 0.14 | (0.12, 0.15) | 0.12 | (0.11, 0.13) | 0.1 | (0.09, 0.11) | −28.8 | −4.4* | |
| Hodgkin lymphoma | 0.04 | (0.03, 0.05) | 0.03 | (0.02, 0.03) | 0.02 | (0.02, 0.02) | −49.1 | −7.6* | |
| Kidney and renal pelvis | 0.07 | (0.06, 0.08) | 0.07 | (0.07, 0.08) | 0.06 | (0.05, 0.07) | −13.2 | −2.1 | |
| Gonads | 0.02 | (0.02, 0.03) | 0.02 | (0.02, 0.03) | 0.02 | (0.01, 0.02) | −30.8 | −4.6* | |
| Liver | 0.07 | (0.06, 0.08) | 0.06 | (0.05, 0.07) | 0.06 | (0.06, 0.07) | −6.3 | −1.1 | |
| 15–19 years | All malignant cancer | 3.59 | (3.44, 3.74) | 3.32 | (3.2, 3.44) | 2.94 | (2.83, 3.06) | −17.9 | −2.6* |
| Leukemia | 1.15 | (1.06, 1.24) | 0.97 | (0.9, 1.03) | 0.83 | (0.77, 0.89) | −27.8 | −4.0* | |
| Acute lymphocytic leukemia | 0.5 | (0.45, 0.56) | 0.41 | (0.36, 0.45) | 0.33 | (0.29, 0.37) | −34.7 | −5.2* | |
| Acute myeloid leukemia | 0.37 | (0.32, 0.42) | 0.32 | (0.29, 0.36) | 0.28 | (0.25, 0.32) | −22.3 | −2.7* | |
| Brain and ONS | 0.52 | (0.46, 0.58) | 0.53 | (0.48, 0.58) | 0.46 | (0.42, 0.51) | −10.9 | −1.7 | |
| Ganglioneuroblastoma | 0.06 | (0.04, 0.09) | 0.06 | (0.05, 0.08) | 0.06 | (0.04, 0.08) | −5.1 | 0 | |
| Bone and joint | 0.48 | (0.42, 0.54) | 0.54 | (0.49, 0.59) | 0.48 | (0.43, 0.53) | 0.4 | −0.4 | |
| Soft tissue including heart | 0.31 | (0.27, 0.36) | 0.32 | (0.28, 0.36) | 0.3 | (0.26, 0.33) | −5.3 | −1 | |
| non-Hodgkin lymphoma | 0.27 | (0.23, 0.31) | 0.24 | (0.2, 0.27) | 0.2 | (0.17, 0.23) | −25.2 | −4.4* | |
| Hodgkin lymphoma | 0.12 | (0.09, 0.15) | 0.07 | (0.06, 0.09) | 0.06 | (0.04, 0.08) | −49.6 | −8.2* | |
| Kidney and renal pelvis | 0.04 | (0.02, 0.06) | 0.04 | (0.03, 0.06) | 0.04 | (0.03, 0.05) | 2.6 | −0.1 | |
| Gonads | 0.08 | (0.06, 0.11) | 0.08 | (0.06, 0.1) | 0.05 | (0.04, 0.07) | −34.8 | −6.1* | |
| Liver | 0.06 | (0.04, 0.08) | 0.05 | (0.04, 0.07) | 0.06 | (0.04, 0.08) | 5.1 | 0.4 | |
| <15 years | All malignant cancer | 2.52 | (2.45, 2.59) | 2.42 | (2.35, 2.48) | 2.21 | (2.15, 2.27) | −12.2 | −1.8* |
| Leukemia | 0.79 | (0.75, 0.83) | 0.74 | (0.7, 0.77) | 0.66 | (0.63, 0.7) | −16.0 | −2.7* | |
| Acute lymphocytic leukemia | 0.36 | (0.34, 0.39) | 0.33 | (0.31, 0.36) | 0.3 | (0.28, 0.32) | −17.8 | −3.3* | |
| Acute myeloid leukemia | 0.24 | (0.22, 0.26) | 0.23 | (0.21, 0.25) | 0.21 | (0.19, 0.23) | −13.7 | −2.1* | |
| Brain and ONS | 0.73 | (0.69, 0.77) | 0.73 | (0.69, 0.76) | 0.69 | (0.65, 0.72) | −5.8 | −0.9* | |
| Ganglioneuroblastoma | 0.29 | (0.26, 0.31) | 0.29 | (0.27, 0.31) | 0.25 | (0.23, 0.27) | −13.9 | −2.0* | |
| Bone and joint | 0.14 | (0.12, 0.16) | 0.12 | (0.11, 0.14) | 0.11 | (0.1, 0.13) | −18.8 | −2.3 | |
| Soft tissue including heart | 0.14 | (0.12, 0.16) | 0.14 | (0.13, 0.16) | 0.14 | (0.13, 0.16) | 3.1 | 0.6 | |
| non-Hodgkin lymphoma | 0.09 | (0.08, 0.11) | 0.08 | (0.07, 0.09) | 0.06 | (0.05, 0.07) | −32.4 | −4.8* | |
| Hodgkin lymphoma | 0.01 | (0.01, 0.02) | 0.01 | (0.01, 0.02) | 0.01 | (0, 0.01) | −47.0 | −4.2 | |
| Kidney and renal pelvis | 0.08 | (0.07, 0.095) | 0.08 | (0.07, 0.11) | 0.07 | (0.06, 0.08) | −15.7 | −2.4 | |
| Gonads | 0.01 | (0.002, 0.01) | 0.01 | (0.004, 0.01) | 0.01 | (0.002, 0.01) | −8.4 | 3.1 | |
| Liver | 0.07 | (0.06, 0.09) | 0.07 | (0.06, 0.08) | 0.07 | (0.06, 0.08) | −9.3 | −1.5 | |
Asterisk indicates statistically significant decrease. Rates are per 100,000.
We next compared mortality in 2000–2002 to that of the last four years for which data are available (2007–2010) to document the absolute decline in mortality rates between the early and latter parts of this time period (Table I). The decline in cancer mortality for patients < 20 years was −14.0%, with a slightly greater decline for the 15–19 year group (−18.1%) compared to the decline for children < 15 years (−12.3%). The decline in mortality for the <20 year age group for leukemias was −19.3%, with a −22.5% decline for ALL and a −14.8% decline for AML. Interestingly, the declines in total leukemia and ALL mortality were greater for 15–19 year olds (−27.8% and −34.0%, respectively) compared to the <15 year old group (−16.5% and −16.7%, respectively). The declines in AML mortality were also numerically greater for the adolescents compared to younger children (−24.3% and −12.5%, respectively). Large percent declines in Hodgkin lymphoma and NHL mortality were noted for the <20 year group (−28.6% and −50.0%, respectively). For solid tumors, the largest percent decline among cancers with significant decreases based on APC was for neuroblastoma (−13.0%).
The pattern of mortality for 2007–2010 differs markedly between the <15 year and the 15–19 year age groups (Figure 1). For the younger age group, leukemias, brain tumors, and neuroblastoma account for nearly three-fourths (71%) of all cancer-related mortality, while these diagnoses account for less than half (46%) of cancer mortality in 15–19 year olds. By contrast bone and soft tissue cancers and NHL account for approximately one-third of mortality in older patients, but less than one-sixth of mortality in younger patients. Kidney and liver tumors account together for 5–6% of mortality in the < 15 year age group, while gonadal tumors and Hodgkin lymphoma are associated with <1% of mortality in this age group. Among 15–19 year olds, these cancers each account for approximately 2% of overall cancer-related mortality.
Figure 1.
Patterns of mortality for children and adolescents < 15 years and 15–19 years for 2007–2010. ALL = acute lymphoblastic leukemia, AML = acute myeloid leukemia, Oth Leuk = other leukemia, NHL = non-Hodgkin lymphoma.
Trends in cancer mortality 1975–2010
We next sought to understand patterns of cancer mortality for 2000 to 2010 in the context of trends in mortality since 1975. Overall childhood cancer mortality declined by 52% from 1975–77 to 2007–2010 (Supplemental Table I). The greatest percentage declines in mortality were for Hodgkin lymphoma and gonadal tumors (−82% and −83%, respectively). Declines exceeding 50% were also noted for leukemias (−64%), renal tumors (−57%), and NHL (−74%). Smaller declines were noted for neuroblastoma (−43%), brain tumors (−29%), and bone tumors (−36%). The smallest declines were observed for liver tumors (−22%) and “soft tissue including heart” (a category primarily including soft tissue sarcomas) (−5%).
Joinpoint analysis was applied to mortality rates for the <20 year age group. With the addition of four years of mortality data (2007–2010) compared to the 1975–2006 data reported previously,1 the best-fitting joinpoint model contained a new joinpoint segment. The new segment corresponded with a period of stable mortality rates from 1998 to 2002 (APC=0.3) that was followed by a period of more pronounced decreasing rates from 2002 to 2010 (APC=−2.4, P≤0.05) (Figure 2). Looking separately at leukemias and lymphomas compared to other cancers (solid tumors and brain cancers), distinctive patterns of declining mortality rates were observed (Figure 3). For leukemias and lymphomas, the APC from 1975–1999 was −3.7%, with a new segment for 1999–2002 with near stable mortality (APC −0.4%), and with a third segment from 2002–2010 with an APC of −3.7% (identical to that observed from 1975–1999). For all other cancer sites combined, declining rates of varying magnitude were observed from 1975 to 1997, followed by a period of near stable mortality from 1997–2003 (APC 0.2%), and then significantly declining rates from 2003–2010 (APC, −2.0%).
Figure 2.

Age-adjusted mortality trends for all malignant cancers among children younger than 20 years of age in the United States from 1975 through 2010 with annual percentage change (APC) by joinpoint segments and estimated averted deaths per year. “*” indicate that the slope of the joinpoint segment is statistically different from zero, P<0.05.
Figure 3.
Age-adjusted mortality trends for all malignant cancers among children younger than 20 years of age in the United States from 1975 through 2010, with annual percentage changes (APCs) for joinpoint segments. “*” indicate that the slope of the joinpoint segment is statistically different from zero, P<0.05. Green line is for leukemias and lymphomas, and blue line is for all other cancer sites.
Looking at more discrete disease groupings, multiple joinpoints were present for leukemias (< 20 years), identifying periods of relatively rapid decline in mortality (APC, −3.2% to −5.0%) for 1975–89, 1992–98, and 2001–10 that were interspersed with periods of relatively stable mortality rates for 1989–92 (−0.8%) and 1998–2001 (APC, +1.1%) (Supplemental Table II). For both Hodgkin lymphoma and NHL (< 20 years), there were no joinpoints, with APCs for each exceeding −4.0% for each for 1975–2010. Among solid tumors, gonadal tumors and bone tumors showed joinpoints, with the first joinpoint period extending from 1975 to approximately 1990 for each with APCs of −8.3% and −3.1%, respectively. This period coincides with the time during which cisplatin-based regimens became a component of frontline standard therapy for pediatric germ cell tumors and osteosarcoma. Subsequently, declines in mortality were slower for both tumor types (−3.2% and −0.3%, respectively). For kidney tumors (< 15 years) there were also two joinpoint segments, with the first from 1975–92 showing a more rapid decline (APC, −3.9%) than the latter from 1992–2010 (APC, −1.3%). For some other solid tumors, there were significant declines in mortality rates from 1975–2010, but without joinpoints, including: neuroblastoma (< 15 years: APC, −1.8%), brain tumors (APC, −1.1%), and liver tumors (APC, −1.1%). Soft tissue cancers showed a significant decline for the period from 1979–2010 (APC, −1.0%).
We also determined the number of deaths averted as a result of advances in treatment for children with cancer since 1975. Assuming that the 1975 baseline persisted, an estimated 47,871 childhood malignant cancer deaths were averted from 1975 through 2010, with over 2300 cancer deaths averted in 2010 (Figure 2). Twenty-six percent of the averted deaths were for the 15–19 year population.
Survival correlates of declines in mortality
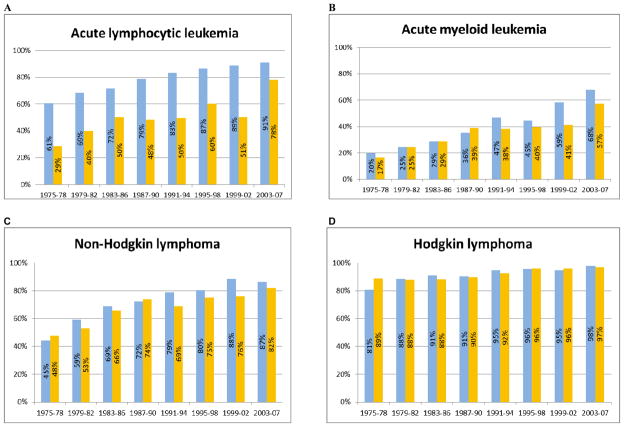
5-year survival rates have increased to 83–84% for all age groups < 20 years of age for 2003–07 (Figure 4). 5-year survival rates for ALL for children < 15 years have increased to 91%, while survival rates have increased to 78% for 15–19 year olds with ALL (Figure 5). Even when patients with ALL are excluded from the survival analyses, 5-year survival remains above 80% (82% for < 20 years of age). For AML, survival rates have increased to 68% for children < 15 years and to 57% for 15–19 year olds, while for Hodgkin lymphoma 5-year survival rates are 98% and 97%, respectively, for the < 15 years and 15–19 years age cohorts. For NHL, 5-year survival rates exceed 80% for all age groups (Figure 5). For solid tumors, 5-year survival rates are approximately 90% or greater for Wilms tumor, neuroblastoma (< 1 year), and germ cell tumors (Supplemental Figure 1). For neuroblastoma > 1year, 5-year survival rates have increased to 68%. Supplemental Figures 2 and 3 provide 5-year survival rates for 1975–78 through 2003–07 for CNS tumors and sarcomas (bone and soft tissue), respectively.
Figure 4.

Five-year relative survival, all malignant cancers combined, children 0–4, 5–14 and 15–19 years of age at diagnosis in SEER 9 registries during four year time periods from 1975–1978 to 2003–2007 with follow-up through 2010.
Figure 5.
Five-year relative survival, hematopoietic cancers among children <15 and 15–19 years of age at diagnosis in SEER 9 registries, 1975–1978 to 2003–2007 with follow-up through 2010: (A) Acute lymphoblastic leukemia; (B) Acute myeloid leukemia; (C) Non-Hodgkin lymphoma; and (D) Hodgkin lymphoma
Discussion
The significant decline in childhood and adolescent cancer mortality in the early years of the 21st century is reassuring in light of concerns that childhood cancer mortality rates had plateaued after decades of consistent decline.1, 2 Our report documents a plateau in mortality rates from 1998–2002, which we interpret as reflecting a period of several years in which more effective treatments were not identified and broadly adopted for childhood cancers. The more recent results highlight both the importance of advances in pediatric oncology research during the past 10 to 15 years, as well as areas of limited progress in which paradigm-shifting therapies are critically needed in order for future progress to occur. The declines in mortality cannot be explained by reduced incidence of childhood cancer during this period, as incidence for childhood cancers trended upward from 2000 to 2010 (Supplemental Figure 4).
Stiller, et al., have noted that while diffusion of breakthrough developments in the treatment of adult cancers can take an extended period, the diffusion of improved treatment regimens occurs quickly for childhood cancers.5 This results in part from the widespread participation of children in clinical trials developed by experts in which the control arm represents best available therapy. Additionally, children not enrolled in clinical trials are likely to be treated as per the control arm of the current (or most recent) clinical trial. The improved outcome for children with ALL likely illustrates this effect, as discoveries from Children’s Oncology Group (COG) clinical trials in the late 1990s identified more effective treatment regimens that then became control arms for clinical trials in 2000 through 2010. These advances included the use of post-induction treatment with intensified courses of methotrexate and asparaginase as well as the use of dexamethasone throughout therapy for children < 10 years of age.6–8 Further advances through COG clinical trials in the past decade included determining that high-dose methotrexate was more effective than an alternative method of methotrexate administration for children with high-risk, B-precursor ALL.9 Additionally, the AALL0031 COG clinical trial conducted from 2002 to 2006 and first reported in 2007 demonstrated that the Bcr-Abl inhibitor, imatinib, markedly improves outcome when added to standard chemotherapy for children with Ph+ ALL.10, 11 For solid tumors, a COG phase 3 trial determined that dose-intensification through interval compression improved outcome for Ewing sarcoma, which may have contributed to the nominal decline in mortality for bone tumors from 2000–2010.12
There have been concerns that the improvements in outcome observed for many childhood cancers have not been observed for adolescents and young adults with cancer.13 The results we report show that from 2000–2010, mortality rates for adolescents with cancer decreased in a similar manner as for children < 15 years of age. Of particular note, adolescents with ALL showed a greater decline in mortality compared to children < 15 years of age. It is likely that broader acceptance of the improved efficacy of pediatric-based treatment approaches compared to adult-based treatment regimens for ALL played a role in this improvement.14, 15
There are clear opportunities for further declines in mortality for ALL, AML, NHL, and Hodgkin lymphoma based on highly active agents in development for these diagnoses. For ALL, antibody-based therapies such as blinatumomab, inotuzumab ozogamicin, and SAR3419 are under development for adult leukemias and lymphomas and have the potential for showing high level activity for pediatric ALL.16 For AML, COG initiated a clinical trial in 2009 combining arsenic trioxide with tretinoin for newly diagnosed APL, and based on adult studies with this combination it is likely that treatment failure will be reduced to a very small percentage of patients achieving remission.17, 18 For anaplastic large cell lymphoma (ALCL), both crizotinib and brentuximab vedotin have shown objective response rates in relapsed/refractory patients well above 50%.19, 20 Incorporating one or both of these agents into standard frontline chemotherapy for ALCL (which is effective for approximately 70% of newly diagnosed patients) has the potential to further improve outcome. Reliably identifying the effectiveness of these novel agents will require well-designed clinical trials that are implemented expeditiously and that are enthusiastically supported by the pediatric oncology research community.
While opportunities for improving outcome are readily apparent for the hematological malignancies, they are less apparent for solid tumors although there are exceptions. For neuroblastoma, the observation that the GD2-targeted monoclonal antibody ch14.18 improved outcome for children with neuroblastoma suggests that declines in neuroblastoma mortality can be anticipated as the impact of widespread use of ch14.18 for high-risk neuroblastoma is realized.21 Developing novel immunotherapy approaches is an important line of future research for childhood solid tumors and brain tumors.22–24
For childhood and adolescent solid tumors and brain cancers, large scale genomic studies are being conducted, in part with the goal of identifying “actionable” recurring mutations for specific cancers that can be used to guide future clinical research for these cancers. However, to date few such mutations have been identified. There are exceptions to this generalization, such as the presence of activating BRAF fusion genes and point mutations in children with low-grade gliomas.25–27 However, the paucity of recurring mutations in kinase genes and other “actionable” genes means that many of the kinase inhibitors and other targeted agents that are being developed for adult cancers may have a limited role for most pediatric cancers. Furthermore, the emerging results from genomic studies highlight the distinctive molecular characteristics of childhood cancers compared to adult cancers, as illustrated by the differing mutations that are found in adult and pediatric high grade gliomas as well as the chromosomal translocations specific for pediatric sarcomas illustrate this point.28–32
Given the distinctive genomic alterations associated with many childhood cancers compared to adult cancers, it will be increasingly important to identify and develop agents that are able to selectively block the oncogenic activity of these unique childhood cancer therapeutic targets. Development of ch14.18 for neuroblastoma, which is now moving towards regulatory approval, may provide a blueprint for how such agents can be developed.21 As well, recent legislative initiatives such as the “Creating Hope Act” may stimulate development of childhood cancer specific agents by providing an incentive to industry by issuing transferrable high priority review vouchers when drugs are developed for specific pediatric rare diseases including cancers.33 More generally regarding the role of FDA, provisions of the 2012 Food and Drug Administration Safety and Innovation Act (FDASIA), including the permanent reauthorization of the Best Pharmaceuticals for Children Act (BPCA) and the Pediatric Research Equity Act (PREA), have the potential to accelerate the evaluation of new therapies for childhood cancers since there is now a requirement to consider and discuss pediatric development plans at “end of phase 2 meetings” for new agents under development for adult malignancies. This should lead to the generation and submission of Written Requests for pediatric evaluations at an earlier point in the drug development timeline.
Reducing childhood cancer mortality is not the sole goal of childhood cancer research, as this goal must be accomplished within the context of high quality of life opportunities for survivors. Some progress has been made in improving the quality of survivorship, including the omission of cranial radiation for most children with ALL, reductions in anthracycline cumulative dose for some cancers and use of cardioprotectants to reduce long-term cardiac toxicity, and reductions in radiation dose and field volume for some lymphomas and solid tumors. That said, a number of children continue to receive treatments that are known to induce cognitive impairment, ototoxicity, bone damage, impaired fertility, and other long-term deleterious effects. As curative treatments are identified for more and more children and adolescents, it is essential to continue research to refine the treatments to reduce the long-term burden of therapy and to monitor survivors for long-term sequelae of their cancer therapy.
In conclusion, significant declines in childhood cancer mortality occurred in the first decade of the 21st century across the pediatric age spectrum. However, the acute and long-term burden of cancer therapy remain high for some children who are cured of their cancer and there remain many challenges to more effectively treating those cancers for which current frontline therapy is not adequate. These remaining challenges highlight the importance of continued cooperative research activities to identify more effective treatments that provide cure and high quality survival for all children with cancer.
Supplementary Material
Acknowledgments
Funding: National Cancer Institute U10CA98543 (Adamson)
Footnotes
The authors have no financial disclosures to declare.
References
- 1.Smith MA, Seibel NL, Altekruse SF, et al. Outcomes for children and adolescents with cancer: challenges for the twenty-first century. J Clin Oncol. 2010;28(15):2625–34. doi: 10.1200/JCO.2009.27.0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pritchard-Jones K, Pieters R, Reaman GH, et al. Sustaining innovation and improvement in the treatment of childhood cancer: lessons from high-income countries. Lancet Oncol. 2013;14(3):e95–e103. doi: 10.1016/S1470-2045(13)70010-X. [DOI] [PubMed] [Google Scholar]
- 3.National Cancer Institute. Surveillance, Epidemiology, and End Results (SEER) [accessed 09/01/2012, 2012];Population Estimates Used in NCI’s SEER*Stat Software. http://seer.cancer.gov/popdata/methods.html.
- 4.Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19(3):335–51. doi: 10.1002/(sici)1097-0258(20000215)19:3<335::aid-sim336>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 5.Stiller CA, Kroll ME, Pritchard-Jones K. Population survival from childhood cancer in Britain during 1978–2005 by eras of entry to clinical trials. Ann Oncol. 2012;23(9):2464–9. doi: 10.1093/annonc/mds183. [DOI] [PubMed] [Google Scholar]
- 6.Bostrom BC, Sensel MR, Sather HN, et al. Dexamethasone versus prednisone and daily oral versus weekly intravenous mercaptopurine for patients with standard-risk acute lymphoblastic leukemia: a report from the Children’s Cancer Group. Blood. 2003;101(10):3809–17. doi: 10.1182/blood-2002-08-2454. [DOI] [PubMed] [Google Scholar]
- 7.Matloub Y, Bostrom BC, Hunger SP, et al. Escalating intravenous methotrexate improves event-free survival in children with standard-risk acute lymphoblastic leukemia: a report from the Children’s Oncology Group. Blood. 2011;118(2):243–51. doi: 10.1182/blood-2010-12-322909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seibel NL, Steinherz PG, Sather HN, et al. Early postinduction intensification therapy improves survival for children and adolescents with high-risk acute lymphoblastic leukemia: a report from the Children’s Oncology Group. Blood. 2008;111(5):2548–55. doi: 10.1182/blood-2007-02-070342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Larsen EC, Salzer WL, Devidas M, et al. Comparison of high-dose methotrexate (HD-MTX) with Capizzi methotrexate plus asparaginase (C-MTX/ASNase) in children and young adults with high-risk acute lymphoblastic leukemia (HR-ALL): A report from the Children’s Oncology Group Study AALL0232. J Clin Oncol. 2011;29(suppl):abstr 3. [Google Scholar]
- 10.Schultz KR, Bowman WP, Aledo A, et al. Improved early event-free survival with imatinib in Philadelphia chromosome-positive acute lymphoblastic leukemia: a children’s oncology group study. J Clin Oncol. 2009;27(31):5175–81. doi: 10.1200/JCO.2008.21.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schultz KR, Bowman WP, Slayton W, et al. Improved Early Event Free Survival (EFS) in Children with Philadelphia Chromosome-Positive (Ph+) Acute Lymphoblastic Leukemia (ALL) with Intensive Imatinib in Combination with High Dose Chemotherapy: Childrens Oncology Group (COG) Study AALL0031. Blood. 2007;110(11):Abstr #4. [Google Scholar]
- 12.Womer RB, West DC, Krailo MD, et al. Randomized controlled trial of interval-compressed chemotherapy for the treatment of localized Ewing sarcoma: a report from the Children’s Oncology Group. J Clin Oncol. 2012;30(33):4148–54. doi: 10.1200/JCO.2011.41.5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferrari A, Montello M, Budd T, Bleyer A. The challenges of clinical trials for adolescents and young adults with cancer. Pediatr Blood Cancer. 2008;50(5 Suppl):1101–4. doi: 10.1002/pbc.21459. [DOI] [PubMed] [Google Scholar]
- 14.Stock W, La M, Sanford B, et al. What determines the outcomes for adolescents and young adults with acute lymphoblastic leukemia treated on cooperative group protocols? A comparison of Children’s Cancer Group and Cancer and Leukemia Group B studies. Blood. 2008;112(5):1646–54. doi: 10.1182/blood-2008-01-130237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nachman JB, La MK, Hunger SP, et al. Young adults with acute lymphoblastic leukemia have an excellent outcome with chemotherapy alone and benefit from intensive postinduction treatment: a report from the children’s oncology group. J Clin Oncol. 2009;27(31):5189–94. doi: 10.1200/JCO.2008.20.8959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kantarjian H, Thomas D, Wayne AS, O’Brien S. Monoclonal Antibody-Based Therapies: A New Dawn in the Treatment of Acute Lymphoblastic Leukemia. J Clin Oncol. 2012 doi: 10.1200/JCO.2012.41.6768. Published online ahead of publication (8/14/2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iland HJ, Bradstock K, Supple SG, et al. All-trans-retinoic acid, idarubicin, and IV arsenic trioxide as initial therapy in acute promyelocytic leukemia (APML4) Blood. 2012;120(8):1570–80. doi: 10.1182/blood-2012-02-410746. [DOI] [PubMed] [Google Scholar]
- 18.Powell BL, Moser B, Stock W, et al. Arsenic trioxide improves event-free and overall survival for adults with acute promyelocytic leukemia: North American Leukemia Intergroup Study C9710. Blood. 2010;116(19):3751–7. doi: 10.1182/blood-2010-02-269621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mosse YP, Balis FM, Lim MS, et al. Efficacy of crizotinib in children with relapsed/refractory ALK-driven tumors including anaplastic large cell lymphoma and neuroblastoma: A Children’s Oncology Group phase I consortium study. J Clin Oncol. 2012;30(suppl):abstr 9500. [Google Scholar]
- 20.Pro B, Advani R, Brice P, et al. Brentuximab Vedotin (SGN-35) in Patients With Relapsed or Refractory Systemic Anaplastic Large-Cell Lymphoma: Results of a Phase II Study. J Clin Oncol. 2012;30(18):2190–6. doi: 10.1200/JCO.2011.38.0402. [DOI] [PubMed] [Google Scholar]
- 21.Yu AL, Gilman AL, Ozkaynak MF, et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med. 2010;363(14):1324–34. doi: 10.1056/NEJMoa0911123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Capitini CM, Gottschalk S, Brenner M, Cooper LJ, Handgretinger R, Mackall CL. Highlights of the second international conference on “Immunotherapy in Pediatric Oncology”. Pediatr Hematol Oncol. 2011;28(6):459–60. doi: 10.3109/08880018.2011.596615. [DOI] [PubMed] [Google Scholar]
- 23.Orentas RJ, Lee DW, Mackall C. Immunotherapy targets in pediatric cancer. Front Oncol. 2012;2:3. doi: 10.3389/fonc.2012.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee DW, Barrett DM, Mackall C, Orentas R, Grupp SA. The future is now: chimeric antigen receptors as new targeted therapies for childhood cancer. Clin Cancer Res. 2012;18(10):2780–90. doi: 10.1158/1078-0432.CCR-11-1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dougherty MJ, Santi M, Brose MS, et al. Activating mutations in BRAF characterize a spectrum of pediatric low-grade gliomas. Neuro Oncol. 2010;12(7):621–30. doi: 10.1093/neuonc/noq007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pfister S, Janzarik WG, Remke M, et al. BRAF gene duplication constitutes a mechanism of MAPK pathway activation in low-grade astrocytomas. J Clin Invest. 2008;118(5):1739–49. doi: 10.1172/JCI33656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schindler G, Capper D, Meyer J, et al. Analysis of BRAF V600E mutation in 1,320 nervous system tumors reveals high mutation frequencies in pleomorphic xanthoastrocytoma, ganglioglioma and extra-cerebellar pilocytic astrocytoma. Acta Neuropathol. 2011;121(3):397–405. doi: 10.1007/s00401-011-0802-6. [DOI] [PubMed] [Google Scholar]
- 28.Khuong-Quang DA, Buczkowicz P, Rakopoulos P, et al. K27M mutation in histone H3. 3 defines clinically and biologically distinct subgroups of pediatric diffuse intrinsic pontine gliomas. Acta Neuropathol. 2012;124(3):439–47. doi: 10.1007/s00401-012-0998-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schwartzentruber J, Korshunov A, Liu XY, et al. Driver mutations in histone H3. 3 and chromatin remodelling genes in paediatric glioblastoma. Nature. 2012;482(7384):226–31. doi: 10.1038/nature10833. [DOI] [PubMed] [Google Scholar]
- 30.Wu G, Broniscer A, McEachron TA, et al. Somatic histone H3 alterations in pediatric diffuse intrinsic pontine gliomas and non-brainstem glioblastomas. Nat Genet. 2012;44(3):251–3. doi: 10.1038/ng.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455(7216):1061–8. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parsons DW, Jones S, Zhang X, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321(5897):1807–12. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Connor E, Cure P. “Creating hope” and other incentives for drug development for children. Sci Transl Med. 2011;3(66):66cm1. doi: 10.1126/scitranslmed.3001707. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.