Abstract
In this study, we compared MSCs from breast and abdominal tissue in terms of their expression of genes deemed important in the support of breast cancer growth and their effect on gene profile of macrophages after coculture. In addition, we investigated the role of MSCs, alone or in combination with macrophages, on proliferation of breast cancer cell lines. Our results show that MSCs derived from breast and abdominal adipose tissues have a comparable gene expression profile, have similar effect on gene expression of macrophages, and are comparable in supporting breast cancer cell line proliferation.
Keywords: Mesenchymal stromal, stem cells, Macrophages, Breast cancer, Tumor cell biology, Immunology, immunobiology
BACKGROUND
Breast cancer arises in an adipose-containing tissue rich in stromal components including mesenchymal stromal/stem cells (MSC; refs.1, 2) and macrophages (3, 4). Both of these cell types are assumed to play a significant role in progression of breast cancer through their interactions with tumor cells (5–7). However, derivation of MSCs from breast adipose tissue remains challenging due to limited availability of samples and, thus, alternative sources such as bone marrow or abdominal adipose MSCs are being used for research purposes. Based on our recent findings on the ability of MSCs to alter phenotypic and functional properties of macrophages (8), we hypothesized that MSCs residing in breast adipose tissue preferentially convert breast tissue macrophages into an immunophenotype that is favorably supporting growth of breast cancer cells and, conversely, that non-breast adipose tissue MSCs (e.g., subcutaneous abdominal adipose tissues) and macrophages together provide an inhospitable microenvironment for the growth of tumor cells.
METHODS
Derivation of MSCs and macrophages
Bone marrow (BM) MSCs were isolated from BM filters as previously described (9). Adipose tissues collected from cosmetic mammary reduction surgeries (breast adipsoe MSCs) or from abdominoplasty surgery (abdominal adipose MSCs) were used for MSC derivation. All of the procedures were approved by the University of Wisconsin Institutional Review Board. Flow cytometry and differentiation assays were conducted on cells at passage 4 to verify their MSC identity according to established criteria (10). MSCs between passage 4 and 6 were used for experiments. Macrophages were derived from peripheral blood monocytes as described previously (8).
Quantitative reverse-transcriptase polymerase chain reaction assay
RNA isolation and cDNA synthesis were carried out as previously described (9). In the case of MSC cocultured macrophages, cells were isolated by CD14-based magnetic bead-based separation. RNA was isolated by the RNeasy micro kit (Qiagen, Germantown, MD, USA) and then converted to cDNA using Quantitect reverse transcriptase kit (Qiagen). Verified primers were purchased from Qiagen and the threshold cycle (Ct) value for each gene was normalized by the average Ct number of three housekeeping genes (RN18S1, GAPDH, and ACTB).
Proliferation assay of breast cancer cells
Breast cancer cell lines MCF7 and MDA-MB-231 were purchased from the American Type Culture Collection (ATCC) and cultured according to the recommended protocol. MSCs were plated into six-well plates at a concentration of 100,000 cells per well. In the case of MSC-macrophage coculture, macrophages were generated by culturing CD14-positive cells for 7 days and, then, MSCs were added (8). Breast cancer cell lines were stained with 10 μmol/L CFSE and were added to MSCs and/or macrophages at a density of 100,000 cells per well and then cultured for 3 days. Three experiments were conducted for each condition. After coculture, cells were harvested using TrypLE (Invitrogen, Carlsbad, CA, USA) and stained with CD14 and CD90 for gating out macrophages and MSCs, respectively. Data acquisition and analyses were conducted with an Accuri C6 Flow Cytometer (Accuri Cytometer, Ann Arbor, MI, USA) and the ModFit Software Program 3.1 (Verity Software, Topsham, ME, USA) to calculate the proliferation index. The proliferation index is the total number of cells divided by the number of cells that underwent division. The relative proliferation index was then calculated by dividing proliferation index by that of the cell line-only control to compensate for variance between batches as described previously (9).
RESULTS
Comparison of breast and abdominal adipose tissue-derived MSCs
Both breast and abdominal adipose MSCs used in this study were shown, by flow cytometry, to express the same cell surface markers as BM MSCs, and were able to differentiate into three of the mesenchymal lineages (bone, fat, and cartilage), confirming their identity as MSCs (11). Real-time qPCR analysis of the expression of genes potentially important to breast cancer progression, by MSCs, showed that the IL-1b expression level was higher in abdominal adipose MSCs (n = 8) compared with breast adipose MSCs (n = 4; Figure 1). However, other genes tested were not expressed at statistically significant different levels between abdominal adipose and breast adipose MSCs.
Figure 1.
Comparison of gene expression between MSCs derived from abdominal or breast adipose tissues. Expression level of genes tested were statistically similar between breast adipose MSCs and abdominal adipose MSCs except for IL-1b, which was expressed at a higher level in abdominal adipose MSCs.
Comparison of gene expression by macrophages cocultured with breast or abdominal adipose MSCs
In addition to MSCs, macrophages are another major component of cancerous tissues, including, breast cancer. Thus, we compared the gene expression profile of macrophages after their coculture with breast (n = 6) or abdominal adipose tissue-derived MSCs (n = 6) using qPCR. Macrophages cultured alone were used as control for comparison (n = 3). Macrophages cocultured with breast and abdominal adipose MSCs expressed higher levels of VEGF A, VEGF C, SER-PINE1, FGF2, IL-1b and IL-6 compared with macrophages alone but in the case of abdominal adipose MSCs increased levels of IL-1b and IL-6 did not reach statistical significance. However, the levels of expression of these genes were not statistically significant between macrophages cocultured with breast versus abdominal adipose tissue-derived MSCs (Figure 2).
Figure 2.
The effect of coculture with MSCs on the macrophage gene expression profile. Coculture of MSCs with macrophages led to increased expression of FGF2, VEGF A, VEGF C, and SERPINE1 in macrophages compared with control macrophages. Coculture of breast adipose MSC induced increased expression of IL-1b and IL-6 in macrophages in addition to the genes mentioned earlier. However, comparison between breast adipose MSC cocultured macrophages and abdominal adipose MSC cocultured macrophages did not find any statistically significant differences.
MSCs and macrophages separately increase proliferation of breast cancer cell lines with, but show no additive effect in, MSC/macrophage cocultures
Compared with the MCF7 cell line-only control [Relative proliferation index (RPI) = 1.00], BM MSCs (1.21 ± 0.09), breast adipose MSCs (1.37 ± 0.16), abdominal adipose MSCs (1.29 ± 0.07), and macrophages (1.28 ± 0.13) all increased proliferation of the MCF7 cell line in a statistically significant manner (Figure 3A). In the case of MDA-MB-231 cell line, a similar increase was noted for BM MSCs (1.14 ± 0.04), breast adipose MSCs (1.09 ± 0.02), abdominal adipose MSCs (1.08 ± 0.02), and macrophages (1.12 ± 0.02; Figure 3B). Although both MSCs and macrophages were capable of increasing proliferation of breast cancer cell lines individually, there was no additive or synergistic effect observed in the MCF7 cell lines. In the case of MDA-MB-231 cells, slight synergistic effect on the proliferation of cancer cell line was observed in cocultures of macrophages with either breast or abdominal adipose MSCs (Figure 3).
Figure 3.
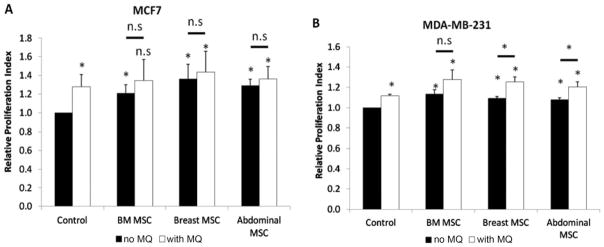
Proliferation of breast cancer cell lines in coculture with MSCs and/or macrophages. MCF7 (A) and MDA-MB-231 (B) cell lines proliferated more in the presence of MSCs or macrophages. Addition of macrophages to MSCs did not increase proliferation in a statistically significant fashion across all conditions for MCF7. Only in the case of MDA-MB-231, addition of macrophages increased their proliferation in coculture with adipose MSCs (both breast and abdominal) compared to coculture with breast and abdominal adipose MSCs alone in a statistically significant way.
DISCUSSION
Similar to other reports, we show that MSCs can support proliferation of breast cancer cells regardless of their origin (5, 12). However, in contrast to our recent finding on the synergistic effect of MSCs and macrophages in the support of multiple myeloma tumor growth (9), the addition of macrophages did not consistently lead to increased proliferation of breast cancer cell lines in this study. We initiated this study based on the fact that breast cancers metastasize more frequently to stroma-rich organs such as bone, lung, liver, and contralateral breast whereas metastasis to non-breast stroma-rich adipose tissues, such as subcutaneous fat, is a rare phenomenon (13–15). However, in retrospect, we acknowledge that our MSC derivation methodology from breast tissue could have favored derivation of breast fat MSCs and not true stromal components of breast ducts or lobules—the actual sites of origination of breast cancer (16, 17). In addition, it is a well-known fact that obesity increases the risk of many cancers, including postmenopausal breast cancer (18). However, to our knowledge, there is no data on whether obesity also increases the risk of metastasis to adipose tissues. We believe investigation of the milieu of adipose tissue environment could provide important clues about the biology of metastasis in breast cancer.
CONCLUSIONS
We hypothesized that breast-derived MSCs have different effect on the growth of breast cancer cell lines compared with non-breast adipose tissue-derived MSCs (i.e., abdominal MSCs) or BM MSCs which, due to ease of access and availability, have been cells of choice in many in vitro assays. However, contrary to our hypothesis, breast and abdominal adipose MSCs were roughly similar in terms of expression of a panel of genes, with the exception of IL-1b; that was higher in abdominal adipose MSCs. Both adipose MSCs upregulated FGF2, SERPINE1, VEGFA, and VEGFC in macrophages after coculture, and those genes have been associated with progression of breast cancer in previous reports (19, 20). However, there were no statistically significant differences between breast adipose MSC cocultured macrophages and abdominal adipose MSC cocultured macrophages (Figure 2). Finally, proliferation assays showed that macrophages and MSCs were both supportive of proliferation of cancer cells with no differences observed between breast and abdominal adipose MSCs. Further investigation of MSCs derived from periductal tissues of breast is warranted.
Acknowledgments
No ethical approval was required for this study. JK, LE, and BD carried out proliferation assay and quantitative PCR analysis. JK and SH conducted isolation of MSCs. JK and PH designed and analyzed experiments. All authors read and approved the final manuscript. The authors thank Kreg Grindle for critical review of the manuscript.
This work was supported by the Department of Defense Concept Award (W81XWH-09–1–0532) to Peiman Hematti. SE Hanson was a recipient of NIH T32 Physician-Scientist Training Grant CA009614.
Footnotes
This work is not subject to United States copyright laws.
DECLARATION OF INTEREST
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Hu M, Polyak K. Molecular characterisation of the tumour microenvironment in breast cancer. Eur J Cancer. 2008;44(18):2760–2765. doi: 10.1016/j.ejca.2008.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhao M, Dumur CI, Holt SE, Beckman MJ, Elmore LW. Multipotent adipose stromal cells and breast cancer development: think globally, act locally. Mol Carcinog. 2010;49(11):923–927. doi: 10.1002/mc.20675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pollard JW. Macrophages define the invasive microenvironment in breast cancer. J Leukoc Biol. 2008;84(3):623–630. doi: 10.1189/jlb.1107762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mantovani A, Marchesi F, Porta C, Sica A, Allavena P. Inflammation and cancer: breast cancer as a prototype. Breast (Edinburgh, Scotland) 2007;16(Suppl 2):S27–S33. doi: 10.1016/j.breast.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 5.Hombauer H, Minguell JJ. Selective interactions between epithelial tumour cells and bone marrow mesenchymal stem cells. Br J Cancer. 2000;82(7):1290–1296. doi: 10.1054/bjoc.1999.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu X, Mulcahy LA, Mohammed RA, Lee AH, Franks HA, Kilpatrick L, Yilmazer A, Paish EC, Ellis IO, Patel PM, Jackson AM. IL-17 expression by breast-cancer-associated macrophages: IL-17 promotes invasiveness of breast cancer cell lines. Breast Cancer Res. 2008;10(6):R95. doi: 10.1186/bcr2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fierro FA, Sierralta WD, Epunan MJ, Minguell JJ. Marrow-derived mesenchymal stem cells: role in epithelial tumor cell determination. Clin Exp Metastasis. 2004;21(4):313–319. doi: 10.1023/b:clin.0000046130.79363.33. [DOI] [PubMed] [Google Scholar]
- 8.Kim J, Hematti P. Mesenchymal stem cell-educated macrophages: a novel type of alternatively activated macrophages. Exp Hematol. 2009;37(12):1445–1453. doi: 10.1016/j.exphem.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim J, Denu RA, Dollar BA, Escalante LE, Kuether JP, Callander NS, Asimakopoulos F, Hematti P. Macrophages and mesenchymal stromal cells support survival and proliferation of multiple myeloma cells. Br J Haematol. 2012;158(3):336–346. doi: 10.1111/j.1365-2141.2012.09154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 11.Hanson SE, Kim J, Hematti P. Comparative analysis of adipose derived mesenchymal stem cells isolated from abdominal and breast tissue. Aesthetic Surg J. 2013;33(6):888–898. doi: 10.1177/1090820X13496115. [DOI] [PubMed] [Google Scholar]
- 12.Yan XL, Fu CJ, Chen L, Qin JH, Zeng Q, Yuan HF, Nan X, Chen HX, Zhou JN, Lin YL, Zhang XM, Yu CZ, Yue W, Pei XT. Mesenchymal stem cells from primary breast cancer tissue promote cancer proliferation and enhance mammosphere formation partially via EGF/EGFR/Akt pathway. Breast Cancer Res Treat. 2012;132(1):153–164. doi: 10.1007/s10549-011-1577-0. [DOI] [PubMed] [Google Scholar]
- 13.Porter GJ, Evans AJ, Pinder SE, James JJ, Cornford EC, Burrell HC, Chan SY, Cheung KL, Robertson JF. Patterns of metastatic breast carcinoma: influence of tumour histological grade. Clin Radiol. 2004;59(12):1094–1098. doi: 10.1016/j.crad.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 14.Leong SP, Cady B, Jablons DM, Garcia-Aguilar J, Reintgen D, Jakub J, Pendas S, Duhaime L, Cassell R, Gardner M, Giuliano R, Archie V, Calvin D, Mensha L, Shivers S, Cox C, Werner JA, Kitagawa Y, Kitajima M. Clinical patterns of metastasis. Cancer Metastasis Rev. 2006;25(2):221–232. doi: 10.1007/s10555-006-8502-8. [DOI] [PubMed] [Google Scholar]
- 15.Krathen RA, Orengo IF, Rosen T. Cutaneous metastasis: a meta-analysis of data. South Med J. 2003;96(2):164–167. doi: 10.1097/01.SMJ.0000053676.73249.E5. [DOI] [PubMed] [Google Scholar]
- 16.Wellings SR, Jensen HM. On the origin and progression of ductal carcinoma in the human breast. J Natl Cancer Inst. 1973;50(5):1111–1118. doi: 10.1093/jnci/50.5.1111. [DOI] [PubMed] [Google Scholar]
- 17.Wellings SR, Jensen HM, Marcum RG. An atlas of sub-gross pathology of the human breast with special reference to possible precancerous lesions. J Natl Cancer Inst. 1975;55(2):231–273. [PubMed] [Google Scholar]
- 18.Hunter DJ, Willett WC. Diet, body size, and breast cancer. Epidemiol Rev. 1993;15(1):110–132. doi: 10.1093/oxfordjournals.epirev.a036096. [DOI] [PubMed] [Google Scholar]
- 19.Dirix LY, Vermeulen PB, Pawinski A, Prove A, Benoy I, De Pooter C, Martin M, Van Oosterom AT. Elevated levels of the angiogenic cytokines basic fibroblast growth factor and vascular endothelial growth factor in sera of cancer patients. Br J Cancer. 1997;76(2):238–243. doi: 10.1038/bjc.1997.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hansen S, Overgaard J, Rose C, Knoop A, Laenkholm AV, Andersen J, Sorensen FB, Andreasen PA. Independent prognostic value of angiogenesis and the level of plasminogen activator inhibitor type 1 in breast cancer patients. Br J Cancer. 2003;88(1):102–108. doi: 10.1038/sj.bjc.6600662. [DOI] [PMC free article] [PubMed] [Google Scholar]




