Abstract
Radiation-induced gastrointestinal (GI) toxicity can be a major source of morbidity and mortality after radiation exposure. There is an unmet need for effective preventative or mitigative treatments against the potentially fatal diarrhea and water loss induced by radiation damage to the GI tract. We report that prolyl hydroxylase inhibition by genetic knockout or pharmacologic inhibition of all PHD isoforms by the small molecule dimethyloxyallylglycine (DMOG) increases HIF expression, improves epithelial integrity, reduces apoptosis, and increases intestinal angiogenesis, all of which are essential for radioprotection. HIF2, but not HIF1, is both necessary and sufficient to prevent radiation-induced GI toxicity and death. Increased VEGF expression contributes to the protective effects of HIF2, since inhibition of VEGF function reversed the radioprotection and radiomitigation afforded by DMOG. Additionally, mortality is reduced from abdominal or total body irradiation even when DMOG is given 24 hours after exposure. Thus, prolyl hydroxylase inhibition represents a new treatment strategy to protect against and mitigate GI toxicity from both therapeutic radiation and potentially lethal radiation exposures.
Introduction
Radiation exposure in a mass casualty setting is an ongoing threat that is a serious military and public health concern (1). Acute radiation syndrome, also known as radiation sickness, describes a constellation of symptoms that occur after total body exposure to radiation. At doses less than 8Gy, fatal injuries are primarily hematopoietic in nature, and can be treated with a bone marrow transplant and supportive care (2). Doses of more than 10Gy universally lead to death, however, because of damage to gastrointestinal (GI) tract (3). At these higher doses of radiation, it is believed that a critical number of intestinal stem cells are irreparably killed, which impairs the regeneration of villi and compromises the epithelial integrity of the entire GI tract (4). The damaged and blunted villi causes malabsorption, fluid loss and electrolyte imbalances which can lead to death (5). Moreover, the loss of epithelial integrity can promote the direct access of enteric pathogens and flora into the bloodstream which can lead to sepsis and death (6). These potentially lethal gastrointestinal symptoms after radiation exposure are sometimes referred to collectively as the radiation-induced gastrointestinal syndrome (RIGS). Unfortunately, few effective treatments exist for radiation-induced GI toxicity. The handful of FDA-approved radioprotectors work by eliminating internally ingested radiation (3), or through free radical scavenging with unfavorable side effect profiles (7) that would not be useful for treating patients on a large scale.
The biology that underlies RIGS has been studied extensively over many decades and is still subject to controversy. The seminal studies of Withers and Elkind (8) established the hypothesis that dose-dependent radiation damage to the intestinal stem cells (ISCs) located in the crypts of Lieberkühn was the primary cause of RIGS (9). Further molecular dissection of these crypt ISCs have demonstrated that while both Lgr5+ (10) and Bmi1+ cells (11) can repopulate the gut, it is the Bmi1+ population of cells that appears to be more critical in the injury response (12). The primacy of the epithelial cell in the radiation response of the gut, however, has been challenged by data showing that genetic (13) or immunologic inhibition (14) of ceramide signaling in the endothelial cells also prevents death from RIGS. Consequently, there is ongoing debate regarding the importance of both epithelial and endothelial cell types in the radiation response of the gut.
Hypoxic signaling through hypoxia-inducible factors-1 and −2 (HIF1 and HIF2) is critical for many aspects of intestinal homeostasis. The intestine naturally exists in a steep physiologic hypoxia gradient, and HIF regulates several genes required for intestinal barrier function such as intestinal trefoil factor (TFF3/ITF) and MDR1 (15). Augmenting HIF expression in the gut with an intestinal-specific knockout of the Von Hippel Lindau (VHL) gene was shown to be protective against infectious or chemical stresses (16). However, the role of HIF in the radiation response of the gut remains unexplored.
The protein stability of the HIF family of transcription factors is regulated by prolyl hydroxylase domain (PHD)-containing proteins. During normoxia, PHD proteins hydroxylate HIF on critical proline residues that enable VHL to bind HIF and target it for proteasomal degradation (17). To date, three major oxygen-dependent prolyl hydroxylase (PHD1-3) have been identified in mammals (25), but their roles in the radiation response of the gut is unknown. We posited that the inhibition of PHD function would stabilize HIF, improve epithelial integrity and possibly reduce radiation toxicity. We show that genetic or pharmacologic inhibition of all three PHD isoforms robustly stabilizes HIF in normoxia and reduces morbidity and mortality from lethal radiation exposure and is also an effective mitigation strategy. Thus, PHD inhibition may be an effective countermeasure for radiation exposure.
Results
Pan-PHD knockout is required for high HIF2 expression and radioprotection of the gut
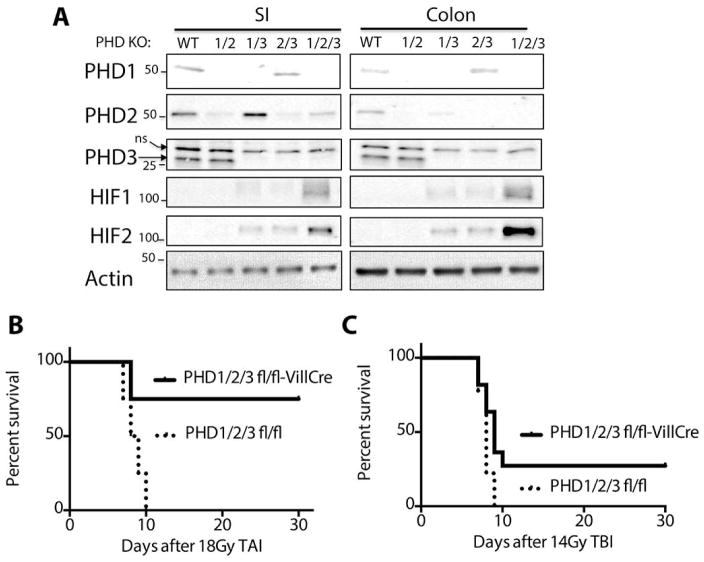
To determine the role of the PHD proteins in the radiation response of the intestinal tract, we created intestine-specific knockouts of all combinations of PHDs by backcrossing triple heterozygous PHD1/2/3 mice (PHD1/2/3fl/+) to create all possible combinations of PHD isoforms (PHD1fl/fl; PHD2fl/fl; PHD3fl/fl; PHD1/2fl/fl; PHD1/3fl/fl; PHD2/3fl/fl; PHD1/2/3fl/fl), as we have done previously (18). These individual homozygous floxed genotypes were then crossed with the Villin-Cre mouse (19) to knockout each combination of PHD genes within the GI epithelium (GI-PHD KO). The expression of PHD1, PHD2 or PHD3 in the small intestine and colon was diminished by 90–95% as determined by Western blot (Figure 1A). HIF1 or HIF2 was not detected in single knockouts of PHD1, −2 or −3, nor was HIF stabilized in PHD1/2 knockout animals (Figure 1A). Mice lacking both PHD1/3 and PHD2/3 in the intestines exhibited modest levels of both HIF1 and HIF2 protein expression, while mice with a knockout of all three PHD isoforms in the gut (GI-PHD1/2/3KO) exhibited robust stabilization of HIF1 and HIF2 in both the small intestine and colon (Figure 1A).
Figure 1. Loss of all PHD isoforms is required for radioprotection.
(A) Western blots from purified epithelial cells of the indicated genotypes and tissues. The wildtype (WT) lane is from the PHD1/2/3 fl/fl animal without Villin-Cre. The various floxed PHD animals were of a mixed C57/BL6-FVB genetic background. (B) Kaplan-Meier analysis of GI-PHD1/2/3 knockout mice and controls after 18Gy total abdominal irradiation (TAI). n=8 per genotype and p=0.005 by log-rank test. (C) Kaplan-Meier analysis of GI-PHD1/2/3 knockout mice and controls after 14Gy total body irradiation (TBI). n=9 per genotype and p=0.02 by log rank test.
To assess the radioprotective effects of PHD proteins, each combination of PHD knockout mice was treated with 18Gy of total abdominal irradiation (TAI), which is a supralethal dose for mice on a mixed genetic background (20). Total abdominal irradiation was achieved with custom irradiation jig (Figure S1), which protects the bone marrow of the upper body and reduces hematopoietic toxicity as a competing cause of death (21). GI-PHD1/2/3KO animals showed a 70% survival rate at 30 days after receiving 18Gy of TAI, while all littermate controls died before 10 days (Figure 1B, table S1). None of the other PHD knockout combinations showed a statistically significant survival advantage after TAI (Figures S2A–F, table S1).
Although TAI is useful in studying gastrointestinal toxicity from radiation, accidental radiation exposure affects the entire body, and not just the lower half. To test whether the knockout of PHD proteins also protected against whole body doses of lethal radiation, we performed total body irradiation (TBI) experiments on GI-PHD1/2/3KO animals at a lethal dose of 14Gy (22). Indeed, GI-PHD1/2/3KO animals exhibited a 27% survival rate (3/11 mice, Figure 1c and table S1) at 30 days compared to a 0% survival rate in the littermate control group (0/9 mice, Figure 1c and table S1), indicating that the loss PHD proteins in the gut is sufficient to protect against radiation-induced gastrointestinal mortality from abdominal and whole body radiation.
Prolyl hydroxylase inhibition stabilizes HIF and protects against radiation-induced gastrointestinal toxicity and death
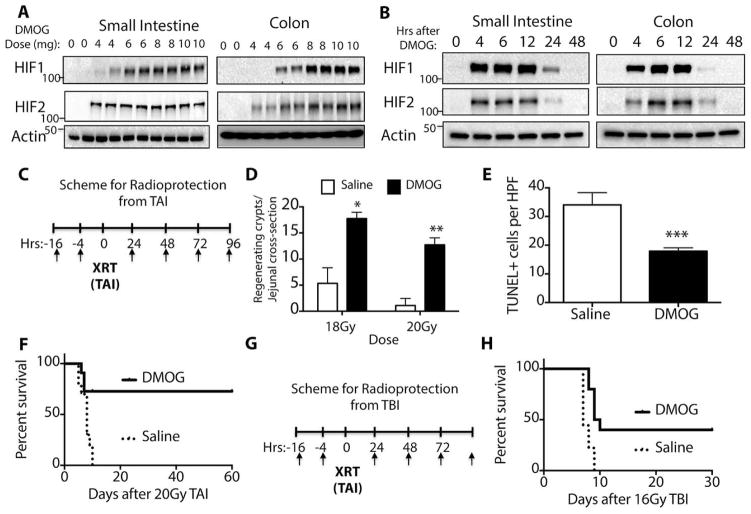
To recapitulate the phenotype of the GI-PHD1/2/3KO mice, we used a small molecule inhibitor of all PHD isoforms called dimethyloxallyl glycine (DMOG), which is an oxoglutarate analog (23). Intraperitoneal administration of DMOG stabilized HIF1 and HIF2 in the small intestine and colon in a dose dependent fashion (Figure 2A). A time course of HIF stabilization after a single 8 mg intraperitoneal injection of DMOG revealed that both HIF1 and HIF2 were fully stabilized by 6 hours after DMOG injection (Figure 2B), which demonstrated that DMOG stabilized HIF1 and HIF2 in the gut for up to 24 hours after a single injection, though expression was diminished at the 24 hour timepoint. Importantly, there was no gross morbidity or mortality from DMOG at the doses and time course used.
Figure 2. Prolyl hydroxylase inhibition by DMOG protects and mitigates against death from potentially lethal abdominal radiation.
All mice in these experiments were 8 week-old, male C57/BL6 mice (see Methods) (A) Dose response of small intestine and colon to DMOG treatment. Epithelial cells were isolated 6 hours after injection and each lane of the Western blot represents a different mouse. (B) Time course of DMOG response of small intestine and colon after an 8mg bolus of DMOG or saline control. (C) Scheme for radioprotection after total abdominal irradiation (TAI), where XRT=18 or 20Gy of TAI as indicated. (D) Regenerating jejunal crypts after 18 Gy (*p=0.0000003 vs saline) or 20Gy (**p= 0.000002, vs saline) by two-tailed t-test. (E) TUNEL+ cells per HPF in the jejunum after 20Gy of radiation. ***p=0.001 vs saline by two-tailed t-test. (F) Kaplan-Meier analysis of mice treated as per (C). n=11/group; p=0.005 by log rank test (G) Scheme for radioprotection after 16Gy total body irradiation (TBI). (H) Kaplan-Meier analysis of mice treated as per (G). n=9 for saline and n=10 for DMOG group; p=0.001 by log rank test.
We assessed if hypoxia played a role in the normal radiation response of the intestines by quantitative PCR for hypoxia-associated genes Glut1 and Pgk1 in the small intestine (Figure S3A and S3B, table S1) and the colon (Figure S3C and S3D, table S1). The expression of hypoxic genes was correlated with detectable levels of HIF1 and HIF2 after radiation alone (Figure S4E), but was 4-fold less than what could be achieved with DMOG (Figure S4E). Interestingly, the intraperitoneal administration of DMOG also increased HIF expression in the liver and kidneys, but not in the lung or peripheral blood (Figure S3F).
While DMOG clearly modulates HIF expression in the gut, it was unknown whether HIF stabilization prior to radiation could reduce mortality after lethal radiation. Towards this end, C57/Bl6 mice were injected with DMOG or saline control prior to and after 20Gy of total abdominal irradiation (TAI), as depicted in Figure 2c. C57/B6 mice have a relatively high radiation tolerance, and 20Gy was found to most reproducibly produce death from GI syndrome in saline treated animals (Figure S4, table S1). Mice were subjected to classical microcolony crypt survival analysis (for representative images, see Figure S5) to determine the survival and regeneration of crypts after radiation (24). DMOG caused a four-fold improvement in crypt survival after 18Gy TAI compared to saline controls (16.2±2.1 vs 4.3±0.9, DMOG vs saline, Figure 2D and table S1) and a twenty-two-fold increase in crypt survival after 20Gy TAI compared to controls (11.0±2.1 vs 0.5±0.5, DMOG vs saline, Figure 2D and table S1). Crypt regeneration was also enhanced in the colon after 20Gy (Figure S6A, table S1). Apoptosis, as determined by TUNEL staining, was decreased in the small intestine (Figure 2E, table S1) and colon (Figure S6B, table S1). There were no differences in γ-H2AX staining (25) between saline and DMOG treated animals, suggesting that PHD inhibition does not alter DNA repair pathways (Fig S7A, quantified in S7B). This improved crypt survival and decreased apoptosis correlated with increased survival, since 67% of mice treated with DMOG survived beyond 60 days after 20Gy of TAI, while none of control mice survived beyond 10 days (Fig. 2F, table S1). Treatment with DMOG also improved mortality after 16 Gy total body irradiation (see scheme in Figure 2G), as 40% of treated mice lived to 30 days while none of the controls survived past 10 days (Figure 2H, table S1).
PHD inhibition mildly increases hematocrit but does not protect tumors
We assessed how DMOG affected hematologic physiology since DMOG is given intraperitoneally and PHD proteins affect the bone marrow niche (18). DMOG mildly improved hematocrit (Figure S8A, table S1), but not hemoglobin levels (Figure S8B, table S1), at 8 days after TAI. Both hematocrit and hemoglobin levels did not change significantly with DMOG after total body irradiation (Figure S8C and S8D, table S1). There were very similar levels of leukopenia and anemia (Table S2), consistent with minimal protection of the bone marrow by DMOG.
Radioprotectors could also have utility in clinical radiotherapy if they could protect normal tissue but not tumors. To determine whether DMOG would also radioprotect tumors, we subcutaneously implanted human colorectal cells (Hct116) or human lung carcinoma cells (A549) into nude mice. The xenografts were grown for 2 weeks before being treated with saline or DMOG for 5 consecutive days concurrent with sham XRT (0Gy × 5) or a clinically relevant course of radiation treatments targeted to the lower abdomen and flank (5Gy × 5, (26)). We find that DMOG treatment did not enhance the growth of HCT116 colorectal derived tumor cells (Fig. S9A, table S1) or A549 cells lung cancer derived cells (Fig. S9B, table S1), as the tumors grew at a similar rate after sham XRT. Importantly, DMOG did not decrease the tumoricidal effect of the 5Gy × 5 radiation treatments when compared to controls (Figs. S9A and B and table S1), indicating that pharmacologic inhibition of PHD proteins does not radioprotect tumors in these xenograft models.
DMOG improves epithelial integrity and GI tract function after radiation
The pathophysiology of the mechanism of radiation-induced gastrointestinal syndrome is tightly linked to the loss of epithelial integrity of the GI tract, which leads to fluid loss, unfettered diarrhea, and electrolyte imbalances, all of which contribute to mortality (3, 27). An effective radioprotectant of the GI tract should thus reduce diarrhea and normalize the amount of formed stool. To determine how prolyl hydroxylase inhibition improved gut physiology after radiation, mice were given saline or DMOG according to the radioprotection protocol depicted previously in Fig. 2C.
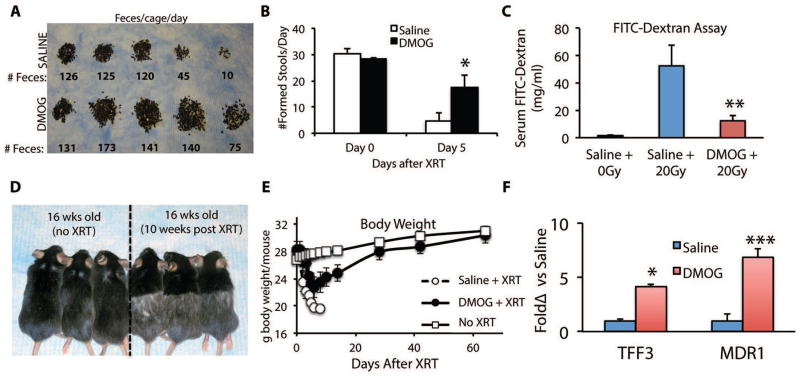
Within three to four days after receiving 20Gy of TAI, mice begin having diarrhea, which was measured as a decrease in formed stool (Figs. S11A and S11B). We quantified formed stool by manually removing droppings from the cage bedding (Figure 3A) and by metabolic cage analysis (Figure 3B, S11A and table S1). In both settings, control animals had almost no formed droppings by Day 5 after radiation, whereas animals in the DMOG cohort exhibited only a 50% decrease in their dropping counts (Fig. 3B, S11A and table S1). These changes were not due to changes in food or water intake, since there were no differences between the saline and DMOG groups (Figs. S11B and S11C, and table S1). Radiation-induced diarrhea led to significant hypernatremia and hyperglycemia in the saline controls, but not the DMOG-treated mice (Table S3). Other electrolytes such as potassium, chloride, bicarbonate, BUN and creatinine were not different between the treated group and controls (Table S3).
Figure 3. DMOG improves epithelial integrity of the lower GI tract.
Mice are treated with DMOG prior to and every day after receiving 20Gy of abdominal XRT (Day 0). (A) ) Dropping counts removed from cage bedding per day from representative cages. (B) Formed stool counts from individual mice in metabolic cages from Day 0 and Day 5 after radiation. n=6 mice per treatment, *p=0.004 Day 5 DMOG vs saline (C) Measurement of epithelial integrity by FITC dextran (4 kD) gavaged at a dose of 0.6mg/kg, five days after treatment per Fig 2C. n=4–6 per group, **p=0.02 vs Saline+20Gy (D) Surviving mice from the DMOG cohort treated with TAI (right) and an age matched control (left). Note the change in fur color in the irradiated lower body. (E) Body weight at the indicated timepoints of mice treated with DMOG/saline and irradiated. Mice were sacrificed if they lost more than 25% of body weight or exhibit any signs of distress. (F) Quantitative PCR for relative mRNA levels of epithelial barrier genes Tff3, and Mdr1 in the jejunum. *p=0.02; **p=0.0006 vs 0 Gy saline, n=6/group.
Death from GI radiotoxicity may also stem from compromised epithelial integrity and barrier functions, which facilitates both electrolyte disturbances and possible parenteral access of enteric pathogens (3). We investigated the epithelial integrity of the GI tract with a FITC-dextran assay, where mice are gavaged with dextran covalently coupled to FITC that cannot cross the GI epithelia unless the epithelial barrier is compromised (28). Four hours after gavage, FITC-dextran levels are measured in the blood. Treatment with DMOG decreased FITC-dextran uptake in the bloodstream of XRT-treated mice by 4-fold over saline treated controls (Fig. 3C and table S1). In contrast, there is almost no uptake in WT mice that did not receive radiation (WT+ 0Gy, Fig 3C, and table S1).
The improved epithelial integrity afforded by DMOG reduced weight loss after radiation (Fig. 3D). Interestingly, the surviving mice from the DMOG cohort gained back most of the weight lost after radiation treatment. By two months after radiation, the DMOG-protected mice that survived 20Gy of ionizing radiation to the abdomen and lower body, were physically indistinguishable in size and weight except for radiation-induced alopecia of the lower body (Figs 3E). These data indicate that the mice treated with DMOG not only survived but had enough functional recovery of their GI tract to display catch up in growth compared to unirradiated age-matched controls.
The expression of intestinal trefoil factor (TFF3/ITF1) and multidrug resistance protein-1 (MDR1) are critical to maintaining epithelial integrity after toxic stimuli (29, 30). DMOG treatment increased TFF3 and MDR1 in the jejunal epithelium by 7- and 3-fold, respectively (Fig. 3F, table S1). Thus, both physiologic and molecular data strongly indicate that increased activation of epithelial barrier function in the small intestine and colon is critical to the radioprotective phenotype.
Enhanced intestinal VEGF expression may play a role in radioprotection
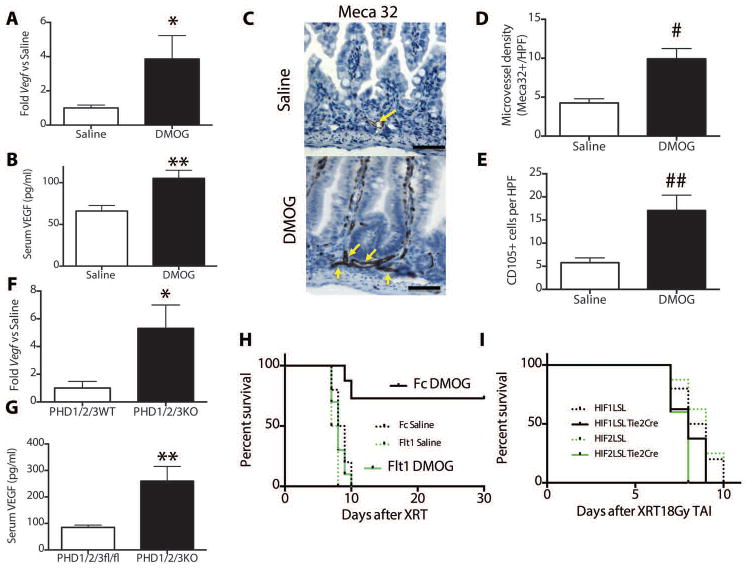
Damage to the gastrointestinal endothelium has also been reported to play a role in the lethality of abdominal radiation since the loss of the vascular endothelium can lead to intestinal ischemia and hypoxia. The endothelial radiation response can be modified by cell signaling mediators such as ceramide (13) or growth factors such as fibroblast growth factor (31) and vascular endothelial growth factor (VEGF (32)). Pharmacologic inhibition of PHD1-3 increased the Vegf expression in isolated epithelia from the jejunum (Figure 4A, table S1) and colon (Figure S12A, table S1) and this correlated with increased serum levels of VEGF (Figure 4B, table S1). DMOG increased microvessel density in the jejunal crypts after radiation as determined by Meca32 immunohistochemistry (Figure 4C and quantified in Figure 4D, table S1). In addition, there were higher levels of CD105+ cells in the intestinal crypts after radiation (Figure 4E, representative images in Fig S12B), which may be an indication of increased angiogenesis (33). Genetic knockout of the PHD1-3 also increased Vegf expression in jejunal epithelia (Figure 4F, table S1) and amounts of VEGF in the serum (Figure 4G, table S1). Serum VEGF was not increased in the other GI-PHD KO animals (Figure S12C).
Fig. 4. Prolyl hydroxylase inhibition increases Vegf.
After treatment with radioprotective protocol per Figure 2c, jejunal epithelium was isolated and assessed by (A) quantitative PCR for relative mRNA levels of Vegf (n=6/treatment, *p=0.0008 vs saline) and (B) Serum VEGF levels measured by ELISA (n=12/treatment, p=0.004 vs saline) (C) Meca32 staining for endothelial cells after 20Gy TAI +/− DMOG. Yellow arrows indicate microvessels. Scale bars=50μm. (D) Microvessel density (# p=0.002 vs 0 Gy saline, n=6 mice per treatment and 4 hpfs per mouse) (E) CD105+ cells per HPF in saline or DMOG controls. (## p=0.0007 vs 0 Gy saline, n=5 mice per treatment and 3 hpfs per mouse) (F) Jejunal Vegf expression (*p=0.005 vs WT, n=6/treatment) and (G) serum VEGF levels (**p=0.006, n=6/treatment) in indicated knockout animals and littermate controls. (H) Kaplan-Meier analysis of mice treated with the indicated adenoviruses and with intraperitoneal saline or DMOG according to Figure 2c. Log rank test showed p=0.002 in Fc DMOG vs Flt1 DMOG. (I) Kaplan-Meier analysis HIF1LSL-Tie2 Cre or HIF2LSL-Tie2 Cre and their littermate controls after 18Gy TAI.
To test the role of VEGF in the radioprotective effects of DMOG, we injected mice with an adenovirus that encodes Flt1 (Ad-Flt1), a soluble VEGF receptor that binds and inhibits the action of VEGF, or Fc (Ad-Fc) controls (34). Five days after treatment with adenovirus, mice were subjected to DMOG treatments and TAI in accordance with the protocol in Figure 2C. The inhibition of VEGF function by Ad-Flt1 eliminated the survival effects of DMOG (Figure 4H, table S1), as DMOG demonstrated a survival advantage in Ad-Fc control animals, but did protect animals treated with Ad-Flt1 (p=0.002, Fc DMOG vs Flt1 DMOG). Saline controls in both groups perished within 10 days. Interestingly, Ad-Flt1 increased hematocrit (Table S4) via known effects on hepatic erythropoieitin production (35), but this had no effect on survival despite a higher hematocrit and hemoglobin (Table S13B and C), suggesting that the prominent effects of Ad-Flt1 on intestinal radiation sensitivity stems from local effects in the gut rather than through modulation of the hematopoietic factors.
Since a pharmacologic PHD inhibitor could potentially affect both epithelial and endothelial cells, we expressed of HIF1 or HIF2 in the endothelial cells to determine if this was sufficient to protect the gut from radiation-induced gastrointestinal syndrome. We used transgenic lox-stop-lox (LSL) mice that expresses either HIF1 of HIF2 only in the presence of a tissue-specific Cre, which removes the stop cassette (36). We bred these LSL mice with Tie2-Cre to create n this case, HIF1LSL Tie2Cre and HIF2LSL Tie2Cre mice which activates either HIF transgene only in endothelial cells (37). Endothelial-specific expression of either HIF1 or HIF2 was not sufficient to protect mice from mortality after 18Gy TAI (Figure 4I, table S1).
HIF2, but not HIF1, mediates the radioprotective effects of PHD inhibition in the GI epithelium
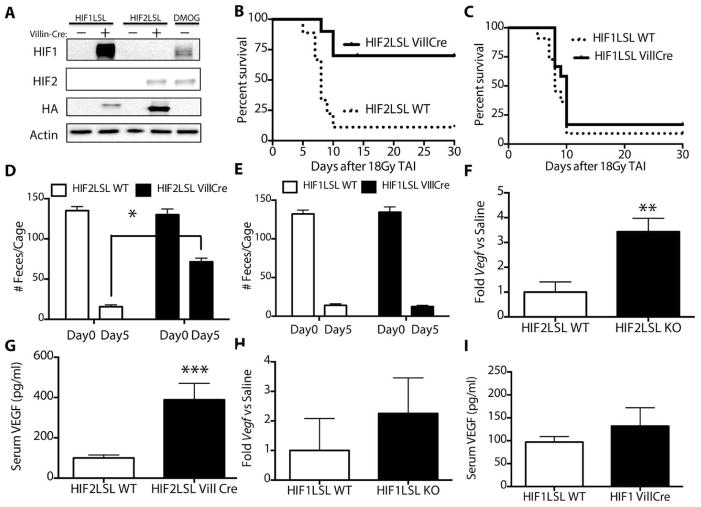
Prolyl hydroxylase inhibition increases levels of both HIF1 and HIF2, but it is unknown whether one or both of these factors were required for radioprotection. To determine whether HIF1 or HIF2 is more important in the radioprotective effect of DMOG in the GI epithelium, we conditionally activated a stabilized HIF1 (HIF1-LSL VillCre) or HIF2 (HIF2-LSL Vill Cre) transgene in the intestinal and colonic epithelia via a lox-stop-lox (LSL) cassette and Villin-Cre (19). We verified expression of HIF1 and HIF2 transgenes by Western blot in the small intestine (Figure 5A) and colon (Figure S14). The expression of HIF1 and HIF2 were comparable to their induction by DMOG treatment (rightmost column in figure 5A).
Fig. 5. Intestinal-specific HIF2 expression is sufficient for lower GI radioprotection and survival.
(A) Western blots from purified epithelial cells from the intestinal tract of the indicated mice and treatments. Kaplan-Meier analysis of mice after 18Gy TAI after intestine-specific overexpression of (B) stabilized HIF2 (n=10/group, p=0.002 by log rank test) or (C) HIF1 (n=11/group, p=0.2 ). Total feces per cage from (D) HIF2-LSLor (E) HIF1-LSL mice on the indicated days after radiation. *p=0.00001 vs Day 5 saline, n=6/group. (F) Jejunal expression of Vegf (*p=0.002, n=6/group) and (G) serum levels of VEGF in HIF2LSL mice bred with Villin-Cre and littermate controls (***p=0.001, n=6/group). (H) Jejunal Vegf expression and (I) serum levels of VEGF HIF1LSL mice bred with Villin-Cre and littermate controls.
HIF1-LSL and HIF2-LSL mice and littermate Cre-negative controls were treated with 18Gy of total abdominal radiation and underwent survival analysis. Mice expressing stabilized HIF2 in the gut had demonstrated improved survival after lethal TAI (Fig. 5B, table S1), while the intestinal-specific overexpression of HIF1 had no effect on survival (Fig. 5C, table S1). Accordingly, HIF2-LSL VillCre mice had a 3-fold improvement of formed stool 5 days after receiving abdominal radiation (Fig. 5D, table S1) while HIF1-LSL VillCre mice showed no changes in formed stools, suggesting that increased epithelial integrity and function contributed to improved survival (Fig. 5E, table S1). HIF2-LSL VillCre mice also showed increased expression of Vegf within the gut epithelia (Figure 5F, table S1) and higher levels of serum VEGF (Figure 5G). Mice with elevated HIF1 in the intestines showed no significant increase in the epithelial Vegf (Figure 5H, table S1) or serum levels of VEGF protein (Figure 5I, table S1)
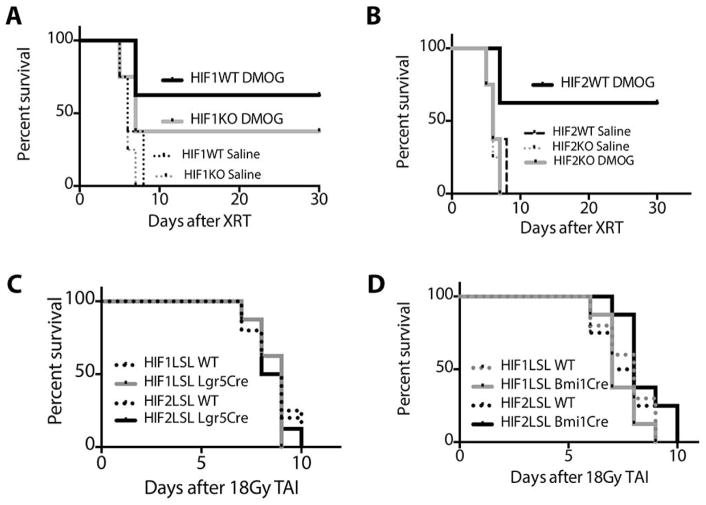
We tested the importance of endogenous HIF1 and HIF2 by generating mice that lacked HIF1 (HIF1KO) or HIF2 (HIF2KO) in the intestines by crossing homozygous HIF1fl/fl or HIF2fl/fl mice with Villin-Cre transgenic animals. These knockout animals and their littermate Cre negative controls (labeled WT) were then treated with saline or DMOG (as in Fig 2C) to determine if either HIF isoform was necessary for radioprotection. Mice lacking HIF1 demonstrated a survival response to DMOG treatment (Figure 6A, table S1), which implied that HIF1 was not required for radioprotection. Though HIF1KO mice treated with DMOG showed a lower survival rate compared to HIF1WT mice treated with DMOG (40% vs 60% at 30days), the difference between these curves were not statistically significant (p=0.7). Conversely, the loss of HIF2 in the intestines nullified the survival effects of DMOG after 18Gy of TAI (Figure 6B), suggesting that HIF2 was necessary to the radioprotection afforded by DMOG. Thus, HIF2 is both necessary and sufficient for the intestinal radioprotection by DMOG, and HIF1 appears to have a minimal role in the radioprotection promoted by PHD inhibition.
Fig. 6. Intestinal HIF2 expression is necessary for radioprotection, but HIF expression limited to the intestinal stem cells is not sufficient for radioprotection.
Kaplan-Meier analysis of (A) HIF1fl/fl (HIF1WT) or HIF1fl/fl-VillCre (HIF1KO) mice (p=0.2 by log rank test between HIF1WT/DMOG and HIF1KO/DMOG, n=8/group) and (B) HIF2fl/fl (HIF2WT) or HIF2fl/fl-VillCre (HIF2KO) mice (p=0.002 by log rank test between HIF2WT/DMOG and HIF2KO/DMOG, n=8/group) treated with either saline or DMOG and (n=8/ group) after 18Gy of abdominal radiation. Both HIF1fl/fl and HIF2fl/fl animals were of a mixed C57/BL/6-FVB background. (C) Kaplan-Meier analysis HIF1LSL-Lgr5 Cre or HIF2LSL-Lgr5 Cre or (D) HIF1LSL-Bmi1 Cre or HIF2LSL-Bmi1 Cre and their littermate controls after 18Gy TAI.
Since Villin-Cre is also expressed in the intestinal stem cell niche, we assessed whether expression of HIF1 or HIF2 in intestinal stem cell populations could recapitulate the effects of expression in all epithelial cells of the gut. We again used the HIF-LSL system and Lgr5-CreERT2 (10) and Bmi1-Cre-ER (38). Lgr5+ cells are the crypt base columnar cells that are interspersed along with Paneth cells throughout the GI tract and are considered a primary stem cell of the GI tract (10). The expression of either HIF1 or HIF2 in Lgr5+ cells, however, was not sufficient to protect mice from lethal TAI (Figure 6C). Bmi1+ cells in the gut have recently been shown to function a reserve pool of intestinal stem cells during intestinal injury (39). Thus, somewhat surprisingly, the expression of HIF1 or HIF2 in the Bmi1+ cells was also not sufficient to protect mice from lethal TAI (Figure 6D).
Animals that survived lethal irradiation live normal lifespans and exhibit minimal morbidity
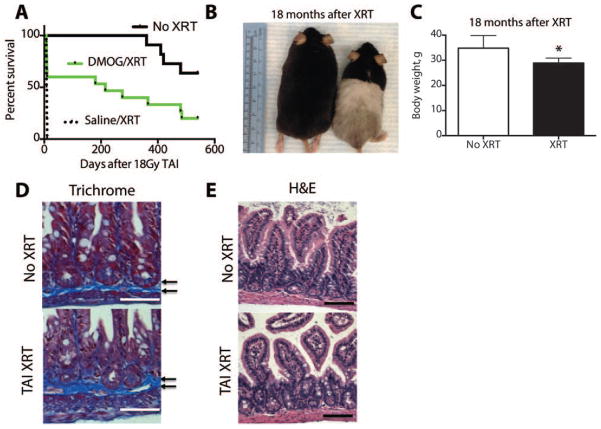
We followed a DMOG-treated cohort of mice that survived 18Gy of total abdominal radiation to determine the levels of chronic morbidity. Kaplan-Meier analysis in Figure 7A revealed that all control mice (10/10) who received saline died within ten days (median survival=8.5 days), while 20% (3/15) of animals who were protected with DMOG lived beyond 20 months of age (median survival=215 days). Sixty-three percent (7/11) of age-matched unirradiated controls lived up to and beyond 20 months (Figure 7A, table S1). Thus, a few mice that would have otherwise perished without DMOG treatment lived a normal lifespan.
Figure 7. Long-term survivors of lethal TAI exhibit lower body weights and microscopic intestinal changes.
(A) Kaplan Meier analysis of mice treated with saline, DMOG or no XRT 18 months after 18Gy TAI. Log rank test showed p=0.01 XRT vs No XRT. (B) Surviving mice from the DMOG cohort treated with TAI (right) and an age matched control (left). Note the change in fur color in the irradiated lower body. (C) Body weight of mice at approximately 20 months of age. (*p=0.02, n=8/group). (D) Trichrome and (E) H&E stains from intestines from mice 18 months after receiving TAI or no radiation. Arrows indicate microscopic fibrotic bands. Scale bars=50μm.
There was surprisingly little morbidity at the age 20 months in the DMOG cohort. The surviving mice appeared smaller (Figure 7B) and weighed less than age-matched unirradiated controls (Figure 7C and Table S1, *p=0.02 vs no XRT), but had no other gross abnormalities other than hypopigmented fur on their lower bodies. Necropsies of sacrificed animals revealed no fistulas, palpable fibrosis or any evidence of malignancy within the abdomen (see example in Figure S15). Microscopic analysis of the gut did not reveal significant fibrosis with trichrome staining (Figure 7D) but did show slightly reduced villi density (Figure 7E). Blood counts revealed that the survivors had a mild anemia, with a hematocrit of 31.0±1.1% in the surviving irradiated animals and 39.7±1.8% in the unirradiated controls (Table S4). This anemia may be partially explained by hypocellularity and fatty marrow replacement in the long bones of the lower body, which were not shielded in TAI (Figure S16). The bone marrow of the humerus exhibited normal morphology, as these limbs were shielded from radiation during TAI (Figure S17).
DMOG mitigates mortality from the GI syndrome
Radioprotectors such as amifostine are effective only when administered before exposure to lethal radiation (40), but not afterwards. On the other hand, a radiation mitigator would reduce the toxicity from radiation when after an exposure, and would thus be useful as a medical countermeasure for a nuclear incident. Currently, there are no FDA-approved radiation mitigators available to treat radiation injury. Towards this end, we tested whether DMOG administered 4 hours after radiation would mitigate mortality from 20Gy of TAI, and found that, indeed, DMOG improved survival versus saline controls (Figure S18A and table S1, 45% vs 0% at 10 days, log rank p=0.002).
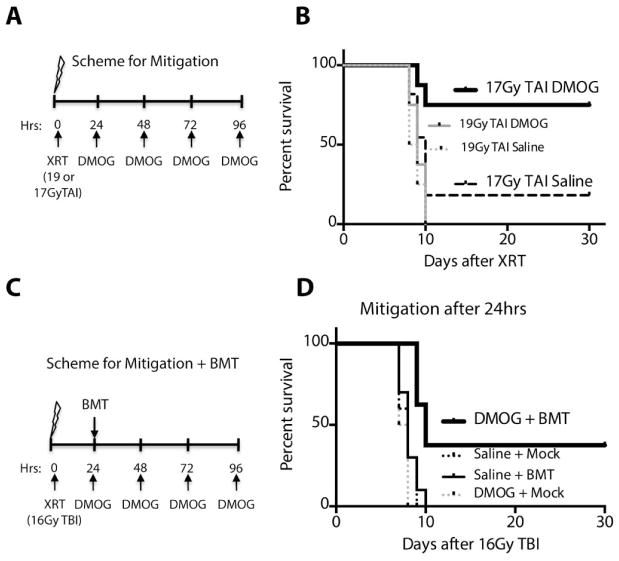
In the event of a potentially lethal radiation exposure, it could take many hours before a patient is diagnosed and properly treated. Thus, we tested whether DMOG could mitigate death from the GI syndrome when administered 24 hours after radiation exposure (40). C57/Bl6 mice were treated with 17 or 19Gy of total abdominal radiation, then the first dose of DMOG or saline was given 24 hours after the radiation exposure as depicted in the scheme in Figure 8A. Kaplan Meier plots shown in Figure 8B reveal that at 19Gy, DMOG exhibits no mitigative properties. At the 17Gy dose, however, DMOG treatment mitigates death from GI syndrome, as 75% of mice (6/8 animals) in the DMOG cohort survived, compared to only 18.2% of the saline cohort (2/11 animals, Figure 8B and table S1, log rank p=0.02).
Figure 8. DMOG mitigates GI radiotoxicity and its effects at high doses require intact bone marrow.
(A) Scheme of radiation mitigation experiments after TAI. (B) Kaplan-Meier analysis of C57/Bl6 mice treated with TAI at the indicated doses then given daily DMOG beginning 24 hours after XRT. (p=0.002 17Gy TAI DMOG vs saline, n=8–11) (C) Scheme of radiation mitigation experiments after TBI and a bone marrow transplant (BMT). (D) Kaplan-Meier analysis of C57/Bl6 mice given saline or DMOG along with a BMT or mock BMT 24 hours after 16Gy TBI. Log-rank analysis shows a p-value of 0.0007 between DMOG + BMT and DMOG + Mock.
To determine if VEGF also played a role in the radiomitigative properties of DMOG, mice were given the VEGF inhibitor Ad-Flt1 or control Ad-Fc five days before receiving 17Gy TAI. DMOG or saline was then injected IP 24 hours after radiation. The inhibition of VEGF function through Ad-Flt1 completely abolished the survival effects of DMOG at this dose (Figure S18B, table S1), indicating that VEGF signaling may also play a role in the mitigative properties of DMOG.
We also tested the ability of DMOG to mitigate GI toxicity 24 hours after total body irradiation (TBI). Since TBI ablates all bone marrow, we also gave a bone marrow transplant at the 24 hours timepoint to reduce hematopoietic death. The scheme for treatment is outlined in Figure 8C. Interestingly, 37.5% of mice (3/8) survived beyond 30 days when given DMOG and a bone marrow transplant 24 hours after radiation (Figure 8D, table S1). DMOG alone without a bone marrow transplant, however, was not sufficient to mitigate death from 16Gy TBI (Figure 8D, table S1).
Discussion
We demonstrate through both genetic and pharmacologic methods that the PHD proteins are critical regulators of radiation sensitivity of the intestinal tract. PHD inhibitors like DMOG have been shown to promote intestinal healing after chemical stresses (41) and sublethal total body irradiation (42), but our study shows that DMOG is capable of robust and reproducible protection against supralethal doses of ionizing radiation to the abdomen or whole body.
We demonstrate that DMOG mitigates radiation injury when given 24 hours after initial exposure to TAI or TBI, which distinguishes prolyl hydroxylase inhibition from many other types of radioprotectors such as free radical scavengers (43), or growth hormones (44) that require administration prior to receiving radiation. These data support the general strategy of PHD inhibition as a potential countermeasure to radiation exposure to mitigate toxicity (45). A caveat to radiomitigation afforded by PHD inhibition is that the protective effects diminish substantially when the drug is given after radiation exposure by TAI, and abrogated completely in lethal TBI exposure, unless a bone marrow transplant is also administered. Treatments with radiomitigative properties in the bone marrow (46) may thus be complementary to radiomitigators of GI toxicity.
The cellular mechanisms of the radiation protection by PHD inhibition are complex, but likely stem from improved epithelial integrity of the GI tract. The increased integrity of the GI tract allows the gut to maintain proper fluid homeostasis and barrier functions, which reduce death by the two most common means: electrolyte disturbances and sepsis (5, 6). By reducing this initial wave of morbidity and mortality, we posit that the intestinal tract and animal are afforded enough time to heal and recover from injury.
The critical role of the epithelial cell in the radiation response of the GI tract is further highlighted by the fact that HIF2 is radioprotective when expressed in all GI epithelial cells. Although endothelial cells (13), Lgr5+ (47), and Bmi1+ (39) cells have been shown to play various roles in intestinal regeneration after radiation injury, the expression of HIF in these specific cell lineages was not sufficient for radioprotection. It is important to note, however, that our data do not rule out these other cell types in the radiation response, but only support the notion that the epithelial cell is the critical to radioprotection by PHD inhibition.
Epithelial VEGF expression likely mediates some the radioprotective effects of PHD inhibition through promoting angiogenesis, and has previously been demonstrated to have powerful radioprotective properties (32).. The growth of new vessels after radiation may further contribute to the improved fluid and nutrient transport after radiation. Although the increased number of CD105+ cells supports angiogenesis, the possibility of reduced endothelial apoptosis or the recruitment of endothelial progenitors cannot be ruled out. Further studies will be needed to understand the precise mechanism involved and whether the endothelium indeed supports the epithelium in recovery after radiation, as has been posited (13), or are merely a marker of HIF activation.
PHD proteins are a pharmacologically tractable target for radioprotection and radiomitigation of toxicity to the GI tract. Pan-PHD inhibition is likely necessary for radioprotection, since no other combination of PHD knockouts showed this effect. Because of their distinct mechanism of action, PHD inhibitors may complement the activity of other published radioprotectors, such as Toll-Like Receptor 5 agonists (48) and anti-ceramide antibodies (14) and may also benefit from being administered along with a radiation mitigator for the hematopoietic system (49).
The major limitation of this preclinical study is that the results are not immediately applicable to human treatment. The amount of radiation to evoke toxicity in a mouse is often more than what is required in human patients (50). Moreover, while DMOG does not radioprotect tumors in a xenograft model, further study is required to determine if PHD inhibition is also protective against conventionally fractionated radiation therapy against spontaneously growing solids tumors. An important point from our study is that experiments are a proof of principle for radioprotection by PHD inhibition, since DMOG may not have the proper pharmacological profile for use in humans (51). With further study and development, however, PHD inhibitors could be rapidly translated to the human use since they are already being developed to treat anemia (52). Such a pharmacologically active PHD inhibitor could conceivably be exploited as a medical countermeasure after a large-scale accidental radiation exposure.
Supplementary Material
Figure S1. Total abdominal radiation (TAI) requires a custom jig.
Figure S2. Single and double PHD knockout combinations are not radioprotective.
Figure S3. Radiation and DMOG both induce HIF in normal tissues.
Figure S4. DMOG radioprotects over a range of doses
Figure S5. Jejunal cross sections are used for crypt counting.
Figure S6. DMOG increased regenerating crypts and decreased apoptosis in the colon after TAI.
Figure S7. γ-H2AX staining in the intestine is not different between saline and DMOG mice after TAI
Figure S8. DMOG mildly increases hematocrit after TAI.
Figure S9. Tumors are not radioprotected by PHD inhibition.
Figure S10. Mouse diarrhea is recognized as loose stools and lack of formed stools.
Figure S11. Metabolic cage measurements quantified complete food and water intake as well as any waste products from an individual mouse.
Figure S12. VEGF and angiogenesis are involved in radioprotection.
Figure S13. VEGF inhibition by Flt1 increases hematocrit.
Figure S14. LSL-Villin Cre animals express HIF in the colon.
Figure S15. Necropsies of long-term survivors reveal no significant abnormalities.
Figure S16. Radiation induced fatty change in tibia bone marrow of long term survivors.
Figure S17. Humerus sections from aged survivors are indistinguishable from age-matched controls.
Figure S18. DMOG mitigates radiation toxicity when administered 4 hours after radiation exposure
Table S1. Original Data (provided as an Excel file)
Table S2. CBC measurements at 5 days after radiation revealed no differences between saline and DMOG treatments.
Table S3. TAI induces hypernatremia and hyperglycemia in saline controls.
Table S4. Long-term survivors after TAI exhibit lower hemoglobin and hematocrit levels compared to unirradiated controls.
Acknowledgments
We would like to acknowledge Kotaru Takeda and Guo-Hua Fong (University of Connecticut) for their generous gift of the PHD1fl/fl, PHD2fl/fl and PHD3fl/fl mice. We also acknowledge Calvin J. Kuo (Stanford) for providing the AdFlt1 and AdFc adenoviruses.
Funding: C.M.T. was supported by RSNA Resident Research Grants 1018 and 1111. C.W. was supported by a training grant from the Canadian Institutes of Health and Research. A.N.D. was supported by a T32 training grant in Comparative Animal Medicine at Stanford University. A.J.G. was supported by grants from NIH CA 67166, 88480, the Silicon Valley Foundation and the Sydney Frank Foundation.
Footnotes
Author contributions: C.M.T., and A.J.G. designed all experiments, wrote and revised the manuscript and shared oversight over this project. C.M.T. performed and analyzed data for most experiments, except as follows: Y.R.M. performed the TUNEL assays, and crypt survival experiments. A.N.D. performed all necropsies and generated crypt survival data. C.W. generated the knockout animals and contributed to design of all animal experiments. C.W. and E.B.R. performed the bone marrow transplant experiments. T.A. and L.X. helped to design the radiation experiments and performed the dosimetry for the mouse jigs.
Competing interests: The authors disclose no competing financial interests.
References
- 1.Singh VK, Ducey EJ, Brown DS, Whitnall MH. A review of radiation countermeasure work ongoing at the Armed Forces Radiobiology Research Institute. International journal of radiation biology. 2012 Apr;88:296. doi: 10.3109/09553002.2012.652726. [DOI] [PubMed] [Google Scholar]
- 2.Dorr H, Meineke V. Acute radiation syndrome caused by accidental radiation exposure - therapeutic principles. BMC medicine. 2011;9:126. doi: 10.1186/1741-7015-9-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Waselenko JK, et al. Medical management of the acute radiation syndrome: recommendations of the Strategic National Stockpile Radiation Working Group. Annals of internal medicine. 2004 Jun 15;140:1037. doi: 10.7326/0003-4819-140-12-200406150-00015. [DOI] [PubMed] [Google Scholar]
- 4.Koenig KL, et al. Medical treatment of radiological casualties: current concepts. Annals of emergency medicine. 2005 Jun;45:643. doi: 10.1016/j.annemergmed.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 5.Gits J, Gerber GB. Electrolyte loss, the main cause of death from the gastrointestinal syndrome? Radiation research. 1973 Jul;55:18. [PubMed] [Google Scholar]
- 6.Jackson WL, Jr, Gallagher C, Myhand RC, Waselenko JK. Medical management of patients with multiple organ dysfunction arising from acute radiation syndrome. BJR supplement / BIR. 2005;27:161. [Google Scholar]
- 7.Hospers GA, Eisenhauer EA, de Vries EG. The sulfhydryl containing compounds WR-2721 and glutathione as radio- and chemoprotective agents. A review, indications for use and prospects. British journal of cancer. 1999 May;80:629. doi: 10.1038/sj.bjc.6690404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Withers HR, Elkind MM. Microcolony survival assay for cells of mouse intestinal mucosa exposed to radiation. Int J Radiat Biol Relat Stud Phys Chem Med. 1970;17:261. doi: 10.1080/09553007014550291. [DOI] [PubMed] [Google Scholar]
- 9.Hendry JH, Potten CS, Roberts NP. The gastrointestinal syndrome and mucosal clonogenic cells: relationships between target cell sensitivities, LD50 and cell survival, and their modification by antibiotics. Radiation research. 1983 Oct;96:100. [PubMed] [Google Scholar]
- 10.Barker N, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007 Oct 25;449:1003. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 11.Sangiorgi E, Capecchi MR. Bmi1 is expressed in vivo in intestinal stem cells. Nature genetics. 2008 Jul;40:915. doi: 10.1038/ng.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tian H, et al. A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature. 2011 Oct 13;478:255. doi: 10.1038/nature10408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paris F, et al. Endothelial apoptosis as the primary lesion initiating intestinal radiation damage in mice. Science. 2001 Jul 13;293:293. doi: 10.1126/science.1060191. [DOI] [PubMed] [Google Scholar]
- 14.Rotolo J, et al. Anti-ceramide antibody prevents the radiation gastrointestinal syndrome in mice. The Journal of clinical investigation. 2012 May 1;122:1786. doi: 10.1172/JCI59920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taylor CT, Colgan SP. Hypoxia and gastrointestinal disease. J Mol Med. 2007 Dec;85:1295. doi: 10.1007/s00109-007-0277-z. [DOI] [PubMed] [Google Scholar]
- 16.Karhausen J, et al. Epithelial hypoxia-inducible factor-1 is protective in murine experimental colitis. The Journal of clinical investigation. 2004 Oct;114:1098. doi: 10.1172/JCI21086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ivan M, et al. HIF-α targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001 Apr 20;292:464. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- 18.Rankin EB, et al. The HIF signaling pathway in osteoblasts directly modulates erythropoiesis through the production of EPO. Cell. 2012 Mar 30;149:63. doi: 10.1016/j.cell.2012.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.el Marjou F, et al. Tissue-specific and inducible Cre-mediated recombination in the gut epithelium. Genesis. 2004 Jul;39:186. doi: 10.1002/gene.20042. [DOI] [PubMed] [Google Scholar]
- 20.Cai WB, Roberts SA, Bowley E, Hendry JH, Potten CS. Differential survival of murine small and large intestinal crypts following ionizing radiation. International journal of radiation biology. 1997 Feb;71:145. doi: 10.1080/095530097144265. [DOI] [PubMed] [Google Scholar]
- 21.Kirsch DG, et al. p53 controls radiation-induced gastrointestinal syndrome in mice independent of apoptosis. Science. 2010 Jan 29;327:593. doi: 10.1126/science.1166202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mason KA, Withers HR, McBride WH, Davis CA, Smathers JB. Comparison of the gastrointestinal syndrome after total-body or total-abdominal irradiation. Radiation research. 1989 Mar;117:480. [PubMed] [Google Scholar]
- 23.Schofield CJ, Ratcliffe PJ. Oxygen sensing by HIF hydroxylases. Nat Rev Mol Cell Biol. 2004 May;5:343. doi: 10.1038/nrm1366. [DOI] [PubMed] [Google Scholar]
- 24.Withers HR, Elkind MM. Radiosensitivity and fractionation response of crypt cells of mouse jejunum. Radiation research. 1969 Jun;38:598. [PubMed] [Google Scholar]
- 25.Redon CE, et al. Histone gammaH2AX and poly(ADP-ribose) as clinical pharmacodynamic biomarkers. Clinical cancer research : an official journal of the American Association for Cancer Research. 2010 Sep 15;16:4532. doi: 10.1158/1078-0432.CCR-10-0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Folkesson J, et al. Swedish Rectal Cancer Trial: long lasting benefits from radiotherapy on survival and local recurrence rate. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005 Aug 20;23:5644. doi: 10.1200/JCO.2005.08.144. [DOI] [PubMed] [Google Scholar]
- 27.Benson AB, 3rd, et al. Recommended guidelines for the treatment of cancer treatment-induced diarrhea. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2004 Jul 15;22:2918. doi: 10.1200/JCO.2004.04.132. [DOI] [PubMed] [Google Scholar]
- 28.Dawson PA, et al. Reduced mucin sulfonation and impaired intestinal barrier function in the hyposulfataemic NaS1 null mouse. Gut. 2009 Jul;58:910. doi: 10.1136/gut.2007.147595. [DOI] [PubMed] [Google Scholar]
- 29.Furuta GT, et al. Hypoxia-inducible factor 1-dependent induction of intestinal trefoil factor protects barrier function during hypoxia. The Journal of experimental medicine. 2001 May 7;193:1027. doi: 10.1084/jem.193.9.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Comerford KM, et al. Hypoxia-inducible factor-1-dependent regulation of the multidrug resistance (MDR1) gene. Cancer research. 2002 Jun 15;62:3387. [PubMed] [Google Scholar]
- 31.Solomon J, Schwarz M. Drug-, toxin-, and radiation therapy-induced eosinophilic pneumonia. Semin Respir Crit Care Med. 2006 Apr;27:192. doi: 10.1055/s-2006-939522. [DOI] [PubMed] [Google Scholar]
- 32.Okunieff P, et al. In vivo radioprotective effects of angiogenic growth factors on the small bowel of C3H mice. Radiation research. 1998 Aug;150:204. [PubMed] [Google Scholar]
- 33.Duff SE, Li C, Garland JM, Kumar S. CD105 is important for angiogenesis: evidence and potential applications. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2003 Jun;17:984. doi: 10.1096/fj.02-0634rev. [DOI] [PubMed] [Google Scholar]
- 34.Lushnikov EF, Stepanenko VF. A pathologist’s radiological culture. Arkhiv patologii. 2008 Jul-Aug;70:58. [PubMed] [Google Scholar]
- 35.Tam BY, et al. VEGF modulates erythropoiesis through regulation of adult hepatic erythropoietin synthesis. Nature medicine. 2006 Jul;12:793. doi: 10.1038/nm1428. [DOI] [PubMed] [Google Scholar]
- 36.Kim WY, et al. Failure to prolyl hydroxylate hypoxia-inducible factor alpha phenocopies VHL inactivation in vivo. EMBO J. 2006 Oct 4;25:4650. doi: 10.1038/sj.emboj.7601300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kisanuki YY, et al. Tie2-Cre transgenic mice: a new model for endothelial cell-lineage analysis in vivo. Developmental biology. 2001 Feb 15;230:230. doi: 10.1006/dbio.2000.0106. [DOI] [PubMed] [Google Scholar]
- 38.Sangiorgi E, Capecchi MR. Bmi1 lineage tracing identifies a self-renewing pancreatic acinar cell subpopulation capable of maintaining pancreatic organ homeostasis. Proceedings of the National Academy of Sciences of the United States of America. 2009 Apr 28;106:7101. doi: 10.1073/pnas.0902508106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yan KS, et al. The intestinal stem cell markers Bmi1 and Lgr5 identify two functionally distinct populations. Proceedings of the National Academy of Sciences of the United States of America. 2012 Jan 10;109:466. doi: 10.1073/pnas.1118857109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brizel DM. Pharmacologic approaches to radiation protection. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2007 Sep 10;25:4084. doi: 10.1200/JCO.2007.11.5816. [DOI] [PubMed] [Google Scholar]
- 41.Cummins EP, et al. The hydroxylase inhibitor dimethyloxalylglycine is protective in a murine model of colitis. Gastroenterology. 2008 Jan;134:156. doi: 10.1053/j.gastro.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 42.Ayrapetov MK, et al. Activation of Hif1alpha by the prolylhydroxylase inhibitor dimethyoxalyglycine decreases radiosensitivity. PloS one. 2011;6:e26064. doi: 10.1371/journal.pone.0026064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weiss JF, Landauer MR. History and development of radiation-protective agents. International journal of radiation biology. 2009 Jul;85:539. doi: 10.1080/09553000902985144. [DOI] [PubMed] [Google Scholar]
- 44.Bhanja P, et al. Protective role of R-spondin1, an intestinal stem cell growth factor, against radiation-induced gastrointestinal syndrome in mice. PloS one. 2009;4:e8014. doi: 10.1371/journal.pone.0008014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Singh VK, Ducey EJ, Brown DS, Whitnall MH. A review of radiation countermeasure work ongoing at the Armed Forces Radiobiology Research Institute. International journal of radiation biology. 2012 Apr;88:296. doi: 10.3109/09553002.2012.652726. [DOI] [PubMed] [Google Scholar]
- 46.Geiger H, et al. Pharmacological targeting of the thrombomodulin-activated protein C pathway mitigates radiation toxicity. Nature medicine. 2012 Jul;18:1123. doi: 10.1038/nm.2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Metcalfe C, Kljavin NM, Ybarra R, de Sauvage FJ. Lgr5(+) stem cells are indispensable for radiation-induced intestinal regeneration. Cell stem cell. 2014 Feb 6;14:149. doi: 10.1016/j.stem.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 48.Burdelya LG, et al. An agonist of toll-like receptor 5 has radioprotective activity in mouse and primate models. Science. 2008 Apr 11;320:226. doi: 10.1126/science.1154986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guinan EC, et al. Bactericidal/permeability-increasing protein (rBPI21) and fluoroquinolone mitigate radiation-induced bone marrow aplasia and death. Science translational medicine. 2011 Nov 23;3:110ra118. doi: 10.1126/scitranslmed.3003126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roderick TH. The Response of Twenty-Seven Inbred Strains of Mice to Daily Doses of Whole-Body X-Irradiation. Radiation research. 1963 Dec;20:631. [PubMed] [Google Scholar]
- 51.Vachal P, et al. 1,3,8-Triazaspiro[4.5]decane-2,4-diones as Efficacious Pan-Inhibitors of Hypoxia-Inducible Factor Prolyl Hydroxylase 1–3 (HIF PHD1-3) for the Treatment of Anemia. Journal of medicinal chemistry. 2012 Feb 27; doi: 10.1021/jm201542d. [DOI] [PubMed] [Google Scholar]
- 52.Higashijima Y, Tanaka T, Nangaku M. Structure-based drug design for hypoxia-inducible factor prolyl-hydroxylase inhibitors and its therapeutic potential for the treatment of erythropoiesis-stimulating agent-resistant anemia: raising expectations for exploratory clinical trials. Expert opinion on drug discovery. 2013 Aug;8:965. doi: 10.1517/17460441.2013.796358. [DOI] [PubMed] [Google Scholar]
- 53.Takeda K, et al. Regulation of adult erythropoiesis by prolyl hydroxylase domain proteins. Blood. 2008 Mar 15;111:3229. doi: 10.1182/blood-2007-09-114561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kiermayer C, Conrad M, Schneider M, Schmidt J, Brielmeier M. Optimization of spatiotemporal gene inactivation in mouse heart by oral application of tamoxifen citrate. Genesis. 2007 Jan;45:11. doi: 10.1002/dvg.20244. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Total abdominal radiation (TAI) requires a custom jig.
Figure S2. Single and double PHD knockout combinations are not radioprotective.
Figure S3. Radiation and DMOG both induce HIF in normal tissues.
Figure S4. DMOG radioprotects over a range of doses
Figure S5. Jejunal cross sections are used for crypt counting.
Figure S6. DMOG increased regenerating crypts and decreased apoptosis in the colon after TAI.
Figure S7. γ-H2AX staining in the intestine is not different between saline and DMOG mice after TAI
Figure S8. DMOG mildly increases hematocrit after TAI.
Figure S9. Tumors are not radioprotected by PHD inhibition.
Figure S10. Mouse diarrhea is recognized as loose stools and lack of formed stools.
Figure S11. Metabolic cage measurements quantified complete food and water intake as well as any waste products from an individual mouse.
Figure S12. VEGF and angiogenesis are involved in radioprotection.
Figure S13. VEGF inhibition by Flt1 increases hematocrit.
Figure S14. LSL-Villin Cre animals express HIF in the colon.
Figure S15. Necropsies of long-term survivors reveal no significant abnormalities.
Figure S16. Radiation induced fatty change in tibia bone marrow of long term survivors.
Figure S17. Humerus sections from aged survivors are indistinguishable from age-matched controls.
Figure S18. DMOG mitigates radiation toxicity when administered 4 hours after radiation exposure
Table S1. Original Data (provided as an Excel file)
Table S2. CBC measurements at 5 days after radiation revealed no differences between saline and DMOG treatments.
Table S3. TAI induces hypernatremia and hyperglycemia in saline controls.
Table S4. Long-term survivors after TAI exhibit lower hemoglobin and hematocrit levels compared to unirradiated controls.