Abstract
Impulsivity, and in particular the negative urgency aspect of this trait, is associated with poor inhibitory control when experiencing negative emotion. Individual differences in aspects of impulsivity have been correlated with striatal dopamine D2/D3 receptor availability and function. This multi-modal pilot study used both positron emission tomography (PET) and functional magnetic resonance imaging (fMRI) to evaluate dopaminergic and neural activity, respectively, using modified versions of the monetary incentive delay task. Twelve healthy female subjects underwent both scans and completed the NEO Personality Inventory Revised to assess Impulsiveness (IMP). We examined the relationship between nucleus accumbens (NAcc) dopaminergic incentive/reward release, measured as a change in D2/D3 binding potential between neutral and incentive/reward conditions with [11C]raclopride PET, and blood oxygen level-dependent (BOLD) activation elicited during the anticipation of rewards, measured with fMRI. Left NAcc incentive/reward dopaminergic release correlated with anticipatory reward activation within the medial prefrontal cortex (mPFC), left angular gyrus, mammillary bodies, and left superior frontal cortex. Activation in the mPFC negatively correlated with IMP and mediated the relationship between IMP and incentive/reward dopaminergic release in left NAcc. The mPFC, with a regulatory role in learning and valuation, may influence dopamine incentive/reward release.
Keywords: Reward, mPFC, NAcc, Dopamine, Impulsivity, PET, fMRI
1. Introduction
Impulsivity has been proposed as a major endophenotype associated with disorders of behavioral control, such as substance use and pathological gambling, as well as co-morbid neuropsychiatric disorders, such as bipolar disorder and borderline personality disorder (Dick et al., 2010; Michalczuk et al., 2011; Zucker et al., 2011). Dimensions of impulsivity include sensation seeking, lack of premeditation, lack of persistence, and urgency (Congdon and Canli, 2005). This latter dimension, representing individual differences in the tendency to engage in ill-considered actions when experiencing intense emotion (Cyders and Smith, 2008), conceptually maps onto models where poor inhibitory control in the face of strong reward impulses leads to heightened motivation to obtain immediate gratification (positive urgency) or avoid immediate negative states (negative urgency; Robinson and Berridge, 2003; Crews and Boettiger, 2009).
As the mesolimbic dopamine (DA) system is associated with motivated responding, such as positive reinforcement of pleasurable effects (Le Moal and Simon, 1991; Fitzgerald et al., 1993; Koob and Le Moal, 2001; Johnson, 2010), recent studies have searched for a neural link relating this system to impulsive behaviors. Positron emission tomography (PET) studies with dopaminergic radioligands allow assessment of the reactivity of the DA system. Strikingly, studies examining amphetamine-induced striatal or ventral striatal (VS) dopamine release have observed a negative association with impulsivity, as measured by the NEO Personality Inventory Revised (NEO-PI-R) (Costa and Crae, 1985) neuroticism facet score of Impulsivity (IMP) (Oswald et al., 2007), but positive associations with impulsivity (Buckholtz et al., 2010b), as measured by the Barratt Impulsivity Scale (BIS-11) (Barratt et al., 1999). These contrasting results are consistent with the multi-dimensional conceptualization of impulsivity (Cyders and Smith, 2008; Zucker et al., 2011), with the BIS-11 reflecting a lack of deliberation or planning, and IMP reflecting urgency, particularly urgency in the face of negative emotions (Whiteside and Lynam, 2001). While both measures show relations to psychopathology, recent research has increasingly recognized the importance of negative urgency for substance use and gambling problems (Castellani and Rugle, 1995; Verdejo-García et al., 2008; Kaiser et al., 2012).
The monetary incentive delay (MID) task has been used in functional magnetic resonance imaging (fMRI) studies to probe incentive-reward responses in striatal regions, including the VS/nucleus accumbens (NAcc; Knutson et al., 2001a). The task requires participants to make rapid responses to gain varying amounts of money. Interestingly, individual differences in IMP have been positively related to VS and frontal cortex responses to reward notification during the MID (Bjork et al., 2008). Further, individual differences in VS activations assessed with fMRI during the MID were positively associated with DA system reactivity in the same region as measured by PET (Schott et al., 2008; Buckholtz et al., 2010a). The study of Schott et al. is of particular interest because rather than using a drug probe to induce DA release in the VS/NAcc, DA release was induced by performance of the MID during PET scanning. This allows for a more direct linkage between DA release and neural activity since both measures are tied to a similar behavioral probe. Correlations were observed between VS/NAcc DA release and fMRI activations during reward anticipation in striatal regions as well as the substantia nigra/ventral tegmental area, the origin of DA neurotransmission and DA release in the striatum (Schott et al., 2008). These findings are consistent with the hypothesis that dopaminergic activity plays a quantitative role in human mesolimbic reward processing such that the amount of DA released determines the incentive level in this circuitry. However, the study of Schott et al. did not test whether these effects were related to trait measures.
DA’s modulation of motivation is proposed to work via conditioning that facilitates consolidation of memory traces by signaling rewards according to prediction errors (Schultz, 1998), which is relevant to reward-related behaviors (Volkow et al., 2009). This bottom-up motivational system competes with, and may be regulated by, top-down cognitive control exerted by the orbitofrontal and dorsolateral regions of the prefrontal cortex (PFC) (Wise, 2002; Bechara, 2005; Volkow et al., 2007). Glutaminergic inputs from the PFC, as well as the thalamus, hippocampus and amygdala, to the NAcc provide presynaptic modulation of DA release in the NAcc. Other glutaminergic afferents from the PFC to the ventral tegmental area (VTA) act as a feedback loop to influence the dopaminergic pathways from the VTA to the NAcc to impact valence of motivation (Phillips et al., 2008). Interindividual variation in this prefrontal influence on ventral basal ganglia DA function has been suggested to underlie variability in impulsive behaviors, and the risk for the development of impulse-control disorders including substance abuse (Congdon and Canli, 2005; Urcelay and Dalley, 2011). Here we hypothesize that the PFC activity will influence DA release in the NAcc during a reward task, and that this activation will be associated with variations in trait impulsivity as measured with the IMP scale.
To test this hypothesis, we used PET and [11C]raclopride to measure DA release in the NAcc during a modified MID task. [11C]Raclopride binds to D2/D3 receptors in the striatum and is sensitive to endogenous striatal DA release (Mawlawi et al., 2001; Seeman et al., 2006) with reductions in receptor availability (BPND) scaling linearly with the amount of endogenous DA release (Breier et al., 1997). Based on this linear relationship, drug- and task-related reductions in DA radiologand binding are typically interpreted as indexing endogenous DA release, although other factors such as receptor internalization may also contribute to the observed effect (Laruelle, 2000; Zald et al., 2004). In the present study, we quantified the difference in D2/D3 BPND between a neutral and a rewarding condition as a measure of incentive/reward DA release. In addition, we examined neural responses in the same striatal regions, and other brain regions including the PFC, by having participants perform a similar MID task during an fMRI scan.
We first examined the association between NAcc incentive/reward DA release and fMRI neural response during reward anticipation. We next examined the relationship between the neuroimaging data and impulsive urgency as assessed by the IMP facet of the NEO-PI-R, which captures the negative urgency dimension associated with risky and addictive behaviors (Smith et al., 2007). To test our main hypotheses, we evaluated whether there was a mediating effect of PFC activity on the relationship between IMP and NAcc incentive/reward DA release.
2. Methods
2.1. Subjects
Twelve healthy right-handed, non-smoking women (age: range, mean ± standard deviation: 19.7–45.7, 30.9 ± 9.0 years) were recruited via advertisement. Participants had no history of, or current medical, neurological, or psychiatric illnesses, including substance abuse or dependence; had alcohol intake of less than five drinks/week; no family history of psychiatric disease in first-degree relatives; no current or recent (6 months) exposure to centrally active prescription or illicit drugs; and were asked not to drink alcohol for 48 h before scanning. Urine drug screens were performed immediately before imaging. The sample was restricted to women owing to known sex differences in striatal DA release (Becker, 1990, 1999; Andersen and Teicher, 2000; Walker et al., 2005; Love et al., 2012) and studied without regard to menstrual cycle phase, based on animal and human data showing that DA uptake and release do not vary across cycles (Nordstrom et al., 1998; Walker et al., 1999). However, we obtained plasma levels of estradiol and progesterone before scanning to examine potential relationships with DA measures. Two subjects reported oral contraceptive use and were excluded from hormone analyses. Protocols were approved by the Investigational Review Board and Radioactive Drug Research Committee of the University of Michigan; written informed consent was obtained from all subjects.
2.2. Impulsivity
Participants were administered the NEO PI-R (Costa and McCrae, 1985) with the impulsiveness facet (IMP) as the primary scale of interest. Subject IMP scores ranged from 9–21, mean 15.4 ± 3.4, consistent with data from population samples of comparable age, mean 15 ± 4 (Costa and McRae, 1992). Individuals endorsing less behavioral control, or lack of reflection, would have higher IMP scores (Fischer et al., 2004).
2.3. Hormone assays
Assays were performed on the IMMULITE 1000 system from Siemens Medical Solutions Diagnostic Division using all solid-phase, competitive chemiluminescent enzyme immunoassays: estradiol - LKE21; progesterone - LKPG1. Results, in units of pg/ml and ng/ml, respectively, were log-converted for normalization.
2.4. Experimental paradigm – PET
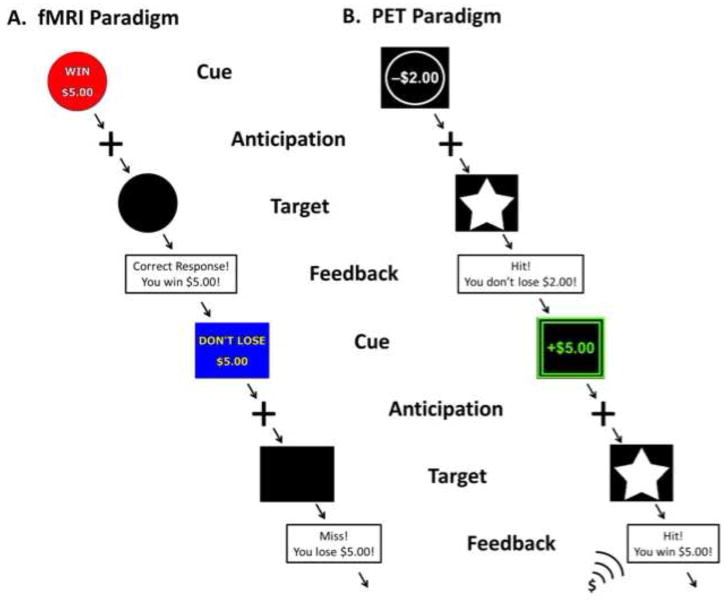
Each subject underwent a single 90-min PET scan with [11C]raclopride, a DA radiotracer with affinity for both D2 and D3 receptors (Seeman et al., 2006), during which they performed a modified version of the MID reward task divided into two conditions, reward and neutral (Pappata et al., 2002; Schott et al., 2008). Trials included an incentive cue, indicating the possibility of reward or the absence of a reward, followed by an anticipation delay. A target then appeared for a variable length of time during which the subject used a mouse-press response in an attempt to gain or avoid losing money; a schematic of the task is presented in Fig. 1. In the reward condition, cues varied in amount ($0.00–5.00) and valence (win or lose), and a feedback message then informed subjects of each trial outcome. To increase attention and reduce adaptation to the reward in the reward condition over time, the feedback included increasing positive sounds (i.e., applause, cash register) with an increasing reward rate over the task. As a control condition, we used a neutral task involving no incentives, where subjects were instructed to respond to a neutral target and feedback was replaced with a message to continue to the next trial without indication of performance. In both conditions, duration of the response target was calculated based on each subject’s reaction time during a practice session before scanning and dynamically adjusted to a mean hit rate of approximately 66%. Each presentation lasted for approximately 30 min without interruption with the neutral condition presented first, beginning at 5 min after tracer injection, followed by the reward condition, beginning at 45 min post-injection. Participants were paid a fixed participation rate and additionally received any money they won during the reward condition. The internal emotional state of subjects was assessed with the Positive and Negative Affective Scale (PANAS) before radiotracer administration and following the neutral and reward conditions (Watson et al., 1988).
Fig. 1.
(A) Schematic of fMRI paradigm: A single trial of 6 s consisted of 2000 ms each for cue; anticipation; and target plus feedback. Subjects complete 2 runs of 5 min each. Reward, loss, and neutral cues were counterbalanced and presented pseudorandomly throughout each run. (B) Schematic of reward condition of PET paradigm: Trials followed the same timing as in the fMRI paradigm, presented in a single run of 30 min with reward and loss cues. Novel changes to cues and feedback were added over time including color and sound to maintain subject interest. A neutral condition presenting a neutral cue and target with no feedback was presented during a separate single 30-min run.
2.5. PET imaging
PET scans were acquired with a Siemens (Knoxville, TN) HR+ scanner in 3-D mode (reconstructed full-width at half maximum (FWHM) resolution (~5.5 mm in-plane and 5.0 mm axially). Radiotracer synthesis and image acquisition, coregistration and reconstruction protocols were identical to those used in previously (Scott et al., 2006; Scott et al., 2007). Briefly, images were reconstructed, attenuation- and motion-corrected, and co-registered to each other (Minoshima et al., 1993). Time points were then decay-corrected during data reconstruction. Approximately 15 mCi was administered for each scan (<40 μg total cold mass for raclopride). Fifty percent of the radiotracer dose was administered as a bolus with the remainder delivered as a continuous infusion to more rapidly achieve steady-state tracer levels. Under these conditions, equilibrium conditions are achieved 35 min after tracer administration (Carson et al., 1997). Twenty-eight image frames were acquired over 90 min with increasing duration (30 s up to 10 min). Dynamic images were transformed, on a voxel-by-voxel basis, into coregistered sets of parametric maps: a tracer transport measure (K1 ratio); and a receptor-related measure at equilibrium BPND (Innis et al., 2007), yielding condition level images obtained from 35–45 min (neutral) and 60–80 min (reward) after tracer administration, using full equilibrium data (Carson, 1991; Carson et al., 1997), with the cerebellum as the non-displaceable reference region.
2.6. PET image processing
Reduction in BPND (i.e., lower levels of in vivo DA D2/D3 receptor availability during the reward condition), calculated as the difference between the neutral and the active task, was interpreted as activation of DA D2/D3 neurotransmission. A second level group paired t-test was mapped into stereotactic space using F maps of statistical significance with SPM8 (Welcome Department of Cognitive Neurology, University College, London) and Matlab software (MathWorks, Natick, MA), using a general linear model (GLM) and correction for multiple comparisons (Friston et al., 1995) and converted to t statistic data using a pooled variance estimate, according to Worsley et al. (Worsley et al., 1996). Calculations were based on absolute Bmax/Kd estimates; only regions with specific DA D2/D3 receptor binding were included in the analyses (voxels with BPND values > 0.2); a three-dimensional Gaussian filter (FWHM 6 mm) was applied to each scan. With a priori interest in the NAcc, 5-mm diameter spherical masks were created using the MarsBaR region of interest (ROI) toolbox (Brett et al., 2002) centered at to [−10 13 −8; 11 13 −8] in Montreal Neurological Institute (MNI) space as specified in previous work (Bjork et al., 2008; Weiland et al., 2013). Data from individual neutral and reward maps were extracted for quantification of regional changes in BPND. Percent change in BP was calculated as ΔBPND = ((BPNDneutral - BPNDreward)/(BPNDneutral – 1))*100. Negative changes in ΔBPND are consistent with the activation of DA D2/D3 neurotransmission induced by the reward condition, compared with the neutral condition. Hereafter we refer to this as incentive/reward DA release, although we acknowledge that the reward condition included loss trials and, due to the temporal resolution of PET, combines all events (i.e., cue presentation, anticipation, outcome, etc.) into a single metric.
2.7. fMRI paradigm
Brain response during anticipation of incentive stimuli was probed during fMRI scanning using a modified MID task (Knutson et al., 2000) similar to the reward condition performed in the PET scan; see Fig. 1. Each session involved 72 6-s trials consisting of four events. Subjects were presented an incentive cue (2000 ms) of seven possible values (gain of $0.20, $1.00 $5.00; loss of $0.20, $1.00 $5.00; or no change $0) followed by a 2000-ms anticipation delay. Next, a target appeared (variable time of 200–300 ms) during which subjects made a button press to gain or avoid losing money; subjects were instructed to respond to neutral targets despite no incentive value and then were given feedback of each trial outcome. Incentive trials were presented in pseudorandom order. Duration of the response target was based on each subject’s reaction time during a practice session before scanning. Success rate was calculated as the percentage of trials in which the subject completed the button press during the target appearance. In this pilot study, the MID version used did not dynamically adjust target duration based on performance. Participants were paid fixed participation rates plus additional money won during the task.
2.8. fMRI imaging
Whole-brain blood oxygen level-dependent (BOLD) functional images were acquired on a 3.0 Tesla GE Signa scanner (Milwaukee, WI) using T2*-weighted single-shot combined spiral in/out sequences (Glover and Law, 2001), parameters: repetition time (TR)=2000 ms, echo time (TE)=30 ms, flip angle (FA)=90°; field-of-view (FOV)=200 mm; matrix size=64 × 64; slice thickness=4 mm, 29 slices. High-resolution anatomical T1 scans were obtained for spatial normalization. Motion was minimized with foam pads and emphasis on the importance of keeping still.
2.9. fMRI image processing
Functional images were reconstructed using an iterative algorithm (Sutton et al., 2003; Fessler et al., 2005) and motion-corrected using statistical parametric mapping (SPM8, Wellcome Institute of Cognitive Neurology, Oxford, UK). All runs for all subjects met the motion-inclusion criterion of less than 2-mm translation or 2° rotation. Images were spatially normalized to MNI space and spatially smoothed with a 6-mm isotropic kernel. A GLM using SPM’s canonical hemodynamic response function, modeled incentive anticipation (Scott et al., 2007), defined as the period between cue and target (Knutson et al., 2001a) with regressors for each condition ($0.20 win, $1.00 win, $5.00 win, $0.20 loss, $1.00 loss, $5.00 loss, $0) and six motion parameters. The design of the MID task used in this study did not incorporate jitter, an omission that prevented a full differentiation between anticipatory and feedback responses, as the latter could be confounded by the anticipatory response. Contrasts for anticipation of combined reward (all three reward trial types: $0.20, $1.00 and $5.00) minus neutral were calculated for each individual for use in second level one-sample t-test and correlation analyses. This contrast was chosen due to a lower than expected success rate with this MID task (mean 41%, see Section 3); striatal response activation to anticipation of wins has been shown to be similar in certain and uncertain conditions, while that of loss is reduced (Cooper and Knutson, 2008). Loss contrasts were not further analyzed for this study.
2.10. Analyses of PET and fMRI data
A whole-brain second-level analysis regressed subjects’ fMRI combined reward minus neutral contrasts incentive/reward DA release for the left and right NAcc separately, using a GLM with correction for multiple comparisons (Friston et al., 1995) and converted to t statistic data using a pooled variance estimate (Worsley et al., 1996). Regions of significant correlation were identified using a voxel-wise threshold of p≤0.001 uncorrected, combined with cluster size threshold of 648 contiguous 1-mm3 isovoxels. This combined threshold provides protection against type I error (Forman et al., 1995) and was estimated with Monte Carlo simulation using AlphaSim (Howard et al., 2000) giving an overall corrected threshold of p<0.05.
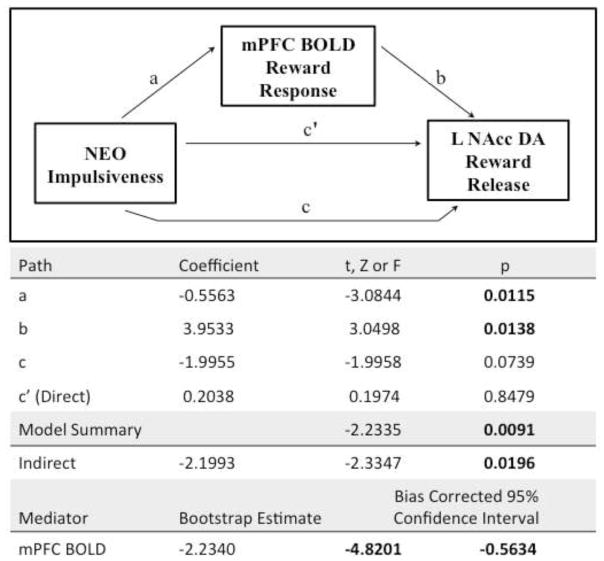
For the NAcc and clusters found in the PFC, activation data were extracted from individual contrast maps for the following analyses: (1) correlation with behavioral measures using Pearson correlations and (2) test of hypothesized model of medial prefrontal cortical (mPFC) activation as a mediator of DA release. The indirect effect of PFC activation was tested with a bias-corrected bootstrapped mediation analysis using an SPSS macro (Preacher and Hayes, 2004). The dependent variable was NAcc incentive/reward DA release, the independent variable was IMP, and the mediator was mPFC BOLD reward response (Fig. 3). This macro reported both the traditional Sobel mediation significance test, as well as a point estimate of the indirect effect with 95% confidence intervals (considered significant when not including zero) from the bootstrapping method (Preacher and Hayes, 2004).
Fig. 3.
Mediation model illustrating the influence of mPFC BOLD activation during reward on the relationship between IMP and left NAcc incentive/reward dopamine release.
3. Results
3.1. MID task during PET
Success rate (%) and reaction times (ms) were as follows: PET reward challenge, 64.7±1.2, 202±13; PET neutral challenge, 63.3±1.2, 227±21. Subjects were faster in the reward than neutral condition (paired t: t=3.963, p=0.003), but success rates for the two conditions were not different (paired t: t=2.060, p=0.064). The PET reward challenge was associated with net increases in PANAS positive affect scores (18.4±62.2%) compared with decreases in the neutral condition (−3.9±26.0%) relative to baseline state.
The NAcc ROIs demonstrated bilateral reductions in the receptor-availability measure, BPND, during the reward condition consistent with the activation of DA D2/D3 neurotransmission (Innis et al., 1992). Average ΔBPND values were −4.8±9.2% and −6.0±11.5% for right and left NAcc (t= −1.810, −1.807; p=0.049, 0.049, respectively, one-tailed), suggestive of increased DA release in response to incentive/reward. Average BPND values for the baseline/neutral condition were 2.07±0.37 and 2.10±0.44, respectively, and for the reward condition were 2.02±0.36 and 2.05±0.46, respectively, for right and left NAcc. There were no significant relationships between BPND neutral and ΔBPND, (r = 0.009, −0.490, p = 0.977, 0.106 for right and left NAcc); therefore, BPND neutral was not included as a covariate in further analyses.
Given the age range of our subjects, we tested for associations between age and both baseline BPND and incentive/reward DA release, finding a trend only in the left NAcc release (R = −0.566, p = 0.055; other p-values > 0.272).
3.2. MID task during fMRI
Overall task average success rate was 41.0±11.2% with success for the combined reward conditions at 46.9 % and for the neutral condition at 39.8% (paired t: t=3.706, p=0.003). Reaction times were 225±13 ms for combined reward and 226±22 ms for neutral conditions. Consistent with previous reports (Knutson et al., 2000), anticipation of monetary gain was associated with activation in NAcc bilaterally, caudate, thalamus, lingual and fusiform gyri, and inferior occipital and temporal lobes. Deactivation was seen in bilateral medial frontal regions, precuneus/cuneus, mid-cingulum, and supplementary motor area (Supplementary Table 1, Supplementary Figs. 1–2).
3.3. Correlation of hormone and PET data
Pearson’s correlations revealed no significant relationships between baseline BPND or ΔBPND in right or left NAcc with progesterone or estradiol levels (all R < 0.45; p-values > 0.189).
3.4. Correlation of PET and fMRI data
The regression of left NAcc incentive/reward DA release with BOLD response during reward anticipation showed positive relationships in the left angular gyrus, mammillary bodies (MB), and medial prefrontal cortex (mPFC) and left superior frontal cortex; negative correlations were found with the right supplemental motor area and dorsal anterior cingulate (Fig. 2 and Table 1). The regression for the right NAcc yielded no clusters meeting significance even at a liberal threshold of p<0.01. In addition, there were no significant relationships between NAcc incentive DA release and the corresponding NAcc ROI BOLD response (p-values>0.100). We found no associations between age and BOLD response during reward anticipation in any of these ROIs (p-values>0.269)
Fig. 2.
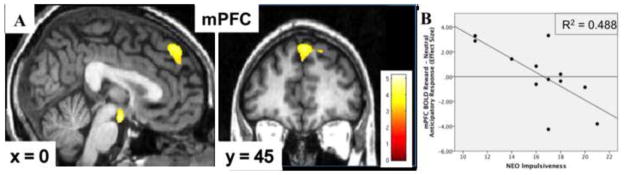
(A) Statistical parametric maps of medial prefrontal cortex (mPFC) BOLD reward anticipation activity positively correlated with left NAcc incentive/reward dopamine release. Color bars represent t-values. (B) Plot of mPFC BOLD activation during reward anticipation versus NEO Impulsiveness.
Table 1.
Linear regression of left nucleus accumbens reward-induce dopamine response with BOLD reward anticipation activity.
| Region | MNI Coordinates x, y, z
|
k
|
Peak T
|
Voxel p, unc
|
|---|---|---|---|---|
| Positive Correlation | ||||
| L Angular Gyrus | −39, −71 48 | 6297 | 5.20 | <0.001 |
| Mammillary Bodies | 3, −7, −19 | 659 | 5.00 | <0.001 |
| mPFC | 0, 45, 47 | 1541 | 4.40 | 0.001 |
| L MFG | −27, 25, 53 | 2706 | 4.38 | 0.001 |
| L SFC | −13, 69, 15 | 672 | 4.20 | 0.001 |
| Negative Correlation | ||||
| R SMA | 4, 6, 74 | 703 | 4.00 | <0.001 |
| Median Cingulate | 1, 20, 36 | 659 | 5.00 | <0.001 |
MNI, Montreal Neurological Institute; k, extent threshold in voxels; L, left; R, right; NAcc, nucleus accumbens; mPFC, medial prefrontal cortex; SFC, superior frontal cortex, SMA, supplementary motor area.
Significance determined at a voxel-wise threshold of p≤0.001 uncorrected, combined with cluster size threshold of 648 voxels.
3.5. Correlation with personality data
Based on previous work, we expected negative relationships between IMP scores and NAcc incentive/reward DA release but found only a trend with the left NAcc and no significant association with right NAcc release or with baseline BPND in either side of the NAcc. We observed a negative relationship between IMP and left mPFC BOLD anticipation of reward, which remained significant when corrected for multiple comparisons (0.05/(3 prefrontal clusters) = 0.017; Fig. 2 and Table 2).
Table 2.
Correlations between NEO-Impulsiveness and NAcc reward-induced dopamine response and region of interest BOLD activation during reward anticipation.
| Region | r | p |
|---|---|---|
| L NAcc DA Reward Release | −0.534 | 0.074 |
| R NAcc DA Reward Release | 0.083 | 0.799 |
| mPFC MID BOLD Response | −0.698 | 0.012 |
| L MFG MID BOLD Response | 0.198 | 0.541 |
| L SFC MID BOLD Response | −0.355 | 0.258 |
L, left; R, right; NAcc, nucleus accumbens; DA, dopamine; MB, mammillary bodies; MID, monetary incentive delay task; BOLD, blood oxygen level-dependent; mPFC, medial prefrontal cortex; SFC, superior frontal cortex.
3.6. Test of prefrontal mediation
The mediation model showed that although the direct effect of IMP on left NAcc incentive/reward DA release was at a trend level, there was a significant indirect effect of IMP on NAcc incentive/reward DA release that was mediated by mPFC anticipatory BOLD response (Fig. 3). Specifically, because mPFC BOLD was positively associated with NAcc DA release, and IMP was negatively associated with mPFC BOLD, the negative relationship between IMP and incentive/reward DA release becomes stronger via mPFC activation.
4. Discussion
This study used multimodal imaging of MID tasks to show that, in healthy young females, left NAcc incentive/reward DA release during PET correlates with fMRI reward-anticipatory activation in frontal, temporal and limbic regions. Importantly, we further show that the hemodynamic anticipatory activity in the mPFC mediates the negative trend-level relationship between negative urgency components of impulsivity and incentive/reward DA release in the left NAcc, providing evidence for an influence of the mPFC within reward circuitry. As the negative urgency component of impulsivity is thought to be a precursive vulnerability marker for inhibitory control disorders (for review, see Verdejo-García et al., 2008), the finding may help elucidate the neural mechanisms of this endophenotype.
A relationship between the striatum and impulsivity has been reported using animal models where NAcc damage was associated with persistent impulsive behaviors, including preference for small immediate over larger delayed reinforcement (Cardinal et al., 2001). Neuroimaging studies in humans have found IMP positively related to ventral striatal (VS) BOLD reward notification using fMRI and negatively related to VS amphetamine-induced DA release using PET, similar to the findings in this study. However, as other research suggests that striatal DA release is itself regulated by the PFC (Louilot et al., 1989; Deutch and Roth, 1991; Olsen and Duvauchelle, 2001; Thompson and Moss, 1995), our results may begin to probe these relationships.
An accumulating literature is elucidating a systematic organization within the PFC with different regions playing distinct roles in cognition (Ridderinkhof et al., 2004; O’Reilly, 2010). Particularly relevant to our study, the dorsal and anterior regions of the mPFC appear to mediate the relationship between personal emotional experience with current environmental context under cognitive demand (Phan et al., 2004) and to encode abstract reinforcement during reward processing (O’Reilly, 2010). Specifically, during decision making, increased activation of the pregenual anterior cingulate and the dorsal mPFC represents reward magnitude with the goal to maximize reinforcement (Rogers et al., 2004). Further, a recent comprehensive study of lesion-symptom mapping found that value-based decision making was associated with both the ventral medial and dorsal anterior PFC regions (Glascher et al., 2012), which overlap with the mPFC region that this study found to be correlated with accumbens DA release. Value-based decision making includes comparing among rewards and setting motivational goals that cognitive control functions can subsequently translate into planning, switching between actions and monitoring responses (Glascher et al., 2012). Evidence drawn from animal models supports this role of the mPFC in influencing reward-based decision making. For example, one study found DA metabolism increased in both the NAcc and mPFC in response to rewarding stimuli (Herman et al., 1982). However, DA metabolism only increased in the latter upon re-exposure to the environment where the stimulus took place, suggesting a conditioning effect that affords the mPFC a controlling function within the central DA system (Herman et al., 1982). Further, studies carried out with a variety of decision-making tasks support a medial prefrontal role in a common valuation system across reward types whether determining salience of appetitive cues, assessing decision strategies, or predicting valuation of potential payoffs (Montague and Berns, 2002).
These studies converge to suggest a regulatory role of the mPFC over the dopaminergic reward response. Our mediation analysis suggests this functional role may be exerted by influencing how one’s impulsiveness drives one’s response to rewarding stimuli, irrespective of whether those stimuli are monetary or drug-related. Animal models of impulsivity have shown that rodents that are more reactive to novel stimuli also develop drug self-administration and also exhibit greater reinforcement by food rewards, effects that depend on dopaminergic function (Dellu et al., 1996). Anatomically, indirect cortico-mesocortical or mesoaccumbens pathways may allow the mPFC to influence NAcc activity through ascending VTA projections (Carr and Sesack, 2000) or this regulation may function in concert with adjoining frontal regions via the cortico-accumbal pathway (Christie et al., 1985). In agreement functionally, a recent study found a negative relationship between the modulation of prefrontal cortical activation during risky decision-making and NAcc D2/D3 receptor availability, supporting an interactive link between mesolimbic and frontal activity (Kohno et al., 2013). Our results suggest a mechanism such that more impulsive individuals would recruit less regulatory prefrontal activation, or even deactivate this region, and likely experience less striatal DA release when experiencing rewarding stimuli.
Interestingly, attempts to detect behaviorally induced DA reward release with the MID have had mixed results to date. For example, Schott et al. found a decrease in [11C]raclopride binding measures in the left ventral striatum using a MID task in which the neutral and reward conditions of the experiment were performed on separate days (Schott et al., 2008). Yet in a more recent report, Urban et al. reported no significant changes in ventral striatal binding potentials but found changes in the posterior caudate (Urban et al., 2012). In this latter study, subjects underwent a baseline scan, which was followed by a MID scan. The task was performed for 24 min outside of the PET scanner, starting 5 min before the second radiotracer injection; the subject was subsequently placed in the scanner and imaging began 40 min after injection. The authors suggested that the timing of the PET imaging, as well as a lower reward:negative outcome ratio in the study of Urban et al. compared with the study of Schott et al. may have decreased the detection of changes in BPND. In our study, we used a single scan approach, with a neutral condition in the first half of the scan, similar to the neutral condition used by Pappata et al. (2002). While it is possible that our subjects had increased endogenous DA release either as an effect of time, carry-over effects from the first half of the scan, or due to the increasing valence in our task, by maintaining consistent condition presentation across subjects, we are, at a minimum, detecting individual differences in these effects. Given the temporal resolution of this PET task, and the observation that both valence and salience contribute to NAcc activation (Cooper and Knutson, 2008), we believe that our results reflect increased DA release associated with reward.
This leads to a discussion of several important limitations of our work, including the version of the MID used during the fMRI study. Unfortunately, this implementation of the MID did not allow the separation of anticipation from receipt of reward, although both anticipation and outcome have been shown to activate reward circuitry (Knutson et al., 2001b). Use of this paradigm may have contributed to the limited number of regions whose BOLD activation was correlated with the striatal incentive/reward DA response; for example, we expected to find a similar ventral tegmental area as that found by Schott et al. (Schott et al., 2008). Our fMRI MID task also did not implement a dynamic adjustment for performance, and our subjects had a lower success rate than we anticipated, much lower than in the PET task. However, recent work by Cooper and Knutson indicates that certainty of reward is not a determinant of anticipatory BOLD activation (Cooper and Knutson, 2008), so all reward trials, both successful and unsuccessful, were included in the contrast used in our fMRI task.
Other limitations of this pilot study include the small sample size, though it is in line with other recent studies combining PET and fMRI imaging (Schott et al., 2008; Urban et al., 2012). Further, the multiple facets of impulsivity (Zucker et al., 2011) inherently limit the generalizability of this study, which used the NEO-IMP to assess negative urgency. This measure has been associated with striatal reward response (Bjork et al., 2008) and substance use (Kaiser et al., 2012), but other measures, evaluating other domains of impulsivity, may be relevant to a wider range of psychopathologies. In addition, in light of work suggesting regionally specific differences in DA release (Riccardi et al., 2006), this pilot study was restricted to females to reduce experimental complexity. Given observations of sex differences in activation of DA neurotransmission (Munro et al., 2006; Urban et al., 2010), future work will include male subjects. Finally, while measurement of D2/D3 receptor BPND with [11C]raclopride has been shown to have high test-retest reliability (Nyberg et al., 1996), future work should also include testing the reproducibility of behaviorally induced dopamine neurotransmission.
In summary, we used two imaging modalities to investigate behaviorally induced reward response. We found that NAcc incentive/reward DA release was associated with increased neural activation in the mPFC during reward anticipation. Our results suggest that the mPFC, a regulatory region associated with learning and valuation in reward circuitry, may mediate between impulsive urgency and NAcc dopaminergic response. Further work is necessary to clarify these interactions.
Supplementary Material
Statistical parametric map of regions activated during the reward minus neutral condition for the fMRI MID task. Color bar represents t-scores.
Statistical parametric map of regions deactivated during the reward minus neutral condition for the fMRI MID task. Color bar represents t-scores.
HIGHLIGHTS.
Our reference: PSYN 10218
Editorial reference: PSYN_PSYN-D-13-00177
This study utilized positron emission tomography (PET) and functional MRI.
A money-based task measured dopamine release (DA) and neural activity in the brain.
Left accumbens DA release correlated with activity in the medial prefrontal cortex (mPFC).
mPFC activity mediated the relationship between Impulsiveness and DA release.
Frontal regulation may influence an individual’s dopaminergic response to reward.
Acknowledgments
This work was supported by NIH grants K01 DA031755 to BJW; K01 DA020088 to MMH; T32 AA07477, R01 AA12217, R37 AA07065 to RAZ, R01 DA022520 to JKZ; a Phil F. Jenkins Foundation award to JKZ; and a NARSAD Brain and Behavior Early Investigator Award to BJW.
We acknowledge and thank Gregory Samanez-Larkin for his contribution in the development of the MID task for the PET study.
Footnotes
The authors have no conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersen SL, Teicher MH. Sex differences in dopamine receptors and their relevance to ADHD. Neuroscience & Biobehavioral Reviews. 2000;24:137–141. doi: 10.1016/s0149-7634(99)00044-5. [DOI] [PubMed] [Google Scholar]
- Barratt ES, Stanford MS, Dowdy L, Liebman MJ, Kent TA. Impulsive and premeditated aggression: a factor analysis of self-reported acts. Psychiatry Research. 1999;86:163–173. doi: 10.1016/s0165-1781(99)00024-4. [DOI] [PubMed] [Google Scholar]
- Bechara A. Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nature Neuroscience. 2005;8:1458–1463. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- Becker JB. Direct effect of 17 beta-estradiol on striatum: sex differences in dopamine release. Synapse. 1990;5:157–164. doi: 10.1002/syn.890050211. [DOI] [PubMed] [Google Scholar]
- Becker JB. Gender differences in dopaminergic function in striatum and nucleus accumbens. Pharmacology, Biochemistry and Behavior. 1999;64:803–812. doi: 10.1016/s0091-3057(99)00168-9. [DOI] [PubMed] [Google Scholar]
- Bjork JM, Smith AR, Hommer DW. Striatal sensitivity to reward deliveries and omissions in substance dependent patients. Neuroimage. 2008;42:1609–1621. doi: 10.1016/j.neuroimage.2008.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breier A, Su TP, Saunders R, Carson RE, Kolachana BS, de Bartolomeis A, Weinberger DR, Weisenfeld N, Malhotra AK, Eckelman WC, Pickar D. Schizophrenia is associated with elevated amphetamine-induced synaptic dopamine concentrations: Evidence from a novel positron emission tomography method. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:2569–2574. doi: 10.1073/pnas.94.6.2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett M, Anton J-L, Valabregue R, Poline JB. Region of interest analysis using an SPM toolbox. 8th International Conference on Functional Mapping of the Human Brain; Sendai, Japan. 2002. [Google Scholar]
- Buckholtz JW, Treadway MT, Cowan RL, Woodward ND, Benning SD, Li R, Ansari MS, Baldwin RM, Schwartzman AN, Shelby ES, Smith CE, Cole D, Kessler RM, Zald DH. Mesolimbic dopamine reward system hypersensitivity in individuals with psychopathic traits. Nature Neuroscience. 2010a;13:419–421. doi: 10.1038/nn.2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckholtz JW, Treadway MT, Cowan RL, Woodward ND, Li R, Ansari MS, Baldwin RM, Schwartzman AN, Shelby ES, Smith CE, Kessler RM, Zald DH. Dopaminergic network differences in human impulsivity. Science. 2010b;329:532. doi: 10.1126/science.1185778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinal RN, Pennicott DR, Sugathapala CL, Robbins TW, Everitt BJ. Impulsive choice induced in rats by lesions of the nucleus accumbens core. Science. 2001;292:2499–2501. doi: 10.1126/science.1060818. [DOI] [PubMed] [Google Scholar]
- Carr DB, Sesack SR. Projections from the rat prefrontal cortex to the ventral tegmental area: target specificity in the synaptic associations with mesoaccumbens and mesocortical neurons. The Journal of Neuroscience. 2000;20:3864–3873. doi: 10.1523/JNEUROSCI.20-10-03864.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson RE. Precision and accuracy considerations of physiological quantitation in PET. Journal of Cerebral Blood Flow & Metabolism. 1991;11:A45–50. doi: 10.1038/jcbfm.1991.36. [DOI] [PubMed] [Google Scholar]
- Carson RE, Breier A, de Bartolomeis A, Saunders RC, Su TP, Schmall B, Der MG, Pickar D, Eckelman WC. Quantification of amphetamine-induced changes in [11c] raclopride binding with continuous infusion. Journal of Cerebral Blood Flow & Metabolism. 1997;17:437–447. doi: 10.1097/00004647-199704000-00009. [DOI] [PubMed] [Google Scholar]
- Castellani B, Rugle L. A comparison of pathological gamblers to alcoholics and cocaine misusers on impulsivity, sensation seeking, and craving. Substance Use & Misuse. 1995;30:275–289. doi: 10.3109/10826089509048726. [DOI] [PubMed] [Google Scholar]
- Christie MJ, James LB, Beart PM. An excitant amino acid projection from the medial prefrontal cortex to the anterior part of nucleus accumbens in the rat. Journal of Neurochemistry. 1985;45:477–482. doi: 10.1111/j.1471-4159.1985.tb04013.x. [DOI] [PubMed] [Google Scholar]
- Congdon E, Canli T. The endophenotype of impulsivity: reaching consilience through behavioral, genetic, and neuroimaging approaches. Behavioral and Cognitive Neuroscience Reviews. 2005;4:262–281. doi: 10.1177/1534582305285980. [DOI] [PubMed] [Google Scholar]
- Cooper JC, Knutson B. Valence and salience contribute to nucleus accumbens activation. NeuroImage. 2008;39:538–547. doi: 10.1016/j.neuroimage.2007.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa P, McRae R. Normal personality assessment in clinical practice: the NEO Personality Inventory. Psychological Assessment. 1992;4:5–13. [Google Scholar]
- Costa PT, Jr, McCrae R. The NEO Personality Inventory Manual. Psychological Assessment Resources; Odessa, FL. 1985. [Google Scholar]
- Crews FT, Boettiger CA. Impulsivity, frontal lobes and risk for addiction. Pharmacology, Biochemistry and Behavior. 2009;93:237–247. doi: 10.1016/j.pbb.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyders MA, Smith GT. Emotion-based dispositions to rash action: positive and negative urgency. Psychological Bullein. 2008;134:807–828. doi: 10.1037/a0013341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellu F, Piazza PV, Mayo W, Le Moal M, Simon H. Novelty-seeking in rats--biobehavioral characteristics and possible relationship with the sensation-seeking trait in man. Neuropsychobiology. 1996;34:136–145. doi: 10.1159/000119305. [DOI] [PubMed] [Google Scholar]
- Deutch AY, Roth RH. The determinants of stress-induced activation of the prefrontal cortical dopamine system. In: Uylings HBM, van Eden CG, de Bruin JPC, Corner MA, Feenstra MGP, editors. Progress in Brain Research. Chapter 19. Elsevier; Amsterdam: 1991. pp. 367–403. [DOI] [PubMed] [Google Scholar]
- Dick DM, Smith G, Olausson P, Mitchell SH, Leeman RF, O’Malley SS, Sher K. Understanding the construct of impulsivity and its relationship to alcohol use disorders. Addiction Biology. 2010;15:217–226. doi: 10.1111/j.1369-1600.2009.00190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fessler J, Lee S, Olafsson V, Shi H, Noll D. Toeplitz-based iterative image reconstruction for MRI with correction for magnetic field inhomogeneity. IEEE Transactions on Signal Processing. 2005;53:3393–3402. [Google Scholar]
- Fischer S, Anderson KG, Smith GT. Coping with distress by eating or drinking: role of trait urgency and expectancies. Psychology of Addictive Behaviors. 2004;18:269–274. doi: 10.1037/0893-164X.18.3.269. [DOI] [PubMed] [Google Scholar]
- Fitzgerald HE, Sullivan LA, Ham HP, Zucker RA, Bruckel S, Schneider AM, Noll RB. Predictors of behavior problems in three-year-old sons of alcoholics: early evidence for the onset of risk. Child Development. 1993;64:110–123. doi: 10.1111/j.1467-8624.1993.tb02898.x. [DOI] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magnetic Resonance in Medicine. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Friston K, Holmes A, Worsley K, Pline J, Frith C, Frackowiak R. Statistical parametric maps in functional imaging: a general linear approach. Human Brain Mapping. 1995;2:189–210. [Google Scholar]
- Glascher J, Adolphs R, Damasio H, Bechara A, Rudrauf D, Calamia M, Paul LK, Tranel D. Lesion mapping of cognitive control and value-based decision making in the prefrontal cortex. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:14681–14686. doi: 10.1073/pnas.1206608109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover GH, Law CS. Spiral-in/out BOLD fMRI for increased SNR and reduced susceptibility artifacts. Magnetic Resonance Medicine. 2001;46:515–522. doi: 10.1002/mrm.1222. [DOI] [PubMed] [Google Scholar]
- Herman JP, Guillonneau D, Dantzer R, Scatton B, Semerdjian-Rouquier L, Le Moal M. Differential effects of inescapable footshocks and of stimuli previously paired with inescapable footshocks on dopamine turnover in cortical and limbic areas of the rat. Life Sciences. 1982;30:2207–2214. doi: 10.1016/0024-3205(82)90295-8. [DOI] [PubMed] [Google Scholar]
- Howard MA, Cowell PE, Boucher J, Broks P, Mayes A, Farrant A, Roberts N. Convergent neuroanatomical and behavioural evidence of an amygdala hypothesis of autism. Neuroreport. 2000;11:2931–2935. doi: 10.1097/00001756-200009110-00020. [DOI] [PubMed] [Google Scholar]
- Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, Holden J, Houle S, Huang SC, Ichise M, Iida H, Ito H, Kimura Y, Koeppe RA, Knudsen GM, Knuuti J, Lammertsma AA, Laruelle M, Logan J, Maguire RP, Mintun MA, Morris ED, Parsey R, Price JC, Slifstein M, Sossi V, Suhara T, Votaw JR, Wong DF, Carson RE. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. Journal of Cerebral Blood Flow & Metabolism. 2007;27:1533–1539. doi: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- Innis RB, Malison RT, al-Tikriti M, Hoffer PB, Sybirska EH, Seibyl JP, Zoghbi SS, Baldwin RM, Laruelle M, Smith EO, et al. Amphetamine-stimulated dopamine release competes in vivo for [123I]IBZM binding to the D2 receptor in nonhuman primates. Synapse. 1992;10:177–184. doi: 10.1002/syn.890100302. [DOI] [PubMed] [Google Scholar]
- Johnson BA. Opportunities, challenges, and successes in the development of medicines for the treatment of addiction. In: Johnson BA, editor. Addiction Medicine: Science and Practice. Springer Science+Business Media; New York: 2010. pp. 1525–1537. [Google Scholar]
- Kaiser AJ, Milich R, Lynam DR, Charnigo RJ. Negative urgency, distress tolerance, and substance abuse among college students. Addictive Behaviors. 2012;37:1075–1083. doi: 10.1016/j.addbeh.2012.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Adams CM, Fong GW, Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. Journal of Neuroscience. 2001a;21:RC159. doi: 10.1523/JNEUROSCI.21-16-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Fong GW, Adams CM, Varner JL, Hommer D. Dissociation of reward anticipation and outcome with event-related fMRI. Neuroreport. 2001b;12:3683–3687. doi: 10.1097/00001756-200112040-00016. [DOI] [PubMed] [Google Scholar]
- Knutson B, Westdorp A, Kaiser E, Hommer D. FMRI visualization of brain activity during a monetary incentive delay task. Neuroimage. 2000;12:20–27. doi: 10.1006/nimg.2000.0593. [DOI] [PubMed] [Google Scholar]
- Kohno M, Ghahremani DG, Morales AM, Robertson CL, Ishibashi K, Morgan AT, Mandelkern MA, London ED. Risk-taking behavior: dopamine D2/D3 receptors, feedback, and frontolimbic activity. Cerebral Cortex. 2013 doi: 10.1093/cercor/bht218. electronic publication 21 August. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Laruelle M. Imaging synaptic neurotransmission with in vivo binding competition techniques: a critical review. Journal of Cerebral Blood Flow and Metabolism. 2000;20:423–451. doi: 10.1097/00004647-200003000-00001. [DOI] [PubMed] [Google Scholar]
- Le Moal M, Simon H. Mesocorticolimbic dopaminergic network: functional and regulatory roles. Physiological Reviews. 1991;71:155–234. doi: 10.1152/physrev.1991.71.1.155. [DOI] [PubMed] [Google Scholar]
- Louilot A, Le Moal M, Simon H. Opposite influences of dopaminergic pathways to the prefrontal cortex or the septum on the dopaminergic transmission in the nucleus accumbens. an in vivo voltammetric study. Neuroscience. 1989;29:45–56. doi: 10.1016/0306-4522(89)90331-x. [DOI] [PubMed] [Google Scholar]
- Love TM, Enoch MA, Hodgkinson CA, Peciña M, Mickey B, Koeppe RA, Stohler CS, Goldman D, Zubieta JK. Oxytocin gene polymorphisms influence human dopaminergic function in a sex-dependent manner. Biological Psychiatry. 2012;72:198–206. doi: 10.1016/j.biopsych.2012.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mawlawi O, Martinez D, Slifstein M, Broft A, Chatterjee R, Hwang DR, Huang Y, Simpson N, Ngo K, Van Heertum R, Laruelle M. Imaging human mesolimbic dopamine transmission with positron emission tomography: I. Accuracy and precision of D2 receptor parameter measurements in ventral striatum. Journal of Cerebral Blood Flow and Metabolism. 2001;21:1034–1057. doi: 10.1097/00004647-200109000-00002. [DOI] [PubMed] [Google Scholar]
- Michalczuk R, Bowden-Jones H, Verdejo-Garcia A, Clark L. Impulsivity and cognitive distortions in pathological gamblers attending the UK National Problem Gambling Clinic: a preliminary report. Psychological Medicine. 2011;41:2625–2635. doi: 10.1017/S003329171100095X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minoshima S, Koeppe RA, Mintun MA, Berger KL, Taylor SF, Frey KA, Kuhl DE. Automated detection of the intercommissural line for stereotactic localization of functional brain images. Journal of Nuclear Medicine. 1993;34:322–329. [PubMed] [Google Scholar]
- Montague PR, Berns GS. Neural economics and the biological substrates of valuation. Neuron. 2002;36:265–284. doi: 10.1016/s0896-6273(02)00974-1. [DOI] [PubMed] [Google Scholar]
- Munro CA, McCaul ME, Wong DF, Oswald LM, Zhou Y, Brasic J, Kuwabara H, Kumar A, Alexander M, Ye W, Wand GS. Sex differences in striatal dopamine release in healthy adults. Biological Psychiatry. 2006;59:966–974. doi: 10.1016/j.biopsych.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Nordstrom AL, Olsson H, Halldin C. A PET study of D2 dopamine receptor density at different phases of the menstrual cycle. Psychiatry Research: Neuroimaging. 1998;83:1–6. doi: 10.1016/s0925-4927(98)00021-3. [DOI] [PubMed] [Google Scholar]
- Nyberg S, Farde L, Halldin C. Test-retest reliability of central [11C]raclopride binding at high D2 receptor occupancy. A PET study in haloperidol-treated patients. Psychiatry Research: Neuroimaging. 1996;67:163–171. doi: 10.1016/0925-4927(96)02921-6. [DOI] [PubMed] [Google Scholar]
- O’Reilly RC. The what and how of prefrontal cortical organization. Trends in Neurosciences. 2010;33:355–361. doi: 10.1016/j.tins.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen CM, Duvauchelle CL. Intra-prefrontal cortex injections of SCH 23390 influence nucleus accumbens dopamine levels 24 h post-infusion. Brain Research. 2001;922:80–86. doi: 10.1016/s0006-8993(01)03152-3. [DOI] [PubMed] [Google Scholar]
- Oswald LM, Wong DF, Zhou Y, Kumar A, Brasic J, Alexander M, Ye W, Kuwabara H, Hilton J, Wand GS. Impulsivity and chronic stress are associated with amphetamine-induced striatal dopamine release. NeuroImage. 2007;36:153–166. doi: 10.1016/j.neuroimage.2007.01.055. [DOI] [PubMed] [Google Scholar]
- Pappata S, Dehaene S, Poline JB, Gregoire MC, Jobert A, Delforge J, Frouin V, Bottlaender M, Dolle F, Di Giamberardino L, Syrota A. In vivo detection of striatal dopamine release during reward: a PET study with [11C]raclopride and a single dynamic scan approach. NeuroImage. 2002;16:1015–1027. doi: 10.1006/nimg.2002.1121. [DOI] [PubMed] [Google Scholar]
- Phan KL, Taylor SF, Welsh RC, Ho SH, Britton JC, Liberzon I. Neural correlates of individual ratings of emotional salience: a trial-related fMRI study. NeuroImage. 2004;21:768–780. doi: 10.1016/j.neuroimage.2003.09.072. [DOI] [PubMed] [Google Scholar]
- Phillips AG, Vacca G, Ahn S. A top-down perspective on dopamine, motivation and memory. Pharmacology, Biochemistry and Behavior. 2008;90:236–249. doi: 10.1016/j.pbb.2007.10.014. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Hayes AF. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behavior Research Methods, Instruments, & Computers. 2004;36:717–731. doi: 10.3758/bf03206553. [DOI] [PubMed] [Google Scholar]
- Riccardi P, Zald D, Li R, Park S, Ansari MS, Dawant B, Anderson S, Woodward N, Schmidt D, Baldwin R, Kessler R. Sex differences in amphetamine-induced displacement of [18F]fallypride in striatal and extrastriatal regions: a PET study. The American Journal of Psychiatry. 2006;163:1639–1641. doi: 10.1176/ajp.2006.163.9.1639. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, van den Wildenberg WPM, Segalowitz SJ, Carter CS. Neurocognitive mechanisms of cognitive control: the role of prefrontal cortex in action selection, response inhibition, performance monitoring, and reward-based learning. Brain and Cognition. 2004;56:129–140. doi: 10.1016/j.bandc.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Addiction. Annual Review of Psychology. 2003;54:25–53. doi: 10.1146/annurev.psych.54.101601.145237. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Ramnani N, Mackay C, Wilson JL, Jezzard P, Carter CS, Smith SM. Distinct portions of anterior cingulate cortex and medial prefrontal cortex are activated by reward processing in separable phases of decision-making cognition. Biological Psychiatry. 2004;55:594–602. doi: 10.1016/j.biopsych.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Schott BH, Minuzzi L, Krebs RM, Elmenhorst D, Lang M, Winz OH, Seidenbecher CI, Coenen HH, Heinze HJ, Zilles K, Duzel E, Bauer A. Mesolimbic functional magnetic resonance imaging activations during reward anticipation correlate with reward-related ventral striatal dopamine release. The Journal of Neuroscience. 2008;28:14311–14319. doi: 10.1523/JNEUROSCI.2058-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Predictive reward signal of dopamine neurons. Journal of Neurophysiology. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- Scott DJ, Heitzeg MM, Koeppe RA, Stohler CS, Zubieta JK. Variations in the human pain stress experience mediated by ventral and dorsal basal ganglia dopamine activity. The Journal of Neuroscience. 2006;26:10789–10795. doi: 10.1523/JNEUROSCI.2577-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott DJ, Stohler CS, Egnatuk CM, Wang H, Koeppe RA, Zubieta JK. Individual differences in reward responding explain placebo-induced expectations and effects. Neuron. 2007;55:325–336. doi: 10.1016/j.neuron.2007.06.028. [DOI] [PubMed] [Google Scholar]
- Seeman P, Wilson A, Gmeiner P, Kapur S. Dopamine D2 and D3 receptors in human putamen, caudate nucleus, and globus pallidus. Synapse. 2006;60:205–211. doi: 10.1002/syn.20298. [DOI] [PubMed] [Google Scholar]
- Smith GT, Fischer S, Cyders MA, Annus AM, Spillane NS, McCarthy DM. On the validity and utility of discriminating among impulsivity-like traits. Assessment. 2007;14:155–170. doi: 10.1177/1073191106295527. [DOI] [PubMed] [Google Scholar]
- Sutton BP, Noll DC, Fessler JA. Fast, iterative image reconstruction for MRI in the presence of field inhomogeneities. IEEE Transactions on Medical Imaging. 2003;22:178–188. doi: 10.1109/tmi.2002.808360. [DOI] [PubMed] [Google Scholar]
- Thompson TL, Moss RL. In vivo stimulated dopamine release in the nucleus accumbens: Modulation by the prefrontal cortex. Brain Research. 1995;686:93–98. doi: 10.1016/0006-8993(95)00429-t. [DOI] [PubMed] [Google Scholar]
- Urban NBL, Kegeles LS, Slifstein M, Xu X, Martinez D, Sakr E, Castillo F, Moadel T, O’Malley SS, Krystal JH, Abi-Dargham A. Sex differences in striatal dopamine release in young adults after oral alcohol challenge: a positron emission tomography imaging study with [11C]raclopride. Biological Psychiatry. 2010;68:689–696. doi: 10.1016/j.biopsych.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban NL, Slifstein M, Meda S, Xu X, Ayoub R, Medina O, Pearlson G, Krystal J, Abi-Dargham A. Imaging human reward processing with positron emission tomography and functional magnetic resonance imaging. Psychopharmacology. 2012;221:67–77. doi: 10.1007/s00213-011-2543-6. [DOI] [PubMed] [Google Scholar]
- Urcelay GP, Dalley JW. Linking ADHD, impulsivity, and drug abuse: a neuropsychological perspective. In: Geyer MA, Ellenbroek BA, Marsden CA, editors. Current Topics in Behavioral Neurosciences. Springer-Verlag; Berlin: 2011. p. 173. [DOI] [PubMed] [Google Scholar]
- Verdejo-García A, Lawrence AJ, Clark L. Impulsivity as a vulnerability marker for substance-use disorders: review of findings from high-risk research, problem gamblers and genetic association studies. Neuroscience & Biobehavioral Reviews. 2008;32:777–810. doi: 10.1016/j.neubiorev.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Baler R, Telang F. Imaging dopamine’s role in drug abuse and addiction. Neuropharmacology. 2009;56:3–8. doi: 10.1016/j.neuropharm.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Swanson JM, Telang F. Dopamine in drug abuse and addiction: results of imaging studies and treatment implications. Archives of Neurology. 2007;64:1575–1579. doi: 10.1001/archneur.64.11.1575. [DOI] [PubMed] [Google Scholar]
- Walker QD, Ray R, Kuhn CM. Sex differences in neurochemical effects of dopaminergic drugs in rat striatum. Neuropsychopharmacology. 2005;31:1193–1202. doi: 10.1038/sj.npp.1300915. [DOI] [PubMed] [Google Scholar]
- Walker QD, Rooney MB, Wightman RM, Kuhn CM. Dopamine release and uptake are greater in female than male rat striatum as measured by fast cyclic voltammetry. Neuroscience. 1999;95:1061–1070. doi: 10.1016/s0306-4522(99)00500-x. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. Journal of Personality and Social Psychology. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Weiland BJ, Welsh RC, Yau WY, Zucker RA, Zubieta JK, Heitzeg MM. Accumbens functional connectivity during reward mediates sensation-seeking and alcohol use in high-risk youth. Drug and Alcohol Dependence. 2013;128:130–139. doi: 10.1016/j.drugalcdep.2012.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteside SP, Lynam DR. The Five Factor Model and impulsivity: using a structural model of personality to understand impulsivity. Personality and Individual Differences. 2001;30:669–689. [Google Scholar]
- Wise RA. Brain reward circuitry: insights from unsensed incentives. Neuron. 2002;36:229–240. doi: 10.1016/s0896-6273(02)00965-0. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston KJ, Evans AC. A unified statistical approach for determining significant signals in images of cerebral activation. Human Brain Mapping. 1996;4:58–73. doi: 10.1002/(SICI)1097-0193(1996)4:1<58::AID-HBM4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Zald DH, Boileau I, El-Dearedy W, Gunn R, McGlone F, Dichter GS, Dagher A. Dopamine transmission in the human striatum during monetary reward tasks. Journal of Neuroscience. 2004;24:4105–4112. doi: 10.1523/JNEUROSCI.4643-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker RA, Heitzeg MM, Nigg JT. Parsing the undercontrol/disinhibition pathway to substance use disorders: a multilevel developmental problem. Child Development Perspectives. 2011;5:248–255. doi: 10.1111/j.1750-8606.2011.00172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Statistical parametric map of regions activated during the reward minus neutral condition for the fMRI MID task. Color bar represents t-scores.
Statistical parametric map of regions deactivated during the reward minus neutral condition for the fMRI MID task. Color bar represents t-scores.